Abstract
Objective
We sought to evaluate outcomes after radical prostatectomy among men with low-risk prostate cancer who would be candidates for active surveillance.
Methods
Using the SEARCH database of men treated with radical prostatectomy at multiple equal-access medical centers between 1988 and 2007, 398 of 2,062 (19%) met our criteria for potential active surveillance: clinical stage T1c or T2a, PSA <10 ng/mL, Gleason sum ≤6, and only 1 or 2 positive cores on at least a sextant biopsy. We examined the risk of adverse pathology, biochemical progression, and PSADT at the time of recurrence. We used a Cox proportional hazards model to determine the significant predictors of PSA progression.
Results
Of the men with low-risk prostate cancer, 85% had organ-confined disease, only 2% had seminal vesicle invasion, and no patient had lymph node metastasis. The 5- and 10-year PSA-free survival rates were 81% (95% CI, 76–86%) and 66% (95% CI, 54–76%). On multivariate analysis, older age (p=0.005), Agent Orange exposure (p=0.02), and obesity (p=0.03) were all significantly associated with biochemical failure. Mean and median PSADT among men who recurred were 37 and 20 months. Only 3 patients recurred with PSADT <9 months.
Conclusions
Most men with low-risk prostate cancer treated with radical prostatectomy experience long-term PSA control. Those who do recur often do so with a long PSADT. Consistent with prior SEARCH database reports, older age, agent orange exposure and obesity increased the risk of recurrence.
Keywords: prostate cancer, upgrading, active surveillance, radical prostatectomy
INTRODUCTION
There has been dramatic stage, grade, and risk migration of newly diagnosed prostate cancer in the prostate-specific antigen (PSA) era.1 Most cases are now diagnosed as clinical stage T1c disease with low serum PSA levels. Because of the long natural history of newly diagnosed low-risk prostate cancer, active surveillance (AS) with selective intervention is considered an option for the management of select patients with low-risk prostate cancer.2 Large series of patients managed with AS suggest that with careful follow-up and selective management, the likelihood of metastatic prostate cancer or death from prostate cancer with such a strategy is low.3–5 However, serious challenges must be overcome before the safety of AS for individual patients can be confirmed. Our ability to risk stratify low-risk patients is imperfect.6 Many patients who are low-risk at diagnosis harbor higher-grade cancer and a smaller proportion have higher-stage prostate cancer at radical prostatectomy than predicted clinically.7, 8 Many patients and clinicians are uncomfortable delaying intervention because even low-risk patients are not guaranteed a complete cure with intervention and the likelihood of a complete cure may decline with time. We examined a large cohort of candidates for AS who underwent radical prostatectomy to evaluate their pathologic and biochemical disease–free outcomes and determine factors associated with recurrence after intervention.
MATERIALS AND METHODS
Study population
After obtaining Institutional Review Board approval from each institution to abstract and combine data, data from patients treated with radical prostatectomy between 1988 to 2007 at the Veterans Affairs Medical Centers in West Los Angeles and Palo Alto, California; Augusta, Georgia; and Durham, North Carolina were added to the Shared Equal Access Regional Cancer Hospital (SEARCH) database.9 This database includes patient age at the time of surgery, race, agent orange exposure, height, weight, clinical stage, grade of cancer on diagnostic biopsies, preoperative PSA, surgical specimen pathology (specimen weight, tumor grade and stage, and surgical margin status), and follow-up PSA data. Patients treated with preoperative androgen deprivation or radiation therapy were excluded. There are data on 1,741 patients in the SEARCH database; we sought to evaluate outcomes of those who would be potential candidates for AS. Therefore, we excluded 583 patients with a PSA ≥10 ng/mL or missing information regarding PSA, 403 men with a biopsy Gleason sum of 7 or higher or missing information, 107 men with clinical stage T2b or higher or unknown clinical stage, 275 men with more than 2 positive cores or missing information, and 27 men who had <6 biopsy cores obtained or missing information. This resulted in a study population of 346. The prostatectomy specimens were sectioned per each institution’s protocol. Biochemical progression was defined as a single PSA >0.2 ng/mL, 2 values at 0.2 ng/mL, or secondary treatment for an elevated PSA.
Statistical analysis
The actuarial risk of biochemical progression was determined using Kaplan-Meier plots and the log-rank test. The significant risk factors for biochemical progression were determined using a Cox proportional hazards regression model. Multivariate analysis was performed using a forwards stepwise regression with p<0.15 determining which variables should be entered into the model at each step. The variable with the highest p value was successively deleted until only variables with p<0.1 remained. The variables considered for entry into the multivariate model included center (categorical term), age (continuous), year of surgery (continuous), preoperative PSA (continuous), race (black vs. nonblack), number of positive cores (1 vs. 2), body mass index (BMI, in kg/m2; <25, 25–29.9, ≥30), and Agent Orange exposure (yes vs. no), regardless of their association (or lack thereof) with recurrence in univariate analysis. Based upon similar risks of recurrence for the BMI groups of <25 and 25–29.9 kg/m2 in univariate analysis, these groups were combined and BMI was examined in multivariate analysis as a dichotomous variable of <30 vs. ≥30 kg/m2. In exploratory analyses, there was no difference in recurrence risk between men with biopsy Gleason 4 or 5 and those with Gleason 6 (p=0.16). Given the limited number of men with lower Gleason scores and the very rare occurrence with which those scores are seen in modern practice, biopsy Gleason score was not included in any formal statistical analyses. Predictive accuracy of the multivariate model to predict PSA recurrence was determined using the concordance index-C.
PSA doubling time (PSADT) was calculated as previously described, assuming first-order kinetics, and computed by dividing the natural log of 2 (0.693) by the slope of the linear regression line of the natural log of PSA over time.10 A PSADT was computed for all patients meeting the definition of recurrence who had a minimum of 2 PSA values, separated by at least 3 months and within 2 years after recurring. Only PSA values before salvage therapy were used to compute doubling time. Four patients with a PSADT <0 (i.e., no increase or decline in PSA) or a very long PSADT (>100 months) were assigned a value of 100 months for ease of calculations. The distribution of all clinicopathological variables was similar among the SEARCH sites. Therefore, data from all centers were combined for analyses. All statistical analyses were performed using STATA 9.2 (Stata Corp., College Station, TX).
RESULTS
The study cohort comprised 346 men who were candidates for AS (Table 1). The mean age was 60.2 ± 6.6 years. The majority of patients had clinical stage T1 disease (73%), biopsy Gleason 6 (85%), and a single core positive (65%). A sizable minority (42%) was black and most patients (72%) were overweight or obese. On pathological analysis, over one-third had Gleason 7 or higher in the prostate and 18% had pathological stage T3 or higher.
TABLE 1.
Pre-operative clinical, postoperative pathological, and follow-up characteristics of men undergoing radical prostatectomy in the SEARCH Database who were potential active surveillance candidates
| No. patients | 347 |
| Mean age ± SD (yr) | 60.3 ± 6.5 |
| Median year of surgery (IQR) | 2001 (1998, 2003) |
| No. Race (%) | |
| Non-black | 202 (58) |
| Black | 145 (42) |
| No. body mass index (%) | |
| Normal weight | 90 (28) |
| Overweight | 126 (39) |
| Obese | 107 (33) |
| No. Agent Orange Exposure (%) | 58 (19) |
| PSA (ng/ml) | |
| Mean ± SD | 5.7 ± 2.2 |
| Median | 5.5 |
| No. biopsy Gleason score (%) | |
| 4 | 19 (5) |
| 5 | 33 (10) |
| 6 | 295 (85) |
| No. clinical stage (%) | |
| T1a | 4 (1) |
| T1c | 251 (72) |
| T2a | 92 (27) |
| Median biopsy cores obtained (IQR) | 8 (6, 10) |
| No. cores positive (%) | |
| 1 | 227 (65) |
| 2 | 120 (35) |
| No. pathological Gleason score (%) | |
| 2–6 | 220 (64) |
| 3+4 | 97 (28) |
| ≥4+3 | 29 (8) |
| No. positive surgical margins (%) | 122 (35) |
| No. extracapsular extension (%) | 56 (16) |
| No. seminal vesicle invasion (%) | 7 (2) |
| No. positive lymph nodes (%) | 0 (0) |
| No. lymph adenectomy not performed (%) | 120 (35) |
| Follow-up length (months) | |
| Mean ± SD | 58 ± 38 |
| Median | 54 |
| No. recurrences (%) | 55 (16) |
| Median PSA doubling time at recurrence* (mo) | 20.2 |
data only available on 27 of the 56 men with recurrence
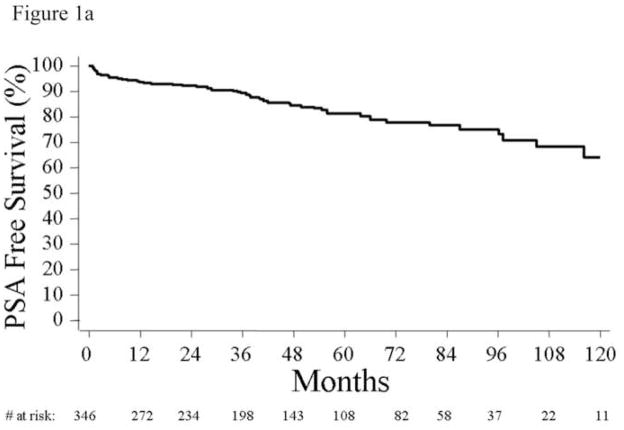
Mean (SD) and median follow-up times among patients who did not recur were 51 (35) and 43 months. During this time, 56 men (17%) developed a PSA recurrence (Figure 1a). The 1-, 5-, and 10-year risk of PSA-free survival was 94% (95% CI, 90%–96%), 81% (95% CI, 75%–86%), and 64% (95% CI, 51%–75%).
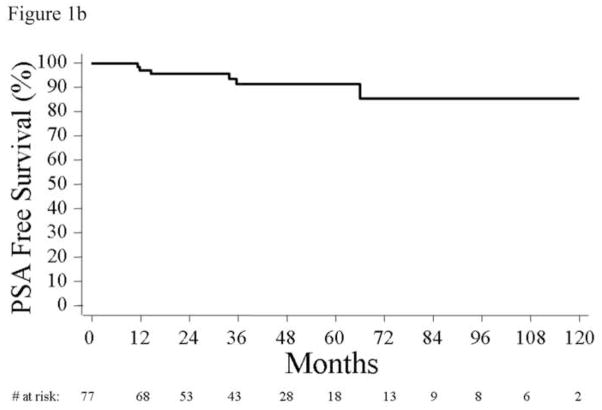
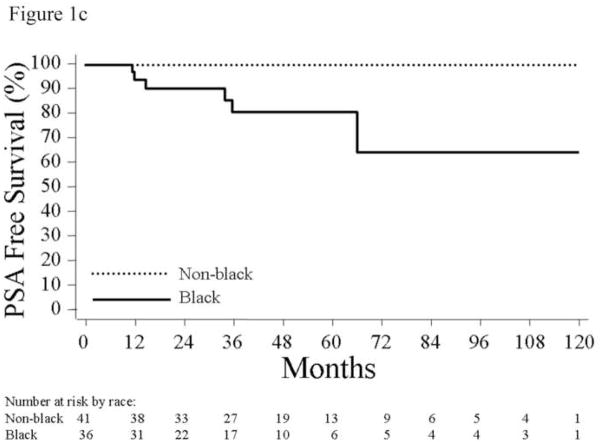
Figure 1.
(a) Actuarial 10-year Kaplan-Meier estimates of biochemical recurrence rates of men who were candidates for active surveillance. (b) Actuarial 10-year Kaplan- Meier estimates of biochemical recurrence rates of men who were candidates for active surveillance with the lowest risk of progression, defined by age <60, not obese, and no Agent Orange exposure. (c) Actuarial 10-year Kaplan-Meier estimates of biochemical recurrence rates of men who were candidates for active surveillance with the lowest risk of progression, defined by age <60, not obese, and no Agent Orange exposure, stratified by age (log-rank, p=0.003).
On univariate analysis, only older age (p=0.01) and Agent Orange exposure (p=0.03) were significantly associated with risk of PSA recurrence (Table 2). On multivariate analysis, older age (p=0.005), Agent Orange exposure (p=0.02), and obesity (p=0.03) were all significantly associated with biochemical failure. The accuracy of this model to predict PSA recurrence (c-index) was 0.72.
TABLE 2.
Cox proportional hazards analysis of factors predicting time to biochemical recurrence following radical prostatectomy among patients who were candidates for active surveillance in the SEARCH database
| Hazards Ratio | 95% CI | p Value | |
|---|---|---|---|
| Univariate analysis: | |||
| Serum PSA | 1.09 | 0.95 – 1.25 | 0.21 |
| Black race | 1.67 | 0.92 – 3.06 | 0.09 |
| Age | 1.07 | 1.02 – 1.13 | 0.01 |
| Year of surgery | 0.97 | 0.88 – 1.06 | 0.48 |
| Clinical stage (T2 vs. T1) | 0.81 | 0.41 – 1.60 | 0.55 |
| Number of cores positive (2 vs. 1) | 1.16 | 0.63 – 2.14 | 0.63 |
| Agent Orange exposure | 2.08 | 1.06 – 4.07 | 0.03 |
| Body mass index (relative to normal weight) | |||
| Overweight | 0.81 | 0.35 – 1.86 | 0.62 |
| Obese | 1.80 | 0.87 – 3.75 | 0.12 |
| Multivariate analysis: | |||
| Age | 1.08 | 1.03 – 1.14 | 0.004 |
| Agent Orange exposure | 2.37 | 1.19 – 4.71 | 0.01 |
| Obesity | 1.86 | 1.01 – 3.40 | 0.045 |
We sought to better define a very low-risk group for progression using the 3 significant risk factors. As age was initially examined as a continuous variable, we performed exploratory analysis to identify the best cut-point within our data to stratify patients into “younger” and “older” groups with differing risk of progression. To accomplish this, patients were stratified by decade of age (≤50, 51–60, 61–70, and >70 years). On exploratory analysis, age did indeed appear to act as a continuous variable in that each older-age group was at higher risk of recurrence than the preceding younger-age group. However, segregating patients into groups of ≤60 and >60 years resulted in the best risk stratification for risk of PSA recurrence (likelihood chi-square ratio=7.32, p=0.01). Of note, this also resulted in near-equal numbers of patients in the younger (n=171, 49%) and older (n=175, 51%) groups.
Among the 77 men with no single high-risk feature (i.e., age <60, not obese, and no Agent Orange exposure) long-term outcomes were very favorable, with only 6 men (8%) developing a PSA recurrence (Figure 1b). Among these 77 men, the 1-, 5-, and 10-year probabilities of PSA-free survival were 97% (95% CI, 89%–99%), 91% (95% CI, 80%–96%), and 86% (95% CI, 66%–94%). Among the 6 men with recurrences, PSADT data at the time of recurrence were available for 5, all of whom had a PSADT >15 months (median 20.9 months).
We sought to further refine the low-risk group (i.e. age <60, not obese, and no Agent Orange exposure) by determining whether any other clinical features could help identify an even lower-risk cohort. Using log-rank analysis for categorical variables (race, clinical stage, number of positive cores) and Cox regression for continuous variables (PSA), we found that the risk of progression among these 77 men varied significantly by race (log-rank, p=0.003). Among the 41 men with all low-risk features who were not black, there were no PSA recurrences, for an estimated PSA-free survival of 100% (Figure 1c).
Of the 56 men who developed a PSA recurrence among the whole cohort of 346 AS candidates, PSADT data were available for 27 (48%). Median PSADT was 19.6 months. Only 1 patient (4%) had a PSADT <3 months and only 2 (7%) had a PSADT between 3 and 9 months. Of the 3 patients with PSADT <9 months, 2 had been exposed to Agent Orange and were black. Thus, only 1 patient (PSADT=5.9 months) who was not black with Agent Orange exposure had a PSADT <9 months. Overall, the majority of men had a PSADT >15 months (n=18, 67%).
COMMENT
This manuscript examines the pathologic outcomes and recurrence after radical prostatectomy of a cohort of patients who potentially would have met criteria to be offered AS. Patients who were candidates for AS and underwent radical prostatectomy had diverse pathological outcomes with over one-third of patients being upgraded to Gleason score 7 or greater. Upgrading has been previously described and is associated with higher PSA values, obesity, higher number of biopsy cores with cancer,11 lower prostate volume,7, 12 and higher patient age. Our ability to optimally select patients who have low-grade and low-volume disease may be improved by only offering surveillance to patients with PSA density under 0.15 ng/mL/gram of prostate tissue, as suggested by some authors.4 We have shown in the SEARCH database that prostate volume is correlated to upgrading and adverse pathology for men who undergo radical prostatectomy.12 So restricting AS to men with larger prostates and/or men with PSA density <0.15 ng/mL may improve the precision of selecting the lowest-risk patients for surveillance. A recent study by Conti et al examined the pathologic upgrading of men who were candidates for AS and found, not unexpectedly, that applying stricter criteria for surveillance yielded a decreased risk of upgrading at radical prostatectomy.13
We only included patients with 1 or 2 positive biopsy cores on at least a sextant biopsy. We did not have information on the length of cancer on each involved core for much of the cohort. Including prostate cancer biopsy core information would further select the lowest-risk patients. Most AS cohorts use biopsy information as inclusion criteria.2 The model proposed by Epstein in 1994 to predict low-volume, low-grade, organ-confined disease included PSA density less than 0.15ng/mL, Gleason grade 6 or less, and less than 3 positive biopsy cores with no more than 50% of any single core involved based upon predominantly sextant biopsy.14 Also, some groups have suggested that in order to fully risk stratify low-risk patients, an extended prostate biopsy with 10, 12, or more cores should be obtained. In the SEARCH database, patients from more recent years have a greater number of biopsies obtained and the median number of cores for the patients in this analysis was 8 (Table 1). Clearly, extended biopsy patterns decrease pathologic upgrading.11, 15 Berglund et al performed extended pattern biopsy on 104 men who were candidates for AS and found higher grade or extent of disease in 27%.6 They routinely repeat biopsy in potential AS candidates. However, even restricting AS to men with <5% of a single core involved with Gleason score 6 prostate cancer yielded upgrading in 18% and upstaging in 8% of men who underwent radical prostatectomy in a recent series16.
Narrowing the selection criteria for AS, of course, restricts the proportion of men who are candidates for surveillance and potentially exposes a greater number of men with insignificant cancer to intervention. Expanded criteria for surveillance may be appropriate for older patients or those with limited life expectancy due to co-morbidities or illness, while strict inclusion criteria may be most appropriate for patients with longevity and/or a strong desire for prostate cancer safety.
When patients are considering therapy with curative intent vs. AS, a common concern is losing the potential for successful treatment during a period of surveillance. The chance of prostate cancer metastasis and mortality is low during periods of surveillance.17 Men with concerning PSA kinetics or higher-volume or higher-grade disease on repeat biopsy can usually be offered curative treatment promptly, thus not losing an opportunity for effective treatment.18 However, a small proportion of men who are candidates for surveillance harbor adverse pathology, including extracapsular disease or seminal vesical invasion, as shown in our analysis. This inability to strongly reassure newly diagnosed, low-risk patients that they indeed harbor low-risk disease is a significant barrier to more widespread adoption of surveillance. We hope that new biomarkers may assist with the substratification of this low-risk cohort.19
Upstaging was fairly uncommon in our cohort, with 17% of patients having pT3 and only 2% pT3b and none node positive. Within the study we were able to identify a very low-risk cohort of patients, defined as having no obesity, no Agent Orange exposure, and age <60, whose 10-year PSA-free survival rate was increased to 86%. We previously described the impact of obesity20 and Agent Orange exposure21 on PSA recurrence risk in the VA population, which is in agreement with our current findings. In the most extensive examination of Agent Orange exposure and prostate cancer risk to date, Chamie et al found that veterans who had been exposed to Agent Orange were twice as likely as unexposed veterans to be diagnosed with prostate cancer, were younger at diagnosis, and had higher-grade disease at diagnosis.22
The impact of age on PSA recurrence is interesting. Multiple groups have shown that younger age is associated with decreased PSA-recurrence risk after radical prostatectomy.23, 24 Older age appears to be associated with higher PSA and grade at diagnosis25 and a worse outcome after radiation therapy.26 Because the relative risk of death from other causes increases with age while the risk of death from prostate cancer remains stable, AS is particularly attractive for older men.27
PSADT after PSA recurrence has also been very highly correlated with prostate cancer–specific mortality with a PSADT >15 months associated with 93%–98% prostate cancer–specific survival.28 In our series, we had an overall median PSADT of 19.6 months. Among men in the very low–risk group defined above, less than 10% developed a PSA recurrence, and those that did recur all had a PSADT >15 months. This is encouraging regarding the safety of AS on this carefully selected group of patients.
Some of the shortcomings of this study include that this was a retrospective study and that the study population is composed entirely of VA patients, who may not correlate well with other populations. VA prostate cancer patients tend to be racially diverse and have lower socioeconomic status than patients cared for in community- or University-based practices.29 This is also a surgical cohort and there may be some unforeseen biases in patient selection for surgical intervention such as family history or patient preference that were not well defined in our series. Also, the radical prostatectomies were performed by many different surgeons with differing degrees of experience, which could have an impact on our findings. Finally, many different pathologists examined the biopsies and radical prostatectomy specimens over the period of the study. There was no central pathology review. There have been well-described interpretive changes on prostate cancer grading over the years.30 This should not have a significant effect on our findings since the grading on both biopsies and radical prostatectomy specimens was concurrent. However, this is the largest examination of pathologic upgrading of potential candidates for AS. It is a multicenter study, which likely makes the results more applicable to clinical practice. Importantly, it is the only examination of the surgical outcomes of potential AS candidates that includes PSA recurrence and PSADT information and a significant follow-up interval. As our study shows for the first time in a large multicenter cohort, the risk of biochemical progression after RP of these potential AS candidates is very low.
CONCLUSIONS
Upgrading at radical prostatectomy is common among men with very low–risk prostate cancer who are candidates for AS. Most men with low-risk prostate cancer treated with radical prostatectomy experience long-term PSA control. Those who do recur often do so with a long PSADT. Older men and obese men were at increased risk of recurrence.
Acknowledgments
Supported by the Department of Veterans Affairs, National Institute of Health R01CA100938 (WJA), NIH Specialized Programs of Research Excellence Grant P50 CA92131-01A1 (WJA), the Georgia Cancer Coalition (MKT), the Department of Defense, Prostate Cancer Research Program, (SJF), and the American Urological Association Foundation/Astellas Rising Star in Urology Award (SJF). Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooperberg MR, Moul JW, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23:8146–8151. doi: 10.1200/JCO.2005.02.9751. [DOI] [PubMed] [Google Scholar]
- 2.Dall’Era MA, Cooperberg MR, Chan JM, et al. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer. 2008;112:1650–1659. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
- 3.Dall’Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112:2664–2670. doi: 10.1002/cncr.23502. [DOI] [PubMed] [Google Scholar]
- 4.Carter HB, Kettermann A, Warlick C, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007;178:2359–2364. doi: 10.1016/j.juro.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klotz LH. Active surveillance for good risk prostate cancer: rationale, method, and results. Can J Urol. 2005;12 (Suppl 2):21–24. [PubMed] [Google Scholar]
- 6.Berglund RK, Masterson TA, Vora KC, et al. Pathological upgrading and up staging with immediate repeat biopsy in patients eligible for active surveillance. J Urol. 2008;180:1964–1967. doi: 10.1016/j.juro.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serkin FB, Soderdahl DW, Cullen J, et al. Patient risk stratification using Gleason score concordance and upgrading among men with prostate biopsy Gleason score 6 or 7. Urol Oncol. 2008 doi: 10.1016/j.urolonc.2008.09.030. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Smith JA., Jr Radical prostatectomy for low risk carcinoma of the prostate. World J Urol. 2008;26:443–446. doi: 10.1007/s00345-008-0293-9. [DOI] [PubMed] [Google Scholar]
- 9.Freedland SJ, Amling CL, Dorey F, et al. Race as an outcome predictor after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Urology. 2002;60:670–674. doi: 10.1016/s0090-4295(02)01847-2. [DOI] [PubMed] [Google Scholar]
- 10.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 11.Freedland SJ, Kane CJ, Amling CL, et al. Upgrading and downgrading of prostate needle biopsy specimens: risk factors and clinical implications. Urology. 2007;69:495–499. doi: 10.1016/j.urology.2006.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turley RS, Hamilton RJ, Terris MK, et al. Small transrectal ultrasound volume predicts clinically significant Gleason score upgrading after radical prostatectomy: results from the SEARCH database. J Urol. 2008;179:523–527. doi: 10.1016/j.juro.2007.09.078. [DOI] [PubMed] [Google Scholar]
- 13.Conti SL, Dall’era M, Fradet V, et al. Pathological outcomes of candidates for active surveillance of prostate cancer. J Urol. 2009;181:1628–1633. doi: 10.1016/j.juro.2008.11.107. [DOI] [PubMed] [Google Scholar]
- 14.Epstein JI, Walsh PC, Carmichael M, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 15.Abouassaly R, Lane BR, Jones JS. Staging saturation biopsy in patients with prostate cancer on active surveillance protocol. Urology. 2008;71:573–577. doi: 10.1016/j.urology.2007.11.094. [DOI] [PubMed] [Google Scholar]
- 16.Thong AE, Shikanov S, Katz MH, et al. A single microfocus (5% or less) of Gleason 6 prostate cancer at biopsy—can we predict adverse pathological outcomes? J Urol. 2008;180:2436–2440. doi: 10.1016/j.juro.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Klotz L. Active surveillance for favorable risk prostate cancer: rationale, risks, and results. Urol Oncol. 2007;25:505–509. doi: 10.1016/j.urolonc.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Khatami A, Damber JE, Lodding P, et al. Does initial surveillance in early prostate cancer reduce the chance of cure by radical prostatectomy?--A case control study. Scand J Urol Nephrol. 2003;37:213–217. doi: 10.1080/00365590310008073. [DOI] [PubMed] [Google Scholar]
- 19.Nakanishi H, Groskopf J, Fritsche HA, et al. PCA3 molecular urine assay correlates with prostate cancer tumor volume: implication in selecting candidates for active surveillance. J Urol. 2008;179:1804–1809. doi: 10.1016/j.juro.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 21.Shah SR, Freedland SJ, Aronson WJ, et al. Exposure to Agent Orange is a significant predictor of prostate-specific antigen (PSA)-based recurrence and a rapid PSA doubling time after radical prostatectomy. BJU Int. 2009;103:1168–1172. doi: 10.1111/j.1464-410X.2009.08405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamie K, DeVere, White RW, et al. Agent Orange exposure, Vietnam War veterans, and the risk of prostate cancer. Cancer. 2008;113:2464–2470. doi: 10.1002/cncr.23695. [DOI] [PubMed] [Google Scholar]
- 23.Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173:1938–1942. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freedland SJ, Presti JC, Jr, Kane CJ, et al. Do younger men have better biochemical outcomes after radical prostatectomy? Urology. 2004;63:518–522. doi: 10.1016/j.urology.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 25.Presti JC, Jr, O’Dowd GJ, Miller MC, et al. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol. 2003;169:125–129. doi: 10.1016/S0022-5347(05)64051-7. [DOI] [PubMed] [Google Scholar]
- 26.D’Amico AV, Cote K, Loffredo M, et al. Advanced age at diagnosis is an independent predictor of time to death from prostate carcinoma for patients undergoing external beam radiation therapy for clinically localized prostate carcinoma. Cancer. 2003;97:56–62. doi: 10.1002/cncr.11053. [DOI] [PubMed] [Google Scholar]
- 27.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293:2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 28.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 29.Cooperberg MR, Lubeck DP, Penson DF, et al. Sociodemographic and clinical risk characteristics of patients with prostate cancer within the Veterans Affairs health care system: data from CaPSURE. J Urol. 2003;170:905–908. doi: 10.1097/01.ju.0000081200.63275.0b. [DOI] [PubMed] [Google Scholar]
- 30.Thompson IM, Canby-Hagino E, Lucia MS. Stage migration and grade inflation in prostate cancer: Will Rogers meets Garrison Keillor. J Natl Cancer Inst. 2005;97:1236–1237. doi: 10.1093/jnci/dji286. [DOI] [PubMed] [Google Scholar]