Abstract
Aim
Using the antiCEA antibody MN14, a LS174T mouse tumor model has been successfully targeted with 99mTc for imaging and 188Re for radiotherapy by a MORF/cMORF pretargeting strategy. This investigation evaluated the antiTAG-72 antibody CC49 as an alternative to MN14 for this application.
Methods
Both CC49 and MN14 were labeled with 111In via SCN-benzyl-DTPA and their biodistributions were compared to that of MN14 labeled via DTPA anhydride. Since the accessibility of the antibody to the effector is required for optimization of pretargeting, the internalization of both MORF-CC49 and MORF-MN14 antibodies in LS174T cells were evaluated in culture. In addition, the accessible concentration of MORF-CC49 antibody in tumor was determined in a series of pretargeting studies with escalating dosages of the 99mTc-cMORF effector. Finally, using these results and our semi-empirical model, an imaging study was performed under optimal pretargeting conditions.
Results
The biodistribution of 111In to trace the MN14 antibody depended significantly on the labeling method. Furthermore, both MORF-CC49 and MORF-MN14 antibodies showed rapid internalization in culture. Fortunately, the accessibility in tumor was found to be less seriously reduced in vivo. In a pretargeting study under optimal conditions, both by imaging and by necropsy, the 99mTc-cMORF effector accumulated predominantly in the tumor of pretargeted mice. Normal tissue accumulations were minimal except in kidneys, liver, and a segment of intestines.
Conclusion
MORF pretargeting with CC49 was equally successful in the LS174T tumor model to the MORF pretargeting with MN14. The MORF-CC49 antibody may therefore be considered for future investigations toward early clinical trials.
Keywords: Tumor, Drug delivery systems, Antibodies, Pretargeting, Radioimmunotargeting
INTRODUCTION
A pretargeting strategy under development uses a pair of phosphorodiamidate morpholino oligomers (MORF/cMORF) for recognition between the pretargeting antibody and the radiolabeled effector administered later. This approach may be superior to alternative pretargeting strategies in that the recognition pair may be varied in chain length and base sequence to optimize binding affinity and pharmacokinetics (1, 2). For example, the base sequence of the radiolabeled cMORF now in use provides decreased kidney levels compared to earlier sequences (3). Using this cMORF effector and the MORF conjugated antiCEA MN14 antibody, both scintigraphic imaging with 99mTc (4) and radiotherapy with 188Re (5) in the LS174T colon tumor mouse model have been reported.
The translation of MORF pretargeting into the clinic may require antibodies directed against different antigens in addition to CEA. For example, the TAG-72 antigen has been reported to be over-expressed on a wide variety of tumor types (6, 7) including the LS174T tumor (8, 9). The CC49 is a second generation antiTAG-72 antibody (10) with higher affinity and tumor accumulation in comparison to the first generation B72.3 antibody (11). The CC49 antibody has been used in humans (12–18) and may be considered a suitable candidate for future clinical applications of MORF pretargeting. This report describes a preclinical study of MORF pretargeting using MORF-CC49 in place of MORF-MN14.
To estimate the optimum dosage of radiolabeled cMORF effector (the dosage just sufficient to saturate this accessible tumor level of MORF), it is necessary to determine accurately the accessibility of the MORF-antibody in tumor for the cMORF effector in pretargeted animals (20). In past studies (19, 20), we have assumed that 111In-DTPA-MN14 serves as an accurate tracer of MORF-MN14 and reported agreement between the accessible MORF-MN14 and the 111In-DTPA-MN14. However, as is shown below, the reliability of using 111In labeled antibody to trace the MORF-antibody as a general approach is questionable. As an alternative and intuitively more accurate method of measuring the accessible MORF levels in tumor, the saturating dosage of 99mTc-cMORF in animals pretargeted with MORF-CC49 was determined by dose escalation.
MATERIALS AND METHODS
As before, both MORF and its complement cMORF were obtained from GeneTools (Philomath, OR) with a primary amine on the 3′-equivalent end. The base sequences were 5′-TCTTCTACTTCACAACTA-linker-amine (MORF) and 5′-TAGTTGTGAAGTAGAAGA-linker-amine (cMORF). The murine anti-CEA antibody MN14 was a gift from Immunomedics (Morris Plains, NJ). The murine antiTAG-72 antibody CC49 was prepared by Strategic Biosolutions (Ramona, CA) from the CC49 murine hybridoma cell line, a gift from Dr Jeff Schlom (Laboratory of Tumor Immunology and Biology, Center for Cancer Research, NCI, NIH, Bethesda, MD). The p-SCN-Benzyl-DTPA was from Macrocyclics (Dallas, TX). The Hydralink kit for the conjugation of MORF to antibody was from Solulink (San Diego, California). The Sephadex G100 resin was from Pharmacia Biotech (Uppsala, Sweden). The 99Mo-99mTc generator and the 111InCl3 were from Perkin Elmer Life Science Inc (Boston, MA). All other chemicals were reagent grade and used without purification.
Preparation of 99mTc-cMORF, MORF-antibodies, and 111In-antibodies
For 111In labeling, the CC49 and MN14 were first conjugated with the bifunctional agent p-SCN-Benzyl-DTPA and, after purification, mixed with 111InCl3 in an acetate buffer (21).
The cMORF was conjugated with MAG3 as previously described (22) and stored at −20 °C. For labeling, a vial was returned to room temperature, 10 μL of 99mTc generator eluate was added to a combined solution of 10 μL (0.3 μg/μL) MAG3-cMORF, 4 μL (50 μg/μL) sodium tartrate 2H2O in a pH 9.2 buffer (0.5 M Na2HCO3, 0.25 M NH4OAc, 0.175 M NH3), and 1.5 μL (4 μg/μL) SnCl2·2H2O in 10 mM HCl-sodium ascorbate. After heating at boiling water temperature for 20 min, the labeling efficiency by size-exclusion HPLC was over 95%.
The antibody was conjugated with MORF using a commercial Hydralink approach (23) in which both CC49 and MN14 were conjugated with succinimidyl 4-hydrazinonicotinate acetone hydrazone (SANH) and the MORF was conjugated with succinimidyl 4-formylbenzoate (SFB). After purification, the hydrazine-modified antibody and the benzaldehyde-modified MORF were combined to form a hydrazone link between the antibody and MORF. Purification of the MORF-antibodies from the free MORF was achieved on a 1.0×50 cm Sephadex G-100 glass Econo-column and the average number of MORFs per antibody (gpm) was determined as described previously (3).
Biodistributions of 111In labeled antibodies
All animal studies were approved by the UMMS Institutional Animal Care and Use Committee. For tumor implantation, 106 LS174T cells were injected into the left thigh of each Swiss NIH nude mouse (Taconic Farms, Germantown, NY). The animals were used 12–13 days later when the tumors were about 0.5 g. Four tumored mice each received intravenously 20 μg (20–30 μCi) of 111In-labeled DTPA-benzyl-CC49 or DTPA-benzyl-MN14. After 48 h, the mice were killed by exsanguination via heart puncture under halothane anesthesia. At necropsy, samples of blood and other organs were removed, weighed, and counted in a NaI(Tl) well counter (Cobra II automatic gamma counter, Packard Instrument Company, CT) along with a standard of the injectate. Blood and muscle were assumed to constitute 7 % and 40 % of body weight respectively. Since the LS174T tumor is difficult to separate from normal tissues, the entire tumor thigh was first excised and as much skin, muscle and bone as possible were removed before counting. Since the radioactivity levels in the normal tissues were negligible, the counts within the tumor samples were attributed to the tumor. After counting, the residual skin, muscle and bone were surgically removed for each tumor sample weighed and the tumor weight determined by subtraction.
Internalization of MORF-conjugated antibodies
The selection of the CC49 antibody for this investigation was partially based on reports that antiTAG-72 antibodies are unlikely to internalize (8, 9). After conjugation with MORF, the internalization property of the CC49 and the MN14 antibodies was examined in LS174T cells using a method described previously (24).
Specifically, about 0.5 ×106 LS174T cells were seeded in each well of 7 twelve-well plates and were used after 1.5–2 days when the cells reached a confluency of greater than 75%. As described previously (24), 150 μL (about 2 μCi) of the 99mTc-cMORF/MORF-antibody complex and 1 mL of PBS/0.5%BSA at 37 °C was added to each well in the direct-targeting group, while 150 μL of MORF-antibody and 1 mL of PBS/0.5%BSA at 37 °C was added to each well of the pretargeting group. After a designated time of incubation at 37 °C, the same amount of 99mTc-cMORF in 10 μL was added to each well of the pretargeting group. Five min later, the cell-associated radioactivity and the radioactivity in the media for both groups were separated and counted. Samples were measured in triplicate for each time-point of each group. The cell-associated radioactivity in the direct-targeting group measured the total antibody accumulation (both internalized and on the surface), while the cell-associated radioactivity in the pretargeting group measured only the antibody on the surface.
Determination of the accessible level of MORF in tumor
Seventeen tumored mice each received intravenously 30 μg of MORF-CC49 (gpm = 0.68) on the 11th day and a dosage of 99mTc-cMORF varying between 1 and 6 μg on the 13th day. The mice were killed for biodistribution 3 h thereafter. At this time, the tumors weights averaged 0.36 ± 0.10 g.
Tumor imaging
As detailed in Appendix, the above measurements were used to estimate the optimum dosage of cMORF effector, the dosage that just saturates the accessible MORF in tumor. A timing schedule identical to that used previously with MN14 could again be used since the CC49 is also an IgG and therefore with similar pharmacokinetics and the since the same cMORF effector was to be used. A single mouse weighing 27 g with a 0.85 g LS174T tumor was administered 53.9 μg MORF-CC49 (gpm = 1.12) 48 h before the injection of 6.3 μg (3.8 mCi) of 99mTc-cMORF and was imaged 3 h later.
Images were obtained on a pinhole HiSPECT system (Bioscan, Washington DC) installed on a three-head PRISM-3000 (25). The mouse was anesthetized by intraperitoneal injection of ketamine-xylazine and placed on a flat Lucite extension clamped to the headrest of the gamma camera bed. The mouse was in the axis of a circular orbit formed by the rotation of three six-pinhole apertures with a radius of 3.7 cm. The radius choice was dictated by our experience in positioning the mouse for convenient data requisition. The acquisition process required 20 min for 36 intervals through 360 degrees resulting in 30 projections. The projection data were reconstructed using commercial software also from Bioscan.
RESULTS
Biodistributions of 111In labeled antibodies
Table 1 lists the biodistributions at 48 h in LS174T tumored mice of CC49 and MN14 antibodies labeled with 111In via DTPA-benzyl group along with historical results for the MN14 antibody radiolabeled with 111In using DTPA (4). As shown, the CC49 accumulated at statistically identical levels (p > 0.05) to those of the MN14 in all normal tissues except for tumor, blood, liver, and spleen. The accumulations in tumor and blood were lower while the accumulations in liver and spleen were higher. When the 111In levels between the MN14 antibody labeled by DTPA and by benzyl-DTPA are compared, the liver, kidney, spleen, tumor, and blood are statistically different (p < 0.05). This was surprising since 111In-DTPA-MN14 accurately traced accessible MORF-MN14 (4). Nevertheless, it appears that 111In as an antibody tracer should be used with caution.
Table 1.
Biodistributions (%ID/g) at 48 h in LS174T tumored mice of 111In-labeled DTPA-benzyl-CC49 and DTPA-Benzyl-MN14. Historical results for 111In-DTPA-MN14 are presented for comparison (4). (Mean ± SD, N=4)
| Organ | 111In-DTPA-Benzyl-CC49 | 111In- DTPA-Benzyl-MN14 | 111In-DTPA-MN14 |
|---|---|---|---|
| Liver | 13.0±1.2 | 6.13±1.29 | 13.0±1.2 |
| Heart | 1.41±0.26 | 1.57±0.29 | 1.37±0.12 |
| Kidney | 5.21±0.54 | 6.00±0.60 | 11.8±0.5 |
| Lung | 2.36±0.32 | 2.59±0.66 | 1.58±0.15 |
| Spleen | 6.38±0.84 | 3.86±1.17 | 5.56±0.63 |
| Muscle | 0.56±0.12 | 0.70±0.14 | 0.51±0.06 |
| Tumor | 21.0±4.7 | 29.0±5.9 | 10.1±0.8 |
| Salivary | 1.64±0.27 | 1.83±0.21 | ----- |
| Blood | 4.02±0.76 | 5.44±1.29 | 1.65±0.08 |
| Tumor size (g) | 0.85±0.27 | 0.90±0.22 | 0.88±0.03 |
Internalization of MORF conjugated antibodies
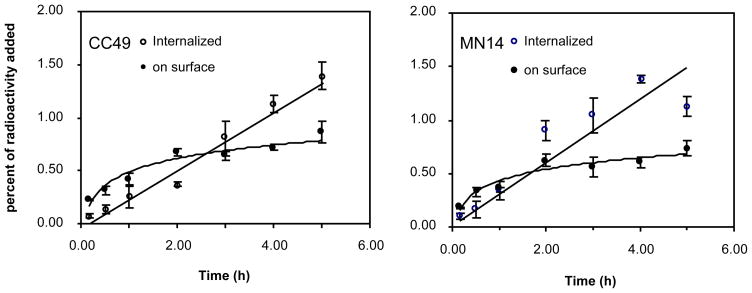
As shown in Fig 1, because of the small number of cells, the radioactivity accumulation in cell culture was below 2.5% within the observation period for either antibody. However, the internalized fraction of cell-associated radioactivity was surprisingly high, reaching approximately 60% at 5 h. A similar degree of internalization in vivo would seriously reduce the accessibility of the MORF-antibody in tumor for the labeled cMORF, an effector with limited ability to cross cell membrane (25). It was therefore necessary to measure the accessible level of MORF-antibody in vivo.
Fig 1.
The percent of the radiolabeled antibodies added to LS174T cells in culture that remained on the cell surface (solid circle) and that became internalized (open circle) over the measurement period, presented separately for the MORF-CC49 antibody (left panel) and the MORF-MN14 antibody (right panel). The solid lines represent the best fit to both curves. The error bars signify one SD of the mean, N =3.
Determination of the accessible level of MORF in tumor
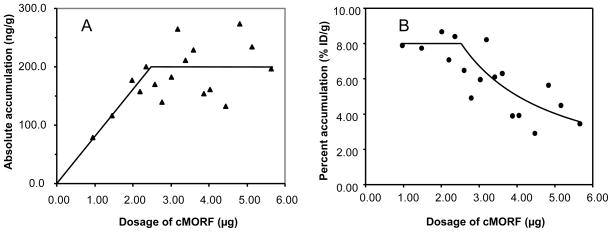
The biodistributions in % ID/g of the labeled cMORF at different dosages in mice pretargeted with 30 μg of MORF-CC49 (gpm = 0.68) 48 h earlier are presented in Table 2. The tumor accumulations from the table are plotted in Fig 2, in both absolute accumulation (ng/g) (panel A) and percent accumulation (%ID/g) (panel B) of labeled cMORF. The accessible level of MORF-antibody in tumor can be determined from these data. As shown in Fig 2A, and in agreement with our semiempirical model (4, 20, 21), the absolute tumor accumulation of effector increases linearly until the MORF-CC49 in tumor becomes saturated at about 2.5 μg and becomes constant thereafter. By averaging the absolute accumulation data in tumor above the dosage of 3.00 μg, the accessible level can be estimated from the graph to be 202 ± 47 ng MORF per gram of tumor as shown by the horizontal line in Fig 2A. The slope before saturation corresponds to the maximum percent tumor accumulation of the effector (MPTA). For tumors that averaged 0.36 g in this study, the MPTA is predicted to be about 8 %ID/g (20) in agreement with the horizontal line at 8 %ID/g in Fig 2B.
Table 2.
Biodistributions in %ID/g of 99mTc-cMORF in tumor and normal organs at 3 h when administered intravenously at different dosages in mice pretargeted with 30 μg MORF-CC49 48 h earlier
| Dosage (μg) | tumor size (g) | %ID/g | ID/organ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor | Blood | Kidney | Liver | Spleen | Lung | Heart | Muscle | Salivary | Stomach | Small intestines | Large intestines | ||
| 0.97 | 0.47 | 7.89 | 3.55 | 3.33 | 1.24 | 0.71 | 1.18 | 0.80 | 0.26 | 0.76 | 0.20 | 0.50 | 2.10 |
| 1.48 | 0.42 | 7.74 | 2.32 | 3.38 | 0.91 | 0.63 | 0.85 | 0.56 | 0.21 | 0.54 | 0.09 | 0.40 | 2.03 |
| 2.01 | 0.29 | 8.66 | 2.06 | 3.44 | 0.84 | 0.49 | 0.70 | 0.43 | 0.18 | 0.50 | 0.09 | 0.35 | 2.34 |
| 2.20 | 0.49 | 7.08 | 1.60 | 4.05 | 0.67 | 0.47 | 0.57 | 0.33 | 0.18 | 0.45 | 0.90 | 1.35 | 2.14 |
| 2.36 | 0.36 | 8.40 | 1.78 | 2.94 | 0.60 | 0.35 | 0.54 | 0.37 | 0.19 | 0.33 | 0.14 | 0.22 | 2.13 |
| 2.60 | 0.46 | 6.48 | 1.91 | 4.49 | 0.76 | 0.44 | 0.90 | 0.37 | 0.18 | 0.53 | 0.22 | 0.51 | 3.00 |
| 2.79 | 0.51 | 4.92 | 1.39 | 3.54 | 0.62 | 0.44 | 0.63 | 0.37 | 0.15 | 0.28 | 0.18 | 0.95 | 2.89 |
| 3.03 | 0.35 | 5.96 | 1.38 | 3.51 | 0.68 | 0.38 | 0.59 | 0.31 | 0.27 | 0.35 | 0.45 | 1.29 | 2.07 |
| 3.19 | 0.25 | 8.22 | 1.76 | 4.56 | 0.62 | 0.38 | 0.58 | 0.32 | 0.15 | 0.34 | 0.15 | 0.46 | 2.10 |
| 3.41 | 0.22 | 6.10 | 1.59 | 2.89 | 0.60 | 0.29 | 0.58 | 0.31 | 0.15 | 0.32 | 0.09 | 0.32 | 1.39 |
| 3.61 | 0.32 | 6.30 | 1.25 | 4.25 | 0.64 | 0.31 | 0.46 | 0.22 | 0.13 | 0.37 | 0.11 | 0.61 | 2.59 |
| 3.88 | 0.44 | 3.90 | 1.05 | 3.00 | 0.51 | 0.28 | 0.38 | 0.22 | 0.09 | 0.28 | 0.23 | 0.29 | 2.39 |
| 4.05 | 0.34 | 3.93 | 0.88 | 3.77 | 0.43 | 0.19 | 0.40 | 0.22 | 0.10 | 0.25 | 0.10 | 0.27 | 1.95 |
| 4.47 | 0.38 | 2.91 | 0.94 | 5.24 | 0.48 | 0.27 | 0.40 | 0.20 | 0.09 | 0.24 | 0.45 | 1.28 | 1.94 |
| 4.83 | 0.37 | 5.64 | 0.84 | 4.19 | 0.46 | 0.34 | 0.35 | 0.19 | 0.11 | 0.19 | 1.74 | 2.16 | 2.75 |
| 5.16 | 0.31 | 4.49 | 1.01 | 4.59 | 0.57 | 0.33 | 0.43 | 0.28 | 0.16 | 0.32 | 0.12 | 1.15 | 2.79 |
| 5.66 | 0.17 | 3.45 | 1.00 | 2.69 | 0.41 | 0.26 | 0.33 | 0.20 | 0.09 | 0.23 | 1.13 | 1.55 | 2.84 |
Fig 2.
The absolute tumor accumulation (panel A) and percent tumor accumulation (panel B) in mice pretargeted with MORF-CC49 vs. the dosage of labeled cMORF, presented individually for each animal. The solid line in both panels was generated by the semiempirical model (see text).
The optimal dosage of effector can be established from these data. Based on the semiempirical model, both the MPTA and the maximum tumor to organ ratios will be obtained when the effector dosage is just equal to that necessary to saturate the accessible MORF levels in tumor, in this case 2.5 μg. Beyond this point, while the absolute accumulation remains constant, the percent tumor accumulation decreases as the excess labeled cMORF delivered to tumor cannot be retained. The decreasing line in Fig 2B is calculated from the absolute accessible level of 202 ng/g [%ID/g = (202*100)/(dosage (μg)*1000)] and as shown is in good agreement with experiment.
Tumor imaging by pretargeting
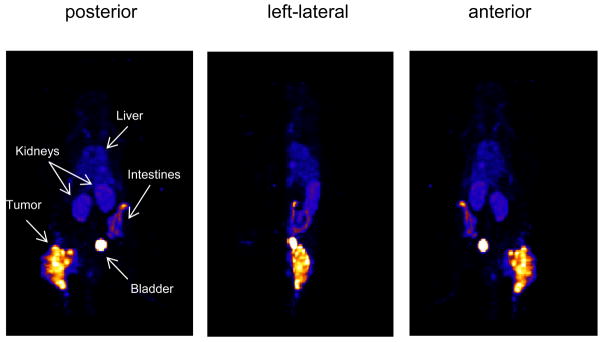
Over 3 h, the whole body radioactivity decreased from 3.8 to 0.5 mCi due to excretion and decay. The dosage of the labeled cMORF (6.3 μg) in this imaging study was estimated for a mouse bearing a tumor of 0.85 g. Three projections of the 3D HiSPECT image are presented in Fig 3. Except for bladder, only tumor, kidney, and a segment of intestine are evident. Levels of radioactivity in other normal organs were confirmed at necropsy to be less than 0.60 %ID/g. The tumor accumulation in this mouse was measured to be 6.67 %ID/g at necropsy, slightly higher than the predicted 5.6 %ID/g but within its ± 2SD range (20). The radioactivity distribution in tumor is obviously inhomogeneous.
Fig 3.
The posterior, left-lateral, and anterior projections of the 3D HiSPECT image obtained 3 h post administration of 99mTc labeled cMORF to a mouse bearing a LS174T tumor and pretargeted 48 h earlier with MORF-CC49.
DISCUSSION
This investigation was conducted to evaluate the antiTAG-72 antibody CC49 as an alternative to the anti-CEA MN14 antibody for future pretargeting studies in part because the TAG-72 antigen, like the CEA antigen, is also over expressed in many tumors (6, 7) and in part because the CC49 antibody has also been extensively used in preclinical studies of streptavidin/biotin pretargeting (26–28).
Since pretargeting antibodies should ideally not internalize, the internalization of MORF-CC49 was investigated along with the MORF-MN14. As shown in Fig 1, about 60% of cell-associated radioactivity for both antibodies was internalized within 5 h in cell culture. However, since both antibodies have been used successfully in pretargeting studies, it may be assumed that internalization occurs to a lesser degree in vivo. In fact, the accessible level measured with the radiolabeled cMORF effector in this study was 25 %ID/g in LS174T tumors of 0.36g (see appendix) and in an unpublished study we found that the total concentration of 111In-DTPA-benzyl-CC49 in the same tumor of about the same size and at the same time was about 36 %ID/g. The small difference between these two values requires that internalization in vivo must have been modest.
The difference in biodistribution of 111In when labeled to MN14 via different linkers (Table 1) is consistent with another recent result from this laboratory (21). Both results suggest that a 111In-antibody may not necessarily trace the MORF-antibody accurately. When coupled with the concerns regarding internalization, in this investigation we considered a novel dosage-escalating method for estimating the accessible tumor level of the antibody.
There are considerable similarities between the pretargeting results of the CC49 and the MN14 antibodies. Under optimal pretargeting conditions, the blood levels of labeled cMORF were approximately the same at 1.7 %ID/g with CC49 (derived from Table 2) and 1.5 %ID/g with MN14 (20). When conjugated with MORF, both antibodies internalize in LS174T cells in culture but less seriously in vivo. The accessible levels of MORF-CC49 at 48 h (25.0 and 14.6 %ID/g for LS174T tumors of 0.36 g and 0.85 g) were comparable to those of MORF-MN14 (17.5 and 8.7 %ID/g for tumors of 0.53 g and 1.00 g) (20). Finally, the normal organ-to-blood ratios of the accessible MORF-MN14 were previously shown to be constant and independent of the dosage ratios of cMORF to MORF-MN14 for the dosages used (20). Examination of the results in Table 2 indicates this is also true for the MORF-CC49 antibody.
CONCLUSION
The pretargeting results using the antiTAG-72 CC49 antibody were similar to those using the antiCEA MN14 antibody. Under optimal conditions, the levels of labeled cMORF in circulation, in tumor, and in normal tissues were comparable in LS174T tumored mice pretargeted with either antibody. The CC49 antibody may therefore be considered as an alternative to the MN14 antibody for MORF/cMORF pretargeting studies in animals and, possible in future clinical pretargeting applications.
Acknowledgments
Financial support: The National Institute of Health (CA94994 and CA107360).
The authors are grateful to Immunomedics, Morris Plains, NJ for providing the MN14 antibody and to Dr Jeffery Schlom (Laboratory of Tumor Immunology and Biology, Center for Cancer Research, NCI, NIH, Bethesda, MD) for providing the CC49 hybridoma. Financial support was from the National Institute of Health (CA94994 and CA107360).
APPENDIX
The dosage of the cMORF effector in the imaging study was estimated based on the measured accessibility, our semiempirical prediction model, and our experience. During imaging, the tumor size, confirmed at sacrifice, was estimated as 0.85 g using a caliper, and therefore larger than the average size of 0.36 g in the dosage escalation study. Since tumor size influences both the percent tumor accumulation of antibody and the MPTA of labeled cMORF, it will influence the optimal dosage of the labeled cMORF. To estimate the optimal dosage for the imaging study, we assumed that the percent tumor accumulation of the accessible MORF-antibody will increase in proportion to the percent tumor accumulation of 111In-DTPA-antibody, independent of tumor size. Thus:
For a tumor of 0.36 g, the %ID/g accessible MORF-CC49 may be calculated to be 25.0 %ID/g from the absolute tumor accessible level of 202 ng/g obtained in this study (= (202 * MCC49 *100)/(1000*McMORF*30*gpm)). The %ID/g 111In-DTPA-benzyl-CC49 in tumors of 0.36g was estimated as 36.0 %ID/g from a previous study in mice (data not presented). For the 0.85 g tumor, the %ID/g 111In-DTPA-benzyl-CC49 is 21.0 %ID/g as shown in Table 1. The %ID/g accessible MORF-CC49 for the 0.85 g tumor may therefore be estimated as 14.6 %ID/g. The optimal dosage of cMORF in the imaging study can be calculated from this value by the following equation, where D is dosage and M is molecular weight.
The MPTA of labeled cMORF for a tumor of 0.85 g is predicted to be 5.6 %ID/g (20), the MORF-CC49 dosage was 53.9 μg and the gpm was 1.12, the molecular weights are 6331 (cMORF) and 160,000 (CC49). Therefore, the optimal dosage of cMORF was calculated to be 6.23 μg and 6.3 μg of 99mTc-cMORF was administered.
Footnotes
Presentation at congress: This paper was presented as part of the oral presentation entitled “Tumor accumulation in mice of the CC49 antibody under different conditions for MORF/cMORF pretargeting” at the SNM meeting in New Orleans, Indiana, USA on June-14–18, 2008,.
References
- 1.Liu G, Zhang S, He J, et al. The influence of chain length and base sequence on the pharmacokinetic behavior of 99mTc-morpholinos in mice. Quarterly J Nucl Med. 2002;46:233–43. [PubMed] [Google Scholar]
- 2.Liu G, He J, Zhang S, et al. Cytosine residues influence kidney accumulations of 99mTc-labeled morpholino oligomers. Antisense Nucleic Acid Drug Dev. 2002;12:393–8. doi: 10.1089/108729002321082465. [DOI] [PubMed] [Google Scholar]
- 3.Liu G, He J, Dou S, et al. Pretargeting in tumored mice with radiolabeled morpholino oligomer showing low kidney uptake. Eur J Nucl Med Mol Imaging. 2004;31:417–24. doi: 10.1007/s00259-003-1393-9. [DOI] [PubMed] [Google Scholar]
- 4.Liu G, He J, Dou S, Gupta S, Rusckowski M, Hnatowich DJ. Further investigations of morpholino pretargeting in mice--establishing quantitative relations in tumor. Eur J Nucl Med Mol Imaging. 2005;32:1115–23. doi: 10.1007/s00259-005-1853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu G, Dou S, Mardirossian G, et al. Successful radiotherapy of tumor in pretargeted mice by 188Re-radiolabeled phosphorodiamidate morpholino oligomer, a synthetic DNA analogue. Clin Cancer Res. 2006;12:4958–64. doi: 10.1158/1078-0432.CCR-06-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loy TS, Nashelsky MB. Reactivity of B72.3 with adenocarcinomas. An immunohistochemical study of 476 cases. Cancer. 1993;72:2495–8. doi: 10.1002/1097-0142(19931015)72:8<2495::aid-cncr2820720830>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Thor A, Ohuchi N, Szpak CA, Johnston WW, Schlom J. Distribution of oncofetal antigen tumor-associated glycoprotein-72 defined by monoclonal antibody B72.3. Cancer Res. 1986;46:3118–24. [PubMed] [Google Scholar]
- 8.Starling JJ, Maciak KJ, Law KL, et al. In vivo antitumor activity of a monoclonal antibody-Vinca alkaloid immunoconjugate directed against a solid tumor membrane antigen characterized by hetemgeneous expression and noninternalization of antibody antigen complexes. Cancer Res. 1991;51:2965–72. [PubMed] [Google Scholar]
- 9.Starling JJ, Law KL, Hinson NA. Internalization studies using a panel of three monoclonal antibodies (MAbs) directed against the human adenocarcinoma-associated antigen, TAG-72. Antibody Immunoconj Radiopharm. 1992;5:403–11. [Google Scholar]
- 10.Gallinger S, Reilly RM, Kirsh JC, et al. Comparative dual label study of first and second generation antitumor-associated glycoprotein-72 monoclonal antibodies in colorectal cancer patients. Cancer Res. 1993;53:271–8. [PubMed] [Google Scholar]
- 11.Johnson VG, Schlom J, Paterson AJ, Bennett J, Magnani JL, Colcher D. Analysis of a humantumor-assodatedglycoprotein(TAG-72) identified by monoclonal antibody B72.3. Cancer Res. 1986;46:850–7. [PubMed] [Google Scholar]
- 12.Forero A, Meredith RF, Khazaeli MB, et al. Phase I study of 90Y-CC49 monoclonal antibody therapy in patients with advanced non-small cell lung cancer: effect of chelating agents and paclitaxel co-administration. Cancer Biother Radiopharm. 2005;20:467–78. doi: 10.1089/cbr.2005.20.467. [DOI] [PubMed] [Google Scholar]
- 13.Shen S, Forero A, LoBuglio AF, et al. Patient-specific dosimetry of pretargeted radioimmunotherapy using CC49 fusion protein in patients with gastrointestinal malignancies. J Nucl Med. 2005;46:642–51. [PubMed] [Google Scholar]
- 14.Robert F, Busby EM, LoBuglio AF. Chemotherapy tolerance after radioimmunotherapy with 90Y-CC49 monoclonal antibody in patients with advanced non-small cell lung cancer: clinical effects and hematologic toxicity. Cancer Biother Radiopharm. 2003;18:317–25. doi: 10.1089/108497803322285071. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez RD, Huh WK, Khazaeli MB, et al. A Phase I study of combined modality 90Yttrium-CC49 intraperitoneal radioimmunotherapy for ovarian cancer. Clin Cancer Res. 2002;8:2806–11. [PubMed] [Google Scholar]
- 16.Tempero M, Leichner P, Baranowska-Kortylewicz J, et al. High-dose therapy with 90Yttrium-labeled monoclonal antibody CC49: a phase I trial. Clin Cancer Res. 2000;6:3095–102. [PubMed] [Google Scholar]
- 17.Meredith RF, Khazaeli MB, Macey DJ, et al. Phase II study of interferon-enhanced 131I-labeled high affinity CC49 monoclonal antibody therapy in patients with metastatic prostate cancer. Clin Cancer Res. 1999;5(10 Suppl):3254s–58s. [PubMed] [Google Scholar]
- 18.Alvarez RD, Partridge EE, Khazaeli MB, et al. Intraperitoneal radioimmunotherapy of ovarian cancer with 177Lu-CC49: a phase I/II study. Gynecol Oncol. 1997;65:94–101. doi: 10.1006/gyno.1996.4577. [DOI] [PubMed] [Google Scholar]
- 19.Liu G, Dou S, Rusckowski M, Hnatowich DJ. An experimental and theoretical evaluation of the influence of pretargeting antibody on the tumor accumulation of effector. Mol Cancer Ther. 2008:5. doi: 10.1158/1535-7163.MCT-07-2203. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu G, Dou S, He J, Liu X, Rusckowski M, Hnatowich DJ. Predicting the biodistribution of radiolabeled cMORF effector in MORF-pretargeted mice. Eur J Nucl Med Mol Imaging. 2007;34:237–46. doi: 10.1007/s00259-006-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu G, Dou S, Pretorius PH, Liu X, Rusckowski M, Hnatowich DJ. Pretargeting CWR22 prostate tumor in mice with MORF-B72.3 antibody and radiolabeled cMORF. Eur J Nucl Med Mol Imaging. 2008;35:272–80. doi: 10.1007/s00259-007-0606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu G, Dou S, He J, et al. Radiolabeling of MAG3-morpholino oligomers with 188Re at high labeling efficiency and specific radioactivity for tumor pretargeting. Appl Radiat Isot. 2006;64:971–8. doi: 10.1016/j.apradiso.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He J, Liu G, Dou S, Gupta S, Rusckowski M, Hnatowich DJ. An improved method for covalently conjugating morpholino oligomers to antitumor antibodies. Bioconjug Chem. 2007;18:983–8. doi: 10.1021/bc060208v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu G, Dou S, Yin D, et al. A novel pretargeting method for measuring antibody internalization in tumor cells. Cancer Biother Radiopharm. 2007;22:33–9. doi: 10.1089/cbr.2006.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schramm NU, Ebel G, Engeland U, Schurrat T, Behe M, Behr TM. High-Resolution SPECT using multi-pinhole collimation. IEEE Trans Nucl Sci. 2003;50:315–20. [Google Scholar]
- 26.Ngai WM, Reilly RM, Polihronis J, Shpitz B. In vitro and in vivo evaluation of streptavidin immunoconjugates of the second generation TAG-72 monoclonal antibody CC49. Nucl Med Biol. 1995;22:77–86. doi: 10.1016/0969-8051(94)e0070-y. [DOI] [PubMed] [Google Scholar]
- 27.Pavlinkova G, Batra SK, Colcher D, Booth BJ, Baranowska-Kortylewicz J. Constructs of biotin mimetic peptide with CC49 single-chain Fv designed for tumor pretargeting. Peptides. 2003;24:353–62. doi: 10.1016/s0196-9781(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 28.Forster GJ, Santos EB, Smith-Jones PM, Zanzonico P, Larson SM. Pretargeted radioimmunotherapy with a single-chain antibody/streptavidin construct and radiolabeled DOTA-biotin: strategies for reduction of the renal dose. J Nucl Med. 2006;47:140–9. [PubMed] [Google Scholar]