Abstract
Background
Regional prefrontal cortex gray matter reductions have been identified in schizophrenia, likely reflecting a combination of genetic vulnerability and disease effects. Few morphometric studies to date have examined regional prefrontal abnormalities in non-psychotic biological relatives who have not passed through the age range of peak risk for onset of psychosis. We conducted a region-of-interest morphometric study of prefrontal subregions in adolescent and young adult relatives of schizophrenia patients.
Methods
Twenty-seven familial high-risk (FHR) first-degree relatives of schizophrenia patients and forty-eight control subjects without a family history of psychosis (ages 13–28) underwent high-resolution magnetic resonance imaging at 1.5 Tesla. The prefrontal cortex was parcellated into polar, dorsolateral, ventrolateral, ventromedial and orbital subregions. The Chapman scales measured subpsychotic symptoms. General linear models examined associations of prefrontal subregion volumes with familial risk and subpsychotic symptoms.
Results
FHR subjects had significantly reduced bilateral ventromedial prefrontal and frontal pole gray matter volumes compared with controls. Ventromedial volume was significantly negatively correlated with magical ideation and anhedonia scores in FHR subjects.
Conclusions
Selective, regional prefrontal gray matter reductions may differentially mark genetic vulnerability and early symptom processes among non-psychotic young adults at familial risk for schizophrenia.
Keywords: schizophrenia, magnetic resonance imaging, prefrontal cortex, genetic risk, adolescence, young adulthood
1. Introduction
Since schizophrenia was first posited to be a brain disorder a century ago (Bleuler, 1911; Kraepelin, 1919), a wealth of postmortem and neuroimaging evidence has provided robust confirmation (Johnstone et al., 1976; Selemon and Goldman-Rakic, 1999; Shenton et al., 2001). Among many brain regions, the prefrontal cortex (PFC) has been implicated by an impressive variety of empirical research, and figures prominently in theoretical accounts of schizophrenia (Keshavan et al., 1994; Seidman, 1983; Weinberger, 1987). Schizophrenia has been associated with reduced prefrontal cortical thickness (e.g., (Selemon et al., 1995)), and with reduced gray matter (GM) in lateral, medial, and orbital prefrontal areas (Gur et al., 2000; Kuperberg et al., 2003; Yamada et al., 2007), although the particular subregions implicated vary across studies and negative reports exist (Chemerinski et al., 2002).
Genetic predisposition accounts for approximately 80% of liability for schizophrenia, and is thought to alter brain development in ways that affect an individual’s probability of developing psychosis (Keshavan and Hogarty, 1999; Tsuang, 2001). Thus, one way of parsing the heterogeneous findings on PFC morphometry is to disambiguate aspects of pathology related to genetic risk for schizophrenia from those associated with the disease process. Studies of patients’ nonpsychotic first-degree relatives can identify brain alterations that mark genetic (familial) loading for schizophrenia.
Anatomical MRI studies of PFC subregions in older adult relatives, who have passed through the age of peak risk for schizophrenia (> age 30), have reported deviations in prefrontal GM integrity, although their regional specificity has varied. One voxel-based morphometry (VBM) investigation found bilateral orbitofrontal cortex (OFC) GM deficits in 36 adult siblings of schizophrenia patients compared with 37 control subjects (McDonald et al., 2004), while two similar sized studies of discordant twin pairs reported a left-sided OFC GM deficit in relation to genetic loading (Hulshoff Pol et al., 2006), or no OFC deficit (Cannon et al., 2002). The latter twin study reported liability-associated GM deficits in the frontal pole and dorsolateral PFC (DLPFC). Some subsequent investigations also found that lateral prefrontal GM was reduced in adult relatives [(McDonald et al., 2006; McIntosh et al., 2006), but see (Borgwardt et al., 2010; McIntosh et al., 2004)], or was inversely correlated with continuous measures of genetic liability (Cannon et al., 2002; McIntosh et al., 2006). However, in the largest VBM study of adult relatives, unaffected siblings showed a trend for increased lateral PFC GM compared with controls, along with significantly decreased medial PFC and frontal pole GM densities (Honea et al., 2008).
The PFC undergoes maturational alterations in gray matter through the third decade of life, and pathological deviations of these processes may occur in association with both inherited risk and emerging psychosis (Cannon, 2005). Yet, there are few morphometric studies of PFC GM abnormalities in relatives younger than 30, who have not passed through the age of peak risk for psychosis onset (Seidman et al., 2006). In the only region-of-interest (ROI) study of the PFC in young relatives, Lawrie and collaborators (2001) found no significant difference in total PFC GM volumes between FHR and control adolescents of the Edinburgh High Risk Project (EHRP); however, within FHR subjects, total PFC volume did correlate with a quantitative estimate of genetic liability. In subsequent VBM studies, medial PFC GM density emerged as significantly lower in FHR adolescents than controls, and significantly higher in FHR adolescents than first-episode schizophrenia patients (Job et al., 2003; Lawrie et al., 2008). In a different cohort, Diwadkar and colleagues (2006) found reduced regional GM densities of the ventral- and dorsal- lateral PFC in FHR adolescents compared with controls; moreover, DLPFC GM deficits were more pronounced among FHR adolescents with subpsychotic symptoms. In combination, these structural MRI studies in young relatives point to a dual association of medial and DLPFC GM with risk and early disease processes.
We report on an ROI morphometry study of PFC subregions in young relatives of schizophrenia patients. Hand-traced ROI-based morphometry, though labor-intensive and time-consuming, is still considered the gold standard for validating the more exploratory findings of automated VBM studies (Giuliani et al., 2005; Honea et al., 2005; Kubicki et al., 2002). This cross-sectional study tested the hypothesis that FHR subjects would show regional reductions in ventromedial and DLPFC GM volumes compared with controls, and that GM volumes of these subregions would be inversely correlated with subpsychotic symptoms in FHR subjects (i.e., smaller volumes, more symptoms).
2. Methods
2.1. Subjects
Subjects were 27 antipsychotic-naïve FHR children and siblings of persons with DSM-IV (APA, 2000) schizophrenia or schizoaffective disorder, depressed type, and 48 children of healthy adults with no family history of psychosis, selected to be comparable on age (13–28 years) and other demographic variables. They were recruited as part of the Harvard Adolescent Genetic Risk Study, previously described in detail (Glatt et al., 2006; Seidman et al., 2006a,b).
Participants were excluded if they had any lifetime history of psychotic illness, substance dependence, neurological disease, head injury or medical illness with demonstrated cognitive sequelae, sensory impairments, current psychotropic medication use, or a full-scale IQ estimate less than 70. Control subjects had an additional exclusion criterion of any first-degree biological relative with lifetime history of psychotic disorder.
Adult patient probands were drawn from respondents to local newspaper advertisements and announcements posted at Boston area hospitals. Adult control probands responded to similar advertisements in the same catchment areas. After probands gave consent, their children and siblings were contacted to determine eligibility and willingness to participate as study subjects. Human research committees of Massachusetts Mental Health Center, Massachusetts General Hospital, and Harvard University approved the study. Subjects 18 years and older gave written informed consent. Subjects younger than 18 gave assent while their legal guardian provided consent.
2.2. Psychiatric assessment
Adult patient and control probands completed the Diagnostic and Family Interviews for Genetic Studies (Maxwell, 1996; Nurnberger Jr. et al., 1994). Relatives of probands were screened for psychosis, substance use, and mood disturbance using the Washington University Kiddie Schedule for Affective Disorders and Schizophrenia (Geller et al., 1996).
2.3. Subpsychotic symptoms
Subjects completed the Chapman psychosis proneness scales. The Revised Physical Anhedonia Scale (RPAS) assesses reduced capacity to experience physical and sensory pleasures (e.g., “I have often felt uncomfortable when friends touch me”) (Chapman et al., 1976). The Perceptual Aberration Scale (PAS) (Chapman et al., 1978) taps perceptual distortions that don’t reach the severity of hallucinations (e.g., “Normal colors sometimes seem much too bright to me”). The Magical Ideation Scale (MIS) (Eckblad and Chapman, 1983) inquires about ideas of reference and odd beliefs (e.g., “I might cause something to happen just by thinking too much about it”). Subpsychotic symptom data from all study subjects were previously published (Glatt et al., 2006) and those for this sample of subjects with MRI data are presented in Table 2.
Table 2.
Scores on Chapman Psychosis Proneness Scales (Mean ± SD) for young adults at familial high-risk (FHR) for schizophrenia and control subjects
| FHR n = 27 |
Controls n = 48 |
T-test | p | ||
|---|---|---|---|---|---|
| Psychosis Proneness Scales | |||||
| Physical Anhedonia (RPAS) | 17.2 ± 8.5 | 13.0 ± 8.1 | 2.06 | .04 | |
| Magical Ideation (MIS) | 6.4 ± 4.5 | 5.9 ± 4.8 | 0.40 | .70 | |
| Perceptual Aberrations (PAS) | 3.8 ± 4.3 | 3.2 ± 4.3 | 0.55 | .58 | |
Note: Higher scores indicate more abnormality.
2.4. MRI methods
2.4.1. Acquisition
Whole brain MR images were collected on a Siemens 1.5 Tesla scanner at the Massachusetts General Hospital (MGH) Martinos Center (Charlestown, Massachusetts). A coronal T2-weighted sequence ruled out clinical neuropathology. Two sagittal 3D MP-RAGE, T1-weighted, non-selective inversion-prepared spoiled gradient echo pulse sequences were used for morphometric analyses (TR/TE/T1/flip=2.73s/3.39ms/1.0s/7, bandwidth=190 Hz/pixel, sampling matrix=256×192 pixels, FOV=256×256 mm, effective slice thickness=1.33mm on a 170mm slab of 128 partitions). Images were coded for blind image analysis and transferred to the MGH Center for Morphometric Analysis (CMA).
2.4.2. Gray and white matter segmentation
Brain images were positionally normalized to overcome variations in head position by using a standard 3-dimensional coordinate system (Filipek et al., 1994). This procedure uses the midpoints of the decussations of the anterior and posterior commissure lines and the midsagittal plane at the level of the posterior commissure as points of reference for rotation and translation. Images then were segmented using a semi-automated intensity contour algorithm for external border definition and signal intensity histogram distributions for delineation of gray-white borders.
2.4.3. Cortical parcellation
The neocortex was divided into 48 parcellation units (PUs) per hemisphere (Caviness et al., 1996; Rademacher et al., 1992). This parcellation system approximates architectonic and functional subdivisions, and is based on specific anatomical landmarks present in all brains. All morphometric measurements were performed blind to identifying information. The first author (IMR) parcellated 60 of the 75 subjects, after achieving excellent interrater reliability with a previously trained technician who had parcellated the other 15 brains (ICCs ≥ .90). Volumes (ml) were calculated by multiplying the area of each PU by slice thickness, and then summing over all slices containing the PU.
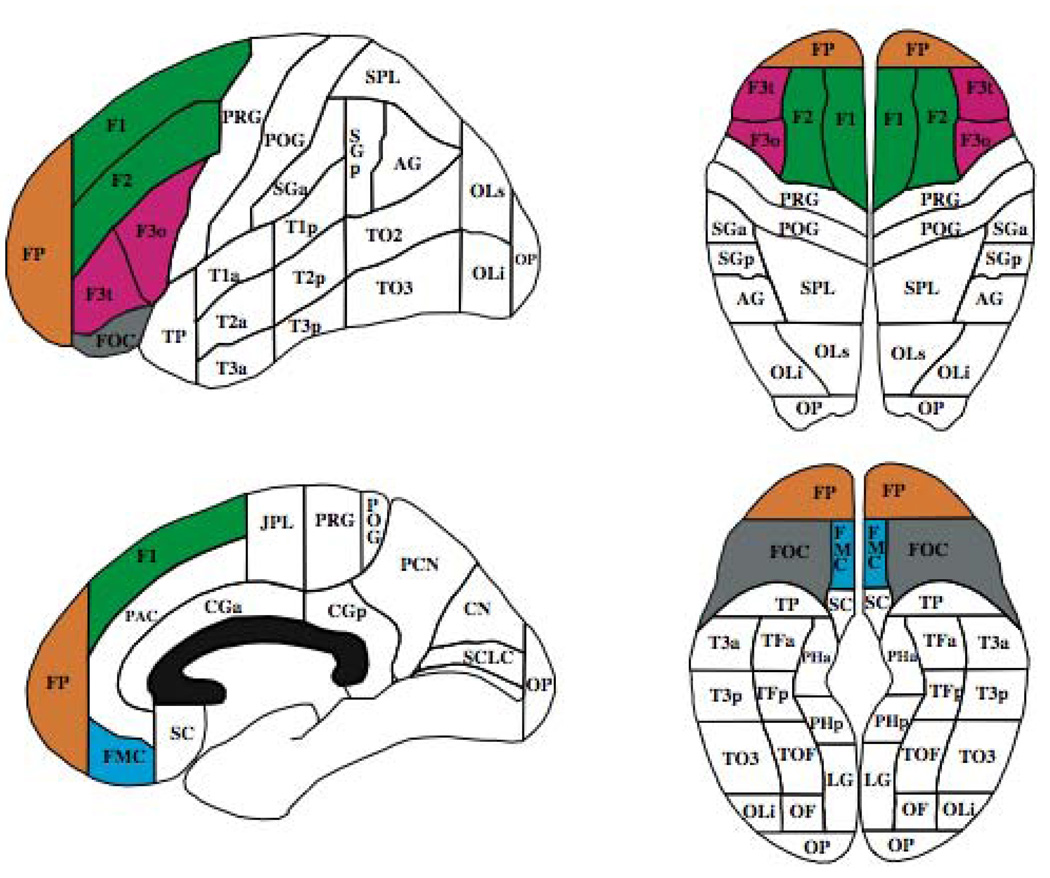
PFC ROIs are shown in Figure 1. The frontal pole (FP) was bordered posteriorly by a coronal plane set at the rostral end of the anterior horizontal ramus of the sylvian fissure (AHRS). FP bordered all other PFC ROIs anteriorly. The dorsolateral PFC (F1+F2) included the superior and middle frontal gyri, with the central sulcus as its posterior border, the inferior frontal sulcus as its lateral inferior border, and the paracingulate sulcus as its medial inferior border. The ventrolateral PFC (F3t+F3o) had as its superior border the inferior frontal sulcus, the AHRS as its lateral inferior border, and the central sulcus as its posterior border. The ventromedial PFC (FMC) was bordered laterally by the olfactory sulcus, superiorly by the paracingulate sulcus, and medially by the interhemispheric fissure. The orbital PFC (FOC) was bordered posteriorly by the basal forebrain, laterally by the olfactory sulcus, and medially by the AHRS.
Figure 1.
Parcellation units comprising the prefrontal cortical (PFC) regions of interest: frontal pole (FP; orange), dorsolateral PFC (F1+F2; green), ventrolateral PFC (F3t+F3o; pink), ventromedial PFC (FMC; blue), orbital PFC (FOC; gray).
2.5. Statistical analyses
All analyses used relative volumes of PFC ROIs (absolute volume/ total cerebral volume *100) to control for scaling effects of brain size. Two sets of hypothesis-driven analyses were conducted: 1) Repeated measures multivariate analysis of covariance (MANCOVA) examined group differences in regional PFC volumes. Prefrontal volumes were the dependent variables using region/ROI (ventromedial, dorsolateral, ventrolateral, orbital, frontal pole) and hemisphere (left, right) as within-subject repeated measures. Familial risk group (FHR, controls) was the independent variable and age was an à priori covariate. Main or interaction effects of group were followed by least square mean contrasts only if they were statistically significant (p ≤.05) at the multivariate level, and after collapsing across any non-significant within-subject dimensions, in order to protect overall Type I error rate. Effect sizes were computed using Cohen’s d (Cohen, 1988). Although sex and its interactions were entered in the initial MANCOVA, they were not included in the final model because their effects were minimal (p’s >.90). For PFC ROIs found to differ significantly between groups, mixed effects ANCOVAs evaluated the effect of familiality; since these mixed models did not alter any findings, their results are not detailed. 2) Pearson’s r examined associations of psychosis proneness with PFC GM within groups, limiting these correlations to ventromedial and dorsolateral subregions as à priori hypotheses. All p-values are two-tailed.
3. Results
Demographic data are presented in Table 1. Groups were comparable except for a significantly lower parental SES in the FHR group.
Table 1.
Demographic characteristics [Mean ± SD or N (%)] of youth at familial high-risk (FHR) for schizophrenia and control subjects
| FHR n = 27 |
Controls n = 48 |
p | |
|---|---|---|---|
| Age (yrs) | 19.0 ± 4.2 | 17.7 ± 3.7 | .15 |
| Female | 12 (44%) | 28 (58%) | .25 |
| Caucasian | 14 (52%) | 29 (60%) | .15 |
| Right-handed | 25 (93%) | 42 (89%) | .64 |
| Education (yrs) | 10.7 ± 2.7 | 11.1 ± 3.3 | .67 |
| Parental SES a | 38 ± 26a | 47 ± 15a | .01 |
| Full-Scale IQ b | 97.4 ± 11.3 | 103.2 ± 15.4 | .10 |
SES: Socioeconomic status, assessed with the Hollingshead Index
Full-Scale IQ: Prorated from 8 subtests of the Wechsler Intelligence Scale for Children-Third Edition (WISC-III)56 or the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III)
3.1. Repeated measures MANCOVA
The main effect of group was nonsignificant (F= 3.023, df= 1/72, p= .09), while the interaction of group with region was statistically significant (F= 2.62, df= 4/69, p= .04), indicating that FHR and control subjects differed on some prefrontal regions but not others. The interactions of group with hemisphere and region-within-hemisphere were not significant (p’s > .20). Age was a significant covariate (F= 20.40, df= 1/72, p<.001) and interacted significantly with region (F= 5.97, df= 4/69, p≤.001), but not with hemisphere or region-within-hemisphere.
Least square mean contrasts for the significant group × region interaction, adjusting for age and collapsing across hemispheres, showed the FHR group had significantly smaller GM volumes than controls for two PFC regions: ventromedial (F= 5.29, df= 1/72, p= .02, d= −0.57) and frontal pole (F= 4.09, df= 1/72, p= .05, d= −0.50) (Table 3).
Table 3.
Absolute and relative regional prefrontal gray matter volumes in familial high-risk (FHR) and control youth
| Absolute Volumesa | Relative Volumesb | |||||
|---|---|---|---|---|---|---|
| FHR n = 27 |
Controls n = 48 |
FHR n = 27 |
Controls n = 48 |
% Difference c |
de | |
| Ventromedial, total | 4.53 ± 0.18 | 4.89 ± 0.12 | 0.38 ± .01 | 0.42 ± .01 d | −9.22 | −0.57 |
| Frontal Pole, total | 67.19 ± 2.25 | 71.07 ± 1.65 | 5.72 ± .17 | 6.14 ± .12 d | −6.90 | −0.50 |
| Dorsolateral, total | 58.75 ± 2.23 | 59.17 ± 1.15 | 4.96 ± .13 | 5.12 ± .09 | −3.07 | −0.25 |
| Ventrolateral, total | 16.93 ± 0.51 | 16.18 ± 0.40 | 1.44 ± 0.04 | 1.40 ± 0.03 | 3.22 | 0.23 |
| Orbitofrontal, total | 13.50 ± 0.54 | 12.57 ± 0.36 | 1.15 ± 0.04 | 1.09 ± 0.03 | 5.52 | 0.27 |
Absolute volumes in cubic centimeters are given as mean ± standard error;
Significant group × region interaction (p ≤ .04) in the repeated measures multivariate analysis of covariance predicting left and right relative prefrontal volumes in FHR vs. control subjects, adjusting for total cerebral volume and age;
Percent differences calculated from volumes using five decimal points for accuracy;
Significant difference between groups in follow-up least square mean contrasts, p ≤ .05;
effect size reported as Cohen’s d.
Follow-up ANCOVAs examined whether significant group differences were affected by entering as covariates two sociodemographic variables that differed significantly (PSES) or marginally (IQ) between groups. The group difference in ventromedial PFC remained significant (F=5.92, df=1/68, p=.02) when covarying for PSES (F= 0.05, df=1/68, p= .82) and IQ (F= 0.76, df= 1/68, p= .39). Similarly, there remained a significant group difference in frontal pole volume (F= 5.09, df= 1/68, p= .03) after covarying for PSES (F= 0.67, df= 1/68, p= .42) and IQ (F= 1.67, df= 1/68, p= .20).
3.2. Correlations of subpsychotic symptoms
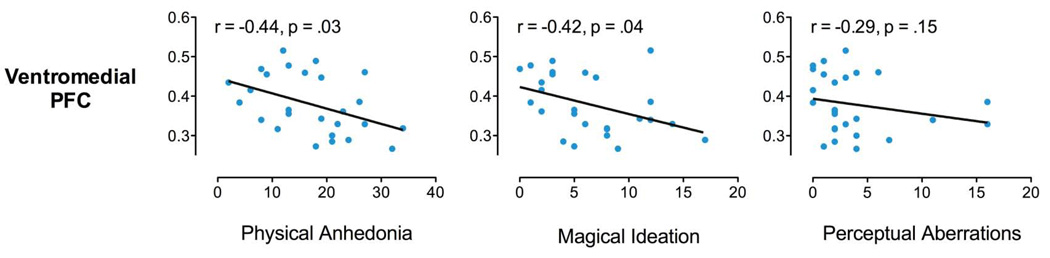
Ventromedial PFC GM volume showed a significant negative correlation with RPAS in FHR subjects (r= −0.44, p = .03) but not in controls (r= .27, p= .07). Ventromedial PFC GM was also significantly negatively associated with MIS scores in FHR subjects (r= −.42, p= .04) but not in controls (r= .04, p= .77). Ventromedial PFC and PAS scores were non-significantly negatively correlated in FHR (r= −0.29, p= .15) and control (r= −.04, p= .77) subjects.
DLPFC GM volume was not significantly associated with RPAS (FHR: r= 0.12, p= .53; controls: r= −.01, p= .94), MIS (FHR: r= 0.13, p= .54; controls: r= −.11, p= .44), or PAS (FHR: r= 0.06, p= .78; controls: r= .03, p= .84) scores.
4. Discussion
We found bilateral reductions of ventromedial and polar PFC GM volumes in adolescent and young adult relatives of schizophrenia patients. Neither deficit was explained by differences in total brain size, SES or IQ. As hypothesized, ventromedial PFC GM was also negatively correlated with subpsychotic symptoms in FHR subjects. Contrary to our expectations, DLPFC GM was not related to familial risk for schizophrenia or subpsychotic symptoms.
Our pattern of findings suggests that ventromedial PFC (BA 11,12) GM reductions are associated with both genetic vulnerability and early disease processes in young relatives of schizophrenia patients. Compared with controls, FHR subjects had less GM volume in a ventromedial PFC area that overlaps with the medial PFC ROI found to be hyperactive in our functional MRI (fMRI) study of a subset of these FHR subjects (Whitfield-Gabrieli et al., 2009), all of whom are included in this report. We therefore have converging anatomical and functional imaging evidence in the same sample that medial PFC abnormalities are associated with familial risk for schizophrenia in young adulthood. Ventromedial PFC volumes also correlated with self-reported anhedonia and magical ideation, subpsychotic symptoms found to predict the emergence of full-blown psychosis in certain high-risk samples (Horan et al., 2008; Meehl, 1962). Thus, we postulate that ventromedial PFC GM deficits may partly mediate the transition to psychosis by becoming more pronounced among FHR adolescents who convert. This would dovetail with VBM findings of the EHRP where adolescents at heightened genetic risk for schizophrenia had a medial PFC GM density intermediate between that of low-risk adolescents and first-episode schizophrenia patients (Job et al., 2003; Lawrie et al., 2008). In addition, Koutsouleris et al. (2009) found more pronounced ventromedial PFC GM loss in the late versus early stage of the schizophrenia prodrome, suggesting this deficit may progress in parallel with emerging disease.
This is the first report of decreased frontal pole (BA 10, 9) GM volume in young biological relatives of schizophrenia patients. Frontal pole deficits have been found in some studies of older biological relatives (Cannon et al., 2002; Honea et al., 2008), including a twin study where GM declined proportionally with degree of genetic loading for schizophrenia (Cannon et al., 2002). Their presence in both young and older adult biological relatives of patients suggests the hypothesis that frontal pole GM deficits may be stable markers of genetic risk for schizophrenia.
The ventromedial PFC and frontal pole mediate an array of behaviors that are compromised in schizophrenia. Both regions have been implicated in socioemotional and self-monitoring functions, including mentalizing (i.e., “theory of mind”) and reality monitoring. The frontal poles are involved in aspects of self/other distinctions, including the ability to distinguish information that is perceived in the environment (other-generated) from information that is imagined (self-generated) (Simons et al., 2006). Deficits in these abilities, in turn, may underlie the genesis of psychotic symptoms (Frith, 1992). Medial PFC involvement is the most replicated finding of functional imaging studies of mentalizing (Brunet-Gouet and Decety, 2006). Moreover, in the only fMRI study of biological relatives of schizophrenia patients performing a mentalizing task, medial PFC activation was positively associated with both genetic risk and subpsychotic symptoms (Marjoram et al., 2006).
A lack of significant familial risk group differences in lateral PFC and OFC volumes may be consistent with research linking anatomical abnormalities in these areas with transition to full-blown psychosis (Smieskova et al., 2010; Wood et al., 2008). In a study of prodromal youth, Pantelis and colleagues (2003) found that subjects who developed psychosis (“converters”) had significantly less baseline right DLPFC GM, and a significant reduction of OFC GM over time compared with non-converters. In similar longitudinal studies, Borgwardt and colleagues (2007, 2008) found more pronounced reductions of lateral and orbital PFC GM in converters relative to non-converters over time, and Sun et al (2009) reported greater contraction of the right DLPFC in association with psychosis onset. Thus, lateral and orbital PFC GM reductions may mark transition to psychotic symptoms, more so than genetic predisposition to schizophrenia in youth. Alternatively, these reductions may appear later in the developmental course of the disorder, since the DLPFC completes maturation later than the frontal pole and ventromedial PFC (Gogtay et al., 2004). Finally, the absence of significant volume differences in lateral and orbital PFC does not preclude functional abnormalities. In fact, abnormal DLPFC activation has been found in two previous fMRI studies of executive functioning in young FHR subjects (Keshavan et al., 2002), including a subsample from the current study (Seidman et al., 2006b).
As with all studies, this investigation has a number of limitations. Due to the labor- and time-intensive nature of ROI methods, our sample size is limited for the detection of small effects. In addition, the FHR subjects have not passed through the age of risk for onset of psychosis, such that information on clinical outcome is not available. The design of the study also precludes separation of genetic effects from shared environmental effects. Nevertheless, our findings encourage further research into PFC GM subregions as markers of risk for schizophrenia, specifically regarding the hypothesis that they may differentially mark inherited vulnerability and early symptom emergence processes in adolescence and young adulthood.
Figure 2.
Relationship of ventromedial prefrontal (VMPFC) GM with scores on three Chapman scales of psychosis proneness in FHR adolescents: A) physical anhedonia; B) magical ideation; C) perceptual aberrations
Acknowledgement
We thank the patients with schizophrenia and their family members, control families, and project staff for their generous contributions to the study. Staff included Lisa Gabel, Anthony Giuliano, Stephen Glatt, Jennifer Koch, Marc Korczykowski, Erica Lee, Virna Merino, Elon Mesholam, Raquelle Mesholam-Gately, Caroline Patterson, Nicole Peace, William Stone, Rosemary Toomey, and Sharon White.
Role of Funding Source
Funding for this study was provided by the Mental Illness and Neuroscience Discovery (MIND) Institute; National Association of Research in Schizophrenia and Depression Stone Award (LJS); National Institute of Mental Health (NIMH) R18 MH 43518 and R01 MH 65562 (MTT, LJS), R01 MH 63951, P50 MH80272 & U01 MH81928 (LJS), R25 MH 60485 (to HWT, Training PI: MTT), K01 MH 06987 (IMR); Commonwealth Research Center, Massachusetts Department of Mental Health (LJS). This work was also supported in part by The National Center for Research Resources (P41RR14075). None of these funding sources had any further role in the study design; in the collection, analysis and interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
These findings were presented at the following meeting: International Congress on Schizophrenia Research, San Diego, CA, March 30th 2009.
Contributors: Drs. Seidman, Faraone and Tsuang designed the study and wrote the protocol. Drs. Thermenos and Brown collected the neuroimaging data and performed the interviews at the imaging session. Mr. Hodge supervised data management and performed some statistical analyses. Drs. Makris, Caviness, and Kennedy developed the parcellation methods and provided expert neuroanatomical consultation. Dr. Rosso performed the cortical parcellations and statistical analyses, and wrote the first draft of the manuscript. Drs. Rosso and Seidman edited drafts of the manuscript. All authors contributed to the manuscript and approved its final version.
Conflict of Interest
All authors declare that they have no conflict of interest.
References
- American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders. Washington DC: Author; 2000. [Google Scholar]
- Bleuler E. In: Dementia Praecox or the Group of Schizophrenias. Zinkin TBJ, editor. New York, NY: International Universities Press; 1911. [Google Scholar]
- Borgwardt SJ, McGuire PK, Aston J, Berger G, Dazzan P, Gschwandtner U, Pfluger M, D'Souza M, Radue EW, Riecher-Rossler A. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. Br. J. Psychiatry Suppl. 2007;51:s69–s75. doi: 10.1192/bjp.191.51.s69. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, McGuire PK, Aston J, Gschwandtner U, Pflüger MO, Stieglitz RD, Radue EW, Riecher-Rössler A. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr. Res. 2008;106:108–114. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Picchioni MM, Ettinger U, Toulopoulou T, Murray R, McGuire PK. Regional gray matter volume in monozygotic twins concordant and discordant for schizophrenia. Biol. Psychiatry. 2010;67:956–964. doi: 10.1016/j.biopsych.2009.10.026. [DOI] [PubMed] [Google Scholar]
- Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res. 2006;148:75–92. doi: 10.1016/j.pscychresns.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Cannon TD. Clinical and genetic high-risk strategies in understanding vulnerability to psychosis. Schizophr. Res. 2005;79:35–44. doi: 10.1016/j.schres.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Thompson PM, van Erp TG, Toga AW, Poutanen VP, Huttunen M, Lonnqvist J, Standerskjold-Nordenstam CG, Narr KL, Khaledy M, Zoumalan CI, Dail R, Kaprio J. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc. Natl. Acad. Sci. U S A. 2002;99:3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb. Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J. Abnorm. Psychol. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Perceptual aberration in schizophrenia. J. Abnorm. Psychol. 1978;87:399–407. doi: 10.1037//0021-843x.87.4.399. [DOI] [PubMed] [Google Scholar]
- Chemerinski E, Nopoulos PC, Crespo-Facorro B, Andreasen NC, Magnotta V. Morphology of the ventral frontal cortex in schizophrenia: relationship with social dysfunction. Biol. Psychiatry. 2002;52:1–8. doi: 10.1016/s0006-3223(01)01363-4. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioural sciences. Hillsdale, NJ: Erlbaum Associates; 1988. [Google Scholar]
- Diwadkar VA, Montrose DM, Dworakowski D, Sweeney JA, Keshavan MS. Genetically predisposed offspring with schizotypal features: an ultra high-risk group for schizophrenia? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:230–238. doi: 10.1016/j.pnpbp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ. Magical ideation as an indicator of schizotypy. J. Consult. Clin. Psychol. 1983;51:215–255. doi: 10.1037//0022-006x.51.2.215. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VR. The young adult human brain: an MRI-based morphometric study. Cereb. Cortex. 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Frith CD. The Cognitive Neuropsychology of Schizophrenia. London: LEA; 1992. [Google Scholar]
- Geller B, Williams M, Zimmerman B, Frazier J. Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) St. Louis, MO: Washington University; 1996. [DOI] [PubMed] [Google Scholar]
- Giuliani NR, Calhoun VD, Pearlson GD, Francis A, Buchanan RW. Voxel-based morphometry versus region of interest: a comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophr. Res. 2005;74:135–147. doi: 10.1016/j.schres.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Glatt SJ, Stone WS, Faraone SV, Seidman LJ, Tsuang MT. Psychopathology, personality traits and social development of young first-degree relatives of patients with schizophrenia. Br. J. Psychiatry. 2006;189:337–345. doi: 10.1192/bjp.bp.105.016998. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF3, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch. Gen. Psychiatry. 2000;57:761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am. J. Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Honea RA, Meyer-Lindenberg A, Hobbs KB, Pezawas L, Mattay VS, Egan MF, Verchinski B, Passingham RE, Weinberger DR, Callicott JH. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol. Psychiatry. 2008;63:465–474. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Reise SP, Subotnik KL, Ventura J, Nuechterlein KH. The validity of Psychosis Proneness Scales as vulnerability indicators in recent-onset schizophrenia patients. Schizophr. Res. 2008;100:224–236. doi: 10.1016/j.schres.2007.12.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Mandl RC, Brans RG, van Haren NE, Baare WF, van Oel CJ, Collins DL, Evans A, Kahn RS. Gray and white matter density changes in monozygotic and same-sex dizygotic twins discordant for schizophrenia using voxel-based morphometry. Neuroimage. 2006;31:482–488. doi: 10.1016/j.neuroimage.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Voxel-based morphometry of grey matter densities in subjects at high risk of schizophrenia. Schizophr. Res. 2003;64:1–13. doi: 10.1016/s0920-9964(03)00158-0. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Crow TJ, Frith CD, Husband J, Kreel L. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet. 1976;2:924–926. doi: 10.1016/s0140-6736(76)90890-4. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J. Psychiatr. Res. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Spencer SM, Harenski KA, Luna B, Sweeney JA. A preliminary functional magnetic resonance imaging study in offspring of schizophrenic parents. Prog Neuropsychopharmacol Biol. Psychiatry. 2002;26:1143–1149. doi: 10.1016/s0278-5846(02)00249-x. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Hogarty GE. Brain maturational processes and delayed onset in schizophrenia. Dev. Psychopathol. 1999;11:525–543. doi: 10.1017/s0954579499002199. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Schmitt GJ, Gaser C, Bottlender R, Scheuerecker J, McGuire P, Burgermeister B, Born C, Reiser M, Möller HJ, Meisenzahl EM. Neuroanatomical correlates of different vulnerability states for psychosis and their clinical outcomes. Br. J. Psychiatry. 2009;195:218–226. doi: 10.1192/bjp.bp.108.052068. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. In: Dementia Praecox and Paraphrenia. Barclay TBR, editor. Huntington, NY: Robert E Krieger Publishing Co; 1919. [Google Scholar]
- Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2002;17:1711–1719. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch. Gen. Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, McIntosh AM, Hall J, Owens DG, Johnstone EC. Brain structure and function changes during the development of schizophrenia: the evidence from studies of subjects at increased genetic risk. Schizophr. Bull. 2008;34:330–340. doi: 10.1093/schbul/sbm158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, Whalley HC, Abukmeil SS, Kestelman JN, Donnelly L, Miller P, Best JJ, Owens DG, Johnstone EC. Brain structure, genetic liability, and psychotic symptoms in subjects at high risk of developing schizophrenia. Biol. Psychiatry. 2001;49:811–823. doi: 10.1016/s0006-3223(00)01117-3. [DOI] [PubMed] [Google Scholar]
- Marjoram D, Job DE, Whalley HC, Gountouna VE, McIntosh AM, Simonotto E, Cunningham-Owens D, Johnstone EC, Lawrie S. A visual joke fMRI investigation into Theory of Mind and enhanced risk of schizophrenia. Neuroimage. 2006;31:1850–1858. doi: 10.1016/j.neuroimage.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Maxwell ME. Family Interview for Genetic Studies: Clinical Neurogenetics Branch, Intramural Research Program. NIMH; 1996. [Google Scholar]
- McDonald C, Bullmore ET, Sham PC, Chitnis X, Wickham H, Bramon E, Murray RM. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch. Gen. Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- McDonald C, Marshall N, Sham PC, Bullmore ET, Schulze K, Chapple B, Bramon E, Filbey F, Quraishi S, Walshe M, Murray RM. Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am. J. Psychiatry. 2006;163:478–487. doi: 10.1176/appi.ajp.163.3.478. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead TW, Harrison LK, Forrester K, Lawrie SM, Johnstone EC. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol. Psychiatry. 2004;56:544–552. doi: 10.1016/j.biopsych.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead WJ, Harrison LK, Whalley HC, Johnstone EC, Lawrie SM. Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006;141B:76–83. doi: 10.1002/ajmg.b.30254. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Schizotaxia, schizotypy, schizophrenia. American Psychologist. 1962;17:827–838. [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH genetics initiative. Arch. Gen. Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Galaburda AM, Kennedy DN, Filipek PA, Caviness VS. Human cerebral cortex: Localization, parcellation and morphometry with magnetic resonance imaging. J. Cogn. Neurosci. 1992;4:352–374. doi: 10.1162/jocn.1992.4.4.352. [DOI] [PubMed] [Google Scholar]
- Seidman LJ. Schizophrenia and brain dysfunction: an integration of recent neurodiagnostic findings. Psychol. Bull. 1983;94:195–238. [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Smith CW, Stone WS, Glatt SJ, Meyer E, Faraone SV, Tsuang MT, Cornblatt B. Neuropsychological functioning in adolescents and young adults at genetic risk for schizophrenia and affective psychoses: results from the Harvard and Hillside Adolescent High Risk Studies. Schizophr. Bull. 2006a;32:507–524. doi: 10.1093/schbul/sbj078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Thermenos HW, Poldrack RA, Peace NK, Koch JK, Faraone SV, Tsuang MT. Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: an fMRI study of working memory. Schizophr. Res. 2006b;85:58–72. doi: 10.1016/j.schres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol. Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch. Gen. Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. discussion 819-20. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr. Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Davis SW, Gilbert SJ, Frith CD, Burgess PW. Discriminating imagined from perceived information engages brain areas implicated in schizophrenia. Neuroimage. 2006;32:696–703. doi: 10.1016/j.neuroimage.2006.04.209. [DOI] [PubMed] [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, Radue EW, McGuire PK, Riecher-Rössler A, Borgwardt SJ. Neuroimaging predictors of transition to psychosis-A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2010 doi: 10.1016/j.neubiorev.2010.01.016. doi:10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, van Erp TG, Thompson PM, Toga AW, Cannon TD, Pantelis C. Progressive brain structural changes mapped as psychosis develops in 'at risk' individuals. Schizophr. Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT. Defining alternative phenotypes for genetic studies: what can we learn from studies of schizophrenia? Am. J. Med. Genet. 2001;105:8–10. [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Pantelis C, Velakoulis D, Yücel M, Fornito A, McGorry PD. Progressive changes in the development toward schizophrenia: studies in subjects at increased symptomatic risk. Schizophr. Bull. 2008;34:322–329. doi: 10.1093/schbul/sbm149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Hirao K, Namiki C, Hanakawa T, Fukuyama H, Hayashi T, Murai T. Social cognition and frontal lobe pathology in schizophrenia: a voxel-based morphometric study. Neuroimage. 2007;35:292–298. doi: 10.1016/j.neuroimage.2006.10.046. [DOI] [PubMed] [Google Scholar]