Abstract
Polychlorinated biphenyls (PCBs) are persistent lipophilic environmental contaminants which are found in fatty tissues of humans and wild-life alike. Maternal transfer of PCBs to offspring is easily achieved across the placenta and via lactation. In male rats, perinatal PCB exposure induces behavioral abnormalities, in addition to hypothyroxinemia and white matter changes. There are sex differences in white matter volume synthesis and density in adult and aged rodents. Yet whether PCB exposure effects on white matter are sex-specific is unclear, because the previous studies were conducted in male offspring. Furthermore, although hypothyroxinemia induced by PCB exposure is thought to trigger white matter changes, PCBs also affect interleukin-6 (IL-6) expression, and IL-6 regulates white matter growth. We hypothesized that perinatal PCB exposure would have sex-specific effects on white matter development associated with altered IL-6 levels. We found that female offspring had higher levels of myelin basic protein (MBP) than males did, at postnatal day (PND) 7, 18 and 21. PCB exposure induced hypothyroxinemia in males and females at PND7, 14, 21, and 42. PCB exposure also increased MBP and reduced glial fibrillary acidic protein (GFAP) levels in males at PND21, but had the opposite effect in females. In addition, at PND 14 and 21, PCB exposure elevated IL-6 levels in male offspring only. The induction of sex-specific changes in white matter proteins, in the absence of sex differences in thyroxine levels after PCB exposure, suggests that serum thyroxine levels do not directly contribute to the white matter alterations. Instead, IL-6 may contribute to increased MBP levels in males, whereas in females estromimetic and thyromimetic PCB metabolites may affect white matter development. This data adds to an increasing body of literature showing that perinatal insults induce sex-specific effects in offspring.
Keywords: Polychlorinated biphenyl, Cerebellum, Oligodendrocyte, Astrocyte, Development, White Matter, Sex
Introduction
Polychlorinated biphenyls (PCBs) are lipophilic environmental contaminants, which were banned in the 1970s, but persist in fatty tissues of wild-life and humans alike (Johnson-Restrepo et al., 2005). Maternal transfer of PCBs is readily achieved via the placenta and also during lactation (Jacobson et al., 1984). In male rats, perinatal PCB exposure induces behavioral hyperactivity, hypothyroxinemia and white matter changes (Holene et al., 1998;Sharlin et al., 2006). Abnormalities in cerebellar white matter tracts are associated with behavioral disorders, and there are sex differences in the activity and density of cerebellar white matter tissues (Berquin et al., 1998;Li et al., 2006;Marin-Husstege et al., 2004;Yang et al., 2008;Volkow et al., 1997;Miller et al., 2010). However, whether PCB exposure induces sex-specific effects on white matter tracts is unclear, because the previous studies were conducted using tissues derived from male offspring (Sharlin et al., 2006). In addition, PCB induced white matter alterations may be attributed to hypothyroxinemia (Sharlin et al., 2008). However, PCBs also affect interleukin-6 synthesis, and IL-6 is a cytokine which not only regulates white matter myelin expression, but is also associated with behavioral disorders (Fujimaki et al., 1997;Valerio et al., 2002;Zhang et al., 2006;Lasky-Su et al., 2008;Neale et al., 2008).
We hypothesized there would be sex-specific effects of PCB exposure on hypothyroxinemia, white matter development and IL-6 levels. To address our hypothesis we exposed developing rats to the Fox River mix of PCBs, which is an environmentally relevant mixture of PCBs similar to those found in fish from the Fox River in Michigan (Wickizer et al., 1981;Kostyniak et al., 2005). We used doses of PCBs similar to those used to induce hypothyroxinemia by other groups (Sharlin et al., 2006), and quantified sera levels of thyroxine, pituitary levels of thyroid stimulating hormone (TSH), cerebellum MBP, GFAP density, and sera IL-6 levels in offspring at PND7, 14, 21 and 42. We chose the aforementioned ages in particular, to determine the effects of PCB exposure prior to and after the reported peaks of myelination (PND10-12) in rodents (Sarlieve et al., 2004;Akiyama et al., 2002). In summary, we describe hypothyroxinemia and sex-specific changes in MBP, GFAP and IL-6 after PCB exposure.
Experimental Procedures
Animals and choice of doses
We first determined the ontogeny of MBP and GFAP in the cerebellum using a cohort of unexposed offspring from timed pregnant Long-Evans (LE) rats (Taconic Farms). Six male and female pups were sacrificed at PND7, 10, 14, 18, 21 and 42. The effects of PCB exposure were next determined using a second cohort of timed pregnant LE rats. Dams were exposed to one of three doseages of Fox River PCBs: 3mg/kg, 6mg/kg or 18mg/kg or corn oil, as a control from gestational day (GD) 6 through weaning. The doses we selected were based on those used by Sharlin et al (2006) to induce hypothyroxinemia and white matter alterations in gestationally exposed LE rats, because we wished to determine if PCB effects on white matter and thyroid hormones was sex-specific. We administered the Fox River PCB mixture by placing a measured amount of PCBs dissolved in corn oil onto a cookie, which was fed to each dam. The volume of the PCB/corn oil mixture was adjusted three times per week based on changes in the dam's weight. Six dams were used per treatment, yielding an n=24 dams in total. Litters comprised of 8-14 pups: who were cross fostered on PND2 within treatments to maintain equal numbers of male and female pups (n=5 of each) per dam, and to ensure equal lactational exposure to the PCB or corn oil as appropriate. Control and dosed dams were housed in clear Plexiglas cages with stainless steel wire lids in a temperature (21–23°C) controlled room and maintained in a sterile pathogen free environment, on a 12:12 h light:dark cycle (lights on at 7:00am). Individual male and female rats were sacrificed at PND 7, 14, 21, 42, and 90. All procedures were IACUC approved and all experiments were conducted in a blinded fashion.
Tissue preparation
Rats were sacrificed by CO2 asphyxiation, followed by decapitation. Brains from exposed and control rats were immediately removed and frozen at -80°C for use in HPLC or Western blot assays. The pituitary gland was extracted at the time of sacrifice, then homogenized in a lysis buffer, containing PBS buffer (pH 7.2) and NP-40 (Sigma-Aldrich) and a protease inhibitor cocktail (Sigma-Aldrich) and then stored at -80°C until use in ELISA assays. Serum was extracted from trunk blood collected after decapitation, and stored at -80°C until use in ELISA assays. Frozen brains were cryo-microdissected on a rostral-caudal gradient into 350μm thick sections, and micro-punches were taken from the cerebellum at Bregma -6mm (Paxinos and Watson, 2007). Micro-punches were suspended in 100μl of lysis buffer and sonicated on ice, prior to use in ELISA and western blot assays. The bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL) was used to calculate protein concentration in cerebellar and pituitary tissues. Brains for immunohistochemistry (IHC) were rapidly immersed in 4% paraformaldehyde (PFA) for 24hours at 4°C followed by a second dissection and immersion step as previously described (Miller et al., 2010). Brain tissue blocks were then embedded in paraffin using a Tissue Tek™ embedder and tissue sections (8μm) were cut using a rotary microtome prior to being mounted on charged pre-coated slides.
ELISAs to determine T4, TSH and IL-6 levels
Commercially available thyroxine (T4) (Calbiotech), IL-6 (R&D Biosystems) and TSH (Shibayagi) ELISAs determined serum T4 and IL-6 levels, and pituitary TSH levels. Sample quantities were as follows, for T4 25μl of sera, for IL-6 75μl of sera and for TSH 25μl of pituitary homogenates containing 1μg/μl of protein were run in triplicate per rat. Samples were run according to kit instructions. To determine the quantity of T4, TSH or IL-6 per sample, plates were loaded with a HRP-conjugated T4, TSH or IL-6 standard, at low to high concentrations supplied with the kit for 1hour RT. The ELISA plates were then incubated with a HRP-TMZ reagent for 20 minutes. After the substrate color was developed an acidic stopping solution provided with the kit was added to each well, and the mean absorbance of the solution per well was read using a spectrophotometric plate reader at 450nm within 15 minutes of stopping the reaction. The concentration of T4, TSH or IL-6 per well was generated by comparing values per sample with the appropriate standard curve. In addition, control samples spiked with T4, IL-6 or TSH standards were used as positive controls in the assay. Data were entered into SPSS v 17 for statistical analysis. For ELISA assays an n=3 animals per group were run in triplicate.
Western Blotting
Cerebellar brain homogenates containing 40μg of protein were diluted with a 1:1 volume of sample buffer containing 10% (w/v) sodium dodecyl sulphate, 30% (v/v) glycerol, 2% (v/v) 2-mercaptoethanol, 0.25% (w/v) bromophenol blue, and Tris Buffered Saline with Tween (TBS-T) (pH 7.2). SDS-PAGE and gel transfer onto Immobilon-P transfer membranes were completed as previously described. Blots were blocked in a 5% fish gelatin solution for 24h, prior to incubation with antibodies to GFAP (Mouse, Calchiochem 1:250) or MBP (Mouse, Millipore 1:250) in the blocking buffer at 4°C for 24h. Gels were also probed with an anti-β Actin antibody (Mouse, Sigma 1:10,000) as a loading control. Gels were then incubated with the an anti-mouse secondary antibody, prior to incubation in streptavidin coupled to HRP as previously described. The blot was developed by incubating it with ECL (Pierce Biotechnology) for 5-10secs and then photographed with a LAS-3000plus gel imaging camera (Fuji). Photomicrographs of blots were analyzed using Image J software to calculate the mean optical density (OD) of the bands in arbitrary units per well per gel (Miller et al., 2010). Individual gels were run per antibody, per age, ie PND 7 or 14 and per sex, i.e male or female. For gel analysis an n=3 animals were run per group, and gels were run in duplicates.
IHC and histology
Tissue sections were cleared in xylene and rehydrated through graded alcohols prior to use. For optimum antigen retrieval, sections were pretreated by boiling in a pH 6.0 citrate buffer- using a microwave for 10 min full power. To quench endogenous peroxidase activity, tissue sections were immersed in a 0.3% H2O2 solution for 15 min. To block non-specific antigen binding, tissue sections were pre-incubated for 30 min with the appropriate blocking serum (horse/goat) from the Vector ABC kit (Vector Labs, Burlingame, CA). Antibodies (polyclonal or monoclonal) were suspended in PBS and 0.1% BSA, incubated for 1h RT. Primary antibodies were: mouse anti-GFAP (Calbiochem, San Diego, CA; 1:500) and mouse anti-MBP for myelin (Millipore 1:500). The specificity of each antibody was demonstrated by using serial sections from the same rats incubated with either ascites/serum alone, antibody in the absence of secondary antibody, or a secondary antibody without the primary antibody. DAB was used to visualize immunostained sections. Sections were dehydrated through graded alcohols to xylene, and then cover-slipped with DPX. All sections were stained in batch assays to minimize inter-assay variation. For IHC analysis an n>5 animals were immunostained per group.
Digital image acquisition and analysis for IHC
Digital photo-micrographs of regions of interest (ROI) were obtained with a Nikon (50i) microscope using 10×, 20× and 60× oil immersion objective lenses coupled to a CCD camera with image grabber software. All images were taken at uniform brightness and in gray-scale to enable densitometric analysis with Image J software. A minimum of 4 images were taken per section, with 20 random sections (out of 40 serial sections) stained per region, per rat. Image J software was used to calculate the OD of antibody immunoreactivity (IR) per immunostained region, in the cerebellum as described in previous publications (Miller et al., 2007;Miller et al., 2009). Non-specific staining in the background was excluded by setting the threshold of detection to between 0-146 which selected only the (dark) immunostained nerve fibers of astrocytes or oligodendrocytes. To ensure consistency in the analysis, the same OD threshold was set for each photomicrograph. Thus, the data for GFAP and MBP IHC analysis represents the mean OD of immunostained fibers per region of interest.
Analysis of PCBs in brain tissues
PCB congeners in brain samples were analyzed following the methods described elsewhere (Johnson-Restrepo et al., 2005). Brain tissues were homogenized with anhydrous sodium sulfate, spiked with PCB congeners 30 (2,4,6-triCB) and 204 (2,2′,3,4,4′,5,6,6′-octaCB) as surrogate standards, and Soxhlet extracted with methylene chloride and hexane for 16 h. An aliquot of the sample extract was spiked with 13C-labeled PCB congeners 3, 15, 31, 52, 118, 153, 180, 194, 206, and 209 and another aliquot was used to determine lipid content by gravimetry. The sample extracts were then purified by passage through a series of layers of silica gel then eluted using 150 mL of 20% dichloromethane in hexane. The extracts were concentrated using a rotary evaporator, treated with sulfuric acid (5 mL) then concentrated to 1 ml for the analysis of PCBs. Extracts were injected into a gas chromatograph (Hewlett-Packard 6890) coupled with a mass-selective detector (Hewlett-Packed, series 5973) for the determination of PCBs. A capillary column coated with RTX-5MS (30 m × 0.25 mm i.d. × 0.25 μm film thickness; Restek Corp, Bellefonte, PA) was used for the separation of individual isomers. The MS was operated in an electron impact (70 eV), selected ion monitoring mode. An equivalent mixture of Kanechlor (KC300, 400, 500, and 600) with known PCB composition was used to identify PCB congeners, subsequently quantified based on external calibration standards containing known concentrations of di- through deca-CB congeners. Individually resolved peaks of PCB isomers were summed to obtain total PCB concentrations. For PCB analysis an n=4 animals were run per group.
Statistics
All analyses were performed in a blinded fashion, and data were uncoded only after experimental analyses were completed. Data per animal per assay was entered into SPSS version 17 database. Significance was set at p<0.05. ANOVA was used to test for the main effects of age, sex and PCB exposure on endpoints of interest. Data were corrected for multiple comparisons using the Bonferroni analysis, a powerful statistical tool.
Results
The effects of PCB exposure on T4 levels
PCB exposure lowered serum T4 levels in both male and female rats at PND7, PND14 and PND21 (p<0.05) but only in the females at PND42 (p<0.05) (Figure 1a-d). There was also a main effect of age on T4 levels in both males and females; the older offspring had higher T4 levels than did the younger offspring (p<0.05).
Figure 1.
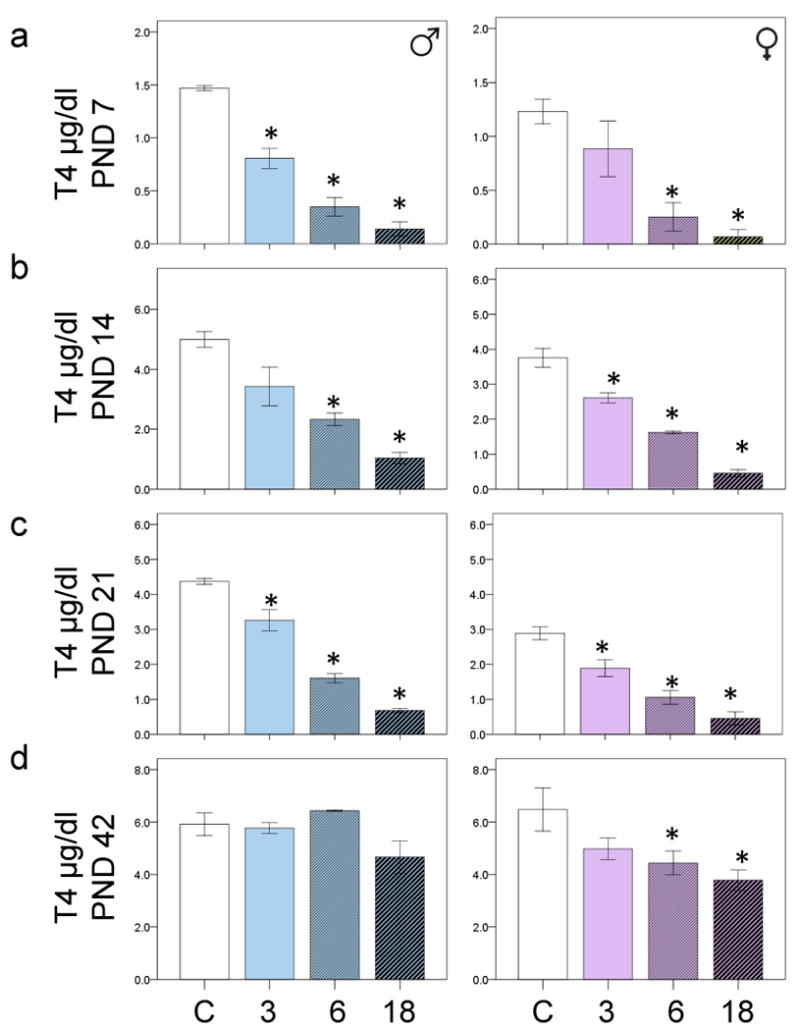
The bar charts show the mean sera T4 levels in control and PCB exposed offspring at a) PND7 b) PND14 c) PND21 and d) PND42. 3= 3mg/kg, 6= 6mg/kg and 18=18mg/kg PCBs. *p<0.05 comparing the PCB exposed group with the appropriate male or female control. Error bars are ±SEM.
ANOVA with Bonferroni correction revealed that all doses PCBs reduced T4 levels in exposed males compared with control males (p<0.05) at PND7. High doses of PCBs reduced T4 levels in exposed females (p<0.05) compared with control females at PND7 (Fig 1a). At PND14 the 6 and 18mg/kg PCB exposed males had lower T4 levels than did control males, and the 3,6 and 18mg/kg PCB exposed females had lower T4 levels than control females (p<0.05) (Fig 1b). At PND21 all doses of PCBs reduced T4 levels in both males and females compared with the appropriate control (p<0.05) (Fig 1c). At PND42, only the 18 mg/kg PCB-exposed females had lower T4 levels than control females (p<0.05) (Fig 1d). There was no main effect of PCB exposure on T4 levels at PND90.
We quantified TSH levels in pituitary tissues from PND7-42 control and 18mg/kg PCB exposed male and female offspring, but found no effect of PCB exposure on TSH levels (data not shown).
The development of MBP fibers in the cerebellum
There was an increase in the density of MBP immunoreactive fibers in the cerebellum between PND7 and PND42 (Figure 2 a). ANOVA revealed a main effect of age and sex on MBP levels (p<0.05). Older rats had higher MBP levels than younger ones; in addition, the females had higher MBP levels than males (Figure 2b/c). Bonferonni analysis revealed that at PND7, PND18 and PND21 females had higher MBP levels than males (p<0.05) (Figure 2c). We confirmed the sex differences in MBP staining derived from IHC analysis, using additional Immunoblot analysis of MBP protein levels in cerebellar brain tissues from PND7 male and female rats. MBP protein levels were higher in females at PND7 compared with males similarly aged (p<0.05) (Figure 2d). We also determined whether there were differences in the isoform composition of MBP between PND14-42, but did not find significant differences in isoform density as a percentage of total myelin with age, rather total myelin content was increased (Supplemental Figure 1).
Figure 2.
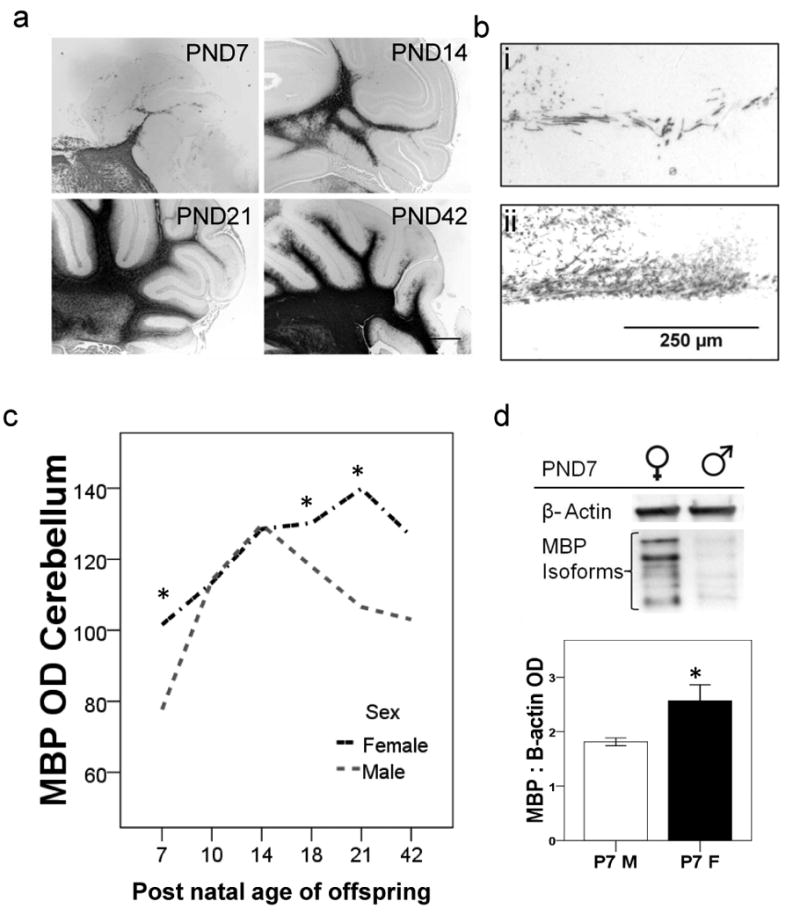
The ontogeny of MBP in cerebellar tissues from unexposed offspring. a) Photomicrographs show an increase in the density of MBP immunoreactive fibers in cerebellar tissues from a female rat, at PND7, 14, 18 and 21. Bar is 1 mm. (bi,bii) There is reduced density of MBP immunoreactive fibers in cerebellar tissue from a male rat as compared with a female rat at PND7. Bar is 100 μm. c) Line graph shows MBP OD values of male and female rats aged PND 7-42. *p<0.05 (n≥5 per group). d) Western blot shows protein MBP levels in cerebellar tissues (40μg) from male and female offspring at PND7. The MBP antibody identified several isoforms which were summed to generate total MBP OD. The bar graph underneath shows mean MBP ODs from male and female offspring at PND7 (n=3 per group). *p<0.05. Error bars are ±SEM.
The effects of PCB exposure on MBP levels
There was no main effect of PCB exposure on MBP levels in male offspring at PND7, but there was a trend towards lower MBP levels in the PCB exposed females (p=0.134) (Figure 3a). At PND14, PCB exposure was associated with a trend towards higher MBP levels (p=0.106) in the male offspring, and a significant reduction in MBP levels in the female offspring (p<0.05) (Figure 3b). At PND14, Bonferonni correction revealed a trend towards elevated MBP levels in the 18mg/kg PCB exposed males (p=0.086) in particular (Figure 3b). At PND21, PCB exposure was associated with an increase in MBP levels in males (p<0.05), but a decrease in MBP levels in females (p<0.05). Bonferonni correction revealed the 18mg/kg PCB exposed males had higher MBP levels than the controls (p<0.05) (Figure 3c). At PND42 PCB exposure was associated with increased MBP levels in the 18mg/kg males (p<0.05), but had no effects on MBP levels in the females (Figure 3d).
Figure 3.
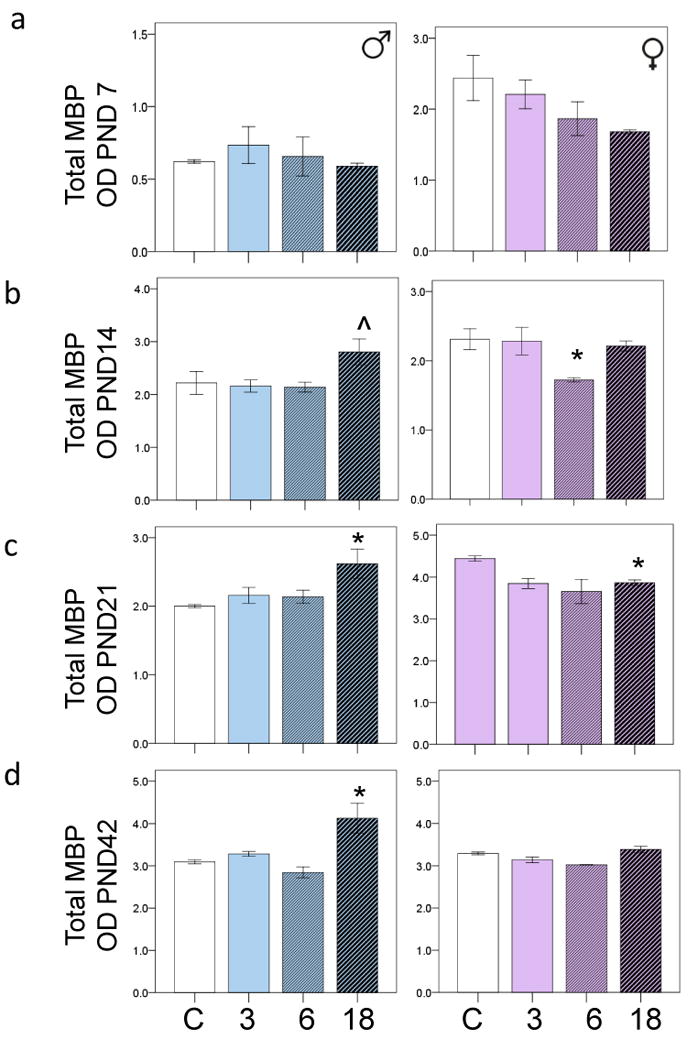
The bar graphs are derived from western blot analysis, and show PCB effects on MBP levels in male and female offspring at a) PND7, b) PND14, c) PND 21 and d) PND 42. *p<0.05. ˆp<0.1. 3PCB= 3mg/kg, 6PCB= 6mg/kg and 18PCB= 18mg/kg. Error bars are ±SEM.
The ontogeny of GFAP in the cerebellum
There was a noticeable decline in GFAP immunoreactivity in cerebellar tissues, between rats at PND7 and PND42 (Figure 4a). There was an association between age and GFAP fiber density in both the male and female offspring (p<0.5). There was no main effect of sex on GFAP levels, either via analysis using IHC derived data, or densometric analysis of western blots run with cerebellar tissues and probed with a GFAP antibody (Figure 4b-d).
Figure 4.
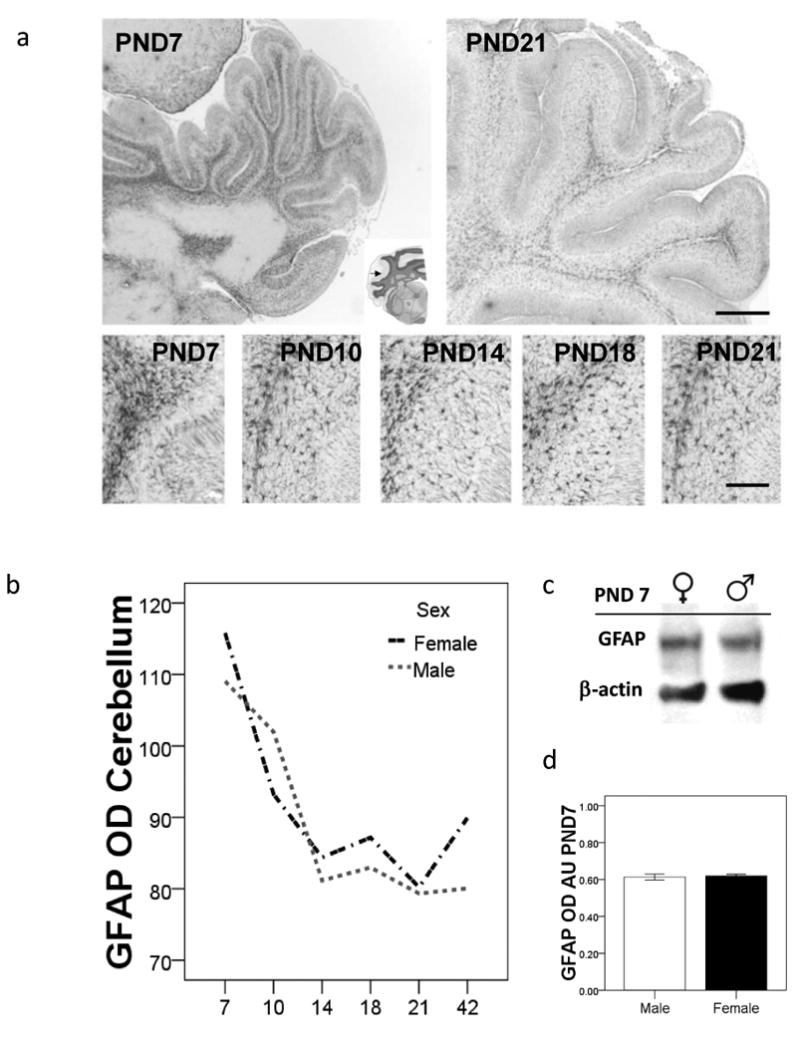
The ontogeny of GFAP in cerebellum tissues from unexposed male and female offspring. a) Photomicrographs of cerebellum tissue sections stained with an anti-GFAP antibody from female rats at PND7 and PND21. Bar is 1mm. The five photomicrographs in the lower panel show a decline in the density of astrocytic fibers with respect to age. Bar is 100μm. b) Line graph shows mean GFAP OD calculated using Image J analysis of tissue sections stained with GFAP, n=6 rats per group. *p<0.05. c) Western blots show protein levels of GFAP at PND7 in cerebellar tissues derived from male and female rats. d) Bar chart shows mean GFAP OD, derived from western blots run using tissues from male and female rats (n=3 per group). Error bars are ±SEM.
The effects of PCB exposure on GFAP levels
There was no effect of PCB exposure on GFAP levels at PND7 or at PND 14. However, at PND21 there was a main effect of PCB exposure on the 55kDa and 70kDa isoforms of the GFAP protein (data not shown). At PND21, PCB exposure was associated with lower GFAP levels in males, but higher GFAP levels in females (p<0.05) (Figure 5a and 5b). At PND42, PCB exposure had no effects on GFAP levels in males, but was associated with lower GFAP levels in females (p<0.05). Bonferroni analysis revealed that at PND42 the 18mg/kg exposed females in particular had lower GFAP levels than the control females (p<0.05) (Figure 5c).
Figure 5.
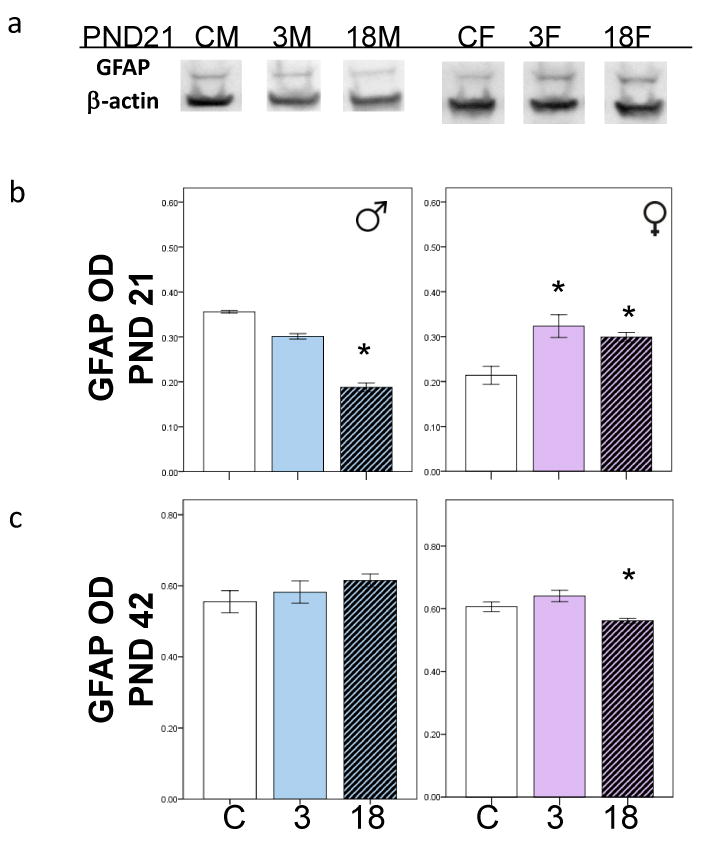
a) Western blots run with tissue samples from control and exposed male and female offspring at PND21, probed with a β-actin (42kDa) and a GFAP (55kDa) antibody. b) Bar charts show mean GFAP OD at PND21 in PCB exposed male and female offspring. c) Bar charts show mean GFAP OD at PND42 in PCB exposed male and female offspring. *p<0.05. Error bars are ±SEM.
The effects of sex and PCB exposure on circulating IL-6 levels
We quantified IL-6 in sera from the PND7, 14 21 and 42 18mg/kg PCB exposed and control male and female offspring. PCB exposure did not affect IL-6 levels in the PND7 or PND42 male or female offspring. At PND14 there was a main effect of sex and PCB exposure (p<0.05) on IL-6 levels, females had higher IL-6 levels than males, and PCB exposure elevated IL-6 levels in the males only (Figure 6a). At PND21 there was a main effect of PCB exposure and sex on IL-6 levels (p<0.05), females had higher IL-6 levels than males, and PCB exposed males had a trend towards higher IL-6 levels than controls (p=0.085) (Figure 6b).
Figure 6.
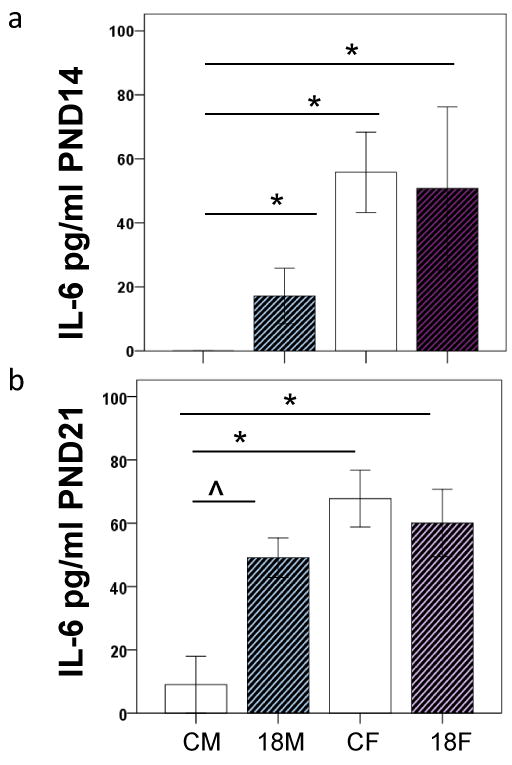
The effects of PCB exposure on serum IL-6 levels in a) PND 14 and b) PND 21 aged male and female rats.CM: control male. 18M: 18mg/kg male. CF: control female. 18F: 18mg/kg female. *p<0.05 ˆp<0.1.
PCB levels in brain tissues
To determine whether the sex-specific effects of PCB exposure were related to differences in the accumulation or penetration of PCB congeners into brain tissues we quantified PCB levels in brain tissues from male and female offspring at PND21 and PND42. We found that PCB levels in both males and females were higher at PND21 than at PND42 likely associated with weaning (p<0.01), but there was no effect of sex on PCB levels (Table 1).
Table 1.
The table shows mean total PCB congener content in brain tissues derived from 18mg/kg PCB exposed male and female offspring at PND21 and PND42. There were n=4 rats per group and data (±SEM) is shown.
| Group | PCBs ng/g wet wt | Lipid % | PCBs ng/g lipid wt | |
|---|---|---|---|---|
| PND 21 18mg/kg | Males | 3152 (±177) | 5.93 (±0.09) | 53272 (±3094) |
| Females | 3256 (±197) | 5.60 (±0.10) | 58233 (±3243) | |
| PND 42 18mg/kg | Males | 522 (±65) | 6.90 (±0.06) | 7565 (±891) |
| Females | 676 (±60) | 6.47 (±0.70) | 10446 (±1024) | |
Discussion
In this study, we found that perinatal exposure to PCBs potently reduced thyroxine levels in both male and female offspring, until PND42. Interestingly, in spite of the fact that thyroxine levels were reduced to the same extent in both males and females, there were sex differences in the effects of PCBs on white matter proteins; MBP and GFAP, particularly at PND21.
In untreated rats there were sex differences in MBP levels, females had higher levels than males at PND7, 18 and 21. After PCB exposure, males had higher MBP levels at PND21 and 42, with reduced GFAP levels found at PND21. Whereas in female offspring, after PCB exposure, reduced MBP levels at PND14 and 21, with elevated GFAP levels found at PND21. This data suggests that either hypothyroxinemia induces sex-specific effects in developing rat offspring, or that serum thyroid hormones are not directly accountable for white matter alterations after PCB exposure.
Reduced thyroxine levels after PCB exposure are commonly attributed to increased expression of hepatic enzymes which promote thyroxine excretion in bile (Martin et al., 2001). Previously other groups have suggested that hypothyroxinemia after PCB exposure reduced MBP, but did not affect GFAP levels in corpus callosum (CC) white matter tissues of male offspring (Sharlin 2006). In the same study, Sharlin et al suggest that hypothyroxinemia and PCB effects are not similar, because exposure to a pharmaceutical agent; 1 methyl-2-mercaptimidazole (MMI) which induces hypothyroxinemia, reduced myelin associated glycoprotein, but also increased GFAP in the CC. Our data appears to conflict with Sharlin et al, because we found similar effects to MMI in female offspring after PCB exposure. This apparent conflict may be due to the use of rodents at different ages; i.e. we determined PCB effects at PND7, 14, 21 and 42, rather than at PND15 as used by Sharlin et al. Furthermore, we determined PCB effects on cerebellar white matter rather than in the CC. However, regardless of the differences between the two studies, the question remains as to whether hypothyroxinemia is responsible for sex-differences in PCB effects on white matter tracts.
We proposed that increased IL-6 synthesis may be associated with increased white matter synthesis after PCB exposure. Our findings show higher IL-6 levels at PND14 and 21, after PCB exposure, only in male offspring. If IL-6 is important for increasing MBP synthesis after PCB exposure, than IL-6 deficient (-/-) male mice should be protected from PCB effects. Previously we found that IL-6-/- aged female mice had lower MBP levels than wild-type females (Miller et al., 2010). Therefore, we expected that lower IL-6 levels would be associated with reduced MBP expression in the PCB exposed females. However, there was no effect of PCB exposure on sera IL-6 levels in the female offspring. Thus, it is likely there are different mechanisms underscoring PCB effects in the male and female offspring.
The sex-specific effects of PCBs on white matter may involve the fact that hydroxylated (OH) PCB metabolites have both thyromimetic and estromimetic effects. Both thyroid hormones and estrogen can affect the proliferation and survival of oligodendrocytes (Gerstner et al., 2009;Franco et al., 2008). There are innate sex differences in oligodendrocyte survival and proliferation in response to gonadal hormones, including estrogen (Marin-Husstege et al., 2004). There are no reports in the literature regarding sex-specific effects of thyroid hormones, or TH receptor (THr) agonists/antagonists on MBP synthesis. However, there are reports that OH-PCBs can interfere with THrβ activity (Marin-Husstege et al., 2004;Amano et al., 2010). Interestingly, THrβ rather than THrα activation is important for inducing MBP transcription (Farsetti et al., 1992). Therefore, we suggest that OH-PCB metabolites may reduce MBP levels via interfering with THrβ regulation of MBP. Yet, whether the thyromimetic or estromimetic OH-PCBs are the determinant factor for reducing MBP synthesis in females, remains unknown.
Bisphenol A is an estromimetic environmental contaminant which can reduce MBP synthesis, via a reduction in THrβ density, suggesting that estromimetic PCBs are equally likely as thyromimetic PCBs to affect MBP levels (Arcaro et al., 1999;Seiwa et al., 2004). To address this novel concept it would be useful to expose oligodencrocyte cells derived from male and female rodents, to OH-PCB metabolites in the presence and absence of THr and estrogen receptor antagonists to determine their efficacy in reducing MBP levels. It is plausible there are different levels of PCB metabolism, with increased OH-PCB synthesis found in female brains. However, we did not find sex differences in PCB parent congener content in brain tissues at PND21 or PND42, suggesting this might not be the case. Thus, we speculate that interactions between estromimetic and thyromimetic PCB metabolites may reduce white matter proteins in female offspring in particular.
It is worth noting that hypothyroxinemia was most prominent during weaning; i.e. while the offspring were still exposed to PCBs via lactation. By PND 90 thyroxine levels returned to those found in control offspring. Although, at PND42 thyroxine levels in PCB exposed females were lower than in controls; suggesting a slower recovery after PCB exposure. In the males at PND42 MBP levels were still higher than in the control males and may be associated with the dysfunctional rotarod behavior (which is regulated by the cerebellum) found after PCB exposure in male but not female offspring (Nguon et al., 2005).
Our data adds to a growing body of studies that describe sex-specific effects of perinatal PCB exposure, and periniatal inflammatory insults on immune responses and behavior in rodent offspring (Nguon et al., 2005;Widholm et al., 2001;Kohman et al., 2008;Onishchenko et al., 2010;Colciago et al., 2009). The interactions between sex-specific factors that regulate brain development, and exposure to toxicants are important because they could bear relevance for explaining part of the sex bias in the risk of developmental disorders. In summary, we describe hypothyroxinemia in male and female offspring after PCB exposure, which is associated with sex-specific alterations in white matter proteins in the cerebellum. We suggest that altered IL-6 levels may be involved with MBP changes in the male offspring, and that estromimetic and thyromimetic PCB metabolites may be directly responsible for altered white matter proteins found in the female offspring.
Supplementary Material
Acknowledgments
We acknowledge assistance from the Biochemistry and Advanced Light Microscopy cores at the Wadsworth Center and thank Tom Zoeller for assistance with the development of Thyroxine assays. This research was supported by NIEHS ES01467502 and ES 015688-01 to RF Seegal.
Footnotes
Conflict of Interest. The authors report no conflict of interest associated with this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Akiyama K, Ichinose S, Omori A, Sakurai Y, Asou H. Study of expression of myelin basic proteins (MBPs) in developing rat brain using a novel antibody reacting with four major isoforms of MBP. J Neurosci Res. 2002;68:19–28. doi: 10.1002/jnr.10188. [DOI] [PubMed] [Google Scholar]
- Amano I, Miyazaki W, Iwasaki T, Shimokawa N, Koibuchi N. The effect of hydroxylated polychlorinated biphenyl (OH-PCB) on thyroid hormone receptor (TR)-mediated transcription through native-thyroid hormone response element (TRE) Ind Health. 2010;48:115–118. doi: 10.2486/indhealth.48.115. [DOI] [PubMed] [Google Scholar]
- Arcaro KF, Yi L, Seegal RF, Vakharia DD, Yang Y, Spink DC, Brosch K, Gierthy JF. 2,2′,6,6′-Tetrachlorobiphenyl is estrogenic in vitro and in vivo. J Cell Biochem. 1999;72:94–102. [PubMed] [Google Scholar]
- Berquin PC, Giedd JN, Jacobsen LK, Hamburger SD, Krain AL, Rapoport JL, Castellanos FX. Cerebellum in attention-deficit hyperactivity disorder: a morphometric MRI study. Neurology. 1998;50:1087–1093. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- Colciago A, Casati L, Mornati O, Vergoni AV, Santagostino A, Celotti F, Negri-Cesi P. Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat: Part 2: Effects on reproductive parameters, on sex behavior, on memory retention and on hypothalamic expression of aromatase and 5alpha-reductases in the offspring. Toxicology and Applied Pharmacology. 2009;239:46–54. doi: 10.1016/j.taap.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Farsetti A, Desvergne B, Hallenbeck P, Robbins J, Nikodem VM. Characterization of myelin basic protein thyroid hormone response element and its function in the context of native and heterologous promoter. J Biol Chem. 1992;267:15784–15788. [PubMed] [Google Scholar]
- Franco PG, Silvestroff L, Soto EF, Pasquini JM. Thyroid hormones promote differentiation of oligodendrocyte progenitor cells and improve remyelination after cuprizone-induced demyelination. Experimental Neurology. 2008;212:458–467. doi: 10.1016/j.expneurol.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Fujimaki H, Shiraishi F, Aoki Y, Saneyoshi K. Modulated cytokine production from cervical lymph node cells treated with BaP and PCB. Chemosphere. 1997;34:1487–93. doi: 10.1016/s0045-6535(97)00445-1. [DOI] [PubMed] [Google Scholar]
- Gerstner B, Lee J, DeSilva TM, Jensen FE, Volpe JJ, Rosenberg PA. 17beta-estradiol protects against hypoxic/ischemic white matter damage in the neonatal rat brain 1. J Neurosci Res. 2009;87:2078–2086. doi: 10.1002/jnr.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holene E, Nafstad I, Skaare JU, Sagvolden T. Behavioural hyperactivity in rats following postnatal exposure to sub-toxic doses of polychlorinated biphenyl congeners 153 and 126. Behav Brain Res. 1998;94:213–224. doi: 10.1016/s0166-4328(97)00181-2. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Fein GG, Jacobson SW, Schwartz PM, Dowler JK. The transfer of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) across the human placenta and into maternal milk. Am J Public Health. 1984;74:378–379. doi: 10.2105/ajph.74.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Restrepo B, Kannan K, Rapaport DP, Rodan BD. Polybrominated diphenyl ethers and polychlorinated biphenyls in human adipose tissue from New York. Environ Sci Technol. 2005;39:5177–5182. doi: 10.1021/es050399x. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Tarr AJ, Sparkman NL, Bogale TMH, Boehm GW. Neonatal endotoxin exposure impairs avoidance learning and attenuates endotoxin-induced sickness behavior and central IL-1[beta] gene transcription in adulthood. Behavioural Brain Research. 2008;194:25–31. doi: 10.1016/j.bbr.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Kostyniak PJ, Hansen LG, Widholm JJ, Fitzpatrick RD, Olson JR, Helferich JL, Kim KH, Sable HJ, Seegal RF, Pessah IN, Schantz SL. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol Sci. 2005;88:400–411. doi: 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Lasky-Su J, et al. Genome-wide association scan of the time to onset of attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1355–1358. doi: 10.1002/ajmg.b.30869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW, Penderis J, Zhao C, Schumacher M, Franklin RJM. Females remyelinate more efficiently than males following demyelination in the aged but not young adult CNS. Experimental Neurology. 2006;202:250–254. doi: 10.1016/j.expneurol.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia-Bonnefil P. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Dev Neurosci. 2004;26:245–254. doi: 10.1159/000082141. [DOI] [PubMed] [Google Scholar]
- Martin LA, Gallo MA, Ruehl CD, Klassen CD. Rapid dissappearance of intravenously administered [125]I-thyroxine (T4) from serum following repeated administration of polychorinated biphenyls (PCBs) Toxicologist. 2001;60:380–381. [Google Scholar]
- Miller VM, Kalaria RN, Hall R, Oakley AE, Kenny RA. Medullary microvessel degeneration in multiple system atrophy. Neurobiol Dis. 2007;26:615–622. doi: 10.1016/j.nbd.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Miller VM, Kenny RA, Oakley AE, Hall R, Kalaria RN, Allan LM. Dorsal motor nucleus of vagus protein aggregates in Lewy body disease with autonomic dysfunction 1. Brain Res. 2009;1286:165–173. doi: 10.1016/j.brainres.2009.05.083. [DOI] [PubMed] [Google Scholar]
- Miller VM, Lawrence DA, Coccaro GA, Mondal TK, Andrews K, Dreiem A, Seegal RF. Sex effects of Interleukin-6 deficiency on neuroinflammation in aged C57Bl/6 mice 1. Brain Res. 2010;8:11–22. doi: 10.1016/j.brainres.2009.12.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, et al. Genome-wide association scan of attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1337–1344. doi: 10.1002/ajmg.b.30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguon K, Baxter MG, Sajdel-Sulkowska EM. Perinatal exposure to polychlorinated biphenyls differentially affects cerebellar development and motor functions in male and female rat neonates 4. Cerebellum. 2005;4:112–122. doi: 10.1080/14734220510007860. [DOI] [PubMed] [Google Scholar]
- Onishchenko N, Fischer C, Wan Ibrahim WN, Negri S, Spulber S, Cottica D, Ceccatelli S. Prenatal Exposure to PFOS or PFOA Alters Motor Function in Mice in a Sex-Related Manner. Neurotox Res. 2010 doi: 10.1007/s12640-010-9200-4. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in stereotaxic coordinates. Burlington, MA: Elsevier; 2007. pp. 0–209. [Google Scholar]
- Sarlieve LL, Rodriguez-Pena A, Langley K. Expression of thyroid hormone receptor isoforms in the oligodendrocyte lineage. Neurochem Res. 2004;29:903–922. doi: 10.1023/b:nere.0000021235.83952.9a. [DOI] [PubMed] [Google Scholar]
- Seiwa C, Nakahara J, Komiyama T, Katsu Y, Iguchi T, Asou H. Bisphenol A exerts thyroid-hormone-like effects on mouse oligodendrocyte precursor cells 1. Neuroendocrinology. 2004;80:21–30. doi: 10.1159/000080663. [DOI] [PubMed] [Google Scholar]
- Sharlin DS, Bansal R, Zoeller RT. Polychlorinated biphenyls exert selective effects on cellular composition of white matter in a manner inconsistent with thyroid hormone insufficiency. Endocrinology. 2006;147:846–858. doi: 10.1210/en.2005-0778. [DOI] [PubMed] [Google Scholar]
- Sharlin DS, Tighe D, Gilbert ME, Zoeller RT. The balance between oligodendrocyte and astrocyte production in major white matter tracts is linearly related to serum total thyroxine. Endocrinology. 2008;149:2527–2536. doi: 10.1210/en.2007-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio A, Ferrario M, Dreano M, Garotta G, Spano P, Pizzi M. Soluble Interleukin-6 (IL-6) Receptor/IL-6 Fusion Protein Enhances in Vitro Differentiation of Purified Rat Oligodendroglial Lineage Cells. Molecular and Cellular Neuroscience. 2002;21:602–615. doi: 10.1006/mcne.2002.1208. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Pappas N, Pascani K, Wong C. Gender differences in cerebellar metabolism: test-retest reproducibility. Am J Psychiatry. 1997;154:119–121. doi: 10.1176/ajp.154.1.119. [DOI] [PubMed] [Google Scholar]
- Wickizer TM, Brilliant LB, Copeland R, Tilden R. Polychlorinated biphenyl contamination of nursing mothers' milk in Michigan. Am J Public Health. 1981;71:132–137. doi: 10.2105/ajph.71.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm JJ, Clarkson GB, Strupp BJ, Crofton KM, Seegal RF, Schantz SL. Spatial Reversal Learning in Aroclor 1254-Exposed Rats: Sex-Specific Deficits in Associative Ability and Inhibitory Control. Toxicology and Applied Pharmacology. 2001;174:188–198. doi: 10.1006/taap.2001.9199. [DOI] [PubMed] [Google Scholar]
- Yang S, Li C, Zhang W, Wang W, Tang Y. Sex differences in the white matter and myelinated nerve fibers of Long-Evans rats. Brain Research. 2008;1216:16–23. doi: 10.1016/j.brainres.2008.03.052. [DOI] [PubMed] [Google Scholar]
- Zhang PL, Izrael M, Ainbinder E, Ben-Simchon L, Chebath J, Revel M. Increased myelinating capacity of embryonic stem cell derived oligodendrocyte precursors after treatment by interleukin-6/soluble interleukin-6 receptor fusion protein. Molecular and Cellular Neuroscience. 2006;31:387–398. doi: 10.1016/j.mcn.2005.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


