Abstract
Background
There is an increased interest in early intervention strategies for severe mental disorders with hopes of mitigating the emergence and impact of the illness. Individuals at clinical high-risk (CHR) for schizophrenia have been primarily identified by the presence of attenuated positive symptoms. Although bipolar disorder and schizophrenia may have overlapping etiologies, few studies have investigated the potential prodrome in bipolar disorder. We sought to determine if there is a prodrome to bipolar disorder and if clinical or neurocognitive measures could distinguish between the bipolar and schizophrenia prodromes.
Methods
We examined subjects who were initially identified as CHR for schizophrenia during the prodromal phase of the illness and followed them prospectively. Unexpectedly, eight subjects developed bipolar disorder. Baseline data from subjects who eventually developed bipolar disorder (pre-BP; N=8), schizophrenia or a psychotic disorder (pre-SZ; N=24) and a non-converter comparison group (NCC; N=115) were compared.
Results
The pre-BP and pre-SZ groups did not differ on attenuated positive symptom severity, global measures of functioning or on the global neurocognitive score. Compared to NCC individuals, both pre-BP and pre-SZ patients reported more severe attenuated positive symptoms and were more likely to be on antipsychotic medication at baseline. The pre-SZ group had a significantly lower current IQ and was significantly more impaired than the NCC group on the overall neurocognitive score.
Conclusions
This study provides preliminary support for a bipolar prodrome, which may be indistinguishable from the schizophrenia prodrome based on clinical and neurocognitive measures currently used in high-risk schizophrenia programs.
Keywords: prodrome, schizophrenia, bipolar disorder, cognition, clinical high-risk, early identification
1. Introduction
Prevention has become an increasingly central interest in psychiatry. To date, research efforts have been directed at developing early interventions for schizophrenia. Although there is considerable question as to whether a clinically-relevant prodromal phase of bipolar disorder can be reliably detected (Skjelstad, Malt et al., 2009), there have been a few retrospective studies showing that individuals with bipolar disorder report subsyndromal symptoms prior to developing the disorder (Conus, Ward et al., 2010; Correll, Penzner et al., 2007a; Egeland, Hostetter et al., 2000; Mantere, Suominen et al., 2008). We found that 100% of adolescents reported prodromal symptoms, which began in 51.9% over a year prior to the first manic episode and in 44.2% between one and twelve months prior (Correll et al., 2007a). Thompson and colleagues (2003) reported three case studies that provided a prospective account of a bipolar prodrome. There is also some evidence that adolescents at genetic high-risk (GHR) for an affective disorder exhibit similar neurocognitive deficits compared to adolescents at GHR for schizophrenia (Maziade, Rouleau et al., 2009; Seidman, Giuliano et al., 2006; Thompson et al., 2003). Although a number of GHR studies have been conducted (Correll, Penzner et al., 2007b), no study to date has prospectively assessed individuals who are at clinical high-risk (CHR) for bipolar disorder.
The goal of this study was to provide initial findings about potential predictors of bipolar disorder in a small, but unique, sample of pre-bipolar adolescents. We hypothesized that there would be a detectable prodrome to bipolar disorder and sought to determine whether differences in the bipolar and schizophrenia prodromes could be detected based on clinical or neurocognitive measures.
2. Methods
Participants included in this report are a subset of the larger Recognition and Prevention (RAP) Program. Written, informed consent was obtained from the patients if they were ≥18 years old, or from their parent (with patient's written assent) if the patient was <18 years. The research protocol was approved by the Institutional Review Board at North Shore-Long Island Jewish Health System (NS-LIJHS). Patients were included in the study if they had a score of moderate or higher on any negative or positive symptom on the Scale of Prodromal Symptoms (SOPS; Miller, McGlashan et al., 1999). Subjects were excluded if they: met DSM-IV criteria for an Axis I schizophrenia-spectrum disorder, major depressive disorder with psychotic features or a bipolar spectrum disorder at baseline; were non-English speaking; had a medical or neurological disorder that could affect brain functioning; drug or alcohol dependence within the past 6 months; or have an estimated IQ below 70.
In the RAP program, 29 CHR patients converted to a schizophrenia spectrum disorder (pre-SZ group; i.e., schizophrenia: N=19; schizoaffective disorder: N=2; schizophreniform disorder: N=2; delusional disorder: N=1; or psychotic disorder NOS: N=5) and 8 converted to a bipolar spectrum disorder (pre-BP group; bipolar I: N=6; bipolar NOS: N=2). Five of the 8 pre-BP subjects reported psychotic features at the time of the first manic episode. Participants missing data from at least 33% of the neurocognitive variables (pre-SZ: N=5) were excluded from the analysis. One hundred and fifteen CHR patients who did not convert to either disorder and who had no missing data were included as a control sample (NCC group). Thus, the final sample consisted of 24 pre-SZ subjects, 8 pre-BP subjects, and 115 NCC subjects.
All participants were assessed at baseline with clinical measures (Table 1) and neurocognitive tests (Table 2). Neurocognitive scores from a healthy control sample (N=38) were used to create a global neuropsychological z-score that was calculated from individual test variables. In all cases, data were statistically evaluated using SPSS (Chicago, Illinois, USA, Version 16.0). Comparisons of demographic variables were performed with an analysis of variance (ANOVA) with group (pre-BP, pre-SZ, NCC) as the between-subjects factor, independent sample t-tests and chi-square analyses. Because there was unequal variance among the three groups and unequal sample size, post-hoc tests were performed using the Games-Howell test of contrasts (Kirk, 1995). Means (and standard deviations) for individual SOPS positive symptom items are presented in Table 5.
Table 1.
Clinical measures
| Clinical Measure | Assessment |
|---|---|
| Schedule for Affective Disorders and Schizophrenia for School-Age Children, Epidemiologic Version (K-SADS-E; Orvaschel and Puig-Antich, 1994) | Axis I diagnoses |
| Structured Interview for Prodromal Syndromes (SIPS) and the Scale of Prodromal Symptoms (SOPS; McGlashan, Miller et al., 2001; Miller et al., 1999) | Prodromal symptoms |
| Global Assessment of Functioning scale (GAF; Hall, 1995) | Global functioning |
| Global Functioning: Social scale (GFS; Auther, Smith et al., 2006; Cornblatt et al., 2007) | Social functioning |
| Global Functioning: Role scale (GFR; Cornblatt et al., 2007; Niendam, Bearden et al., 2006) | Role functioning |
Table 2.
Neurocognitive tests and dependent measures.
| Test | Dependent Measure |
|---|---|
| IQ Measures | |
| Premorbid IQ | |
| WRAT-3 (Wilkinson, 1993) | Age scaled score for words read correctly |
| Current IQa | |
| WISC-III (Wechsler, 1991)/WAIS-R (Wechsler, 1981) | Sum of age scaled score for Vocabulary and Block Design subscales |
| Neurocognitive Battery | |
| Boston Naming Test (Kaplan, Goodglass et al., 1983) | Number of correctly named items |
| CVLT Adult (Delis, Kramer et al., 1987) | |
| CVLT Child (Delis, Kramer et al., 1994) | |
| Total for trials 1–5 | Words recalled in trials 1-5 |
| Short delay free recall | Words recalled after delay |
| CPT-IP (Cornblatt and Keilp, 1994) | d' (for all stimulus sets) |
| 2-, 3-, 4-digits and shapes | |
| COWAT (Benton and Hamsher, 1989) | Words produced in three 60 second trials |
| Finger Tapping Test, Dominant and Nondominant Hand Scores (Reitan, 1979) | Taps in 10 seconds, over 5 trials |
| Grooved Pegboard Test, Dominant and Nondominant Hand Scores (Matthews and Klove, 1964) | Time to place pegs |
| Judgment of Line Orientation (Benton, Sivan et al., 1983) | Lines accurately matched |
| Letter-Number Span (Gold, Carpenter et al., 1997) | Number of correct trials |
| Ruff Figural Fluency Test (Ruff, 1987) | Number of unique designs generated |
| Trail Making Test, Parts A and B (Reitan, 1979) | Time to complete trails |
| WAIS-R/WISC-III (Wechsler, 1981, 1991) | |
| Digit Span, Forward and Backward | Digit sequences recalled |
| Block Design | Correctly reconstruct blocks to recreate patterns on cards |
| Vocabulary | Number of words correctly defined |
| Information | Number of correct responses |
| WMS-R (Wechsler, 1987) | |
| Logical Memory, Immediate and Delayed | Story elements recalled |
| Visual Reproduction, Immediate and Delayed | Number of correctly reproduced figures |
| WSCT (Heaton, Chelune et al., 1993) | Number of perseverative errors |
| WRAT-3 (Wilkinson, 1993) | Total score for words read correctly |
Abbreviations: WRAT-3, Wide-Range Achievement Test, 3rd Edition; WISC-III, Wechsler Intelligence Scale for Children, 3rd Edition; WAIS-R, Wechsler Adult Intelligence Scale, Revised; CVLT, California Verbal Learning Test; CPT-IP, Continuous Performance Test-Identical Pairs; COWAT, Controlled Oral Word Association Test; WMS-R, Wechsler Memory Scale, Revised; WSCT, Wisconsin Card Sorting Test, Version 2.
Estimated current full-scale IQ was derived from the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) for three subjects (BP: N=1; SZ: N=2).
Table 5.
SOPS positive symptom item means ± standard deviations for the pre-BP, pre-SZ and NCC groups.
| Pre-BP Group (N = 8) |
Pre-SZ Group (N = 24) |
NCC Group (N = 115) |
|
|---|---|---|---|
| SOPS Unusual Thought Content | 2.88 ± 2.23 | 2.67 ± 2.35 | 1.77 ± 1.88 |
| SOPS Suspiciousness | 3.25 ± 1.75 | 3.88 ± 2.23 | 2.36 ± 1.71 |
| SOPS Grandiose Ideas | 0.25 ± 0.46 | 1.67 ± 1.93 | 0.44 ± 1.09 |
| SOPS Perceptual Abnormalities | 3.25 ± 2.87 | 2.54 ± 2.21 | 1.50 ± 1.93 |
| SOPS Disorganized Communication | 1.25 ± 1.58 | 2.50 ± 1.72 | 1.18 ± 1.40 |
Abbreviations: BP, bipolar disorder; SZ, schizophrenia; NCC, non-converter comparison group; SOPS, Scale of Prodromal Symptoms.
3. Results
All results are presented in Table 3 and Table 4. There were no differences among the groups on age, gender or ethnicity. The pre-SZ group had a significantly lower current IQ than the NCC group (p<0.05); however, there was no difference between the pre-BP and pre-SZ groups, nor between the pre-BP and NCC groups' premorbid or current IQ. When comparing the pre-BP and pre-SZ groups, the age of disorder onset was similar, however pre-BP subjects took longer to develop the full-blown disorder after being identified as a high-risk subject than the pre-SZ subjects (p<0.05). The pre-BP and pre-SZ groups did not differ on what medication they were taking at baseline, but the NCC group was significantly less likely to be on an antipsychotic medication at baseline.
Table 3.
Demographic data means ± standard deviations for the pre-BP, pre-SZ and NCC groups.
| Pre-BP Group (N = 8) | Pre-SZ Group (N = 24) | NCC Group (N = 115) | F/t/χ2 | p | Post-hoc tests (p-value) | |||
|---|---|---|---|---|---|---|---|---|
| Pre-BP vs. Pre-SZ | Pre-BP vs. NCC | Pre-SZ vs. NCC | ||||||
| Age | 15.85 ± 1.87 | 17.11 ± 2.17 | 16.03 ± 2.18 | 2.61 | ns | - | - | - |
| Sex, N (%) male | 5 (62.5%) | 20 (83.3%) | 76 (66.1%) | 2.90 | ns | - | - | - |
| Ethnicity, N (%) Caucasian | 6 (75%) | 17 (70.8%) | 96 (83.5%) | 3.33 | ns | - | - | - |
| Current IQ | 94.50 ± 23.21 | 96.30 ± 12.51 | 104.96 ± 16.67 | 3.77 | < 0.05 | ns | ns | < 0.05 |
| Premorbid IQ | 103.12 ± 14.54 | 102.38 ± 15.65 | 106.43 ± 11.75 | 1.19 | ns | - | - | - |
| Age of disorder onset | 18.43 ± 3.08 | 18.41 ± 2.29 | N/A | 0.02 | ns | - | - | - |
| Disorder onset after identified as high-risk (days) | 941.88 ± 641.24 | 475.50 ± 447.20 | N/A | 2.29 | < 0.05 | - | - | - |
| On antipsychotic at baseline, N (%) | 4 (50%) | 14 (58.3%) | 17 (14.8%) | 23.96 | < 0.001 | ns | < 0.01 | < 0.001 |
| On antidepressant at baseline, N (%) | 2 (25%) | 5 (20.8%) | 28 (24.3%) | 0.14 | ns | - | - | - |
| On mood stabilizer at baseline, N (%) | 1 (12.5%) | 1 (4.2%) | 4 (3.5%) | 1.56 | ns | - | - | - |
| On stimulant at baseline, N (%) | 1 (12.5%) | 0 (0.0%) | 8 (7%) | 2.27 | ns | - | - | - |
| On anxiolytic at baseline, N (%) | 2 (25%) | 4 (16.7%) | 10 (8.7%) | 3.04 | ns | - | - | - |
Abbreviations: BP, bipolar disorder; SZ, schizophrenia; NCC, non-converter comparison group; ns, non-significant; N/A, not applicable.
Table 4.
Clinical data means ± standard deviations for the pre-BP, pre-SZ and NCC groups.
| Pre-BP Group (N = 8) | Pre-SZ Group (N = 24) | NCC Group (N = 115) | F | p | Post-hoc tests (p-value) | |||
|---|---|---|---|---|---|---|---|---|
| Pre-BP vs. Pre-SZ | Pre-BP vs. NCC | Pre-SZ vs. NCC | ||||||
| SOPS Total Positive Score | 10.88 ± 3.40 | 13.25 ± 7.04 | 7.25 ± 5.01 | 13.48 | < 0.001 | ns | < 0.05 | <0.001 |
| GAF Score | 48.62 ± 12.34 | 41.78 ± 10.32 | 46.80 ± 8.53 | 3.28 | < 0.05 | ns | ns | = 0.09 |
| Global Functioning: Role Score | 5.25 ± 2.96 | 5.29 ± 2.16 | 5.63 ± 2.17 | 0.32 | ns | ns | ns | ns |
| Global Functioning: Social Score | 6.12 ± 2.75 | 5.42 ± 1.35 | 6.04 ± 1.40 | 1.83 | ns | ns | ns | ns |
| Global Neurocognitive Score | -2.85 ± 3.86 | -2.32 ± 2.18 | -0.73 ± 1.69 | 10.12 | < 0.001 | ns | ns | < 0.01 |
Abbreviations: BP, bipolar disorder; SZ, schizophrenia; NCC, non-converter comparison group; SOPS, Scale of Prodromal Symptoms; GAF, Global Assessment of Functioning; ns, non-significant.
Both the pre-BP and pre-SZ groups reported significantly more severe positive symptoms than the NCC group (p<0.05 and p<0.001, respectively), without differences between the pre-BP and pre-SZ groups. There were no group differences on the functioning measures, with the exception of a trend (p=0.09) towards the pre-SZ group having a lower GAF score than the NCC group.
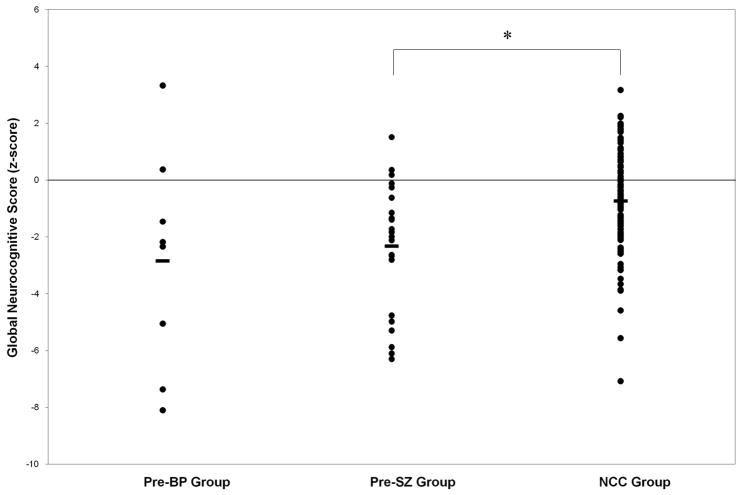
The pre-BP group did not differ from either the pre-SZ or NCC groups on the global neurocognitive score. However, the pre-SZ group was significantly more impaired than the NCC group (Table 3, Figure 1), even when covarying for current IQ (F(2,142) = 5.78, p < 0.01).
Figure 1.
Individual global neurocognitive scores for pre-BP, pre-SZ and NCC subjects. Group means are represented by a solid horizontal bar. The line at the zero z-score indicates the mean for the healthy control group used to normalize the data. The asterisk indicates that the pre-SZ group had significantly lower global neurocognitive scores than the NCC group (p < 0.01).
4. Discussion
This is the first study to assess clinical and neurocognitive characteristics during the prodromal phase of bipolar disorder compared to the prodromal phase of schizophrenia. There was no difference between the pre-BP and pre-SZ subjects on total positive symptom scores at baseline. Although most individual items were similar across the two groups (Table 5), grandiosity was low in the pre-BP group. On average, the pre-BP group reported more perceptual abnormalities, whereas the pre-SZ group reported more disorganized communication. Overall, the NCC group scored lower on the total positive symptom score compared to both groups and was less likely to be on antipsychotics at baseline. These trends may be informative for future research to differentiate risk for bipolar disorder and schizophrenia.
All three groups were impaired on social and role functioning. Overall, these findings are consistent with the literature, which shows that individuals in the prodromal phase of schizophrenia have impaired social and role functioning (Cornblatt, Auther et al., 2007). Additionally, bipolar disorder patients do exhibit some variability in social and role functioning (Goldberg and Harrow, 2004). Thus, the data show that deficits in functioning are present prior to the development of the full-blown disorder, however these deficits were not specific to conversion in this sample.
Pre-SZ subjects had significant neurocognitive deficits compared to NCC subjects, yet the NCC subjects were almost one standard deviation below healthy controls. This is consistent with the literature, with some neurocognitive deficits in CHR subjects, but more extensive neurocognitive deficits in subjects who eventually converted to psychosis (Eastvold, Heaton et al., 2007; Keefe, Perkins et al., 2006; Lencz, Smith et al., 2006). However, we did not find a difference between pre-BP subjects and the NCC group on neurocognitive function. On average, though, the pre-BP group performed worse than the NCC group. It is difficult to know the extent to which neurocognitive deficits are a characteristic of the bipolar prodrome. Furthermore, the data from the bipolar group had a large degree of variance, which is consistent with what is observed in cognitive performance in bipolar patients (Martino, Strejilevich et al., 2008).
The main limitation of this study is its small sample size, particularly in the pre-BP group. Most of the pre-BP subjects received their diagnosis less than 6 months after disorder onset (2 subjects were diagnosed over 2 years later), therefore long term follow-up is also needed to confirm the diagnosis of bipolar disorder and not schizoaffective disorder. Further limitations include an ascertainment bias towards individuals who were recruited for a study assessing the prodrome to schizophrenia, and limited measures specific to bipolar disorder. However, the results are strengthened by the fact that they are based on prospective data in adolescents with a true prodrome. Given that only 35% of CHR individuals develop psychosis (Cannon, Cadenhead et al., 2008), results from other high-risk studies are likely skewed by false-positives.
Results from this study support the notion that patients can be identified during the bipolar prodrome, and that at least some of its features may overlap clinically and neurocognitively with the schizophrenia prodrome. Although not the central focus of this paper, we found that individuals who convert to full-blown bipolar disorder or schizophrenia can be differentiated from non-converters based on clinical severity, and in the case of schizophrenia, neurocognitive deficits. Overall, these data are preliminary, but they suggest that the CHR approach is feasible in bipolar disorder, and calls for the need to develop prodromal measures specific for defining CHR for bipolar disorder.
Acknowledgments
The authors would like to thank Pradeep Nagachandran, M.D. and Ruth Olsen, B.S. for their assistance in carrying out this study.
Role of Funding Source
Funding for this study was provided by National Institute of Mental Health (NIMH) grants MH-079326 and MH-61523 awarded to Dr. Cornblatt and MH-074543 to Dr. John Kane for the Zucker Hillside Hospital NIMH Advanced Center for Intervention and Services Research for the Study of Schizophrenia. NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
Dr. Correll has been a consultant and/or advisor to Actelion, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Janssen/J&J, GSK, Hoffmann-La Roche, Lundbeck, Otsuka, Pfizer, Schering-Plough, Supernus, Takeda and Vanda. Dr. Cornblatt was the original developer of the CPT-IP and has been an advisor for Merck. All other authors declare that they have no conflicts of interest.
Contributors
Doreen M. Olvet undertook the statistical analysis and wrote the manuscript. Walter H. Stearns assisted in writing the manuscript. Danielle McLaughlin collected the data and edited the manuscript. Andrea M. Auther designed the study, collected the data and edited the manuscript. Christoph U. Correll designed the study and edited the manuscript. Barbara A. Cornblatt designed the study and edited the manuscript.
References
- Auther A, Smith CW, et al. Global Functioning: Social Scale (GF: Social) Zucker Hillside Hospital; Glen Oaks, NY: 2006. [Google Scholar]
- Benton AL, Hamsher K. Multilingual Aphasia Examination. AJA Associates; Iowa City, IA: 1989. [Google Scholar]
- Benton AL, Sivan AB, et al. Judgement of Line Orientation, Form H. Oxford University Press; New York: 1983. [Google Scholar]
- Cannon TD, Cadenhead K, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conus P, Ward J, et al. Characterisation of the prodrome to a first episode of psychotic mania: Results of a retrospective study. J Affect Disord. 2010 doi: 10.1016/j.jad.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Auther AM, et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33(3):688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull. 1994;20(1):31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- Correll CU, Penzner JB, et al. Differentiation in the preonset phases of schizophrenia and mood disorders: evidence in support of a bipolar mania prodrome. Schizophr Bull. 2007a;33(3):703–714. doi: 10.1093/schbul/sbm028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Penzner JB, et al. Early identification and high-risk strategies for bipolar disorder. Bipolar Disord. 2007b;9(4):324–338. doi: 10.1111/j.1399-5618.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, et al. California Verbal Learning Test Manual. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Delis DC, Kramer JH, et al. Manual for the California Verbal Learning Test Manual-Children's Version. The Psychological Corporation; San Antonio, TX: 1994. [Google Scholar]
- Eastvold AD, Heaton RK, et al. Neurocognitive deficits in the (putative) prodrome and first episode of psychosis. Schizophr Res. 2007;93(1-3):266–277. doi: 10.1016/j.schres.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland JA, Hostetter AM, et al. Prodromal symptoms before onset of manic-depressive disorder suggested by first hospital admission histories. J Am Acad Child Adolesc Psychiatry. 2000;39(10):1245–1252. doi: 10.1097/00004583-200010000-00011. [DOI] [PubMed] [Google Scholar]
- Gold JM, Carpenter C, et al. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54(2):159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Goldberg JF, Harrow M. Consistency of remission and outcome in bipolar and unipolar mood disorders: a 10-year prospective follow-up. J Affect Disord. 2004;81(2):123–131. doi: 10.1016/S0165-0327(03)00161-7. [DOI] [PubMed] [Google Scholar]
- Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36(3):267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, et al. The Wisconsin Card Sorting Test Manual-Revised and Expanded. Psychological Assessment Resources, Inc; Odessa, FL: 1993. [Google Scholar]
- Kaplan EF, Goodglass H, et al. The Boston Naming Test. 2nd. Lea & Febiger; Philadelphia, PA: 1983. [Google Scholar]
- Keefe RS, Perkins DO, et al. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophr Res. 2006;88(1-3):26–35. doi: 10.1016/j.schres.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. 3rd. Brooks/Cole Publishing; New York: 1995. [Google Scholar]
- Lencz T, Smith CW, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59(9):863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Mantere O, Suominen K, et al. Only half of bipolar I and II patients report prodromal symptoms. J Affect Disord. 2008;111(2-3):366–371. doi: 10.1016/j.jad.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Martino DJ, Strejilevich SA, et al. Heterogeneity in cognitive functioning among patients with bipolar disorder. J Affect Disord. 2008;109(1-2):149–156. doi: 10.1016/j.jad.2007.12.232. [DOI] [PubMed] [Google Scholar]
- Matthews CG, Klove H. Instruction Manual for the Adult Neuropsychology Test Battery. University of Wisconsin Medical School; Madison, WI: 1964. [Google Scholar]
- Maziade M, Rouleau N, et al. Shared neurocognitive dysfunctions in young offspring at extreme risk for schizophrenia or bipolar disorder in eastern quebec multigenerational families. Schizophr Bull. 2009;35(5):919–930. doi: 10.1093/schbul/sbn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan T, Miller TJ, et al. Instrument for the Assessment of Prodromal Symptoms and States. In: Miller TJ, Mednick SA, editors. Early Intervention in Psychotic Disorders. Springer-Verlag; New York: 2001. pp. 135–149. [Google Scholar]
- Miller TJ, McGlashan TH, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70(4):273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Niendam T, Bearden CE, et al. Global Functioning: Role Scale (GF: Role) University of California, Los Angeles; Los Angeles, CA: 2006. [Google Scholar]
- Orvaschel H, Puig-Antich J. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Epidemiologic Version. Center for Psychological Studies, Nova Southeastern University; Fort Lauderdale, FL: 1994. [Google Scholar]
- Reitan RM. Manual for Administration of Neuropsychological Test Batteries for Adults and Children. Reitan Neuropsychology Laboratories, Inc.; Tuscon: 1979. [Google Scholar]
- Ruff RM. The Ruff Figural Fluency Test: A normative study with adults. Developmental Neuropsychology. 1987;3:37–52. [Google Scholar]
- Seidman LJ, Giuliano AJ, et al. Neuropsychological functioning in adolescents and young adults at genetic risk for schizophrenia and affective psychoses: results from the Harvard and Hillside Adolescent High Risk Studies. Schizophr Bull. 2006;32(3):507–524. doi: 10.1093/schbul/sbj078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjelstad DV, Malt UF, et al. Symptoms and signs of the initial prodrome of bipolar disorder A systematic review. J Affect Disord. 2009 doi: 10.1016/j.jad.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Thompson KN, Conus PO, et al. The initial prodrome to bipolar affective disorder: prospective case studies. J Affect Disord. 2003;77(1):79–85. doi: 10.1016/s0165-0327(02)00100-3. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale, Revised. The Psychological Corporation; New York, NY: 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale, Revised. The Psychological Corporation; New York, NY: 1987. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd. The Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Intelligence Scale (WASI) The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test Administration Manual. Wide Range, Inc; Wilmington, DE: 1993. [Google Scholar]