Abstract
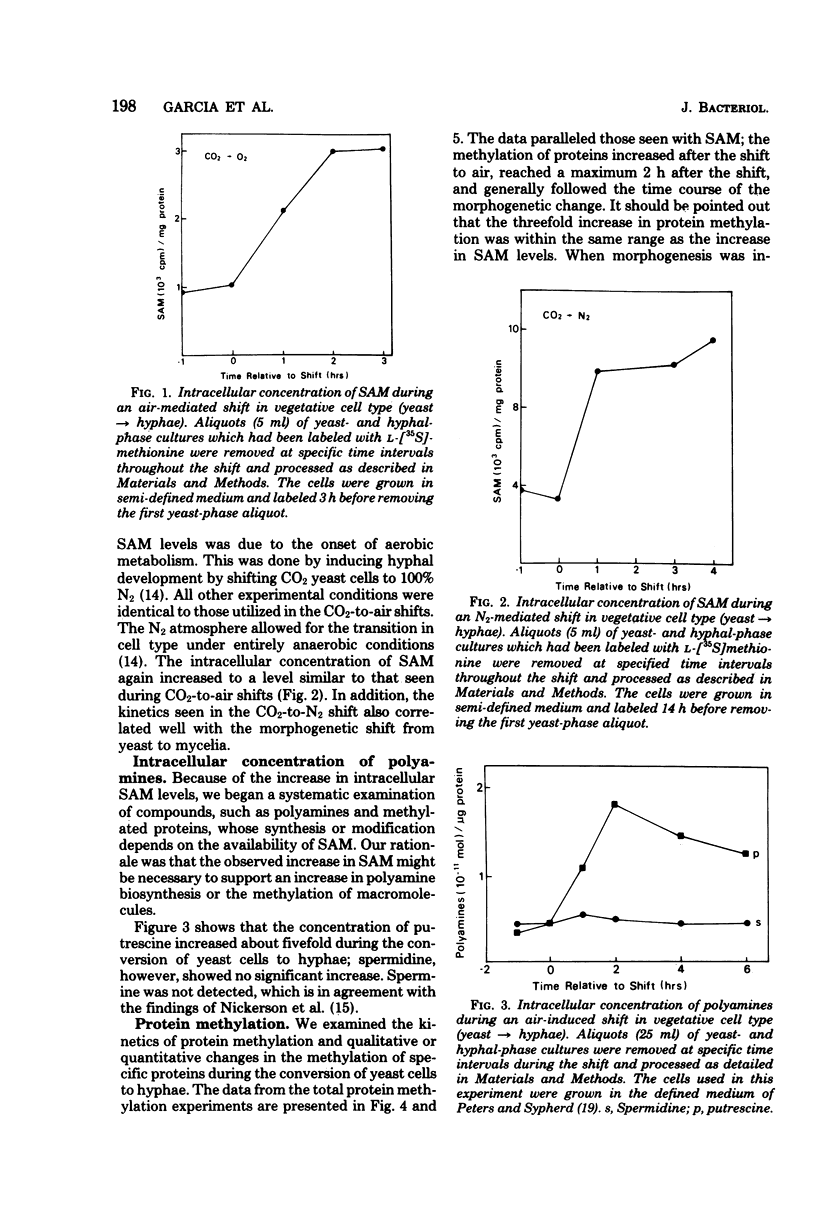
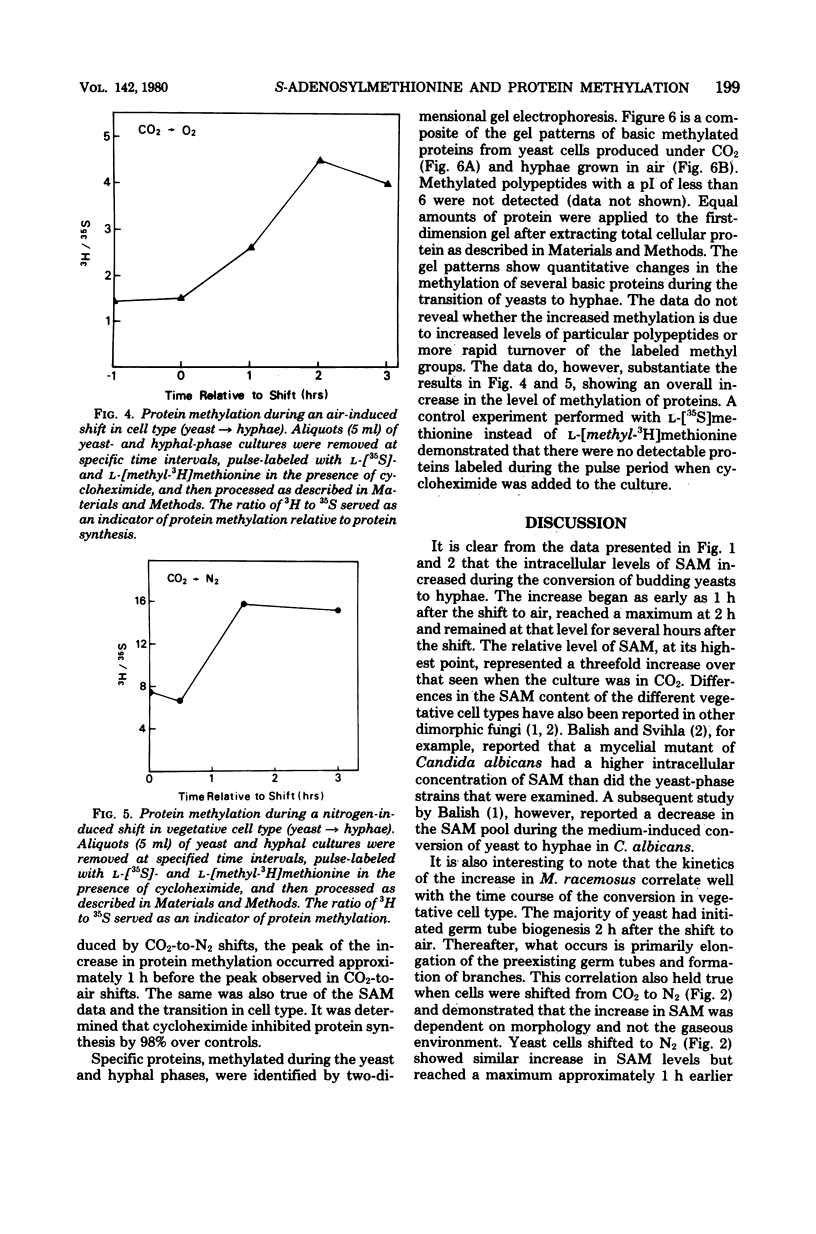
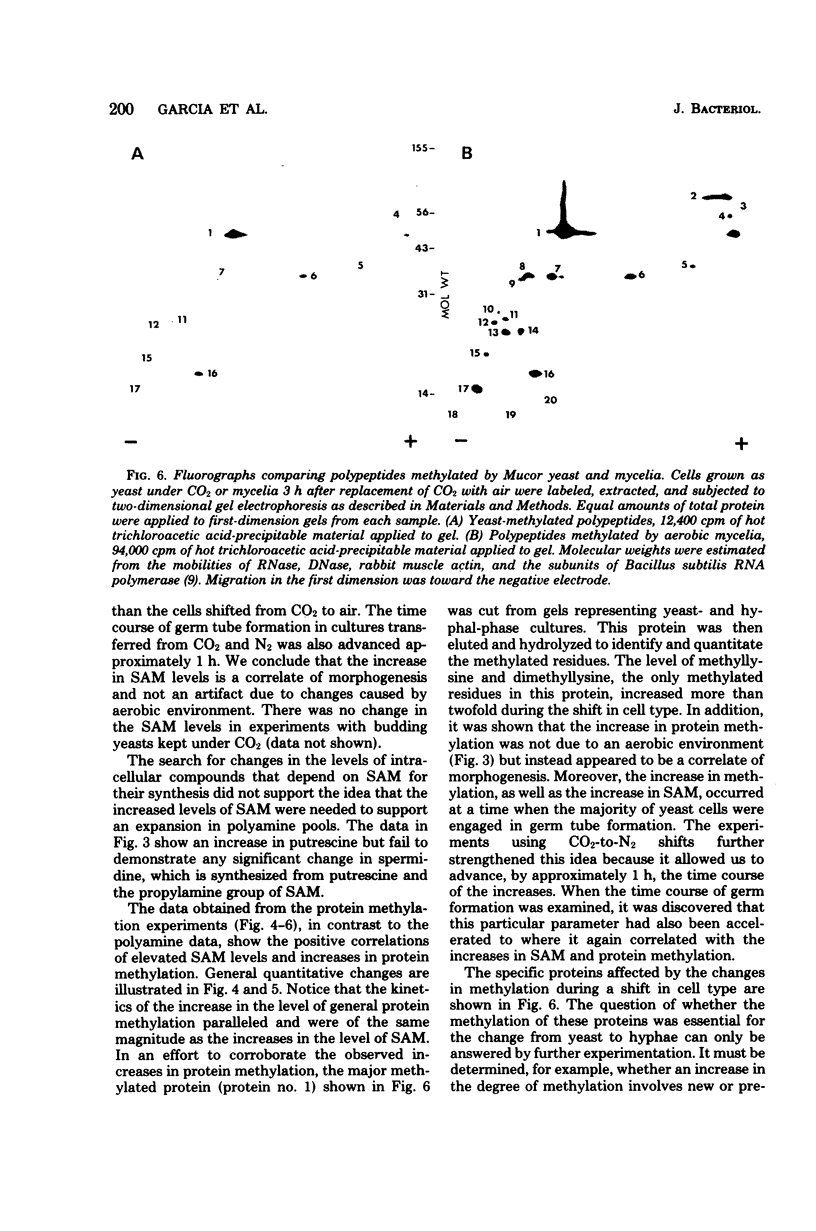
The intracellular level of S-adenosylmethionine increased as the yeast-phase cells of Mucor racemosus were induced to convert to hyphae. This increase correlated well with time course of the conversion in cell type and was independent of the metabolic changes caused by the shift to aerobic conditions. There was no significant change in the intracellular level of spermidine, a polyamine synthesized from putrescine and the propylamine group of S-adenosylmethionine. Spermine was not detected. An examination of protein methylation revealed an increase in the methylation of total protein during the shift in cell type and possible qualitative as well as quantitative changes in specific base proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTNICKI-GARCIA S., NICKERSON W. J. Induction of yeast-like development in Mucor by carbon dioxide. J Bacteriol. 1962 Oct;84:829–840. doi: 10.1128/jb.84.4.829-840.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Nutrition, growth, and morphogenesis of Mucor rouxii. J Bacteriol. 1962 Oct;84:841–858. doi: 10.1128/jb.84.4.841-858.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balish E. Methionine biosynthesis and S-adenosylmethionine degradation during an induced morphogenesis of Candida albicans. Can J Microbiol. 1973 Jul;19(7):847–853. doi: 10.1139/m73-135. [DOI] [PubMed] [Google Scholar]
- Balish E., Svihla G. Ultraviolet microscopy of Candida albicans. J Bacteriol. 1966 Dec;92(6):1812–1820. doi: 10.1128/jb.92.6.1812-1820.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Control of dimorphism in Mucor by hexoses: inhibition of hyphal morphogenesis. J Bacteriol. 1968 Nov;96(5):1586–1594. doi: 10.1128/jb.96.5.1586-1594.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D. Development of respiration and mitochondria in Mucor genevensis after anaerobic growth: absence of glucose repression. J Bacteriol. 1972 Jan;109(1):399–408. doi: 10.1128/jb.109.1.399-408.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diliberto D. J., Jr, Veiveros O. H., Axelrod J. Subcellualr distribution of protein carboxymethylase and its endogenous substrates in the adrenal medulla: possible role in excitation-secretion coupling. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4050–4054. doi: 10.1073/pnas.73.11.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt W. R., Whiteley H. R. Translation of RNAs synthesized in vivo and in vitro from bacteriophage SP82 DNA. J Virol. 1978 Feb;25(2):616–629. doi: 10.1128/jvi.25.2.616-629.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inderlied C. B., Cihlar R. L., Sypherd P. S. Regulation of ornithine decarboxylase during morphogenesis of Mucor racemosus. J Bacteriol. 1980 Feb;141(2):699–706. doi: 10.1128/jb.141.2.699-706.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. I. METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI B. J Biol Chem. 1963 Sep;238:2919–2922. [PubMed] [Google Scholar]
- Kondoh H., Ball C. B., Adler J. Identification of a methyl-accepting chemotaxis protein for the ribose and galactose chemoreceptors of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):260–264. doi: 10.1073/pnas.76.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Mooney D. T., Sypherd P. S. Volatile factor involved in the dimorphism of Mucor racemosus. J Bacteriol. 1976 Jun;126(3):1266–1270. doi: 10.1128/jb.126.3.1266-1270.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson K. W., Dunkle L. D., Van Etten J. L. Absence of spermine in filamentous fungi. J Bacteriol. 1977 Jan;129(1):173–176. doi: 10.1128/jb.129.1.173-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Paveto C., Epstein A., Passeron S. Studies on cyclic adenosine 3' ,5'-monophosphate levels, Adenylate cyclase and phosphodiesterase activities in the dimorphic fungus Mucor rouxii. Arch Biochem Biophys. 1975 Aug;169(2):449–457. doi: 10.1016/0003-9861(75)90187-3. [DOI] [PubMed] [Google Scholar]
- Paznokas J. L., Sypherd P. S. Respiratory capacity, cyclic adenosine 3',5'-monophosphate, and morphogenesis of Mucor racemosus. J Bacteriol. 1975 Oct;124(1):134–139. doi: 10.1128/jb.124.1.134-139.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N. Identification and quantitation of amines by thin-layer chromatography. J Chromatogr. 1971 Dec 9;63(1):97–112. doi: 10.1016/s0021-9673(01)85620-x. [DOI] [PubMed] [Google Scholar]




