Abstract
Colorectal cancer is the third most common form of malignancy, behind prostate and lung cancers. Despite recent advances in medicine, mortality from colorectal cancer remains high, highlighting the need for improved therapies. Numerous studies have demonstrated increased activation of EGFR and its family members (EGFRs), IGF-1R as well as c-Src in colorectal cancer. The current study was undertaken to examine the effectiveness of combination therapy of dasatinib (BMS-354825; Bristol-Myers Squibb), a highly specific inhibitor of Src family kinases (SFK) and a non-toxic dietary agent; curcumin (diferuloylmethane), in colorectal cancer in in vitro and in vivo experimental models. For the latter we utilized C57BL/6J-ApcMin+/− mice. Initial in vitro studies revealed synergistic interactions between the two agents. Additionally, we have observed that combination treatment causes a much greater inhibition of the following metastatic processes than either agent alone: (a) colony formation (b) invasion through extracellular matrix (c) tubule formation by endothelial cells. Dasatinib affects the cell adhesion phenotype of colon cancer HCT-116 cells whereas the combination therapy enhances this effect to a greater extent. Preclinical investigation revealed that the combination therapy to be highly effective causing an over 95% regression of intestinal adenomas in ApcMin+/− mice, which could be attributed to decreased proliferation and increased apoptosis. In conclusion, our data suggest that combination treatment of dasatinib and curcumin could be a potential therapeutic strategy for colorectal cancer.
Keywords: Combination therapy, synergistic interactions, cell signaling, cell invasion and angiogenesis, tumor regression
Introduction
Despite the use of aggressive surgical resection and chemotherapy, nearly 50% of patients with colorectal carcinoma develop recurrent disease, highlighting the need for improved therapies 1. Recent advances in the understanding of the molecular pathogenesis of cancer have aided in formulating both preventive and/or therapeutic strategies. Accumulating evidence indicates that the development and progression of many malignancies, including colorectal cancer are associated with constitutive activation of multiple signaling pathways that promote proliferation, inhibit apoptosis and induce metastasis 2.
EGF-receptor (EGFR) and/or some of its family members, specifically ErbB-2/HER-2 and ErbB-3/HER-3 [referred to as EGFRs] have been shown to play a crucial role in regulating a number of pathways that affect tumor cell survival, angiogenesis, motility and invasiveness 3–5. Abnormal receptor activity has been associated with the development and progression of many malignancies, including that of the colorectal cancer 6–8. A majority of solid tumors, including those in the colon express one or more members of the EGFR family. There is evidence to suggest that development of enhanced drug resistance is often associated with expression of more than one member of the EGFR family 9. In addition, a growing number of studies have implicated the insulin-like growth factor (IGF)/IGF-receptor-1 (IGF-1R) system as well as c-Src, a non-receptor tyrosine kinase, in the development and progression of colorectal cancer 3, 10–13.
Since multiple signal transduction pathways become dysfunctional in most malignancies, including colorectal cancer, it is likely that the maximal and most durable therapeutic benefit against tumor growth will be achieved with combination therapies that affect several targets. Thus, agent(s)/regimen(s) that target EGFRs, IGF-1R and c-Src should be more effective than narrowly focused therapies as they are likely to impact several aspects of tumor progression.
Dasatinib (BMS-354825) was identified as a highly potent, ATP-competitive inhibitor of Src and Abl kinases with antiproliferative activity in both hematologic and solid tumor cell lines 14. Dasatinib inhibits the kinase activity of Bcr-Abl mutants found in chronic myeloid leukemia patients with acquired resistance to imatinib 15 and has promising activity in phase I/II clinical evaluation in patients with imatinib-resistant chronic myeloid leukemia 16. Dasatinib also inhibits Src kinase activity in epithelial cell lines 17–18 and is currently in clinical trials for the treatment ofsolid tumors 19–20. Dasatinibmay have multiple effects on solid tumors, demonstrating inhibition of cell proliferation, migration and invasion 14, 17–18. However, it remains unclear which of these mechanisms will become more relevant in the clinical application of dasatinibin solid tumors of epithelial origin.
Curcumin [diferuloylmethane; I,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione], the major pigment in turmeric powder, possesses anti-inflammatory and anti-oxidant properties 21. With no discernable toxicity, curcumin has been shown to inhibit the growth of transformed cells and colon carcinogenesis at the initiation, promotion and progression stages in carcinogen-induced rodent models 22–24. Development of azoxymethane-induced preneoplastic and neoplastic lesions of the colon is also inhibited in experimental animals fed a diet containing 0.2–1.6% curcumin 23–24. In addition, curcumin has been reported to prevent adenoma development in the intestinal tract of Min −/+ mice, a model of human familial adenomatous polyposis 25. In a Phase I clinical trial, curcumin was shown to be effective in inhibiting tumor growth 26. We reported that curcumin in combination with ERRP, a pan-erbB inhibitor 27 causes a greater inhibition of the growth of colon cancer cells that either agent alone 28. We have also reported that curcumin acts synergistically with FOLFOX (5-fluorouracil plus oxaliplatin) in inhibiting growth of colon cancer cells in vitro 29. These and other relevant observations have prompted us to undertake the current investigation.
Our working hypothesis, therefore, is that a combination of dasatinib and curcumin will be an effective therapeutic strategy for colorectal neoplasia and/or cancer. We further hypothesize that this enhanced effectiveness is the result of an attenuation of multiple signaling pathways leading to inhibition of transformation properties of colon cancer cells.
METHODS AND MATERIALS
Cell lines and cell culture
Human colon cancer HCT-116 p53 wild type (wt), HT-29, and HCT-116 p53 null (HCT-116 p53−/−) and SW-620 cells were used to investigate efficacy of combined therapy of dasatinib in and curcumin in growth inhibition. HCT-116 (wt), HT-29 and SW-620 cells were obtained from American Type Culture Collection (ATCC, Rockville, MD), whereas HCT-116 p53 null cells, originally generated in Dr. Bert Vogelstein laboratory at John Hopkins University, Baltimore, MD, were obtained from Dr Ping Dou at Karmanos Cancer Institute. The cells were maintained in tissue culture flasks in Dulbecco’s modified Eagle medium (DMEM) in a humidified incubator at 37 °C in an atmosphere of 95% air and 5% CO2. The cell culture medium was supplemented with 5% FBS and 1% antibiotic/antimycotic. Human umbilical vein endothelial cells (HUVEC), a kind gift from Dr. Fazlul Sarkar at the Karmanos Cancer Institute, Detroit, MI, were used for angiogenesis assay. Endothelial growth medium with nutrient supplements were bought from Lonza Walkersville Inc. (Walkersville, MD). Additionally, the cell culture medium was supplemented with 5% FBS and 1% antibiotic/antimycotic. Medium was changed three times a week and cells were passaged using trypsin/EDTA.
Chemicals
Dulbecco’s modified Eagle medium (DMEM), fetal bovine serum (FBS), and antibiotic/antimycotic were obtained from GIBCO BRL (Bethesda, MD). Dasatinib was purchased from LC laboratories (Woburn, MA). Protease inhibitor cocktail, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and all other chemicals were obtained from Sigma (St. Louis, MO). Anti p-EGFRs (tyr-845, -1068-), p-HER2 (877), p-HER3 (1289), p-Src(tyr-416), Src, p-Akt (473), p-Erk(1/2), BclXL and Cox-2 p-IGF-1R, IGF-1, IGFBP3 and Rb were purchased from Cell Signaling (Beverley, MA). Antibodies to β-actin antibody was purchased from Sigma (USA). Chemiluminescence detection of proteins was conducted with the use of a kit from Amersham Biosciences/Amersham Pharmacia Biotech (Piscataway, NJ). Recombinant TGF-α was purchased from Oncogene (San Diego, CA).
Growth inhibition assay
Inhibition of cell growth in response to dasatinib and or curcumin was examined by 3-(4,5-dimethyl-thiazol-2yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay as described previously 30. Briefly, cells were dispersed by trypsin-EDTA treatment and resuspended in appropriate culture medium containing 5% of FBS and 5,000 cells/well were seeded into 96-well culture plates with six replicates. After 24 hrs of plating, incubation was continued for another 48 h in the absence (control) or presence of different drugs as described in the legends to the figures. At the end of the treatment period, cells were incubated with 10% of 5 mg/ml stock of MTT. The mitochondrial oxidation reaction was allowed to proceed for 3 h at 37 °C. The culture medium was then removed. The formazan crystals were then dissolved by adding 0.1 ml of dimethyl sulfoxide (DMSO). The intensity of the color developed, which is proportional to the number of live cells, was measured at a wavelength of 570 nm. All values were compared to the corresponding controls. All assays were performed with six replicates.
Analysis of interaction between curcumin and dasatinib
Combination Indices (CI) method adapted for in vitro anti-cancer drug testing was employed to determine the nature of interaction between the two agents. This method utilizes multiple drug-effect equation originally derived from enzyme kinetics model, where the output is represented as combination indices (CI) and/or isobologram analysis. CI analysis was performed by utilizing Calcusyn software (Biosoft, Ferguson, MO). Based on CI values extent of synergism/antagonism may be determined. In general, CI values below 1 suggest synergy, whereas CI above 1 indicates antagonism between the drugs. CI values in the range of 0.9 – 1.10 would mainly indicate additive effects of the drugs, those between 0.9 and 0.85 would suggest slight synergy, and values in the range of 0.7 – 0.3 are indicative of moderate synergy. Any value less than 0.3 would suggest strong synergistic interactions between the drugs.
Western-blot analysis
Western blot analysis was performed as described previously 30. Briefly, aliquots of cell lysates containing 80 μg of protein were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Electrophoresed proteins were transferred onto nitrocellulose membranes and detected using specific primary and secondary antibodies. The protein bands were visualized by enhanced chemiluminescence (ECL) detection kit (Amersham Biosciences/Amersham Pharmacia Biotech Piscataway, NJ). The membranes were reprobed for β-actin as loading control. All Western blots were performed at least three times for each experiment. Densitometric measurements of the scanned bands were performed using the digitized scientific software program UN-SCAN-IT. Data were normalized to β-actin.
Electrophoretic mobility shift assay (EMSA)
Nuclear protein extracts were prepared according to the method described earlier by Banerjee et al. 31. Briefly, HCT-116 cells treated with dasatinib and/or curcumin were lysed and nuclear proteins were extracted as described previously 31. EMSA was performed by incubating 8 μg of nuclear protein extract with IRDye™ –700 labelled NF-κB oligonucleotide. The incubation mixture included 2μg of poly (dI-dC) in a binding buffer. The DNA-protein complex formed was separated from free oligonucleotide on an 8.0% native polyacralyamide gel using buffer containing 50mM Tris, 200mM glycine, pH 8.5, and 1mM EDTA, and then visualized by Odyssey Infrared Imaging System using Odyssey Software Release 1.1 (Li-COR Inc, Lincoln, Nebraska). Anti-Rb immunoblotting with nuclear proteins was done as loading control.
Morphological changes
HCT-116 cells were seeded in 6-well cell culture plates and allowed to form colonies for 5 days in the absence (control) or presence of dasatinib and/or curcumin. At the end of exposure, one set of experiment was terminated by fixing in 70% ethanol, subsequently stained with 0.1% crystal violet. The colonies formed in response to different treatments were photographed. The cells were allowed to grow further in medium without any drugs, fixed, stained and photographed after 8 and 13 days to observe changes in colony formation and morphology of the cells. Each experiment was conducted at least 3 times.
Invasion assay
Invasion assay was performed using a colorimetric assay from the Chemicon International Inc. (Temecula, CA, USA) according to the manufacturer’s instructions. In brief, 20,000 HCT-116 cells were seeded with or without dasatinib (1μM), incubated at 37 °C for 72 h. At the end of the incubation, non-invading cells were gently removed using a cotton-tipped swab from interior of the inserts. The invasive cells on the lower surface of inserts were stained and photographed.
Tubule formation assay
Tubule formation by HUVECs, a measure of angiogenesis, was carried out utilizing In vitro angiogenesis assay kit from Chemicon International Inc. according to the manufacturer’s instructions. The assay was performed in 96-well plate. Briefly, 15 × 103 cells/well were seeded on ECMatrix™ that consisted of laminin, collagen type IV, heparin sulfate, proteoglycan, entactin and nidogen as well as various growth factors. Cellular network structures, in the absence (control) or presence of dasatinib were allowed to develop over 12 h. Each well was photographed using an inverted microscope with digital camera as mentioned above for migration study.
Preclinical efficacy analysis
Female Min mice (5 weeks; female C57BL/6J-APCMin+/−) were obtained from The Jackson Laboratory. After two weeks of acclimatization, the mice were randomly assigned into four groups and given various treatments by gavage. At this time, all tumors have been formed but continue to grow in size 32. Group-1 received the vehicle (DMSO), Group- 2 received dasatinib (10mg/kg body weight), Group-3 received curcumin (250 mg/kg body weight)) and Group-4 received both dasatinib and curcumin. The treatment was given for 5 consecutive days a week for four weeks. At the end of respective treatments, the mice were killed by CO2 asphyxiation; the intestinal tract was excised, and 10 cm from the proximal (jejunum) and distal (ileum) small intestine were removed, opened longitudinally, and then rinsed with ice-cold PBS. They were fixed overnight in formalin, and the number of the intestinal tumors was recorded using a dissecting microscope with 4X to 10X magnification. Subsequently, the residual tumors were excised, fixed in buffered-formalin and processed for immunohistochemistry 33–34. All procedures involving animals were approved by the Animal Investigation Committee at Wayne State University School of Medicine.
Immunohistochemical analysis
Paraffin-embedded tumor remnants were sectioned and analysed for proliferation and apoptosis as described previously 33–34. Proliferation was determined by counting mitotic bodies in H&E stained sections. TUNEL assay was performed to detect apoptotic cells using the in situ cell Death Detection kit from Roche Applied Science (Indianapolis, IN) according to the manufacturer’s instructions as described previously 33–34. 3-amino-9-ethylcarbazole was used as chromagen, and the sections were counterstained with hematoxylin. Apoptotic cell nuclei appeared as red stained structures against a blue-violet background. The mitotic or apoptotic cells were counted for 4–6 microscopic fields under a 10× objective.
Statistical Analysis
Unless otherwise stated, data were expressed as mean ± SD. Where applicable, the results were compared by using the unpaired, two-tailed Student t-test, as implemented by Excel 2000 (Microsoft Corp., Redmond, WA). P values smaller than 0.05 were considered statistically significant.
Results
Curcumin synergizes with dasatinib to inhibit the growth of colon cancer cells
We have postulated that curcumin in combination with dasatinib will be a superior therapeutic strategy for colorectal cancer. As a first step in testing this hypothesis, we examined the effects of incremental doses of curcumin and dasatinib, each alone or in combination on the growth of different human colon cancer cells. We have reported previously that curcumin inhibits the growth of both HCT-116 and HT-29 cells, which are p53 positive and p53 mutant, respectively, suggesting that the growth inhibitory properties of curcumin are independent of p53 status 29. In the current investigation, we examined the effects of curcumin and dasatinib, each alone or in combination, on the growth of HCT-116 cells containing either p53 (p53+/+) or devoid of p53 (p53−/−), HT-29 and SW-620 cells. Cellular growth, as determined by MTT assays, revealed that, both dasatinib and/or curcumin were effective in inhibiting the growth of p53-positive and p53-negative colon cancer cells in a dose-dependent manner (Figs. 1AD). Dose response curves were generated for the drugs in colon cancer cells using Calcusyn software (Biosoft, Ferguson, MO) (Figs. 1A–D). In each colon cancer cell line, the combination therapy caused a significantly greater growth inhibition compared to that achieved in response to a single agent (Figs. 1A–D). While curcumin (1μM) and dasatinib (10 μM), each alone caused a 20–30% reduction, the combination therapy caused a marked inhibition of 81% in growth of the p53-positive HCT-116 cells (Figure 1A).
Figure 1.
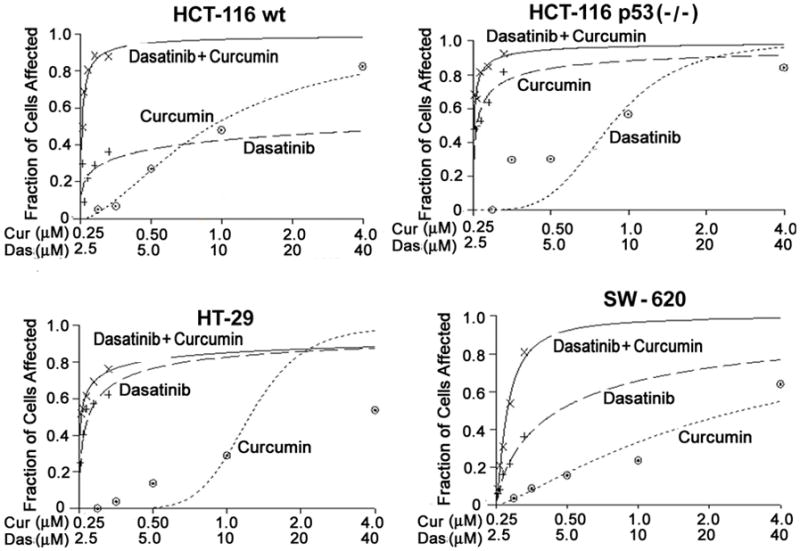
Effects of dasatinib and/or curcumin on the growth of different colon cancer cells: Growth as determined by MTT assay after 48 hrs incubation with incremental doses of curcumin and/or dasatinib in colon cancer cells (A) HCT-116 (wt), (B) HCT-116 p53 (−/−) (C) HT-29 and (D) SW-620. Dose response curves were generated for the drugs using Calcusyn software.
The fraction of cells affected in response to each treatment was thus utilized to perform synergy analysis with Calcusyn. The Combination Index (CI) as formulated by the software, revealed values of less than 1.0 indicating a synergistic interaction between the two agents at most of the dose combinations tested (Table 1). The results suggest that curcumin act synergistically with dasatinib to inhibit the growth in colon cancer cells. However, the synergy was not observed at high combinatorial doses of curcumin and dasatinib. This could be due to the fact that since the maximal inhibition by either curcumin or dasatinib was also achieved with high doses, CI values for the corresponding combination failed to demonstrate synergy. Since the synergistic interaction between dasatinib and curcumin, observed at lower doses, is not p53 dependent, subsequent experiments were carried out with the wild type (p53+/+) HCT-116 cells. In all further in vitro studies 10 μM curcumin and 1 μM dasatinib were used.
Table 1.
“Calcusyn” analysis reveals synergistic interactions between dasatinib and curcumin in variety of human colon cancer cells
| COMBINATION THERAPY | COMBINATION INDEX (CI) | ||||
|---|---|---|---|---|---|
| Das (μM) | Cur (μM) | HCT-116 (p53 wt) | HCT-116 (p53 null) | HT-29 | SW-620 |
| 0.25 | 2.5 | 0.14 | 0.25 | 0.29 | 1.09 |
| 0.5 | 5.0 | 0.17 | 0.56 | 0.51 | 0.71 |
| 1.0 | 10.0 | 0.23 | 0.53 | 0.77 | 0.86 |
| 2.0 | 20.0 | 0.32 | 0.92 | 1.16 | 0.71 |
| 4.0 | 40.0 | 0.65 | 1.2 | 1.86 | 0.45 |
CI; Combination Index < 1.0 suggests synergy
Curcumin and/or dasatinib treatment attenuates EGFRs, IGF-1R and c-Src signaling
Previously, we reported that the marked growth inhibition of colon cancer cells in response to the combination of curcumin and ERRP, a pan-erbB inhibitor 27, was associated with attenuation of EGFR, HER-2, HER-3 and IGF-1R activation and signaling 28. Similar changes were noted with HCT-116 cell growth inhibition with the combination of curcumin and FOLFOX 29. To determine whether and to what extent the signal transduction pathways activated by the receptor and non-receptor tyrosine kinases would be affected by curcumin and/or dasatinib, we examined the constitutive levels of activated (tyrosine phosphorylation) forms of EGFR, HER-2 and HER-3, IGF-1R as well as c-Src in HCT-116 (wt) cells following treatment with curcumin or dasatinib, or a combination of both for 48 h. As can be seen from the densitometric analysis (percent of control), although curcumin or dasatinib significantly decreased (50%–90%) the levels of activated (phosphorylated) EGFR (tyr 845) and (tyr 1068), HER-2 (tyr 877) and HER-3 (tyr 1289), curcumin together with dasatinib resulted in a much greater reduction when compared to the controls (Fig. 2A). As expected, dasatinib caused a 77% reduction in c-Src activation, as determined by phosphorylation of tyrosine residue at 416 (Fig. 2A). Curcumin had a minor (14%) effect but the combination treatment inhibited c-Src phosphorylation by 85%, when compared with the controls (Fig. 2A). Interestingly, dasatinib was found to be slightly more effective in reducing IGF-1R phosphorylation than curcumin, and the combination of curcumin and dasatinib caused further reduction (Fig. 2A).
Figure 2.
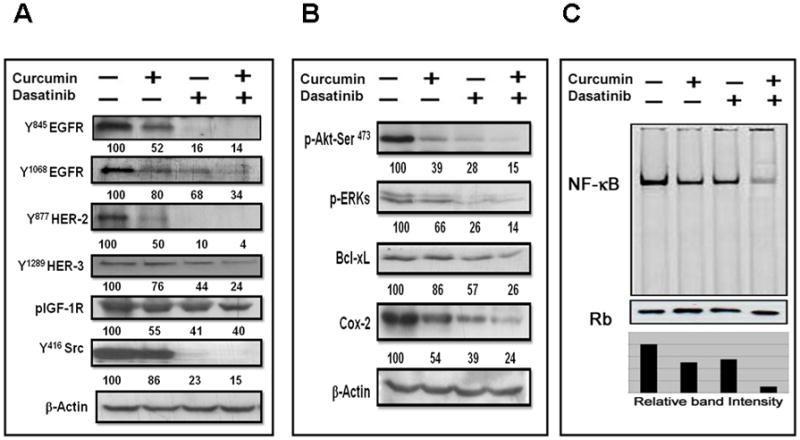
Effects of dasatinib and/or cucrcumin (A) on levels of phosphorylated forms of EGFR, HER-2, HER-3, IGF-1R and c-Src, (B) on downstream signaling effector proteins and (C) NFκ B activity, in colon cancer HCT-116 (wt) cells following 48h of treatment.
Curcumin and/or dasatinib inhibits downstream effectors and NFκB activity
We then examined the effect of the current treatment strategy on Akt and Erk activation and expression of BcLxL and COX-2, which are critically involved in cell survival 35. Although curcumin and dasatinib, each alone, markedly decreased the phosphorylated (activated) forms of Akt and Erks, the magnitude of this reduction was found to be much greater in response to the combination therapy than either agent alone (Fig. 2B). Similar changes were noted for BcLxL and Cox-2 expression (Fig. 2B).
Further, to unravel the molecular mechanism of therapeutic benefit observed by the combinatorial regimen in potentiating the anti-tumor effect, we performed electromobility shift assays (EMSA) to examine the status of the transcription factor NF-κB in HCT-116 cells following curcumin and/dasatinib treatment. Our results revealed that, whereas curcumin or dasatinib caused a minor 30–35% reduction in DNA binding activity of NF-κB, curcumin together with dasatinib produced a marked 88% attenuation of the same, when compared with the controls (Fig. 2C).
Curcumin and/or dasatinib inhibits colony formation and induces morphological changes in colon cancer cells
To determine whether combination therapy is effective in inhibiting cell transformation properties, we carried out colony formation assay. Combined therapy significantly inhibited colony formation in anchorage-dependent settings (Figs. 3A–C). It should also be noted that the combined therapy not only reduced the size but also the number of colonies formed by HCT-116 cells. Drastic change in the morphology of the cells was seen in dasatinib and combined treatment groups. Dasatinib essentially caused rounding off of the cells (Fig. 3A). The cells were allowed to revive after pre-treatment with dasatinib and/or curcumin. The cells continued to proliferate as round floating balls rather than growing as adherent monolayers (Fig. 3B). After 3 weeks of revival period, these ball-like structures started adhering and forming layers on the culture plates. (Fig. 3C). This morphological change was more significant in response to combined treatment.
Figure 3.
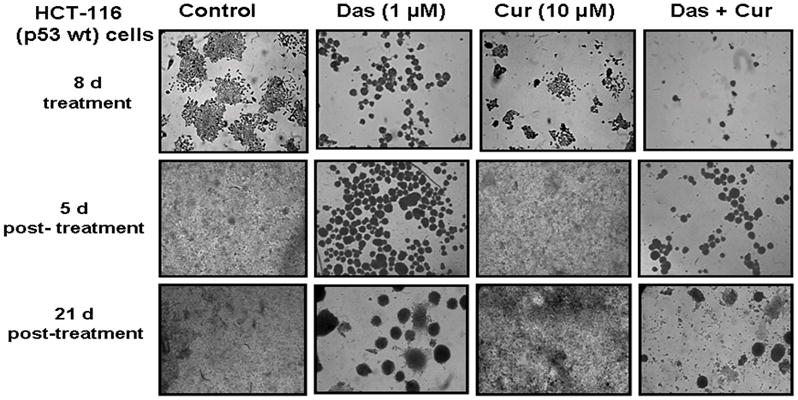
Effects of dasatinib and/or curcumin on morphology as well as anchorage-dependent growth of human colon cancer cells. (A) Photomicrographs depicting changes in the number and size of colonies formed after 8 days of treatment with dasatinib and/or curcumin. One set of treated cells were stained with 0.1% crystal violet stain and photographed at the end of treatment. Other sets of cells were allowed to grow after the removal of drugs, subsequently stained with 0.1% crystal violet stain and photographed after (B) Colony formation after 5 days of recovery (C) Effects of dasatinb and/or curcumin on colony formation and morphological changes after 16 days post-treatment. The experiment was repeated at least three times.
Dasatinib and curcumin inhibit metastatic potential of colon cancer cells
To examine the effectiveness of combination therapy in inhibiting metastatic processes, cell invasion through extracellular matrix and changes in tubule formation by HUVECs, a parameter of angiogenesis, were investigated. Although the cell invasive properties of HCT-116 cells, as determined by their ability to pass through the extracellular matrix, were inhibited by dasatinib, the combination treatment was found to have a greater effect than either agent alone (Figs. 4A). On the other hand, curcumin alone was found to be highly effective in abrogating the sprouting and tubule formation by HUVEC cells. At the end of 12h treatment, HUVECs had completely failed to form closed vesicles that represent the neo-angiogenic potential of the cancer cells (Fig. 4B). Taken together, the results suggest that the combination therapy may be effective in modulating multiple processes of metastasis, by differential inhibition of the processes by dasatinib and curcumin. Curcumin is shown to exert its anti-angiogenic action through inhibition of key effectors of angiogenic process: VEGF and b-FGF 36–37. An indirect role of curcumin in inhibiting angiogenesis is thought to be via inhibition of EGFR and/or its family members and matrix-metallo proteinases (MMPs) 38.
Figure 4.
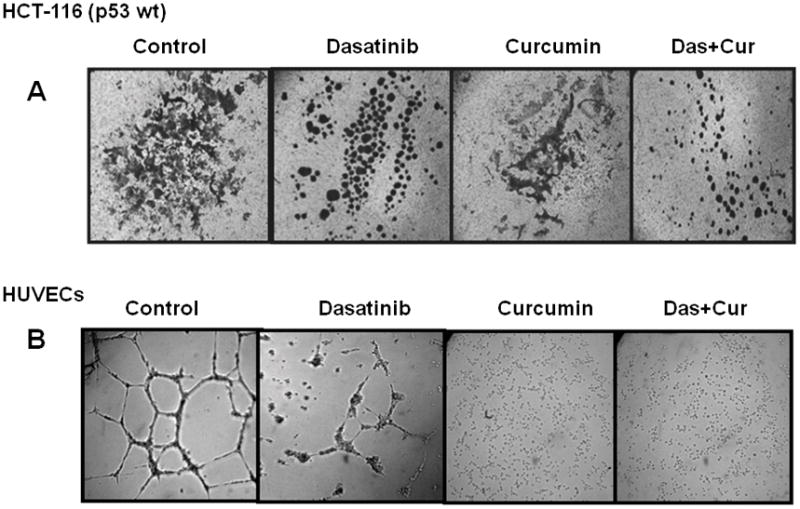
Effects of curcumin and/or dasatinib on (A) extra cellular invasion by colon cancer HCT-116 cells as determined by ECM invasion assay and (B) neo-angiogenesis as determined by tubule formation by HUVECs in the absence (control) or presence of drugs.
Dasatinib and/or curcumin lead to regression of intestinal adenomas in APCMin+/− mice
Next, we determined the therapeutic effectiveness of the combination therapy in regression of adenomas in C57BL/6J-APCMin+/− mice. The APC Min+/− mice, harboring a truncating mutation in codon 850 of the Apc gene 39 develop spontaneous intestinal adenoma are widely used as a model for colorectal cancer. Treatment of Min mice began when most, if not all, tumors had already developed. As shown in Fig. 5A, dasatinib and curcumin, each alone caused a significant (50–85%) regression of tumors in both small intestine and colon. On the other hand, combination therapy caused 90–99% regression of intestinal tumors (Fig 5A). To determine whether the regression of adenomas in response to these treatments could at least in part be due to inhibition of proliferation and stimulation of apoptosis, we analyzed the formalin-fixed intestinal tissues for changes in proliferative activity and apoptosis. While the changes in proliferative activity were examined by counting mitotic bodies in H&E stained sections, apoptosis was determined by TUNEL assay. As shown in Fig 5B, the combination therapy significantly decreased the mitosis and induced apoptosis in the intestinal adenomas.
Figure 5.
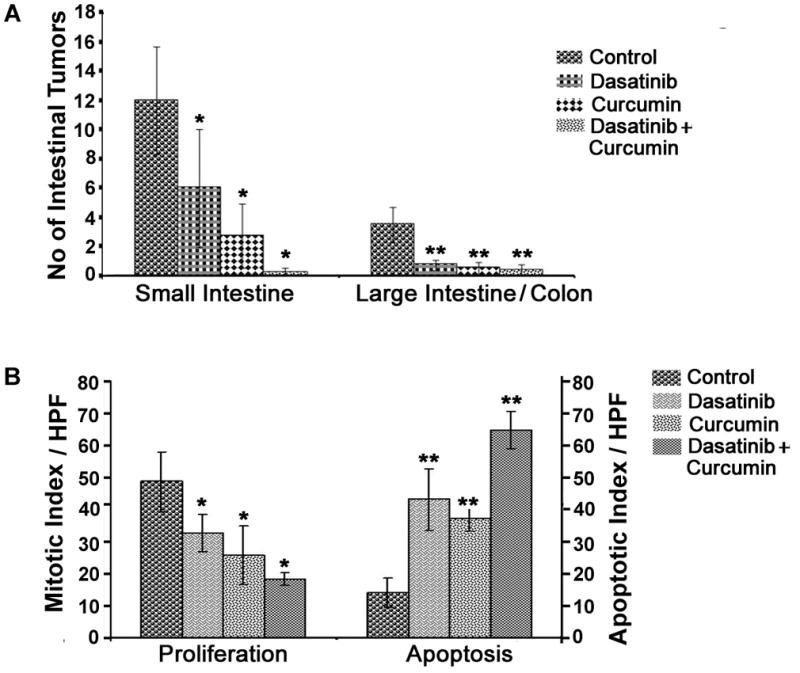
(A) The effects of dasatinib and/or curcumin on regression of intestinal tumors in ApcMin+/− mice. (B) Effects of dasatinib and/or curcumin on proliferation and apoptosis in the tissue remnants from adenomas.
Discussion
Several Src inhibitors including dasatinib, have been tested in solid tumors with limited success 17–18, 40, which could partly be attributed to the presence and dominance of compensatory pathways in the cancer cells. For instance, STAT-3 pathway is inhibited by dasatinib transiently and through a compensatory pathway 41, and is re-activated as early as 24h 42. It has been suggested that STAT-3 inhibitors show synergistic interactions with dasatinib in HNSCC 42. Therefore, in order to achieve a better therapeutic efficacy, targeting multiple pathways simultaneously is warranted. We have reported that dietary agent curcumin enhances the efficacy of Folfox and the pan-erbB inhibitor ERRP in colon cancer cells in vitro 28–29. In the current investigation we further demonstrate that curcumin also synergizes with c-Src targeting therapy; dasatinib and is effective in inhibiting different transformation properties of human colon cancer cells.
Our current observation that curcumin inhibits growth of colon cancer cells that are either p53 functional or mutant in a dose dependent manner is in agreement with what we noted earlier in colon cancer HCT-116 and HT-29 cells 28–29. Interestingly, the growth inhibitory effect of curcumin was found to be greater in colon cancer cells that were p53-negative (HCT-116 p53-null and HT-29) than those that had functional p53. This observation is similar to that reported by Howells et al. 43. Although the reasons for increased sensitivity of p53-negative colon cancer cells to curcumin is not known, it has been suggested by Howells et al. that curcumin exerts its growth inhibitory effect on p53-negative cells by targeting a different pathway 43. Interestingly our data also show for the first time, that the growth inhibitory properties of dasatinib are independent on p53 status, in that both p53-wild type and p53 null colon cancer HCT -116 cells are responsive to the growth inhibitory effect of dasatinib. Additionally, we have also observed that the growth inhibitory effect is more pronounced in response to combination of curcumin and dasatinib at most of the doses tested, but the synergistic interaction appears to be independent of p53 status. Similar p53-independent synergistic interactions of curcumin with oxaliplatin; a standard chemotherapy for colon cancer, had been reported by Howells et al 43. The fact that the synergy between dasatinib and curcumin is independent of p53 status in cancer cells, provides a rationale for utilizing such a combination as a therapeutic strategy for colorectal cancer which harbors 40–50% p53 mutation.
Aberrant activation of growth factor receptors as well as non-receptor tyrosine kinases is often implicated in initiation and progression of cancer 6–8. The combination therapy was found to be effective in inhibiting the activation of EGFRs at different tyrosine residues. The combination therapy inhibited the activation of EGFR in c-Src dependent (tyr-845) as well as c-Src independent manner tyr-1068 and tyr-1173). Cancer cells develop resistance to anti-cancer therapies through overexpression/coexpression of EGFR and/or other HER family receptors 9. Our current observation that the combination and dasatinib also inhibits the activation of HER-2 and HER-3 in colon cancer cells suggests that the combination therapy could be a superior therapeutic strategy for colon cancer. In addition, IGF-1R is often overexpressed in colon cancer 12. The fact that the current combination therapy also causes a marked inhibition of IGF-1R activation in colon cancer cells suggests that the IGF-1R signaling could be effectively attenuated by the combination of curcumin and dasatinib. The mechanisms for attenuation of IGF-1R activation by the combination of curcumin and dasatnib have not been fully elucidated.
The current combination therapy leads to a marked attenuation of downstream signaling, as evidenced by a greater reduction in the levels of the phosphorylated (activated) form of Akt and Erks (1/2), accompanied by a concomitant decrease in the levels of anti-apoptotic protein Bcl-XL and Cox-2. Several in vivo and in vitro studies, including our own have demonstrated that curcumin inhibits COX-2 expression and activity, leading to a reduction in prostaglandin synthesis and loss of cancer cell growth 28, 44–45.
Akt mediated stimulation of cell survival is transduced, in part, by activation of NF-κB 35, 46, which induces the expression of pro-survival genes including Bcl2 47. Several studies have demonstrated that curcumin-mediated growth inhibition of several epithelial cancer cells, including those in the colon is associated with decreased activity of NF-κB 28, 48. Earlier, we reported that the inhibition of growth of colon cancer cells in vitro in response to either curcumin or curcumin together with ERRP is associated with a concomitant inhibition of NF-κB activity 28. The current observation is in line with our previous observation and further demonstrates that the combination therapy causes a greater reduction in DNA binding activity of NF-κB in colon cancer HCT-116 cells than either agent alone.
Curcumin has been reported to affect several processes of cell transformation and metastasis by targeting multiple effector molecules 36. Similarly, dasatinib has been shown to inhibit such properties of cancer cells, primarily by modulating Src family kinases 49. Dasatinib has been reported to inhibit c-Src signaling and thus inhibit cell invasion, migration and invasion in a variety of cancers 17–18, 40–41, 49–52. Our current study demonstrates that dasatinib and curcumin inhibit transformation properties of colon cancer cells differentially. However, the combination treatment of colon cancer cells shows a greater inhibition of several transformation properties like colony formation, cell adhesion and invasion as well as angiogenesis. The combination therapy was also found to be highly effective in regressing adenomas in the small and large intestine in APCMin+/− mice. This could be attributed in part due to modulation of cellular growth involving decreased proliferation and increased apoptosis.
The poor systemic availability of curcumin has raised concerns about its use for the chemoprevention or treatment of malignancies remote from the site of absorption 25. However, for gastrointestinal cancers, it has been suggested that orally administered curcumin may exert its inhibitory effects primarily via luminal and/or intra-mucosal routes [although negligible levels were absorbed into the circulation via this route] 53. Therefore, poor systemic availability would not preclude its use in prevention/treatment of gastrointestinal malignancies 54, as curcumin distribution in the gastrointestinal mucosa is to a great extent, independent of systemic availability 25. In fact the accumulation of curcumin in the intestinal mucosa of mice was shown to be much higher than other organs following feeding of curcumin 25, 55. Our current observation of a significant 77% and 86% reduction in adenomas in the small and large intestine, respectively, in response to curcumin supports the contention that curcumin could be an effective preventive/therapeutic agent for gastrointestinal cancers.
In conclusion, our data show that the combination treatment of dasatinib and curcumin is highly effective in inhibiting the growth of colon cancer cells, in p53 independent manner. Combination therapy leads to attenuation of growth factor receptor (EGFR and IGF-1R) and non-receptor (c-Src) signaling. The combination therapy results in decreased activation of downstream signaling pathways {(Akt and Erk(s)}, associated with decreased NF-κB activity. Our data also show that the two agents affect transformation properties differentially and that the combination of dasatinib and curcumin is a better strategy in inhibiting metastasis. Furthermore, the combination therapy is highly effective in modulating cellular growth leading to regression of intestinal adenomas in preclinical investigations. The data presented above clearly demonstrate that the combination of curcumin and dasatinib is highly effective in suppressing EFGRs, IGF-R and c-Src signaling pathways and processes of development and progression of colon cancers.
Acknowledgments
A part of the work presented in this communication has been supported by grants to Dr Majumdar by the Department of Veterans Affairs (VA Merit Review) and NIH/NIA (AG014343).
Footnotes
Novelty and Impact of the Current Study
Dysregulation of several signal pathways is implicated in the processes of initiation and progression of many malignancies, including colorectal cancer. Numerous studies have demonstrated increased activation of EGFR and its family members (EGFRs), IGF-1R as well as c-Src in colorectal cancer. Therefore targeting multiple signaling pathways forms the rationale for development of therapeutic strategy. FOLFOX remains the mainstay of colorectal cancer treatment, but with limited success. Moreover, the continued use of FOLFOX can lead to additional toxicities, underscoring the need for development of therapeutic strategy that combines non-toxic natural agents to achieve a better clinical outcome.
Our study for the first time demonstrates synergistic interactions between a targeted therapeutic (dasatinib, a c-Src inhibitor) and a non-toxic dietary ingredient (curcumin). Clearly, data derived from our current investigation demonstrate the effectiveness of combining dasatinib with curcumin in the treatment of colon cancer.
References
- 1.Wils J, O’Dwyer P, Labianca R. Adjuvant treatment of colorectal cancer at the turn of the century: European and US perspectives. Ann Oncol. 2001;12:13–22. doi: 10.1023/a:1008357725209. [DOI] [PubMed] [Google Scholar]
- 2.McCarty MF. Targeting multiple signaling pathways as a strategy for managing prostate cancer: multifocal signal modulation therapy. Integr Cancer Ther. 2004;3:349–80. doi: 10.1177/1534735404270757. [DOI] [PubMed] [Google Scholar]
- 3.Harari PM, Huang SM. Modulation of molecular targets to enhance radiation. Clin Cancer Res. 2000;6:323–5. [PubMed] [Google Scholar]
- 4.Messa C, Russo F, Caruso MG, Di Leo A. EGF, TGF-alpha, and EGF-R in human colorectal adenocarcinoma. Acta Oncol. 1998;37:285–9. doi: 10.1080/028418698429595. [DOI] [PubMed] [Google Scholar]
- 5.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 6.Barnard JA, Beauchamp RD, Russell WE, Dubois RN, Coffey RJ. Epidermal growth factor-related peptides and their relevance to gastrointestinal pathophysiology. Gastroenterology. 1995;108:564–80. doi: 10.1016/0016-5085(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 7.Malecka-Panas E, Kordek R, Biernat W, Tureaud J, Liberski PP, Majumdar AP. Differential activation of total and EGF receptor (EGF-R) tyrosine kinase (tyr-k) in the rectal mucosa in patients with adenomatous polyps, ulcerative colitis and colon cancer. Hepatogastroenterology. 1997;44:435–40. [PubMed] [Google Scholar]
- 8.Relan NK, Saeed A, Ponduri K, Fligiel SE, Dutta S, Majumdar AP. Identification and evaluation of the role of endogenous tyrosine kinases in azoxymethane induction of proliferative processes in the colonic mucosa of rats. Biochim Biophys Acta. 1995;1244:368–76. doi: 10.1016/0304-4165(95)00024-6. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Yeung TK, Wang Z. Enhanced Drug Resistance in Cells Coexpressing ErbB2 with EGF Receptor or ErbB3. Biochemical and Biophysical Research Communications. 2000;277:757–63. doi: 10.1006/bbrc.2000.3731. [DOI] [PubMed] [Google Scholar]
- 10.Adachi Y, Lee CT, Coffee K, Yamagata N, Ohm JE, Park KH, Dikov MM, Nadaf SR, Arteaga CL, Carbone DP. Effects of genetic blockade of the insulin-like growth factor receptor in human colon cancer cell lines. Gastroenterology. 2002;123:1191–204. doi: 10.1053/gast.2002.36023. [DOI] [PubMed] [Google Scholar]
- 11.Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta. 2002;1602:114–30. doi: 10.1016/s0304-419x(02)00040-9. [DOI] [PubMed] [Google Scholar]
- 12.Hakam A, Yeatman TJ, Lu L, Mora L, Marcet G, Nicosia SV, Karl RC, Coppola D. Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol. 1999;30:1128–33. doi: 10.1016/s0046-8177(99)90027-8. [DOI] [PubMed] [Google Scholar]
- 13.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–58. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 14.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–61. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 15.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 16.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O’Brien S, Nicaise C, Bleickardt E, Blackwood-Chirchir MA, Iyer V, et al. Dasatinib in Imatinib-Resistant Philadelphia Chromosome-Positive Leukemias. N Engl J Med. 2006;354:2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 17.Johnson FM, Saigal B, Talpaz M, Donato NJ. Dasatinib (BMS-354825) Tyrosine Kinase Inhibitor Suppresses Invasion and Induces Cell Cycle Arrest and Apoptosis of Head and Neck Squamous Cell Carcinoma and Non-Small Cell Lung Cancer Cells. Clin Cancer Res. 2005;11:6924–32. doi: 10.1158/1078-0432.CCR-05-0757. [DOI] [PubMed] [Google Scholar]
- 18.Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, Mirosevich J, Lee FY, Jove R. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–89. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- 19.Lind JS, Herder GJ, Smit EF. [New therapies for the treatment of advanced non-small cell lung cancer: inhibitors of the epidermal growth factor receptor and angiogenesis] Ned Tijdschr Geneeskd. 2008;152:928–32. [PubMed] [Google Scholar]
- 20.Liu B, Fang M, Lu Y, Mills GB, Fan Z. Involvement of JNK-mediated pathway in EGF-mediated protection against paclitaxel-induced apoptosis in SiHa human cervical cancer cells. Br J Cancer. 2001;85:303–11. doi: 10.1054/bjoc.2001.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toda S, Miyase T, Arichi H, Tanizawa H, Takino Y. Natural antioxidants. III. Antioxidative components isolated from rhizome of Curcuma longa L. Chem Pharm Bull (Tokyo) 1985;33:1725–8. doi: 10.1248/cpb.33.1725. [DOI] [PubMed] [Google Scholar]
- 22.Huang MT, Wang ZY, Georgiadis CA, Laskin JD, Conney AH. Inhibitory effects of curcumin on tumor initiation by benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1992;13:2183–6. doi: 10.1093/carcin/13.11.2183. [DOI] [PubMed] [Google Scholar]
- 23.Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–66. [PubMed] [Google Scholar]
- 24.Rao CV, Simi B, Reddy BS. Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis. 1993;14:2219–25. doi: 10.1093/carcin/14.11.2219. [DOI] [PubMed] [Google Scholar]
- 25.Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, Sharma RA, Williams ML, Steward WP, Gescher AJ. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2002;11:535–40. [PubMed] [Google Scholar]
- 26.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Yu Y, Marciniak D, Rishi AK, Sarkar FH, Kucuk O, Majumdar AP. Epidermal growth factor receptor (EGFR)-related protein inhibits multiple members of the EGFR family in colon and breast cancer cells. Mol Cancer Ther. 2005;4:435–42. doi: 10.1158/1535-7163.MCT-04-0280. [DOI] [PubMed] [Google Scholar]
- 28.Reddy S, Rishi AK, Xu H, Levi E, Sarkar FH, Majumdar AP. Mechanisms of curcumin- and EGF-receptor related protein (ERRP)-dependent growth inhibition of colon cancer cells. Nutr Cancer. 2006;55:185–94. doi: 10.1207/s15327914nc5502_10. [DOI] [PubMed] [Google Scholar]
- 29.Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, Rishi AK, Majumdar AP. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122:267–73. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 30.Nautiyal J, Majumder P, Patel BB, Lee FY, Majumdar AP. Src inhibitor dasatinib inhibits growth of breast cancer cells by modulating EGFR signaling. Cancer Lett. 2009 doi: 10.1016/j.canlet.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee S, Hussain M, Wang Z, Saliganan A, Che M, Bonfil D, Cher M, Sarkar FH. In vitro and in vivo molecular evidence for better therapeutic efficacy of ABT-627 and taxotere combination in prostate cancer. Cancer Res. 2007;67:3818–26. doi: 10.1158/0008-5472.CAN-06-3879. [DOI] [PubMed] [Google Scholar]
- 32.Schmelz EM, Xu H, Sengupta R, Du J, Banerjee S, Sarkar FH, Rishi AK, Majumdar AP. Regression of early and intermediate stages of colon cancer by targeting multiple members of the EGFR family with EGFR-related protein. Cancer Res. 2007;67:5389–96. doi: 10.1158/0008-5472.CAN-07-0536. [DOI] [PubMed] [Google Scholar]
- 33.Levi E, Mohammad R, Kodali U, Marciniak D, Reddy S, Aboukameel A, Sarkar FH, Kucuk O, Rishi AK, Majumdar AP. EGF-receptor related protein causes cell cycle arrest and induces apoptosis of colon cancer cells in vitro and in vivo. Anticancer Res. 2004;24:2885–91. [PubMed] [Google Scholar]
- 34.Marciniak DJ, Moragoda L, Mohammad RM, Yu Y, Nagothu KK, Aboukameel A, Sarkar FH, Adsay VN, Rishi AK, Majumdar AP. Epidermal growth factor receptor-related protein: a potential therapeutic agent for colorectal cancer. Gastroenterology. 2003;124:1337–47. doi: 10.1016/s0016-5085(03)00264-6. [DOI] [PubMed] [Google Scholar]
- 35.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 36.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Letters. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: A short review. Life Sciences. 2006;78:2081–87. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Rishi AK, Parikh R, Wali A, Durko L, Zhang L, Yu Y, Majumdar AP. EGF receptor-related protein (ERRP) inhibits invasion of colon cancer cells and tubule formation by endothelial cells in vitro. Anticancer Res. 2006;26:1029–37. [PubMed] [Google Scholar]
- 39.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–4. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 40.Trevino JG, Summy JM, Lesslie DP, Parikh NU, Hong DS, Lee FY, Donato NJ, Abbruzzese JL, Baker CH, Gallick GE. Inhibition of Src Expression and Activity Inhibits Tumor Progression and Metastasis of Human Pancreatic Adenocarcinoma Cells in an Orthotopic Nude Mouse Model. Am J Pathol. 2006;168:962–72. doi: 10.2353/ajpath.2006.050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsao AS, He D, Saigal B, Liu S, Lee JJ, Bakkannagari S, Ordonez NG, Hong WK, Wistuba I, Johnson FM. Inhibition of c-Src expression and activation in malignant pleural mesothelioma tissues leads to apoptosis, cell cycle arrest, and decreased migration and invasion. Mol Cancer Ther. 2007;6:1962–72. doi: 10.1158/1535-7163.MCT-07-0052. [DOI] [PubMed] [Google Scholar]
- 42.Johnson FM, Saigal B, Tran H, Donato NJ. Abrogation of Signal Transducer and Activator of Transcription 3 Reactivation after Src Kinase Inhibition Results in Synergistic Antitumor Effects. Clin Cancer Res. 2007;13:4233–44. doi: 10.1158/1078-0432.CCR-06-2981. [DOI] [PubMed] [Google Scholar]
- 43.Howells LM, Mitra A, Manson MM. Comparison of oxaliplatin- and curcumin- mediated antiproliferative effects in colorectal cell lines. Int J Cancer. 2007;121:175–83. doi: 10.1002/ijc.22645. [DOI] [PubMed] [Google Scholar]
- 44.Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001;172:111–8. doi: 10.1016/s0304-3835(01)00655-3. [DOI] [PubMed] [Google Scholar]
- 45.Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, Dannenberg AJ. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis. 1999;20:445–51. doi: 10.1093/carcin/20.3.445. [DOI] [PubMed] [Google Scholar]
- 46.Moghal N, Sternberg PW. Multiple positive and negative regulators of signaling by the EGF-receptor. Curr Opin Cell Biol. 1999;11:190–6. doi: 10.1016/s0955-0674(99)80025-8. [DOI] [PubMed] [Google Scholar]
- 47.Wang CY, Guttridge DC, Mayo MW, Baldwin AS., Jr NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923–9. doi: 10.1128/mcb.19.9.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siwak DR, Shishodia S, Aggarwal BB, Kurzrock R. Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer. 2005;104:879–90. doi: 10.1002/cncr.21216. [DOI] [PubMed] [Google Scholar]
- 49.Serrels A, Macpherson IRJ, Evans TRJ, Lee FY, Clark EA, Sansom OJ, Ashton GH, Frame MC, Brunton VG. Identification of potential biomarkers for measuring inhibition of Src kinase activity in colon cancer cells following treatment with dasatinib. Mol Cancer Ther. 2006;5:3014–22. doi: 10.1158/1535-7163.MCT-06-0382. [DOI] [PubMed] [Google Scholar]
- 50.Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, Gelovani JG, Kim S-J, Wang Z, Gallick GE. Targeting Src Family Kinases Inhibits Growth and Lymph Node Metastases of Prostate Cancer in an Orthotopic Nude Mouse Model. Cancer Res. 2008;68:3323–33. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- 51.Shor AC, Keschman EA, Lee FY, Muro-Cacho C, Letson GD, Trent JC, Pledger WJ, Jove R. Dasatinib Inhibits Migration and Invasion in Diverse Human Sarcoma Cell Lines and Induces Apoptosis in Bone Sarcoma Cells Dependent on Src Kinase for Survival. Cancer Res. 2007;67:2800–08. doi: 10.1158/0008-5472.CAN-06-3469. [DOI] [PubMed] [Google Scholar]
- 52.Song L, Morris M, Bagui T, Lee FY, Jove R, Haura EB. Dasatinib (BMS-354825) Selectively Induces Apoptosis in Lung Cancer Cells Dependent on Epidermal Growth Factor Receptor Signaling for Survival. Cancer Res. 2006;66:5542–48. doi: 10.1158/0008-5472.CAN-05-4620. [DOI] [PubMed] [Google Scholar]
- 53.Barnes CJ, Lee M. Chemoprevention of spontaneous intestinal adenomas in the adenomatous polyposis coli Min mouse model with aspirin. Gastroenterology. 1998;114:873–7. doi: 10.1016/s0016-5085(98)70305-1. [DOI] [PubMed] [Google Scholar]
- 54.Aggarwal BB, Ichikawa H, Garodia P, Weerasinghe P, Sethi G, Bhatt ID, Pandey MK, Shishodia S, Nair MG. From traditional Ayurvedic medicine to modern medicine: identification of therapeutic targets for suppression of inflammation and cancer. Expert Opin Ther Targets. 2006;10:87–118. doi: 10.1517/14728222.10.1.87. [DOI] [PubMed] [Google Scholar]
- 55.Padhye S, Banerjee S, Chavan D, Pandye S, Swamy KV, Ali S, Li J, Dou QP, Sarkar FH. Fluorocurcumins as Cyclooxygenase-2 Inhibitor: Molecular Docking, Pharmacokinetics and Tissue Distribution in Mice. Pharm Res. 2009 doi: 10.1007/s11095-009-9955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


