Abstract
High-throughput drug screening methods against the intracellular stage of Leishmania have been facilitated by the development of in vitro models of infection. The use of cell lines rather than primary cells facilitates these methods. Peripheral blood mononuclear cell (PBMC) derived macrophages and THP-1 cells were infected with stationary phase egfp transfected Leishmania amazonensis parasites and then treated with anti-leishmanial compounds. Drug activity was measured using a flow cytometric approach, and toxicity was assessed using either the MTT assay or trypan blue dye exclusion. Calculated EC50’s for amphotericin B, sodium stibogluconate, and miltefosine were 0.1445±0.0005 μg/ml, 0.1203± 0.018 mg/ml, and 26.71 μM using THP-1 cells, and 0.179±0.035 μg/ml 0.1948±0.0364 mg/ml, and 13.77±10.74 μM using PBMC derived macrophages, respectively. We conclude that a flow cytometric approach using egfp transfected Leishmania spp. can be used to evaluate anti-leishmanial compounds against the amastigote stage of the parasite in THP-1 cells with excellent concordance to human PBMC derived macrophages.
Keywords: Leishmania amazonensis, parasite, amastigote, drug screening, THP-1, flow cytometry
Introduction
Although there are as many as 12 million cases of leishmaniasis worldwide (WHO, 1984), the therapeutic armamentarium for the disease is limited, and the drugs in use are all associated with undesirable side effects. High throughput drug screening facilitates the rapid testing of chemical libraries for compounds with anti-leishmanial activity. However, the complex life-cycle of the parasite involves an extracellular and an intracellular stage with differing metabolic profiles. It is the intracellular stage, or amastigote form, that produces human disease. Screening of drugs against this stage has been facilitated by the availability of in vitro models of infection using macrophages from human peripheral blood, mouse peritoneal cavity, or cell lines.
The sources of macrophages from animals and from immortalized cell lines for the in vitro models have their respective merits, and the variability among them may contribute to their differences in susceptibility to Leishmania infection. Monocytic cell lines readily provide a homogeneous population of macrophages, in comparison to those collected from animals or humans, e. g. primary human peripheral blood monocyte (PBMC) derived macrophages(Auwerx, 1991; Prieto et al., 1994; Matthews et al., 2001). The latter are less homogeneous and also known to vary with the sources and from one batch to another. Monocytic cell lines of human origin include U937 and THP-1, which resemble tissue macrophages to various extents(Auwerx, 1991; Prieto et al., 1994; Matthews et al., 2001). The THP-1 cell line, derived from the blood of a human with acute monocytic leukemia, has been shown to support the infection by promastigotes of several Leishmania species (Ogunkolade et al., 1990). L. donovoni and L. infantum were found to be as susceptible to anti-leishmanials in these cells as in mouse peritoneal macrophages (Gebre-Hiwot et al., 1992), except when different lipid formulations of amphotericin B were used. (Yardley and Croft, 2000).
We report here that the THP-1 cell line is a suitable surrogate for human PBMC derived macrophages for the in vitro screening of antileishmanial compounds against L. amazonensis using flow cytometry.
Methods
Culture of parasites
Leishmania mexicana amazonensis transfected episomally with a modified p6.5 plasmid encoding egfp and a tunicamycin resistance gene was used (Kawazu et al., 1997). The egfp transfected strain was maintained in culture at 24°C in M199 medium plus 10% fetal bovine serum and 10 μg/ml tunicamycin. Stationary phase promastigotes were harvested and washed in phosphate buffered saline prior to use.
Culture of macrophages and cell lines
Peripheral blood mononuclear cells were collected using standard Ficoll gradients from healthy volunteers obtained after informed consent (UCSD IRB# 051038). THP-1 cells were maintained in culture at 2×105–1×106 cells/mL in RPMI 1640 with 10% heat-inactivated fetal bovine serum at 37°C in humidified atmosphere with 5% CO2. Cells were stimulated with autologous platelet poor serum or 20 nM phorbol myristic acid (PMA) for PBMC and THP-1 cells respectively, prior to infection with promastigotes.
Quantification of intracellular infection
Parasites were added to wells containing macrophages at a ratio of 10:1 in fresh media. Cells were placed in a 34°C incubator. After 1 day, cells were washed to remove extracellular parasites and then anti-leishmanial compounds were added. After further incubation for 1–4 additional days (optimized according to cell type and anti-leishmanial compound used), cells were harvested from wells using Cell Stripper (Mediatech Inc., Manassas, VA), washed and then fixed in 1% EM grade formaldehyde. Fixed cells were analyzed on a Becton Dickinson FACSCanto® cytometer (BD, Franklin Lakes, NJ) with an excitation wavelength of 488 nm and an emission filter of 530/30 nm. Five thousand cells were analyzed for each measurement using the FACSDiva® software (BD).
Drug Screening
The activity of amphotericin B, miltefosine and pentavalent antimony was screened using infected THP-1 cells and primary monocyte derived macrophages (MDM) and the EC50, CC50, and selective index were determined for each of the therapeutic condition applied to both cell types. Six to eight serial dilutions of each of the anti-leishmanial agents in media were added to the wells containing infected cells. Starting concentrations were: 1) amphotericin B - 0.8 μg/ml followed by serial 2 fold dilutions, 2) sodium stibogluconate – 33.33 mg/ml followed by serial 3 fold dilutions, and 3) miltefosine – 100 μM followed by serial 3 fold dilutions. The untreated cells received an equivalent amount of media in place of diluted drug. The EC50 was defined as the concentration of drug which reduced the infection rate to 50% of that seen in the untreated cells based on GFP fluorescence. All assays were performed in three separate experiments for both cell types, and the results from the two of three experiments with the best infection rates in each cell line were chosen for analysis. Samples were normalized to untreated controls, and then a non-linear regression curve fit with a sigmoidal dose-response curve with variable slope model was employed in Graphpad PRISM® to calculate the EC50 and CC50.
Cytotoxicity Assay
The CC50 was defined as the concentration of drug which reduced the viability of the macrophages such that only 50% of the cells survived when compared to the untreated group. The MTT assay, which measures the reduction of the yellow MTT dye to purple formazan by living cells, was used to assess cytotoxicity of amphotericin B and miltefosine to macrophages. However, since sodium stibogluconate itself was found to affect the MTT assay, the standard trypan blue dye exclusion test was used for that drug. In this method, viable (clear) and non-viable (blue) cells were counted using a hemocytometer to determine cell viability in percent. All cytotoxicity assays were performed in duplicate. CC50 was calculated using Graphpad PRISM® as described above.
Selective Index Calculation
The selective index(SI) was calculated by determining the ratio of the EC50 to the CC50.
Results
Flow cytometric evaluation of infected cells for GFP fluorescence revealed infection rates equivalent to 44.1 ± 16.3 % and 63.8 ± 12.9% for untreated PBMC derived macrophages and untreated THP-1 cells, respectively. The mean EC50, CC50, and SI calculated for each anti-leishmanial compound in each cell line is shown in Table 1. The effect of anti-leishmanial compounds on this infection is demonstrated by Figure 1, which is a representative set of histograms demonstrating that the median fluorescence intensity, as a measure of infection, decreased with increasing concentrations of amphotericin B. Amphotericin B was found to be slightly more effective than sodium stibogluconate in the human macrophages (EC50 of 0.179±0.035 μg/ml and 0.1948±0.0364 mg/ml, respectively), and slightly less effective in THP-1 cells (EC50 of 0.1445±0.0005 μg/ml and 0.1203± 0.018 mg/ml respectively) while miltefosine was much less active in both cell types. Amphotericin B had a much larger selective index(SI) than did either sodium stibogluconate, or miltefosine in both human macrophages (SI of 355.9, 32.34, and 6.13, respectively) and THP-1 cells (SI of 105.2, 38.24, and 1.51 respectively). Measurement of infection of the EC50 of amphotericin B was also performed using standard Giemsa staining of cells with same conditions used for the flow cytometry experiments. An infection rate of 46% was seen, and a EC50 of 0.074 μg/ml was calculated, similar to what was seen using our flow cytometric system.
Table 1.
EC50, CC50, and Selective Index (SI) of sodium stibogluconate, amphotericin B and miltefosine in THP-1 cells and human PBMC derived macrophages
| THP-1 cell | Human Macrophage | |||||
|---|---|---|---|---|---|---|
| EC50 | CC50 | SI | EC50 | CC50 | SI | |
| Sodium stibogluconate (mg/ml) | 0.1203± 0.018 | 4.6±1.1 | 38.24 | 0.1948±0.0364 | 6.3±2.8 | 32.34 |
| Amphotericin B (μg/ml) | 0.1445±0.0005 | 15.2±0.7 | 105.2 | 0.179±0.035 | 63.7±18.2 | 355.9 |
| Miltefosine (μM) | 26.71 | 40.5±12.0 | 1.51 | 13.77±10.74 | 84.5±6.9 | 6.13 |
Figure 1.
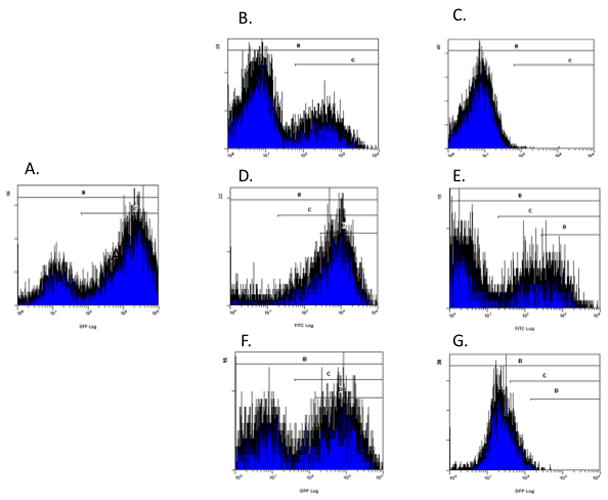
Histograms of THP-1 cells cultured with L. amazonensis for A) untreated cells, and B) treated with 0.1 μg/ml of amphotericin B, C) treated with 0.8 μg/ml of amphotericin B, D) treated with 0.39 μM miltefosine, E) treated with 100 μM miltefosine, F) treated with 0.4 μg/ml of sodium stibogluconate, and G) treated with 50 μg/ml of sodium stibogluconate. These histograms contain two peaks corresponding to uninfected cells on the left and infected cells on the right. Increasing concentrations of amphotericin B, miltefosine, and sodium stibogluconate demonstrate shifting of the population so that the right peak diminishes in size and the left peak increases in size. Of note, for miltefosine, cells were harvested at 4 days after drug exposure as opposed to 1 or 2 days (depending on cell type) with amphotericin B and sodium stibogluconate. Thus, the histogram with the low level miltefosine exposure demonstrating increased shift to the right had undergone an additional two days in culture before harvest.
Discussion
The drug susceptibilities determined in this in vitro system were comparable between the THP-1 cell line and isolated human macrophages, and correlated very well with those reported in the literature (Yardley and Croft, 2000; Ayres et al., 2008; Varela et al., 2009). The flow cytometric method used reported previously for screening anti-leishmanial compounds against the clinically relevant amastigote stage of GFP-transfected Leishmania (Viannia) panamensis (Varela et al., 2009) in the U937 cell line. In that report CD33 was used as a marker for macrophages and level of GFP fluorescence used as a measure of parasitic infection, and the authors found that two anti-leishmanial compounds, meglumine antimoniate and amphotericin B gave EC50’s of 20 and 0.078 μg/ml respectively, which were comparable to those previously determined by conventional methods with animal-derived macrophages.(Yardley and Croft, 2000).
Our data (Table 1) are consistent with, albeit not identical to, those previously reported for L. amazonensis amastigotes in murine peritoneal macrophages, i.e. CC50’s of 30.4, 0.5, and 16.0 μg/ml for glucantime, amphotericin B and miltefosine, respectively(Ayres et al., 2008). Differences in experimental conditions make absolute comparisons difficult.
We also calculated a therapeutic index (SI) for each of the three drugs. The SI values calculated after day 1 of therapy for amphotericin B and sodium stibogluconate were fairly high in human macrophages(356 and 32 respectively). Interestingly, other groups have found that the peak concentration of sodium stibogluconate during standard clinical therapy (10 mg/kg of Sb) resulted in peak serum concentrations of 0.008–0.01 mg/ml which is well below the EC50 we calculated in this study (Chulay et al., 1988; al et al., 1995). On the other hand, work by Atkinson and Bennett showed that standard doses of amphotericin B(0.3 mg/kg on days 1 and 2 followed by increasing doses to 0.5 mg/kg) during clinical therapy are expected to maintain a serum level of this drug above 0.2 μg/ml, which was just above our calculated EC50(Atkinson, Jr. and Bennett, 1978). We did notice that during our experiment a longer miltefosine exposure was required in human macrophages to reduce the infection. Therefore for miltefosine, we treated infected human macrophages with drug for 4 days in attempt to improve the therapeutic effect, but significant toxicity against the macrophages was seen. The calculated SI for miltefosine was very low (1.51 for THP-1 cells, and 6.13 for PBMC derived macrophages), suggesting a narrow therapeutic window. Miltefosine’s mode of action against Leishmania was postulated to be via interference with its cellular carrier systems and/or ether-lipid biosynthesis leading to induction of apoptotic DNA fragmentation in the parasites. Miltefosine is known to be teratogenic to mammalian cells, and it is likely that there is some induction of apoptosis in the macrophages as well (Verma and Dey, 2004). Nonetheless several large scale clinical trials of miltefosine performed in India have shown excellent anti-leishmanial efficacy, and tolerable side effects. A recent pharmokinetic study performed on Dutch soldiers returning from Afghanistan with cutaneous leishmaniasis and treated with a 28 day course of miltefosine (150 mg daily), found a median concentration during the last week of treatment of 3.08 mg/ml (Dorlo et al., 2008) which is close to the CC50 of 75 uM calculated in our system. It is clear from the human pharmokinetic studies that in vitro models can be used to find lead compounds, but are not necessarily predictive of the effective serum drug levels for clinical application.
We conclude that Leishmania species, such as L. amazonensis, episomally transfected to produce GFP with the p6.5 expression vector, can be used to evaluate anti-leishmanial compounds against the amastigote stage of the parasite in THP-1 cells with excellent concordance to human PBMC derived macrophages. Although this in vitro system is not fully predictive of plasma levels required for clinical responses, the method should facilitate the screening of anti-leishmanial drugs for promising lead compounds against clinically relevant amastigotes.
Acknowledgments
Support for this work was provided by the UCSD Center for AIDS Research and by NIAID Institutional Postdoctoral Training Grant #5T32AI007036. KPC is supported by NIH AI-20486 and AI-68835.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.The leishmaniases. Report of a WHO Expert Committee. World Health Organization Technical Report Series 701. :1–140. [PubMed] [Google Scholar]
- 2.al JM, el-Yazigi A, Kojan M, Croft SL. Skin uptake, distribution, and elimination of antimony following administration of sodium stibogluconate to patients with cutaneous leishmaniasis. Antimicrobial Agents and Chemotherapy. 1995;39:516–519. doi: 10.1128/aac.39.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson AJ, Jr, Bennett JE. Amphotericin B pharmacokinetics in humans. Antimicrobial Agents and Chemotherapy. 1978;13:271–276. doi: 10.1128/aac.13.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auwerx J. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia. 1991;47:22–31. doi: 10.1007/BF02041244. [DOI] [PubMed] [Google Scholar]
- 5.Ayres DC, Pinto LA, Giorgio S. Efficacy of pentavalent antimony, amphotericin B, and miltefosine in Leishmania amazonensis-infected macrophages under normoxic and hypoxic conditions. Journal of Parasitology. 2008;94:1415–1417. doi: 10.1645/GE-1613.1. [DOI] [PubMed] [Google Scholar]
- 6.Chulay JD, Fleckenstein L, Smith DH. Pharmacokinetics of antimony during treatment of visceral leishmaniasis with sodium stibogluconate or meglumine antimoniate. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1988;82:69–72. [PubMed] [Google Scholar]
- 7.Dorlo TP, van Thiel PP, Huitema AD, Keizer RJ, de Vries HJ, Beijnen JH, de Vries PJ. Pharmacokinetics of miltefosine in Old World cutaneous leishmaniasis patients. Antimicrobial Agents and Chemotherapy. 2008;52:2855–2860. doi: 10.1128/AAC.00014-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gebre-Hiwot A, Tadesse G, Croft SL, Frommel D. An in vitro model for screening antileishmanial drugs: the human leukaemia monocyte cell line, THP-1. Acta Tropica. 1992;51:237–245. doi: 10.1016/0001-706x(92)90042-v. [DOI] [PubMed] [Google Scholar]
- 9.Kawazu S, Lu HG, Chang KP. Stage-independent splicing of transcripts two heterogeneous neighboring genes in Leishmania amazonensis. Gene. 1997;196:49–59. doi: 10.1016/s0378-1119(97)00190-x. [DOI] [PubMed] [Google Scholar]
- 10.Matthews JB, Green TR, Stone MH, Wroblewski BM, Fisher J, Ingham E. Comparison of the response of three human monocytic cell lines to challenge with polyethylene particles of known size and dose. Journal of Materials Science: Materials in Medicine. 2001;12:249–258. doi: 10.1023/a:1008967200706. [DOI] [PubMed] [Google Scholar]
- 11.Ogunkolade BW, Colomb-Valet I, Monjour L, Rhodes-Feuillette A, Abita JP, Frommel D. Interactions between the human monocytic leukaemia THP-1 cell line and Old and New World species of Leishmania. Acta Tropica. 1990;47:171–176. doi: 10.1016/0001-706x(90)90023-s. [DOI] [PubMed] [Google Scholar]
- 12.Prieto J, Eklund A, Patarroyo M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunology. 1994;156:191–211. doi: 10.1006/cimm.1994.1164. [DOI] [PubMed] [Google Scholar]
- 13.Varela MR, Munoz DL, Robledo SM, Kolli BK, Dutta S, Chang KP, Muskus C. Leishmania (Viannia) panamensis: an in vitro assay using the expression of GFP for screening of antileishmanial drug. Experimental Parasitology. 2009;122:134–139. doi: 10.1016/j.exppara.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma NK, Dey CS. Possible mechanism of miltefosine-mediated death of Leishmania donovani. Antimicrobial Agents and Chemotherapy. 2004;48:3010–3015. doi: 10.1128/AAC.48.8.3010-3015.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yardley V, Croft SL. A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasis. International Journal of Antimicrobial Agents. 2000;13:243–248. doi: 10.1016/s0924-8579(99)00133-8. [DOI] [PubMed] [Google Scholar]


