Abstract
In the Tropics, there is substantial temporal and spatial overlap of diseases propagated by anthropophilic mosquito vectors (such as malaria and dengue) and human helminth diseases (such as onchocerciasis and lymphatic filariasis) that are treated though mass drug administrations (MDA). This overlap will result in mosquito vectors imbibing significant quantities of these drugs when they blood feed on humans. Since many anthelmintic drugs have broad anti-invertebrate effects, the possibility of combined helminth control and mosquito-borne disease control through MDA is apparent. It has been previously shown that ivermectin can reduce mosquito survivorship when administered in a blood meal, but more detailed examinations are needed if MDA is to ever be developed into a tool for malaria or dengue control. We examined concentrations of drugs that follow human pharmacokinetics after MDA and that matched with mosquito feeding times, for effects against the anthropophilic mosquito vectors Anopheles gambiae s.s. and Aedes aegypti. Ivermectin was the only human-approved MDA drug we tested that affected mosquito survivorship, and only An. gambiae s.s. were affected at concentrations respecting human pharmacokinetics at indicated doses. Ivermectin also delayed An. gambiae s.s. re-feeding frequency and defecation rates, and two successive ivermectin-spiked blood meals following human pharmacokinetic concentrations compounded mortality effects compared to controls. These findings suggest that ivermectin MDA in Africa may be used to decrease malaria transmission if MDAs were administered more frequently. Such a strategy would broaden the current scope of polyparasitism control already afforded by MDAs, and which is needed in many African villages simultaneously burdened by many parasitic diseases.
Keywords: Anopheles gambiae, malaria, Aedes aegypti, dengue, ivermectin, mass drug administration
1. Introduction
Mass drug administrations (MDAs) of human populations are performed worldwide with several different anthelmintics to control numerous parasitic diseases. The African Program for Onchocerciasis Control (APOC) and the Onchocerciasis Elimination Program for the Americas both rely on once or twice yearly MDA of ivermectin (IVM) for the control of onchocerciasis in Africa and Latin America, respectively (Hotez, 2007). The Global Program for the Elimination of Lymphatic Filariasis distributes various combinations and amounts of IVM, diethylcarbamazine (DEC), or albendazole (ALB) via MDA for the control of lymphatic filariasis worldwide (Ottesen et al., 2008). Pyrantel (PYL) has been distributed by MDA for the control of hookworms, roundworm and whipworm (Reddy et al., 2007).
There is substantial geographic and temporal overlap of helminth-derived neglected tropical diseases (NTDs) and mosquito-borne parasitic and viral diseases such as malaria, dengue, and yellow fever (Brooker et al., 2007). There is a call for an integrated approach to controlling some of these diseases (Hotez et al., 2006). Many of the Anopheles vectors of human Plasmodium spp., particularly those in sub-Saharan Africa such as Anopheles gambiae s.s., exhibit frequent and anthropophilic blood feeding preferences (Beier, 1996; Dia et al., 2003; Scott et al., 2006). Aedes aegypti, the primary vector of dengue viruses (Flaviviridae, Flavivirus: DENV) and yellow fever virus (Flaviviridae, Flavivirus: YFV), also feeds frequently and almost exclusively on humans (De Benedictis et al., 2003; Edman et al., 1992; Scott et al., 2000). Due to these anthropophilic feeding preferences and the spatial overlap where MDAs are performed, these mosquito vectors will ingest varying concentrations of IVM, PYL, ALB or DEC when they blood feed on treated humans. While these drugs vary in their molecular targets, all show specific activity against invertebrate helminths (Jones and Sattelle, 2008), and often affect orthologous targets in blood feeding vectors (Bloomquist, 2003; Mounsey et al., 2007). Previous studies have determined that IVM (and possibly DEC) can reduce the survivorship of various laboratory-raised mosquito species (Cartel et al., 1991; Foley et al., 2000; Fritz et al., 2009; Jones et al., 1992; Pampiglioni et al., 1985; Tesh and Guzman, 1990). Furthermore, Bockarie et al. (Bockarie et al., 1999) demonstrated that IVM MDA for the control of Wuchereria bancrofti reduced the survivorship of wild, blood fed Anopheles punctulatus in Papua New Guinea.
The daily probability of mosquito survival (p) is the most influential variable in mosquito vectorial capacity (V) (Black and Moore, 2005; Garrett-Jones, 1964). If IVM, PYL, ALB or DEC reduce mosquito longevity and thereby reduce the daily probability of mosquito survival, then MDA of humans will reduce the vectorial capacity of mosquitoes. The second most influential variable in the vectorial capacity equation is the daily probability of a mosquito feeding on a human (a) which is a factor of the mosquito host preference index and feeding frequency. If ingestion of IVM, PYL, ALB or DEC in a blood meal leads to a delay in re-feeding frequency, then the delay would further reduce the vectorial capacity of anthropophilic mosquitoes for pathogen transmission.
In the present study, An. gambiae s.s. and Ae. aegypti were chosen for experiments based upon their anthropophilic blood feeding tendencies, importance as disease vectors, and occurrence in regions of the world where IVM, DEC, PYL, and ALB are administered by MDA. IVM, DEC, PYL, and the primary metabolite of ALB, albendazole sulfoxide, (ALB SOx) (Mathew and Kalyanasundaram, 2007) were examined in this study because they are already approved for human use and are administered throughout the world by MDA (Hotez, 2007; Ottesen et al., 2008; Reddy et al., 2007). The drugs were serially diluted and mixed with human blood that was imbibed from artificial membrane feeders by laboratory-reared An. gambiae s.s. and Ae. aegypti. The concentrations at which these four drugs could affect mosquito survivorship and re-blood feeding frequency were determined, with particular respect to the drug concentrations found in human blood following MDAs (Elkassaby, 1991; Fasanmade et al., 1994; Shenoy et al., 2002).
2. Materials and methods
2.1. Mosquitoes
Anopheles gambiae s.s. G3 strain (origin The Gambia) were raised at 28º–31ºC, 80% relative humidity, and a 14:10 light: dark cycle. Larvae were raised on a diet of ground Tetramin® fish food. Adults were provided water and 10% sucrose solution ad libitum. Aedes aegypti Rexville D strain (origin Puerto Rico) were raised at 26º–28ºC, 75% relative humidity, and a 14:10 light: dark cycle. Larvae were raised on a diet of ground Tetramin® fish food mixed with ground mouse food. Adults were provided water and 10% sucrose solution or raisins ad libitum.
2.2. Drugs
Powdered formulations of IVM, DEC, and PYL (pyrantel pamoate) were obtained from Sigma Aldrich (St. Louis, USA). Powdered ALB SOx was obtained from WITEGA (Berlin, Germany). Powdered formulations of each drug were diluted in dimethylsulfoxide (DMSO) to concentrations of 10 mg/ml and aliquots were frozen at −20ºC.
2.3. In vitro blood feeds
Human blood was drawn into 3.2% sodium citrate tubes by a phlebotomist at the Colorado State University Hartshorn health clinic. Previous experiments showed this blood preparation did not affect mosquito survivorship (data not shown). Human blood, not more than two weeks post-drawn, was mixed with various concentrations of drugs. Drugs frozen in DMSO were thawed and serially diluted in phosphate buffered saline prior to addition to blood meals. Ten μl of varied concentrations of drug in PBS were added to 990 μl of blood to reach the final concentrations that were offered to mosquitoes. Control mosquitoes were fed 990 μl of blood with 10 μl of PBS alone. A priori experiments showed that DMSO:blood at ratio ≤ 1:100 did not affect mosquito survivorship, re-feeding or physiologic behavior (data not shown), and all drug concentrations tested in mosquitoes had DMSO:blood ratios ≤ 1×10−4:100. Blood meals were pipetted into glass bell feeders (Lillie Glass Feeders; Smyrna, GA, USA) sealed with hog sausage casing and warmed to 37 ºC with a circulating-heating water pump.
2.4. LC50 determination
Multiple concentrations of drugs were fed to mosquitoes between 2–8 days post-emergence to determine the lethal concentration that killed 50% of the mosquitoes (LC50). After blood feeding, mosquitoes were chilled in a refrigerator, sorted on ice-chilled Petri dishes, and only fully engorged mosquitoes were retained for survivorship analysis. Mosquitoes were held for five days post blood feed in four L cages with access to water and raisins as a sugar source. A priori experiments demonstrated that ingested drugs affected mosquito survivorship within four days post ingestion. Survivorship was monitored every twenty-four hours and dead mosquitoes were removed from the cage at each time point. Wing lengths of a subset of mosquitoes were measured to determine if adult mosquito size (Lounibos et al., 1995) affected susceptibility to the drugs.
A non-linear mixed model with probit analysis was used to calculate LC50s due to ~20% mortality rate in control An. gambiae s.s. This non-linear mixed model not only corrected for background control mortality but it also assessed replicate effects. The model is fully described in the Supporting Text.
2.5. Effects of drugs on mosquito re-feeding frequency and defecation
Age matched adult mosquitoes were taken from 55 liter rearing bins and moved into four liter cages and held with access to raisins and water until two or eight days post emergence. In vitro blood meals containing diluted drug in human blood were offered to these mosquitoes at two or eight days post emergence. After blood feeding ten fully engorged female mosquitoes from the control and experimental groups were aspirated and placed individually into 50 ml tubes. The remaining fully engorged female mosquitoes were held in four liter cages with access to sugar and water sources to assess survival rates (see below). The 50 ml tubes were covered with organdy. Mosquitoes were held without access to sugar for the remainder of the experiment and a wet cotton ball was placed at the bottom of each 50 ml tube to maintain humidity and provide access to water. Individually held mosquitoes were offered a human arm laid on top of the tubes for five minutes, at 24 hour intervals following their initial blood feed. The same person who donated blood for the in vitro feed, fed the mosquitoes in subsequent re-feedings. Human subjects research training was received by the CSU Intitutional Review Board for these experiments and these experiments were approved under CSU-IRB protocol 09-1148H. Following the daily chance to re-blood feed, mosquitoes were immediately scored for survival, defecation and re-feeding. The experiment ended for each mosquito when it had either re-blood fed or died. Three independent replicates were performed for each drug concentration fed to the mosquitoes. All mosquitoes in these experiments had their wing lengths measured at the end of the experiment. Concentrations of DEC, PYL and ALB SOx were offered to mosquitoes at the maximum concentrations found in human plasma after ingestion of standard clinical dose regimens: DEC = 6mg/kg dose, maximum plasma level = 2100 ng/ml; PYL = 750 mg, maximum plasma level = 50 ng/ml; ALB = 400 mg, maximum plasma level of ALB SOx = 700 ng/ml (Fasanmade et al., 1994; Shenoy et al., 2002). Maximal concentrations of DEC, PYL, and ALB SOx failed to change the re-feeding frequency of An. gambiae s.s. or Ae. aegypti (data not shown) and so lower concentrations of the drugs were not investigated. Re-feeding experiments were conducted using IVM concentrations based on the average time of mosquito host seeking for each species and where this matched with the pharmacokinetic curve of IVM in human blood after ingestion of a single oral dose of 150 μg/kg of IVM as measured by Elkassaby (Elkassaby, 1991) (see results).
2.6. Effect of mosquito age on IVM susceptibility
The remaining fully engorged mosquitoes that were not used for the re-blood feeding frequency experiments were maintained in groups in four liter cages with access to water and raisins. Daily mortality was recorded over the course of five days as described above in order to determine if mosquito age altered IVM susceptibility. All mosquitoes in these experiments had their wing lengths measured as a correlate for adult size to determine if adult size affected mosquito susceptibility to IVM.
2.7. Effects of multiple IVM blood meals on mosquito survivorship
To measure the effects of cumulative IVM exposures in a blood meal, two day post emergence adult An. gambiae s.s. were offered two consecutive blood meals, three days apart. The blood contained IVM concentrations that were predicted based on average mosquito feeding times matched to the pharmacokinetic curve of IVM in humans following a single oral dose of 150 μg/kg of IVM. Immediately following their first blood meal, mosquitoes were sorted, and only fully engorged females were retained in a four liter cage, without sugar, but with access to water. The second blood meal was offered to these mosquitoes three days later, control mosquitoes for each group were given only blood containing 10 μl of PBS. Following their second blood meal, individual fully engorged mosquitoes were placed in 50 ml tubes and maintained with access to water, but not a sugar source, until they died. Mortality was checked daily. Experiments with groups receiving primary blood meals containing 11.26 ng/ml and 6.58 ng/ml IVM were replicated twice and the data were pooled. Due to the high death rate and inhibited re-blood feeding frequency of mosquitoes that ingested a primary blood meal that contained 26.21 ng/ml of IVM, this experiment was performed only once.
2.8. Survival analysis
Survival analysis was performed on the re-feeding frequency experiments, time to first defecation and the consecutive blood feeding experiments. In the former two experiments, mosquitoes that died instead of re-blood feeding or depositing feces over the course of the experiment were censored data (up-ticks marked on each graph line). Replicates were pooled and analyzed by the Logrank Test (Mantel-Haenszel method; proportional hazards model) and the hazard ratio with 95% confidence intervals. All survival analysis data was analyzed in Prism (GraphPad Software, Inc.)
3. Results
3.1. LC50 determination
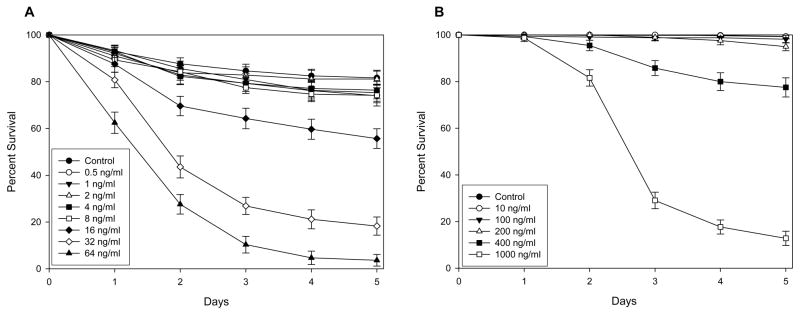
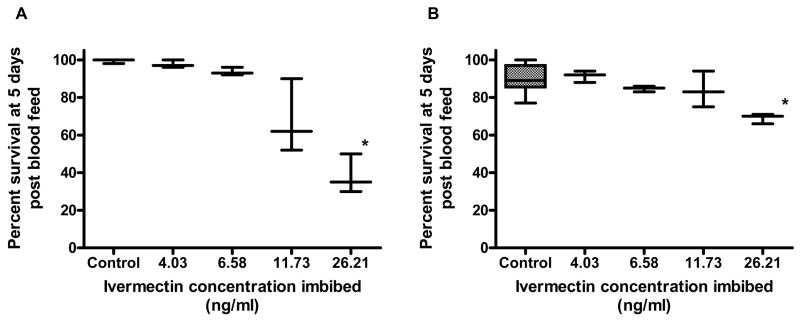
Ivermectin reduced the survivorship of both An. gambiae s.s. and Ae. aegypti (Fig. 1). The LC50 of IVM fed to An. gambiae s.s. was calculated as LC50 22.4 ng/ml [18.0, 26.9] (n = 2013, 8 replicates), while the LC50 of IVM fed to Ae. aegypti was calculated as 601.3 ng/ml [506.6, 712.9] (n = 1669, 3 replicates). PYL, ALB SOx, or DEC did not reduce the survivorship of An. gambiae s.s. or Ae. aegypti (supplementary figures S1–S3). Due to the lack of effect on mosquito survivorship for these drugs, LC50 values could not be calculated. The combination of IVM and ALB SOx also did not reduce survivorship of An. gambiae s.s. when compared IVM alone (supplementary figure S4). Adult size of An. gambiae s.s. did not alter susceptibility to IVM (P = 0.5935, n = 307).
Figure 1. Percent survivorship of mosquitoes that imbibed ivermectin (A) An. gambiae s.s. (B) Ae. aegypti.
An. gambiae s.s. and Ae. aegypti were fed varied concentrations of IVM to determine if IVM reduced mosquito survivorship. IVM reduced the survivorship of both An. gambiae s.s. (LC50 22.4 ng/ml [18.0, 26.9]) and Ae. aegypti (LC50 601.3 ng/ml [506.6, 712.9]).
3.2. Modeling IVM pharmacokinetics with mosquito biting times
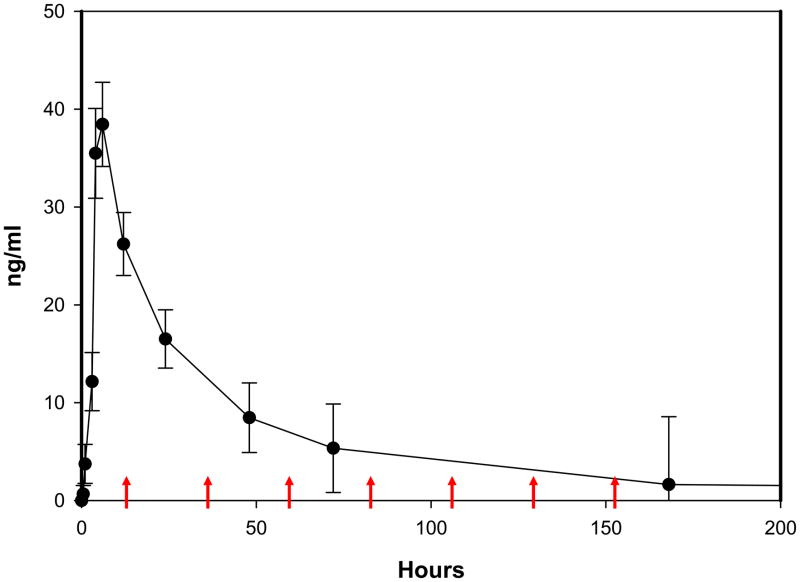
To predict the effects of IVM against mosquitoes in the field, mosquitoes were offered blood meals with concentrations of IVM that would be predicted during normal mosquito blood feeding times following MDA. Elkassaby (Elkassaby, 1991) measured plasma levels of IVM over time in ten Sudanese adults infected with Onchocerca volvulus after ingestion of a single standard oral dose (150 μg/kg). Mean plasma concentrations of ivermectin from Elkassaby (Elkassaby, 1991) were plotted and these data were used to estimate the concentration of IVM found in human blood post IVM MDA. IVM concentrations from these data were selected based on a typical noontime MDA in the field (personal observations by MS, BDF and KCK from APOC directed MDA to villages in Southeastern Senegal). The LC50 of Ae. aegypti was more than 10 fold the mean maximal concentration (~46 ng/ml) of that can be expected in human blood following the standard oral IVM dose (Elkassaby, 1991) therefore Ae. aegypti were not further evaluated. For An. gambiae s.s., peak biting frequency is at approximately 24:00 hrs (Mathenge et al., 2001), which corresponds to 12 hrs. post-MDA when the mean plasma concentration is 26.21 ng/ml (Figure 2). The decay portion of the pharmacokinetic curve was then fitted by non-linear regression to the Two-phase Exponential decay equation (Y=Span1*exp[−K1*X] + Span2*exp[−K2*X] + Plateau) (Goodness of Fit, R2 = 0.9999) and subsequent concentrations of IVM given to An. gambiae s.s. were interpolated at 24 hr time points thereafter from this curve (36 hrs. = 11.73 ng/ml, 60 hrs. = 6.58 ng/ml, 84 hrs. = 4.03 ng/ml, 108 hrs. = 2.75 ng/ml, 132 hrs. = 2.1 ng/ml).
Figure 2. The pharmacokinetic curve of IVM with mosquito blood feeding times and interpolated IVM doses.
The IVM pharmacokinetic curve was constructed by plotting mean data points (± SEM) from data previously published by Elkassaby (Elkassaby, 1991). IVM MDA was modeled to begin at 12:00 hrs. (Time = 0 hrs.). Mean mosquito feeding times were then matched to the curve and used to interpolate doses imbibed at these same times by fitting the decay portion of the pharmacokinetic curve to the Two-phase Exponential Decay model with non-linear regression (Goodness of Fit R2 = 0.9999). Modeled times of An. gambiae s.s. blood feeding are designated by the red arrows (12 hrs. = 26.21 ng/ml, 36 hrs. = 11.73 ng/ml, 60 hrs. = 6.58 ng/ml, 84 hrs. = 4.03 ng/ml, 108 hrs. = 2.75 ng/ml, 132 hrs. = 2.1 ng/ml, 156 hrs. = 1.78 ng/ml).
3.3. Effects of drugs on mosquito re-feeding frequency and defecation
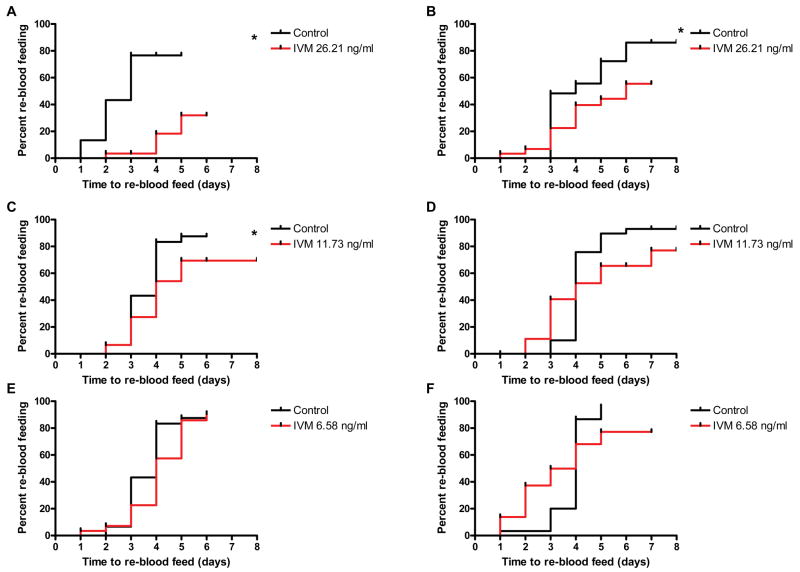
Re-blood feeding was delayed in 2 day post emergence An. gambiae s.s. that ingested a 26.21 ng/ml IVM concentration (P < 0.0001, Hazard ratio = 7.656 [3.487, 18.63]) and a 11.73 ng/ml IVM concentration (P = 0.0499, Hazard ratio = 1.603 [1, 4.459]), but not the 6.58 ng/ml concentration (P = 0.1536). Re-blood feeding was delayed in 8 day post emergence An. gambiae s.s. that ingested the 26.21 ng/ml IVM concentration (P = 0.0513, Hazard ratio = 1.784 [0.9956, 4.825]) but not the 11.73 ng/ml concentration (P = 0.4684, Hazard ratio = 1.19 [0.6247, 2.782]), nor the 6.58 ng/ml concentration (P = 0.7231) (Fig. 3). Maximum plasma concentrations of PYL (50 ng/ml), ALB SOx (700 ng/ml) and DEC (2100 ng/ml) did not delay the re-blood feeding frequency of An. gambiae s.s. regardless of age (data not shown).
Figure 3. Re-blood feeding frequency of An. gambiae s.s. after a primary blood meal that contained IVM.
Re-blood feeding frequency was assessed for 2 days post emergence (left panels - A, C & E) and 8 days post emergence (right panels - B, D & F) An. gambiae s.s. that initially fed on 26.21 ng/ml (A & B), 11.73 ng/ml (C & D), and 6.58 ng/ml (E & F) IVM concentrations. Panels in which mosquitoes exhibited significantly altered re-feeding frequencies compared to controls are marked with an asterisk (Logrank Test).
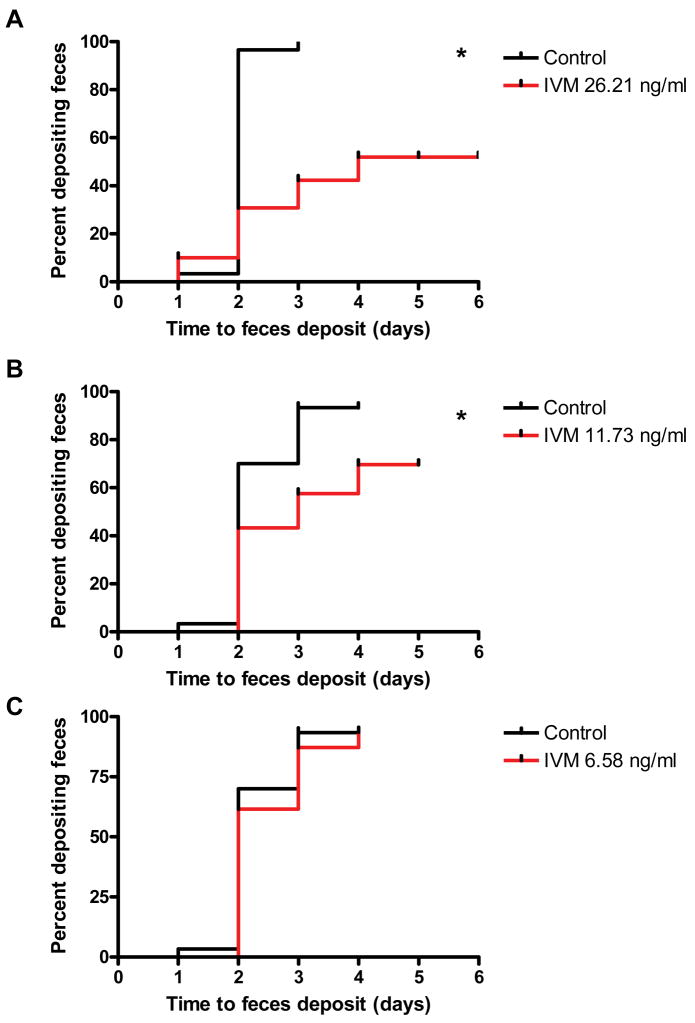
In these same experiments, time to first defecation was also recorded. Time to defecation following the initial blood meal was delayed for 2 day post-emergence An. gambiae s.s. that ingested 26.21 ng/ml IVM (P < 0.0001, Hazard ratio = 2.62 [3.679, 23.78]) and 11.73 ng/ml IVM (P = 0.0028, Hazard ratio = 1.836 [1.541, 8.059]), but not the 6.58 ng/ml concentration (P = 0.4337, Hazard ratio = 1.141 [0.5771, 3.6]) (Fig. 4). However, none of the 8 day post-emergence An. gambiae s.s. exhibited delayed defecation compared to controls (data not shown).
Figure 4. Time to defecation of 2 day post emergence An. gambiae s.s. following a primary blood meal that contained IVM.
Time to defecation was assessed for 2 days post emergence An. gambiae s.s. that initially fed on 26.21 ng/ml (A), 11.73 ng/ml (B), and 6.58 ng/ml (C) IVM concentrations. Panels in which mosquitoes exhibited significantly altered defecation times compared to controls are marked with an asterisk (Logrank Test).
3.4. Effect of mosquito age on IVM susceptibility
For An. gambiae s.s., significant differences were found in the median survival of the 2 day post-emergence mosquitoes blood fed on the different concentrations of IVM (Kruskal Wallis test statistic = 12.97, P = 0.0114), but only the IVM 26.21 ng/ml group was significantly different from controls (Dunn s Multiple Comparison post-test, difference in rank sum = 11.33, P < 0.01). Similar results were obtained with the 8 day post-emergence An. gambiae s.s., whereby significant differences were found in the median survival between groups (Kruskal Wallis test statistic = 10.21, P = 0.037), but again, only the IVM 26.21 ng/ml group was significantly different from controls (Dunn s Multiple Comparison post-test, difference in rank sum = 11.44, P < 0.05) (Fig. 5).
Figure 5. Percent survivorship of An. gambiae s.s. that fed on interpolated IVM doses.
Two day post emergence (A) and 8 days post emergence (B) An. gambiae s.s. survivorship after feeding on interpolated IVM doses. Data are presented as box and whiskers plots and represent 3–8 independent replicates per group, each containing between 10–100 mosquitoes/replicate.
3.5. Effects of multiple IVM blood meals on mosquito survivorship
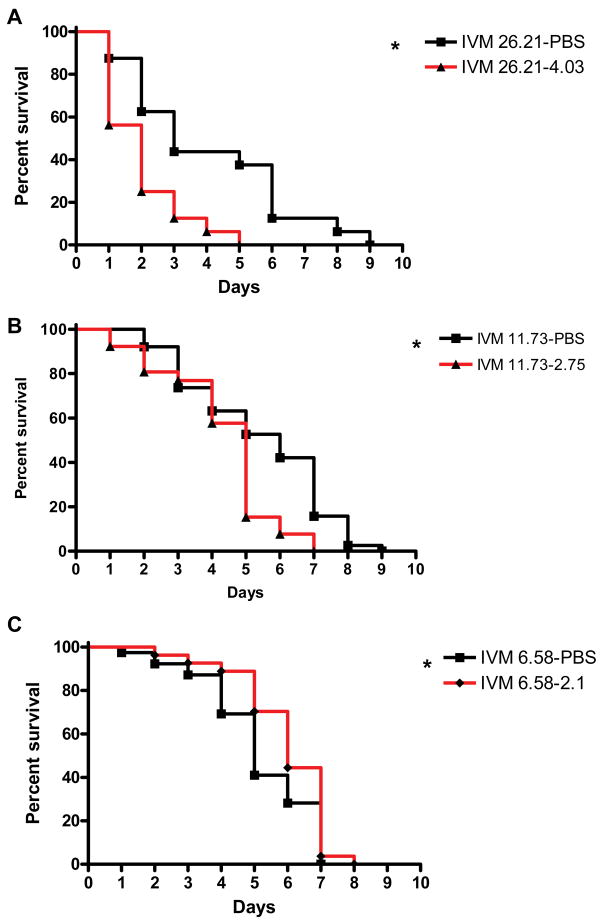
Anopheles gambiae s.s. that survived their primary blood meal containing IVM concentrations predicted on the first night (26.21 ng/ml) and second night (11.73 ng/ml) post-MDA, had significantly reduced survivorship when re-blood fed on the predicted IVM concentration 3 days later compared to controls ( IVM 26.21-PBS (n = 16) versus IVM 26.21–4.03 (n = 16), P = 0.0027, Hazard ratio = 0.449 [0.086, 0.598]); ( IVM 11.73-PBS (n = 38) versus IVM 11.73–2.75 (n = 26), P < 0.0061, Hazard ratio = 0.571 [0.2, 0.766]). Anopheles gambiae s.s. that fed on an initial IVM concentration predicted at three days (6.58 ng/ml) post-MDA, and then re-blood fed on a concentration of IVM predicted three days later (2.1 ng/ml), had elevated survivorship compared to their PBS controls ( IVM 6.58-PBS (n = 39) versus IVM 6.58–2.1 (n = 27), P = 0.045, Hazard ratio = 1.429 [1.016, 3.922] (Fig 6), but this latter effect is not biologically relevant because the mosquitoes were all dead at the same time point whereas the mosquitoes that fed on the other two initial (26.21 and 11.73 ng/ml) concentrations died at different time points relative to their controls.
Figure 6. Percent survivorship of An. gambiae s.s. after imbibing two consecutive blood meals that contained IVM.
Two days post emergence An. gambiae s.s. were given two consecutive blood meals (3 days apart) that contained IVM concentrations corresponding to those interpolated from the IVM pharmacokinetic curve, and then monitored for survivorship over time. Panel A represents one experiment and Panels B and C represent the sum of two replicates. Mosquitoes that exhibited significantly reduced survival (Logrank Test) compared to controls (in which the 2º blood meal contained only PBS) are marked with an asterisk.
4. Discussion
These experiments were designed to evaluate the effects of oral anthelmintics, currently administered via MDA, on the survivorship and re-feeding behavior of frequent blood feeding and anthropophilic mosquito disease vectors. The experiments were performed in a laboratory environment and using membrane-feeding so that we could conduct controlled experiments aimed at precisely testing these effects. Of the four drugs tested, only IVM affected An. gambiae s.s. survival and re-blood feeding behavior; Ae. aegypti was not affected by ivermectin levels that would be present in humans after MDA (data not shown). Anopheles gambiae s.s. exhibited decreased survivorship and delayed re-blood feeding behavior when the mosquitoes ingested IVM concentrations that would be present in venous human blood for a period of several days post IVM MDA. Overall, these data should help to predict how IVM MDA might eventually be used to help control malaria transmission by An. gambiae s.s. in Africa.
Ivermectin is a macrocyclic lactone that specifically agonizes glutamate-gated chloride (GluCl) anion channels in parasitic worms, causing their paralysis and death (Wolstenholme and Rogers, 2005). There is additional evidence that ivermectin interacts with GABA-gated and pH-sensitive anion channels in some arthropods (Bloomquist, 2003; Kane et al., 2000; Mounsey et al., 2007). While the effects of IVM against various mosquito species has been shown previously (Bockarie et al., 1999; Cartel et al., 1991; Foley et al., 2000; Fritz et al., 2009; Gardner et al., 1993; Jones et al., 1992; Pampiglioni et al., 1985; Tesh and Guzman, 1990), our study differs from these previous reports in that it specifically addresses IVM pharmacokinetics in humans following standard indicated IVM MDA (150 μg/kg) coupled to a focus on anthropophilic, frequently feeding mosquitoes. Furthermore, to our knowledge we are the first to measure age differences, changes in blood feeding frequency and defecation times, and the compounding mortality effects of serial IVM doses on mosquitoes. It is reasonable to think that these physiologic and cumulative sub-lethal drug effects may be important in field situations; for example, delays in blood-feeding frequency may compound mosquito mortality in the field due to decreased nutrition.
The lab based in vitro blood meal strategy employed in this report has the advantage of feeding specified concentrations to mosquitoes that match mean pharmacokinetic plasma levels found in humans post ingestion of the indicated IVM dose (150 μg/kg) (Elkassaby, 1991). Indeed, specific IVM concentrations in any one person following MDA in the field can vary by more than 3-fold and one can not determine the blood concentration in humans or in biting mosquitoes without difficult and costly analysis by HPLC or mass spectrometry. One potential disadvantage of our in vitro experiments was that the drugs were solubilized in DMSO and mixed with blood drawn in anticoagulant; however, a priori experiments sought to address this issue, and we determined that there were no detrimental effects from this experimental treatment on mosquito blood meal digestion, behavior or survival. An additional caveat of our experiments is that that the blood pharmacokinetics of IVM in humans have only been measured from venous-drawn plasma samples, but a mosquito feeding on an IVM treated human may potentially ingest a different amount of IVM when they imbibe blood from sub-dermal capillaries, especially because ivermectin is lipophilic and is found at higher concentrations in fat and dermal tissue than in venous plasma (Baraka et al., 1996). Thus the concentration of IVM in venous blood compared to the concentration of ivermectin ingested by a mosquito should be explored in the future. Lipophilic IVM properties may explain the surprising results reported by Foley et al. (Foley et al., 2000) where groups of Anopheles farauti exhibited significantly reduced survivorship when fed on a single human who ingested a non-standard 250 μg/kg dose of IVM even when blood fed on this person 44 days post IVM administration. In contrast, Bockarie et al. (Bockarie et al., 1999) found that wild An. punctulatus exhibited reduced survival only when blood fed on villagers within <4 days after the villagers received IVM MDA of an even higher non-standard dose (400 μg/kg). The contradiction of these and our own data, which showed that most effects against Anopheles gambiae s.s. would be restricted to IVM concentrations present in humans for several days post MDA, are likely due to a combination of factors including Anopheles species differences, different pharmacokinetics following different IVM doses, and wild vs. laboratory mosquitoes. Detailed knowledge concerning the specific length of time that clinically indicated doses of IVM can affect blood feeding mosquitoes in the field will be crucial for assessing IVM s potential for malaria control.
Our data suggest that An. gambiae s.s. adult size did not appear to alter susceptibility to IVM but adult age might alter susceptibility to IVM. Conversely, other investigators have shown that older An. gambiae s.s. are more susceptible to DDT and pyrethroids (Hodjati and Curtis, 1999; Lines and Nassor, 1991). For proper comparison to our two day post emergence groups, our eight day post emergence An. gambiae s.s. were not offered multiple blood meals prior to the ingestion of a blood meal that contained IVM. However such a situation is highly unlikely in the field, and the lack of multiple blood feeding from the eight day post emergence groups may have influenced our results. Examining the effects of mosquito senescence on IVM susceptibility will be important to assess in future studies.
Pyrantel, ALB, and DEC had no effect on either mosquito species. ALB SOx disrupts microtubule assembly in worm cells (Martin et al., 1997) and DEC likely activates the innate immune response of the host, causing rapid sequestration of microfilaria by granulocytes (Maizels and Denham, 1992; Martin et al., 1997; McGarry et al., 2005). The efficacy of DEC against various filarioid nematodes is restricted to in vivo experiments (McGarry et al., 2005; Vickery et al., 1985), and so the lack of reduced survivorship of An. gambiae s.s. or Ae. aegypti fed DEC in our in vitro study does not necessarily mean that DEC will not have an effect on mosquitoes that blood feed directly on treated humans. It is somewhat surprising that PYL showed inactivity against mosquitoes. This drug affects nicotinic acetylcholine-gated receptors (nAChRs) at nematode neuromuscular junctions (Martin et al., 1997), and these nAChRs are also used by arthropods for fast cholinergic synaptic transmission (Bloomquist, 2003). However, multiple subunit genes in this family are common in invertebrate genomes. Ten nAChR paralogues have been identified in An. gambiae s.s. (Jones et al., 2005), and this redundancy may prevent PYL mortality effects even if it cross-targeted one or more mosquito nAChRs. Other drugs that target nematode nAChRs, such as levamisole, oxantel, and morantel are approved for human use (Reddy et al., 2007), but these drugs were not examined for activity against mosquitoes in this study because they are not commonly used in current human MDA regimens. Neonicotinoids such as nitenpyram and imidacloprid are specifically designed to target arthropod nAChRs, and may be more effective against mosquito vectors, but are currently only used in veterinary practice (Meinke, 2001) so they are not as relevant in the context of anthropophilic mosquitoes.
In this paper, we only examined physiologic mosquito effects that directly relate to malaria parasite transmission (adult survival and re-blood feeding frequency), as opposed to physiologic effects that indirectly relate to transmission (fecundity effects that possibly would affect the abundance of subsequent mosquito generations). However, it is important to note that low concentrations of IVM in a blood meal have been found to reduce fecundity and egg viability of surviving Anopheles spp. (Fritz et al., 2009; Gardner et al., 1993). The size of mosquito populations relative to vertebrate hosts in malaria-endemic regions of Africa can be very large, and density-dependent development of An. gambiae larvae has been documented (Gimnig et al., 2002). Thus, it is not certain that fewer or less viable eggs laid by a subset of females after an IVM MDA would result in significantly fewer adult mosquitoes in subsequent generations or less parasite transmission. Field examinations of larval abundance and larval development in breeding sites following IVM MDAs will be important to address these issues in the future.
Our data suggest that IVM, if given on a more frequent MDA schedule, may be able to negatively influence malaria transmission in sub-Saharan Africa by temporarily reducing An. gambiae s.s. survival and re-blood feeding frequencies. However, there are important questions that should be answered before human IVM MDA can become a realistic weapon in the arsenal of malaria control strategies. Do these laboratory findings accurately reflect field situations where there are wild, mixed populations of Anopheles spp. and inconsistent treatment of people in villages? If so, how many IVM MDAs are needed, and at what interval, to observe a reduction in malaria transmission? Can a balance be struck in that interval where adult mosquito survival is significantly reduced but fecundity effects are minimized so that malaria control is achieved without putting undue pressure on the mosquito population to develop IVM resistance? The potential benefits of using IVM MDA for malaria control are apparent, including a different mode of action (Wolstenholme and Rogers, 2005) and delivery from the currently used carbamates, pyrethroids, and organochlorines (Hemingway and Ranson, 2005), the ability of IVM to be able to affect both endophagic and exophagic vectors, and the likely reduction in polyparasitism that will occur with more frequent IVM MDA (Geary et al., 2010; Ranque et al., 2001; Wen et al., 2008). The difficulties associated with community-directed IVM MDA have already been identified and streamlined by current onchocerciasis control efforts (Boatin and Richards, 2006; Diawara et al., 2009) so this malaria control strategy could be feasible in the near future. Given the massive burden that malaria has on the developing world, creating and implementing new malaria control measures is an important task.
Supplementary Material
An. gambiae s.s. and Ae. aegypti were fed varied concentrations of PYL to determine if PYL reduced mosquito survivorship, but the drug had no effect on An. gambiae s.s. nor Ae. aegypti.
An. gambiae s.s. and Ae. aegypti were fed varied concentrations of ALB SOx to determine if ALB SOx reduced mosquito survivorship, but the drug had no effect on An. gambiae s.s. nor Ae. aegypti.
An. gambiae s.s. and Ae. aegypti were fed varied concentrations of DEC to determine if DEC reduced mosquito survivorship, but the drug had no effect on An. gambiae s.s. nor Ae. aegypti.
An. gambiae s.s. were fed varied concentrations of IVM and ALB SOx to determine if the addition of ALB SOx to IVM reduced mosquito survivorship, but the drug combination did not affect mosquito survival over IVM alone.
Acknowledgments
We would like to acknowledge the help of the AIDL support staff and the phlebotomists at the CSU Hartshorn Health clinic. We would also like to acknowledge Phillip Chapman with the Colorado State University Department of Statistics for his advice. This work was supported by grant AI079528, and by contract N01-AI-25489 from the U.S. National Institutes of Allergy and Infectious Diseases, and by the Bill and Melinda Gates Foundation Grand Challenges Explorations program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott WS. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology. 1925;18:265–276. [Google Scholar]
- Baraka OZ, Mahmoud BM, Marschke CK, Geary TG, Homeida MMA, Williams JF. Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus. European Journal of Clinical Pharmacology. 1996;50:407–410. doi: 10.1007/s002280050131. [DOI] [PubMed] [Google Scholar]
- Beier JC. Frequent blood-feeding and restrictive sugar-feeding behavior enhance the malaria vector potential of Anopheles gambiae s.l. and An. funestus (Diptera:Culicidae) in western Kenya. J Med Entomol. 1996;33:613–8. doi: 10.1093/jmedent/33.4.613. [DOI] [PubMed] [Google Scholar]
- Black WCI, Moore CG. Population Biology as a Tool to Study Vector-Borne Diseases. In: Marquardt WC, editor. Biology of Disease Vectors. Elsevier Academic Press; San Diego, CA: 2005. pp. 187–206. [Google Scholar]
- Bloomquist JR. Chloride channels as tools for developing selective insecticides. Arch Insect Biochem Physiol. 2003;54:145–56. doi: 10.1002/arch.10112. [DOI] [PubMed] [Google Scholar]
- Boatin BA, Richards FO. Control of Onchocerciasis. Advances in Parasitology. 2006;61:349–394. doi: 10.1016/S0065-308X(05)61009-3. [DOI] [PubMed] [Google Scholar]
- Bockarie MJ, Hii JL, Alexander ND, Bockarie F, Dagoro H, Kazura JW, Alpers MP. Mass treatment with ivermectin for filariasis control in Papua New Guinea: impact on mosquito survival. Med Vet Entomol. 1999;13:120–3. doi: 10.1046/j.1365-2915.1999.00159.x. [DOI] [PubMed] [Google Scholar]
- Brooker S, Akhwale W, Pullan R, Estambale B, Clarke SE, Snow RW, Hotez PJ. Epidemiology of plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg. 2007;77:88–98. [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information - Theoretic Approach. Springer-Verlag; New York City: 2002. [Google Scholar]
- Cartel JL, Sechan Y, Spiegel A, Nguyen L, Barbazan P, Martin PM, Roux JF. Cumulative mortality rates in Aedes polynesiensis after feeding on polynesian Wuchereria bancrofti carriers treated with single doses of ivermectin, diethylcarbamazine and placebo. Trop Med Parasitol. 1991;42:343–5. [PubMed] [Google Scholar]
- Collett D. Modelling Binary Data. Chapman and Hall; London: 1991. [Google Scholar]
- De Benedictis J, Chow-Shaffer E, Costero A, Clark GG, Edman JD, Scott TW. Identification of the people from whom engorged Aedes aegypti took blood meals in Florida, Puerto Rico, using polymerase chain reaction-based DNA profiling. Am J Trop Med Hyg. 2003;68:437–46. [PubMed] [Google Scholar]
- Dia I, Diop T, Rakotoarivony I, Kengne P, Fontenille D. Bionomics of Anopheles gambiae Giles, An. arabiensis Patton, An. funestus Giles and An. nili (Theobald) (Diptera: Culicidae) and transmission of Plasmodium falciparum in a Sudano-Guinean zone (Ngari, Senegal) J Med Entomol. 2003;40:279–83. doi: 10.1603/0022-2585-40.3.279. [DOI] [PubMed] [Google Scholar]
- Diawara L, Traore MO, Badji A, Bissan Y, Doumbia K, Goita SF, Konate L, Mounkoro K, Sarr MD, Seck AF, Toe L, Touree S, Remme JH. Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: first evidence from studies in Mali and Senegal. PLoS Negl Trop Dis. 2009;3:e497. doi: 10.1371/journal.pntd.0000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman JD, Strickman D, Kittayapong P, Scott TW. Female Aedes aegypti (Diptera: Culicidae) in Thailand rarely feed on sugar. J Med Entomol. 1992;29:1035–8. doi: 10.1093/jmedent/29.6.1035. [DOI] [PubMed] [Google Scholar]
- Elkassaby MH. Ivermectin uptake and distribution in the plasma and tissue of Sudanese and Mexican patients infected with Onchocerca volvulus. Trop Med Parasitol. 1991;42:79–81. [PubMed] [Google Scholar]
- Fasanmade AA, Akanni AO, Olaniyi AA, Fasanmade AA, Tayo F. Bioequivalence of Pyrantel Pamoate Dosage Forms in Healthy-Human Subjects. Biopharmaceutics & Drug Disposition. 1994;15:527–534. doi: 10.1002/bdd.2510150610. [DOI] [PubMed] [Google Scholar]
- Fieller EC. The biological standardization of insulin. Supplement to the Journal of the Royal Statistical Society. 1940;7:1–64. [Google Scholar]
- Foley DH, Bryan JH, Lawrence GW. The potential of ivermectin to control the malaria vector Anopheles farauti. Trans R Soc Trop Med Hyg. 2000;94:625–8. doi: 10.1016/s0035-9203(00)90211-6. [DOI] [PubMed] [Google Scholar]
- Fritz ML, Siegert PY, Walker ED, Bayoh MN, Vulule JR, Miller JR. Toxicity of bloodmeals from ivermectin-treated cattle to Anopheles gambiae s.l. Ann Trop Med Parasitol. 2009;103:539–47. doi: 10.1179/000349809X12459740922138. [DOI] [PubMed] [Google Scholar]
- Gardner K, Meisch MV, Meek CL, Biven WS. Effects of ivermectin in canine blood on Anopheles quadrimaculatus, Aedes albopictus and Culex salinarius. J Am Mosq Control Assoc. 1993;9:400–2. [PubMed] [Google Scholar]
- Garrett-Jones C. Prognosis for Interruption of Malaria Transmission through Assessment of the Mosquito's Vectorial Capacity. Nature. 1964;204:1173–5. doi: 10.1038/2041173a0. [DOI] [PubMed] [Google Scholar]
- Geary TG, Woo K, McCarthy JS, Mackenzie CD, Horton J, Prichard RK, de Silva NR, Olliaro PL, Lazdins-Helds JK, Engels DA, Bundy DA. Unresolved issues in anthelmintic pharmacology for helminthiases of humans. Int J Parasitol. 2010;40:1–13. doi: 10.1016/j.ijpara.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM, Walker ED. Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. J Med Entomol. 2002;39:162–72. doi: 10.1603/0022-2585-39.1.162. [DOI] [PubMed] [Google Scholar]
- Hemingway J, Ranson H. Chemical control of vectors and mechanisms of resistance. In: Marquardt WC, editor. Biology of Disease Vectors. Elsevier Academic Press; San Diego: 2005. pp. 627–647. [Google Scholar]
- Hodjati MH, Curtis CF. Evaluation of the effect of mosquito age and prior exposure to insecticide on pyrethroid tolerance in Anopheles mosquitoes (Diptera: Culicidae) Bulletin of Entomological Research. 1999;89:329–337. [Google Scholar]
- Hotez PJ. Control of onchocerciasis--the next generation. Lancet. 2007;369:1979–80. doi: 10.1016/S0140-6736(07)60923-4. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Ehrlich Sachs S, Sachs JD. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Med. 2006;3:e102. doi: 10.1371/journal.pmed.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AK, Grauso M, Sattelle DB. The nicotinic acetylcholine receptor gene family of the malaria mosquito, Anopheles gambiae. Genomics. 2005;85:176–87. doi: 10.1016/j.ygeno.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Jones AK, Sattelle DB. The cys-loop ligand-gated ion channel gene superfamily of the nematode, Caenorhabditis elegans. Invert Neurosci. 2008;8:41–7. doi: 10.1007/s10158-008-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Meisch MV, Meek CL, Bivin WS. Lethal effects of ivermectin on Anopheles quadrimaculatus. J Am Mosq Control Assoc. 1992;8:278–80. [PubMed] [Google Scholar]
- Kane NS, Hirschberg B, Qian S, Hunt D, Thomas B, Brochu R, Ludmerer SW, Zheng Y, Smith M, Arena JP, Cohen CJ, Schmatz D, Warmke J, Cully DF. Drug-resistant Drosophila indicate glutamate-gated chloride channels are targets for the antiparasitics nodulisporic acid and ivermectin. Proc Natl Acad Sci U S A. 2000;97:13949–54. doi: 10.1073/pnas.240464697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines JD, Nassor NS. DDT resistance in Anopheles gambiae declines with mosquito age. Medical and Veterinary Entomology. 1991;1991:261–265. doi: 10.1111/j.1365-2915.1991.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Nishimura N, Conn J, Lourenco-de-Oliveira R. Life history correlates of adult size in the malaria vector Anopheles darlingi. Memorias do Instituto Oswaldo Cruz. 1995;90:769–774. doi: 10.1590/s0074-02761995000600020. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Denham DA. Diethylcarbamazine (Dec) - Immunopharmacological Interactions of an Anti-Filarial Drug. Parasitology. 1992;105:S49–S60. doi: 10.1017/s0031182000075351. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Robertson AP, Bjorn H. Target sites of anthelmintics. Parasitology. 1997;114:S111–S124. [PubMed] [Google Scholar]
- Mathenge EM, Gimnig JE, Kolczak M, Ombok M, Irungu LW, Hawley WA. Effect of permethrin-impregnated nets on exiting behavior, blood feeding success, and time of feeding of malaria mosquitoes (Diptera : Culicidae) in western Kenya. Journal of Medical Entomology. 2001;38:531–536. doi: 10.1603/0022-2585-38.4.531. [DOI] [PubMed] [Google Scholar]
- Mathew N, Kalyanasundaram M. Antifilarial agents. Expert Opinion on Therapeutic Patents. 2007;17:767–789. [Google Scholar]
- McGarry HF, Plant LD, Taylor MJ. Diethylcarbamazine activity against Brugia malayi microfilariae is dependent on inducible nitric-oxide synthase and the cyclooxygenase pathway. Filaria J. 2005;4:4. doi: 10.1186/1475-2883-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke PT. Perspectives in animal health: old targets and new opportunities. J Med Chem. 2001;44:641–59. doi: 10.1021/jm990564h. [DOI] [PubMed] [Google Scholar]
- Mounsey K, Dent J, Holt D, McCarthy J, Currie B, Walton S. Molecular characterisation of a pH-gated chloride channel from Sarcoptes scabei. Invertebrate Neuroscience. 2007;7:149–156. doi: 10.1007/s10158-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Ottesen EA, Hooper PJ, Bradley M, Biswas G. The global programme to eliminate lymphatic filariasis: health impact after 8 years. PLoS Negl Trop Dis. 2008;2:e317. doi: 10.1371/journal.pntd.0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampiglioni S, Majori G, Petrangeli G, Romi R. Avermectins, MK-933 and MK-936, for mosquito control. Trans R Soc Trop Med Hyg. 1985;79:797–9. doi: 10.1016/0035-9203(85)90121-x. [DOI] [PubMed] [Google Scholar]
- Ranque S, Chippaux JP, Garcia A, Boussinesq M. Follow-up of Ascaris lumbricoides and Trichuris trichiura infections in children living in a community treated with ivermectin at 3-monthly intervals. Ann Trop Med Parasitol. 2001;95:389–93. doi: 10.1080/00034980120065822. [DOI] [PubMed] [Google Scholar]
- Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Oral drug therapy for multiple neglected tropical diseases: a systematic review. Jama. 2007;298:1911–24. doi: 10.1001/jama.298.16.1911. [DOI] [PubMed] [Google Scholar]
- Scott TW, Githeko AK, Fleisher A, Harrington LC, Yan G. DNA profiling of human blood in anophelines from lowland and highland sites in Western Kenya. Am J Trop Med Hyg. 2006;75:231–7. [PubMed] [Google Scholar]
- Scott TW, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Zhou H, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. J Med Entomol. 2000;37:77–88. doi: 10.1603/0022-2585-37.1.77. [DOI] [PubMed] [Google Scholar]
- Shenoy RK, Suma TK, John A, Arun SR, Kumaraswami V, Fleckenstein LL, Na-Bangchang K. The pharmacokinetics, safety and tolerability of the co-administration of diethylcarbamazine and albendazole. Annals of Tropical Medicine and Parasitology. 2002;96:603–614. doi: 10.1179/000349802125001663. [DOI] [PubMed] [Google Scholar]
- Tesh RB, Guzman H. Mortality and infertility in adult mosquitoes after the ingestion of blood containing ivermectin. Am J Trop Med Hyg. 1990;43:229–33. doi: 10.4269/ajtmh.1990.43.229. [DOI] [PubMed] [Google Scholar]
- Vickery AC, Nayar JK, Tamplin ML. Diethylcarbamazine-Mediated Clearance of Brugia-Pahangi Microfilariae in Immunodeficient Nude-Mice. American Journal of Tropical Medicine and Hygiene. 1985;34:476–483. doi: 10.4269/ajtmh.1985.34.476. [DOI] [PubMed] [Google Scholar]
- Wen LY, Yan XL, Sun FH, Fang YY, Yang MJ, Lou LJ. A randomized, double-blind, multicenter clinical trial on the efficacy of ivermectin against intestinal nematode infections in China. Acta Trop. 2008;106:190–4. doi: 10.1016/j.actatropica.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Wolstenholme AJ, Rogers AT. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology. 2005;131(Suppl):S85–95. doi: 10.1017/S0031182005008218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An. gambiae s.s. and Ae. aegypti were fed varied concentrations of PYL to determine if PYL reduced mosquito survivorship, but the drug had no effect on An. gambiae s.s. nor Ae. aegypti.
An. gambiae s.s. and Ae. aegypti were fed varied concentrations of ALB SOx to determine if ALB SOx reduced mosquito survivorship, but the drug had no effect on An. gambiae s.s. nor Ae. aegypti.
An. gambiae s.s. and Ae. aegypti were fed varied concentrations of DEC to determine if DEC reduced mosquito survivorship, but the drug had no effect on An. gambiae s.s. nor Ae. aegypti.
An. gambiae s.s. were fed varied concentrations of IVM and ALB SOx to determine if the addition of ALB SOx to IVM reduced mosquito survivorship, but the drug combination did not affect mosquito survival over IVM alone.