Abstract
The progression of prostate cancer to the castration-recurrent phenotype remains a major problem medically. The present study examined the transcriptomics of de novo androgen synthesis as a potential mechanism to escape from dependence on circulating androgen. VCaP, LNCaP and LAPC4 cells were acclimated to 1 nM testosterone for 5 generations before subjecting them to a reduced level of 0.03 nM testosterone. Changes in gene expression were quantified using qRT-PCR. Analyses of the cholesterol biosynthesis pathway and the Δ4, Δ5 and backdoor steroidogenic pathways were carried out. VCaP cells showed no change in the transcriptome of cholesterol biosynthesis. However, several receptors for cholesterol transport were up-regulated. The Δ4 and Δ5 steroidogenic pathways, but not the backdoor pathway, were stimulated. Additionally, androgen receptor (AR) expression was increased. Taken together, the above changes might allow recovery of AR activity to a near normal level. In contrast, LNCaP cells showed only minimal adjustment in the transcriptome of steroidogenesis. LAPC4 cells were equally unresponsive to boosting the machinery of androgen production. In brief, our results suggest that the VCaP model is an appropriate model for further investigation of targeting the androgen-AR axis to block the emergence of castration-resistant prostate cancer.
Keywords: prostate cancer, androgen reduction, de novo steroidogenesis, cholesterol biosynthesis, transcriptomics, gene expression, cholesterol uptake
Introduction
Androgen is essential to the growth and differentiation of the prostate. Testosterone is the major androgen in the circulation, and is converted to dihydrotestosterone (DHT) in prostate tissue. Both testosterone and DHT bind to the androgen receptor (AR) to cause its activation, although DHT has a slower dissociation rate from AR compared to testosterone. Once activated, AR serves as a transcription factor in regulating the expression of a variety of genes that are involved either directly or indirectly in anabolism.1 Prostate cancer cells are notoriously dependent on AR signaling for proliferation and survival.2
For decades, androgen deprivation therapy (ADT) has been a treatment modality of choice for advanced prostate cancer. Surgical or medical castration is used to eliminate testicular androgen production in order to reduce the supply of androgen to the prostate. However, after a median remission of 2–3 years following ADT, the majority of prostate cancer begins to progress. The late stage disease is known as androgen refractory or castration-recurrent prostate cancer. Interestingly, most of these tumors maintain a functional AR signal despite the decrease of circulating testosterone.3–5 Several hypotheses have been proposed to explain the phenomenon, including a hypersensitive AR, a promiscuous AR, or an outlaw AR.6,7
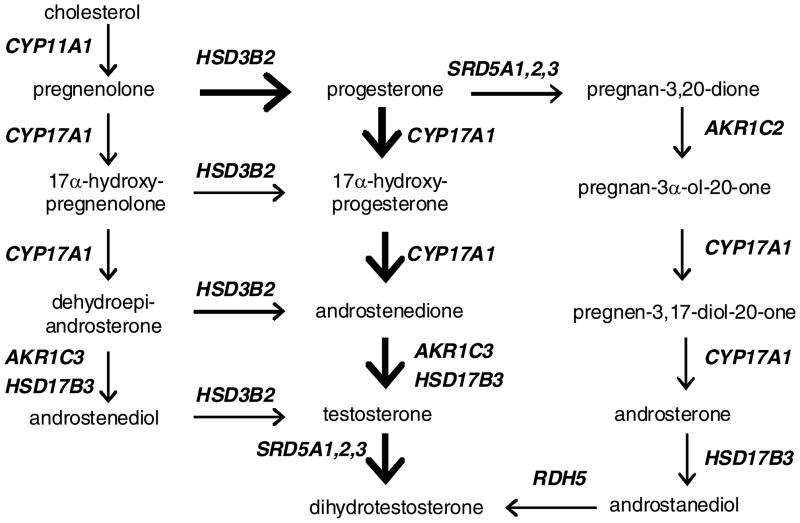
Recently, increasing attention has been focused on the ability of prostate cancer to synthesize androgen in situ. Mohler’s group5,8 and then Nelson’s group9 independently reported that the level of testosterone in castration-recurrent prostate cancer is comparable to, if not higher than, that found in androgen-stimulated prostate cancer or benign prostatic hyperplasia. Enhanced expression of transcripts encoding many of the steroidogenic enzymes was also demonstrated in castration-recurrent tumors excised from either local soft tissue or distant metastatic sites.9,10 With the use of radiolabeled cholesterol as the precursor substrate, another study provided direct evidence of de novo testosterone synthesis by high-passage LNCaP cells.11 Cells usually obtain cholesterol either by uptake from the circulation via lipoprotein receptors or by making their own from acetyl CoA. There are three known enzymatic routes by whichtestosterone and DHT are synthesized from cholesterol; these are referred to commonly as the Δ4 pathway, the Δ5 pathway, and the backdoor pathway (Fig. 1). To date, scanty information is available on how prostate cancer cells respond to decreases of androgen supply to acquire cholesterol (receptor-mediated uptake versus de novo synthesis) and produce androgen (Δ4, Δ5 or backdoor pathway).
Figure 1.
Schematic diagram of androgen biosynthesis. The left side is the Δ5 pathway, the middle is the Δ4 pathway, and the right side is the backdoor pathway.
Nearly all androgen-sensitive prostate cancer cell lines used in research are propagated routinely in a medium supplemented with 10% fetal bovine serum (FBS). Commercial FBS contains about 0.3 nM testosterone (data provided by vendors, and confirmed by our own HPLC-MS analysis). Thus, these androgen-sensitive cells are accustomed to an environment of 0.03 nM testosterone. This level of testosterone is much lower than what has been reported for circulating testosterone in castrated males.12–14 In order to simulate more closely the pre-ADT condition, three different androgen-sensitive prostate cancer cell lines, VCaP, LNCaP and LAPC4, were grown in 1 nM testosterone for at least 5 generations before returning them to the 10% FBS medium containing 0.03 nM testosterone. The above protocol is referred to in the text as “testosterone reduction”. The transcriptomics of cholesterol replenishment and androgen synthesis following testosterone reduction were characterized. In this in vitro model, the only variable is the level of exogenous testosterone. The model therefore allows us to evaluate the impact of testosterone alone in eliciting gene expression changes that are involved directly in repleting the supply of androgen in the cancer epithelium. The ultimate goal is to identify potential targets that might be exploited for novel prostate cancer treatment.
Results
The transcriptome of cholesterogenesis and steroidogenesis in testosterone-trained cells
As pointed out in the Introduction, most prostate cancer cell lines are propagated in a medium containing 0.03 nM testosterone. In the present study, cells were “trained” for 5 generations in 1 nM testosterone in order to re-familiarize them to a more physiologically relevant environment. The gene expression changes involved in cholesterol and androgen production due to testosterone training were examined by quantitative real time-PCR as a first step. The information is summarized in Table 1. The data are expressed as fold of change observed in 1 nM testosterone-trained cells relative to the value observed in cells exposed to 0.03 nM testosterone. The genes are divided into three categories in the table. The top portion contains the genes involved in cholesterol biosynthesis, and the middle portion contains the genes that code for the receptors for cholesterol uptake. Values in bold face denote a significant change of >2-fold. A minus sign indicates a decrease relative to the control value. Testosterone conditioning produced only minimal changes in the mRNA level of cholesterol biosynthesis genes in VCaP cells, and caused modest increases of many of the same genes in LNCaP and LAPC4 cells. The LNCaP and LAPC4 data suggest that testosterone stimulation up-regulates the transcription of genes involved in cholesterol synthesis, a finding which is consistent with that reported previously in the literature.15,16 The cholesterol receptor data told a different story. VCaP cells exhibited sizable decreases of cholesterol receptor expression in the presence of 1 nM testosterone, especially for LOX1, SREC and STAR. In contrast, LNCaP cells raised the expression of SR-BI, LDLR and STAR, while LAPC4 cells were unresponsive.
Table 1.
Changes in expression of cholesterol biosynthesis and uptake genes and steroidogenic genes in cells acclimated to 1 nM testosterone exposure. The data are expressed as fold of change relative to the values in control cells. The numbers in bracket represent the range converted from the ΔΔCt values (mean ± SD).
| Gene\Model | VCaP | LNCaP | LAPC4 | |
|---|---|---|---|---|
| Cholesterol synthesis | ACAT2 | 1.0 | 3.3 (3.1–3.4)** | 2.1 (1.9–2.3)* |
| HMGCS1 | − 1.1 | 2.1 (2.0–2.1)** | 2.8 (2.7–2.9)** | |
| HMGCR | 1.6 | 1.9 | 2.1 (2.0–2.1)** | |
| MVK | 1.5 | 2.3 (1.9–2.6)* | 2.4 (2.3–2.5)** | |
| PMVK | 1.6 | 2.1 (2.0–2.2)** | 1.8 | |
| MVD | 1.5 | 2.0 (1.9–2.0)** | 2.6 (2.5–2.6)** | |
| FDFT1 | 1.3 | 3.2 (3.1–3.3)** | 2.4 (2.3–2.5)** | |
| SQLE | 1.9 | 1.7 | 2.1 (2.0–2.1)** | |
| uptake Cholesterol | SR-BI | 1.5 | 4.0 (3.8–4.2)** | 1.6 |
| LDLR | 1.6 | 2.5 (2.5–2.5)** | 1.5 | |
| LOX1 | −9.0 (8.1–10.0) ** | 1.5 | 1.9 | |
| SREC | − 4.7 (3.8–5.8) * | 1.2 | 1.5 | |
| STAR | − 4.7 (3.9–5.6) ** | 2.3 (2.1–2.4)** | 1.8 | |
| steroidogenesis | CYP11A1 | − 8.2 (5.6–11.8) * | 1.3 | 1.7 |
| HSD3B2 | 3.9 (3.6–4.3) ** | 1.2 | 1.9 | |
| CYP17A1 | − 11.3(8.9–14.4)** | − 2.5 (2.4–2.6)** | − 1.9 | |
| AKR1C3 | − 1.3 | − 1.3 | 6.9 (5.0–9.6)* | |
| HSD17B3 | − 3.1 (1.6–5.7) * | − 5.6 (2.8–11.0)* | 1.8 | |
| SRD5A1 | 1.1 | 1.9 | 3.0 (2.8–3.3)** | |
| SRD5A2 | − 12.4(9.5–16.3)** | − 7.5 (5.2–10.8)* | 1.0 | |
| SRD5A3 | − 1.8 | 2.2 (2.0–2.3)** | 1.6 | |
| AKR1C2 | − 2.5 (2.3–2.8)** | − 1.8 | 5.6 (3.5–8.8)* | |
| RDH5 | 1.0 | 2.3 (2.1–2.5)** | 1.3 | |
p<0.05;
p<0.005, paired student’s t-test.
The bottom portion of Table 1 summarizes the changes in steroidogenesis gene expression in testosterone-trained cells. In general, VCaP and LNCaP cells showed a similar pattern qualitatively, although there were some quantitative differences. Notably, the transcript level of a few key genes, such as CYP17A1, HSD17B3 and SRD5A2, was suppressed significantly by testosterone. These changes were not observed in LAPC4 cells. Instead, testosterone promoted the expression of AKR1C3, SRD5A1, and AKR1C2.
Thus, regardless of the availability of testosterone, the transcriptomes of cholesterol and androgen biosynthesis are intact in prostate cancer cells. Since testosterone stimulation may produce divergent responses in different cell lines, the baseline data must be taken into consideration when evaluating the effect of testosterone reduction.
Changes of gene expression in cholesterol biosynthesis after decreasing the supply of testosterone
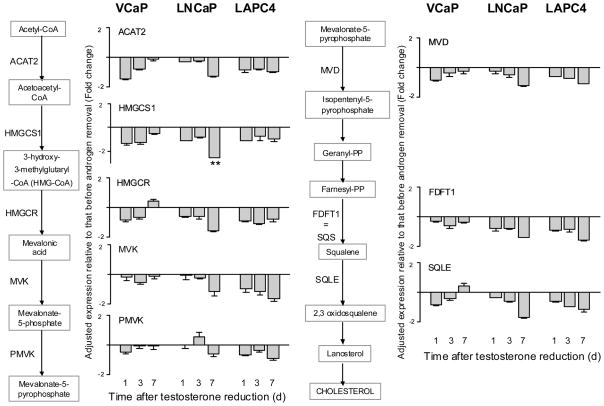
The biosynthesis of cholesterol from acetyl CoA involves many steps. Figure 2 shows the biochemical pathway and the results of the transcriptomic changes following testosterone reduction. The data, which are presented in a bar graph format, are expressed as fold of change relative to the value observed before testosterone reduction. Bars above or below the X-axis signify an increase or a decrease, respectively. As noted in Methods, a 2-fold change minimum in either direction is considered statistically significant. In looking at the entire data set of Figure 2, there were no significant changes with respect to any of the cholesterogenesis genes in all 3 cell lines. The results suggest that endogenous production of cholesterol is unlikely to be geared up in order to provide more precursor substrate for the synthesis of androgen.
Figure 2.
Changes in gene expression of cholesterol biosynthesis after testosterone reduction. Results are expressed as fold of change relative to the value obtained before testosterone reduction (mean ± SD, n=3). Values above or below the X-axis represent an increase or a decrease, respectively. **P<0.005, paired Student’s t-test.
The above conclusion is based on comparing cells undergoing testosterone reduction to those exposed continuously to 1 nM testosterone. As shown in Table 1, testosterone stimulates the expression of a number of genes involved in cholesterogenesis, especially in LNCaP and LAPC4 cells. It is possible that at the time immediately before testosterone is removed from the culture, the cholesterol biosynthesis machinery is already operating at near maximum capacity. Consequently, there is little elasticity for expansion even though there is a demand for endogenous androgen production to compensate for the loss of exogenous androgen.
Changes of gene expression in cholesterol uptake and transport after decreasing the supply of testosterone
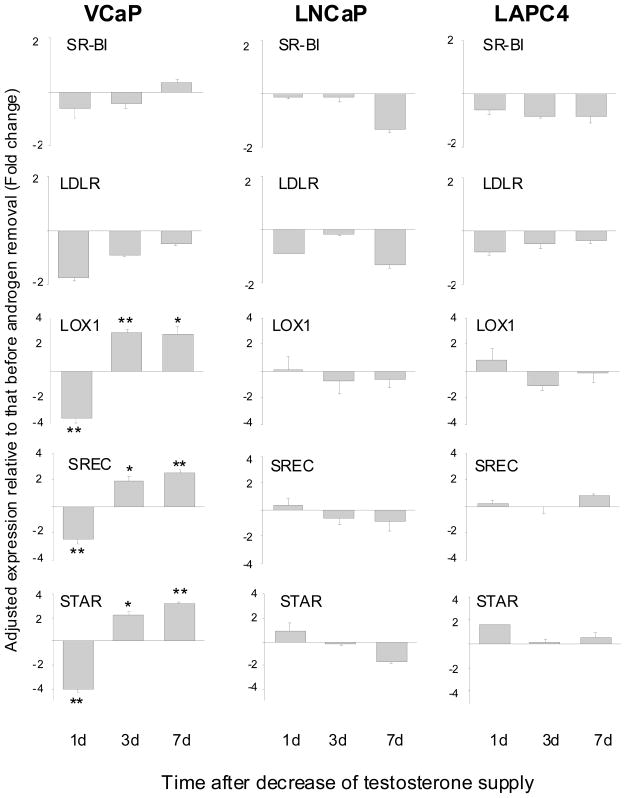
In addition to synthesizing its own cholesterol, cells may acquire cholesterol from an outside source. A variety of receptors participate in bringing cholesterol from the outside to the inside of cells. Cholesterol is present as lipoprotein complexes in the circulation. SR-B1 is a receptor which facilitates the uptake of cholesterol in high density lipoprotein into cells.17 LDLR is the low density lipoprotein receptor, and is the major receptor for cholesterol uptake in the body.18 LOX1 and SREC are members of the scavenger receptor family.19,20 They transport lipoprotein complexes into cells in order to process the release of cholesterol. STAR is a carrier which mediates the transport of cholesterol from the outer mitochondrial membrane to the inner mitochondrial membrane to facilitate the conversion of cholesterol to pregnenolone.21 The transcript level of the above receptors was analyzed before and after testosterone reduction (Fig. 3). The expression of several receptors/transporters, including LOX1, SREC and STAR, was up-regulated in VCaP cells, but not in LNCaP or LAPC4 cells. The increase in VCaP cells happened on day 3 of testosterone reduction and thereafter, following an initial drop on day 1. The reason for the early decrease, which was observed for all the genes, is unclear.
Figure 3.
Changes in gene expression of cholesterol uptake/transport receptors after testosterone reduction. *P<0.05, **P<0.005, paired Student’s t-test.
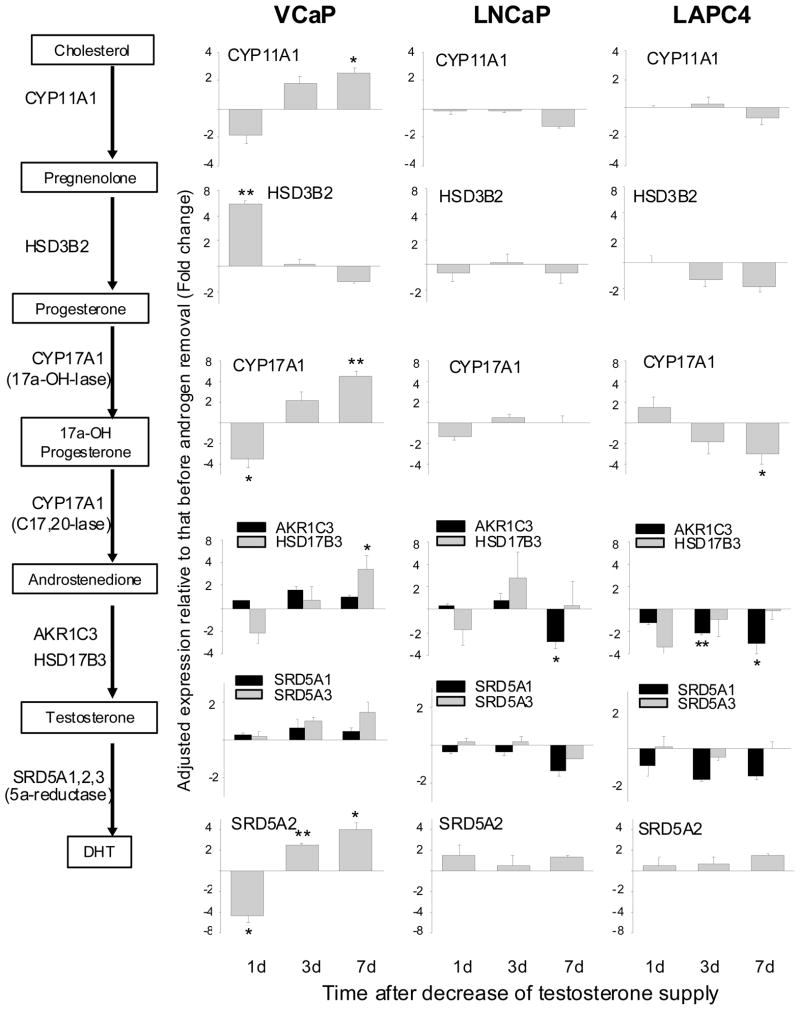
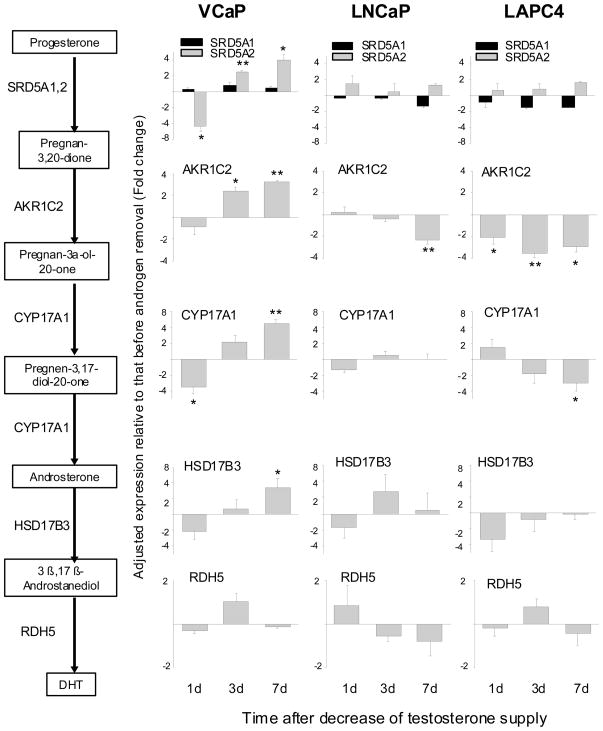
Changes of gene expression in the Δ4 pathway of androgen biosynthesis after decreasing the supply of testosterone
In view of the fact that the Δ4 and Δ5 pathways share the same enzymes at each corresponding step (Fig. 1), we are only presenting the Δ4 pathway data as illustrated in Figure 4. Compared to VCaP cells, LNCaP and LAPC4 cells showed much less striking changes after testosterone reduction. In general, the changes in the latter two cell models were <2-fold, and they fluctuated either above or below the baseline with no distinctive trend. VCaP cells, on the other hand, showed more robust and consistent changes.
Figure 4.
Change in gene expression of Δ4 androgen biosynthesis pathway after testosterone reduction. *P<0.05, **P<0.005, paired Student’s t-test.
In VCaP cells, several steroidogenic enzymes, including CYP11A1, CYP17A1, HSD17B3 and SRD5A2, were significantly up-regulated by day 7 after testosterone reduction. Of particular interest is CYP17A1. It is a rate limiting enzyme, and catalyzes two consecutive steps: progesterone → 17α-hydroxyprogesterone → androstenedione. A 4-fold increase in the transcript level of this enzyme should boost output in a profitable way. As an aside, the primary regulation for most steroidogenic enzymes is at the transcriptional level, and not by post-translational modification.22 In contrast to the above, HSD3B2 showed a different pattern of change. The transcript level of HSD3B2 was up-regulated as early as day 1 after testosterone reduction. The increase receded quickly, and by day 3, the level was back to the control. The rapid, although short-lived, increase of HSD3B2 may be of some significance. In addition to catalyzing the conversion of pregnenolone to progesterone, HSD3B2 is also critical in converting dehydroepiandrosterone to androstenedione (Fig. 1). The latter in turn is converted to testosterone by HSD17B3 (as noted above, HSD17B3 was induced by testosterone reduction). Since dehydroepiandrosterone is a major adrenal steroid, prostate cancer cells may be able to use adrenal dehydroepiandrosterone for the synthesis of androgen as a quick-fix remedy.
Androstenedione is converted to testosterone by either AKR1C3 or HSD17B3, while testosterone is converted to DHT by SRD5A1, SRD5A2 or SRD5A3. In prostate cancer cells, AKR1C3 is predominant to HSD17B3, and SRD5A1 and SRD5A3 are predominant to SRD5A2.23–25 The relative abundance of all 5 transcripts was examined. There was indeed much more AKR1C3, SRD5A1 and SRD5A3 than HSD17B3 or SRD5A2 (data not shown). And yet, HSD17B3 and SRD5A2 were the ones which were significantly up-regulated after testosterone reduction. SRD5A3 showed a gradual increase with time, but did not reach the 2-fold threshold by day 7.
Change of gene expression in the backdoor pathway of and androgen biosynthesis after decreasing the supply of testosterone
The genes in the Δ4, Δ5, and the backdoor pathways are identified in Figure 1. There are genes common to all three, except AKR1C2 and RDH5, which are unique to the backdoor pathway.26,27 AKR1C2 and RDH5 are responsible for converting pregnan-3,20-dione to pregnan-3α-ol-20-one, and androstanediol to DHT, respectively. The other genes in the backdoor pathway perform similar functions in either the Δ4 or Δ5 pathway. Despite the overlap, the entire data set of the backdoor pathway is presented in Figure 5 in order to fully capture its contribution to androgen biosynthesis. As expected, LNCaP and LAPC4 cells were not particularly responsive to an up-regulation of the backdoor pathway after testosterone reduction. VCaP cells, on the other hand, showed an enhanced expression of the genes engaged in the first 5 steps, starting from progesterone and extending to 3β,17β-androstanediol. However, RDH5, which is responsible for the production of DHT from 3β,17β-androstanediol, was not changed. The finding suggests that the backdoor pathway may not play an important role in replenishing DHT in VCaP cells after testosterone reduction.
Figure 5.
Change in gene expression of backdoor androgen biosynthesis pathway after testosterone reduction. *P<0.05, **P<0.005, paired Student’s t-test.
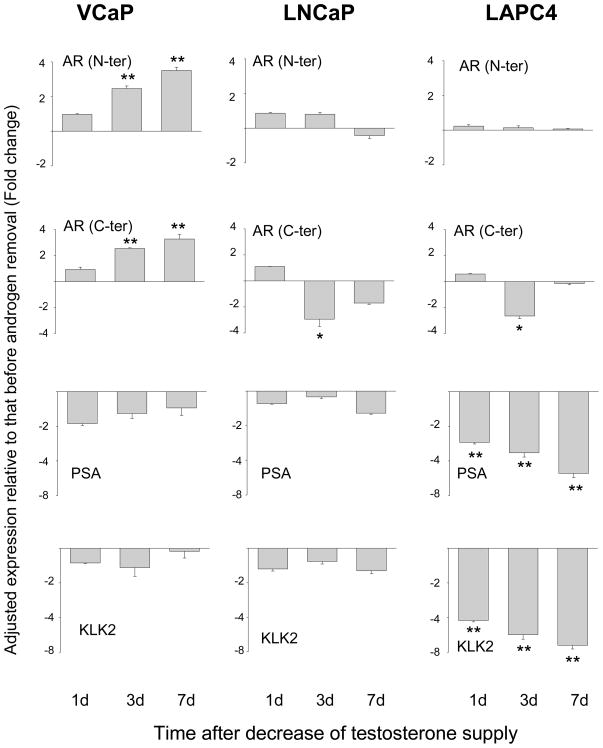
Expression of AR and AR target genes after decreasing the supply of testosterone
Since new AR spliced variants lacking the ligand binding domain have been reported,28,29 two sets of primers were designed to detect changes in AR expression. They are directed against either the N-terminal transactivation domain (thereby recognizing both the full length and truncated AR), or the C-terminal ligand binding domain (thereby recognizing only the full length AR). PSA and KLK2 were also examined as markers of AR target genes. The results are shown in Figure 6. In VCaP cells, the level of AR transcript was increased after testosterone reduction. The increase was observed with either the N-terminal or C-terminal primers. The transcripts for PSA and KLK2 were decreased only slightly, suggesting that the compensatory reinforcement by the Δ4 and Δ5 pathways and increased AR expression helped to keep AR functioning at a near normal capacity. The expression pattern of AR in LNCaP and LAPC4 cells was different. No change was detected with the AR N-terminal primers. With the C-terminal primers, there was a decrease on day 3, followed by a recovery on day 7. The expression of PSA and KLK2 was not changed in LNCaP cells. In contrast, the expression of both AR target genes was decreased markedly in LAPC4 cells.
Figure 6.
Change in gene expression of AR, PSA and KLK2 after testosterone reduction. *P<0.05, **P<0.005, paired Student’s t-test.
Discussion
In this study, the transcriptome of androgen production was characterized in 3 different androgen-sensitive prostate cancer cell lines after decreasing the supply of testosterone. The changes were examined in the context of the androgen-AR axis in an attempt to (a) spotlight a cell model which may help us understand the transition from androgen dependence to androgen independence, and (b) identify potential targets which might be incorporated in a new strategy for the treatment of advanced prostate cancer. Historically, all three cell lines used in this project are propagated in a medium containing 0.03 nM testosterone. In this environment, each cell line may have acquired a genetic constitution that would allow it to survive in a very low androgen condition. In the present experiments, the cells were first exposed to 1 nM testosterone for several generations. The purpose is to re-introduce the cells to a testosterone-rich condition reminiscent of pre-ADT. A brief explanation of the 1 nM testosterone training condition is in order. Total circulating testosterone level in intact males is in the range of 12–15 nM.30 Most of it is bound to steroid hormone binding globulin. This pool of testosterone may not be available for uptake. Free testosterone is estimated to be around 0.5 nM. On the other hand, prostate tissue level of testosterone is between 2–3 nM.8 Many prostate cancer cell models, including those used in the present study, do not grow well at testosterone levels greater than 2 nM. Our design of using 1 nM testosterone is a compromise between what is found in the prostate microenvironment and what is favored by the cells to support optimal growth. The in vitro testosterone training procedure clearly cannot mimic the in vivo situation in every aspect. Nonetheless, the approach does yield certain changes which are largely in line with the way cells would be expected to behave when the availability of androgen is abundant (see Table 1).
Our results suggest that the VCaP model is an appropriate model to study how androgen metabolism and androgen signaling adjust to testosterone availability. During the “testosterone training” period, the cholesterol uptake system is down-regulated (Table 1), since there is apparently little need for additional supply of cholesterol as a precursor substrate for androgen. The expression of a number of genes involved in steroidogenesis is also decreased. Many of the same genes are up-regulated when testosterone is reduced in the culture (Figs. 3 and 4). The reversible effect lends confidence to the specificity of the response to changes in androgen levels. The key findings are summarized in Table 2 for a quick review. To compensate for the loss of exogenous testosterone, the cholesterol uptake/transport system, the Δ4 and Δ5 androgen biosynthesis pathways, and the expression of AR are up-regulated. There seems to be a concerted effort to keep the activity of AR from being depressed too much. Previous publications have focused mainly on the ability of prostate cancer cells to synthesize androgen from cholesterol, but few have examined the preference of replenishing the supply of cholesterol, i.e. endogenous versus exogenous sources. The present research highlighting the cholesterol uptake system is novel and could potentially arouse new interest in the area. An increased capacity to synthesize androgen de novo could contribute to castration resistance. VCaP cells, which are derived from bone metastasis, may have a high demand for androgen because they over-express the wild type AR. The up-regulation of steroidogenic gene expression has also been reported previously in distant soft tissue metastasis of prostate cancer.9
Table 2.
Summary of the responses of prostate cancer cells to testosterone withdrawal
| Model\Response | VCaP | LNCaP | LAPC4 |
|---|---|---|---|
| Cholesterol biosynthesis | minimal change | slight decrease with time | small but persistent decrease |
| Cholesterol uptake/transport | up-regulation of several receptors after an initial drop | minimal change | minimal change |
| Delta 4 and delta 5 steroidogenic pathways | significant increase of certain key enzymes, esp. CYP17A1 | mostly remain unchanged | slight decrease with time |
| Backdoor steroidogenic pathway | changes similar to that of Δ4 pathway, except no change in RDH5 | minimal change | minimal change |
| AR expression | increase verified by N-ter and C-ter primers | no change detected by N-ter primer, decrease detected by C-ter primer | same as observed in LNCaP |
| AR target gene expression | small small decrease followed by gradual recovery with time | slight decrease | significant and progressive decrease with time |
In contrast to VCaP cells, LNCaP and LAPC4 cells do not show an up-regulation of the cholesterol or androgen biosynthesis pathway after testosterone reduction (Table 2). Apparently, these cells do not perceive a need to synthesize more androgen even when the environmental supply of androgen is reduced. Part of the reason could be due to the fact that both cell types have a lower expression of AR compared to VCaP cells. They may be able to keep the AR signal operative, although in a diminished capacity, with a basal level of androgen. The above explanation is consistent with the observation from an in vivo LNCaP xenograft study that the up-regulation of steroidogenic genes is modest at best following castration of the host.27 LNCaP cells have a point mutation in the ligand binding domain of the AR gene.31 The mutation may allow AR to be activated by non-androgen ligands.32,33 One interesting point to note about the LAPC4 cells is that the expression of AR target genes is significantly depressed as a result of testosterone reduction, and yet the growth rate is not affected (data not shown). The findings suggest that LAPC4 cells no longer depend on high levels of testosterone to maintain their growth.
The period immediately after ADT might represent a window of opportunity to block the emergence of castration-recurrent prostate cancer. The VCaP model offers several targets which could be exploited for this purpose. Cholesterol, in addition to providing precursor substrate for the biosynthesis of androgen, is metabolized to other intermediates which are important for the post-translational modification of certain growth and survival signaling molecules.34 Previously, the use of a cholesterol-lowering statin drug has been reported to reduce the risk of advanced prostate cancer.35,36 The present research also suggests that the cholesterol uptake/transport system could be an area worthy of further investigation. CYP17A1 is a rate-limiting enzyme in the androgen biosynthesis pathway. Our results indicate that the expression of CYP17A1 is significantly increased after testosterone reduction. More than half of castration-resistant prostate cancer patients respond to Abiraterone acetate,37,38 which is a specific CYP17A1 inhibitor. The data is consistent with previous findings that metastatic prostate cancer generally has a high level of CYP17A1.9 According to the VCaP results, HSD3B2, HSD17B3, SRD5A1, SRD5A2 and SRD5A3 might represent additional candidates for intervention. In summary, the present study has generated a treasure of clues. These clues are expected to lead to further in-depth studies. Specifically, the functional significance of many of the gene expression changes will be investigated, as the next step, as well as the underlying mechanism of their regulation. The present study is an important contribution to our understanding of how prostate cancer cells transition to androgen independence and how an effective strategy might be developed to block the progression of the disease.
Materials and Methods
Cell lines and treatment with testosterone
VCaP and LNCaP cells were obtained from ATCC (Rockville, MD). LAPC4 cells were a gift from Dr. Charles Sawyers at the Memorial Sloan Kettering Cancer Center, New York City. VCaP cells were cultured in phenol red-free DMEM, while LNCaP and LAPC4 cells were cultured in RPMI 1640 medium. All 3 cell lines were propagated routinely in 10% FBS. The concentration of testosterone in a medium containing 10% FBS is ~0.03 nM. Cells with <10 passage number were used in all experiments. All cultures were maintained in a humidified 5% CO2 incubator at 37°C.
Preliminary studies showed that 1 nM testosterone provides an optimal condition for the growth of all 3 cell lines. Consequently, the cells were grown in 1 nM testosterone for at least 5 generations before subjecting them to testosterone reduction. The idea was to acclimate the cells to a more physiologically relevant level of testosterone and then return them to an environment of 0.03 nM testosterone (i.e. 10% FBS only with no added testosterone) in order to study their response to testosterone reduction. Cells were seeded in 6-well plates at a density of 2 × 105/ml. Analysis of transcriptome expression (as determined by quantitative real time-PCR) was performed at 1, 3, 5 or 7 days following the removal of testosterone. Cells that were cultured continuously in 1 nM testosterone were used as time-matched controls.
Quantitative real time-PCR and data analysis
Total RNA was isolated with the RNEASY Mini Kit from Qiagen (Valencia, CA). cDNA was generated by using the SuperScript VILO cDNA synthesis kit from Invitrogen (Carlsbad, CA) according to the manufacturer’s instruction. The real time-PCR experiments were carried out by using a Tecan Freedom Evo 200 robotics system (Tecan Group, Switzerland) in a 384-well PCR format, and with the SYBR Green qPCR supermix from Invitrogen (Carlsbad, CA). Amplification was achieved in an Applied Biosystems 7900HT fast real time-PCR system (Foster City, CA). The PCR cycle was programmed as follows. The PCR mix was set at 50°C for 2 min for the Uracil DNA Glycosylase incubation, and at 95°C for 2 min for “hot start” activation. This was followed by 40 cycles of amplification which includes denaturation at 95°C for 15 sec, and annealing and extension at 60°C for 1 min. Melting point analysis was performed after real time-PCR in order to verify the specificity of the amplification reaction.
The primer pairs for each transcript are shown in Supplemental Table 1. The RPL13A gene was chosen as the internal control for normalization, since it has less variability than the β-actin gene. All gene expression experiments were repeated three times.
The relative expression of each gene was calculated using the −ΔΔCt method. First, the value obtained at each time point, either in the presence or absence of testosterone, was calibrated against the zero hour baseline value, with normalization to the reference RPL13A1 gene, in determining the −ΔΔCt value. Next, the −ΔΔCt value from the continuous testosterone-treated sample was subtracted from the −ΔΔCt value of the testosterone-deprived sample. The samples were matched according to the same length of time in culture. The net gain or loss was calculated as a positive or negative fold-change relative to the testosterone-treated value. Following the convention of gene expression analysis, a minimum of 2-fold difference in either direction is considered a cutoff for statistical significance.
Supplementary Material
Acknowledgments
This work was supported by grant P01CA126804 (C. Ip), Grant P01CA77739 (J. L. Mohler) from the National Cancer Institute; Grant 62-2388-01 from the Roswell Park Alliance Foundation (C. Ip) and partially supported by the Roswell Park Cancer Center Support Grant 1P30-CA016056 from the National Cancer Institute.
Abbreviations
- AR
androgen receptor
- DHT
dihydrotestosterone
- ADT
androgen deprivation therapy
- FBS
fetal bovine serum
Footnotes
There are no conflicts of interest or financial disclosure to report.
References
- 1.Ma C, Yoshioka M, Boivin A, Gan L, Takase Y, Labrie F, St Amand J. Atlas of dihydrotestosterone actions on the transcriptome of prostate in vivo. Prostate. 2009;69:293–316. doi: 10.1002/pros.20883. [DOI] [PubMed] [Google Scholar]
- 2.Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62:1008–13. [PubMed] [Google Scholar]
- 3.Gregory CW, Hamil KG, Kim D, Hall SH, Pretlow TG, Mohler JL, French FS. Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res. 1998;58:5718–24. [PubMed] [Google Scholar]
- 4.Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, Ryan C, Smith S, Scher H, Scardino P, Reuter V, Gerald WL. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–27. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohler JL, Gregory CW, Ford OH, III, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–8. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 6.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 7.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 8.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–7. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 11.Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295:115–20. doi: 10.1016/j.mce.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben Josef E, Mendelson DS, Middleton R, Sharp SA, Smith TJ, Talcott J, Taplin M, Vogelzang NJ, Wade JL, III, Bennett CL, Scher HI. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 13.Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, Knudsen B, Hess DL, Nelson CC, Matsumoto AM, Bremner WJ, Gleave ME, Nelson PS. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–41. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 14.Page ST, Lin DW, Mostaghel EA, Hess DL, True LD, Amory JK, Nelson PS, Matsumoto AM, Bremner WJ. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91:3850–6. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- 15.Heemers H, Maes B, Foufelle F, Heyns W, Verhoeven G, Swinnen JV. Androgens stimulate lipogenic gene expression in prostate cancer cells by activation of the sterol regulatory element-binding protein cleavage activating protein/sterol regulatory element-binding protein pathway. Mol Endocrinol. 2001;15:1817–28. doi: 10.1210/mend.15.10.0703. [DOI] [PubMed] [Google Scholar]
- 16.Swinnen JV, Van Veldhoven PP, Esquenet M, Heyns W, Verhoeven G. Androgens markedly stimulate the accumulation of neutral lipids in the human prostatic adenocarcinoma cell line LNCaP. Endocrinology. 1996;137:4468–74. doi: 10.1210/endo.137.10.8828509. [DOI] [PubMed] [Google Scholar]
- 17.Krieger M. The "best" of cholesterols, the "worst" of cholesterols: a tale of two receptors. Proc Natl Acad Sci U S A. 1998;95:4077–80. doi: 10.1073/pnas.95.8.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown MS, Goldstein JL. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc Natl Acad Sci U S A. 1979;76:3330–7. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta JL, Li DY. Identification and autoregulation of receptor for OX-LDL in cultured human coronary artery endothelial cells. Biochem Biophys Res Commun. 1998;248:511–14. doi: 10.1006/bbrc.1998.9004. [DOI] [PubMed] [Google Scholar]
- 20.Tamura Y, Osuga J, Adachi H, Tozawa R, Takanezawa Y, Ohashi K, Yahagi N, Sekiya M, Okazaki H, Tomita S, Iizuka Y, Koizumi H, Inaba T, Yagyu H, Kamada N, Suzuki H, Shimano H, Kadowaki T, Tsujimoto M, Arai H, Yamada N, Ishibashi S. Scavenger receptor expressed by endothelial cells I (SREC-I) mediates the uptake of acetylated low density lipoproteins by macrophages stimulated with lipopolysaccharide. J Biol Chem. 2004;279:30938–44. doi: 10.1074/jbc.M313088200. [DOI] [PubMed] [Google Scholar]
- 21.Sugawara T, Holt JA, Driscoll D, Strauss JF, III, Lin D, Miller WL, Patterson D, Clancy KP, Hart IM, Clark BJ. Human steroidogenic acute regulatory protein: functional activity in COS-1 cells, tissue-specific expression, and mapping of the structural gene to 8p11.2 and a pseudogene to chromosome 13. Proc Natl Acad Sci U S A. 1995;92:4778–82. doi: 10.1073/pnas.92.11.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–70. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 23.El Alfy M, Luu-The V, Huang XF, Berger L, Labrie F, Pelletier G. Localization of type 5 17beta-hydroxysteroid dehydrogenase, 3beta-hydroxysteroid dehydrogenase, and androgen receptor in the human prostate by in situ hybridization and immunocytochemistry. Endocrinology. 1999;140:1481–91. doi: 10.1210/endo.140.3.6585. [DOI] [PubMed] [Google Scholar]
- 24.Lin HK, Steckelbroeck S, Fung KM, Jones AN, Penning TM. Characterization of a monoclonal antibody for human aldo-keto reductase AKR1C3 (type 2 3alpha-hydroxysteroid dehydrogenase/type 5 17beta-hydroxysteroid dehydrogenase); immunohistochemical detection in breast and prostate. Steroids. 2004;69:795–801. doi: 10.1016/j.steroids.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Titus MA, Gregory CW, Ford OH, III, Schell MJ, Maygarden SJ, Mohler JL. Steroid 5alpha-reductase isozymes I and II in recurrent prostate cancer. Clin Cancer Res. 2005;11:4365–71. doi: 10.1158/1078-0432.CCR-04-0738. [DOI] [PubMed] [Google Scholar]
- 26.Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15:432–8. doi: 10.1016/j.tem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, Ettinger SL, Gleave ME, Nelson CC. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–15. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 28.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–13. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Endogenous Hormones and Prostate Cancer Collaborative Group. Endogenous sex hormones and prostate cancer: A collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–83. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veldscholte J, Ris-Stalpers C, Kuiper GG, Jenster G, Berrevoets C, Claassen E, van Rooij HC, Trapman J, Brinkmann AO, Mulder E. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173:534–40. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 32.Culig Z, Hobisch A, Cronauer MV, Cato AC, Hittmair A, Radmayr C, Eberle J, Bartsch G, Klocker H. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol Endocrinol. 1993;7:1541–50. doi: 10.1210/mend.7.12.8145761. [DOI] [PubMed] [Google Scholar]
- 33.Culig Z, Hobisch A, Hittmair A, Peterziel H, Cato AC, Bartsch G, Klocker H. Expression, structure, and function of androgen receptor in advanced prostatic carcinoma. Prostate. 1998;35:63–70. doi: 10.1002/(sici)1097-0045(19980401)35:1<63::aid-pros9>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 34.Hager MH, Solomon KR, Freeman MR. The role of cholesterol in prostate cancer. Curr Opin Clin Nutr Metab Care. 2006;9:379–85. doi: 10.1097/01.mco.0000232896.66791.62. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton RJ, Goldberg KC, Platz EA, Freedland SJ. The influence of statin medications on prostate-specific antigen levels. J Natl Cancer Inst. 2008;100:1511–8. doi: 10.1093/jnci/djn362. [DOI] [PubMed] [Google Scholar]
- 36.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16:2226–32. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 37.Antonarakis ES, Eisenberger MA. Is abiraterone acetate well tolerated and effective in the treatment of castration-resistant prostate cancer? Nat Clin Pract Oncol. 2009;6:12–13. doi: 10.1038/ncponc1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–71. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.