Abstract
Fractalkine (CX3CL1) to fractalkine receptor (CX3CR1) interactions in the brain are involved in the modulation of microglial activation. Our recent findings indicate that there is microglial hyperactivity in the aged brain during an inflammatory challenge. The underlying cause of this amplified microglial response in the aged brain is unknown. Therefore, the purpose of this study was to determine the degree to which age-associated impairments of CX3CL1 and CX3CR1 in the brain contribute to exaggerated microglial activation after intraperitoneal (i.p.) injection of lipopolysaccharide (LPS). Here we show that CX3CL1 protein was reduced in the brain of aged (18–22 mo) BALB/c mice compared to adult (3–6 mo) controls. CX3CL1 protein, however, was unaltered by LPS injection. Next, CX3CR1 levels were determined in microglia (CD11b+/CD45low) isolated by Percoll-density gradient separation at 4 and 24 h after LPS injection. Flow cytometric and mRNA analyses of these microglia showed that LPS-injection caused a marked decrease of CX3CR1 and a simultaneous increase of IL-1β at 4 h after LPS injection. While surface expression of CX3CR1 was enhanced on microglia of adult mice by 24 h, it was still significantly downregulated on a subset of microglia from aged mice. This protracted reduction of CX3CR1 corresponded with a delayed recovery from sickness behavior, prolonged IL-1β induction, and decreased TGFβ expression in the aged brain. In the last set of studies BV2 microglia were used to determine effect of TGFβ on CX3CR1. These results showed that TGFβ enhanced CX3CR1 expression and attenuated the LPS-induced increase in IL-1β expression.
Keywords: Microglia, Aging, Brain, Cytokines, Fractalkine, Inflammation
1. Introduction
Microglia are part of the innate immune system in the CNS and play an important role in interpreting and propagating inflammatory signals in response to activation of the peripheral immune system (Davalos et al., 2005; Nguyen et al., 2002; Nimmerjahn et al., 2005). Several studies using older rodents, non-human primates, and humans indicate that the profiles of microglia and astrocytes become more inflammatory with age. For example, markers of glial reactivity or priming, such as major histocompatibility complex (MHC) class II, are increased in the brain during normal aging (Godbout and Johnson, 2006; Perry et al., 2003; Wynne et al., 2009). Recent studies in older rodents indicate that a potential consequence of a reactive glial profile is an exaggerated neuroinflammatory cytokine response to either central (Abraham et al., 2008; Huang et al., 2008) or peripheral (Chen et al., 2008; Godbout et al., 2005b; Henry et al., 2008; Henry et al., 2009) innate immune challenge. An exaggerated microglial response with age (Henry et al., 2009) is relevant because it is coupled with a myriad of complications including cognitive impairment (Barrientos et al., 2009; Barrientos et al., 2006; Chen et al., 2008), altered febrile response (Barrientos et al., 2009), exaggerated sickness behavior (Abraham et al., 2008; Godbout et al., 2005a; Huang et al., 2008), and protracted depressive-like behavior (Godbout et al., 2008). Collectively, these data point to an impaired ability to regulate microglia in the aged brain.
Recent data has begun to establish fractalkine (CX3CL1) to fractalkine receptor (CX3CR1) interactions as an important mechanism of microglial regulation. CX3CL1 is a member of the CX3C family of chemokines (Harrison et al., 1998; Nishiyori et al., 1998; Pan et al., 1997) and is one of the few chemokines that is highly expressed in the brain (Pan et al., 1997; Sunnemark et al., 2005; Tarozzo et al., 2003). CX3CL1 exists both as a ~95 kDa membrane-bound ligand and a ~65 kDa soluble glycoprotein (Harrison et al., 1998). Complementary expression of CX3CL1 on neurons and CX3CR1 on microglia (Hughes et al., 2002; Maciejewski-Lenoir et al., 1999; Nishiyori et al., 1998; Pan et al., 1997) has led to the hypothesis of a unique communication system where neurons constitutively express and release CX3CL1 to modulate activation of microglia (Cardona et al., 2006).
In acute inflammatory conditions, CX3CL1 and CX3CR1 interact to attenuate microglial activation. For example, pretreatment with neutralizing anti-CX3CL1 antibody exaggerates neuroinflammation after i.c.v. injection of LPS (Zujovic et al., 2001). In cultured microglia or mixed glia cultures, soluble CX3CL1 attenuates LPS-induced production of TNFα, IL-6, and IL-1β (Mizuno et al., 2003; Zujovic et al., 2000). Moreover, CX3CR1-deficiency amplifies microglial IL-1β expression, neurotoxicity, and mortality in CX3CR1−/− mice compared to CX3CR1+/− mice after repeated i.p. injections of lipopolysaccharide (LPS) (Cardona et al., 2006). Furthermore, in ex vivo microglial cultures, CX3CR1 expression is up-regulated by the anti-inflammatory cytokine, transforming-growth-factor β (TGFβ) (Chen et al., 2002), and is downregulated by LPS (Boddeke et al., 1999). The reduction in CX3CR1 expression after exposure to LPS is associated with decreased sensitivity to CX3CL1 (Boddeke et al., 1999). Collectively, these data indicate that CX3CL1-CX3CR1 interactions in the brain function to regulate the activation of microglia following an acute immune challenge.
Our recent findings indicate that there is amplified microglial activation in the aged brain after i.p. challenge with LPS (Henry et al., 2009). We hypothesize that age-associated changes in neural expression of CX3CL1, microglial expression of CX3CR1, or both, contribute to impaired regulation of microglia in the aged brain after LPS challenge. Therefore, the purpose of this study was to determine the degree to which expression of CX3CL1 or CX3CR1 was affected in the brain of adult and aged mice by peripheral LPS challenge. Here we show that CX3CL1 was decreased in the brain of aged BALB/c mice, but was not altered by LPS injection. Moreover, we show that injection of LPS reduced surface expression of CX3CR1 on microglia of aged and adult mice and caused protracted downregulation of CX3CR1 on microglia of aged mice. This LPS-induced reduction of CX3CR1 on aged microglia corresponded with a delayed recovery from sickness behavior, prolonged microglial activation, and decreased TGFβ expression in the brain of aged mice compared to adults. Furthermore, data from cultured BV2 microglia showed that TGFβ enhanced CX3CR1 expression and attenuated the LPS-induced increase in IL-1β expression.
2. Materials and Methods
2.1 Animals
Adult (3–6 months-old) BALB/c mice were obtained from a breeding colony kept in barrier-reared conditions in a specific-pathogen-free facility at the Ohio State University. Mice were individually housed in polypropylene cages and maintained at 25° C under a 12 h light/12 h dark cycle with ad libitum access to water and rodent chow. For age comparisons, male BALB/c mice (3–6 and 18–22 mo) were purchased from the National Institute on Aging specific-pathogen-free colony (maintained at Charles River Laboratories, Inc., MA). The median lifespan for BALB/c mice is approximately 26 months (Morley and Trainor, 2001). To investigate changes that occur from adulthood to what is considered aged, 3–6-month-old (young adult) and 18–22-month-old (aged) male mice were used. Upon arrival, mice were individually housed as described above. All procedures were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee.
2.2 Experimental Protocols
In the first study, adult (3–6 mo) BALB/c mice received an intraperitoneal (i.p.) injection of saline or Escherichia coli LPS (0.33 mg/kg; serotype 0127:B8, Sigma, St. Louis, MO) and were sacrificed by CO2 asphyxiation 4, 8, or 24 h later (n=6). This LPS dosage was selected because it elicits a proinflammatory cytokine response in the brain resulting in a transient sickness response in adult mice (Berg et al., 2004; Godbout et al., 2005b; Henry et al., 2008). The entire cortex and hippocampus were dissected out of the brain and homogenized separately for total RNA isolation/quantitative PCR (n=7–8) or CX3CL1 protein analysis (n=4). In a related experiment, adult (3–6 mo) and aged (18–22 mo) male BALB/c mice were injected i.p. with saline or LPS (0.33 mg/kg) and a 1 mm coronal brain section (+0.38 mm from bregma) (Paxinos and Franklin, 2004) was used for CX3CL1 protein analyses (n=6). The brain section was collected by cutting the intact brain using a rodent brain matrix (ASI instruments, Warren, MI) as a guide.
In the second study, adult (3–6 mo) BALB/c mice received an intraperitoneal (i.p.) injection of saline or LPS (0.33 mg/kg or 3.3 mg/kg) and were sacrificed by CO2 asphyxiation 4 h later (n=6). Brains were homogenized and microglia were isolated by discontinuous Percoll density gradient. Microglia were used for total RNA isolation/quantitative PCR (n= 8) or for flow cytometric analyses of CD11b, IL-1β, and CX3CR1 expression (n=6–8).
In the third study, adult (3–6 mo) and aged (18–22 mo) male BALB/c mice were injected i.p. with saline or LPS (0.33 mg/kg) and social exploratory behavior was determined 0, 4, 8, 12, and 24 hours after i.p. injections (n=4). At 4 or 24 h after injections, mice were sacrificed and microglia were isolated by discontinuous Percoll density gradient. Microglia were used for total RNA isolation/quantitative PCR (n=6–8) or for flow cytometric analyses for CD11b and CX3CR1 (n=7–8). In a subset of mice, a 1 mm coronal brain section (+0.38 mm from bregma) was collected and was used for CX3CL1 protein analysis or mRNA analysis (IL-1β, TGFβ, IL-10, and IL-4) (n=8–10).
In the final study, BV2 microglia were pre-treated with vehicle or recombinant human TGFβ (0.1 ng/ml; R&D Systems, MN) for 30 minutes and then treated with saline or LPS (100 ng/ml). BV2 microglia were collect 4 h later and used for total RNA isolation/quantitative PCR (IL-1β and CX3CR1) or immunocytochemistry for CX3CR1 surface expression (n=12, three independent experiments).
2.3 BV2 Cell culture and treatment
BV2 microglia cell lines were cultured in growth medium (DMEM supplemented with 10% FBS, sodium bicarbonate 3.7 g/L, 200 mM glutamine, 100 U/ml penicillin G, 100 μg/ml streptomycin, 0.25 μg/ml fungizone) as previously described (Abraham et al., 2008). Cultures were maintained at 37°C with 95% humidity and 5% CO2 and growth medium was replenished every third day until confluence. For RNA isolation/qPCR experiments, cells were seeded at 5x105 cells per well in 12-well plates and allowed to adhere for 20 h. For immunocytochemistry experiments, cells were seeded at 2x105 cells per well in 24-well plates on poly-L-lysine coated glass coverslips and allowed to adhere for 20 h. Immediately before treatment, cultures were washed twice and supplemented with warm growth medium containing experimental treatments. Cell viability was measured by the MTS cell proliferation assay according to the manufacturer’s instructions (Promega, Madison, WI).
2.4 Isolation of Microglia
Microglia were isolated from whole brain homogenates as described previously (Frank et al., 2006b; Henry et al., 2009). In brief, brains were homogenized in Hank’s Balanced Salt Solution (HBSS, pH 7.4) by passing through a 70 μm nylon cell strainer. Resulting homogenates were centrifuged at 600 g for 6 min. Supernatants were removed and cell pellets were re-suspended in 70% isotonic Percoll (GE-healthcare, Uppsala, Sweden) at room temperature. A discontinuous Percoll density gradient was layered as follows: 70%, 50%, 35%, and 0% isotonic Percoll. The gradient was centrifuged for 20 minutes at 2000 g and microglia were collected from the interphase between the 70% and 50% Percoll layers (Frank et al., 2006b). Cells were washed and then re-suspended in sterile HBSS. The number of viable cells was determined using a hemocytometer and 0.1% Trypan blue staining. Each brain extraction yielded approximately 3 x 105 viable cells. We have previously characterized these cells as approximately 85% CD11b+/CD45low microglia with less than 1% CD11b+/CD45high macrophages (Henry et al., 2009). These relative percentages of cells do not change with age or LPS (Henry et al., 2009). Based on this previous characterization, cells isolated by Percoll density separation are referred to as “enriched microglia”.
2.4 RNA isolation and qPCR
RNA was isolated from discrete brain regions (e.g., cortex or hippocampus), 1mm coronal brain section (+0.38 mm from bregma), BV2 microglia or enriched microglia extracted by Percoll density gradient. For brain samples and BV2 microglia, total RNA was isolated using the Tri-Reagent protocol (Sigma, MO) and subjected to the DNA-free™ RNA clean up procedure (Ambion, TX). For Percoll isolated cells, RNA was isolated using the RNeasy plus mini kit (Qiagen, CA). In all RNA isolation procedures, RNA concentration was determined by spectrophotometry (Eppendorf, NY) and RNA was reverse transcribed to cDNA using an RT RETROscript kit (Ambion, TX).
Quantitative PCR was performed using the Applied Biosystems (Foster, CA) Taqman® Gene Expression assay as previously described (Godbout et al., 2005b). In brief, cDNA was amplified by real time PCR where a target cDNA (e.g., CD11b, IL-1β, CX3CL1, CX3CR1, TGFβ1, IL-4, and IL-10) and a reference cDNA (glyceraldehyde-3-phosphate dehydrogenase) were amplified simultaneously using an oligonucleotide probe with a 5' fluorescent reporter dye (6-FAM) and a 3' quencher dye (NFQ). Fluorescence was determined on an ABI PRISM 7300-sequence detection system (Applied Biosystems, CA). Data were analyzed using the comparative threshold cycle (Ct) method and results are expressed as fold difference from control.
2.5 Protein Analysis of CX3CL1
Protein levels of CX3CL1 in a 1 mm coronal brain section (+0.38 mm from Bregma) or the whole hippocampus were determined by ELISA (R&D Systems, MN). Brain tissue was homogenized in ice-cold lysis buffer containing 1% Triton-X-100, 100 mM NaCl, 50 mM NaF, 1mM EGTA, 25 mM benzamidine, 2 mM sodium orthovanadate, 1 mM dithiothreitol, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 1 mM PMSF, and 50 mM Tris, pH 7.4. Lysates were clarified by centrifugation and total protein concentration was determined by Bio-Rad protein assay. Clarified lysates were assayed for CX3CL1 according to the manufacturer’s instructions. Absorbance (450 nm) was determined using a Bio-Tek synergy HT microplate reader (Bio-Tek Instruments, VT). Assays were sensitive to ~0.20 ng/ml of CX3CL1 and inter- and intra-assay coefficients of variation were less than 0.1.
2.6 Microglial Staining and Flow Cytometry
Microglial surface antigens were stained as previously described (Henry et al., 2008; Henry et al., 2009). In brief, Fc receptors were blocked with anti-CD16/CD32 antibody (eBioscience, CA). Next, enriched microglia were incubated with rabbit anti-mouse CX3CR1 and rat anti-mouse CD11b-APC antibodies (eBioscience, CA). Microglia were washed and then incubated with a FITC-conjugated goat anti-rabbit IgG secondary antibody (eBioscience, CA). For isotype controls, cells from each treatment group were combined and stained with non-specific, isotype-matched antibody (APC-conjugated anti-rat IgG or FITC-conjugated goat anti-rabbit IgG). Cells were washed and then resuspended in FACS buffer for analysis.
Staining for intracellular IL-1β was performed using the BD Cytofix/Cytoperm Plus fixation/permeabilization protocol (BD Biosciences, CA). To block secretion of cytokines, microglia were incubated in 10% FBS DMEM media containing brefeldin-A (BD Biosciences, CA) for 4 h at 37 °C. Next, enriched microglia were washed in FACS buffer (2% FBS in HBSS with 1mg/ml sodium azide) and Fc receptors were blocked with anti-CD16/CD32 antibody (eBioscience, CA). After blocking, microglia were stained with a rat anti-mouse CD11b-APC antibody. Microglia were then fixed and permeabilized with BD Fixation/Permeablization buffer for 20 min. Microglia were washed with BD Perm/Wash™ buffer, re-suspended in BD Perm/Wash™ buffer, and incubated with rabbit anti-mouse IL-1β-FITC (eBioscience, CA) for 30 min. Non-specific binding was assessed by using non-specific, isotype matched-conjugated antibodies. Microglia were washed twice in BD Perm/Wash buffer and resuspended in FACS buffer for analysis.
Antigen expression was determined using a Becton-Dickinson FACSCaliber four color flow cytometer. Ten thousand events were recorded. For each antibody, gating was determined based on appropriate negative isotype stained controls. Flow data were analyzed using FlowJo software (Tree Star, CA). For analysis of CX3CR1 expression, cells were gated for positive CD11b expression and expression levels of CX3CR1 were determined on this population. CD11b+ cells that exhibited FITC-fluorescence below the levels of the isotype control were considered CX3CR1low microglia. In some analyses, an additional gate was placed to divide the remaining cells into medium and high-expressing cells (CX3CR1med and CX3CR1high). All samples were analyzed with identical gates.
2.7 Immunocytochemistry on cultured BV2 microglia
BV2 microglia were plated in 24-well plates on poly-L-lysine coated glass coverslips and treated as described above. After treatment, media was aspirated and cells were washed 2 times. Cells were blocked for 10 minutes with anti-CD16/CD32 antibody in PBS supplemented with 1% BSA and 5% normal goat serum. Cells were then incubated with rabbit anti-mouse CX3CR1 (eBioscience, CA) diluted 1:250 in PBS for 20 minutes. Cells were then fixed in 4% paraformaldehyde for 10 minutes at room temperature before staining with alexafluor 594-conjugated secondary antibody (1:500) for 20 minutes. After staining, glass coverslips were mounted on superfrost plus glass slides (Fisher Scientific, PA) with fluoromount G (EMS, PA).
Images were captured at 20x using an epifluorescent Leica DM5000B microscope equipped with a Leica DFC300 FX camera and Leica imaging software. Fluorescent images were captured with identical exposure, gamma, and gain settings. Fluorescence intensity was measured using Image-Pro software (Media Cybernetics, Bethesda, MD).
2.8 Social Exploratory Behavior
To assess the motivation to engage in social exploratory behavior, a novel juvenile conspecific was introduced into the test subject’s home cage for a 10-min period. Behavior was videotaped and the cumulative amount of time the subject engaged in social investigation was determined from the video records by a trained observer who was blind to the experimental treatments. Baseline social behavior was measured immediately before experimental treatment (time 0). Social behavior was determined as the amount of time that the experimental subject spent investigating (e.g., anogenital sniffing, trailing) the juvenile. Results are expressed as percent decrease in time engaged in social behavior compared to respective baseline measures.
2.9 Statistical Analysis
To ensure a normal distribution, data were subjected to Shapiro-Wilk test using Statistical Analysis Systems (SAS) statistical software (Cary, NC). Observations greater than 3 interquartile ranges from the first and third quartile were excluded in the subsequent analysis. To determine significant main effects and interactions between main factors, data were analyzed using one- (Age, Treatment, Time), two- (Age × Treatment, Treatment x Time) or three- (Age × Treatment x Time) way ANOVA using the General Linear Model procedures of SAS. When appropriate, differences between treatment means were evaluated by an F-protected t-test using the Least-Significant Difference procedure of SAS. All data are expressed as treatment means ± standard error of the mean (SEM).
3. Results
3.1 Age associated reduction of CX3CL1 protein in the brain of aged mice
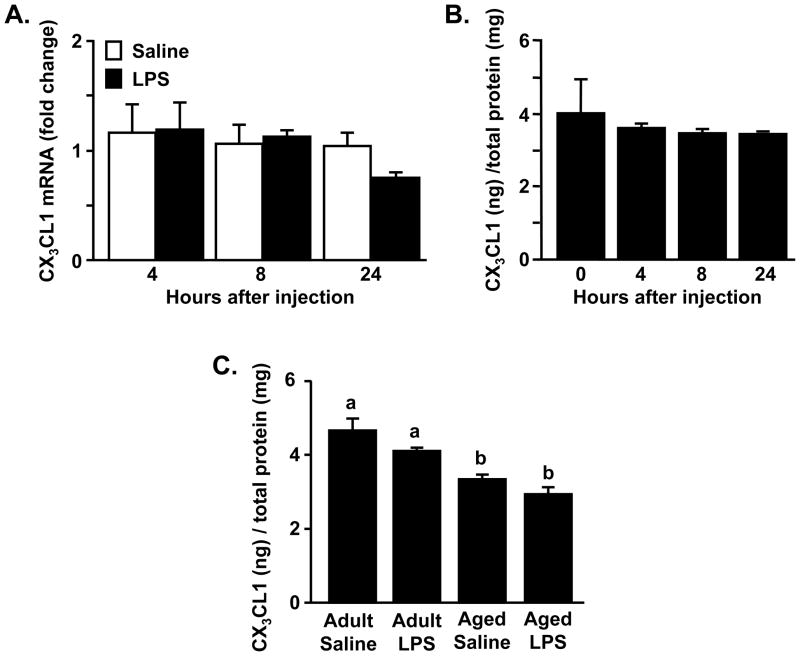
To determine the degree to which CX3CL1 levels were altered in the brain following LPS injection, adult mice were injected i.p. with saline or LPS, and mRNA expression was determined in the hippocampus or cortex collected 4, 8, or 24 h later. The hippocampus and cortex were selected because the neurons of these regions express the highest levels of CX3CL1 (Sunnemark et al., 2005; Tarozzo et al., 2003). CX3CL1 mRNA and total protein levels were unchanged by LPS injection at the times tested in the hippocampus (Fig.1A&B) and cortex (data not shown). Previous findings indicate that membrane-bound CX3CL1 (~95kd) can be released from glutamate-overstimulated neurons as a soluble glycoprotein (~65 kDa) independent of mRNA transcription or total protein increase (Limatola et al., 2005). To exclude this possibility in our model, western blot analysis for CX3CL1 was also performed. This assay did not reveal any significant change in membrane-bound or soluble CX3CL1 protein (data not shown).
Fig. 1. Total CX3CL1 was reduced in the aged brain.
Adult mice (3–6 mo) were injected with saline or LPS i.p. and A) CX3CL1 mRNA or B) CX3CL1 protein levels were determined in the hippocampus collected 4, 8, or 24 h later. Bars represent the mean ± SEM (n=6). C) Adult (3–6 mo) and aged (18–22 mo) BALB/c mice were injected i.p. with saline or LPS (0.33 mg/kg) and protein levels of CX3CL1 were determined from a 1 mm coronal brain section collected 24 h later. Bars represent the mean ± SEM (n=6). Means with different letters (a or b) are significantly different (p <0.05) from each other.
Next, we sought to determine CX3CL1 protein levels in the brain of aged mice after LPS injection. In this related but separate experiment, adult and aged mice were injected with saline or LPS i.p. and total CX3CL1 protein levels were determined in brain homogenate from a 1 mm coronal brain section through the prefrontal cortex (+0.38 mm from bregma) collected either 4 or 24 h later. The 24 h time point corresponds with the time when older mice show extended behavioral symptoms of sickness after LPS injection compared to adults (Godbout et al., 2005a; Godbout et al., 2008). The coronal brain section samples were collected prior to microglial extraction and corresponding analyses (shown in the subsequent figures). This protocol allowed for comparison of CX3CL1 protein levels in the same mice that social exploratory behavior and microglial mRNA and protein analyses were determined.
Fig. 1C shows that there was a significant age-dependent decrease in CX3CL1 protein in the brain of aged mice collected 24 h later (Age, F(1,23)= 32.7, p < 0.0001). Similar results were also obtained at 4 h after LPS (data not shown). Although there was a main effect of age on CX3CL1 levels at the 4 and 24 h time points, there was not a significant main effect of LPS treatment. Collectively, these data indicate that CX3CL1 protein is decreased in the brain of aged mice and that i.p. injection of 0.33 mg/kg LPS does not affect mRNA or total protein levels of CX3CL1.
3.2 Peripheral injection of LPS decreased CX3CR1 and increased IL-1β expression in microglia
CX3CR1 is highly expressed on microglia and plays a role in modulating their responses (Boddeke et al., 1999; Boehme et al., 2000; Hughes et al., 2002; Mizuno et al., 2003), the effect of peripheral injection of LPS on microglial CX3CR1 expression, however, has not been determined in vivo. Thus, we investigated the extent to which CX3CR1 was altered on microglia following peripheral challenge with LPS. In this experiment, adult mice were injected with LPS and mRNA levels of several microglia-related genes (CD11b, IL-1β, and CX3CR1) were determined in enriched microglia collected 4 h later. This 4 h time point after LPS injection was selected because it is a time when microglia actively produce cytokines (Henry et al., 2009) and when mice display the behavioral symptoms of sickness (Godbout et al., 2005b). Fig.2A shows that CX3CR1 mRNA expression in enriched microglia was markedly reduced after peripheral LPS injection (LPS, F(1,30)= 26.82, p < 0.001). Moreover, peripheral injection of LPS increased IL-1β mRNA expression in enriched microglia at 4 h after injection (Fig.2B; LPS, F(1,30)= 33.11, p < 0.0001). Fig.2C shows that CD11b mRNA expression was unaffected by LPS injection.
Fig. 2. Peripheral LPS injection increased IL–1β mRNA expression and decreased CX3CR1 expression in microglia.
Adult mice (3–6 mo) were injected with saline or LPS i.p. and A) CX3CR1, B) IL-1β, and C) CD11b mRNA levels were determined from enriched microglia isolated 4 h later (n=8). Means with different letters (a or b) are significantly different (P <0.05) from each other. Representative bivariate dot plots of Percoll isolated cells stained with antibodies for D) CD11b and CX3CR1 or E) CD11b and intracellular IL-1β. Cells were gated for CD11b expression. F) Average percentage of cells that were CD11b+/CX3CR1Low. Bars represent the mean ± SEM (n=8). Means with different letters (a, b, or c) are significantly different (P < 0.05) from each other. G) Representative histogram of CX3CR1-FITC fluorescence in microglia from a saline and LPS (3.3mg/kg) treated mouse. H) Average percentage of cells that were CD11b+/IL-1β+. Bars represent the mean ± SEM (n=4). Means with different letters (a, b, or c) are significantly different (P<0.05) from each other.
Because peripheral LPS injection caused a marked reduction of CX3CR1 mRNA in enriched microglia at 4 h (Fig.2A), we next determined CX3CR1 surface expression on microglia after LPS injection. In this experiment adult mice were injected i.p. with saline or LPS (0.33 or 3.3 mg/kg) and CX3CR1 surface expression was determined by flow cytometry 4 h later. Fig.2D shows representative bivariate dot plots of CD11b and CX3CR1 staining. LPS injection caused a dose-dependent decrease in CX3CR1 surface protein on microglia (Fig.2F; LPS, F(2,23)= 23.9, p < 0.001). The representative histogram of CX3CR1-FITC mean fluorescence intensity (MFI) confirmed that CX3CR1 expression was reduced on a subset of microglia from that received LPS (3.3mg/kg) (Fig.2G). Intracellular IL-1β staining was also determined in CD11b+ microglia (Fig.2E). Fig.2E&H show that LPS injection caused a dose-dependent increase in IL-1β protein levels in microglia (LPS, F(2,11)= 16.6, p < 0.001). Taken together, these findings indicate that LPS injection led to increased intracellular IL-1β protein and decreased CX3CR1 surface expression on microglia in the adult brain.
3.3 Peripheral LPS injection caused protracted sickness behavior and prolonged downregulation of CX3CR1 mRNA on microglia of aged mice
We have previously reported that LPS injection caused exaggerated IL-1β production by microglia of aged mice (Henry et al., 2009) and that the behavioral symptoms of sickness (i.e., anorexia, lethargy, and social withdrawal) persist longer in aged mice compared to adults (Godbout et al., 2005b). Because CX3CL1 modulates the activation of microglia, impairment in the regulation of CX3CR1 may contribute to exaggerated and prolonged microglial activation in response to inflammatory stimuli. To confirm the age-associated differences in the recovery from sickness behavior, a subset of adult and aged mice (n= 4) were injected i.p. with LPS and social exploratory behavior was determined 0, 4, 8, 12, and 24 h after injection. As expected, aged mice showed an impaired recovery from the LPS-induced sickness behavior (Fig.3A; Age x LPS x Time, F (4,15)=6.29, p < 0.001). Fig.3B shows CD11b mRNA levels in enriched microglia were unaffected by either LPS or age. Fig.3C shows that enriched microglia from the brain of aged mice 24 h after LPS had the highest levels of IL-1β mRNA compared to all other treatments (Age x LPS interaction, F(1,31)= 4.1, p < 0.05).
Fig. 3. LPS injection caused a reduction of CX3CR1 mRNA in microglia of adult and aged mice.
Adult (3–6 mo) and aged (18–22 mo) BALB/c mice were injected i.p. with saline or LPS (0.33 mg/kg) and A) social exploratory behavior was determined 0, 4, 8, 12, and 24 h later (n=4). Means marked with * are significantly different from baseline controls and means marked with ‡ are significantly different from Adult LPS (p < 0.05). Adult and aged mice were injected i.p. with saline or LPS and. B) CD11b and C) IL-1β mRNA levels were determined from microglia isolated 24 h later. Mice were treated as above and CX3CR1 mRNA expression was determined from microglia isolated D) 4 h or E) 24 h after LPS injection. Bars represent the mean ± SEM (n=7–8). Means with different letters (a, b, or c) are significantly different (p < 0.05) from each other.
Next, mRNA expression of CX3CR1 in microglia of adult and aged mice 4 and 24 h after LPS injection was determined. Fig.3D shows that LPS injection decreased CX3CR1 mRNA expression in microglia independent of age (LPS, F(1,30)= 17.15, p < 0.005) at 4 h after injection. At 24 h after LPS injection, CX3CR1 mRNA expression returned to baseline in microglia of adult mice, but it was still reduced in microglia of aged mice (Fig.3E; Tendency for Age x LPS interaction, F(1,27)= 2.4, p = 0.1).
3.4 Prolonged downregulation of CX3CR1 surface expression on aged microglia at 24 h after peripheral injection of LPS
Based on the mRNA results we next determined surface expression of CX3CR1 in adult and aged microglia after LPS challenge. In this experiment adult and aged mice were injected with saline or LPS, CD11b and CX3CR1 levels were determined on microglia collected by Percoll separation 4 or 24 h after injection. Fig.4A shows that the same percentage of CD11b+ cells were used in the analysis for each treatment and age group. Representative bivariate dot plots of CD11b and CX3CR1 staining at 4 and 24 h after LPS injection are shown in Fig.4B&C, respectively. At 4 h after LPS injection, both adult and aged mice showed a significant increase of CX3CR1low microglia (Fig.4B&D); main effect of LPS, F(1,29)= 257.85, p < 0.0001). At 24 h after LPS injection, CX3CR1 expression was recovered to baseline levels in adult mice, but was still markedly reduced on microglia of aged mice (Fig.4C; Age x LPS, F(1,29) = 14.66, p < 0.001). Furthermore, this significant age x LPS interaction was dependent on time after LPS injection (Age x LPS x time interaction, F(1,51)= 10.49, p < 0.01). Taken together, these data indicate that the reduction of CX3CR1 on the surface of microglia was protracted in the aged brain
Fig. 4. Protracted reduction of CX3CR1 surface expression on microglia of aged mice after LPS injection.
Adult (3–6 mo) and aged (18–22 mo) BALB/c mice were injected i.p. with saline or LPS (0.33 mg/kg) and CX3CR1 surface expression was determined on microglia. A) Percentage of CD11b positive microglia from each treatment group that were used in the analysis. Bars represent the mean ± SEM (n=7). Representative bivariate dot plots of Percoll isolated cells stained with antibodies for CD11b and CX3CR1 and gated for positive CD11b expression at B) 4 and C) 24 h after LPS injection. D) Average percentage of cells that were CD11b+/CX3CR1Low. Bars represent the mean ± SEM (n=7). Means with different letters (a or b) are significantly different (p < 0.05) from each other
3.5 Enhanced expression of CX3CR1 on microglia of adult mice at 24 h after LPS injection
In addition to a recovery to baseline levels, the bivariate dot plots in Fig.4C indicated that total expression of CX3CR1 on microglia of adult mice was enhanced at 24 h after LPS injection. To confirm this observation, CX3CR1-FITC fluorescence was determined and gated based on low, medium, and high CX3CR1-FITC staining. Representative histograms of adult and aged microglia at 24 h after LPS injection with the low, medium, and high designations are shown in Fig.5A&B. Fig.5C illustrates the relative percentages of CX3CR1-expressing microglia for each treatment group. The average MFI of CX3CR1-FITC fluorescence and the average relative percentages of CX3CR1-expressing microglia for each treatment group are provided in Fig.5D. There was a significant increase of CX3CR1 MFI on microglia of adult mice at 24 h after LPS compared to all other groups (Age x LPS interaction, F(1,37)= 4.3, p < 0.05). Consistent with Fig.4C, a significant population of CX3CR1low microglia was still present from aged mice treated with LPS (Fig.5B&D). It is important to mention that there was also a modest increase in the CX3CR1high population in aged mice 24 h after LPS (9.6 ± 1.3%) compared to the adult (1.3 ± 0.2%) and aged saline controls (4.7 ± 0.6%). The percentage of CX3CR1high microglia, however, was highest in the microglia of adult mice (15.6 ± 2.4%) and this Adult LPS group was the only group that exhibited an increase in overall MFI (Fig.5D, Age x LPS interaction, F(1,37)= 9.15, p < 0.05). Taken together, these data indicate that while CX3CR1 surface expression was globally enhanced on microglia of adult mice 24 h after LPS injection, it was still significantly reduced on a subset of microglia of aged mice.
Fig. 5. Enhanced CX3CR1 surface expression on microglia of adult mice after LPS injection.
Adult (3–6 mo) and aged (18–22 mo) BALB/c mice were injected i.p. with saline or LPS (0.33 mg/kg) and CX3CR1 surface expression was determined on microglia. Representative histograms of CX3CR1-FITC fluorescence on CD11b+ cells from A) adult and B) aged mice 24 h after injection. C) Relative percentages of CX3CR1-low, medium, or high-expressing microglia. D) Mean fluorescence intensity (MFI) and percentage of CX3CR1-low, medium, or high-expressing microglia. Values represent the mean ± SEM (n=6–8). Means with different letters (a, b, or c) are significantly different (p < 0.05) from each other.
3.6 TGFβ mRNA levels were augmented in the adult brain 24 h after LPS
Surface expression of CX3CR1 was still significantly lower in a subset of microglia from aged mice 24 h after LPS challenge, while it was enhanced on microglia of adult mice at this time. Because CX3CR1 is modulated by cytokines, mRNA levels of several cytokines (IL-1β, IL-4, IL-10, and TGFβ) were determined in brain homogenate derived from a coronal brain section (+0.38 mm from bregma) collected prior to the extraction of brain microglia used in the above experiments (Fig.4). Coronal brain sections were collected from the same mice used in the experiments shown in Figs.3&4. As expected, IL-1β mRNA was the highest in the brain of aged mice 24 h after LPS (Fig.6A; Tendency for Age x LPS interaction, F(1,22)= 3.7, p = 0.07). Fig.6B shows that LPS decreased IL-4 mRNA levels independent of age (LPS, F(1,28)= 6.48, p < 0.05). IL-10 mRNA levels were increased by LPS, independent of age (Fig.6C). It is important to note that IL-10 mRNA was undetected in the brain of saline-treated mice. Fig.6D shows that TGFβ was increased in the brain of adult mice 24 h after LPS (LPS, F(1,27)= 19.27, P < 0.001), but TGFβ mRNA expression was not increased in the brain of aged mice 24 h after LPS (age x LPS interaction, F(1,27)= 6.28 p < 0.02). These data indicate that 24 h after LPS there were higher mRNA levels of IL-1β , but lower mRNA levels of TGFβ in the brain of aged mice compared to adults.
Fig. 6. Attenuated TGFβ mRNA levels in the aged brain 24 h after LPS injection.
Adult (3–6 mo) and aged (18–22 mo) BALB/c mice were injected i.p. with saline or LPS (0.33 mg/kg) and A) IL-1β, B) IL-4, C) IL-10, and D) TFGβ mRNA levels were determined from a 1 mm coronal brain section collected 24 h later. Bars represent the mean ± SEM (n=6–8). Means with different letters (a, b, or c) are significantly different (p < 0.05) from each other.
3.7 TGFβ enhanced CX3CR1 expression and attenuated the LPS-induced increase in IL-1β in BV2 microglia
To begin to understand the potential effect of less TGFβ in the brain of aged mice after LPS injection, a series of cell culture experiments using BV2 microglia were completed. In the first experiment, BV2 cells were treated with recombinant human TGFβ and mRNA levels of CX3CR1 and IL-1β were determined. Fig.7A&B shows that TGFβ treatment simultaneously increased CX3CR1 mRNA expression (LPS, F(5,23)= 9.9, p < 0.0001) and decreased IL-1β mRNA expression in a dose-dependent manner (LPS, F(5,23)= 3.85 , p < 0.05). In the second experiment, BV2 cells were incubated with TGFβ and then were treated with LPS (Fig.7C&D). As anticipated, mRNA of levels CX3CR1 were enhanced by TGFβ (TGFβ, F(1,36)= 56.4, p < 0.0001) and reduced by LPS (LPS, F(1,36)= 10.3, p < 0.01). Moreover, TGFβ pretreatment reversed the LPS-induced reduction in CX3CR1. In fact, the enhancement of CX3CR1 mRNA by TGFβ was still maintained in the LPS-treated BV2 cells. Fig.7D shows that TGFβ pretreatment attenuated the LPS-induced increase in IL-1β (TGFβ x LPS, F(1,31)= 163.65, p < 0.0001).
Fig. 7. TGFβ enhanced CX3CR1 expression and attenuated IL-1β expression in BV2 microglia after LPS stimulation.
BV2 microglia were incubated with TGFβ (0–1.0 ng/ml) and A) CX3CR1 and B) IL-1β mRNA expression was determined 4 h later. Bars represent the mean ± SEM (n=6). Means with different letters (a, b, or c) are significantly different (p < 0.05) from each other. In a related study, BV2 cells were incubated with vehicle or TGFβ (0.1 ng/ml) for 30 min before LPS (100 ng/ml) treatment and C) CX3CR1 and D) IL-1β mRNA levels were determined in cells collected 4 h later. Bars represent the mean ± SEM (n=8, 2 independent experiments). Means with different letters (a, b, or c) are significantly different from each other (p < 0.05). BV2 cells were treated as above and CX3CR1 surface expression was determined 4 h later. E) Representative CX3CR1 staining in saline-treated (panels i & ii), LPS-treated (panels iii & iv), and TGFβ/LPS-treated BV2 microglia (panels v & vi). Top panels show fluorescent images of CX3CR1+ staining and the bottom panels show corresponding bright field images. The inset images in the bottom panels represent merged fluorescent and brightfield images of the white outlined boxes. Scale bar = 100 μm. F) Average fluorescence intensity of CX3CR1 staining. Bars represent the mean ± SEM (n=12, 3 independent experiments). Means with different letters (a, b, or c) are significantly different from each other (p < 0.05).
Next, surface expression of CX3CR1 was determined on BV2 cells after TGFβ and LPS treatments. Fig.7E shows representative staining for CX3CR1. In saline-treated BV2 cells, CX3CR1 was detected as a distinct ring around the membrane of the cell (Fig.7E.i). In LPS-treated BV2 cells, there was less CX3CR1 on the membrane of the cells compared to saline controls (Fig.7E.iii&F; LPS, F(1,48)= 6.79, p < 0.05). Moreover, TGFβ enhanced CX3CR1 surface expression (Fig.7E.v&F; TGFβ, F(1,48)=26.46, p < 0.0001) and post hoc analysis revealed that TGFβ attenuated the LPS-induced reduction of CX3CR1 surface expression (p < 0.05).
4. Discussion
We have previously reported that stimulation of the peripheral innate immune system in aged BALB/c mice caused exaggerated microglial activation with amplified mRNA and intracellular protein expression of IL-1β (Henry et al., 2009). The exaggerated neuroinflammation and microglial activation was paralleled by prolonged sickness behavior (Godbout et al., 2005b), impaired working memory (Chen et al., 2008), and protracted depressive-like behavior (Godbout et al., 2008). The results of the current study support our hypothesis that impaired CX3CL1-CX3CR1 mediated regulation of microglia contributes to the exaggerated microglial activation in the brain of aged mice after peripheral immune challenge. First, we show there was significantly less CX3CL1 protein in the brain of aged mice compared to adults (Fig.1). Second, peripheral challenge with LPS caused a simultaneous reduction of CX3CR1 (mRNA and surface expression) and an induction of IL-1β (mRNA and intracellular protein expression) in microglia (Figs.2–4). Third, our data indicate that i.p. injection of LPS caused an extended sickness response, prolonged induction of IL-1β, and a protracted reduction of CX3CR1 on microglia of aged mice compared to adults (Figs.3–5). Fourth, TGFβ mRNA was increased in the brain of adult mice 24 h after LPS, but was not up-regulated in the brain of aged mice (Fig.6). Last, studies with BV2 microglia show that TGFβ enhanced surface expression of CX3CR1 and attenuated IL-1β expression following an LPS challenge (Fig.7).
Several reports using rodent models of aging indicate that the brain environment becomes more proinflammatory with age (Frank et al., 2006a; Godbout et al., 2005a; Henry et al., 2009; Lyons et al., 2009; Sierra et al., 2007). Consistent with this hypothesis, we show that there was an age-dependent reduction in CX3CL1 protein (Fig.1C). A potential explanation for less CX3CL1 in the brain of aged mice is a reduction in total fractalkine produced by neurons. This may be caused by increased neuronal loss with aging (Woodruff-Pak et al., 2010) or functional changes in aged neurons associated with impaired oxidative or calcium homeostasis (Toescu, 2005). Whatever the cause, reduced levels of CX3CL1 in the brain of aged mice may contribute to a reduced ability to regulate microglial activation after LPS challenge. In support of this notion, other studies show that soluble CX3CL1 attenuated LPS-induced production of TNFα, IL-6, and IL-1β in microglia, mixed glia, and neuron/glia co-cultures (Mizuno et al., 2003; Zujovic et al., 2000). Moreover, in vivo studies show that pretreatment with a neutralizing anti-CX3CL1 antibody led to exaggerated neuroinflammation (i.e., TNFα and 8-isoprostane production) after i.c.v. injection of LPS (Zujovic et al., 2001). Our results are also consistent with recent studies showing that CX3CL1 was decreased in the brain of 22 mo Wistar rats (Lyons et al., 2009) and 22 mo Fisher 344 rats (Bachstetter et al., 2009). In these studies, the age-associated increase in MHC II+ microglia was attenuated following either a single i.c.v. injection of CX3CL1 (Lyons et al., 2009) or a 7-day infusion of CX3CL1 (Bachstetter et al., 2009). We have reported an age-associated increase of MHC II expression on microglia of BALB/c mice (CD11b+/CD45low) and that LPS challenge elicited a robust induction of IL-1β in these MHC II+ microglia (Henry et al., 2009). Thus, a reduction in CX3CL1 protein in older BALB/c mice may contribute to the increased number of MHC II+ microglia detected in our model (Henry et al., 2009). In addition, we interpret these current findings to suggest that a reduction in CX3CL1 in the aged brain (Fig.1C) contributes to the amplified microglial activation following peripheral LPS injection in a BALB/c model of aging (Figs.3&4).
It is important to note that the function of CX3CL1 is dependent on the nature of the inflammatory stimulus. For instance, expression of CX3CL1 is markedly enhanced in several rodent models of neurodegenerative diseases (Huang et al., 2006; Sunnemark et al., 2005), ischemia (Denes et al., 2008) and traumatic CNS injury (Harrison et al., 1998; Rancan et al., 2004). In these examples, increased CX3CL1 is associated with recruitment of peripheral immune cells into the CNS. Moreover, intra-parenchymal injection of high levels of CX3CL1 is associated with increased microglial activation (Hughes et al., 2002; Shan et al., 2009). Therefore, neuronal or glial release of significant levels of CX3CL1 may be a mechanism by which microglia and other CX3CR1+ immune cells are recruited to damaged areas within the CNS. In the present study, however, LPS injection did not increase total mRNA or protein levels of CX3CL1 in the hippocampus (or cortex) at 4, 8, or 24 h after LPS injection (Fig.1). Furthermore, we did not detect a significant influx in either macrophages (CD45high, CD11b+) or T-cells (CD3+, CD45+) in Percoll isolated cells from adult mice at 24 or 72 h after LPS injection (data not shown). Thus, in the context of an acute immune activation, our data support the idea that CX3CL1-CX3CR1 interactions function to regulate the activation of microglia (Cardona et al. 2006; Lyons et al. 2009a).
Microglia are modulated by CX3CL1 and therefore a change in the ligand/receptor interaction must occur to circumvent this regulation by neurons. A relevant finding of this study was that activation of microglia in response to a peripheral injection of LPS was associated with decreased surface expression of CX3CR1 (Fig.2). Independent of the age comparisons, this microglial specific reduction in surface expression of CX3CR1 after i.p. injection of LPS is a novel finding. Our data indicate that there is less CX3CR1 on the surface of microglia after LPS injection (Figs.2, 4&5) to respond to the constitutively produced CX3CL1 (Fig.1). These findings are consistent with a previous study showing that LPS caused a marked reduction of CX3CR1 mRNA expression and decreased sensitivity to CX3CL1 in ex vivo microglial cultures (Boddeke et al., 1999). Moreover, in our studies, peripheral injection of LPS caused a dose-dependent reduction in CX3CR1 that corresponded with an induction of IL-1β in microglia of adult mice (Fig.2). This inverse relationship between IL-1β and CX3CR1 was also apparent in the studies with BV2 microglia (Fig.7). Taken together, we interpret these data to suggest that the CX3CR1low microglia represent a more active subset of microglia.
A key finding of this study was that downregulation of CX3CR1 surface expression on microglia of aged mice was protracted compared to adults 24 h after LPS injection (Fig.4C and Fig.5). The protracted down-regulation of CX3CR1 on microglia of aged mice was associated with extended IL-1β expression (Fig.3C) and prolonged sickness behavior (Fig.3A). We hypothesize that protracted reduction of CX3CR1 on microglia plays a direct role in the prolonged production of IL-1β and subsequent neurobehavioral deficits in aged mice. In support of this notion, microglia from LPS-treated CX3CR1-deficient (CX3CR1−/−) mice express higher levels of IL-1β mRNA compared to CX3CR1−/+ controls. Injection of microglia isolated from LPS-treated CX3CR1−/− mice into the brain of a wild-type mouse caused neuronal cell death, which was blocked by co-injection of IL-1 receptor antagonist (Cardona et al., 2006). Another study showed that blocking CX3CR1 in the brain of adult rats with an anti-CX3CR1 antibody caused increased IL-1β production that was paralleled by deficits in neurogenesis (Bachstetter et al., 2009). These collective findings indicate that either loss or significant downregulation of CX3CR1 on microglia leads to extended expression of IL-1β after the microglia have become activated.
It is important to mention that along with an increased CX3CR1low population there was also an increased CX3CR1high population in aged mice 24 h after LPS compared to saline treated mice (Fig.5D). This is not unexpected considering the 24 h time-point after LPS in the aged brain represents a transitional time when inflammation is still present, but is moving towards resolution (Godbout et al, 2005). The CX3CR1 staining in microglia supports this conclusion. For example, in the adult brain at 24 h after LPS, the inflammation has dissipated and microglial expression of CX3CR1 is uniformly higher than in any other group (Fig.5D). In the aged mice, brain inflammation and sickness behavior are lower than they were at 4 h but both are still present 24 h after LPS (Fig.3A&C, Fig.6A; (Godbout et al., 2005b) and this is paralleled by the presence of a significant CX3CR1low population of microglia (Figs.4C & 5D). This is relevant because a persistent population of CX3CR1low microglia in the brain of aged mice after LPS is consistent with an active phenotype. Collectively these data support the hypothesis that impaired fractalkine-mediated regulation of microglia in the brain of aged mice contributes to exaggerated neuroinflammation and the corresponding extension of sickness and depressive-like behaviors (Godbout et al., 2005; Godbout et al., 2008).
It is important to highlight that along with transcriptional regulation, translation and translocation into the membrane are also important levels of regulation for chemokine receptors (Kershaw et al., 2009). This may explain the apparent inconsistency between CX3CR1 mRNA and protein data. For example, while there was a marked increase of CX3CR1 surface expression on adult microglia 24 h after LPS (Fig.5), this protein increase was detected in the absence of a corresponding increase in mRNA expression (Fig.3E). Other chemokine receptors, including CXCR4, are sequestered in intracellular vesicles and can be translocated to the membrane independent of transcription (Forster et al., 1998). It is plausible that a similar mechanism can increase CX3CR1 surface expression on microglia.
The cause of the protracted reduction of CX3CR1 expression on microglia of aged mice 24 h after LPS is unknown, but it may be related to an imbalance of pro- and anti-inflammatory cytokines. For example, higher IL-1β mRNA was detected in microglia (Fig.3C) as well as in homogenate derived from a coronal brain section (Fig.6A). Because several anti-inflammatory cytokines, including IL-10, IL-4, and TGFβ, attenuate microglial activation (Wynne et al., 2009) mRNA levels of these genes were determined in homogenate derived from a coronal brain section at 24 h after LPS (Fig.6). Of the three, only TGFβ mRNA levels were significantly affected by both LPS and age. For example, TGFβ mRNA levels were increased in brain homogenate from adult mice 24 h after LPS, but were not increased by LPS in aged mice (Fig.6D). Moreover, the enhancement of TGFβ mRNA levels in the adult brain at 24 h after LPS corresponded with an overall increase in MFI of CX3CR1 surface expression that was not present in the microglia of aged mice (Figs.5D). In a previous study using microglia cultures, TGFβ enhanced mRNA levels of CX3CR1 (Chen et al., 2002). Consistent with this previous finding, pretreatment with TGFβ directly increased CX3CR1 mRNA and protein expression on BV2 microglia (Fig.7A&E). Furthermore, these data show that pretreatment with TGFβ enhanced CX3CR1 expression and attenuated the LPS-induced increase in IL-1β (Fig.7C&D). Last, a recent study showed that lower TGFβ expression in the brain of aged mice was associated with exaggerated IL-1β and prostaglandin E2 levels in a model of chronic inflammatory arthritis (Wu et al., 2008). Taken together, restoration or enhancement of CX3CR1 on the surface of microglia after activation is compromised in the brain of aged mice and it is plausible that this is dependent on the levels of TGFβ.
In conclusion, the present study demonstrates that there was a reduction of CX3CL1 protein in the aged brain and that LPS induced a protracted reduction of CX3CR1 surface expression on microglia in the aged brain. We interpret these data to indicate that there are two potential age-associated problems in CX3CL1-CX3CR1 mediated regulation of microglia. First, decreased levels of CX3CL1 are likely associated with the exaggerated pro-inflammatory response of microglia after LPS injection. Second, a failure to restore CX3CR1 receptor expression after it has been downregulated leads to an incomplete resolution of the microglia-driven inflammatory signal.
Acknowledgments
This work is supported by NIH grants: R21-MH077817 and R01 AG-033028-01 to J.P.G. C.J.H. was supported by an NIH T32-AI-05-5411 Training Grant. The authors thank Dr. John Sheridan (OSU, Dept of Oral Biology) for the use of a Becton-Dickinson FACS Caliber four color Cytometer, Dr. Ron Glaser (OSU, Dept of MVIMG) for the use of an Applied Biosystems PRISM 7300 sequence detection system, and Dr. Virginia Sanders (OSU, Dept of MVIMG) for the use of FlowJo software. The authors also thank Dr. Andrew Fischer (OSU, Dept of Neuroscience) for technical assistant with the Leica DM5000B microscope and data analyses of immunofluorescent staining of BV2 cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29(4):614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX(3)CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain, behavior, and immunity. 2009;23(1):46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27(5):723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Berg BM, Godbout JP, Kelley KW, Johnson RW. Alpha-tocopherol attenuates lipopolysaccharide-induced sickness behavior in mice. Brain, behavior, and immunity. 2004;18(2):149–157. doi: 10.1016/S0889-1591(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Boddeke EW, Meigel I, Frentzel S, Biber K, Renn LQ, Gebicke-Harter P. Functional expression of the fractalkine (CX3C) receptor and its regulation by lipopolysaccharide in rat microglia. Eur J Pharmacol. 1999;374(2):309–313. doi: 10.1016/s0014-2999(99)00307-6. [DOI] [PubMed] [Google Scholar]
- Boehme SA, Lio FM, Maciejewski-Lenoir D, Bacon KB, Conlon PJ. The chemokine fractalkine inhibits Fas-mediated cell death of brain microglia. J Immunol. 2000;165(1):397–403. doi: 10.4049/jimmunol.165.1.397. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9(7):917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain, behavior, and immunity. 2008;22(3):301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Luo D, Streit WJ, Harrison JK. TGF-beta1 upregulates CX3CR1 expression and inhibits fractalkine-stimulated signaling in rat microglia. J Neuroimmunol. 2002;133(1–2):46–55. doi: 10.1016/s0165-5728(02)00354-5. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab. 2008;28(10):1707–1721. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- Forster R, Kremmer E, Schubel A, Breitfeld D, Kleinschmidt A, Nerl C, Bernhardt G, Lipp M. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J Immunol. 1998;160(3):1522–1531. [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006a;27(5):717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. J Neurosci Methods. 2006b;151(2):121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Berg BM, Krzyszton C, Johnson RW. Alpha-tocopherol attenuates NFkappaB activation and pro-inflammatory cytokine production in brain and improves recovery from lipopolysaccharide-induced sickness behavior. J Neuroimmunol. 2005a;169(1–2):97–105. doi: 10.1016/j.jneuroim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005b;19(10):1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Neurol Clin. 2006;24(3):521–538. doi: 10.1016/j.ncl.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, JOC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33(10):2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95(18):10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. Journal of neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain, behavior, and immunity. 2009;23(3):309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Shi FD, Jung S, Pien GC, Wang J, Salazar-Mather TP, He TT, Weaver JT, Ljunggren HG, Biron CA, Littman DR, Ransohoff RM. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. Faseb J. 2006;20(7):896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29(11):1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PM, Botham MS, Frentzel S, Mir A, Perry VH. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, during acute and chronic inflammation in the rodent CNS. Glia. 2002;37(4):314–327. [PubMed] [Google Scholar]
- Kershaw T, Wavre-Shapton ST, Signoret N, Marsh M. Analysis of chemokine receptor endocytosis and intracellular trafficking. Methods Enzymol. 2009;460:357–377. doi: 10.1016/S0076-6879(09)05218-5. [DOI] [PubMed] [Google Scholar]
- Limatola C, Lauro C, Catalano M, Ciotti MT, Bertollini C, Di Angelantonio S, Ragozzino D, Eusebi F. Chemokine CX3CL1 protects rat hippocampal neurons against glutamate-mediated excitotoxicity. J Neuroimmunol. 2005;166(1–2):19–28. doi: 10.1016/j.jneuroim.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Lyons A, Lynch AM, Downer EJ, Hanley R, O'Sullivan JB, Smith A, Lynch MA. Fractalkine-induced activation of the phosphatidylinositol-3 kinase pathway attentuates microglial activation in vivo and in vitro. J Neurochem. 2009;110(5):1547–1556. doi: 10.1111/j.1471-4159.2009.06253.x. [DOI] [PubMed] [Google Scholar]
- Maciejewski-Lenoir D, Chen S, Feng L, Maki R, Bacon KB. Characterization of fractalkine in rat brain cells: migratory and activation signals for CX3CR-1-expressing microglia. J Immunol. 1999;163(3):1628–1635. [PubMed] [Google Scholar]
- Mizuno T, Kawanokuchi J, Numata K, Suzumura A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979(1–2):65–70. doi: 10.1016/s0006-8993(03)02867-1. [DOI] [PubMed] [Google Scholar]
- Morley AA, Trainor KJ. Lack of an effect of vitamin E on lifespan of mice. Biogerontology. 2001;2(2):109–112. doi: 10.1023/a:1011589218219. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3(3):216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, Kume T, Akaike A, Satoh M. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429(2):167–172. doi: 10.1016/s0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos JC, Gearing D. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387(6633):611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. 2 2004. [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4(2):103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Rancan M, Bye N, Otto VI, Trentz O, Kossmann T, Frentzel S, Morganti-Kossmann MC. The chemokine fractalkine in patients with severe traumatic brain injury and a mouse model of closed head injury. J Cereb Blood Flow Metab. 2004;24(10):1110–1118. doi: 10.1097/01.WCB.0000133470.91843.72. [DOI] [PubMed] [Google Scholar]
- Shan S, Hong-Min T, Yi F, Jun-Peng G, Yue F, Yan-Hong T, Yun-Ke Y, Wen-Wei L, Xiang-Yu W, Jun M, Guo-Hua W, Ya-Ling H, Hua-Wei L, Ding-Fang C. NEW evidences for fractalkine/CX3CL1 involved in substantia nigral microglial activation and behavioral changes in a rat model of Parkinson's disease. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55(4):412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Sunnemark D, Eltayeb S, Nilsson M, Wallstrom E, Lassmann H, Olsson T, Berg AL, Ericsson-Dahlstrand A. CX3CL1 (fractalkine) and CX3CR1 expression in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis: kinetics and cellular origin. Journal of neuroinflammation. 2005;2:17. doi: 10.1186/1742-2094-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarozzo G, Bortolazzi S, Crochemore C, Chen SC, Lira AS, Abrams JS, Beltramo M. Fractalkine protein localization and gene expression in mouse brain. J Neurosci Res. 2003;73(1):81–88. doi: 10.1002/jnr.10645. [DOI] [PubMed] [Google Scholar]
- Toescu EC. Normal brain ageing: models and mechanisms. Philos Trans R Soc Lond B Biol Sci. 2005;360(1464):2347–2354. doi: 10.1098/rstb.2005.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Foy MR, Akopian GG, Lee KH, Zach J, Nguyen KP, Comalli DM, Kennard JA, Agelan A, Thompson RF. Differential effects and rates of normal aging in cerebellum and hippocampus. Proc Natl Acad Sci U S A. 2010;107(4):1624–1629. doi: 10.1073/pnas.0914207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Tokuda Y, Zhang XW, Nakanishi H. Age-dependent responses of glial cells and leptomeninges during systemic inflammation. Neurobiol Dis. 2008;32(3):543–551. doi: 10.1016/j.nbd.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Godbout JP. Immune and behavioral consequences of microglial reactivity in the aged brain. Integrative and Comparative Biology. 2009;49(3):254–266. doi: 10.1093/icb/icp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zujovic V, Benavides J, Vige X, Carter C, Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29(4):305–315. [PubMed] [Google Scholar]
- Zujovic V, Schussler N, Jourdain D, Duverger D, Taupin V. In vivo neutralization of endogenous brain fractalkine increases hippocampal TNFalpha and 8-isoprostane production induced by intracerebroventricular injection of LPS. J Neuroimmunol. 2001;115(1–2):135–143. doi: 10.1016/s0165-5728(01)00259-4. [DOI] [PubMed] [Google Scholar]