Abstract
Malignant gliomas, which include glioblastomas and anaplastic astrocytomas, are the most common primary tumors of the brain. Over the past 30 years, the standard treatment for these tumors has evolved to include maximal safe surgical resection, radiation therapy and temozolomide chemotherapy. While the median survival of patients with glioblastomas has improved from 6 months to 14.6 months, these tumors continue to be lethal for the vast majority of patients. There has, however, been recent substantial progress in our mechanistic understanding of tumor development and growth. The translation of these genetic, epigenetic and biochemical findings into therapies that have been tested in clinical trials is the subject of this review.
1. Introduction
Gliomas are the most common primary tumors of the brain and are classified on the basis of two histological criteria, the resemblance of tumor cells to normal glial cells and the relative degree of malignancy. Astrocytomas, for example, are comprised of tumor cells that resemble astrocytes while oligodendroglial tumors contain neoplastic cells that resemble oligodendrocytes. Malignancy is graded using a progressive 4 tier scale in which grades I and II are assigned to low grade or benign tumors and grades III and IV are assigned to high grade or malignant tumors (CBTRUS, 2007; Louis et al., 2007).
Grade I astrocytomas are generally benign and frequently curable with complete surgical removal. Grade II astrocytomas also demonstrate slow growth and a high degree of cellular differentiation, but frequently infiltrate surrounding brain tissues. The median overall survival (OS) time after surgical diagnosis ranges from 6–8 years and is reflective of the time required for tumors to transform into higher grade lesions. Grade III astrocytomas, also known as anaplastic astrocytomas (AA), are diffusely infiltrating lesions with focal or dispersed regions of anaplasia and marked proliferative potential. The median OS time ranges from 2–3 years and is also generally determined by the amount of time required for the progression of tumors to grade IV (Louis et al., 2007). Grade IV astrocytomas, also known as glioblastoma multiforme or glioblastomas (GBM), are the most common and malignant glioma subtype. GBMs typically contain cellular polymorphism, nuclear atypia, brisk mitotic activity, neovascular proliferation, and areas of frank necrosis. Additionally, the aggressive invasion and diffuse infiltration of tumor cells into the surrounding brain tissue negate any possibility for a complete surgical tumor removal.
Over the past 30 years, significant changes in the standard treatment of malignant gliomas have been limited. Prior to the 1980’s, the median OS of patients with malignant gliomas was 6 months. In 1980, a prospective randomized trial was reported in which 467 patients with malignant gliomas were randomized to one of four treatment groups: semustine (MeCCNU), radiotherapy (XRT), carmustine (BCNU) plus XRT, or semustine plus XRT. Toxicities included acceptable skin reactions secondary to XRT and thrombocytopenia due to chemotherapy. Patients who received XRT alone or in combination with a nitrosourea (carmustine or semustine) had significantly improved OS compared to patients treated with semustine alone. The median OS of the carmustine plus XRT group (51 weeks) was greater than that of the semustine plus XRT (42 weeks) and XRT alone (36 weeks) groups, but the differences were not statistically significant (Walker et al., 1980).
In 1996, the FDA approved a polyanhydride biodegradable polymer wafer containing BCNU, known as Gliadel®, for the treatment of recurrent gliomas. Patients with recurrent tumors who had wafers placed at the time of their second surgeries were found to have an 8 week survival benefit (Brem et al., 1995). In patients undergoing primary resections for newly diagnosed tumors, the survival benefit of wafer placement was 2.3 months (Westphal et al., 2003). BCNU wafer therapy is currently being studied in combination with other systemic therapies.
In 2001, a retrospective analysis of 416 patients with GBM was reported. Patients who had undergone resection of 98% or more of their tumor volume had a significantly longer median OS (13 months, 95% confidence interval [CI] 11.4–14.6 months) than those who had undergone less than 98% (8.8 months, 95% CI 7.4–10.2 months) (p < 0.0001)(Lacroix et al., 2001). Based on these results, the standard care in the U.S. for patients with malignant gliomas had been maximal safe surgical resection followed by XRT and nitrosourea chemotherapy, commonly carmustine or lomustine (CCNU). More recently, a prospective analysis of surgical resection also demonstrated a survival benefit from maximal surgical resection, especially for patients in recursive partitioning analysis (RPA) classes IV and V (Pichlmeier et al., 2008).
The most significant advance in malignant glioma therapy since radiation therapy has been the administration of temozolomide (TMZ). A prospective randomized trial of 573 patients from 85 centers compared XRT alone with XRT plus TMZ, given concomitantly with and after XRT. The median OS was 14.6 months with XRT plus TMZ and 12.1 months with XRT alone with an unadjusted hazard ratio for death in the XRT-plus-TMZ group of 0.63 (95% CI, 0.52 to 0.75; P<0.001 by the log-rank test). Additionally, the two-year survival rate was 26.5% with XRT plus TMZ and 10.4% with XRT alone. Grade 3 or 4 hematologic toxic effects occurred in 7% of patients treated with XRT and TMZ (Stupp et al., 2005). Five year follow-up results of this study confirmed the benefits of adjuvant TMZ with radiotherapy (Stupp et al., 2009) Since the publication of these results, the standard of care for malignant gliomas has been maximal safe surgical resection followed by XRT plus TMZ, given concomitantly with and after XRT.
Although the median OS of 14.6 months following treatment with XRT and TMZ is a distinct improvement over the 6 months seen prior to the routine use of XRT, malignant gliomas remain a lethal tumor type. There have been significant advances in our basic understanding of tumor pathogenesis and the translation of these findings has resulted in a variety of treatment approaches. The following is a summary of the scientific findings that have resulted in phase I, II, or III clinical trials and the outcomes of those trials when available. Completed trials are listed in Table 1 and those still in progress are listed in Table 2. “Primary” GBM, AA or malignant gliomas are newly diagnosed tumors that have not been previously treated whereas “recurrent” tumors are those that have been previously treated, usually with surgery, XRT and either a nitrosourea or TMZ.
Table 1.
Summary of discussed clinical trials
| Historical Studies | XRT + TMZ | P | III | 287 | 24 | 58.4 | Stupp et al., 2005 | |||
| Meta analysis | R | II | 375 | 10 | 30 | Wong et al., 1999 | ||||
| Receptor tyrosine kinase and downstream signaling pathway inhibitors | EGFR | SMI | Erlotinib | +/− TMZ | R | I | 57 | 8 | N/A | Prados et al., 2006 |
| TMZ + XRT | P | I/II | 97 | 28.8 | 61.2 | Brown et al. 2008 | ||||
| Carboplatin | R | II | 43 | 9 | 30 | Groot et al., 2008 | ||||
| TMZ + XRT | P | II | 65 | 32.8 | 77.2 | Prados et al., 2009b | ||||
| v. TMZ/BCNU | R | II | 100 | 7.2 (Erlotinib); 9.6 (TMZ/BCNU) | 30.8 (Erlotinib); 29.2 (TMZ/BCNU) | van den Bent et al., 2009 | ||||
| Erlotinib/Gefitinib | R | I | 21 | 12.2 | 20.5 | Preusser et al., 2008 | ||||
| Gefitinib | P | II | 28 | 8.4 | 24.6 | Franceschi et al., 2007 | ||||
| R | II | 57 | 8.1 | 39.4 | Rich et al., 2004 | |||||
| Lapatinib | R | I/II | 24 | N/A | N/A | Thiessen et al., 2009 | ||||
| VEGFR | SMI | Cediranib | R | II | 16 | 15.9 | 30.1 | Batchelor et al., 2007 | ||
| Vatalanib | Imatinib + HU | R | I | 37 | 12 | 48 | Reardon et al., 2009 | |||
| TMZ + XRT | P | I | 19 | 18.4+ | N/A | Batchelor et al., 2009 | ||||
| TMZ + XRT | P | I/II | 22 | 27.7 | 69.2 | Brandes et al., 2009 | ||||
| PDGFR | SMI | Imatinib | HU | P | I | 30 | 10 | 19 | Dresemann, 2005 | |
| TMZ | R | I | 65 | 26.6 | 47.6 | Reardon et al., 2008 | ||||
| R | I/II | 105 | N/A | N/A | Wen et al., 2006 | |||||
| HU | R | II | 33 | 14.4 | 48.9 | Reardon et al., 2005 | ||||
| R | II | 112 | 7.2 (GBM); 7.6 (AOD/OA); 7.2 (AA/rLGA) | 23.6 (GBM); 21.2 (AOD/OA); 20 (AA/rLGA) | Raymond et al., 2008 | |||||
| HU | R | II | 39 | 10.9 | 33.3 | Desjardins et al., 2007 | ||||
| HU v. HU alone | R | III | 240 | 6 | 21 | Dresemann et al., 2009 | ||||
| PI3K/Akt/mTOR pathway | Macrolide | Sirolimus | R | I | 15 | N/A | N/A | Cloughesy et al., 2008 | ||
| Erlotinib/gefitinib | R | I | 28 | 12 | N/A | Doherty et al., 2006 | ||||
| Gefitinib | R | I | 34 | 8.2 | N/A | Reardon et al., 2006 | ||||
| Erlotinib | R | II | 32 | N/A | N/A | Reardon et al., 2010 | ||||
| Temsirolimus | R | II | 43 | 9 | N/A | Chang et al., 2005 | ||||
| R | II | 65 | 9.2 | 17.6 | Galanis et al., 2005 | |||||
| Everolimus | Gefitinib | R | I | 22 | 10.4 | 23.2 | Kreisl et al., 2009b | |||
| TMZ | P/R | I | 25 | N/A | N/A | Mason et al., 2009 | ||||
| Ras/MAPK pathway | SMI | Tipifarnib | R | I | 23 | 7 | N/A | Cloughesy et al., 2005 | ||
| XRT | P | I | 13 | N/A | 48 | Cohen-Jonathan Moyal et al., 2007 | ||||
| R | II | 89 | non-EIAED: 8 (AG), 9 (GBM); EIAED: 8 (AG), 6 (GBM) | N/A | Cloughesy et al., 2006 | |||||
| P | II | 28 | 6 | 30.8 | Lustig et al., 2008 | |||||
| Lonafarnib | TMZ | R | I | 15 | 14 | N/A | Gilbert et al., 2006 | |||
| PKC | SMI | Enzastaurin | R | I | 26 | 5.6 | 22.8 | Kreisl et al., 2009a | ||
| TMZ | P/R | I | 15 | N/A | N/A | Rampling et al., 2009 | ||||
| R | II | 85 | N/A | N/A | Fine et al., 2005 | |||||
| v. CCNU | R | III | 226 | 6 | 26.4 | Wick et al., 2010 | ||||
| Multi-target | SMI | Tandutinib | R | I | 19 | N/A | N/A | Supko et al., 2009 | ||
| Vandetanib | P | I | 13 | N/A | N/A | Drappatz et al., 2009 | ||||
| Sorafenib | R | I | 35 | N/A | N/A | Nabors et al., 2007 | ||||
| Erlotinib | R | I/II | 26 | N/A | N/A | Prados et al., 2009a | ||||
| Temsirolimus | R | I/II | 13 | N/A | N/A | Wen et al., 2009 | ||||
| Sunitinib | R | II | 21 | 6.4 | 15.2 | Neyns et al., 2009 | ||||
| XL184 | R | II | 46 | N/A | N/A | de Groot et al., 2009 | ||||
| R | II | 46 | N/A | N/A | DePrimo et al., 2009 | |||||
| R | II | 46 | N/A | N/A | Sorensen et al., 2009 | |||||
| Biological Therapies | Oncolytic Virus | HSV1716 | R\P | I | 12 | N/A | N/A | Harrow et al., 2004 | ||
| HSV-G206 | P | I | 21 | N/A | N/A | Markert et al., 2000 | ||||
| HSV-G207 | R | I | 9 | N/A | 30.4 | Karrasch et al., 2009 | ||||
| ONYX-015 | R | I | 24 | 6.5 | 24.8 | Chiocca et al., 2004 | ||||
| Reolysin | R | I | 12 | 4.3 | 21 | Forsyth et al., 2008 | ||||
| Gene Therapy | Ad5CMV-p53 | R | I | 15 | 13 | 43 | Lang et al., 2003 | |||
| Adenovirus-hIFN-β | R | I | 11 | 9.3 | 17.9 | Chiocca et al., 2008 | ||||
| HSV-tk | R | I | 2 | N/A | N/A | Izquierdo et al., 1997 | ||||
| P | III | 248 | 25.7 | 51.4 | Rainov, 2000 | |||||
| adenoviral HSV-tk | R\P | II | 17 | N/A | 62.4 | Immonen et al., 2004 | ||||
| lipo-HSV1-Tk | R | I\II | 8 | N/A | N/A | Reszka et al., 2005 | ||||
| IL13Rα2 | Receptor-ligand targeted toxins | IL13-PE38QQR | R | I | 51 | N/A | 42.7 | Kunwar et al., 2007 | ||
| R | I\II | 67 | N/A | 37.1 (intratumoral); 45.9 (peritumroal) | Prados et al., 2005 | |||||
| R | III | 184 | 17.7 | 36.4 | Debinski and Tatter, 2009 | |||||
| IL-4R | IL-4PE | R | I | 9 | N/A | N/A | Rand et al., 2000 | |||
| R | I | 31 | N/A | 32.8 | Weber et al., 2003 | |||||
| EGFR | TP-38 | R | II | 20 | N/A | 23 | b has shown effective | |||
| R | II | 15 | 14.9 | 28 | Sampson et al., 2008 | |||||
| TfR | Tf-CRM107 | R | III | 323 | N/A | N/A | Celtic Pharma, 2007 | |||
| MMP-2 | TM-601 | R | I | 18 | N/A | 27 | Mamelak et al., 2006 | |||
| EGFR | Antibody | cetuximab | R | II | 58 | 7.9 | 20 | Neyns et al., 2009 | ||
| Nimotuzumab | P | I\II | 29 | N/A | 88.68 | Ramos et al., 2006 | ||||
| 188Re- nimotuzumab | R | I | 9 | N/A | N/A | Torres et al., 2008 | ||||
| mAb-425 | P | II | 180 | N/A | 90 (GBM); 260 (AA) | Quang and Brady, 2004 | ||||
| VEGF | Antibody | Bevicizumab | Irinotecan | R | I | 21 | N/A | N/A | Stark-Vance, 2005 | |
| R | II | 48 | 16 | 31 | Kreisl et al., 2008 | |||||
| R | II | 85 | N/A | 38.8 (bevacizuma b); 35.6 (irinotecan) | Cloughesy et al., 2008 | |||||
| R | II | 85 (beva cizu mab); 82 (beva cizu mb + irinot ecan) | 16.8 (bevacizumab); 22.4 (bevacizumab + irinotecan) | 36.8 (bevacizuma b); 34.8 (bevacizuma b + irinotecan) | Friedman, et al., 2009 | |||||
| Etoposide (metronomic) | R | II | 59 | N/A | 63.2 (AA), 44.4 (GBM) | Reardon et al., 2009 | ||||
| Irinotecan | R | II | 32 | 23 | 40 (GBM); N/A (AA) | Vredenburgh et al., 2007 | ||||
| Irinotecan | R | II | 19 | 16.8 | 28 | Bokstein et al., 2008 | ||||
| Irinotecan | R | II | 55 | 19.3 | N/A | Norden et al., 2008 | ||||
| Irinotecan | R | II | 82 | 22.4 | 34.8 | Friedman, et al., 2009 | ||||
| TMZ + XRT | P | II | 10 | N/A | N/A | La et al., 2008 | ||||
| TMZ + XRT | P | II | 16 | 48 | 64 | Liebross et al., 2009 | ||||
| TMZ + XRT | P | II | 72 | N/A | N/A | Kirkpatrick et al., 2009 | ||||
| Decoy receptor | Aflibercept | R | II | 48 | N/A | N/A | De Groot et al., 2008 | |||
| Tenascin | Antibody | 131I-81C6 | TMZ + XRT | P | II | 15 | N/A | 90.6 | Reardon et al., 2008 | |
| BC-4 | R | II | 48 | N/A | 44 | Paganelli et al., 1999 | ||||
| P | II | 37 | 336 | N/A (AA); 134 (GBM) | Grana et al., 2002 | |||||
| TMZ | R | II | 38 | 20 (BC4); (40 BC4 + TMZ) | 70 (BC4); 100 (BC4 + TMZ) | Bartolomei et al., 2004 | ||||
| Histone H1 | Antibody | Cotara | R\P | I/II | 12 | N/A | 37.9 | Patel et al., 2005 | ||
| Immunotherapy | WCL | ATV-NDV | P | II | 23 | 40 | 100 | Steiner et al., 2004 | ||
| Formalin fixed lysate | R | II | 12 | N/A | 42.8 | Ishikawa et al., 2007 | ||||
| Vitespen | R | II | 12 | N/A | 42 | Wood and Mulders, 2009 | ||||
| HUVEC | R | II | 9 | N/A | N/A | Okaji et al., 2008 | ||||
| Peptide vaccine | CDX-110 | P | II | 12 | 56.8 | 104 | Heimberger et al., 2006 | |||
| TMZ | P | II | 8 | 60.8 | 92.8 | Heimberger and Sampson, 2009 | ||||
| WT1 | R | II | 21 | 20 | N/A | Izumoto et al., 2008 | ||||
| multiple peptide | P | I | 17 | 13.3 | 88.8 | Yajima et al., 2005 | ||||
| Dendritic cell therapy | Lysate-pulsed DC | R | I | 8 | N/A | 133 | Yu et al., 2004 | |||
| Lysate-pulsed DC + IL-4 | R | I | 5 | 6 | N/A | Okada et al., 2007 | ||||
| mRNA-pulsed DC | R | I | 7 | N/A | N/A | Caruso et al., 2004 | ||||
| Fused DC − glioma cells | R | I | 8 | N/A | N/A | Kikuchi et al., 2001 | ||||
| Fused DC − glioma cells + IL-12 | R | II | 15 | N/A | N/A | Kikuchi et al., 2004 | ||||
| Lysate-pulsed DC | R | I/II | 24 | N/A | 68.5 | Yamanaka, et al., 2005 | ||||
| Lysate-pulsed DC | R | II | 56 | 12 | 38.4 | De Vleeschouwer et al., 2008 | ||||
| Immune system stimulants | poly ICIC | P | II | 30 | 18 | 69.4 | Butowski et al., 2008 | |||
| R | II | 45 | N/A | 43 | Butowski et al., 2009 | |||||
| CpG ODNs | R | II | 24 | N/A | 28.8 | Carpentier et al., 2006 | ||||
| AP 12009 | R | II | 12 | N/A | 136 (AA), N/A (GBM) | Bogdahn et al., 2008a, Bogdahn et al., 2008b | ||||
| Adoptive immune therapy | PBMCs | R | I | 4 | N/A | N/A | Steinbok et al., 1984 | |||
| LAK + IL-2 | R | I | 10 | N/A | N/A | Jacobs et al., 1986 | ||||
| R | I | 23 | N/A | N/A | Yoshida et al., 1988 | |||||
| R | I | 15 | N/A | 53 | Hayes, et al., 1995 | |||||
| R | I | 9 | N/A | N/A | Barba et al., 1989 | |||||
| R | I | N/A | N/A | N/A | Blacklock and Grimm, 1989 | |||||
| R | I | 13 | N/A | N/A | Merchant et al., 1988 | |||||
| R | II | 11 | 42 | 63 | Lillehei et al., 1991 | |||||
| LAK infusion | R | II | 31 | N/A | 70 | Dillman et al., 2004 | ||||
| P | II | 33 | N/A | 82 | Dillman et al., 2009 | |||||
| MAK | R | I | 19 | N/A | 51 (mean) | Ingram, et al., 1987 | ||||
| R | II | 83 | N/A | N/A | Ingram et al., 1990 | |||||
| R | II | 16 | N/A | 30 | Jeffes et al., 1993 | |||||
| CTL | R | I | 5 | N/A | N/A | Kitahara et al., 1987 | ||||
| R | I | 5 | N/A | N/A | Virasch and Kruse, 2001 | |||||
| Other antiangiogenic therapies | CD36R | CD36R agonist | ABT-510 | P | I | 23 | 31.4 | 60.3 | Kekan et al., 2009 | |
| COX-2, ? | COX-2 inhibitors | Rofecoxib | TMZ + XRT | P | I | 13 | 32 | 64 | Tuettenberg et al., 2004 | |
| Pioglitazone | R | I | 14 | 7 | N/A | Hau et al., 2007 | ||||
| Celecoxib | P | II | 35 | N/A | 64 (EIAED); 46 (non- EIAED) | Grossman et al., 2008 | ||||
| 13-cis-retinoic acid | R | II | 25 | 24 | N/A | Levin et al., 2006a | ||||
| Irinotecan | R | II | 37 | 11 | 31.5 | Reardon et al., 2005b | ||||
| TMZ + Thalidomide | R | II | 50 | 23.6 | 50.4 | Kesari et al., 2008 | ||||
| HIF-1α | HIF-1α inhibitors | 2ME2 | R | II | 16 | N/A | N/A | Kirkpatrick et al., 2007 | ||
| Thalidomide and derivatives | Thalidomide | R | II | 36 | 10 | 28 | Fine et al., 2000 | |||
| R | II | 38 | N/A | 31 | Marx et al., 2001 | |||||
| R | II | 18 | N/A | 10 | Short et al., 2001 | |||||
| P | II | 44 | N/A | 63 (Thalidomide ); 103 (Thalidomide + TMZ) | Baumann et al., 2004 | |||||
| BCNU | R | II | 40 | N/A | 14.2 | Fine et al., 2003 | ||||
| Cyclophosphamide + etoposide + celecoxib | R | II | 48 | 26 (AA); 11 (GBM) | 41.5 (AA), 21 (GBM) | Kesari et al., 2007 | ||||
| Irinotecan | P/R | II | 26 | 16 (P); 8 (R) | N/A | Fadul et al., 2008 | ||||
| Irinotecan | R | II | 32 | 13 | 36 | Puduvalli et al., 2008 | ||||
| TMZ | R | II | 43 | 15 | N/A | Groves et al., 2007 | ||||
| TMZ | P | II | 67 | 22 | 73 | Chang et al., 2004 | ||||
| TMZ | P | II | 23 | N/A | 48 (Thalidomide ); 52 (Thalidomide + TMZ) | Riva et al., 2007 | ||||
| Lenalidomide | R | I | 27 | 7 | 24 | Fine et al., 2007 | ||||
| P | I | 20 | 20 | 44 | Drappatz et al., 2009 | |||||
| Anti-invasive agents | Integrin inhibitors | Cilengitide | R | II | 41 (500 mg); 40 (2000 mg) | 7.9 (500mg); 8.1 (2000mg) | 26 (500mg); 39.6 (2000mg) | Reardon et al., 2008 | ||
| R | I | 51 | N/A | 22.4 | Nabors et al., 2007 | |||||
| TMZ | P | I/II | 52 | N/A | N/A | Stupp et al., 2007 | ||||
| MMP antagonists | Marimastat | P | II | 83 | 17.1 | 42.9 | Levin et al., 2006 | |||
| TMZ | R | II | 44 | 12 | 45 | Groves et al., 2002 | ||||
| TMZ | R | II | 46 | 24 | 69 | Groves et al., 2006 | ||||
| Prinomastat | TMZ | II | 43 | 25.2 | 60 | Levin et al., 2002 | ||||
| Epigenetic therapies | MGMT inhibitor | O6-BG | TMZ | R | I | 38 | N/A | N/A | Quinn et al., 2005 | |
| TMZ | R | I | 28 | N/A | N/A | Quinn et al., 2009b | ||||
| BCNU | R | II | 18 | N/A | N/A | Quinn et al., 2002 | ||||
| BCNU | R | II | 52 | N/A | 50.3 | Quinn et al., 2009a | ||||
| TMZ | R | II | 66 | 7.9 | 24.7 | Quinn et al., 2009c | ||||
| HDAC inhibitor | SAHA | R | II | 52 | N/A | 22.8 | Galanis et al., 2009 | |||
| Cellular development, maintenance, and apoptosis | Topoisomerase inhibitors | Irinotecan | BCNU | R | I | 73 | N/A | N/A | Quinn et al., 2004 | |
| TMZ | R | I | 31 | N/A | N/A | Loghin et al., 2007 | ||||
| TMZ | R | I | 106 | 11.7 | N/A | Reardon et al., 2005 | ||||
| BCNU | R\P | II | 76 | N/A | 51.3 (P); | Reardon et al., 2004 | ||||
| 31.3 (R) | ||||||||||
| BCNU | R | II | 42 | 35.3 | 46.8 | Brandes et al., 2004 | ||||
| TMZ | P | II | 22 | 30.8 | 51.2 | Fountzilas et al., 2006 | ||||
| TMZ | R | II | 32 | 29 (AA); 22 (GBM) | N/A | Gruber and Buster, 2004 | ||||
| VM-26 | Irinotecan | R | II | 25 | N/A | N/A | Feun et al., 2007 | |||
| Gimatecan | R | II | 29 | 12 | N/A | Hu et al., 2009 | ||||
| RTA 744 | P | I | 20 | 6 | N/A | Conrad et al., 2007 | ||||
| PEG-DOX | R | I | 40 | N/A | 74 | Hau et al., 2004 | ||||
| RTA 744 | R | I | 20 | N/A | N/A | Kazerooni et al., 2007 | ||||
| Proteasome inhibitors | Bortezomib | TMZ | R\P | I | 27 | N/A | 17.4 | Kubicek et al., 2009 | ||
| BCL-2 inhibitors | AT-101 | TMZ + XRT | P | I | 16 | N/A | 15.2–18.2 | Fiveash et al., 2009 |
Tumors categorized as primary (P), progressive/recurrent (R), or both (P/R)
Median PFS and OS published in months converted to weeks (x4)
2ME2: 2-methoxyestradiol; AA: anaplastic astrocytoma; Ad5CMV-p53: p53 adenovirus; Adenovirus-hIFN-β: human interferon beta adenovirus; AdV: adenoviral vector; ATV-NDV: Newcastle virus; BC-4: anti-tenascin monoclonal antibody; BCL-2: B-cell lymphoma 2 protein; BCNU: carmustine; CCNU: lomustine CD36R: CD36 receptor; CDX-110: EGFRvIII vaccine; CMV: cytomegalovirus; COX-2: cyclooxygenase-2; CTL: cytotoxic T lymphocyte; DC: dendritic cell; EGFR: epidermal growth factor receptor; HDAC: histone deacetylase; HIF-1α: hypoxia-inducible factor 1 alpha; HSV: herpes simplex virus; HSV-tk: herpes simplex virus thymidine kinase; HU: hydroxyurea; 131I-81C6: IL-2: interleukin-2; IL4-PE: IL-4 conjugated pseudomonas exotoxin; IL-4R: interleukin-4 receptor; IL13-PE38QQR: IL-13 conjugated pseudomonas exotoxin; IL13Rα2: interleukin-13 receptor alpha 2; LAK: lymphokine-activated killer cell; Lipo-HSV-tk: liposome herpes simplex virus-tk; mAb: monoclonal antibody; MAK: mitogen-activated killer cells; MAPK: mitogen-activated protein kinase; MGMT: O-6-methylguanine-DNA methyltransferase; MMP: matrix metalloproteinase;; mOS: median overall survival; mPFS: median progression free survival; mTOR: mammalian target of rapamycin; mTTP: median time to progression; NP GBM: non-progressive GBM; PEG-DOX: pegylated doxorubicin; PDGFR: platelet-derived growth factor receptor; PI3K: phosphoinositide 3-kinase; PKC: protein kinase C; poly ICIC: polyriboinosinic-polyribocytidylic acid-polylysine carboxymethylcellulose; SAHA: suberoylanilide hydroxamic acid; SMI: small molecular inhibitor; TfR: transferring receptor; TMZ: temozolomide; TP38-PE: transforming growth factor alpha-conjucated pseudomonas exotoxin; VEGF: vascular endothelial growth factor; VEGFR: vascular endothelial growth factor receptor; VM-26: teniposide; WCL: whole-cell lysate; XRT: radiation therapy.
Table 2. Ongoing clinical trials in patients with malignant gliomas.
Search conducted using clinicaltrials.gov using query “glioma [drug name].”
| Receptor tyrosine kinase and downstream signaling pathway inhibitors | EGFR | SMI | Erlotinib | I | R | NCT00227032 | |
| Dasatinib | I | R | NCT00609999 | ||||
| I/II | R | NCT00301418 | |||||
| Sirolimus | I/II | R | NCT00509431 | ||||
| Sorafenib | I/II | R | NCT00335764 | ||||
| Temsirolimus | I/II | R | NCT00112736 | ||||
| XRT | I/II | P | NCT00124657 | ||||
| II | R | NCT00054496 | |||||
| Bevacizumab | II | R | NCT00671970 | ||||
| Bevacizumab | II | P | NCT00720356 | ||||
| Bevacizumab + TMZ | II | P | NCT00525525 | ||||
| Sirolimus | II | R | NCT00672243 | ||||
| TMZ + XRT | II | P | NCT00274833 | ||||
| TMZ + XRT | II | P | NCT00039494 | ||||
| TMZ + XRT | II | P | NCT00187486 | ||||
| TMZ/BCNU | II | R | NCT00086879 | ||||
| XRT | II | P/R | NCT00360854 | ||||
| Gefitinib | Irinotecan | I | R | NCT00132158 | |||
| Everolimus | I/II | R | NCT00085566 | ||||
| XRT | I/II | P | NCT00052208 | ||||
| XRT | II | P | NCT00042991 | ||||
| Lapatinib | II | R | NCT00095940 | ||||
| Pazopanib | II | R | NCT00350727 | ||||
| BIBW-2992 | XRT +/− TMZ | I | P | NCT00977431 | |||
| TMZ | II | R | NCT00727506 | ||||
| VEGFR | SMI | Cediranib | I | R | NCT00326664 | ||
| Cilengitide | I | R | NCT00979862 | ||||
| Bevacizumab | I | R | NCT00458731 | ||||
| TMZ + XRT | I/II | P | NCT00662506 | ||||
| II | R | NCT00305656 | |||||
| CCNU | III | R | NCT00777153 | ||||
| Vatalanib | TMZ + XRT | I | P | NCT00385853 | |||
| TMZ + XRT | I/II | P | NCT00128700 | ||||
| PDGFR | SMI | Imatinib | TMZ | I | P/R | NCT00354068 | |
| Sirolimus + | I | R | NCT00613132 | ||||
| HU | |||||||
| I/II | R | NCT00049127 | |||||
| II | R | NCT00039364 | |||||
| Vandetanib + HU | II | R | NCT00613054 | ||||
| HU | II | R | NCT00615927 | ||||
| PI3K/Akt/mTOR pathway | Macrolide | Sirolimus | Vandetanib | I | R | NCT00821080 | |
| TMZ | I | P/M | NCT00784914 | ||||
| Erlotinib | I/II | R | NCT00509431 | ||||
| I/II | P | NCT00411619 | |||||
| Erlotinib | II | R | NCT00672243 | ||||
| III | R | NCT00789828 | |||||
| Temsirolimus | TMZ | I | P/M | NCT00784914 | |||
| TMZ + XRT | I | P | NCT00316849 | ||||
| Perifosine | I/II | R | NCT01051557 | ||||
| Erlotinib | I/II | R | NCT00112736 | ||||
| Sorafenib, Erlotinib, Tipifarnib | I/II | R | NCT00335764 | ||||
| Sorafenib | I/II | R | NCT00329719 | ||||
| TMZ + XRT | II | P | NCT01019434 | ||||
| Bevacizumab | II | R | NCT00800917 | ||||
| Everolimus | Imatinib + HU | I | R | NCT00613132 | |||
| TMZ | I | P/R | NCT00387400 | ||||
| I/II | P | NCT00411619 | |||||
| TMZ + XRT | I/II | P | NCT00553150 | ||||
| Gefitinib | I/II | P | NCT00085566 | ||||
| AEE788 | I/II | R | NCT00107237 | ||||
| II | R | NCT00782626 | |||||
| II | R | NCT00823459 | |||||
| II | R | NCT00831324 | |||||
| TMZ + XRT, then bevacizumab | II | P | NCT00805961 | ||||
| Ras/MAPK pathway | SMI | Tipifarnib | TMZ + XRT | I | P | NCT00049387 | |
| XRT | I/II | P | NCT00079339 | ||||
| Sorafenib, Erlotinib, Temsirolimus | I/II | R | NCT00335764 | ||||
| XRT | II | P | NCT00209989 | ||||
| Lonafarnib | TMZ | I | R | NCT00083096 | |||
| TMZ | I | R | NCT00102648 | ||||
| PKC | SMI | Enzastaurin | I | R | NCT00112788 | ||
| TMZ | I | P | NCT00516607 | ||||
| Carboplatin | I | R | NCT00438997 | ||||
| I | P | NCT00503724 | |||||
| Bevacizumab | II | R | NCT00559923 | ||||
| Bevacizumab | II | R | NCT00586508 | ||||
| XRT | II | P | NCT00509821 | ||||
| v. CCNU | III | R | NCT00295815 | ||||
| Multi-target | SMI | Dasatinib | Vandetanib + XRT | I | P | NCT00996723 | |
| Erlotinib | I | R | NCT00609999 | ||||
| Bevacizumab | I/II | R | NCT00892177 | ||||
| TMZ + XRT | I/II | P | NCT00895960 | ||||
| CCNU | I/II | R | NCT00948389 | ||||
| II | R | NCT00423735 | |||||
| Pazopanib | I | R | NCT00929903 | ||||
| Lapatinib | II | R | NCT00350727 | ||||
| Sorafenib | I | P | NCT00884416 | ||||
| I | R | NCT00093613 | |||||
| XRT | I | P/M | NCT00639262 | ||||
| TMZ + XRT | I/II | P | NCT00734526 | ||||
| Erlotinib, Tipifarnib, or Temsirolimus | I/II | R | NCT00335764 | ||||
| Temsirolimus | I/II | R | NCT00329719 | ||||
| TMZ | II | R | NCT00597493 | ||||
| TMZ + XRT | II | P | NCT00544817 | ||||
| Bevacizumab | II | R | NCT00621686 | ||||
| Sunitinib | Irinotecan | I | R | NCT00611728 | |||
| II | R | NCT00923117 | |||||
| II | R | NCT00713388 | |||||
| II | R | NCT00499473 | |||||
| II | R | NCT00606008 | |||||
| II | R | NCT00535379 | |||||
| N/A | R | NCT00864864 | |||||
| Tandutinib | I/II | R | NCT00379080 | ||||
| Bevacizumab | II | R | NCT00667394 | ||||
| Vandetanib | Etoposide | I | R | NCT00613223 | |||
| Dasatinib + XRT | I | P | NCT00996723 | ||||
| XRT | I | P | NCT00472017 | ||||
| I | R | NCT00721292 | |||||
| XRT | I | R | NCT00822887 | ||||
| Sirolimus | I | R | NCT00821080 | ||||
| I/II | R | NCT00293566 | |||||
| TMZ + XRT | I/II | P | NCT00441142 | ||||
| Carboplatin | II | R | NCT00995007 | ||||
| Imatinib | II | R | NCT00613054 | ||||
| XL184 | TMZ + XRT | I | P | NCT00960492 | |||
| II | R | NCT00704288 | |||||
| Biological Therapies | Oncolytic virus | Reolysin | I/II | R | NCT00528684 | ||
| MV-CEA | I | R | NCT00390299 | ||||
| Delta-24- RGD | Surgery | I | R | NCT00805376 | |||
| Gene therapy | AdV-tk | Valacyclovir | I | P | NCT00751270 | ||
| II | P | NCT00589875 | |||||
| II | N/A | NCT00870181 | |||||
| MMP-2 | Receptor-ligand targeted toxins | TM-601 | I | R | NCT00591058 | ||
| I/II | R | NCT00683761 | |||||
| II | R | NCT00114309 | |||||
| EGFR | Antibody | Nimotuzumab | TMZ + XRT | III | P | NCT00753246 | |
| MAb-425 | TMZ + XRT | II | P | NCT00589706 | |||
| Cetuximab | Irinotecan | II | P | NCT01012609 | |||
| TMZ + XRT | II | P | NCT00311857 | ||||
| TMZ + XRT | II | P | NCT01044225 | ||||
| VEGF | Antibody | Bevacizumab | Fosbretabulin | I | R | NCT01052363 | |
| I | R | NCT00968240 | |||||
| Pabinostat | I/II | R | NCT00859222 | ||||
| metronic TMZ | II | R | NCT00501891 | ||||
| Erlotinib | II | R | NCT00671970 | ||||
| BCNU + TMZ + XRT | II | P | NCT00660621 | ||||
| Irinotechan + Carboplatin | II | R | NCT00953121 | ||||
| Tandutinib | II | R | NCT00667394 | ||||
| Bortezomib | II | R | NCT00611325 | ||||
| BCNU | II | R | NCT00795665 | ||||
| Enzasturin | II | R | NCT00559923 | ||||
| Irinotecan + BCNU | II | R | NCT00735436 | ||||
| TMZ + topotechan + XRT | II | P | NCT01004874 | ||||
| TMZ + irinotechan | II | P | NCT00612339 | ||||
| Irinotechan | II | R | NCT00921167 | ||||
| TMZ | II | R | NCT00883298 | ||||
| TMZ or irinotechan | II | P | NCT00817284 | ||||
| TMZ + XRT | II | P | NCT00590681 | ||||
| III | P | NCT00943826 | |||||
| TMZ + XRT | III | P | NCT00884741 | ||||
| TMZ + XRT | I | P | NCT00650923 | ||||
| Decoy receptor | Aflibercept | II | R | NCT00369590 | |||
| II | R | NCT00427440 | |||||
| SF/HGF | Antibody | AMG-102 | II | R | NCT00677716 | ||
| Histone H1 | Antibody | Cotara | TMZ | II | P | NCT00458601 | |
| Immunotherapy | EGFRvIII peptide vaccine | CDX-110 | TMZ + XRT | II | P | NCT00639639 | |
| Dendritic cell therapy | CMV DC | TMZ + XRT | II | P | NCT01006044 | ||
| Pep DC | TMZ + XRT | II | P | NCT00045968 | |||
| TMZ + XRT | I | P | NCT00890032 | ||||
| RNA DC | TMZ + XRT | I/II | P | NCT00846456 | |||
| v. TMZ/BCNU | III | R | NCT00761280 | ||||
| Immune system stimulants | AP 12009 | v. Prolifeprosan + Gliadel Wafer | II | P | NCT00814593 | ||
| Adoptive immune therapy | LAK cells | I/II | R | NCT00990496 | |||
| CTL | Bevacizumab + irinotecan | I | R | NCT00762255 | |||
| Epigenetic therapies | HDAC | HDAC inhibitors | Vorinostat | Bevacizumab + TMZ | I\II | R | NCT00939991 |
| carboplatin + isotretinoin | I\II | R | NCT00555399 | ||||
| Vorinostat | II | R | NCT00641706 | ||||
| II | R | NCT00238303 | |||||
| I\II | R | NCT00085540 | |||||
| Romidepsin | TMZ + XRT | II | P | NCT00313664 | |||
| Valproic acid | II | R | NCT00679354 | ||||
| Anti-invasive agents | Integrin | Integrin inhibitors | Celingitide | II | R | NCT00093964 | |
| Cediranib | II | R | NCT00979862 | ||||
| TMZ + XRT | II | P | NCT00813943 | ||||
| TMZ + XRT | II | P | NCT00689221 | ||||
| TMZ + XRT | II | P | NCT01044225 | ||||
| I | R | NCT00822458 | |||||
| Cellular development, maintenance, and apoptosis | SHH signaling | SMI | GDC-0449 | II | R | NCT00939484 | |
| Surgery | II | R | NCT00980343 |
Tumors categorized as primary (P), progressive/recurrent (R), or both (P/R)
+: with; −: without; AA: anaplastic astrocytomas; AdV-tk: Adenovirus mediated herpes simplex virus thymidine kinase; CCNU: lomustine; CNS: central nervous system; Cotara: 131I-chTNT-1/BmAb; CMV DC: Cytomegalovirus activated dendritic cell; CTL: cytotoxic T lymphocytes; EIAED: enzyme-inducing antiepileptic drug; EGFR: epidermal growth factor receptor; GBM: glioblastoma multiforme; HDAC: Histone deacetylase; HDACi: Histone deacetylase inhibitor; LAK: lymphokine activated killer cell; MGMT: O-6-methylguanine-DNA methyltransferase; MMP-2: matrix metalloproteinase– 2; mTOR: mammalian target of rapamycin; MV-CEA: measles virus producing carcinoembryonic antigen; NCT ID: national clinical trial identifier; Pep DC: peptide activated dendritic cell; PDGFR: platelet-derived growth factor receptor; PI3K: phosphoinositide 3-kinase; PKC: protein kinase C; RNA DC: RNA-activated dendritic cell; SF/HGF: scatter factor/hepatocyte growth factor; SHH: sonic hedgehog homolog; SMI: small molecule inhibitor; TMZ: temozolomide; v.: versus; VEGF: vascular endothelial growth factor; VEGFR: vascular endothelial growth factor receptor; XRT: radiotherapy
2. Receptor tyrosine kinase and downstream signaling pathway inhibitors
Receptor tyrosine kinases (RTK) are high affinity cell surface receptors for a variety of polypeptide growth factors, cytokines and hormones. The majority of RTKs exist as single subunit receptors that have an extracellular N-terminal ligand binding domain, a hydrophobic transmembrane spanning domain, and a kinase containing intracellular C-terminal domain. Ligand binding causes subunit dimerization and receptor autophosphorylation in which a phosphate group is transferred from adenosine triphosphate (ATP) to a cytoplasmic domain tyrosine. This leads to receptor activation, initiation of downstream signaling cascades, and changes in gene transcription. Under physiologic conditions, they are key regulators of normal cellular processes. Under pathologic conditions, however, they are critically involved in the development and growth of tumors. RTKs are therefore logical targets for the treatment of cancers.
2.1. Epidermal growth factor receptor
The most studied RTK for malignant glioma therapy is the epidermal growth factor receptor (EGFR). EGFR and its downstream signaling pathways – primarily Ras/MAPK and PI3K/Akt/mTOR – play important roles in regulating cell survival, proliferation, angiogenesis, migration, and tumorigenicity (Figure 1). EGFR is amplified or overexpressed in over 40% of GBMs (Maher et al., 2001). Furthermore, 25% of GBMs express a constitutively active mutant EGFR, EGFRvIII, which lacks the extracellular ligand-binding domain (van den Bent et al., 2009). In preclinical mouse models of human xenografts, EGFRvIII expression is associated with increased tumorigenicity (Nishikawa et al., 1994). EGFR overactivity or overexpression is also associated with increased PI3K/Akt/mTOR and Ras/MAPK signaling and may in this way promote tumor growth and inhibit apoptosis (Chan, 2004). Due to its specificity, EGFR inhibition is an attractive target in the treatment of malignant gliomas.
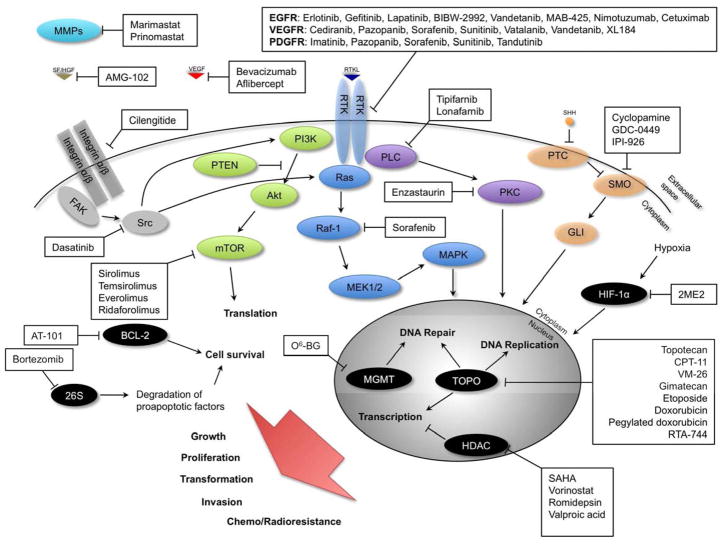
Figure 1. Molecular targeted therapies.
Signaling pathways and their constituent molecules implicated in glioma transformation, growth, proliferation, invasion, and resistance to chemotherapy and XRT. Locations of action of experimental therapies targeting specific molecules and/or signaling pathways are indicated.
Arrows represent activation, bars represent inhibition.
2ME2: 2-methoxyestradiol; 26S: 26S proteasome; Akt: protein kinase B; BCL-2: B-cell lymphoma protein 2; CPT-11: Irinotecan; EGF: epidermal growth factor; CTL: CMV-specific cytotoxic T cells; EGFR: epidermal growth factor receptor; FAK: focal adhesion kinase; GLI: Gli transcription factor family; HDAC: histone deacetylase; HIF-1α: hypoxia-inducible factor 1 alpha; MAPK: mitogen-activated protein kinase; MEK1/2: mitogen-activated protein kinase kinase; MGMT: O-6-methylguanine-DNA methyltransferase; MMP-2: matrix metalloproteinase 2; mTOR: mammalian target of rapamycin; O6-BG: O6-benzylguanine; PDGFR: platelet-derived growth factor receptor; PI3K: phosphoinositide 3-kinase; PKC: protein kinase C; PLC: phospholipase C; PTC: patched hedgehog receptor; PTEN: phosphatase and tensin homolog; Ras: Ras GTPase; Raf: serine/threonine kinase; Rb: retinoblastoma protein; RTK: receptor tyrosine kinase; RTKL: receptor tyrosine kinase ligand; SAHA: suberoylanilide hydroxamic acid; SF/HGF: scatter factor/hepatocyte growth factor; SHH: sonic hedgehog homolog; SMO: smoothened hedgehog receptor; Src: sarcoma tyrosine kinase; TOPO: topoisomerase; VEGF: vascular endothelial growth factor; VEGFR: vascular endothelial growth-factor; VM-26: teniposide.
Erlotinib, Gefitinib, Lapatinib
Three small molecule inhibitors of EGFR, erlotinib, gefitinib, and lapatinib, have been evaluated preclinically and clinically for the treatment of malignant gliomas. These inhibitors act as ATP mimetics, binding to the ATP-binding site on the intracellular domains and inhibiting receptor autophosphorylation and activation (Raizer, 2005; van den Bent et al., 2009). Lapatinib is also a dual inhibitor of EGFR and Her2/neu kinases (Eskens et al., 2008; Geyer et al., 2006).
EGFR small molecule inhibitors have shown promise in preclinical models. Erlotinib inhibition of EGFR has been shown to induce cell cycle arrest and apoptosis in human tumors in vitro and in vivo in mouse xenograft models (Chinnaiyan et al., 2003; Moyer et al., 1997; Pollack et al., 1999; Raizer, 2005). In addition, gefitinib has been shown to sensitize U251 MG cells to radiation treatment in vitro (Raizer, 2005; Stea et al., 2003). Lapatinib has been demonstrated to inhibit proliferation and migration, and promote apoptosis of U87 and M059K human MG cells in vitro (Giannopoulou et al., 2009). Erlotinib and gefitinib have been approved by the FDA for use in patients with advanced or metastatic non-small cell lung cancer (NSCLC) (Raizer, 2005).
In recent clinical trials, however, these drugs have shown limited efficacy as single-agent therapies for malignant gliomas. A randomized phase II study compared erlotinib monotherapy to TMZ or BCNU and found erlotinib to be less effective than either: six-month progression free survival (PFS) was 11.4% in the erlotinib arm, versus 24% in the TMZ or BCNU arm. Three point seven percent of patients in the erlotinib arm experienced partial response and 16.7% experienced stable disease while 9.6% of patients in the TMZ or BCNU arm experienced partial response and 34.6% experienced stable disease. Similar OS rates were observed in both arms. Median PFS and OS were 1.8 and 7.7 months, respectively in the erlotinib arm and 2.4 and 7.3 months, respectively in the TMZ/BCNU arm. Within the erlotinib arm, 50% of patients with low phospho-Akt (p-Akt) expression had a 6 month PFS, whereas no correlation was observed between p-Akt expression and PFS in the control arm. Furthermore, EGFRvIII expression correlated with poor OS in patients treated with erlotinib, but not in the control arm (P = .003 vs. P = .714). No patients whose tumors co-expressed EGFRvIII and PTEN had a 6 month PFS. Grade 2 or greater skin toxicity in response to erlotinib was associated with improved PFS and OS (van den Bent et al., 2009).
Single-agent erlotinib therapy was recently evaluated in phase I and II NABTC trials. In the phase I trial which included 32 patients (30 with recurrent MG, one atypical meningioma, and one non-progressive GBM), DLTs included grade 3 rash and grade 3 deep venous thrombosis and pulmonary embolism (Raizer et al., 2010b). In the phase II trial which included 104 patients, 96 of whom were evaluable for response, the most common DLTs were grade 3 rash, fatigue, neutropenia, and gastrointestinal toxicities. One patient experienced grade 4 hypomagnesemia and another patient experienced grade 5 seizure. Among evaluable patients with recurrent GBM, median PFS and OS were 2 months and 6 months, respectively. Among evaluable patients with AG, median PFS and OS were 2 months and 7 months, respectively. Six month PFS rates for recurrent GBM and AG patients were 3% and 27%, respectively. Within these two groups, one patient (6.7%) with AG achieved complete response and another (6.7%) achieved partial response. Disease stabilization was observed in three (7.9%) GBM and two (13.3%) AG patients. Among patients with non-progressive GBM, one-year PFS, one-year OS, and median OS were 9%, 53%, and 14 months, respectively (Raizer et al., 2010a). Erlotinib in combination with bevacizumab was assessed in a phase II trial in 56 patients with heavily pretreated recurrent MG (24 GBM, 32 AG) and was shown to have modest clinical efficacy. Severe toxicities included pulmonary embolism, intestinal perforation, ischemic stroke, gastric bleeding, nasal perforation, and grade 3 rash. Six month PFS was 25% and 50% for GBM and AG, respectively (Sathornsumetee et al., 2009).
A phase II trial of gefitinib monotherapy for recurrent GBM resulted in a 6 month PFS of 13.2%. Median event free survival (EFS) was 8.1 weeks and median OS was 39.4 weeks. Forty two percent of patients achieved stable disease. Although EGFR expression did not correlate with EFS or OS, diarrhea was found to predict favorable OS (Rich et al., 2004). Another phase II study of gefitinib in progressive high-grade gliomas yielded a 6 month PFS of 14.3% and a 12 month PFS of 7.1%. Seventeen point nine percent of patients experienced stable disease. The median PFS and OS for all patients in the study were 8.4 and 24.6 weeks, respectively. EGFR and p-Akt expression did not predict response to gefitinib (Franceschi et al., 2007).
An exploratory study of erlotinib or gefitinib monotherapy for recurrent or progressive malignant glioma observed a 19% 6 month PFS rate in patients treated with either gefitinib or erlotinib. Partial response was observed in 1/18 (5.6%) patients receiving erlotinib and in 2/3 (66.7%) of patients receiving gefitinib. Median TTP and survival time for all patients were 3.05 months and 5.13 months, respectively. Six month PFS and OS at 6 months were 19% and 29%, respectively. Expression of EGFR, EGFRvIII, PTEN, or p-Akt did not significantly correlate with PFS or OS. Though the authors concluded that EGFR inhibition had not been effective in their study, they acknowledged the bias associated with selecting patients with advanced and heavily pre-treated disease (Preusser et al., 2008). Similarly, lapatinib monotherapy, in a phase II trial, failed to produce an objective response in patients with recurrent GBM, though stable disease was achieved in 24% of patients (Thiessen et al., 2010).
EGFR signaling regulates anti-apoptotic pathways and promotes resistance of malignant gliomas to radiation in preclinical models (Chakravarti et al., 2002; Raizer, 2005). Because inhibition of these pathways may sensitize tumors to cytotoxic therapy, several studies have examined EGFR inhibition in combination with chemotherapy and/or XRT (de Groot et al., 2008a; Prados et al., 2009b; Prados et al., 2006). However, as with EGFR inhibitor monotherapy, these studies have shown limited clinical efficacy. In a phase I study of erlotinib administered with TMZ, 8/57 (14%) of evaluable patients achieved partial response, and 6/57 (10.5%) achieved a 6 month PFS. Median TTP was 8 weeks. Adverse effects were mild to moderate and the therapy was well-tolerated (Prados et al., 2006). A phase II study of erlotinib combined with carboplatin resulted in a median PFS of 9 weeks, and a 6 month PFS rate of 14%. One patient out of 43 experienced a partial response, 20 experienced stable disease for an average of 12 weeks, and median OS was 30 weeks. No association was found between EGFR, PTEN, or Akt expression and PFS and OS (de Groot et al., 2008a).
The combination of EGFR inhibition, chemotherapy and XRT has also failed to show widespread clinical efficacy. A phase II trial of erlotinib in combination with TMZ and XRT did not find a clinical benefit from the addition of erlotinib to the treatment regimen when compared to the TMZ/XRT arm of the European Organization for Research and Treatment of Cancer/National Cancer Institute of Canada trial 26981/22981. Median PFS and OS were 7.2 months and 15.3 months, respectively. Although the study was not able to identify factors significantly predictive of outcome, high-level EGFR amplification – greater than a doubling in EGFR copy number as assessed by fluorescence in situ hybridization – correlated with improved PFS (10.1 months vs. 5.9 months, P = .155) and OS (19.4 months vs. 14.2 months, P = .103). In addition to a lack of evidence of clinical benefit, the combination of erlotinib, TMZ, and XRT produced significant toxicity, including grade 5 non-neutropenic pneumonias in two patients (Brown et al., 2008). In a more recent phase II trial, however, the addition of erlotinib to TMZ and XRT produced a clinical benefit in patients with newly-diagnosed GBM or gliosarcoma: median survival was 19.3 months, versus 14.1 in historical controls (TMZ plus XRT), with a hazard ratio for survival of .64 (treated versus control). Median PFS was 8.2 months. Interestingly, MGMT promoter methylation was associated with PTEN expression and with improved survival (25.5 months versus 14.6 months), suggesting tumors of this molecular phenotype may be sensitive to EGFR inhibition (Prados et al., 2009b).
As the above mentioned studies show, EGFR inhibition benefits a small subset – 10 to 20% – of malignant glioma patients (Mellinghoff et al., 2005). Identification of a molecular phenotype predictive of sensitivity to EGFR inhibition has been attempted. In one study, tumors obtained at initial surgery were analyzed for EGFR overexpression, gene amplification and mutations, as well as phosphorylated Akt levels. Low levels of phosphorylated Akt were found to be borderline significantly associated (P = .068) with improved outcome in patients treated with erlotinib. Moreover, EGFRvIII expression to correlated with poor survival in patients treated with erlotinib (P = .003), but not in patients treated with TMZ/BCNU (P = .714) (van den Bent et al., 2009). This is in contrast to a separate study that identified EGFRvIII and PTEN expression as significant predictors of response to EGFR inhibition. The response rate among patients whose tumors expressed EGFRvIII was 50%, compared to 7% in patients with EGFRvIII-negative tumors. Similarly, 54% of patients whose tumors expressed PTEN responded to EGFR inhibition, whereas no patients with EGFRvIII-negative tumors responded. Furthermore, coexpression of EGFRvIII and PTEN sensitized U87MG cells to erlotinib in vitro (Mellinghoff et al., 2005). The challenge in future clinical trials will be the a priori identification of patients most likely to benefit from EGFR inhibition and other targeted therapies. Ongoing trials of EGFR inhibitors are listed in Table 2.
BIBW-2992
BIBW-2992 is an orally-bioavailable selective and irreversible inhibitor of EGFR and HER-2/neu (Eskens et al., 2008; Minkovsky and Berezov, 2008). BIBW-2992 covalently binds the Cys 773 and 805 residues in the ATP-binding sites of EGFR and HER-2/neu, respectively, and prevents their activation and downstream signaling (Li et al., 2008; Minkovsky and Berezov, 2008). In preclinical studies, the drug inhibited the growth of several solid tumors in vitro and in vivo (Minkovsky and Berezov, 2008). In clinical studies, however, BIBW-2992 has yet to demonstrate efficacy. No patients achieved complete or partial response in a phase I trial of 38 patients with advanced solid non-CNS tumors. Seven of 38 (18.4%) patients did achieve stable disease for at least four treatment cycles and dose limiting toxicities (DLTs) included grade 3 fatigue, ALT elevation, diarrhea and skin rash (Eskens et al., 2008; Minkovsky and Berezov, 2008). BIBW-2992 is currently being evaluated in two ongoing phase I and II trials alone and in combination with TMZ and/or XRT (NCT00727506, NCT00977431) (Table 2).
2.2. Vascular endothelial growth factor receptor
Malignant gliomas constitute some of the most highly-vascularized solid tumors. The expression level of vascular endothelial growth factor (VEGF) correlates directly with tumor grade and malignancy, with a greater than 10-fold difference in expression between high and low grade gliomas (Norden et al., 2009). Because angiogenesis is uncommon in adults and is typically limited to malignancies, inhibition of the RTK VEGF receptor (VEGFR), and consequently tumor angiogenesis, is an attractive potential treatment strategy (Dietrich et al., 2009; Rich and Bigner, 2004).
Cediranib
The small-molecule cediranib is an orally-bioavailable potent pan-VEGFR inhibitor that acts by competitively binding the ATP-binding site of VEGFR as well as those of PDGFRα/β and c-Kit (Dietrich et al., 2009; Wedge et al., 2005). Preclinical analysis has shown cediranib to be a potent inhibitor of VEGFR2 and other VEGF receptors, such as Flt-1 and Flt-4, as well as c-Kit and PDGFRβ, in several human tumor cell lines. In a fibroblast and endothelial cell co-culture model, cediranib inhibited vascular tubule growth, resulting in smaller vessels with reduced branching. In vivo, cediranib inhibited both normal and malignant processes dependent on vascular growth. Furthermore, cediranib-mediated inhibition of VEGFR inhibited growth in human breast, colon, lung, and ovarian tumor xenografts and induced regression of tumor vasculature in Calu-6 xenografts in nude mice (Wedge et al., 2005).
In a phase II trial of recurrent GBM patients who had failed standard therapy, cediranib treatment was well tolerated, and on MRI volume analysis, produced a greater than 50% reduction in tumor enhancement in 9/16 patients (56.3%) and between 25 and 50% reduction in 3/16 patients (18.8%). Median PFS was 111 days, and median OS was 211 days (versus a historical value of 175 days). Furthermore, cediranib treatment significantly reduced vasogenic edema during the course of treatment, as determined by T2-FLAIR, ADC, and ve MRI. This was accompanied by a reduction in mass effect in 14/15 patients. Cediranib treatment also reduced or eliminated the need for steroid use in study patients: 5/16 did not require steroids at the start of the study, 8/16 had a reduction in dose, and 3/16 discontinued steroid use. Once cediranib was discontinued, all patients required steroids for the management of edema. Finally, the study identified potential biomarkers indicative of tumor responsiveness to VEGF inhibition. While serum levels of VEGF and placental growth factor (PlGF) were significantly elevated during cediranib treatment, tumor progression during cediranib treatment was associated with reduced plasma levels of PlGF and increased plasma levels of basic FGF and SDF1α. Furthermore, there were increased blood levels of viable circulating endothelial cells (CECs) when tumors escaped treatment and circulating progenitor cells (CPCs) when tumors progressed. This was attributed to the activation of alternative angiogenic signaling pathways. Expression of VEGFR, PDGFR, or c-Kit in tumor samples obtained at initial biopsy did not correlate with response to cediranib (Batchelor et al., 2007).
To determine if the benefits of cediranib were due to edema control and/or an anti-tumor effect, a murine orthotopic GBM model was used. It was found that cediranib monotherapy significantly prolonged survival despite continued tumor growth. Intravital microscopy revealed a significant cediranib-induced reduction in blood vessel diameter and permeability, normalization of tumor vasculature, and a thinning of the vascular basement membrane, all leading to reduced edema (Kamoun et al., 2009).
It has been postulated that the vascular normalization induced by cediranib treatment may improve the efficacy of conventional therapies by reducing interstitial fluid pressure and allowing for better drug penetration as well as by reducing hypoxia (Dietrich et al., 2009). As such, several ongoing and recently-completed clinical trials are examining the efficacy of cediranib in combination with CCNU (NCT00503204; NCT00777153), the integrin inhibitor cilengitide (NCT00979862), TMZ and XRT (NCT00662506), and bevacizumab (NCT00458731). Other ongoing trials are further examining cediranib as a monotherapy in adult (NCT00305656) and pediatric (NCT00326664) brain tumor patients (Table 2).
Vatalanib
Vatalanib is an orally administered small molecule inhibitor of the VEGF receptor family, including VEGF1, VEGF2, and VEGFR3, and, at higher concentrations, PDGFRβ, c-Kit, and c-Fms (Goldbrunner et al., 2004; Reardon et al., 2009b). Vatalanib acts by competitively and reversibly binding to the ATP-binding pocket of the VEGFR tyrosine kinase domains, inhibiting their autophosphorylation in response to the binding of VEGF ligand (Goldbrunner et al., 2004; Scott et al., 2007). In preclinical models, vatalanib has shown effectiveness in inhibiting glioma growth and neovascularization. In a C6 rat glioma model, for instance, vatalanib administration led to a significant reduction in tumor volume and vessel density, and a 1.7-fold increase in intratumoral necrosis (Goldbrunner et al., 2004).
A recent phase I trial of vatalanib in combination with imatinib and hydroxyurea for recurrent malignant gliomas demonstrated it to be well-tolerated and patients had a median OS of 48 weeks, a median PFS of 12 weeks, and a 6 month PFS of 25% (Reardon et al., 2009b). In a phase I trial of patients with newly-diagnosed GBM, vatalanib combined with TMZ and XRT was well-tolerated and without increased toxicity relative to TMZ and RT alone. Median PFS and OS were 6.8 and 17.3 months, respectively (Brandes et al., 2010). A study seeking to determine the maximum tolerated dose (MTD) of vatalanib within this treatment regimen in patients taking enzyme-inducing anti-epileptic drugs (EIAED) is currently underway. Thus far, observed adverse effects include grade 3–4 elevated ALT, thrombocytopenia, leukopenia, neutropenia, depressed consciousness, fatigue, and asymptomatic intracerebral hemorrhage. Of the patients who completed therapy, 2/13 (15.4%) achieved partial response and 7/13 (53.9%) achieved stable disease. Median PFS was at least 18.4 months (Batchelor et al., 2009). Vatalanib in combination with TMZ and radiation therapy is also being evaluated in another ongoing phase I trial (NCT00385853).
2.3. Platelet-derived growth factor receptor
Another well-studied RTK implicated in malignant glioma pathogenesis is the platelet-derived growth factor receptor (PDGFR). PDGFR is amplified in more than 20% of GBMs (Dresemann et al., 2010; Joensuu et al., 2005) and, like EGFR, is upstream of the PI3K/Akt/mTOR and Ras/MAPK signaling pathways, which are involved in cell survival, proliferation, and transformation (Holmen and Williams, 2005; Konings et al., 2009). In addition, expression of PDGF ligand is frequently upregulated in gliomas (Guha et al., 1995; Hermanson et al., 1992), implicating a role for PDGF autocrine signaling in gliomagenesis (Hermanson et al., 1992). PDGFR signaling also plays a role in angiogenesis (Raymond, 2009). As a result, PDGFR is an attractive therapeutic target.
Imatinib
Imatinib is a small molecule inhibitor of PDGFR-α and β, c-Kit, Abl, and ARG (Dresemann, 2005; Holdhoff et al., 2005). In preclinical models, imatinib treatment has shown promising antitumor activity. In addition to its direct antitumor effects, imatinib has been demonstrated to sensitize human GBM cells to radiation as well as cytotoxic drugs. Through vascular normalization, it may also facilitate the tumor penetration of cytotoxic drugs (Holdhoff et al., 2005; Pietras et al., 2001; Pietras et al., 2002; Reardon et al., 2008a; Russell et al., 2003; Slupianek et al., 2001).
Early clinical trials of imatinib in malignant glioma patients showed the drug to be well-tolerated but of limited clinical efficacy as a monotherapy. A phase I/II study of imatinib mesylate for recurrent malignant gliomas determined an MTD of 800 mg/day imatinib for patients not on EIAEDs, with DLTs including neutropenia, rash, and elevated ALT. No MTD was determined for patients on EIAEDs, even though they received up to 1200 mg/day. In the phase I component of the study, 1/23 (4.4%) non-EIAED patients experienced a partial response, and 10/23 (43.5)% non-EIAED and 9/27 (33.3%) EIAED patients experienced stable disease at 8 weeks. In the phase II component of the study 5/54 (9.3%) patients developed intratumoral hemorrhages after treatment with 800 mg/day imatinib, though one of the five patients had a prior history of hemorrhage and another had grade 3 thrombocytopenia at the time of hemorrhage. No patients exhibited complete response, 2/54 (3.7%) (GBM) had partial response, and 11/55 (20.4%) (6/33 GBM, 5/21 AA) had stable disease. Six month PFS was 3% for GBM and 10% for AA, both lower than historical rates (Wen et al., 2006).
A more recent phase II study of imatinib in patients with recurrent, post-XRT gliomas obtained similar results. Six month PFS in GBM, AA, and OD patients were 5/31 (16.1%), 2/22 (9.1%) and 1/27 (3.7%), respectively. Median PFS and OS for all patients were as follows: in GBM patients, 1.8 months and 5.9 months, respectively; in patients with AO or OA (oligoastrocytoma), 1.9 months and 5.3 months, respectively; in patients with AA or recurrent low-grade glioma, 1.8 months and 5.0 months, respectively. Two out of 80 patients experienced partial response, leading the study authors to conclude that although well-tolerated, imatinib monotherapy at the doses tested was ineffective at inhibiting tumor growth in patients with recurrent malignant gliomas (Raymond et al., 2008). The lack of efficacy of imatinib monotherapy may be attributable to its poor penetration of the blood-brain barrier (Takayama et al., 2002).
Several studies have examined the use of imatinib in combination with conventional chemotherapy. In a preliminary study of imatinib and hydroxyurea in patients with progressive, chemotherapy and XRT refractory GBM, the combined treatment regimen was well-tolerated and demonstrated some efficacy. Six of 30 (20%) patients exhibited complete or partial response, and 17/30 (57%) patients experienced a clinical benefit of response or stable disease for at least 3 months. No grade 3 or 4 toxicities were observed and median time to progression, median OS, 6 month PFS, and 24 month PFS were 10 weeks, 19 weeks, 32%, and 16%, respectively. Three of 30 (10%) patients survived for at least 106 weeks (Dresemann, 2005). Similarly promising results were obtained in a phase II study of imatinib plus hydroxyurea in adults with recurrent GBM following prior chemotherapy and XRT. Three of 33 (9%) patients achieved radiographic response, and 14/33 (42%) achieved stable disease. Six month PFS, median PFS, and median OS were 27%, 14.4 weeks, and 48.9 weeks respectively (Reardon et al., 2005a).
A phase II study further evaluated the efficacy of imatinib and hydroxyurea in patients with recurrent grade III malignant gliomas. Thirty nine patients received imatinib and hydroxyurea, and the treatment regimen was well-tolerated. Overall, 4/39 (10.3%) of patients achieved radiographic response and 13/39 (33.3%) achieved stable disease. Among these 17 patients, the median PFS was 26.9 weeks, and the 6 month PFS and 12 month PFS were 53% and 29%, respectively. Of 37 patient evaluable for survival, the overall median PFS, 6 month PFS, 12 month PFS, and median OS were 10.9 weeks, 24%, 14%, and 33.3 weeks, respectively (Desjardins et al., 2007).
However, a recent phase III study of imatinib in combination with hydroxyurea in patients with progressive, recurrent GBM has cast doubt on the clinical benefit of this treatment regimen versus hydroxyurea alone. Two hundred forty patients were randomly assigned to receive hydroxyurea either alone or in combination with imatinib. A similar median PFS of 6 weeks was observed for both arms of the study. Likewise, 6 month PFS for the hydroxyurea and the combination arms were 7% and 5%, respectively. Six month OS rates were 37% and 40% in the hydroxyurea and combination arms, respectively. Overall response rates – stable disease or better – were approximately 25% in each arm. Median OS was 19 weeks and 21 weeks for the hydroxyurea arm and the combination arm, respectively. The authors recommend against the use of combined imatinib and hydroxyurea therapy at first relapse in GBM patients (Dresemann et al., 2010).
The combination of imatinib and TMZ has also been recently examined in a phase I study in adults with malignant gliomas. DLTs included grade 3 neutropenia, nausea/emesis, hypokalemia, acute renal failure (in patients on EIAEDs), and hyperbilirubemia, as well as grade 4 hypocalcemia and hypophosphatemia. Median PFS, 6 month PFS, and OS were 26.6 weeks, 52.3%, and 47.6 weeks, respectively. Eight of 64 (12.5%) evaluable patients achieved radiographic response, including 6/51 (11.8%) GBM patients and 2/13 (15.4%) AA patients. Complete response occurred in one patient with AA who enrolled in the study with stable disease following radiation and TMZ (Reardon et al., 2008a). Recently completed and ongoing clinical trials are further evaluating the safety and clinical efficacy of imatinib alone or in combination with other therapies for the treatment of malignant gliomas, and are summarized in Table 2.
2.4. PI3K/Akt/mTOR pathway
In response to signals from activated RTKs such as EGFR, VEGFR, FGFR, PDGFR, and c-Kit, the PI3K/Akt/mTOR pathway effects changes in protein synthesis via the mTOR-mediated phosphorylation of the ribosomal protein p70S6K and the 4E binding protein 1 (4E-BP1). Phosphorylation of p70S6K is important for translation of mRNAs encoding for ribosomal and translational complex proteins (Hu et al., 2005; Volarevic and Thomas, 2001) while phosphorylation of 4E-BP1 releases its inhibition of eukaryotic translation initiation factor 4 E (eIF4E). eIF4E in turn regulates cap-dependent mRNA translation (Culjkovic et al., 2005; Graff et al., 2008) and appears to preferentially facilitate the translation of mRNAs involved in cell proliferation and transformation, such as cyclin D1 and c-Myc (Culjkovic et al., 2005, 2006; Graff et al., 2008). The PI3K/Akt/mTOR pathway is negatively regulated by the phosphatase and tensin homolog (PTEN) protein, which is deleted, inactivated, or mutated in approximately 40–70% of GBMs (Cloughesy et al., 2008; Doherty et al., 2006; Hu et al., 2005; Minniti et al., 2009). Whether through loss of PTEN activity or aberrant activation of growth factor receptors, malignant gliomas frequently have upregulation of the PI3K/Akt/mTOR pathway (Chakravarti et al., 2004; Doherty et al., 2006). Inhibition of this pathway may thus be a promising treatment strategy.
Sirolimus
Sirolimus is an orally administered blood-brain barrier permeable (Dancey, 2010) macrolide that binds the cytosolic protein FKBP12, and the resulting complex in turn inhibits mTOR (Chan, 2004; Yuan et al., 2009). In a phase I trial of 15 patients with recurrent PTEN-deficient GBM tumors, sirolimus was administered daily for one week prior to surgery and following recovery from surgery until disease progression. No patients experienced grade 3 or 4 adverse effects and tumor growth was retarded in some patients. Specimens from patients following sirolimus treatment showed a marked reduction in p70S6K phosphorylation as well as mTOR inhibition. However, sirolimus induced significant activation of Akt in 7/14 (50%) patients, potentially resulting in a reduced time to progression. The authors suggested combined inhibition of mTOR and Akt to overcome this problem (Cloughesy et al., 2008).
Consistent with this, a prior pilot study evaluated combined inhibition of both EGFR and mTOR. Twenty eight patients with heavily-retreated recurrent malignant glioma (22 GBM, 6 AA) received sirolimus plus erlotinib or gefitinib and DLTs included grade 3 rash and infection. Five of 26 (19.2%) evaluable patients achieved a partial response, and 14/26 (53.8%) achieved stable disease at 2 months. Median TTP and six month PFS were 12 weeks and 26%, respectively (Doherty et al., 2006). In a phase I trial of sirolimus in combination with gefitinib in 34 patients with recurrent malignant glioma, DLTs included mucositis, thrombocytopenia, rash, hypertriglyceridemia, and diarrhea. Two of 34 (5.9%) patients had a partial response and 13/34 (38.2%) experienced disease stabilization. The median PFS and 6 month PFS were 8.2 weeks and 23.5%, respectively (Reardon et al., 2006).
The combination of sirolimus and erlotinib was further evaluated in a recent phase II study. Thirty two patients with heavily-pretreated recurrent GBM received sirolimus and erlotinib and DLTs were uncommon. No patients achieved objective response, but 15/32 (46.9%) patients did achieve disease stabilization. Six month PFS for all patients was 3.1%, and PFS was higher among patients not taking EIAEDs (Reardon et al., 2010). Ongoing phase I and II trials are further evaluating sirolimus in combination with other molecular target inhibitors, such as erlotinib (NCT00509431) and vandetanib (NCT00821080) (Table 2).
Temsirolimus
Temsirolimus (CCI-179) is an intravenously-administered ester analog of sirolimus with improved aqueous solubility and pharmacokinetics (Galanis et al., 2005). In a phase II trial of 43 patients with recurrent GBM, temsirolimus was well-tolerated but did not show clinical efficacy as a monotherapy. Among assessable patients, 2/41 (4.9%) achieved partial response and 20/41 (48.8%) achieved stable disease, although durability of response was short. One of 41 (2.4%) patients had a 6 month PFS, and median TTP was 9 weeks (Chang et al., 2005).
A phase II study by the North Central Cancer Treatment Group yielded similar results. Sixty five patients received temsirolimus intravenously and DLTs included grade 3 or 4 hypercholesterolemia, hypertriglyceridemia, and hyperglycemia. Objective response was not observed in any patients, although 36% of patients had radiographic improvement. Median TTP and OS were 2.3 months and 4.4 months, respectively. Radiographic response was associated with the presence of phosphorylated p70S6K in baseline tumor, and with development of grade 2 or greater hyperlipidemia during the initial two treatment cycles (Galanis et al., 2005). Temsirolimus is currently in phase I and II clinical trials in patients with malignant gliomas, and its use is being investigated in conjunction with erlotinib (NCT00112736), TMZ (NCT00784914), radiation therapy (NCT01019434), TMZ and radiation therapy (NCT00316849), bevacizumab (NCT00800917), and the multi-kinase inhibitors sorafenib and tipifarnib (NCT00335764; NCT00329719) (Table 2).
Everolimus
Everolimus is a sirolimus analogue that can be administered orally (Yuan et al., 2009). In a currently ongoing phase I trial, 27 patients with recurrent or newly-diagnosed post-radiation GBM received everolimus and TMZ. DLTs included neutropenia and thrombocytopenia and there are plans to further escalate everolimus and TMZ doses in patients taking EIAEDs (Mason et al., 2009). A recent phase I/II trial evaluated the combination of everolimus and gefitinib and no DLTs were observed. Three of 22 (13.6%) patients achieved partial response and 8/22 (36.4%) patients achieved stable disease. One of 22 (4.5%) patients had a 6 month PFS and the median PFS and OS were 2.6 months and 5.8 months, respectively (Kreisl et al., 2009b).
Current trials are underway to further assess the safety and efficacy of everolimus alone (NCT00823459; NCT00831324) and in combination with other therapies, such as imatinib and hydroxyurea (NCT00613132), TMZ and/or radiation therapy (NCT00553150; NCT00387400), gefitinib (NCT00085566), and bevacizumab (NCT00805961) (Table 2).
Ridaforolimus
Ridaforolimus is a newly-developed, intravenously-administered sirolimus analogue (Konings et al., 2009; Vignot et al., 2005). Clinical trials in patients with various solid tumors have shown ridaforolimus to be well-tolerated (Vignot et al., 2005), but the drug has not yet been approved for any indication (Yuan et al., 2009). Ridaforolimus has reached phase III investigation in patients with soft-tissue sarcoma (NCT00538239). A phase I study of ridaforolimus in the treatment of patients with progressive or recurrent glioma was recently completed, though data has not yet been made available (NCT00087451) (Table 2).
2.5. Ras/MAPK pathway
The Ras/MAPK signaling pathway transmits signals from several RTKs, including EGFR, VEGFR, PDGFR, FGFR, and IGFR, and therefore regulates cell proliferation, cell cycle progression, migration, and malignant transformation (Argyriou and Kalofonos, 2009; Holmen and Williams, 2005; Rich and Bigner, 2004; Wen and Kesari, 2008). Ras/MAPK activity also modulates PI3K/Akt/mTOR signaling (Argyriou and Kalofonos, 2009). Furthermore, upregulated Ras/MAPK activity, due in part to increased growth factor receptor activation, is commonly observed in malignant gliomas (Argyriou and Kalofonos, 2009). An important regulator of Ras/MAPK activity is the enzyme farnesyl transferase, which farnesylates Ras proteins and anchors them to the cell membrane, where they are able to transduce growth factor receptor signals (Rich and Bigner, 2004).
Tipifarnib
Tipifarnib is an orally-bioavailable farnesyl transferase inhibitor (Newton, 2003). In preclinical models, inhibition of Ras farnesylaion with tipifarnib and other agents demonstrated promising antitumor activity (Rich and Bigner, 2004). In addition, tipifarnib sensitized glioma cells to radiation and reduced hypoxia and matrix metalloproteinase 2 expression in human GBM xenografts in mice (Delmas et al., 2003; Delmas et al., 2002; Wang et al., 2006).
In a phase I trial of tipifarnib monotherapy, grade 3 toxicities included rash, fatigue, and headache. Objective response was not seen in any patients, although 2/23 (8.7%) patients experienced disease stabilization for at least 6 months. Median PFS was 7 weeks and analysis of patient tumor samples showed potent inhibition of farnesyl transferase activity (Cloughesy et al., 2005). A subsequent phase II study showed good tolerability and evidence of clinical benefit in patients with recurrent GBM. Two of 22 (9.1%) patients with AA and 8/67 (11.9%) patients with GBM experienced a PFS of greater than 6 months. Median PFS were as follows: for patients not on EIAEDs, 8 weeks (AG) and 9 weeks (GBM); for patients on EIAEDs, 8 weeks (AG) and 6 weeks (GBM) (Cloughesy et al., 2006).
As a result of preclinical studies showing a radiosensitizing effect of tipifarnib, several trials have assessed tipifarnib in combination with XRT and have demonstrated the regimen to be well-tolerated and encouragingly efficacious. In a phase I trial, 13 patients received concurrent XRT and tipifarnib, the latter given one week prior to and continuously during XRT. Toxicities included grade 2 thrombocytopenia, grade 3 phlebitis and diarrhea, and grade 4 neutropenia. One patient experienced pulmonary embolism and later died of respiratory failure. One of nine (11.1%) evaluable patients achieved partial response and 4/9 (44.4%) achieved disease stabilization. Median OS was 12 months (Cohen-Jonathan Moyal et al., 2007). Tipifarnib administered prior to radiation therapy has also recently been evaluated in a phase II trial in patients with newly-diagnosed GBM, but yielded unacceptable adverse effects. Median PFS and OS were 42 days and 234.5 days, respectively (Lustig et al., 2008). Current and recently completed phase I and II trials are further assessing the safety and efficacy of tipifarnib in combination with TMZ and/or radiation (NCT00050986; NCT00049387; NCT00209989), and with inhibitors of EGFR, mTOR, and other kinases (NCT00335764) (Table 2).
Lonafarnib
Lonafarnib is a farnesyl transferase inhibitor that has been shown in vitro to inhibit the growth of several glioma cell lines in a dose-dependent manner. It stimulates G2 arrest and inhibits MAPK phosphorylation (Basso et al., 2005; Feldkamp et al., 2001; Glass et al., 2000) and has been shown to inhibit components of the mTOR signaling pathway, resulting in reduced p70S6K phosphorylation and enhanced docetaxel-induced cytotoxicity (Basso et al., 2005). Moreover, lonafarnib causes substantial growth inhibition of human GBM xenografts in mice (Feldkamp et al., 2001).
In a phase I study of lonafarnib in combination with TMZ, no DLTs were observed, although grade 3/4 leukopenia, neutropenia, thrombocytopenia, lymphonemia, pneumonitis, diarrhea, esophagitis, hypokalemia, and fatigue were observed. Four of 14 (28.6%) evaluable patients achieved partial response and 3/14 (21.4%) achieved disease stabilization. Six month PFS and median PFS were 33% and 14 weeks, respectively (Gilbert et al., 2006). Lonafarnib, in combination with TMZ for the treatment of malignant gliomas, is being further assessed in additional phase I trials (NCT00083096; NCT00102648) (Table 2).
2.6. Protein kinase C
Protein kinase C (PKC) enzymes are a family of serine/threonine kinases that act as downstream effectors of several RTKs, including VEGFR and PDGFR (Graff et al., 2005). Through activation of topoisomerase II, drug efflux pumps, and regulation of the Ras/MAPK and anti-apoptotic pathways, PKC has been implicated in the resistance of malignant gliomas to chemotherapy and XRT (da Rocha et al., 2002). Furthermore, PKC is commonly overexpressed in malignant gliomas and transformed astrocytes compared to normal astrocytes (Bredel and Pollack, 1997; da Rocha et al., 2002; Sharif and Sharif, 1999). These data make PKC an attractive molecular target for the treatment of malignant gliomas.
Enzastaurin
Enzastaurin is a lipid soluble, orally bioavailable selective inhibitor of PKCβ that acts by competitively binding the ATP-binding site of PKCβ. In vitro, enzastaurin inhibits proliferation and induces apoptosis in GBM cells by decreasing PKCβ-mediated phosphorylation and activation of glycogen synthase kinase 3 beta (GSK3β), Akt, and p70S6K (Graff et al., 2005). Furthermore, inhibition of GSK3β has been shown to inhibit glioma migration and invasion (Nowicki et al., 2008).
In a phase II trial of enzastaurin in patients with recurrent high-grade gliomas, the drug was well-tolerated and of promising efficacy in highly-pretreated patients. Of 79 patients evaluable for response, 14 (17.7%) achieved radiographic response, including one complete response), and 13 (16.5%) achieved stable disease for at least 3 months (Fine et al., 2005). In a subsequent phase I study of 26 patients with recurrent gliomas, twice daily dosing was found to double the average drug concentration of enzastaurin under steady-state conditions compared with daily dosing enzastaurin. The drug was, however, poorly tolerated at all dose levels evaluated with thrombocytopenia and prolonged QTc as DLTs. One (5.3%) patient had a complete response, one (5.3%) had a partial response, eight (42.1%) patients had stable disease and two (10.5%) patients achieved long-term disease control with PFS of greater than 150 weeks. Median PFS and OS for all 26 patients were 1.4 months and 5.7 months, respectively. In six patients evaluable for GSK3β phosphorylation, five (83.3%) had reduced levels of phospho-GSK3β. Moreover, all five of these patients achieved stable disease or complete or partial response, whereas the patient with elevated phospho-GSK3β had disease progression after 3 weeks of treatment. Finally, the study showed enzastaurin to have a direct antitumor effect in vitro and related it to inhibition of GSK3β activation. GSK3β phosphorylation may thus be a reliable marker of PKCβ inhibition (Kreisl et al., 2009a). In a recent phase III trial comparing enzastaurin with CCNU in 266 patients with recurrent GBM, enzastaurin was found to be well tolerated but without additional clinical benefit. Response rates were similar between treatment groups, with 2.9% of patients given enzastaurin and 4.3% of patients given CCNU experiencing objective response, and 38.5% of patients given enzastaurin and 35.9% of patients given CCNU experiencing disease stabilization. In the enzastaurin arm, median PFS and OS were 1.5 months and 6.6 months, respectively; in the CCNU arm, median PFS and OS were 1.6 months and 7.1 months, respectively. Four of 174 patients (2.3%) given enzastaurin experienced lethal adverse effects, of which one was related to treatment (Wick et al., 2010).
A recent and ongoing phase I trial is examining enzastaurin in combination with TMZ for the treatment of malignant glioma. No DLTs were observed, although 7/15 (46.7%) patients experienced modest thrombocytopenia and 5/15 (33.3%) patients developed neutropenia. The death of one patient was recorded, but was unrelated to treatment. Two of 15 (13.3%) patients achieved partial response and 6/15 (40%) patients achieved stable disease, suggesting a limited degree of clinical efficacy (Rampling et al., 2009). Several other ongoing clinical trials are further examining the safety and efficacy of enzastaurin alone and in combination with chemotherapy and XRT for the treatment of malignant gliomas, and are summarized in Table 2.
2.7. Multi-target inhibitors
As discussed above, the greatest clinical benefit from molecular targeted therapies will likely be obtained from the concurrent inhibition of multiple aberrant signaling pathways. While this may be done with combination therapies, newer-generation small molecule inhibitors offer the promise of multiple-target inhibition with a single agent.
Dasatinib
Dasatinib is an inhibitor of the Src kinase family, PDGFRβ, c-Kit, and Bcr-Abl, and has demonstrated potent anti-tumor and anti-migratory activity in vitro (de Groot and Milano, 2009; Dumont et al., 2009; Lu et al., 2009). In vivo, dasatinib inhibited tumor growth, promoted tumor apoptosis, and prolonged survival in mice with human GBM xenografts, effects that were augmented with concurrent EGFR inhibition (Lu et al., 2009) and TMZ treatment (Milano et al., 2009). In mice, dasatinib has been shown to penetrate the blood-brain barrier (Porkka et al., 2008).
Dasatinib has been tested in phase III trials of patients with a variety of cancers, including CML, ALL, prostate, and gastrointestinal stromal tumors (de Groot and Milano, 2009). Dasatinib as a therapy for malignant gliomas is currently being investigated as a monotherapy in phase I and II trials (NCT00423735) and in combination with vandetanib and radiation therapy (NCT00996723), bevacizumab (NCT00892177), erlotinib (NCT00609999), TMZ and radiation therapy (NCT00895960), and CCNU (NCT00948389) (Table 2).
Pazopanib
Pazopanib, an orally-bioavailable inhibitor of VEGFR1/2/3, PDGFRα/β, and c-Kit, binds and inactivates the ATP-binding site of the receptors’ tyrosine kinase domains (Bukowski et al., 2010; Harris et al., 2008; Kumar et al., 2007). In vitro, pazopanib is a potent inhibitor of ligand-induced phosphorylation and activation of VEGFR2, PDGFRβ, and c-Kit. While pazopanib inhibits the VEGF- and bFGF- induced proliferation of human umbilical vein endothelial cells (HUVEC), no similar anti-proliferative effect has been observed in tumor cell lines. In vivo, pazopanib inhibited the growth of tumor xenografts in a dose-dependent manner and also inhibited ocular angiogenesis (Kumar et al., 2007).
Clinically, pazopanib has been shown to be well-tolerated, but with only limited efficacy in several solid tumors (Hurwitz et al., 2009). Nevertheless, current phase I and II clinical studies are examining pazopanib alone (NCT00929903, NCT00459381) and in combination with the EGFR inhibitor lapatinib (NCT00350727) (Table 2).
Sorafenib
Sorafenib is an orally-administered small molecule inhibitor of Raf, PDGFR and VEGFR, and has been shown to have antiproliferative and antiangiogenic effects in vitro and in vivo (Jane et al., 2006; Nabors et al., 2007b), and is able to cross the blood-brain barrier (Kane et al., 2006). Although generally well tolerated, sorafenib monotherapy did not demonstrate any efficacy in a phase I trial. This prompted its evaluation in combination with other molecular targeting drugs (Prados et al., 2009a; Wen et al., 2009). In a phase I/II trial, 13 patients received sorafenib in combination with temsirolimus and DLTs included grade 3 thrombocytopenia and grade 3 or 4 transaminitis, hypophosphatemia, fatigue, hyperlipidemia, and diarrhea. In the phase II portion of the trial, no patients experienced response or disease control (Wen et al., 2009). Another phase I/II trial has examined sorafenib in combination with erlotinib. In the phase I portion, 17 patients received sorafenib and erlotinib and DLTs included grade 3 or 4 elevated lipase, transaminitis, hypertension, and hypophosphatemia. Pharmacokinetic analysis suggested sorafenib expedited erlotinib clearance. No data is currently available for the phase II portion of the trial (Prados et al., 2009a). Ongoing and recently completed trials are evaluating sorafenib alone (NCT00884416) and in combination with TMZ (NCT00597493), XRT (NCT00639262), TMZ and XRT (NCT00734526; NCT00544817), erlotinib (NCT00445588; NCT00335764), temsirolimus (NCT00329719; NCT00335764), bevacizumab (NCT00621686), and tipifarnib (NCT00335764) (Table 2).
Sunitinib
Sunitinib is an orally-bioavailable and blood-brain barrier-permeable (Patyna and Peng, 2006) small molecule inhibitor of VEGFR and PDGFR that inhibits cell proliferation and migration and induces apoptosis in a dose-dependent manner (Giannopoulou et al., 2009). Additionally, sunitinib-induced vascular normalization increased the tumor distribution of TMZ in mice transplanted with human glioma cells (Zhou and Gallo, 2009; Zhou et al., 2008). In a recent phase II trial of patients with TMZ-refractory high grade gliomas, however, sunitinib monotherapy did not provide any significant clinical benefit. Median TTP and OS were 1.6 and 3.8 months, respectively. However, three patients with secondary GBM achieved partial response with combination sunitinib and CCNU therapy and median TTP and OS in this group were 2.8 months and 9 months, respectively (Neyns et al., 2009a). Additional phase I and II clinical trials in patients with malignant gliomas are underway to examine the safety and efficacy of sunitinib alone (NCT00923117, NCT00713388, NCT00499473, NCT00606008, NCT00535379, NCT00864864) or in combination with irinotecan (NCT00611728) in this patient population (Table 2).
Tandutinib
Tandutinib is an orally-bioavailable and blood-brain barrier-permeable ATP-competitive inhibitor of multiple tyrosine kinases, including PDGFR, Flt3, and c-Kit (Hibner et al., 2008; Supko et al., 2009). In a C6 rat glioma model, tandutinib inhibited PDGFR autophosphorylation and tumor growth; the latter effect was augmented by the addition of TMZ (Hibner et al., 2008). A recent phase I trial examined the safety of tandutinib monotherapy in patients with recurrent GBM and DLTs included grade 3 fatigue, somnolence and weakness. A phase II trial is in progress (Supko et al., 2009;NCT00379080) and tandutinib is also being evaluated in combination with bevacizumab (NCT00667394) (Table 2).
Vandetanib
Vandetanib is an orally-bioavailable, multi-kinase small molecule inhibitor that acts on EGFR and VEGFR2/3 as an ATP mimetic (Sandstrom et al., 2008). Vandetanib treatment of human glioma cell lines in vitro and in several xenograft models resulted in the inhibition of proliferation, VEGF expression and angiogenesis and the induction of apoptosis (Damiano et al., 2005; Rich et al., 2005; Sandstrom et al., 2004; Sandstrom et al., 2008). Furthermore, these effects were enhanced with concurrent XRT (Damiano et al., 2005; Sandstrom et al., 2008), TMZ (Sandstrom et al., 2008), and the histone deacetylase inhibitor suberoylanalide hydroxamic acid (Jane et al., 2009). Tumors resistant to EGFR inhibition or VEGFR inhibition alone responded to treatment with vandetanib, supporting further investigation into concurrent inhibition of multiple molecular targets (Rich et al., 2005).
The clinical safety and efficacy of vandetanib, alone and in combination with other therapeutic agents, have been and are currently being evaluated in several phase I and II trials of patients with malignant gliomas. One such phase I trial in patients with newly-diagnosed GBMs examined the safety and efficacy of combining vandetanib with TMZ and radiation therapy. DLTs included grade 5 gastrointestinal hemorrhage, grade 4 neutropenia and grade 3 thrombocytopenia. Of evaluable patients, 1/10 (10%) achieved a 25 – 50% reduction in enhancement that was maintained for 8 weeks and 8/10 (80%) patients achieved stable disease (Drappatz et al., 2009). Other ongoing studies are summarized in Table 2.
XL184
XL184 is an orally-administered and blood-brain barrier permeable (Zhang et al., 2010) inhibitor of the VEGFR2, RET, and MET tyrosine kinases (Salgia et al., 2007). In a multicenter phase II trial of XL184 in patients with recurrent or progressive malignant gliomas, grade 3 and 4 adverse effects possibly related to treatment included elevated troponin I, myocarditis, dehydration, nausea, fatigue, elevated ALT, pulmonary embolism, and CNS hemorrhage. With regard to efficacy, 38% of patients experienced a greater than 50% reduction in bidimensional contrast-enhancing tumor measurements, 35% of patients achieved tumor measurement changes that ranged from a 24% increase to a 49% reduction, and 27% of patients experienced a greater than 25% growth in tumor size. Among patients who had not received prior anti-angiogenic therapy, 53% achieved a greater than 50% reduction in tumor size (De Groot et al., 2009). Further investigation into biomarkers indicative of response to XL184 showed a modulation in the plasma levels of VEGF-A, soluble MET, soluble VEGFR2, soluble KIT, and PlGF (DePrimo et al., 2009). XL184 is currently being investigated for use in patients with GBM in a phase I trial as a monotherapy (NCT00704288) and in combination with TMZ (NCT00960492) (Table 2).
3. Biologic therapies
3.1. Oncolytic viruses
In 1910, De Pace presented to the International Cancer Congress a patient who had cervical tumor regression after receiving an attenuated rabies vaccine for a dog bite (De Pace, 1912). He attributed this remission to virus-induced oncolysis and went on to treat several cervical cancer patients with the rabies vaccine, but without success. In 1941, Pack encountered a malignant melanoma patient with prolonged survival who had received the rabies vaccine following a dog bite. Based on this finding, he administered the vaccine to twelve patients, but only two developed disease remission (Pack, 1950). Due to the lack of success of these treatment attempts, the theory of viral oncolysis was abandoned for a time. The first experiments involving oncolysis for gliomas began in the early 1990’s with the development of technologies to genetically modify viruses. The first virus used for oncolysis was the Herpes Simplex-1 virus (HSV) with a modified tyrosine kinase promoter, which allowed it to replicate only in dividing cells, but also left it resistant to acyclovir. Mice treated with the mutated HSV had decreases in xenograft tumor size, but also showed signs of encephalopathy upon examination (Martuza et al., 1991). These first attempts raised initial safety concerns, but also provided early proof of concept that oncolysis with a suitably engineered virus could be used in the treatment of gliomas.
Herpes Simplex virus
After the tyrosine kinase deficient model described above proved promising, several mutations were tried in order to maintain susceptibility to antiviral agents. The first mutations involved a lacZ insertion into the UL39 gene which normally encodes for a viral ribonucleotide reductase. This insertion was initially hypothesized to prevent the virus from synthesizing nucleotides in non-dividing cells, allowing it to replicate only in rapidly dividing cells (Boviatsis et al., 1994a; Boviatsis et al., 1994b; Mineta et al., 1994). Later studies have shown, however, that disruption of the UL39 gene allows for viral replication in both quiescent and dividing cells with homozygous p16INK4α gene deletions, a mutation that occurs in approximately 30% of gliomas (Aghi et al., 2008). Another tested mutation was in the RL1 gene that controls γ134.5 protein production. In wild type virus, this protein enhances viral protein synthesis. Following mutation, it shuts off protein synthesis and inhibits viral replication in infected cells (Cassady et al., 1998; He et al., 1997). These mutations have made the virus specific for glioma cells and also retained its susceptibility to anti-viral agents such as acyclovir, thus allowing it to be used clinically.
HSV1716 is an HSV mutant first identified because of its lack of neurovirulence in mice (MacLean et al., 1991). Experiments since have revealed that the lack of neurovirulence is because of a mutation in the RL1 gene. Testing of the virus on glioma cell lines, along with primary tumor biopsies, proved that the virus caused cytotoxicity in both tissue types (McKie et al., 1996). Further experimentation involved animal trials and two different phase I trials, one in primary gliomas and the other in recurrent gliomas. In these trials, HSV1716 was proven to be safe and without adverse side effects or pathologic indication of encephalitis (Papanastassiou et al., 2002; Rampling et al., 2000). Because of the small patient groups within each trial, efficacy could not be measured. However, in one trial for recurrent gliomas, 4 of 9 patients were alive 14–24 months following treatment (Rampling et al., 2000). A larger Phase II study was conducted on 12 patients, who received injections of HSV1716 into the surgical cavity following the resection of a newly diagnosed or recurrent glioma. Throughout the study, there were no signs of clinical toxicity. One of six (16.6%) treated recurrent glioma patients was alive without progression after 22 months. Of the newly diagnosed glioma patients, two patients were alive with stable disease after 15 months (Harrow et al., 2004). HSV1716 is currently in a phase I trial for non-CNS solid tumors (NCT00931931).
HSV-G207 is a vector containing mutations in both the γ134.5 and UL39 genes. These mutations give HSV-G207 the ability to target and replicate within rapidly dividing cells and cells with a p16INK4α gene deletion such as glioma cells. In order to test this mutant’s virulence, two known HSV susceptible animals, owl monkeys and HSV susceptible mice, were inoculated with the double mutated virus. HSV susceptible mice inoculated with 1×107 PFU of the mutated virus did not show adverse effects, while mice inoculated with 1×103 PFU of wild type HSV died. The owl monkeys were also without signs of HSV infection following inoculation with the G207 virus (Mineta et al., 1995). Phase I trials further provided evidence of G207’s safety. In several of these trials, G207 never achieved dose related toxicity, even at doses of 3×109 PFU (Markert et al., 2009; Markert et al., 2000). These same recurrent glioma patients had a mean time from inoculation to disease progression of 3.5 months (range, 0–20; n = 21), a mean time from inoculation to death of 6.2 months, and mean time of diagnosis to death of 15.9 months (Markert et al., 2000). Use of G207 in post radiation recurrent glioma patients resulted in a median OS time of 7.6 months for nine patients (Karrasch et al., 2009).
G47Δ is based on the G207 virus with an additional deletion encompassing the α47 gene and the promoter of the US11 gene. Loss of the α47 gene product abrogates the down-regulation of MHC class I expression that occurs in HSV-infected human cells and thus enhances the recognition of infected cells by cytotoxic T lymphocytes. Loss of the US11 gene promoter places transcription of the late US11 gene under the control of the immediate-early α47 promoter. This change enhances viral growth in a variety of cell lines and increases the cytopathic effects in tumor cells. G47Δ had significantly greater efficacy than G207 at inhibiting tumor growth in both immune-competent and immune-deficient animal models, but was as safe as G207 (Todo et al., 2001). Because of these promising preclinical studies, G47Δ is now undergoing clinical trials in Japan.
Many additional HSV subtypes are now being engineered to not only cause selective oncolysis in tumor cells, but also to deliver genes. Some of these new variants include genes coding for murine forms of IL-4, IL-10, and IL-12. The IL-12 variant (M002) showed the greatest antitumor efficacy and was used to treat a neuroblastoma murine xenograft model. Additional testing of M002 also found it was efficacious against the U251 glioma cell line (Parker et al., 2000). The M032 variant has insertion of the human IL-12 gene and both M002 and M032 are under review for phase I clinical trials. HSV vectors are a growing area of possible treatments and thus there are many more under clinical investigation that are not mentioned in this review.
Adenovirus
Adenoviruses were tried in the oncolytic therapy of cervical cancer due to their ability to replicate selectively in epithelial cells. The response was similar to that seen in earlier vaccine trials. There were occasional early responses followed by uniform later disease progression (Huebner et al., 1956). In the late 1990’s, the adenovirus was revisited as an oncolytic agent due to the easy manipulation of its genome. Non-replicating mutants were generated that carried pro-apoptotic genes. The viruses worked well in vitro but in vivo efficacy was limited, most likely due to poor viral delivery (Curiel et al., 2000). More recently, conditionally-replicating viruses have been generated to help overcome this difficulty.
The first genetically modified, conditionally replicating adenovirus was Delta 24. This virus was named for a 24 nucleotide deletion in the transcript of the E1A protein, which normally binds to the host cell cycle-inhibiting protein Rb. Wild type E1A allows for cell cycle progression and replication of the adenovirus (Dyson and Harlow, 1992), but the mutated E1A protein in Delta-24 lacks binding affinity for Rb and thus inhibits viral replication in normal Rb producing cells. In gliomas, Rb levels are often decreased or non-existent due to genetic mutations and so similar inhibitory effects on replication are not present, in turn allowing for selective replication. The selectivity of Delta-24 was shown both in vitro using human glioma cell lines and in vivo using murine xenograft models (Fueyo et al., 2000). Delta-24 has been further modified to carry the RGD-4C peptide, which allows for entry into cancer cells that do not express the normal adenovirus entry receptor, coxsackie-adenovirus receptor (CAR). Expression of the RGD-4C peptide greatly enhanced the experimental in vivo tumor killing ability of the Delta-24 mutants (Fueyo et al., 2003). The Delta-24-RGD mutant has also shown efficacy in glioma stem cell lines and a phase I study for recurrent gliomas is currently recruiting patients (Jiang et al., 2007; NCT00805376). As Delta-24-RGD can also decrease expression of the DNA repair enzyme O(6)-methylguanine-DNA methyltransferase (MGMT) and theoretically make TMZ more effective, concurrent treatment with TMZ and Delta-24-RGD treatment is being considered (Alonso et al., 2007; Hegi et al., 2005).
Onyx-015 is an adenoviral vector missing the gene for E1B-55k, a viral protein that inactivates the tumor suppressing host protein p53. Loss of p53 deregulates DNA damage checkpoints and contributes to neoplastic transformation. P53 is mutated in as many as 30% of gliomas (Bögler et al., 1995). The loss of p53 inactivation by Onyx-015 allows for its replication in p53 underexpressing cells, such as gliomas with p53 mutations. A phase I clinical trial of Onyx-015 in recurrent malignant gliomas has been completed, but efficacy was less than expected (Chiocca et al., 2004). Phase II clinical trials in the US in which cisplatin and 5-fluorouracil combined with Onyx-015 led to recession of head and neck tumors has suggested the use of Onyx-015 as an adjunct to chemotherapy (Lamont et al., 2000).
Reovirus
Reovirus is a replication competent virus with a propensity to replicate in cells overexpressing the intracellular growth factor signaling protein ras. Many glioma cell lines overexpress ras, as well as associated upstream and downstream signaling proteins, making gliomas an attractive target for reoviral oncolytic therapy (Gerosa et al., 1989). Reolysin, a type III reovirus, was first tried on several glioma cell lines with great success. The virus caused lysis in 20 out of 24 different cell lines, and was also 100% successful in causing lysis in primary culture cell lines (Gerosa et al., 1989). Additionally, human xenografts in nude mice were found to disappear after injection of the live virus without significant toxic effects (Wilcox, 2001). The virus subsequently made it to phase I trials for recurrent gliomas and was found to be safe and well tolerated (Forsyth et al., 2008). There is currently an ongoing phase II to study the efficacy of the reovirus in recurrent gliomas (NCT00528684).
Measles virus
In the 1970’s, several case reports were published in which infection with the wild-type measles virus led to regression of hematological malignancy (Msaouel et al., 2009). Concerns regarding toxicity delayed initial testing of the virus as a formal oncolytic treatment, but eventually a live attenuated strain (MV-Edm) was shown to prevent in vivo progression of both Burkett lymphoma cells and follicular lymphoma human xenografts in immunodeficient mice(Grote et al., 2001). Furthermore, the MV-Edm strain was found to efficiently enter cells through CD46, an often upregulated receptor in several malignancies including gliomas (Anderson et al., 2004). The MV-Edm virus was additionally altered by adding the carcinoembryonic antigen gene (MV-CEA), allowing for monitoring of viral replication via CEA levels (Peng et al., 2002). MV-CEA virus caused syncytia formation and apoptosis of U87 glioma cells in vitro. In vivo treatment of orthotopic U87 transplants in immunodeficient mice prevented tumor progression and extended overall survival compared to non-treated controls. The safety of the virus was demonstrated by intracranial injections of the MV-CEA virus into a measles virus susceptible mouse strain as well as Rhesus macaques. In both animals, there were no signs of neurotoxicity or viral replication even with a viral inoculation 35 times the expected required human dose (Allen et al., 2008; Myers et al., 2008). MV-CEA is now undergoing a phase I clinical trial in recurrent glioma patients (NCT00390299).
3.2. Gene therapy
In contrast to the use of conditionally replicating viruses for the induction of oncolytic activity, non-replicating viruses have been used for the delivery of therapeutic genes, such as those with presumed tumor suppressing or immune system activating functions.
Ad5CMV-p53
P53 is a tumor suppressing gene causing cell cycle arrest and apoptosis in the context of DNA damage. In gliomas, this pathway is often altered through mutations in or allelic loss of the p53 gene on chromosome 17p (Bögler et al., 1995). This led to the hypothesis that restoration of p53 in gliomas could be used to induce apoptosis. Transfection of glioma cell lines with the p53 gene caused decreased glioma cell growth (Mercer et al., 1990) and so a non-replicating adenovirus capable of transfering the p53 gene (Ad5CMV-p53) to tumor cells was generated and tested. This new virus was shown to be highly effective in preventing growth in five of six glioma cell lines and in flank tumors in nude mice (Köck et al., 1996). Studies on additional human cell lines provided evidence that the adenovirus-mediated transfer of p53 was effective in causing apoptosis in glioma cell lines carrying mutated but not wild type p53 (Lang et al., 1999; Li et al., 1999). The success in animal models led to a phase I clinical trial in which toxicity was found to be minimal. Gene transfer occurred only short distances from the site of convection-enhanced delivery (CED), however, and further clinical trials have not been completed (Lang, 2003).
Adenovirus-hIFN-β
Adenovirus-hIFN-β (Ad.hINF-β) is a non-replicating adenovirus carrying the human interferon-β (INF-β) gene. Interferons are a group of immune modulating proteins found to be efficacious against several malignancies including hairy cell leukemia, renal cell carcinoma, and chronic mylogenous leukemia (Quesada, 1987; Swanson and Quesada, 1988). In studies of both glioma cell lines and human glioma xenografts in mice, treatment with INF-β stalled tumor growth and induced tumor apoptosis (Nakamura et al., 1986; Rosenblum et al., 1990). A phase I trial, however, demonstrated systemically administered INF-β to be unacceptably toxic, with two deaths in the study (Yung et al., 1991). Glioma growth was not inhibited at the evaluated doses. Ad.hINF-β was therefore used to directly deliver high intratumoral doses of INF-β to avoid systemic toxicity. In murine models, Ad.hINF-β caused glial xenograft regression in immune-compromised mice as well as in immune-competent mice (Qin et al., 2001; Qin et al., 1998). In a phase I trial of direct intratumoral delivery, Ad.hINF-β showed minimal toxicity while causing apoptosis and necrosis in injected areas. The median PFS was 9.3 weeks (95% CI 4.0–14.1), and the median OS was 17.9 weeks (Chiocca et al., 2008). At the time of this review there were no active trials using Ad.hINF-β against gliomas.
Herpes simplex type-1 thymidine kinase gene with gancyclovir
Transduction of cells with the HSV thymidine kinase (HSV-tk) gene causes the expression of HSV-tk protein, which converts gancyclovir (GCV) to its monophosphate form, which is then enzymatically converted to GCV-triphosphate, an inhibitor of DNA synthesis (Oliver et al., 1985). GCV- triphosphate is toxic to cells that generate it as well as to neighboring cells connected via gap junctions (Elshami et al., 1996). This so-called bystander effect occurred in murine studies of glioma xenografts in which a 10% transduction rate was enough to cause full tumor regression (Freeman et al., 1993; Rubsam et al., 1999).
A variety of viral vectors have been made for the purpose of transducing cells with the HSV-tk gene. Replication deficient retroviruses carrying the HSV-tk gene showed promise in rat glioma models, but failed to have an effect in xenogeneic human tumor models unless they were of a small size (Izquierdo et al., 1997; Short et al., 1990). The retroviral delivery system was tested in phase III clinical trials, but treated patients did not show improved survival compared to controls (Rainov, 2000). There has been further evaluation of the retroviral vector in conjunction with TMZ treatment, but clinical trials have yet to occur (Rainov et al., 2001).
Both replication deficient and conditionally replicating adenoviral vectors containing the HSV-tk gene have also been generated. In a European phase II trial, replication-deficient adenoviral vector injections increased mean survival time from 39 weeks in non-treated patients to 70.6 weeks in virus-treated patients (Immonen et al., 2004). There are several ongoing phase I and II studies using replication-deficient adenoviral vectors as adjuncts to XRT for the treatment of recurrent and newly diagnosed gliomas (NCT00751270, NCT00870181, NCT00589875). Conditionally replication-competent adenoviruses with the HSV-tk gene have also been evaluated preclinically, but the initiation of clinical trials has not been warranted due to the lack of improved efficacy compared to replication-deficient viruses (Hakkarainen et al., 2006).
The immunogenicity and inflammation seen with viral vectors has led to the creation of non-viral delivery methods for the HSV-tk gene. A liposome form of the HSV-tk gene was developed and a Phase I/II study showed that (CED) of lipo-HSV-tk was safe and caused >50% regression in two out of eight patients (Reszka et al., 2005).
3.3. Receptor-ligand based toxin therapy
Interleukin-13 receptor α2
Interleukin-13 receptor α2 (IL-13Rα2) is a cell surface receptor subunit overexpressed in the majority of malignant gliomas but not in normal brain (Debinski et al., 1995; Joshi et al., 2000). As a glioma-specific marker, IL-13Rα2 serves as a promising target for toxin-based therapy. The first toxin developed against IL-13Rα2 was a fusion protein composed of the IL-13 ligand and PE38QQR, a mutated pseudomonas exotoxin (Debinski et al., 1995). IL-13-PE38QQR was found to be highly toxic to IL-13Rα2 expressing glioma cell lines. In vivo studies showed regression of murine U251 xenografts following intratumoral injection of IL-13-PE38QQR (Husain et al., 2001). Toxicity studies showed that IL-13-PE38QQR could be given safely without causing damage to the normal brain tissue of mice and rats (Husain and Puri, 2003). A phase I/II trial showed that patients undergoing recurrent glioma resection lived up to 70 weeks after surgery with optimally placed cytotoxin delivery catheters. Side effects included headache (31%), hemiparesis (16%), and fatigue (11%) (Prados et al., 2005). A combined look at three phase I trials found that patients receiving IL-13-PE38QQR lived 42.7–55.6 weeks after glioma resection, depending on catheter placement (Kunwar et al., 2007). However, in a subsequent phase III trial, patients receiving IL-13-PE38QQR had a life expectancy of 36.4 weeks compared to 35.3 weeks for patients receiving a carmustine wafer after an efficacy endpoint of 215 patients (Debinski and Tatter, 2009). Possible explanations for the lack of significant difference in life expectancy between the two treatment groups include poor intratumoral delivery of toxin (Sampson et al., 2009) and lack of IL-13Rα2 overexpression by tumor cells. In a study of primary glioma biopsy specimens using two independent methods, microarray analysis and quantitative real-time PCR, approximately half of gliomas expressed IL-13Rα2 (Jarboe et al., 2007). Optimization of delivery and prescreening of tumors for IL-13Rα2 overexpression may identify patients likely to be susceptible to this promising targeted toxin.
IL-4 receptor
IL-4 receptor (IL-4R) is another cell surface receptor differentially overexpressed in gliomas and selected solid tumors compared to normal tissue. IL-4 was first found on murine sarcoma cells, and a fusion protein comprised of IL-4 and pseudomonas exotoxin (IL-4PE) was generated (Puri et al., 1991). IL-4PE was found to be cytotoxic to murine sarcoma cells and other IL-4R expressing tumors were therefore sought. IL4-R was found to be expressed by several glioma cell lines, and as expected, IL-4PE was found to be toxic to these cells as well (Puri et al., 1994). In murine xenograft studies, the cytotoxin was found to target tumor tissue, and toxicity studies in monkeys showed that intrathecal injections were non-toxic to normal brain at low doses (Puri, 1999). Several different phase I trials for intratumoral injection of IL-4PE for recurrent gliomas have since been completed. In one phase I trial, 84% of patients experienced seizures, although no long-term side effects were observed (Kawakami et al., 2003). In another phase I trial, however, six out of nine patients had severe edema requiring craniotomies to relieve pressure. On autopsy, the patients were found to have evidence of tumor necrosis, but normal brain tissue necrosis could not be ruled out (Rand et al., 2000). With regard to efficacy, there has been one case report of a patient living three years after IL-4PE infusion before tumor recurrence led to death, but phase I data showed an average survival of 5.8 months (Kawakami et al., 2003; Rainov and Heidecke, 2004; Weber et al., 2003). This apparent lack of efficacy has kept the IL-4PE toxin from advancing to further trials.
Epidermal growth factor receptor
Transforming growth factor alpha (TGF-α) is an extracellularly secreted protein that can serve as a ligand for the epidermal growth factor receptor (EGFR). Increased expression of EGFR has been found in both glioma cell lines and primary glioma samples and is usually due to a gain of chromosome 7 or an amplification of the EGFR gene (Bigner et al., 1988; Wong et al., 1987). A toxin targeting the EGFR receptor was created by attaching TGF-α to a mutated pseudomonas exotoxin (TP38-PE). In preclinical testing, this cytotoxin was found to be toxic to seven out of eight glioma cell lines, and caused significant tumor regression in murine subcutaneous human glioma xenografts (Kunwar et al., 1993). TP38-PE dose titrations have been carried out in mice, rats, and rhesus macaques in order to assess safety. The preclinical efficacy of TP38-PE was assessed by transplanting the epidermoid carcinoma cell line A431 into the caudate nuclei of athymic mice and treating the tumors with TP38-PE. In the TP38-PE treatment groups, 90–100% of the athymic mice lived past 19 days, depending on the dosage group, while none of the animials in the control group lived past 19 days (Sampson et al., 2003). A phase I/II study of recurrent glioma patients showed that TP38-PE had an acceptable safety profile and was associated with a mean survival of 23 weeks. Three out of 15 patients with residual tumor at time of treatment showed disease regression (Sampson et al., 2003). Measurement of drug distribution revealed the inefficiency of cytotoxin delivery as a possible cause for the decreased efficacy of TP-38-PE. Many patients were noted to have heterogeneous distribution of TP38-PE, but those with homogeneous distribution showed better responses (Sampson et al., 2008).
Transferrin receptor
The transferrin receptor (TfR) is expressed on brain tumor cells but not on normal neural cells, and has correlative staining with Ki67 (Prior et al., 1990; Recht et al., 1990). The high expression of TfR in malignant gliomas in particular made TfR an ideal target for therapy. Because of the broad expression of TfR in other organs and on the endothelial cells of blood capillaries (Jefferies et al., 1984), intratumoral injection rather than system administration was proposed to deliver a transferrin protein tagged with a mutated diphtheria toxin (Tf-CRM107). Mutation of the diphtheria toxin reduced its receptor binding by 8000 fold, but still allowed for the blocking of elongation factor 2 and the subsequent inhibition of protein synthesis and cell death (Greenfield et al., 1987). Tf-CRM107 treatment of nude mice with intraperitoneal U251 tumor grafts caused significant reduction in tumor size and increase in life span (Laske et al., 1994). However, injection of rat brains with Tf-CRM107 caused encephalomalacia, underscoring the need for local and specific delivery in clinical trials (Laske et al., 1994). Phase I clinical trials were conducted in which Tf-CRM107 was administered using CED. Responders in this trial had an increased median survival time compared to non-responders, 74 weeks versus 36 weeks, respectively (Laske et al., 1997). Systemic toxicities were also minimal with only transient elevations in transaminases and mild hypoalbunemia observed (Laske et al., 1997). In the sole Phase II study of Tf-CRM107, thirty-five percent of patients responded, either partially or completely, to treatment (Weaver and Laske, 2003). Of those who responded, median OS was 74 weeks. A phase III clinical trial was started shortly after the release of the phase II trial results, but was terminated early due to disappointing unreleased preliminary results.
TM-601
TM-601 is a synthetically produced neurotoxin similar to chlorotoxin, the venom of the giant yellow Israeli scorpion Leiurus quinquestriatus. This toxin has been found to bind to glioma cells specifically, possibly through binding of matrix metalloproteinase 2 (MMP-2) or associated chloride channels (Deshane et al., 2003). Preclinical studies showed that TM-601 bound to tumor biopsy specimens, but not normal brain tissue. In addition, repeated injections of TM-601 into rabbits did not result in antibody formation (Mamelak and Jacoby, 2007). A phase I study of recurrent glioma patients was conducted evaluating the direct injection of iodine tagged TM-601 (131I-TM-601) into tumor cavities two weeks after resection. In this trial, 131I-TM-601 was found to be safe, with very little radioactivity found outside the injected cavity. Of the 18 patients treated, four (22.2%) had radiographically stable disease after 180 days of follow up, one (5.6%) had a partial response in that same time period, and two (11.1%) were without evidence of disease for more than 30 months (Mamelak et al., 2006). There are several ongoing phase I and II trials to follow up on these promising results (NCT00114309, NCT00683761, NCT00591058).
3.4. Antibodies
Anti-epidermal growth factor receptor
As stated above, EGFR is often highly expressed in glioma specimens compared to normal brain tissue. It is also frequently mutated in gliomas and the most common mutant variant, EGFRvIII, lacks amino acids 6–273 of the receptor’s extracellular domain. As a result of this truncation, EGFRvIII lacks the ability to bind EGF and TGF-α, but in contrast to the wild type receptor, it is constitutively phosphorylated and active. Glioma cells expressing both EGFR and EGFRvIII have greater tumorigenic activity than glioma cells expressing EGFR alone when transplanted into nude mice either subcutaneously or intracranially (Nishikawa et al., 1994). Because of the increased expression of EGFR and EGFRvIII in gliomas and the role of EGFR in tumorigenicity, several antibodies against the two receptors have been developed.
Cetuximab is a mouse anti-human EGFR antibody that binds to both wild type and EGFRvIII receptors. Binding to the wild type receptor causes competitive inhibition of EGF and TGF-α binding and internalization of the antibody receptor complex. Antibody dependent cellular cytotoxicity (AADC) is also observed in both the mutant and wild type EGFR expressing cell lines following treatment with cetuximab (Fukai et al., 2008; Kimura et al., 2007). In vivo studies showed that mice with flank and brain xenografts had increased survival after receiving intraperitoneal cetuximab and XRT compared to XRT alone (Eller et al., 2005). Clinically, there was a case report of three patients with high EGFR expressing tumors who were refractory to other treatments but responded to cetuximab as a single agent (Belda-Iniesta et al., 2006). In a phase II trial, there were no adverse side effects reported following cetuximab treatment, but there was also no difference in survival between patients with EGFR overexpressing tumors compared to those with tumors showing normal EGFR expression profiles. There was, however, disease control in 42.9% of patients with EGFR amplification and only 29.6% of patients without EGFR amplification (Neyns et al., 2009b). Cetuximab is currently being investigated in three phase II clinical trials in combination with other therapies for primary gliomas (NCT01012609, NCT00311857, NCT01044225).
Nimotuzumab is a humanized mouse monoclonal antibody with high affinity for the human EGFR (Mateo et al., 1997). Phase I trials were first carried out on nine recurrent glioma patients, eight (88.9%) of whom were without disease progression at 6 months and two (22.2%) remained alive after 4 years (Crombet et al., 2001). Phase I/II studies were then conducted using nimotuzumab in combination with XRT. Patients in this study tolerated I.V. administration of the antibody, and there were no serious adverse events. The median survival time for all patients was 22.17 months (17.7 in GBM patients, and no median survival time was reached with AA). The control group received XRT only and had a median OS of 17.8 months (15.0 months for GBM and 20.2 months for AA) (Ramos et al., 2006). 88Re-nimotuzumab, a radiolabeled form of the antibody, was used to treat patients by local injection immediately following resection in a phase I trial. In this trial, doses were well tolerated, and signs of systemic antibody distribution were minimal, warranting phase II investigation (Torres et al., 2008). There is currently a phase III trial using nimotuzumab in conjunction with standard treatment for primary gliomas (NCT00753246). There is also an ongoing phase II study looking at pediatric high-grade gliomas (NCT00600054).
MAb-425 is a mouse anti-human EGFR monoclonal antibody developed using the A431 vulvar carcinoma cell line (Rodeck et al., 1987). Following tagging with 125iodine, the antibody delivers a low dose of radiation to EGFR expressing cells and also causes AADC. In the first phase I trial of patients with AA, mAb-425 was administered via intra-arterial injection. Of the 15 patients in this study, one (6.7%) had a complete response, two (13.3%) had partial responses, and five (33.3%) patients had stable disease (Brady et al., 1990). There have been a number of phase II studies of GBM and anaplastic astrocytoma patients since that initial report, and a cumulative review of 180 patients revealed that patients under 40 years old and with a KPS of greater than 70 had median survivals of 22.5 months and 65 months, respectively, for the two tumor types (Quang and Brady, 2004). Of the 115 GBM patients included in this analysis, 10 (8.7%) lived for more than five years. There is ongoing recruitment to a phase II study in high-grade glioma patients (NCT00589706).
Anti-vascular endothelial growth factor
Vascular endothelial growth factor (VEGF) is a major mediator of angiogenesis in GBM and its expression level often correlates with tumor grade, blood vessel density, invasiveness, and poor prognosis (Zhou et al., 2003). A method to decrease VEGF-induced angiogenesis has been the antibody-mediated sequestration of VEGF.
Bevacizumab, a humanized monoclonal antibody against VEGF-A, was shown in early glioma preclinical studies to cause a reduction in the size of human xenograft tumors in mice (Stefanik et al., 2001). It may in part act by destroying the niche for brain tumor stem cells (Calabrese et al., 2007). In 2004, bevacizumab was approved for the treatment of colorectal cancer in combination with irinotecan (CPT-11), fluorouracil, and leucovorin after improvements in treatment response rate and median OS were found (Hurwitz et al., 2004). Given this promising efficacy, bevacizumab was tried in combination with irinotecan in phase II trials for recurrent and primary gliomas. Irinotecan was chosen due to its ability to cross the blood-brain barrier and its mild success in previous glioma clinical trials (Friedman et al., 1999). The first study included 21 recurrent glioma patients, and one (4.8%) had a complete response, eight (38.1%) had partial responses, and 11 (52.4%) had stable disease for 7 months of treatment. Two of the patients died from known complications of the combination treatment, one from intracranial hemorrhage and the other from intestinal perforation (Stark-Vance, 2005). The next phase II trial of irinotecan and bevacizumab included 32 recurrent grade III and IV glioma patients. This study found a response rate of 63% with a PFS of 23 weeks and a 6 month survival of 72% (Vredenburgh et al., 2007a). However, there was a high rate of toxicity with 28% of patients having to be removed from the study due to adverse effects (Vredenburgh et al., 2007a). In an attempt to reduce toxicity, a follow up trial was conducted in which the dosing frequency of bevacizumab was decreased. This trial consisted of 12 recurrent glioma patients and had a drop out rate of 31% (Vredenburgh et al., 2007b). Longer term follow up of both cohorts revealed a 2 year survival rate of 22% (33% for grade III patients and 15% for grade IV patients) (Wagner et al., 2008). Additional studies showed similar response rates and toxicities (Bokstein et al., 2008; Norden et al., 2008). In an effort to further decrease its toxicity, bevacizumab was administered alone instead of in combination with irinotecan. In a study of 48 patients, 71% experienced an objective radiographic response with a median PFS of approximately 16 weeks and a 6-month PFS of approximately 29%. A dropout rate of 12.5% (5 thromboembolic events, one bowel perforation) was also noted (Kreisl et al., 2008). In a separate phase II trial of 167 recurrent glioma patients, half received bevacizumab monotherapy and the other half bevacizumab and irinotecan combination therapy. In the monotherapy and combination therapy groups, estimated 6-month PFS rates were 42.6% and 50.3%, respectively; objective response rates were 28.2% and 37.8%, respectively; median OS were 9.2 months and 8.7 months, respectively (Friedman et al., 2009). The results of Kreisl et al. and Friedman et al. led to the accelerated approval of bevacizumab as a single agent for recurrent gliomas in May 2009 (Cohen et al., 2009). In a more recent trial with recurrent glioma patients, the combination of metronomic etoposide plus bevacizumab resulted in a mean OS of 44.4–63.1 weeks, depending on the grade of tumor, and response rates were 22%–37%, which are similar to previous responses to bevacizumab alone and in combination with irinotecan (Reardon et al., 2009a).
Bevacizumab, in combination with standard radiation therapy and TMZ, has also been evaluated for the treatment of newly diagnosed gliomas. In a pilot study of 10 patients, myelotoxicity, wound healing complications, and venous thromboembolic events occurred with a frequency of at least 20%, but only one patient had a PFS < 40 weeks (Lai et al., 2008). A 16 patient trial of bevacizumab plus standard TMZ and radiation reported a median PFS of 12 months and a median OS of 16 months for resectable and non-resectable primary gliomas (Liebross et al., 2009). A third trial of 34 patients also had promising results with 71% having 12 month PFS (Kirkpatrick et al., 2009). As a result of these findings, there are a number of ongoing trials evaluating the treatment of newly diagnosed tumors with bevacizumab, in combination with standard and additional experimental therapies (Table 2).
Aflibercept is an engineered protein comprised of the extracellular domains of human VEGF receptor 1 (VEGFR1) and human VEGF receptor 2 (VEGFR2) fused to the constant region (Fc) of human IgG (Holash, 2002). This decoy receptor has been found to have a hundred times greater binding affinity for VEGF-A than does bevacizumab, making it a possible candidate for glioma treatment (Konner and Dupont, 2004). Preclinical studies have shown that aflibercept has the ability to stop U87 xenograft growth when used in combination with XRT (Wachsberger et al., 2007). Additionally, aflibercept was efficacious in treating initial and advanced tumor xenografts as evidenced by a significant increase in survival (Gomez-Manzano et al., 2008). Preliminary results from a phase II trial showed a radiographic response rate similar to bevacizumab, 50% for AA and 30% for GBM, but the 6 month PFS rate has not yet been reported. Toxicity was concerning, with 25% of patients discontinuing therapy within two months of starting the trial (De Groot et al., 2008b). There are currently two ongoing trials, one for recurrent gliomas and the other for newly diagnosed gliomas (NCT00650923, NCT00369590) (Table 2).
Anti- scatter factor/hepatocyte growth factor
Scatter factor/hepatocyte growth factor (SF/HGF) is the ligand for the RTK c-Met. HGF was first identified for its mitogenic activity in hepatocytes (Nakamura et al., 1984). SF was identified for its ability to increase epithelial cell motility (Stoker and Perryman, 1985). Both proteins were later found to be the same and were recognized as the ligand of the c-Met (Bottaro et al., 1991). Grade III-IV gliomas have significantly higher expression of c-Met receptor than low-grade tumors (Lamszus et al., 1998) and c-Met is known to activate several intracellular pathways involved in tumorigenicity and tumor progression through its tyrosine kinase activity. Activation of the ERK/MAPK pathway by c-Met leads to cell cycle progression and cell migration. Akt is also activated by c-Met, leading to stimulation of survival pathways and evasion of apoptosis (Abounader and Laterra, 2005). Angiogenesis in rat xenografts increased with glioma cells transfected with SF/HGF versus non-transfected glioma cells, providing evidence that c-Met can also play a role in angiogenesis (Laterra et al., 1997).
AMG-102 is a fully human antibody to SF/HG that has entered phase I trials after promising preclinical testing. Antibodies to SF/HGF were first tested on U87 and U118 xenografts in mice. AMG-102 decreased growth of both U87 and U118 tumors while also causing cell death in U87 tumors (Burgess et al., 2006). Further preclinical studies on non-human primates demonstrated the safety of the antibody (Kakkar et al., 2007). There are now ongoing phase II trials of AMG 102 in the treatment of recurrent gliomas (NCT00427440) (Table 2).
Anti-tenascin
Tenascin is a glycoprotein highly expressed in gliomas compared to normal brain (Bourdon et al., 1983). Immunostaining with an anti-tenascin antibody revealed that 10/11 GBM, 0/3 cerebrum, and 0/1 cerebellum specimens contained tenascin (Bourdon et al., 1983). The identification of tenascin as a tumor specific antigen has made it a candidate for antibody treatment.
81C6 is an anti-tenascin monoclonal antibody created following immunization of mice with U251 cells (Bourdon et al., 1983). Conjugation of 81C6 with 131I allows for the delivery of radiation to tumors as well as the imaging of 81C6 distribution and localization (Bourdon et al., 1984). 131I-81C6 was tested in murine xenograft models and found to decrease tumor burden in both brain and subcutaneous tumors (Lee et al., 1988). Phase I trials with intravenous administration of 131I-81C6 showed that the radioisotope was localized to glioma tissue compared to normal tissue by a ratio of 25:1, but the level of radiation in the tumor was not enough to be efficacious (Zalutsky et al., 1989). A more recent phase I study found the combination of 131I-81C6 and standard XRT and TMZ treatment to be safe with the worst attributable toxicity limited to mild reversible grade 3 neutropenia or thrombocytopenia. The patients also fared well in phase I trials, with 87% living past one year (Reardon et al., 2008c).
BC-2 and BC-4 are anti-tenascin monoclonal antibodies used in a multistep process of intravenous injections to localize radiation to tumors. In the first step, biotinylated BC antibody is administered and any antibody not bound to tumor is allowed to clear from the body for 48 hours. In the second step, avidin, which has extremely high affinity for biotin, is injected. A few hours later, in the final step, 90Y-biotin is administered and either binds to the avidin previously bound to biotinylated BC antibodies or is quickly cleared from the body. This three step process delivers 15 times more radioactivity to tumors than to vital organs and minimizes systemic toxicity (Paganelli et al., 1999). In a phase I trial, significant tumor reduction occurred in 25% of patients and 52% of patients were without tumor progression after 2 months of treatment (Paganelli et al., 1999). In a trial of grade III and IV glioma patients who had recently received conventional radiation therapy, the overall median disease-free interval of BC treated patients was 28 months in grade IV patients and 56 months in grade III patients while that of controls was 8 months (Grana et al., 2002). A phase II trial combining locoregional BC-4 radiation treatment with TMZ showed even more promise. Patients who had previously failed systemic BC-4 therapy were administered local BC-4 radiation either alone or in combination with TMZ. The respective OS times were 17 months and 25 months. (Bartolomei et al., 2004). Importantly, both trials included only patients whose tumors stained positive for tenascin. Phase III trial data have yet to be reported.
Anti-histone H1
131I-chTNT-1/BmAb is a radiolabeled chimeric antibody against histone H1 complexed to deoxyribonucleic acid, which is exposed at the core of necrotic tumors. The delivery of 131I-chTNT-1/B mAb to a tumor core causes the release of radiation to surrounding live tumor tissue but not enough radiation to damage normal brain tissue (Shapiro et al., 2006). A phase I study of recurrent glioma patients demonstrated the safety of this approach and the median OS of patients receiving the accepted safe dose of total radiation was 37.9 weeks (Patel et al., 2005). A phase II study for recurrent gliomas is currently ongoing (Table 2).
4. Immunotherapy
The relative lack of immune response to gliomas has stimulated the development of methods to activate the immune system to target glioma-specific antigens.
4.1 Vaccination
Whole cells
Whole cell lysates were used in the first anti-glioma vaccines because they theoretically avoided some of the limitations of single peptide vaccines, such as the need to identify tumor specific peptides and the limited antigenic repertoire of immune responses to single peptides. The first whole cell lysate vaccines were composed of either U251 or D-54MG cells and the immune stimulant levamisole. In a proof of principle study, patients receiving U251 injections lived significantly longer than patients receiving D-54MG and historical controls (Mahaley et al., 1983).
More recent vaccination attempts have utilized cells from patient’s own tumors. One approach has been to take patient cell lines and infect them with Newcastle disease virus, a virus known to stimulate an active immune response (Vonhoegen, Zawatzky, & V Schirrmacher, 1990). In one phase I trial, 11 patients underwent standard gross total resection and XRT followed by vaccination with autologous Newcastle virus infected cells. While the vaccination strategy appeared to be without significant side effects and elicited an immune response, survival was not improved compared to chemotherapy (Schneider et al., 2001). In a follow up trial of 23 patients, a more robust immune response was elicited by Newcastle virus treated cell lysates and the average PFS was 40 weeks in patients treated with surgery, XRT and vaccination versus 26 weeks in control patients who were treated with only surgery and XRT (log-rank test, P = .024). In addition, mean OS was 100 weeks for the vaccinated patients and 49 weeks for the controls (log-rank test, P<.001)(Steiner et al., 2004).
Another strategy has been intradermal injection of a patient’s formalin fixed tumor cells. In a study of eight recurrent and four residual primary glioma patients, there was one (8.3%) complete response, one (8.3%) partial response, two (16.7%) minor responses. One patient (8.3%) had stable disease for 24 months and seven (58.3%) patients had progressive disease. Patients who responded better tended to have higher MHC I class expression and lower p53 expression (Ishikawa et al., 2007).
Vitespen is a heat-shock protein (gp96)–tumor lysate complex taken up by immune cells via CD91 receptors. Once inside cells, the complex is dissociated and tumor lysate peptides are presented on MHC class I proteins to initiate an immune response. Vitespin has been shown to be efficacious in animal models of melanoma, colon cancer, and glioma. Phase I and II trials of melanoma patients have been completed and phase III trials are currently ongoing. A phase I/II clinical trial of glioma patients has shown this vaccine to be safe and capable of initiating an immune response in 11/12 (91.7%) patients. In addition, all patients lived passed 26 weeks with a median OS of 42 weeks (Wood and Mulders, 2009). Currently, there is one ongoing phase II trial for primary glioma treatment (Table 2).
In an attempt to target tumor blood vessels, a vaccine was made from glutaraldehyde-fixed human umbilical vein endothelial cells (HUVECs). This treatment was hypothesized to be more effective against tumors than tumor lysate vaccines because vascular endothelium is more exposed to the immune system than are actual tumors. In addition, endothelial markers on HUVECs, such as VEGF receptor-2 and αvβ3, are homologous to those on glioma tumor endothelium (Chen et al., 2006). In a phase I trial, six patients with recurrent gliomas were treated with glutaraldehyde-fixed HUVECs. Results were promising with three (50%) of the patients showing complete decrease in contrast enhancement on MRI (Okaji et al., 2008). There are now plans to use this vaccine in combination with chemotherapy.
Peptides
Even though whole cell lysates have thus far not been associated with any major side effects, there is a theoretical risk of developing undesired autoimmune reactions. The processing of individual patients’ tumors is also a time consuming process, and may not be feasible for patients with a time-limited disease. Single and oligo peptide vaccines have been developed to avoid these issues. Synthetic peptides are less likely to cause autoimmune reactions and can be readily available for patient administration.
The CDX-110 peptide vaccine consists of the keyhole limpet hemocyanin (KLH) protein covalently attached to an EGFRvIII derived peptide. In a phase I trial of patients with primary EGFRvIII positive gliomas, intradermally vaccinated patients had a significantly longer median time to progression (14.2 months) than did matched historical controls of XRT alone (6.2 months) (Heimberger et al., 2006). In another trial testing vaccine efficacy in combination with TMZ, vaccinated patients had median TTP of 15.2 months versus 6.4 months for historical controls who received TMZ alone (p = 0.0004), and median OS of 23.2 months versus 15.2 months, respectively (p = 0.0004) (Heimberger and Sampson, 2009). A phase II/III trial of this treatment is ongoing but has stopped recruiting patients (NCT00458601) (Table 2).
Increased expression of Wilms’ tumor gene (WT1) protein was found to be present in 70/73 glial tumor specimens and was significantly (p < 0.001) associated with MIB-1 staining index (Hashiba et al., 2007). Based on these results, an HLA-A*2402–restricted, modified 9-mer WT1 peptide vaccine was created for intradermal injection. A phase II trial of patients with WT1 expressing recurrent or progressive gliomas demonstrated a median PFS of 20.0 weeks and a 6 month PFS rate of 33.3% (Izumoto et al., 2008).
There have also been attempts at immunization with patient specific peptide vaccines. In one phase I study, HLA-A24+ and HLA-A2+ patients had their peripheral blood mononuclear cells (PBMC) tested for responses to a peptide panel. Patients were then vaccinated with up to four personally reactive peptides. All patients had an IgG response to the vaccinated peptides and the mean survival time of the GBM patients was 622 days (Yajima et al., 2005).
4.2. Dendritic cell therapy
Dendritic cells (DCs) have been used in immunization strategies because they can easily be generated in vitro from either myeloid precursors or PBMCs. DCs can also be activated in vitro by pulsing them with peptide antigens or fusing them to glioma cells. In the pulsation technique, DCs are exposed to the peptide(s) of interest, and matured using cytokines prior to intradermal injection. In an initial phase I trial of DCs pulsed with autologous MHC class I peptides, 4/7 (57.1%) patients showed cytotoxic T cell-mediated immunological responses without adverse effects. In four patients that underwent additional resection after treatment, two had signs of cytotoxic (CD8+) and memory (CD45RO+) T-cell infiltration in areas of intracranial tumor. This provided proof of principle that peripherally administered peptide-pulsed DCs could elicit a robust intracranial immune response (Yu et al., 2001). In a second phase I trial of recurrent glioma patients, vaccination with whole tumor cell lysate-pulsed DCs elicited significant cytotoxic responses against tumor in 6/10 (60%) patients, as determined by quantitative PCR analysis of IFN- message in re-stimulated PBMCs. Cytotoxic responses were also seen in 3/6 (50%) patients undergoing a second resection. Median OS was 133 weeks in DC-treated patients versus 30 weeks in the control group patients who underwent surgical resection and XRT (Mantel Cox log-rank test, P = 0.0013)(Yu et al., 2004).
In an effort to make the pulsation strategy more effective, IL-4 transfected fibroblasts have been injected into patients along with autologous tumor lysate-pulsed DCs. IL-4 was chosen because it had previously been shown to prevent tumor growth and to extend survival in a rat glioma model (Okada et al., 2001). In addition, when injected alone, it can increase interferon-γ levels, an indicator of a strong immune reaction (Okada et al., 1999). In a pilot study of a single patient injected with both lysate pulsed DCs and IL-4 transfected fibroblasts, infiltration levels of CD4, CD8 and CD1a positive cells increased proportionally to the amount of IL-4 produced at each injection site. The patient lived 10 months following treatment (Okada et al., 2003) and a larger phase I study ensued. Five patients with newly diagnosed GBM who had been treated with gross total resection and XRT were enrolled. All patients tolerated the treatment well, and PFS ranged from 4–10 months (Okada et al., 2007).
DCs have also been pulsed with mRNA isolated from primary glioma cells. In a phase I study of seven pediatric patients with an assortment of gliomas, there were no adverse effects but also limited efficacy. Only two (28.6%) patients had stable disease after treatment, and only one (14.3%) had a strong clinical response (Caruso et al., 2004). There are currently two ongoing clinical trials using DCs pulsed with mRNA from CD133+ cells in an attempt to target glioma stem cells (NCT00846456, NCT00890032).
Clinical trials have investigated the optimal location and timing of DC injection. In a phase I study of autologous tumor lysate pulsed DCs, 18 patients with recurrent gliomas underwent either intradermal only or intratumoral and intradermal injections. Survival was greater in the latter group (Yamanaka et al., 2005). In a separate phase I study, 53 GBM patients were assigned to one of three different vaccination schedules. Patients on the shortest vaccination schedule had significantly longer PFS than the other two cohorts (p=0.0008)(De Vleeschouwer et al., 2008).
A second strategy to activate DCs has been to use polyethylene glycol to fuse them with glioma cells. In an initial phase I trial, eight patients with primary malignant gliomas were treated with intradermal administration of the fused cells (Kikuchi et al., 2001). Although adverse reactions were minimal, the study observed limited anti-tumor response. In a second phase I study, IL-12 was injected along with fused cells to provide supplementary stimulation to T and natural killer (NK) cells. There were no significant side effects and of the 15 enrolled patients, four (26.7%) had tumor regression of greater than 50% and at least one other patient had a mixed response (Kikuchi et al., 2004).
4.3. Immune system stimulants
Poly ICLC
Additional methods have been used to overcome immune system suppression induced by gliomas. Toll-like receptors are thought to be critical in anti-viral immune responses. Toll-like receptor 3 (TLR3), for example, is activated upon binding of dsRNA ligands and initiates a transcriptional cascade through Toll-IL-1 receptor (TIR) domain-containing adaptor molecule-1 (TICAM-1) which in turn induces type I INF (especially INF-β) and DC maturation (Matsumoto and Seya, 2008). Poly ICLC is a dsRNA molecule known to activate TLR3 and lead to stimulation of T cells, natural killer cells, and myeloid DCs. In a trial initiated before the standardization of TMZ therapy, patients receiving poly ICLC had a median survival of 65 weeks compared to historical controls of 57 weeks for chemotherapy and 40 weeks for XRT alone (Butowski et al., 2008). In a phase II trial of patients with recurrent astrocytomas, 10 patients were found to have a median survival of 43 weeks after treatment with poly ICLC alone, which was comparable to historical controls (Butowski et al., 2009).
CpG ODNs
Synthetic phosphorothioate oligodeoxy nucleotides (ODNs) containing unmethylated CpG dinucleotides are ligands for the immune stimulating toll-like receptor-9 (TLR9). Activation of TLR9 drives the immune response toward the T helper type 1 (Th1) phenotype, which promotes CD8+ cellular cytotoxicity (Krieg, 2004). In a phase I trial of patients with second or third glioma recurrence, direct intratumoral administration of CpG ODN was well tolerated with only one patient experiencing DLT. Of the 24 enrolled patients, a minor response was observed in two (8.3%) patients with an median OS of 7.2 months (Carpentier et al., 2006).
AP 12009
TGF-β2 is a cytokine that decreases stimulation of B and T cells, inhibits lymphokine activated killer cells, and increases production of the inhibitory IL-10 by macrophages (Espevik et al., 1987; Kehrl et al., 1986a; Kehrl et al., 1986b; Maeda et al., 1995; Rook et al., 1986). It is highly expressed by gliomas and may contribute to the immunosuppression seen in patients (Wrann et al., 1987). In addition, it is a known angiogenic peptide that may promote tumor growth (Pepper, 1997). The combination of these effects may explain why increased TGF-β2 levels are correlated with high grade lesions and poor clinical prognoses (Kjellman et al., 2000).
AP 12009, an 18-mer phosphorothioate oligodeoxynucleotide complementary to TGF-β2 mRNA, inhibits TGF-β2 protein production. It was administered intratumorally via CED to avoid systemic toxicity and because it does not cross the blood brain barrier. Tumor cell lines established from malignant gliomas showed a 73% decrease in TGF-β2 production as well as decreased migration and sphere formation when treated with AP 12009 in vitro (Hau et al., 2009). Intrathecal administration was locally tolerated in both monkeys and rabbits. In a trial of 24 recurrent glioma patients, seven (29.2%) showed a response with two (8.3%) of the responders having complete remission (Hau et al., 2007a). In a phase II trial for recurrent GBM and AA, patients were found to do well on the lowest given dose of AP 12009, with an overall response rate of 7% at 12 months and 42% at 14 months, respectively, for the two tumor types (Bogdahn et al., 2008a; Bogdahn et al., 2008b). There is now an ongoing phase III trial for AA and one planned for GBMs (NCT00761280) (Table 2).
4.4. Adoptive immune therapy
In adoptive immune therapy, a patient’s immune cells are collected, manipulated, and reintroduced either systemically and/or intratumorally. Several different cell types including PBMCs, lymphokine-activated killer (LAK) cells, mitogen activated killer (MAK) cells, and tumor infiltrating lymphocytes have been attempted.
PBMCs were the first immune cells to be used and in one of the first clinical trials, peripheral PBMCs were harvested and reinfused intratumorally without any activation. Although safe, there were no signs of efficacy (Steinbok et al., 1984). After the finding that in vitro IL-2-activated PBMCs could become LAK cells and subsequently lyse murine sarcoma cells (Grimm et al., 1982), a number of glioma clinical trials were initiated. The first trial involved harvesting PBMCs, activating them in vitro, and injecting them back into the surgical resection cavity. Injection of IL-2 alone into the surgical resection cavity was also undertaken in this study. While the dose escalation of either IL-2 or LAK cells were with few side effects, signs of efficacy were not seen in the 10 patients (Jacobs et al., 1986). Several follow-up studies were conducted and in one, nine patients had improvements in mental status, gait, or motor function. Tumor regression was observed on CT scintigrams in six (66.7%) cases (Yoshida et al., 1988). Another study reported a median survival after reoperation of 53 weeks and a one year survival rate of 53% for GBM patients treated with immunotherapy. The corresponding survival values for 18 contemporary patients treated with chemotherapy were 25.5 weeks and 6%, respectively. A confounding factor, however, was that 6/15 (40%) immunotherapy group patients underwent additional surgery or chemotherapy (Hayes et al., 1995).
Multiple other LAK cell studies have not noted significant responses (Barba et al., 1989; Blacklock and Grimm, 1989; Lillehei et al., 1991; Merchant et al., 1988). In a more recent phase I trial, 31 recurrent glioma patients had a median survival from the date of original diagnosis of 17.5 months compared to 13.6 months for a control group of 41 contemporary GBM patients (p = 0.012) (Dillman et al., 2004). LAK cells have also been used as an adjuvant therapy for the treatment of newly diagnosed gliomas. The median survival from the date of original diagnosis was 20.5 months with a 1-year survival rate of 75% (Dillman et al., 2009). There is now an ongoing phase II study comparing carmustine wafers to intralesional injection of LAK cells (Table 2).
There have also been attempts to increase the potency of adoptive therapy by selectively proliferating cytotoxic cells. MAK cells were generated ex vivo using phytohemagglutin or anti-CD3 antibodies and IL-2. In vitro, these cells showed increased non-MHC restricted cytotoxicity. The first phase I studies found that patients treated with MAK cells fared better than those treated with the conventional therapy of the time, and there was a greater than 90% response rate in one study (Ingram et al., 1990; Ingram et al., 1987). Another MAK cell study found a median OS of 30 weeks after therapy in 19 patients (Jeffes et al., 1993). A more recent variation on this strategy has been the generation of cytokine-induced CD3+CD56+ killer cells (CIKs) by culturing peripheral blood lymphocytes (PBLs) in the presence of IFN-gamma, IL-2, anti-CD3 monoclonal antibodies, and IL-1a (Schmidt-Wolf et al., 1997). These cells have been tested preclinically and clinically on a variety of cancers (Kim et al., 2007a; Kim et al., 2009; Kim et al., 2007b). A phase III clinical trial of CIKs administered intravenously after the surgical resection of primary gliomas is now underway in Korea.
In an effort to increase the selectivity of adoptive therapy, cytotoxic T lymphocytes (CTLs) were generated by the stimulation of PBLs with IL-2 and autologous glioma cells. Two of five (40%) patients responded to this treatment with at least a 50% reduction in glioma size (Kitahara et al., 1987). Follow up strategies have attempted to generate antigen specific CTLs against MHC class I proteins, which are moderately expressed in gliomas, but not in normal brain tissue. In a study of five patients, one (20%) was alive after 40 months, and two others (40%) survived greater than 80 months on treatment (Virasch and Kruse, 2001).
Recent studies have demonstrated the overexpression of the cytomegalovirus (CMV) in malignant gliomas in comparison to normal brain (Mitchell et al., 2008; Scheurer et al., 2008). In addition, the amount of tissue infected with CMV appears to correlate with tumor grade (Scheurer et al., 2008). There are now ongoing trials of treatments involving CMV antigen-pulsed DCs and CMV specific T-cells (NCT00693095, NCT00990496) (Table 2).
5. Other antiangiogenic therapies
Angiogenesis is the development of new blood vessels necessary for the growth and progression of tumors. Therapies targeting the angiogenesis mediators VEGF, PDGFR, and HGF/SF have been reviewed above. The following section discusses therapies that target additional mediators of angiogenesis.
5.1. CD36 receptor agonist
Thrombospondin-1 (TSP-1) binds to CD36 on endothelial cells and inhibits their proliferation and migration. This naturally occurring angiogenesis inhibitor is down regulated in many gliomas and normalization of TSP-1 levels in mouse xenografts causes decreased angiogenesis and tumorigenicity (Hsu et al., 1996; Tenan et al., 2000). However, TSP-1 is difficult and costly to produce on a large scale due to its high molecular weight, and is likely immunogenic. ABT-510 is a TSP-1 mimetic drug that interacts with CD36, has anti-angiogenic properties, and induces apoptosis (Dawson et al., 1997). In vivo testing in mouse xenograft models demonstrated that ABT-510 is able to inhibit tumor growth, decrease microvessel density, and cause apoptosis of endothelial cells (Anderson et al., 2007). In a phase I trial of patients with primary gliomas, ABT-510 used as an adjunct to standard radiation therapy and TMZ had very few side effects and no maximum tolerated dose was reached. Time to disease progression of ABT-510 treated patients was 220 days and the median OS was 422 days (Kekan et al., 2009).
5.2. COX-2 inhibitors
Cyclo-oxygenase (COX) proteins are the rate limiting enzymes in prostaglandin production. The COX-1 isoform is constitutively expressed, while COX-2 expression is increased in the presence of pro-inflammatory agents such as lipopolysaccharides, cytokines, and growth factors (Hinz and Brune, 2002). Selective inhibitors of COX-2 such as celecoxib and rofecoxib have been widely used for the treatment of arthritis, acute pain and dysmenorrhea. The expression of COX-2 is increased in many cancers, and in gliomas, this increase is associated with higher grade and worse prognosis (Shono et al., 2001). In a murine xenograft model, celecoxib enhanced radiosensitivity and prolonged survival by inhibiting angiogenesis and augmenting tumor necrosis (Kang et al., 2007). In one of the first phase II trials of COX-2 inhibition, rofecoxib was used as an adjunct to TMZ and XRT for the treatment of primary gliomas. Patients experienced no significant toxicities and had a median TTP of 8 months and a median OS of 16 months (Tuettenberg et al., 2004). Additional preclinical studies demonstrated that the combination of celecoxib and irinotecan (CPT-11) was more efficacious than each drug alone (Trifan et al., 2002). In a phase II trial of this combination in patients with recurrent malignant gliomas, six patients (16%), all with recurrent GBM, achieved an objective radiographic response and an additional 13 patients (35%) achieved stable disease. The median PFS was 11.0 weeks, the 6-month PFS was 25.1% and the median OS was 31.5 weeks. (Reardon et al., 2005c). Celecoxib or rofecoxib has also been tested in combination with pioglitazone (a PPAR- agonist), thalidomide, 13-cis-retinoic acid, or cyclophosphamide. While significant safety issues did not arise in any of these trials, the added efficacy was limited (Grossman et al., 2008; Hau et al., 2007b; Kesari et al., 2008; Levin et al., 2006a). Interestingly, it has been shown that celecoxib derivatives without COX-2 inhibiting effects can also prevent angiogenesis in vitro. This suggests that mechanisms other than, or in addition to, COX-2 inhibition may be responsible for the anti-angiogenic actions of celecoxib (Schonthal, 2006).
5.3. HIF-1α inhibitors
Hypoxia inducible factor alpha (HIF-1α) is a constitutively expressed positive regulator of angiogenesis. Under normoxic conditions, it is hydroxylated by prolyl hydroxylase, ubiquitinated and degraded in proteasomes (Harris, 2002). Under hypoxic conditions, HIF-1α is not hydroxylated, and the intact protein translocates to the nucleus where it combines with the aryl hydrocarbon receptor nuclear translocator (ARNT), CBP/p300 and the DNA polymerase II (Pol II) complex. This protein assembly then binds to hypoxia-responsive elements (HREs) in the promoter regions of target genes such as erythropoietin (EPO), VEGF, lactate dehydrogenase (LDH), inducible nitric oxide synthase (iNOS), GLUT-1, and enolase-1 (ENO)(Harris, 2002). In gliomas, HIF-1α is expressed in hypoxic pseudopalisading cells and in neoplastic cells at the leading edge of tumors, promoting angiogenesis and invasion (Zagzag et al., 2000). 2-methoxyestradiol (2ME2), an inhibitor of HIF-1α production, has been tested in a phase I trial as a monotherapy for patients with recurrent glioma. There were no adverse effects and 38% of the patients had stable disease while one patient had a minor response (Kirkpatrick et al., 2007). A recent phase II trial combining 2ME2 with TMZ has been completed, but the results have not as yet been released (NCT00481455).
5.4. Thalidomide and derivatives
Thalidomide is a drug first used as an anesthetic and subsequently as an antiemetic for pregnant women, the latter with tetratogenic consequences. The teratogenicity of thalidomide is due to the blocking of vasculogenesis (D’Amato et al., 1994), a desired property in the treatment of gliomas. In the first phase I trial of recurrent glioma patients, thalidomide was found to be well tolerated and 6% of patients had partial responses, 6% had minor responses, and 33% had stable disease for 8 weeks (Fine et al., 2000). Two subsequent trials showed similar results (Marx et al., 2001; Short et al., 2001). Thalidomide has also been tried in combination with other drugs. Recurrent glioma patients who received thalidomide in combination with BCNU had a median PFS of 100 days and an objective radiographic response rate of 24%, which was higher than historical controls at the time (Fine et al., 2003). Once TMZ was found to have efficacy, a phase II study of recurrent glioma patients compared thalidomide alone to thalidomide with TMZ. Patients who received both drugs had a median survival of 103 weeks compared to 63 weeks for the thalidomide only treated group (Baumann et al., 2004). In another large trial of thalidomide with TMZ, combination therapy resulted in a six month PFS of 24%, comparable to TMZ alone (Groves et al., 2007). In a study of primary glioma patients, patients receiving combined TMZ and thalidomide had a median OS of 73 weeks, greater than that of historical control patients who did not receive adjuvant chemotherapy treatment or who received BCNU as an adjuvant (Chang et al., 2004). Thalidomide has also been tested in combination with irinotecan, with one trial for patients with recurrent gliomas and the other for patients with either primary or recurrent gliomas. There was a significant dropout rate due to toxicity and there was only a slight increase in 6 month PFS compared to historical data from other phase II trials and compared to irinotecan alone (Fadul et al., 2008; Puduvalli et al., 2008). Thalidomide and celecoxib have also been combined with metronomic etoposide in a clinical trial. This combination did not significantly improve OS in either recurrent or primary glioma patients (Kesari et al., 2007; Kesari et al., 2008).
Lenalidomide, a thalidomide analog with antitumor activity in multiple myeloma and a promising safety profile, has also been assessed clinically. In a phase II trial of recurrent glioma patients, lenalidomide was well tolerated but no more efficacious than other agents previously tested in phase II trials (Galea et al., 2007).
6. Anti-invasion agents
6.1. Integrin inhibitors
Integrins, a family of heterodimeric cell adhesion molecules composed of non-covalently associated α and β chains, mediate a wide array of cellular responses such as adhesion, migration, proliferation, cell survival, and apoptosis (Ruoslahti, 1991). The expression of αvβ3 and αvβ5 heterodimers in particular is increased in gliomas, and they are thought to regulate angiogenesis and migration (Bello et al., 2001). Cilengitide binds to and antagonizes both αvβ3 and αvβ5 with nanomolar affinity. In preclinical studies, cilengitide induced apoptosis of U87 cells grown on vitronectin coated plates and inhibited xenograft tumor growth in mice (Taga et al., 2002; Yamada et al., 2006). In a pilot study of recurrent glioma patients, the median survival was 5.6 months and there were no significant toxicities (Nabors et al., 2007a). A follow up phase II study tested two different doses, 500mg and 2000mg, of cilengitide as a single agent against recurrent gliomas. Overall, the patients who received 2000mg cilengitide had minimal difference in toxicity when compared to the 500mg dose and had a longer median OS, although the difference was not significant. Comparison between the patients receiving the 2000mg dose and previous TMZ studies at first GBM relapse showed similar 6 moth PFS values (Reardon et al., 2008b). Newly diagnosed GBM patients have also been evaluated by adding 500mg of cilengitide to the standard TMZ/XRT therapy. In a phase I/IIa study, 65.4% of patients treated with this combinational therapy reached 6 month PFS. Additionally, patients who had MGMT promoter methylation tended to have better outcomes than patients without MGMT promoter methylation(Stupp et al., 2007). There are now several ongoing trials testing the efficacy and safety of combinational cilengitide therapy (Table 2).
ATN-161, a five–amino acid peptide that binds and antagonizes several integrins including α5β1 and αvβ3, may also prevent glioma progression (Doñate et al., 2008) and a phase II clinical trial of ATN-161 and carboplatin has recently been completed with results yet to be released.
6.2. Matrix metalloproteinase antagonists
Matrix metalloproteinases (MMP) are a family of 26 endoproteases that cleave a variety of extracellular matrix substrates and enhance angiogenesis and glioma cell invasion. Several types of MMPs are overexpressed in gliomas, especially in regions adjacent to newly formed blood vessels (Raithatha et al., 2000). Marimastat, a broad MMP family inhibitor, was combined with TMZ in a phase II trial for recurrent glioma. However, the combination was not significantly more efficacious than TMZ alone and joint pain occurred in a majority of patients (Groves et al., 2006; Groves et al., 2002). A trial of newly diagnosed gliomas found that the survival of patients treated with marimastat did not significantly differ from that of patients treated with placebo (Levin et al., 2006b). A trial of the selective MMP-2 and MMP-9 inhibitor, prinomastat, in combination with TMZ, was also carried out for patients with newly diagnosed gliomas. Combination treated patients did no better than placebo or TMZ treated patients. In addition to no treatment advantage, prinomastat was associated with severe toxicities (Levin et al., 2002).
7. Epigenetic therapies
Epigenetic regulatory mechanisms such as DNA methylation, histone methylation and acetylation, and expression of microRNAs leads to heritable alterations in gene expression without changes in primary DNA sequence.
7.1. O6-methylguanine-DNA methyltransferase inhibitor
Increased expression of O6-methylguanine–DNA methyltransferase (MGMT), an enzyme that repairs DNA damage caused by drugs such as TMZ and carmustine, has been associated with increased resistance to both drugs in several clinical trials (Belanich et al., 1996; Friedman et al., 1998; Hotta et al., 1994; Jaeckle et al., 1998). Promoter methylation prevents MGMT gene expression and is associated with better response to TMZ and XRT leading to longer survival (Hegi et al., 2005). O6-benzylguanine (O6-BG), a substrate for MGMT, inactivates the enzyme and has therefore been tried in combination with either BCNU or TMZ. In a phase II study, the combination of BCNU and O6-BG caused myelosuppression and only subtherapeutic doses of BCNU could be administered safely (Quinn et al., 2002). In a phase I trial of TMZ with a single dose of O6-BG in recurrent glioma patients, myelosuppression was not as profound but a 50% reduction in TMZ dose was still needed (Quinn et al., 2005). A subsequent phase II trial of this combination reported a response rate of only 16% in recurrent AA patients and 3% in recurrent GBM patients. In addition, 48% of patients had grade 4 hematological events (Quinn et al., 2009b). In an effort to reduce toxicity, local administration of BCNU using intracavitary wafers has also been tried. Median OS was 50.3 weeks, but there was an increased incidence of CSF leaks, hydrocephalus, and CNS infections (Quinn et al., 2009a).
7.2. Histone deacetylase inhibitors
Histone deacetylase (HDAC) inhibitors promote an open chromatin structure and allow for gene transcription. In the context of glioma treatments, they could provide DNA damaging chemotherapies better access to DNA and facilitate the expression of genes leading to cell cycle arrest and apoptosis (Nagarajan and Costello, 2009). Suberoylanilide hydroxamic acid (SAHA) targets class I and II HDACs and in U87 cells increases p21 promoter histone acetylation and decreases proliferation. SAHA also inhibits the growth of GL26 tumors in mice (Yin et al., 2007) and contributes to chemotherapy and radiation sensitivity (Chinnaiyan et al., 2005; Kim et al., 2003). In a clinical trial of recurrent glioma patients, SAHA treated patients met primary efficacy endpoints, and showed no significant toxicities (Galanis et al., 2009). The median OS of patients in that study was 5.7 months. The results of four other SAHA trials are pending. Other HDAC inhibitors currently undergoing clinical testing are valproic acid, an anti-epileptic medication, and romidepsin, a bicyclic depsipeptide antibiotic isolated from the bacterium Chromobacterium violaceum.
8. Cellular development, maintenance and apoptosis
8.1. Sonic hedgehog
Expression of hedgehog (Hh) proteins, important regulators of embryogenesis and stem cell development, is often increased in a variety of primary brain tumors. The pathway between extracellular binding and intracellular signaling is the same for all three Hh protein family members, which includes Desert Hedgehog (Dhh), Indian Hedgehog (Ihh), and Sonic Hedgehog (Shh). Signaling begins by binding of the ligand Hh to its receptor, Patched1 (PTCH1). Activation of PTCH1 causes release of its constitutive inhibition of smoothened (SMO). With SMO inhibition removed, the glioma-associated oncogene homolog (Gli) family of proteins (Gli1, Gli2, and Gli3) is activated, causing the onset or termination of transcription. Changes in transcription can then result in downregulation of tumor suppressor genes or upregulation of oncogenes (Jiang and Hui, 2008).
The Gli proteins, effectors of the Hh pathway, are also involved in the pathogenesis of many cancers, including gliomas. Over expression of Gli1 was first found in gliomas leading to its name as the glioma-associated oncogene homolog (Kinzler et al., 1987). A possible role for Gli1 in oncogenesis was demonstrated in experiments in which its ectopic expression in the embryonic frog epidermis resulted in the development of tumors that expressed endogenous Gli1. Similar effects have been shown in the tadpole CNS as well (Dahmane et al., 1997; Dahmane et al., 2001). More recently, Shh-Gli1 signaling has been shown to regulate human glioma growth, cancer stem cell self-renewal, and tumorigenicity (Clement et al., 2007). These findings have prompted the development of Hh pathway inhibitors for the treatment of CNS tumors.
Cyclopamine
Shepherds in Idaho found that sheep eating the corn lily had ewes born with just one eye, leading to further investigation and discovery of the steroidal alkaloid cyclopamine. Cyclopamine’s mechanism of action is antagonism of the PTCH1 receptor, which prevents the activation of SMO (Incardona et al., 1998). Cyclopamine was the first Hh antagonist to be used in in vitro and in vivo cancer models. In vivo animal model results were promising, with reductions in tumor volume of both medulloblastomas and following treatment (Berman et al., 2002; Clement et al., 2007). However, cyclopamine’s unfavorable pharmacokinetic properties – acid sensitivity, poor solubility and weak potency relative to direct chemical derivatives – have limited its transition to clinical trials (Chen et al., 2002; Lipinski et al., 2008).
IPI-926
IPI-926 is a direct derivative of cyclopamine and has greater acid stability and aqueous solubility (Chen et al., 2002; Lipinski et al., 2008; Tremblay et al., 2009). Due to its ability to cause complete remission in a medulloblastoma allograft model, IPI-926 is now being tested in a phase I clinical trial in solid tumor patients (Tremblay et al., 2009, NCT00761696).
GDC-0449
GDC-0449 is a derivative of benzimidazole, a drug found to decrease Gli1 activity in a high-throughput drug screen. GDC-0449 was found to exhibit improved potency, solubility, and metabolic stability relative to benzimidazole and other related compounds. Further testing showed efficacy in both in vitro (CALU-6 cell line) and in vivo (medulloblastoma xenograft) models (Robarge et al., 2009). In a phase I clinical trial, drug-related adverse events included grade 2 or less dysguesia in 16% of patients, and grade 3 hyponatremia and fatigue in 10.5% and 5% of patients respectively, with no dose related toxicity observed (Molckovsky and Siu, 2008). More recently, GDC-0449 was tested on a 26 year old patient with refractory and metastatic medulloblastoma (Rudin et al., 2009). GDC-0449 caused remission of most of his metastases within 3 months of treatment. However, the patient died 6 months following treatment due to an Hh pathway mutation within the tumor. GDC-0449 is now being tested in several phase II clinical trials for refractory and recurrent medulloblastomas as well as for recurring gliomas (NCT00939484, NCT00980343, NCT00822458) (Table 2).
8.2. Topoisomerase inhibitors
Topoisomerase enzymes wind and unwind DNA during DNA repair, transcription, and replication. Type I topoisomerases, such as topo I, release DNA torsion by nicking one of the two DNA strands in an energy independent manner, while type II topoisomerases, such as topo II, cut both strands and require ATP (Wang, 2002).
Type I inhibitors are derived from camptothecin (CPT), an extract from the Camptotheca acuminate tree that had antileukemic activity in animal models but was toxic in clinical trials (Wall et al., 1966). The development of water-soluble CPT analogues such as irinotecan (CPT-11) revived interest in topo I inhibitors (Slichenmyer et al., 1994). CPT-11 has been tested in clinical trials as a monotherapy for recurrent gliomas and as a combination therapy for both recurrent and newly diagnosed gliomas. As a monotherapy, 6 month PFS ranged from 0% to at best 23% for AA. In one trial, combination treatment with BCNU was no better than irinotecan alone and had greater toxicity (Quinn et al., 2004; Reardon et al., 2004). In a trial of patients who had specifically failed first line TMZ and XRT, the combination did result in a 6 month PFS of 30% (Brandes et al., 2004). CPT-11 in combination with TMZ has also been tested using a variety of dosing regimens, although no studies thus far have been able to achieve an increase in efficacy without an increase in toxicity (Fountzilas et al., 2006; Gruber and Buster, 2004; Loghin et al., 2007; Reardon et al., 2005b). Due to observations that topo II levels increase after inhibition of topo I, a combination of VM-26, a topo II inhibitor, and CPT-11 has also been tested. The combination appeared efficacious in vitro, but patients with recurrent gliomas fared no better than historical controls of each agent administered alone (Ciesielski and Fenstermaker, 1999; Feun et al., 2007). CPT-11 has also been tried in combination with the angiogenesis inhibitors celecoxib, thalidomide and bevacizumab. Two other topo I inhibitors, topotecan and gimatecan, have also shown only limited success against gliomas (Feun and Savaraj, 2008; Hu et al., 2009).
The prototype topo II inhibitors are etoposide and doxorubicin. Both of these drugs have been tested in clinical trials as monotherapies, albeit with limited success (Parney and Chang, 2003). Doxorubicin derivatives, such as pegylated doxorubicin and RTA 744, which crosses the blood-brain barrier (BBB) and has efficacy in animal models (Conrad et al., 2006), have yet to show clinical efficacy (Conrad et al., 2007; Hau et al., 2004; Kazerooni et al., 2007).
8.3. Proteasome inhibitors
Bortezomib is a proteasome inhibitor that decreases proliferation, enhances the activity of chemotherapy and radiation, and reverses chemoresistance in a variety of hematologic and solid malignancies in vitro and in vivo. It also induces apoptosis of tumor cells by stabilizing p53, p21, p27, Bax, and IκBα, resulting in nuclear factor κB inhibition (Koschny et al., 2007; Ludwig et al., 2005; Styczynski et al., 2006). Bortezomib has been approved for the treatment of multiple myeloma and mantle cell lymphoma (Fisher et al., 2006; Richardson et al., 2005). In phase I trials of recurrent and newly diagnosed glioma patients, bortezomib combined with TMZ after radiation resulted in no grade 4 toxicities and only a small number of grade 3 toxicities. In addition, the median OS was 16.9 months for newly diagnosed glioma patients and 14.4 for recurrent glioma patients (Kubicek et al., 2009). This study did not, however, take into account the biodistribution of bortezomib. Rat experiments using radiolabeled bortezomib revealed high levels of radioactivity in the gastrointestinal tract 24 h after injection, but no radioactivity was detected in the brain (Hemeryck et al., 2007). Consistent with this, one study reported the absence of activity of bortezomib in a patient with cerebral involvement of myeloma (Mele et al., 2007).
8.4. BCL-2 inhibitors
The Bcl-2 family proteins regulate outer mitochondrial membrane permeability and can be either pro-apoptotic or anti-apoptotic. Bcl-2 proper is anti-apoptotic and inhibition of its function can lead to cell death. AT-101, a polyphenolic compound isolated from cottonseeds, antagonizes Bcl-2, Bcl-xL and Mcl-1 through direct binding, as well as upregulation, of Noxa and Puma (Wang et al., 2009). In a phase I trial of 16 patients with newly diagnosed GBM, one arm received AT-101 concurrently with TMZ and XRT and the other arm received AT-101 as a component of TMZ adjuvant therapy after chemoradiation. At the time of analysis, six of the patients were still alive with median survival times of 15.2 months and 18.2 months for the two arms, respectively (Fiveash et al., 2009).
9.0. Conclusion
Malignant gliomas are highly aggressive tumors in need of improved treatments. Our knowledge of the genetic, epigenetic, biochemical and cellular mechanisms underlying tumor development and growth has expanded tremendously over the past few years, and efforts to translate this knowledge into meaningful therapies are vigorously ongoing as evidenced by the variety of clinical trials. Drugs and strategies such as bevacizumab have shown great promise, but progress is still measured in terms of months, rather than years, of additional survival and legitimate studies reporting reproducible cures are non-existent. There are a variety of possible explanations for this. Among them are the genetic and biochemical heterogeneity of patient tumors classified simply as malignant gliomas, the use of tumor cells and lines grown using serum rather stem cell promoting conditions in most in vitro and animal studies, and the challenges of delivering therapies not only across the blood-brain barrier but to infiltrating tumor cells nestled within otherwise normal brain tissues. Continued basic science and translational research, as well as enrollment of patients in clinical trials, are necessary to make further strides against these lethal tumors.
Figure 2. Immunotherapies.
AP 12009 is an immunostimulant that acts through inhibition of TGF-β2 production by both gliomas and immature dendritic cells. TGF-β2 promotes immune suppression due to downregulation of MHC II & B7 expression, repression of T helper proliferation, and induction of T regulatory cell proliferation. Adoptive T cells help overcome immune suppression through the use of stimulated T helper and cytotoxic cells that are specific to tumor antigens. These T cells are in turn capable of activating B cells for antibody production as well as cytotoxic cells. CpG ODNS are capable of activating the otherwise suppressed T cells. Peptide and whole cell vaccines stimulates the systemic immune system to produce glioma-specific antigens, a process that is otherwise suppressed in tumors. Poly ICLCs stimulate natural killer and dendritic cell activation. Dendritic cell vaccines introduce into the patient stimulated dendritic cells that activate an immune response to the tumor.
Figure 3. Neovascularization and glioma invasion associated peptides.
HIF-1α expression is increased in response to hypoxia, which increases transcription of other pro-angiogenic peptides. 2ME2 is known to decrease HIF-1α expression. MMPs are proteinases that breakdown the extracellular matrix and open the Blood-brain barrier. The degradation of these barriers enhances glioma neovascularization and invasion. MMPs are targeted inhibited by marimastat and prinomastat. COX-2 expression is increased in gliomas. Pharmacological use of COX-2 inhibitors, such as celecoxib and rofecoxib, decreases neovascularization of gliomas.
TSP-1 is an endogenous inhibitor of neovascularization, and it is often down regulated in gliomas. ABT-510 is a TSP-1 mimetic peptide that, like TSP-1, is an agonist for CD36. VEGF is a potent angiogenic peptide that is a target for the antibody bevacizumab and the decoy receptor aflibrecept. Additionally, the VEGFR tyrosine kinase receptor, expressed on endothelial cells, is targeted by cediranib, pazopanib, sorafenib, sunitinib, vatalanib, vandetanib, and XL184.
PDGFR is also a pro-angiogenic receptor expressed on both endothelial cells and their surrounding pericytes. PDGFR is inhibited by imatinib, pazopanib, sorafenib, sunitinib, tandutinib. SF/HGF, a growth factor, has been implicated in both angiogenesis and tumor invasion. SF/HGF is inhibited by the antibody AMG-102. C-Met, the receptor for SF/HGF, has yet to be targeted.
Acknowledgments
This work was supported by the intramural research programs of the National Institute of Neurological Disorders and Stroke and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH, and the Howard Hughes Medical Institute-National Institutes of Health Research Scholars Program.
Abbreviations
- AA
anaplastic astrocytoma
- AADC
antibody dependent cellular cytotoxicity
- AG
anaplastic glioma
- ALT
alanine amino transferase
- AO
anaplastic oligodendroglioma
- APC
antigen presenting cell
- ATP
adenosine triphosphate
- BCNU
1,3-bis-(2-chloroethyl)-1-nitrosourea
- CI
confidence interval
- CNS
central nervous system
- COX
cyclo-oxygenase
- DC
dendritic cell
- DLT
dose-limiting toxicity
- EFS
event free survival
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EIAED
enzyme-inducing anti-epileptic drug
- FC
fusion cell
- GBM
glioblastoma multiforme
- Gli
glioma-associated oncogene homolog
- HIF-1α
hypoxia induxible factor alpha
- HUVEC
human umbilical vein endothelial cells
- INF
interferon
- IL-2
Interleukin-2
- LAK
lymphokine-activated killer cell
- MAPK
mitogen-activated protein kinase
- MG
malignant glioma
- MGMT
O6-methylguanine-DNA methyltransferase
- MHC
major histocompatability complex
- MTD
maximum tolerated dose
- NK
natural killer cell
- NSCLC
non-small-cell lung cancer
- O6-BG
O6-Benzylguanine
- OD
oligodendroglioma
- OS
overall survival
- p-Akt
phosphorylated Akt
- PDGF
platelet-derived growth factor
- PDGFR
platelet-derived growth factor receptor
- PI3K
phosphoinosotide 3′-kinase
- PKC
protein kinase C
- PFS
progression free survival rate
- PTCH1
patched1
- PBMC
peripheral blood mononuclear cell
- PTEN
phosphatase and tensin homolog
- RTK
receptor tyrosine kinase
- SMO
smoothend
- TMZ
temozolomide
- TTP
time to progression
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Abounader R, Laterra J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro Oncol. 2005;7(4):436–451. doi: 10.1215/S1152851705000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghi M, Visted T, Depinho RA, Chiocca EA. Oncolytic herpes virus with defective ICP6 specifically replicates in quiescent cells with homozygous genetic mutations in p16. Oncogene. 2008;27(30):4249–4254. doi: 10.1038/onc.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen C, Paraskevakou G, Liu C, Iankov ID, Msaouel P, Zollman P, et al. Oncolytic measles virus strains in the treatment of gliomas. Expert Opin Biol Ther. 2008;8(2):213–220. doi: 10.1517/14712598.8.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso MM, Gomez-Manzano C, Bekele BN, Yung WKA, Fueyo J. Adenovirus-Based Strategies Overcome Temozolomide Resistance by Silencing the O6-Methylguanine-DNA Methyltransferase Promoter. Cancer Res. 2007;67(24):11499–11504. doi: 10.1158/0008-5472.CAN-07-5312. [DOI] [PubMed] [Google Scholar]
- Anderson BD, Nakamura T, Russell SJ, Peng KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64(14):4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- Anderson JC, Grammer JR, Wang W, Nabors LB, Henkin J, Stewart JE, et al. ABT-510, a modified type 1 repeat peptide of thrombospondin, inhibits malignant glioma growth in vivo by inhibiting angiogenesis. Cancer Biol Ther. 2007;6(3):454–462. doi: 10.4161/cbt.6.3.3630. [DOI] [PubMed] [Google Scholar]
- Argyriou AA, Kalofonos HP. Molecularly targeted therapies for malignant gliomas. Mol Med. 2009;15(3–4):115–122. doi: 10.2119/molmed.2008.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba D, Saris SC, Holder C, Rosenberg SA, Oldfield EH. Intratumoral LAK cell and interleukin-2 therapy of human gliomas. J Neurosurg. 1989;70(2):175–182. doi: 10.3171/jns.1989.70.2.0175. [DOI] [PubMed] [Google Scholar]
- Bartolomei M, Mazzetta C, Handkiewicz-Junak D, Bodei L, Rocca P, Grana C, et al. Combined treatment of glioblastoma patients with locoregional pre-targeted 90Y-biotin radioimmunotherapy and temozolomide. Q J Nucl Med Mol Imaging. 2004;48(3):220–228. [PubMed] [Google Scholar]
- Basso AD, Mirza A, Liu G, Long BJ, Bishop WR, Kirschmeier P. The farnesyl transferase inhibitor (FTI) SCH66336 (lonafarnib) inhibits Rheb farnesylation and mTOR signaling. Role in FTI enhancement of taxane and tamoxifen anti-tumor activity. J Biol Chem. 2005;280(35):31101–31108. doi: 10.1074/jbc.M503763200. [DOI] [PubMed] [Google Scholar]
- Batchelor T, Eichler AF, Plotkin SR, Drappatz J, Wen P, Sorensen AG, et al. Phase I trial of vatalanib (PTK787) in combination with standard radiation and temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol (Meeting Abstracts) 2009;27(15S):2035. [Google Scholar]
- Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann F, Bjeljac M, Kollias SS, Baumert BG, Brandner S, Rousson V, et al. Combined thalidomide and temozolomide treatment in patients with glioblastoma multiforme. J Neurooncol. 2004;67(1–2):191–200. doi: 10.1023/b:neon.0000021803.01170.03. [DOI] [PubMed] [Google Scholar]
- Belanich M, Pastor M, Randall T, Guerra D, Kibitel J, Alas L, et al. Retrospective study of the correlation between the DNA repair protein alkyltransferase and survival of brain tumor patients treated with carmustine. Cancer Res. 1996;56(4):783–788. [PubMed] [Google Scholar]
- Belda-Iniesta C, Carpeño JdC, Saenz EC, Gutiérrez M, Perona R, Barón MG. Long term responses with cetuximab therapy in glioblastoma multiforme. Cancer Biol Ther. 2006;5(8):912–914. doi: 10.4161/cbt.5.8.3118. [DOI] [PubMed] [Google Scholar]
- Bello L, Francolini M, Marthyn P, Zhang J, Carroll RS, Nikas DC, et al. Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery. 2001;49(2):380–389. doi: 10.1097/00006123-200108000-00022. [DOI] [PubMed] [Google Scholar]
- Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297(5586):1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- Bigner SH, Mark J, Burger PC, Mahaley MS, Bullard DE, Muhlbaier LH, et al. Specific Chromosomal Abnormalities in Malignant Human Gliomas. Cancer Res. 1988;48(2):405–411. [PubMed] [Google Scholar]
- Blacklock JB, Grimm EA. Lymphokine-activated killer lymphocytes: LAK and interleukin-2 in the treatment of malignancies of the central nervous system. Immunol Ser. 1989;48:93–99. [PubMed] [Google Scholar]
- Bogdahn U, Hau P, Olyushin V, Mahapatra AK, Parfenov V, Venkataramana NK, et al. Targeted therapy with AP 12009 in recurrent or refractory glioblastoma patients: Results of a phase Iib study. ASCO Meeting Abstracts. 2008a;26(15_suppl):2018–2018. [Google Scholar]
- Bogdahn U, Mahapatra AK, Olyushin V, Parfenov V, Stockhammer G, Ludwig S, et al. Results of a phase Iib active-controlled study with AP 12009 for patients with recurrent or refractory anaplastic astrocytoma. ASCO Meeting Abstracts. 2008b;26(15_suppl):2076–2076. [Google Scholar]
- Bögler O, Huang HJ, Kleihues P, Cavenee WK. The p53 gene and its role in human brain tumors. Glia. 1995;15(3):308–327. doi: 10.1002/glia.440150311. [DOI] [PubMed] [Google Scholar]
- Bokstein F, Shpigel S, Blumenthal DT. Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer. 2008;112(10):2267–2273. doi: 10.1002/cncr.23401. [DOI] [PubMed] [Google Scholar]
- Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251(4995):802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- Bourdon MA, Coleman RE, Blasberg RG, Groothuis DR, Bigner DD. Monoclonal antibody localization in subcutaneous and intracranial human glioma xenografts: paired-label and imaging analysis. Anticancer Res. 1984;4(3):133–140. [PubMed] [Google Scholar]
- Bourdon MA, Wikstrand CJ, Furthmayr H, Matthews TJ, Bigner DD. Human Glioma-Mesenchymal Extracellular Matrix Antigen Defined by Monoclonal Antibody. Cancer Res. 1983;43(6):2796–2805. [PubMed] [Google Scholar]
- Boviatsis EJ, Park JS, Sena-Esteves M, Kramm CM, Chase M, Efird JT, et al. Long-term survival of rats harboring brain neoplasms treated with ganciclovir and a herpes simplex virus vector that retains an intact thymidine kinase gene. Cancer Res. 1994a;54(22):5745–5751. [PubMed] [Google Scholar]
- Boviatsis EJ, Scharf JM, Chase M, Harrington K, Kowall NW, Breakefield XO, et al. Antitumor activity and reporter gene transfer into rat brain neoplasms inoculated with herpes simplex virus vectors defective in thymidine kinase or ribonucleotide reductase. Gene Ther. 1994b;1(5):323–331. [PubMed] [Google Scholar]
- Brady LW, Markoe AM, Woo DV, Rackover MA, Koprowski H, Steplewski Z, et al. Iodine125 labeled anti-epidermal growth factor receptor-425 in the treatment of malignant astrocytomas. A pilot study. J Neurosurg Sci. 1990;34(3–4):243–249. [PubMed] [Google Scholar]
- Brandes AA, Stupp R, Hau P, Lacombe D, Gorlia T, Tosoni A, et al. EORTC study 26041–22041: phase I/II study on concomitant and adjuvant temozolomide (TMZ) and radiotherapy (RT) with PTK787/ZK222584 (PTK/ZK) in newly diagnosed glioblastoma. Eur J Cancer. 2010;46(2):348–354. doi: 10.1016/j.ejca.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Brandes AA, Tosoni A, Basso U, Reni M, Valduga F, Monfardini S, et al. Second-Line Chemotherapy With Irinotecan Plus Carmustine in Glioblastoma Recurrent or Progressive After First-Line Temozolomide Chemotherapy: A Phase II Study of the Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) J Clin Oncol. 2004;22(23):4779–4786. doi: 10.1200/JCO.2004.06.181. [DOI] [PubMed] [Google Scholar]
- Bredel M, Pollack IF. The role of protein kinase C (PKC) in the evolution and proliferation of malignant gliomas, and the application of PKC inhibition as a novel approach to anti-glioma therapy. Acta Neurochir (Wien) 1997;139(11):1000–1013. doi: 10.1007/BF01411552. [DOI] [PubMed] [Google Scholar]
- Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345(8956):1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- Brown PD, Krishnan S, Sarkaria JN, Wu W, Jaeckle KA, Uhm JH, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26(34):5603–5609. doi: 10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski RM, Yasothan U, Kirkpatrick P. Pazopanib. Nat Rev Drug Discov. 2010;9(1):17–18. doi: 10.1038/nrd3073. [DOI] [PubMed] [Google Scholar]
- Burgess T, Coxon A, Meyer S, Sun J, Rex K, Tsuruda T, et al. Fully Human Monoclonal Antibodies to Hepatocyte Growth Factor with Therapeutic Potential against Hepatocyte Growth Factor/c-Met-Dependent Human Tumors. Cancer Res. 2006;66(3):1721–1729. doi: 10.1158/0008-5472.CAN-05-3329. [DOI] [PubMed] [Google Scholar]
- Butowski N, Chang SM, Junck L, DeAngelis LM, Abrey L, Fink K, et al. A phase II clinical trial of poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: a North American Brain Tumor Consortium (NABTC01–05) J Neurooncol. 2008;91(2):175–182. doi: 10.1007/s11060-008-9693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowski N, Lamborn KR, Lee BL, Prados MD, Cloughesy T, DeAngelis LM, et al. A North American brain tumor consortium phase II study of poly-ICLC for adult patients with recurrent anaplastic gliomas. J Neurooncol. 2009;91(2):183–189. doi: 10.1007/s11060-008-9705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Carpentier A, Laigle-Donadey F, Zohar S, Capelle L, Behin A, Tibi A, et al. Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma. Neuro Oncol. 2006;8(1):60–66. doi: 10.1215/S1522851705000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso DA, Orme LM, Neale AM, Radcliff FJ, Amor GM, Maixner W, et al. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro Oncol. 2004;6(3):236–246. doi: 10.1215/S1152851703000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady KA, Gross M, Roizman B. The second-site mutation in the herpes simplex virus recombinants lacking the gamma134.5 genes precludes shutoff of protein synthesis by blocking the phosphorylation of eIF-2alpha. J Virol. 1998;72(9):7005–7011. doi: 10.1128/jvi.72.9.7005-7011.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CBTRUS. Statistical Report: Primary Brain Tumors in the United States, 2000–2004. Central Brain Tumor Registry of the United States; 2007. [Google Scholar]
- Chakravarti A, Chakladar A, Delaney MA, Latham DE, Loeffler JS. The epidermal growth factor receptor pathway mediates resistance to sequential administration of radiation and chemotherapy in primary human glioblastoma cells in a RAS-dependent manner. Cancer Res. 2002;62(15):4307–4315. [PubMed] [Google Scholar]
- Chakravarti A, Zhai G, Suzuki Y, Sarkesh S, Black PM, Muzikansky A, et al. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol. 2004;22(10):1926–1933. doi: 10.1200/JCO.2004.07.193. [DOI] [PubMed] [Google Scholar]
- Chan S. Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer. 2004;91(8):1420–1424. doi: 10.1038/sj.bjc.6602162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SM, Lamborn KR, Malec M, Larson D, Wara W, Sneed P, et al. Phase II study of temozolomide and thalidomide with radiation therapy for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2004;60(2):353–357. doi: 10.1016/j.ijrobp.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Chang SM, Wen P, Cloughesy T, Greenberg H, Schiff D, Conrad C, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23(4):357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A. 2002;99(22):14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Zhang W, Wu S, Bi F, Su YJ, Tan XY, et al. Vaccination with Viable Human Umbilical Vein Endothelial Cells Prevents Metastatic Tumors by Attack on Tumor Vasculature with Both Cellular and Humoral Immunity. Clin Cancer Res. 2006;12(19):5834–5840. doi: 10.1158/1078-0432.CCR-06-1105. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan P, Huang S, Armstrong E, Harari PM. Radiosensitization following EGFR signaling inhibition by erlotinib (Tarceva(TM)) Int J Radiat Oncol Biol Phys. 2003;57(2, Supplement 1):S294–S294. [doi: DOI: 10.1016/S0360-3016(03)01153-2] [Google Scholar]
- Chinnaiyan P, Vallabhaneni G, Armstrong E, Huang SM, Harari PM. Modulation of radiation response by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys. 2005;62(1):223–229. doi: 10.1016/j.ijrobp.2004.12.088. [DOI] [PubMed] [Google Scholar]
- Chiocca EA, Abbed K, Tatter S, Louis D, Hochberg F, Barker F, et al. A Phase I Open-Label, Dose-Escalation, Multi-Institutional Trial of Injection with an -Attenuated Adenovirus, ONYX-015, into the Peritumoral Region of Recurrent Malignant Gliomas, in the Adjuvant Setting. Mol Ther. 2004;10(5):958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Chiocca EA, Smith KM, McKinney B, Palmer CA, Rosenfeld S, Lillehei K, et al. A Phase I Trial of Ad.hIFN-β Gene Therapy for Glioma. Mol Ther. 2008;16(3):618–626. doi: 10.1038/sj.mt.6300396. [DOI] [PubMed] [Google Scholar]
- Ciesielski MJ, Fenstermaker RA. Synergistic cytotoxicity, apoptosis and protein-linked DNA breakage by etoposide and camptothecin in human U87 glioma cells: dependence on tyrosine phosphorylation. J Neurooncol. 1999;41(3):223–234. doi: 10.1023/a:1006129119460. [DOI] [PubMed] [Google Scholar]
- Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 Signaling Regulates Human Glioma Growth, Cancer Stem Cell Self-Renewal, and Tumorigenicity. Curr Biol. 2007;17(2):165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloughesy TF, Kuhn J, Robins HI, Abrey L, Wen P, Fink K, et al. Phase I trial of tipifarnib in patients with recurrent malignant glioma taking enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol. 2005;23(27):6647–6656. doi: 10.1200/JCO.2005.10.068. [DOI] [PubMed] [Google Scholar]
- Cloughesy TF, Wen PY, Robins HI, Chang SM, Groves MD, Fink KL, et al. Phase II Trial of Tipifarnib in Patients With Recurrent Malignant Glioma Either Receiving or Not Receiving Enzyme-Inducing Antiepileptic Drugs: A North American Brain Tumor Consortium Study. J Clin Oncol. 2006;24(22):3651–3656. doi: 10.1200/JCO.2006.06.2323. [DOI] [PubMed] [Google Scholar]
- Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5(1):e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Jonathan Moyal E, Laprie A, Delannes M, Poublanc M, Catalaa I, Dalenc F, et al. Phase I Trial of Tipifarnib (R115777) Concurrent With Radiotherapy in Patients with Glioblastoma Multiforme. Int J Radiat Oncol Biol Phys. 2007;68(5):1396–1401. doi: 10.1016/j.ijrobp.2007.02.043. [doi: DOI: 10.1016/j.ijrobp.2007.02.043] [DOI] [PubMed] [Google Scholar]
- Cohen MH, Shen YL, Keegan P, Pazdur R. FDA Drug Approval Summary: Bevacizumab (Avastin(R)) as Treatment of Recurrent Glioblastoma Multiforme. Oncologist. 2009;14(11):1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- Conrad CA, Maher E, Cloughesy T, Groves M, Gilbert M, Madden T, et al. Final results of a phase I trial with the novel anthracycline derivative RTA 744 in patients with primary brain tumors. ASCO Meeting Abstracts. 2007;25(18_suppl):2062–2062. [Google Scholar]
- Conrad CA, Meyer C, Madden T, Priebe W, Lang FF. Survival study of RTA 744 (currently a single agent phase I study) alone and in combination with temozolomide in orthotopic model of glioma. ASCO Meeting Abstracts. 2006;24(18_suppl):1577–1577. [Google Scholar]
- Crombet T, Torres O, Rodríguez V, Menéndez A, Stevenson A, Ramos M, et al. Phase I clinical evaluation of a neutralizing monoclonal antibody against epidermal growth factor receptor in advanced brain tumor patients: preliminary study. Hybridoma. 2001;20(2):131–136. doi: 10.1089/02724570152057634. [DOI] [PubMed] [Google Scholar]
- Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL. eIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3′UTR. J Cell Biol. 2005;169(2):245–256. doi: 10.1083/jcb.200501019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL. eIF4E is a central node of an RNA regulon that governs cellular proliferation. J Cell Biol. 2006;175(3):415–426. doi: 10.1083/jcb.200607020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel DT, Alemany R, Balagué C. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18(7):723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91(9):4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha AB, Mans DR, Regner A, Schwartsmann G. Targeting protein kinase C: new therapeutic opportunities against high-grade malignant gliomas? Oncologist. 2002;7(1):17–33. doi: 10.1634/theoncologist.7-1-17. [DOI] [PubMed] [Google Scholar]
- Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389(6653):876–881. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- Dahmane N, Sánchez P, Gitton Y, Palma V, Sun T, Beyna M, et al. The Sonic Hedgehog-Gli pathway regulates dorsal brain growth and tumorigenesis. Development. 2001;128(24):5201–5212. doi: 10.1242/dev.128.24.5201. [DOI] [PubMed] [Google Scholar]
- Damiano V, Melisi D, Bianco C, Raben D, Caputo R, Fontanini G, et al. Cooperative antitumor effect of multitargeted kinase inhibitor ZD6474 and ionizing radiation in glioblastoma. Clin Cancer Res. 2005;11(15):5639–5644. doi: 10.1158/1078-0432.CCR-05-0174. [DOI] [PubMed] [Google Scholar]
- Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol. 2010;7(4):209–219. doi: 10.1038/nrclinonc.2010.21. [DOI] [PubMed] [Google Scholar]
- Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138(3):707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot J, Milano V. Improving the prognosis for patients with glioblastoma: the rationale for targeting Src. J Neurooncol. 2009;95(2):151–163. doi: 10.1007/s11060-009-9916-2. [DOI] [PubMed] [Google Scholar]
- de Groot JF, Gilbert MR, Aldape K, Hess KR, Hanna TA, Ictech S, et al. Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J Neurooncol. 2008a;90(1):89–97. doi: 10.1007/s11060-008-9637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot JF, Prados M, Urquhart T, Robertson S, Yaron Y, Sorensen AG, et al. A phase II study of XL184 in patients (pts) with progressive glioblastoma multiforme (GBM) in first or second relapse. J Clin Oncol (Meeting Abstracts) 2009;27(15S):2047. [Google Scholar]
- De Groot JF, Wen PY, Lamborn K, Chang S, Cloughesy TF, Chen AP, et al. Phase II single arm trial of aflibercept in patients with recurrent temozolomide-resistant glioblastoma: NABTC 0601. J Clin Oncol (Meeting Abstracts) 2008b;26(15S):2020. [Google Scholar]
- De Pace NG. Sulla scomparsa di un enorme cancro vegetante del collo dell’utero senza cura chirurgica. Ginecologia. 1912;9:82–88. [Google Scholar]
- De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14(10):3098–3104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res. 1995;1(11):1253–1258. [PubMed] [Google Scholar]
- Debinski W, Tatter SB. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother. 2009;9(10):1519–1527. doi: 10.1586/ern.09.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas C, End D, Rochaix P, Favre G, Toulas C, Cohen-Jonathan E. The farnesyltransferase inhibitor R115777 reduces hypoxia and matrix metalloproteinase 2 expression in human glioma xenograft. Clin Cancer Res. 2003;9(16 Pt 1):6062–6068. [PubMed] [Google Scholar]
- Delmas C, Heliez C, Cohen-Jonathan E, End D, Bonnet J, Favre G, et al. Farnesyltransferase inhibitor, R115777, reverses the resistance of human glioma cell lines to ionizing radiation. Int J Cancer. 2002;100(1):43–48. doi: 10.1002/ijc.10439. [DOI] [PubMed] [Google Scholar]
- DePrimo S, Wu B, Huang S, Bautista R, Cancilla B, Vysotskaia V, et al. Correlative tumor molecular profiling and plasma biomarker analysis in a phase II study of XL184 in patients with progressive or recurrent glioblastoma multiforme (GBM) J Clin Oncol (Meeting Abstracts) 2009;27(15S):2049. [Google Scholar]
- Deshane J, Garner CC, Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003;278(6):4135–4144. doi: 10.1074/jbc.M205662200. [DOI] [PubMed] [Google Scholar]
- Desjardins A, Quinn JA, Vredenburgh JJ, Sathornsumetee S, Friedman AH, Herndon JE, et al. Phase II study of imatinib mesylate and hydroxyurea for recurrent grade III malignant gliomas. J Neurooncol. 2007;83(1):53–60. doi: 10.1007/s11060-006-9302-2. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Wang D, Batchelor TT. Cediranib: profile of a novel anti-angiogenic agent in patients with glioblastoma. Expert Opin Investig Drugs. 2009;18(10):1549–1557. doi: 10.1517/13543780903183528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman RO, Duma CM, Ellis RA, Cornforth AN, Schiltz PM, Sharp SL, et al. Intralesional lymphokine-activated killer cells as adjuvant therapy for primary glioblastoma. J Immunother. 2009;32(9):914–919. doi: 10.1097/CJI.0b013e3181b2910f. [DOI] [PubMed] [Google Scholar]
- Dillman RO, Duma CM, Schiltz PM, DePriest C, Ellis RA, Okamoto K, et al. Intracavitary placement of autologous lymphokine-activated killer (LAK) cells after resection of recurrent glioblastoma. J Immunother. 2004;27(5):398–404. doi: 10.1097/00002371-200409000-00009. [DOI] [PubMed] [Google Scholar]
- Doherty L, Gigas DC, Kesari S, Drappatz J, Kim R, Zimmerman J, et al. Pilot study of the combination of EGFR and mTOR inhibitors in recurrent malignant gliomas. Neurology. 2006;67(1):156–158. doi: 10.1212/01.wnl.0000223844.77636.29. [DOI] [PubMed] [Google Scholar]
- Doñate F, Parry GC, Shaked Y, Hensley H, Guan X, Beck I, et al. Pharmacology of the Novel Antiangiogenic Peptide ATN-161 (Ac-PHSCN-NH2): Observation of a U-Shaped Dose-Response Curve in Several Preclinical Models of Angiogenesis and Tumor Growth. Clin Cancer Res. 2008;14(7):2137–2144. doi: 10.1158/1078-0432.CCR-07-4530. [DOI] [PubMed] [Google Scholar]
- Drappatz J, Norden AD, Wong ET, Lassman AB, Doherty L, LaFrankie D, et al. Phase I study of vandetanib with radiation therapy and temozolomide for newly diagnosed glioblastoma. J Clin Oncol (Meeting Abstracts) 2009;27(15S):2031. [Google Scholar]
- Dresemann G. Imatinib and hydroxyurea in pretreated progressive glioblastoma multiforme: a patient series. Ann Oncol. 2005;16(10):1702–1708. doi: 10.1093/annonc/mdi317. [DOI] [PubMed] [Google Scholar]
- Dresemann G, Weller M, Rosenthal MA, Wedding U, Wagner W, Engel E, et al. Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. J Neurooncol. 2010;96(3):393–402. doi: 10.1007/s11060-009-9976-3. [DOI] [PubMed] [Google Scholar]
- Dumont RA, Hildebrandt I, Su H, Haubner R, Reischl G, Czernin JG, et al. Noninvasive imaging of alphaVbeta3 function as a predictor of the antimigratory and antiproliferative effects of dasatinib. Cancer Res. 2009;69(7):3173–3179. doi: 10.1158/0008-5472.CAN-08-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N, Harlow E. Adenovirus E1A targets key regulators of cell proliferation. Cancer Surv. 1992;12:161–195. [PubMed] [Google Scholar]
- Eller JL, Longo SL, Kyle MM, Bassano D, Hicklin DJ, Canute GW. Anti-epidermal growth factor receptor monoclonal antibody cetuximab augments radiation effects in glioblastoma multiforme in vitro and in vivo. Neurosurgery. 2005;56(1):155–162. doi: 10.1227/01.neu.0000145865.25689.55. discussion 162-155-162; discussion 162. [DOI] [PubMed] [Google Scholar]
- Elshami AA, Saavedra A, Zhang H, Kucharczuk JC, Spray DC, Fishman GI, et al. Gap junctions play a role in the ‘bystander effect’ of the herpes simplex virus thymidine kinase/ganciclovir system in vitro. Gene Ther. 1996;3(1):85–92. [PubMed] [Google Scholar]
- Eskens FA, Mom CH, Planting AS, Gietema JA, Amelsberg A, Huisman H, et al. A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br J Cancer. 2008;98(1):80–85. doi: 10.1038/sj.bjc.6604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espevik T, Figari IS, Shalaby MR, Lackides GA, Lewis GD, Shepard HM, et al. Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J Exp Med. 1987;166(2):571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadul CE, Kingman LS, Meyer LP, Cole BF, Eskey CJ, Rhodes CH, et al. A phase II study of thalidomide and irinotecan for treatment of glioblastoma multiforme. J Neurooncol. 2008;90(2):229–235. doi: 10.1007/s11060-008-9655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldkamp MM, Lau N, Roncari L, Guha A. Isotype-specific Ras.GTP-levels predict the efficacy of farnesyl transferase inhibitors against human astrocytomas regardless of Ras mutational status. Cancer Res. 2001;61(11):4425–4431. [PubMed] [Google Scholar]
- Feun L, Savaraj N. Topoisomerase I inhibitors for the treatment of brain tumors. Expert Rev Anticancer Ther. 2008;8(5):707–716. doi: 10.1586/14737140.8.5.707. [DOI] [PubMed] [Google Scholar]
- Feun LG, Marini A, Landy H, Markoe A, Heros D, Robles C, et al. Clinical trial of CPT-11 and VM-26/VP-16 for patients with recurrent malignant brain tumors. J Neurooncol. 2007;82(2):177–181. doi: 10.1007/s11060-006-9261-7. [DOI] [PubMed] [Google Scholar]
- Fine HA, Figg WD, Jaeckle K, Wen PY, Kyritsis AP, Loeffler JS, et al. Phase II trial of the antiangiogenic agent thalidomide in patients with recurrent high-grade gliomas. J Clin Oncol. 2000;18(4):708–715. doi: 10.1200/JCO.2000.18.4.708. [DOI] [PubMed] [Google Scholar]
- Fine HA, Kim L, Royce C, Draper D, Haggarty I, Ellinzano H, et al. Results from phase II trial of enzastaurin ( LY317615) in patients with recurrent high grade gliomas. J Clin Oncol (Meeting Abstracts) 2005;23(16_suppl):1504. [Google Scholar]
- Fine HA, Wen PY, Maher EA, Viscosi E, Batchelor T, Lakhani N, et al. Phase II Trial of Thalidomide and Carmustine for Patients With Recurrent High-Grade Gliomas. J Clin Oncol. 2003;21(12):2299–2304. doi: 10.1200/JCO.2003.08.045. [DOI] [PubMed] [Google Scholar]
- Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, et al. Multicenter Phase II Study of Bortezomib in Patients With Relapsed or Refractory Mantle Cell Lymphoma. J Clin Oncol. 2006;24(30):4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- Fiveash JB, Chowdhary SA, Peereboom D, Mikkelsen T, Nabors LB, Lesser GJ, et al. NABTT-0702: A phase II study of R-(−)-gossypol (AT-101) in recurrent glioblastoma multiforme (GBM) ASCO Meeting Abstracts. 2009;27(15S):2010–2010. [Google Scholar]
- Forsyth P, Roldán G, George D, Wallace C, Palmer CA, Morris D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16(3):627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- Fountzilas G, Karkavelas G, Kalogera-Fountzila A, Karina M, Ignatiadis M, Koukoulis G, et al. Post-operative combined radiation and chemotherapy with temozolomide and irinotecan in patients with high-grade astrocytic tumors. A phase II study with biomarker evaluation. Anticancer Res. 2006;26(6C):4675–4686. [PubMed] [Google Scholar]
- Franceschi E, Cavallo G, Lonardi S, Magrini E, Tosoni A, Grosso D, et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Br J Cancer. 2007;96(7):1047–1051. doi: 10.1038/sj.bjc.6603669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL, et al. The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53(21):5274–5283. [PubMed] [Google Scholar]
- Friedman HS, McLendon RE, Kerby T, Dugan M, Bigner SH, Henry AJ, et al. DNA mismatch repair and O6-alkylguanine-DNA alkyltransferase analysis and response to Temodal in newly diagnosed malignant glioma. J Clin Oncol. 1998;16(12):3851–3857. doi: 10.1200/JCO.1998.16.12.3851. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Petros WP, Friedman AH, Schaaf LJ, Kerby T, Lawyer J, et al. Irinotecan Therapy in Adults With Recurrent or Progressive Malignant Glioma. J Clin Oncol. 1999;17(5):1516. doi: 10.1200/JCO.1999.17.5.1516. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- Fueyo Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95(9):652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- Fueyo Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19(1):2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- Fukai J, Nishio K, Itakura T, Koizumi F. Antitumor activity of cetuximab against malignant glioma cells overexpressing EGFR deletion mutant variant III. Cancer Sci. 2008;99(10):2062–2069. doi: 10.1111/j.1349-7006.2008.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis E, Buckner JC, Maurer MJ, Kreisberg JI, Ballman K, Boni J, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23(23):5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- Galanis E, Jaeckle KA, Maurer MJ, Reid JM, Ames MM, Hardwick JS, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol. 2009;27(12):2052–2058. doi: 10.1200/JCO.2008.19.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28(1):12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Gerosa MA, Talarico D, Fognani C, Raimondi E, Colombatti M, Tridente G, et al. Overexpression of N-ras Oncogene and Epidermal Growth Factor Receptor Gene in Human Glioblastomas. J Natl Cancer Inst. 1989;81(1):63–67. doi: 10.1093/jnci/81.1.63. [DOI] [PubMed] [Google Scholar]
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus Capecitabine for HER2-Positive Advanced Breast Cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- Giannopoulou E, Dimitropoulos K, Argyriou AA, Koutras AK, Dimitrakopoulos F, Kalofonos HP. An in vitro study, evaluating the effect of sunitinib and/or lapatinib on two glioma cell lines. Invest New Drugs. 2009 doi: 10.1007/s10637-009-9290-0. [DOI] [PubMed] [Google Scholar]
- Gilbert MR, Gaupp P, Liu V, Conrad C, Colman H, Groves M, et al. A phase I study of temozolomide (TMZ) and the farnesyltransferase inhibitor (FTI), lonafarnib (Sarazar, SCH66336) in recurrent glioblastoma. J Clin Oncol (Meeting Abstracts) 2006;24(18_suppl):1556. [Google Scholar]
- Glass TL, Liu TJ, Yung WK. Inhibition of cell growth in human glioblastoma cell lines by farnesyltransferase inhibitor SCH66336. Neuro Oncol. 2000;2(3):151–158. doi: 10.1093/neuonc/2.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbrunner RH, Bendszus M, Wood J, Kiderlen M, Sasaki M, Tonn JC. PTK787/ZK222584, an inhibitor of vascular endothelial growth factor receptor tyrosine kinases, decreases glioma growth and vascularization. Neurosurgery. 2004;55(2):426–432. doi: 10.1227/01.neu.0000129551.64651.74. discussion 432. [DOI] [PubMed] [Google Scholar]
- Gomez-Manzano C, Holash J, Fueyo J, Xu J, Conrad CA, Aldape KD, et al. VEGF Trap induces antiglioma effect at different stages of disease. Neuro Oncol. 2008;10(6):940–945. doi: 10.1215/15228517-2008-061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff JR, Konicek BW, Carter JH, Marcusson EG. Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 2008;68(3):631–634. doi: 10.1158/0008-5472.CAN-07-5635. [DOI] [PubMed] [Google Scholar]
- Graff JR, McNulty AM, Hanna KR, Konicek BW, Lynch RL, Bailey SN, et al. The protein kinase Cbeta-selective inhibitor, Enzastaurin ( LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65(16):7462–7469. doi: 10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- Grana C, Chinol M, Robertson C, Mazzetta C, Bartolomei M, De Cicco C, et al. Pretargeted adjuvant radioimmunotherapy with yttrium-90-biotin in malignant glioma patients: a pilot study. Br J Cancer. 2002;86(2):207–212. doi: 10.1038/sj.bjc.6600047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield L, Johnson VG, Youle RJ. Mutations in diphtheria toxin separate binding from entry and amplify immunotoxin selectivity. Science. 1987;238(4826):536–539. doi: 10.1126/science.3498987. [DOI] [PubMed] [Google Scholar]
- Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SA, Olson J, Batchelor T, Peereboom D, Lesser G, Desideri S, et al. Effect of phenytoin on celecoxib pharmacokinetics in patients with glioblastoma. Neuro Oncol. 2008;10(2):190–198. doi: 10.1215/15228517-2007-055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote D, Russell SJ, Cornu TI, Cattaneo R, Vile R, Poland GA, et al. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97(12):3746–3754. doi: 10.1182/blood.v97.12.3746. [DOI] [PubMed] [Google Scholar]
- Groves MD, Puduvalli VK, Chang SM, Conrad CA, Gilbert MR, Tremont-Lukats IW, et al. A North American brain tumor consortium (NABTC 99–04) phase II trial of temozolomide plus thalidomide for recurrent glioblastoma multiforme. J Neurooncol. 2007;81(3):271–277. doi: 10.1007/s11060-006-9225-y. [DOI] [PubMed] [Google Scholar]
- Groves MD, Puduvalli VK, Conrad CA, Gilbert MR, Yung WKA, Jaeckle K, et al. Phase II trial of temozolomide plus marimastat for recurrent anaplastic gliomas: a relationship among efficacy, joint toxicity and anticonvulsant status. J Neurooncol. 2006;80(1):83–90. doi: 10.1007/s11060-006-9160-y. [DOI] [PubMed] [Google Scholar]
- Groves MD, Puduvalli VK, Hess KR, Jaeckle KA, Peterson P, Yung WKA, et al. Phase II trial of temozolomide plus the matrix metalloproteinase inhibitor, marimastat, in recurrent and progressive glioblastoma multiforme. J Clin Oncol. 2002;20(5):1383–1388. doi: 10.1200/JCO.2002.20.5.1383. [DOI] [PubMed] [Google Scholar]
- Gruber ML, Buster WP. Temozolomide in combination with irinotecan for treatment of recurrent malignant glioma. Am J Clin Oncol. 2004;27(1):33–38. doi: 10.1097/01.coc.0000045852.88461.80. [DOI] [PubMed] [Google Scholar]
- Guha A, Dashner K, Black PM, Wagner JA, Stiles CD. Expression of PDGF and PDGF receptors in human astrocytoma operation specimens supports the existence of an autocrine loop. Int J Cancer. 1995;60(2):168–173. doi: 10.1002/ijc.2910600206. [DOI] [PubMed] [Google Scholar]
- Hakkarainen T, Hemminki A, Curiel DT, Wahlfors J. A conditionally replicative adenovirus that codes for a TK-GFP fusion protein (Ad5Delta24TK-GFP) for evaluation of the potency of oncolytic virotherapy combined with molecular chemotherapy. Int J Mol Med. 2006;18(4):751–759. [PMC free article] [PubMed] [Google Scholar]
- Harris AL. Hyoxia - A Key Regulatory Factor in Tumor Growth. Nat Rev Cancer. 2002;2(1):38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Harris PA, Boloor A, Cheung M, Kumar R, Crosby RM, Davis-Ward RG, et al. Discovery of 5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-m ethyl-benzenesulfonamide (Pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor. J Med Chem. 2008;51(15):4632–4640. doi: 10.1021/jm800566m. [DOI] [PubMed] [Google Scholar]
- Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11(22):1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- Hau P, Fabel K, Baumgart U, Rümmele P, Grauer O, Bock A, et al. Pegylated liposomal doxorubicin-efficacy in patients with recurrent high-grade glioma. Cancer. 2004;100(6):1199–1207. doi: 10.1002/cncr.20073. [DOI] [PubMed] [Google Scholar]
- Hau P, Jachimczak P, Bogdahn U. Treatment of malignant gliomas with TGF-beta2 antisense oligonucleotides. Expert Rev Anticancer Ther. 2009;9(11):1663–1674. doi: 10.1586/era.09.138. [DOI] [PubMed] [Google Scholar]
- Hau P, Jachimczak P, Schlingensiepen R, Schulmeyer F, Jauch T, Steinbrecher A, et al. Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides. 2007a;17(2):201–212. doi: 10.1089/oli.2006.0053. [DOI] [PubMed] [Google Scholar]
- Hau P, Kunz-Schughart L, Bogdahn U, Baumgart U, Hirschmann B, Weimann E, et al. Low-Dose Chemotherapy in Combination with COX-2 Inhibitors and PPAR-Gamma Agonists in Recurrent High-Grade Gliomas – A Phase II Study. Oncology. 2007b;73(1–2):21–25. doi: 10.1159/000120028. [DOI] [PubMed] [Google Scholar]
- Hayes RL, Koslow M, Hiesiger EM, Hymes KB, Moore EJ, Pierz DM, et al. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer. 1995;76(5):840–852. doi: 10.1002/1097-0142(19950901)76:5<840::aid-cncr2820760519>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A. 1997;94(3):843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- Heimberger AB, Hussain SF, Aldape K, Sawaya R, Archer GA, Friedman H, et al. Tumor-specific peptide vaccination in newly-diagnosed patients with GBM. ASCO Meeting Abstracts. 2006;24(18_suppl):2529–2529. [Google Scholar]
- Heimberger AB, Sampson JH. The PEPvIII-KLH (CDX-110) vaccine in glioblastoma multiforme patients. Expert Opin Biol Ther. 2009;9(8):1087–1098. doi: 10.1517/14712590903124346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemeryck A, Geerts R, Monbaliu J, Hassler S, Verhaeghe T, Diels L, et al. Tissue distribution and depletion kinetics of bortezomib and bortezomib-related radioactivity in male rats after single and repeated intravenous injection of 14 C-bortezomib. Cancer Chemother Pharmacol. 2007;60(6):777–787. doi: 10.1007/s00280-007-0424-9. [DOI] [PubMed] [Google Scholar]
- Hermanson M, Funa K, Hartman M, Claesson-Welsh L, Heldin CH, Westermark B, et al. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52(11):3213–3219. [PubMed] [Google Scholar]
- Hibner B, Iartchouk N, Robertson R, Guo R, Smith M, Bradley D, et al. Activity of Tandutinib (MLN0518) in xenograft models of glioma. Paper presented at the Neuro Oncol.2008. [Google Scholar]
- Hinz B, Brune K. Cyclooxygenase-2—10 Years Later. J Pharmacol Exp The. 2002;300(2):367–375. doi: 10.1124/jpet.300.2.367. [DOI] [PubMed] [Google Scholar]
- Holash J. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdhoff M, Kreuzer KA, Appelt C, Scholz R, Na IK, Hildebrandt B, et al. Imatinib mesylate radiosensitizes human glioblastoma cells through inhibition of platelet-derived growth factor receptor. Blood Cells Mol Dis. 2005;34(2):181–185. doi: 10.1016/j.bcmd.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Holmen SL, Williams BO. Essential role for Ras signaling in glioblastoma maintenance. Cancer Res. 2005;65(18):8250–8255. doi: 10.1158/0008-5472.CAN-05-1173. [DOI] [PubMed] [Google Scholar]
- Hotta T, Saito Y, Fujita H, Mikami T, Kurisu K, Kiya K, et al. O6-alkylguanine-DNA alkyltransferase activity of human malignant glioma and its clinical implications. J Neurooncol. 1994;21(2):135–140. doi: 10.1007/BF01052897. [DOI] [PubMed] [Google Scholar]
- Hsu SC, Volpert OV, Steck PA, Mikkelsen T, Polverini PJ, Rao S, et al. Inhibition of Angiogenesis in Human Glioblastomas by Chromosome 10 Induction of Thrombospondin-1. Cancer Res. 1996;56(24):5684–5691. [PubMed] [Google Scholar]
- Hu J, Wen PY, Abrey LE, Fadul C, Drappatz J, Salem N, et al. Phase II trial of oral gimatecan in adults with recurrent glioblastoma. ASCO Meeting Abstracts. 2009;27(15S):2009–2009. [Google Scholar]
- Hu X, Pandolfi PP, Li Y, Koutcher JA, Rosenblum M, Holland EC. mTOR promotes survival and astrocytic characteristics induced by Pten/AKT signaling in glioblastoma. Neoplasia. 2005;7(4):356–368. doi: 10.1593/neo.04595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner RJ, Rowe WP, Schatten WE, Smith RR, Thomas LB. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956;9(6):1211–1218. doi: 10.1002/1097-0142(195611/12)9:6<1211::aid-cncr2820090624>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Hurwitz HI, Dowlati A, Saini S, Savage S, Suttle AB, Gibson DM, et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. 2009;15(12):4220–4227. doi: 10.1158/1078-0432.CCR-08-2740. [DOI] [PubMed] [Google Scholar]
- Husain SR, Joshi BH, Puri RK. Interleukin-13 receptor as a unique target for anti-glioblastoma therapy. Int J Cancer. 2001;92(2):168–175. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1182>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Husain SR, Puri RK. Interleukin-13 receptor-directed cytotoxin for malignant glioma therapy: from bench to bedside. J Neurooncol. 2003;65(1):37–48. doi: 10.1023/a:1026242432647. [DOI] [PubMed] [Google Scholar]
- Immonen A, Vapalahti M, Tyynelä K, Hurskainen H, Sandmair A, Vanninen R, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004;10(5):967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Gaffield W, Kapur RP, Roelink H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125(18):3553–3562. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- Ingram M, Buckwalter JG, Jacques DB, Freshwater DB, Abts RM, Techy GB, et al. Immunotherapy for recurrent malignant glioma: an interim report on survival. Neurol Res. 1990;12(4):265–273. doi: 10.1080/01616412.1990.11739955. [DOI] [PubMed] [Google Scholar]
- Ingram M, Jacques S, Freshwater DB, Techy GB, Shelden CH, Helsper JT. Salvage Immunotherapy of Malignant Glioma. Arch Surg. 1987;122(12):1483–1486. doi: 10.1001/archsurg.1987.01400240131025. [DOI] [PubMed] [Google Scholar]
- Ishikawa E, Tsuboi K, Yamamoto T, Muroi A, Takano S, Enomoto T, et al. Clinical trial of autologous formalin-fixed tumor vaccine for glioblastoma multiforme patients. Cancer Sci. 2007;98(8):1226–1233. doi: 10.1111/j.1349-7006.2007.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo M, Cortés ML, Martín V, de Felipe P, Izquierdo JM, Pérez-Higueras A, et al. Gene therapy in brain tumours: implications of the size of glioblastoma on its curability. Acta Neurochir Suppl. 1997;68:111–117. doi: 10.1007/978-3-7091-6513-3_21. [DOI] [PubMed] [Google Scholar]
- Izumoto S, Tsuboi A, Oka Y, Suzuki T, Hashiba T, Kagawa N, et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg. 2008;108(5):963–971. doi: 10.3171/JNS/2008/108/5/0963. [DOI] [PubMed] [Google Scholar]
- Jacobs SK, Wilson DJ, Kornblith PL, Grimm EA. Interleukin-2 or autologous lymphokine-activated killer cell treatment of malignant glioma: phase I trial. Cancer Res. 1986;46(4 Pt 2):2101–2104. [PubMed] [Google Scholar]
- Jaeckle KA, Eyre HJ, Townsend JJ, Schulman S, Knudson HM, Belanich M, et al. Correlation of tumor O6 methylguanine-DNA methyltransferase levels with survival of malignant astrocytoma patients treated with bis- chloroethylnitrosourea: a Southwest Oncology Group study. J Clin Oncol. 1998;16(10):3310–3315. doi: 10.1200/JCO.1998.16.10.3310. [DOI] [PubMed] [Google Scholar]
- Jane EP, Premkumar DR, Addo-Yobo SO, Pollack IF. Abrogation of mitogen-activated protein kinase and Akt signaling by vandetanib synergistically potentiates histone deacetylase inhibitor-induced apoptosis in human glioma cells. J Pharmacol Exp Ther. 2009;331(1):327–337. doi: 10.1124/jpet.109.155705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane EP, Premkumar DR, Pollack IF. Coadministration of sorafenib with rottlerin potently inhibits cell proliferation and migration in human malignant glioma cells. J Pharmacol Exp Ther. 2006;319(3):1070–1080. doi: 10.1124/jpet.106.108621. [DOI] [PubMed] [Google Scholar]
- Jarboe JS, Johnson KR, Choi Y, Lonser RR, Park JK. Expression of Interleukin-13 Receptor 2 in Glioblastoma Multiforme: Implications for Targeted Therapies. Cancer Res. 2007;67(17):7983–7986. doi: 10.1158/0008-5472.CAN-07-1493. [DOI] [PubMed] [Google Scholar]
- Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312(5990):162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- Jeffes EW, Beamer YB, Jacques S, Silberman RS, Vayuvegula B, Gupta S, et al. Therapy of recurrent high grade gliomas with surgery, and autologous mitogen activated IL-2 stimulated killer (MAK) lymphocytes: I. Enhancement of MAK lytic activity and cytokine production by PHA and clinical use of PHA. J Neurooncol. 1993;15(2):141–155. doi: 10.1007/BF01053935. [DOI] [PubMed] [Google Scholar]
- Jiang H, Gomez-Manzano C, Aoki H, Alonso MM, Kondo S, McCormick F, et al. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst. 2007;99(18):1410–1414. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- Jiang J, Hui C. Hedgehog Signaling in Development and Cancer. Dev Cell. 2008;15(6):801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensuu H, Puputti M, Sihto H, Tynninen O, Nupponen NN. Amplification of genes encoding KIT, PDGFRalpha and VEGFR2 receptor tyrosine kinases is frequent in glioblastoma multiforme. J Pathol. 2005;207(2):224–231. doi: 10.1002/path.1823. [DOI] [PubMed] [Google Scholar]
- Joshi BH, Plautz GE, Puri RK. Interleukin-13 Receptor {{alpha}} Chain: A Novel Tumor-associated Transmembrane Protein in Primary Explants of Human Malignant Gliomas. Cancer Res. 2000;60(5):1168–1172. [PubMed] [Google Scholar]
- Kakkar T, Ma M, Zhuang Y, Patton A, Hu Z, Mounho B. Pharmacokinetics and safety of a fully human hepatocyte growth factor antibody, AMG 102, in cynomolgus monkeys. Pharm Res. 2007;24(10):1910–1918. doi: 10.1007/s11095-007-9316-2. [DOI] [PubMed] [Google Scholar]
- Kamoun WS, Ley CD, Farrar CT, Duyverman AM, Lahdenranta J, Lacorre DA, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27(15):2542–2552. doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12(24):7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- Kang KB, Wang TT, Woon CT, Cheah ES, Moore XL, Zhu C, et al. Enhancement of glioblastoma radioresponse by a selective COX-2 inhibitor celecoxib: inhibition of tumor angiogenesis with extensive tumor necrosis. Int J Radiat Oncol Biol Phys. 2007;67(3):888–896. doi: 10.1016/j.ijrobp.2006.09.055. [DOI] [PubMed] [Google Scholar]
- Karrasch M, Gillespie GY, Braz E, Liechty PG, Nabors LB, Lakeman AD, et al. Treatment of recurrent malignant glioma with G207, a genetically engineered herpes simplex virus-1, followed by irradiation: Phase I study results. ASCO Meeting Abstracts. 2009;27(15S):2042–2042. [Google Scholar]
- Kawakami M, Kawakami K, Puri RK. Interleukin-4-Pseudomonas exotoxin chimeric fusion protein for malignant glioma therapy. J Neurooncol. 2003;65(1):15–25. doi: 10.1023/a:1026294416718. [DOI] [PubMed] [Google Scholar]
- Kazerooni R, Conrad C, Baud C, Johansen M, Thapar N, Meyer C, et al. Phase I clinical pharmacokinetics of RTA 744: A blood brain barrier penetrating anthracycline active against high-grade glioma. ASCO Meeting Abstracts. 2007;25(18_suppl):2045–2045. [Google Scholar]
- Kehrl JH, Roberts AB, Wakefield LM, Jakowlew S, Sporn MB, Fauci AS. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986a;137(12):3855–3860. [PubMed] [Google Scholar]
- Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, et al. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986b;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekan M, Fiveash J, Markert JM, Gillespie GY, Kuo H, Meleth S, et al. A phase I study of ABT 510 and concurrent temozolamide and radiotherapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol (Meeting Abstracts) 2009;27(15S):2023. [Google Scholar]
- Kesari S, Schiff D, Doherty L, Gigas DC, Batchelor TT, Muzikansky A, et al. Phase II study of metronomic chemotherapy for recurrent malignant gliomas in adults. Neuro Oncol. 2007;9(3):354–363. doi: 10.1215/15228517-2007-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesari S, Schiff D, Henson JW, Muzikansky A, Gigas DC, Doherty L, et al. Phase II study of temozolomide, thalidomide, and celecoxib for newly diagnosed glioblastoma in adults. Neuro Oncol. 2008;10(3):300–300. doi: 10.1215/15228517-2008-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Akasaki Y, Abe T, Fukuda T, Saotome H, Ryan JL, et al. Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother. 2004;27(6):452–459. doi: 10.1097/00002371-200411000-00005. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Akasaki Y, Irie M, Homma S, Abe T, Ohno T. Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother. 2001;50(7):337–344. doi: 10.1007/s002620100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Kang JS, Lim J, Park SK, Lee K, Yoon YD, et al. Inhibition of human ovarian tumor growth by cytokine-induced killer cells. Arch Pharm Res. 2007a;30(11):1464–1470. doi: 10.1007/BF02977372. [DOI] [PubMed] [Google Scholar]
- Kim HM, Lim J, Kang JS, Park SK, Lee K, Kim JY, et al. Inhibition of human cervical carcinoma growth by cytokine-induced killer cells in nude mouse xenograft model. Int Immunopharmacol. 2009;9(3):375–380. doi: 10.1016/j.intimp.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Kim HM, Lim J, Park SK, Kang JS, Lee K, Lee CW, et al. Antitumor activity of cytokine-induced killer cells against human lung cancer. Int Immunopharmacol. 2007b;7(13):1802–1807. doi: 10.1016/j.intimp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Kim MS, Blake M, Baek JH, Kohlhagen G, Pommier Y, Carrier F. Inhibition of Histone Deacetylase Increases Cytotoxicity to Anticancer Drugs Targeting DNA. Cancer Res. 2003;63(21):7291–7300. [PubMed] [Google Scholar]
- Kimura H, Sakai K, Arao T, Shimoyama T, Tamura T, Nishio K. Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci. 2007;98(8):1275–1280. doi: 10.1111/j.1349-7006.2007.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Bigner SH, Bigner DD, Trent JM, Law ML, O’Brien SJ, et al. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987;236(4797):70–73. doi: 10.1126/science.3563490. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick J, Desjardins A, Quinn J, Rich J, Vredenburgh J, Sathornsumetee S, et al. Phase II open-label, safety, pharmacokinetic and efficacy study of 2-methoxyestradiol nanocrystal colloidal dispersion administered orally to patients with recurrent glioblastoma multiforme. J Clin Oncol (Meeting Abstracts) 2007;25(18S):2065. [Google Scholar]
- Kirkpatrick JP, Desjardins A, Reardon DA, Friedman AH, Friedman HS, Vredenburgh JJ. Radiotherapy, Temozolomide and Bevacizumab followed by Irinotecan, Temozolomide and Bevacizumab Appears Well-tolerated and Efficacious in Newly Diagnosed Glioblastoma Multiforme: Results from a Phase II Trial. Int J Radiat Oncol Biol Phys. 2009;75(3, Supplement 1):S102-S103–S102-S103. [Google Scholar]
- Kitahara T, Watanabe O, Yamaura A, Makino H, Watanabe T, Suzuki G, et al. Establishment of interleukin 2 dependent cytotoxic T lymphocyte cell line specific for autologous brain tumor and its intracranial administration for therapy of the tumor. J Neurooncol. 1987;4(4):329–336. doi: 10.1007/BF00195603. [DOI] [PubMed] [Google Scholar]
- Kjellman C, Olofsson SP, Hansson O, Von Schantz T, Lindvall M, Nilsson I, et al. Expression of TGF-beta isoforms, TGF-beta receptors, and SMAD molecules at different stages of human glioma. Int J Cancer. 2000;89(3):251–258. doi: 10.1002/1097-0215(20000520)89:3<251::aid-ijc7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Köck H, Harris MP, Anderson SC, Machemer T, Hancock W, Sutjipto S, et al. Adenovirus-mediated p53 gene transfer suppresses growth of human glioblastoma cells in vitro and in vivo. Int J Cancer. 1996;67(6):808–815. doi: 10.1002/(SICI)1097-0215(19960917)67:6<808::AID-IJC9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Konings IR, Verweij J, Wiemer EA, Sleijfer S. The applicability of mTOR inhibition in solid tumors. Curr Cancer Drug Targets. 2009;9(3):439–450. doi: 10.2174/156800909788166556. [DOI] [PubMed] [Google Scholar]
- Konner J, Dupont J. Use of soluble recombinant decoy receptor vascular endothelial growth factor trap (VEGF Trap) to inhibit vascular endothelial growth factor activity. Clin Colorectal Cancer. 2004;4(Suppl 2):S81–85. doi: 10.3816/ccc.2004.s.013. [DOI] [PubMed] [Google Scholar]
- Koschny R, Holland H, Sykora J, Haas TL, Sprick MR, Ganten TM, et al. Bortezomib Sensitizes Primary Human Astrocytoma Cells of WHO Grades I to IV for Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand–Induced Apoptosis. Clin Cancer Res. 2007;13(11):3403–3412. doi: 10.1158/1078-0432.CCR-07-0251. [DOI] [PubMed] [Google Scholar]
- Kreisl TN, Kim L, Moore K, Duic P, Kotliarova S, Walling J, et al. A phase I trial of enzastaurin in patients with recurrent gliomas. Clin Cancer Res. 2009a;15(10):3617–3623. doi: 10.1158/1078-0432.CCR-08-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II Trial of Single-Agent Bevacizumab Followed by Bevacizumab Plus Irinotecan at Tumor Progression in Recurrent Glioblastoma. J Clin Oncol. 2008;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl TN, Lassman AB, Mischel PS, Rosen N, Scher HI, Teruya-Feldstein J, et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM) J Neurooncol. 2009b;92(1):99–105. doi: 10.1007/s11060-008-9741-z. [DOI] [PubMed] [Google Scholar]
- Krieg AM. Antitumor applications of stimulating toll-like receptor 9 with CpG oligodeoxynucleotides. Curr Oncol Rep. 2004;6(2):88–95. doi: 10.1007/s11912-004-0019-0. [DOI] [PubMed] [Google Scholar]
- Kubicek GJ, Werner-Wasik M, Machtay M, Mallon G, Myers T, Ramirez M, et al. Phase I trial using proteasome inhibitor bortezomib and concurrent temozolomide and radiotherapy for central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2009;74(2):433–439. doi: 10.1016/j.ijrobp.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Knick VB, Rudolph SK, Johnson JH, Crosby RM, Crouthamel MC, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6(7):2012–2021. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- Kunwar S, Pai LH, Pastan I. Cytotoxicity and antitumor effects of growth factor-toxin fusion proteins on human glioblastoma multiforme cells. J Neurosurg. 1993;79(4):569–576. doi: 10.3171/jns.1993.79.4.0569. [DOI] [PubMed] [Google Scholar]
- Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM, et al. Direct Intracerebral Delivery of Cintredekin Besudotox (IL13-PE38QQR) in Recurrent Malignant Glioma: A Report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25(7):837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- Lai A, Filka E, McGibbon B, Nghiemphu PL, Graham C, Yong WH, et al. Phase II Pilot Study of Bevacizumab in Combination with Temozolomide and Regional Radiation Therapy for Up-Front Treatment of Patients With Newly Diagnosed Glioblastoma Multiforme: Interim Analysis of Safety and Tolerability. Int J Radiat Oncol Biol Phys. 2008;71(5):1372–1380. doi: 10.1016/j.ijrobp.2007.11.068. [DOI] [PubMed] [Google Scholar]
- Lamont JP, Nemunaitis J, Kuhn JA, Landers SA, McCarty TM. A prospective phase II trial of ONYX-015 adenovirus and chemotherapy in recurrent squamous cell carcinoma of the head and neck (the Baylor experience) Ann Surg Oncol. 2000;7(8):588–592. doi: 10.1007/BF02725338. [DOI] [PubMed] [Google Scholar]
- Lamszus K, Schmidt NO, Jin L, Laterra J, Zagzag D, Way D, et al. Scatter factor promotes motility of human glioma and neuromicrovascular endothelial cells. Int J Cancer. 1998;75(1):19–28. doi: 10.1002/(sici)1097-0215(19980105)75:1<19::aid-ijc4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Lang FF. Phase I Trial of Adenovirus-Mediated p53 Gene Therapy for Recurrent Glioma: Biological and Clinical Results. J Clin Oncol. 2003;21(13):2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- Lang FF, Yung WK, Sawaya R, Tofilon PJ. Adenovirus-mediated p53 gene therapy for human gliomas. Neurosurgery. 1999;45(5):1093–1104. doi: 10.1097/00006123-199911000-00016. [DOI] [PubMed] [Google Scholar]
- Laske DW, Ilercil O, Akbasak A, Youle RJ, Oldfield EH. Efficacy of direct intratumoral therapy with targeted protein toxins for solid human gliomas in nude mice. J Neurosurg. 1994;80(3):520–526. doi: 10.3171/jns.1994.80.3.0520. [DOI] [PubMed] [Google Scholar]
- Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3(12):1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- Laterra J, Nam M, Rosen E, Rao JS, Lamszus K, Goldberg ID, et al. Scatter factor/hepatocyte growth factor gene transfer enhances glioma growth and angiogenesis in vivo. Lab Invest. 1997;76(4):565–577. [PubMed] [Google Scholar]
- Lee YS, Bullard DE, Zalutsky MR, Coleman RE, Wikstrand CJ, Friedman HS, et al. Therapeutic Efficacy of Antiglioma Mesenchymal Extracellular Matrix 131I-Radiolabeled Murine Monoclonal Antibody in a Human Glioma Xenograft Model. Cancer Res. 1988;48(3):559–566. [PubMed] [Google Scholar]
- Levin VA, Giglio P, Puduvalli VK, Jochec J, Groves MD, Yung WK, et al. Combination chemotherapy with 13-cis-retinoic acid and celecoxib in the treatment of glioblastoma multiforme. J Neurooncol. 2006a;78(1):85–90. doi: 10.1007/s11060-005-9062-4. [DOI] [PubMed] [Google Scholar]
- Levin VA, Phuphanich S, Glantz MJ, Mason WP, Groves MD, Recht LD, et al. Randomized phase II study of temozolomide (TMZ) with and without the matrix metalloprotease (MMP) inhibitor prinomastat in patients (pts) with glioblastoma multiforme (GBM) following best surgery and radiation therapy. Paper presented at the Procedings American Society Clinical Oncology.2002. [Google Scholar]
- Levin VA, Phuphanich S, Yung WKA, Forsyth PA, Maestro RD, Perry JR, et al. Randomized, double-blind, placebo-controlled trial of marimastat in glioblastoma multiforme patients following surgery and irradiation. J Neurooncol. 2006b;78(3):295–302. doi: 10.1007/s11060-005-9098-5. [DOI] [PubMed] [Google Scholar]
- Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Alonso-Vanegas M, Colicos MA, Jung SS, Lochmuller H, Sadikot AF, et al. Intracerebral adenovirus-mediated p53 tumor suppressor gene therapy for experimental human glioma. Clin Cancer Res. 1999;5(3):637–642. [PubMed] [Google Scholar]
- Liebross RH, Leagre C, Schultz S, Payner T, Sartorius C, Young R, et al. A Feasibility Trial of Concurrent Radiation, Temozolomide, and Bevacizumab followed by Temozolomide and Bevacizumab for Resectable and Unresectable Glioblastoma Multiforme of the Brain. Int J Radiat Oncol Biol Phys. 2009;75(3, Supplement 1):S125. [Google Scholar]
- Lillehei KO, Mitchell DH, Johnson SD, McCleary EL, Kruse CA. Long-term follow-up of patients with recurrent malignant gliomas treated with adjuvant adoptive immunotherapy. Neurosurgery. 1991;28(1):16–23. doi: 10.1097/00006123-199101000-00003. [DOI] [PubMed] [Google Scholar]
- Lipinski RJ, Hutson PR, Hannam PW, Nydza RJ, Washington IM, Moore RW, et al. Dose- and route-dependent teratogenicity, toxicity, and pharmacokinetic profiles of the hedgehog signaling antagonist cyclopamine in the mouse. Toxicol Sci. 2008;104(1):189–197. doi: 10.1093/toxsci/kfn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loghin ME, Prados MD, Wen P, Junck L, Lieberman F, Fine H, et al. Phase I study of temozolomide and irinotecan for recurrent malignant gliomas in patients receiving enzyme-inducing antiepileptic drugs: a north american brain tumor consortium study. Clin Cancer Res. 2007;13(23):7133–7138. doi: 10.1158/1078-0432.CCR-07-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KV, Zhu S, Cvrljevic A, Huang TT, Sarkaria S, Ahkavan D, et al. Fyn and SRC are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients. Cancer Res. 2009;69(17):6889–6898. doi: 10.1158/0008-5472.CAN-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig H, Khayat D, Giaccone G, Facon T. Proteasome inhibition and its clinical prospects in the treatment of hematologic and solid malignancies. Cancer. 2005;104(9):1794–1807. doi: 10.1002/cncr.21414. [DOI] [PubMed] [Google Scholar]
- Lustig R, Mikkelsen T, Lesser G, Grossman S, Ye X, Desideri S, et al. Phase II preradiation R115777 (tipifarnib) in newly diagnosed GBM with residual enhancing disease. Neuro Oncol. 2008;10(6):1004–1009. doi: 10.1215/15228517-2008-070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean AR, ul-Fareed M, Robertson L, Harland J, Brown SM. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the ‘a’ sequence. J Gen Virol. 1991;72(Pt 3):631–639. doi: 10.1099/0022-1317-72-3-631. [DOI] [PubMed] [Google Scholar]
- Maeda H, Kuwahara H, Ichimura Y, Ohtsuki M, Kurakata S, Shiraishi A. TGF-beta enhances macrophage ability to produce IL-10 in normal and tumor-bearing mice. J Immunol. 1995;155(10):4926–4932. [PubMed] [Google Scholar]
- Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15(11):1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- Mamelak AN, Jacoby DB. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601) Expert Opin Drug Deliv. 2007;4(2):175–186. doi: 10.1517/17425247.4.2.175. [DOI] [PubMed] [Google Scholar]
- Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17(1):199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7(10):867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- Martuza R, Malick A, Markert J, Ruffner K, Coen D. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252(5007):854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- Marx GM, Pavlakis N, McCowatt S, Boyle FM, Levi JA, Bell DR, et al. Phase II study of thalidomide in the treatment of recurrent glioblastoma multiforme. J Neurooncol. 2001;54(1):31–38. doi: 10.1023/a:1012554328801. [DOI] [PubMed] [Google Scholar]
- Mason WP, MacNeil M, Easaw J, McIntosh L, Lwin Z, Chen E, et al. A phase I study of temozolomide (TMZ) and RAD001 in patients (pts) with glioblastoma multiforme (GBM) J Clin Oncol (Meeting Abstracts) 2009;27(15S):2036. [Google Scholar]
- Mateo C, Moreno E, Amour K, Lombardero J, Harris W, Pérez R. Humanization of a mouse monoclonal antibody that blocks the epidermal growth factor receptor: recovery of antagonistic activity. Immunotechnology. 1997;3(1):71–81. doi: 10.1016/s1380-2933(97)00065-1. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Seya T. TLR3: Interferon induction by double-stranded RNA including poly(I:C) Adv Drug Deliv Rev. 2008;60(7):805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- McKie EA, MacLean AR, Lewis AD, Cruickshank G, Rampling R, Barnett SC, et al. Selective in vitro replication of herpes simplex virus type 1 (HSV-1) ICP34.5 null mutants in primary human CNS tumours--evaluation of a potentially effective clinical therapy. Br J Cancer. 1996;74(5):745–752. doi: 10.1038/bjc.1996.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele G, Pinna S, Alloro E, Brocca MC, Coppi MR, Quarta G. Inefficacy of bortezomib therapy for CNS involvement of refractory multiple myeloma. Leuk Res. 2007;31(5):721–723. doi: 10.1016/j.leukres.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- Mercer WE, Shields MT, Amin M, Sauve GJ, Appella E, Romano JW, et al. Negative growth regulation in a glioblastoma tumor cell line that conditionally expresses human wild-type p53. Proc Natl Acad Sci U S A. 1990;87(16):6166–6170. doi: 10.1073/pnas.87.16.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant RE, Grant AJ, Merchant LH, Young HF. Adoptive immunotherapy for recurrent glioblastoma multiforme using lymphokine activated killer cells and recombinant interleukin-2. Cancer. 1988;62(4):665–671. doi: 10.1002/1097-0142(19880815)62:4<665::aid-cncr2820620403>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Milano V, Piao Y, LaFortune T, de Groot J. Dasatinib-induced autophagy is enhanced in combination with temozolomide in glioma. Mol Cancer Ther. 2009;8(2):394–406. doi: 10.1158/1535-7163.MCT-08-0669. [DOI] [PubMed] [Google Scholar]
- Mineta T, Rabkin S, Yazaki T, Hunter W, Martuza R. Attenuated multi–mutated herpes simplex virus–1 for the treatment of malignant gliomas. Nat Med. 1995;1(9):938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- Mineta T, Rabkin SD, Martuza RL. Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutant. Cancer Res. 1994;54(15):3963–3966. [PubMed] [Google Scholar]
- Minkovsky N, Berezov A. BIBW-2992, a dual receptor tyrosine kinase inhibitor for the treatment of solid tumors. Curr Opin Investig Drugs. 2008;9(12):1336–1346. [PubMed] [Google Scholar]
- Minniti G, Muni R, Lanzetta G, Marchetti P, Enrici RM. Chemotherapy for glioblastoma: current treatment and future perspectives for cytotoxic and targeted agents. Anticancer Res. 2009;29(12):5171–5184. [PubMed] [Google Scholar]
- Mitchell DA, Xie W, Schmittling R, Learn C, Friedman A, McLendon RE, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol. 2008;10(1):10–18. doi: 10.1215/15228517-2007-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molckovsky A, Siu LL. First-in-class, first-in-human phase I results of targeted agents: Highlights of the 2008 American Society of Clinical Oncology meeting. J Hematol Oncol. 2008;1:20–20. doi: 10.1186/1756-8722-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JD, Barbacci EG, Iwata KK, Arnold L, Boman B, Cunningham A, et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57(21):4838–4848. [PubMed] [Google Scholar]
- Msaouel P, Dispenzieri A, Galanis E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview. Curr Opin Mol Ther. 2009;11(1):43–53. [PMC free article] [PubMed] [Google Scholar]
- Myers R, Harvey M, Kaufmann TJ, Greiner SM, Krempski JW, Raffel C, et al. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum Gene Ther. 2008;19(7):690–698. doi: 10.1089/hum.2008.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, et al. Phase I and Correlative Biology Study of Cilengitide in Patients With Recurrent Malignant Glioma. J Clin Oncol. 2007a;25(13):1651–1657. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabors LB, Rosenfeld M, Chamberlain M, Phuphanich S, Batchelor T, Supko J, et al. A phase I trial of sorafenib (BAY 43–9006) for patients with recurrent or progressive malignant glioma (NABTT 0401) J Clin Oncol (Meeting Abstracts) 2007b;25(18_suppl):2058. doi: 10.1093/neuonc/nor145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan RP, Costello JF. Epigenetic mechanisms in glioblastoma multiforme. Semin Cancer Biol. 2009;19(3):188–197. doi: 10.1016/j.semcancer.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Nakamura O, Maruo K, Ueyama Y, Nomura K, Takakura K. Interactions of human fibroblast interferon with chemotherapeutic agents and radiation against human gliomas in nude mice. Neurol Res. 1986;8(3):152–156. doi: 10.1080/01616412.1986.11739747. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122(3):1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- Newton HB. Molecular neuro-oncology and development of targeted therapeutic strategies for brain tumors. Part 1: Growth factor and Ras signaling pathways. Expert Rev Anticancer Ther. 2003;3(5):595–614. doi: 10.1586/14737140.3.5.595. [DOI] [PubMed] [Google Scholar]
- Neyns B, Chaskis C, Dujardin M, Everaert H, Sadones J, Nupponen NN, et al. Phase II trial of sunitinib malate in patients with temozolomide refractory recurrent high-grade glioma. J Clin Oncol (Meeting Abstracts) 2009a;27(15S):2038. [Google Scholar]
- Neyns B, Sadones J, Joosens E, Bouttens F, Verbeke L, Baurain JF, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol. 2009b;20(9):1596–1603. doi: 10.1093/annonc/mdp032. [DOI] [PubMed] [Google Scholar]
- Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci U S A. 1994;91(16):7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden AD, Drappatz J, Wen PY. Antiangiogenic therapies for high-grade glioma. [10.1038/nrneurol.2009.159] Nat Rev Neurol. 2009;5(11):610–620. doi: 10.1038/nrneurol.2009.159. [DOI] [PubMed] [Google Scholar]
- Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–779. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- Nowicki MO, Dmitrieva N, Stein AM, Cutter JL, Godlewski J, Saeki Y, et al. Lithium inhibits invasion of glioma cells; possible involvement of glycogen synthase kinase-3. Neuro Oncol. 2008;10(5):690–699. doi: 10.1215/15228517-2008-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Giezeman-Smits KM, Tahara H, Attanucci J, Fellows WK, Lotze MT, et al. Effective cytokine gene therapy against an intracranial glioma using a retrovirally transduced IL-4 plus HSVtk tumor vaccine. Gene Ther. 1999;6(2):219–226. doi: 10.1038/sj.gt.3300798. [DOI] [PubMed] [Google Scholar]
- Okada H, Lieberman FS, Edington HD, Witham TF, Wargo MJ, Cai Q, et al. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of recurrent glioblastoma: preliminary observations in a patient with a favorable response to therapy. J Neurooncol. 2003;64(1–2):13–20. doi: 10.1007/BF02700016. [DOI] [PubMed] [Google Scholar]
- Okada H, Lieberman FS, Walter KA, Lunsford LD, Kondziolka DS, Bejjani GK, et al. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas. J Transl Med. 2007;5(1):67. doi: 10.1186/1479-5876-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Villa L, Attanucci J, Erff M, Fellows WK, Lotze MT, et al. Cytokine gene therapy of gliomas: effective induction of therapeutic immunity to intracranial tumors by peripheral immunization with interleukin-4 transduced glioma cells. Gene Ther. 2001;8(15):1157–1166. doi: 10.1038/sj.gt.3301496. [DOI] [PubMed] [Google Scholar]
- Okaji Y, Tsuno NH, Tanaka M, Yoneyama S, Matsuhashi M, Kitayama J, et al. Pilot study of anti-angiogenic vaccine using fixed whole endothelium in patients with progressive malignancy after failure of conventional therapy. Eur J Cancer. 2008;44(3):383–390. doi: 10.1016/j.ejca.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Oliver S, Bubley G, Crumpacker C. Inhibition of HSV-transformed murine cells by nucleoside analogs, 2′-NDG and 2′-nor-cGMP: mechanisms of inhibition and reversal by exogenous nucleosides. Virology. 1985;145(1):84–93. doi: 10.1016/0042-6822(85)90203-x. [DOI] [PubMed] [Google Scholar]
- Pack GT. Note on the experimental use of rabies vaccine for melanomatosis. AMA Arch Derm Syphilol. 1950;62(5):694–695. doi: 10.1001/archderm.1950.01530180083015. [DOI] [PubMed] [Google Scholar]
- Paganelli G, Grana C, Chinol M, Cremonesi M, De Cicco C, De Braud F, et al. Antibody-guided three-step therapy for high grade glioma with yttrium-90 biotin. Eur J Nucl Med. 1999;26(4):348–357. doi: 10.1007/s002590050397. [DOI] [PubMed] [Google Scholar]
- Papanastassiou V, Rampling R, Fraser M, Petty R, Hadley D, Nicoll J, et al. The potential for efficacy of the modified (ICP 34.5-) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9(6):398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, Markert JM. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci U S A. 2000;97(5):2208–2213. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parney IF, Chang SM. Current chemotherapy for glioblastoma. Cancer J. 2003;9(3):149–156. doi: 10.1097/00130404-200305000-00003. [DOI] [PubMed] [Google Scholar]
- Patel SJ, Shapiro WR, Laske DW, Jensen RL, Asher AL, Wessels BW, et al. Safety and Feasibility of Convection-enhanced Delivery of Cotara for the Treatment of Malignant Glioma: Initial Experience in 51 Patients. Neurosurgery. 2005;56(6):1243–1253. doi: 10.1227/01.neu.0000159649.71890.30. [DOI] [PubMed] [Google Scholar]
- Patyna S, Peng G. Distribution of sunitinib and its active metabolite in brain and spinal cord tissue following oral or intravenous administration in rodents and monkeys. Ejc Supplements. 2006;4(12):21–21. [Google Scholar]
- Peng KW, Facteau S, Wegman T, O’Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med. 2002;8(5):527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8(1):21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Pichlmeier U, Bink A, Schackert G, Stummer W. Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol. 2008;10(6):1025–1034. doi: 10.1215/15228517-2008-052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras K, Ostman A, Sjoquist M, Buchdunger E, Reed RK, Heldin CH, et al. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 2001;61(7):2929–2934. [PubMed] [Google Scholar]
- Pietras K, Rubin K, Sjoblom T, Buchdunger E, Sjoquist M, Heldin CH, et al. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62(19):5476–5484. [PubMed] [Google Scholar]
- Pollack VA, Savage DM, Baker DA, Tsaparikos KE, Sloan DE, Moyer JD, et al. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J Pharmacol Exp Ther. 1999;291(2):739–748. [PubMed] [Google Scholar]
- Porkka K, Koskenvesa P, Lundan T, Rimpilaeinen J, Mustjoki S, Smykla R, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood. 2008;112(4):1005–1012. doi: 10.1182/blood-2008-02-140665. [DOI] [PubMed] [Google Scholar]
- Prados M, Gilbert M, Kuhn J, Lamborn K, Cloughesy T, Lieberman F, et al. Phase I/II study of sorefenib and erlotinib for patients with recurrent glioblastoma (GBM) (NABTC 05–02) J Clin Oncol (Meeting Abstracts) 2009a;27(15S):2005. [Google Scholar]
- Prados M, Kunwar S, Lang FF, Ram Z, Westphal M, Barnett G, et al. Final results of phase I/II studies of IL13-PE38QQR administered intratumorally (IT) and/or peritumorally (PT) via convection-enhanced delivery (CED) in patients undergoing tumor resection for recurrent malignant glioma. J Clin Oncol (Meeting Abstracts) 2005;23(16S):1506. [Google Scholar]
- Prados MD, Chang SM, Butowski N, DeBoer R, Parvataneni R, Carliner H, et al. Phase II Study of Erlotinib Plus Temozolomide During and After Radiation Therapy in Patients With Newly Diagnosed Glioblastoma Multiforme or Gliosarcoma. J Clin Oncol. 2009b;27(4):579–584. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados MD, Lamborn KR, Chang S, Burton E, Butowski N, Malec M, et al. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro Oncol. 2006;8(1):67–78. doi: 10.1215/S1522851705000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preusser M, Gelpi E, Rottenfusser A, Dieckmann K, Widhalm G, Dietrich W, et al. Epithelial Growth Factor Receptor Inhibitors for treatment of recurrent or progressive high grade glioma: an exploratory study. J Neurooncol. 2008;89(2):211–218. doi: 10.1007/s11060-008-9608-3. [DOI] [PubMed] [Google Scholar]
- Prior R, Reifenberger G, Wechsler W. Transferrin receptor expression in tumours of the human nervous system: relation to tumour type, grading and tumour growth fraction. Virchows Arch A Pathol Anat Histopathol. 1990;416(6):491–496. doi: 10.1007/BF01600299. [DOI] [PubMed] [Google Scholar]
- Puduvalli VK, Giglio P, Groves MD, Hess KR, Gilbert MR, Mahankali S, et al. Phase II trial of irinotecan and thalidomide in adults with recurrent glioblastoma multiforme. Neuro Oncol. 2008;10(2):216–222. doi: 10.1215/15228517-2007-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri RK. Development of a recombinant interleukin-4-Pseudomonas exotoxin for therapy of glioblastoma. Toxicol Pathol. 1999;27(1):53–57. doi: 10.1177/019262339902700111. [DOI] [PubMed] [Google Scholar]
- Puri RK, Leland P, Kreitman RJ, Pastan I. Human neurological cancer cells express interleukin-4 (IL-4) receptors which are targets for the toxic effects of IL4-pseudomonas exotoxin chimeric protein. Int J Cancer. 1994;58(4):574–581. doi: 10.1002/ijc.2910580421. [DOI] [PubMed] [Google Scholar]
- Puri RK, Ogata M, Leland P, Feldman GM, FitzGerald D, Pastan I. Expression of High-Affinity Interleukin 4 Receptors on Murine Sarcoma Cells and Receptor-mediated Cytotoxicity of Tumor Cells to Chimeric Protein between Interleukin 4 and Pseudomonas Exotoxin. Cancer Res. 1991;51(11):3011–3017. [PubMed] [Google Scholar]
- Qin XQ, Beckham C, Brown JL, Lukashev M, Barsoum J. Human and mouse IFN-beta gene therapy exhibits different anti-tumor mechanisms in mouse models. Mol Ther. 2001;4(4):356–364. doi: 10.1006/mthe.2001.0464. [DOI] [PubMed] [Google Scholar]
- Qin XQ, Tao N, Dergay A, Moy P, Fawell S, Davis A, et al. Interferon-beta gene therapy inhibits tumor formation and causes regression of established tumors in immune-deficient mice. Proc Natl Acad Sci U S A. 1998;95(24):14411–14416. doi: 10.1073/pnas.95.24.14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quang TS, Brady LW. Radioimmunotherapy as a novel treatment regimen: 125I-labeled monoclonal antibody 425 in the treatment of high-grade brain gliomas. Int J Radiat Oncol Biol Phys. 2004;58(3):972–975. doi: 10.1016/j.ijrobp.2003.09.096. [DOI] [PubMed] [Google Scholar]
- Quesada JR. Alpha interferons in hairy cell leukaemia: a clinical model of biological therapy for cancer. Interferon. 1987;8:111–134. [PubMed] [Google Scholar]
- Quinn JA, Desjardins A, Weingart J, Brem H, Dolan ME, Delaney SM, et al. Phase I Trial of Temozolomide Plus O6-Benzylguanine for Patients With Recurrent or Progressive Malignant Glioma. J Clin Oncol. 2005;23(28):7178–7187. doi: 10.1200/JCO.2005.06.502. [DOI] [PubMed] [Google Scholar]
- Quinn JA, Jiang SX, Carter J, Reardon DA, Desjardins A, Vredenburgh JJ, et al. Phase II trial of Gliadel plus O6-benzylguanine in adults with recurrent glioblastoma multiforme. Clin Cancer Res. 2009a;15(3):1064–1068. doi: 10.1158/1078-0432.CCR-08-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JA, Jiang SX, Reardon DA, Desjardins A, Vredenburgh JJ, Gururangan S, et al. Phase 1 trial of temozolomide plus irinotecan plus O6-benzylguanine in adults with recurrent malignant glioma. Cancer. 2009b;115(13):2964–2970. doi: 10.1002/cncr.24336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JA, Pluda J, Dolan ME, Delaney S, Kaplan R, Rich JN, et al. Phase II trial of carmustine plus O(6)-benzylguanine for patients with nitrosourea-resistant recurrent or progressive malignant glioma. J Clin Oncol. 2002;20(9):2277–2283. doi: 10.1200/JCO.2002.09.084. [DOI] [PubMed] [Google Scholar]
- Quinn JA, Reardon DA, Friedman AH, Rich JN, Sampson JH, Vredenburgh J, et al. Phase 1 trial of irinotecan plus BCNU in patients with progressive or recurrent malignant glioma. Neuro Oncol. 2004;6(2):145–153. doi: 10.1215/S1152851703000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11(17):2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- Rainov NG, Fels C, Droege JW, Schäfer C, Kramm CM, Chou TC. Temozolomide enhances herpes simplex virus thymidine kinase/ganciclovir therapy of malignant glioma. Cancer Gene Ther. 2001;8(9):662–668. doi: 10.1038/sj.cgt.7700355. [DOI] [PubMed] [Google Scholar]
- Rainov NG, Heidecke V. Long Term Survival in a Patient with Recurrent Malignantc Glioma Treated with Intratumoral Infusion of an IL4-Targeted Toxin (NBI-3001) J Neurooncol. 2004;66(1/2):197–201. doi: 10.1023/b:neon.0000013478.27604.01. [DOI] [PubMed] [Google Scholar]
- Raithatha SA, Muzik H, Rewcastle NB, Johnston RN, Edwards DR, Forsyth PA. Localization of gelatinase-A and gelatinase-B mRNA and protein in human gliomas. Neuro Oncol. 2000;2(3):145–150. doi: 10.1093/neuonc/2.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizer JJ. HER1/EGFR tyrosine kinase inhibitors for the treatment of glioblastoma multiforme. J Neurooncol. 2005;74(1):77–86. doi: 10.1007/s11060-005-0603-7. [DOI] [PubMed] [Google Scholar]
- Raizer JJ, Abrey LE, Lassman AB, Chang SM, Lamborn KR, Kuhn JG, et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol. 2010a;12(1):95–103. doi: 10.1093/neuonc/nop015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizer JJ, Abrey LE, Lassman AB, Chang SM, Lamborn KR, Kuhn JG, et al. A phase I trial of erlotinib in patients with nonprogressive glioblastoma multiforme postradiation therapy, and recurrent malignant gliomas and meningiomas. Neuro Oncol. 2010b;12(1):87–94. doi: 10.1093/neuonc/nop017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos TC, Figueredo J, Catala M, González S, Selva JC, Cruz TM, et al. Treatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3: report from a phase I/II trial. Cancer Biol Ther. 2006;5(4):375–379. doi: 10.4161/cbt.5.4.2522. [DOI] [PubMed] [Google Scholar]
- Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7(10):859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- Rampling R, Sanson M, Taal W, Lai C, Stoffregen C, Munoz M, et al. Phase 1 study of LY317615 (enzastaurin) and temozolomide in patients with gliomas - EORTC trial 26054. J Clin Oncol (Meeting Abstracts) 2009;27(15S):e13005. doi: 10.1093/neuonc/nor221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand RW, Kreitman RJ, Patronas N, Varricchio F, Pastan I, Puri RK. Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin Cancer Res. 2000;6(6):2157–2165. [PubMed] [Google Scholar]
- Raymond E. PDGFR inhibition in brain tumours--oft expectation fails where most it promises. Eur J Cancer. 2009;45(13):2236–2238. doi: 10.1016/j.ejca.2009.05.031. [DOI] [PubMed] [Google Scholar]
- Raymond E, Brandes AA, Dittrich C, Fumoleau P, Coudert B, Clement PM, et al. Phase II study of imatinib in patients with recurrent gliomas of various histologies: a European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J Clin Oncol. 2008;26(28):4659–4665. doi: 10.1200/JCO.2008.16.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DA, Desjardins A, Vredenburgh JJ, Gururangan S, Friedman AH, Herndon JE, 2nd, et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J Neurooncol. 2010;96(2):219–230. doi: 10.1007/s11060-009-9950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DA, Desjardins A, Vredenburgh JJ, Gururangan S, Sampson JH, Sathornsumetee S, et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: a phase II study. Br J Cancer. 2009a doi: 10.1038/sj.bjc.6605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DA, Desjardins A, Vredenburgh JJ, Sathornsumetee S, Rich JN, Quinn JA, et al. Safety and pharmacokinetics of dose-intensive imatinib mesylate plus temozolomide: phase 1 trial in adults with malignant glioma. Neuro Oncol. 2008a;10(3):330–340. doi: 10.1215/15228517-2008-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DA, Egorin MJ, Desjardins A, Vredenburgh JJ, Beumer JH, Lagattuta TF, et al. Phase I pharmacokinetic study of the vascular endothelial growth factor receptor tyrosine kinase inhibitor vatalanib (PTK787) plus imatinib and hydroxyurea for malignant glioma. Cancer. 2009b;115(10):2188–2198. doi: 10.1002/cncr.24213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DA, Egorin MJ, Quinn JA, Rich JN, Gururangan S, Vredenburgh JJ, et al. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol. 2005a;23(36):9359–9368. doi: 10.1200/JCO.2005.03.2185. [DOI] [PubMed] [Google Scholar]
- Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O’Neill A, Plotkin S, et al. Randomized Phase II Study of Cilengitide, an Integrin-Targeting Arginine-Glycine-Aspartic Acid Peptide, in Recurrent Glioblastoma Multiforme. J Clin Oncol. 2008b;26(34):5610–5617. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- Reardon DA, Quinn JA, Rich JN, Desjardins A, Vredenburgh J, Gururangan S, et al. Phase I trial of irinotecan plus temozolomide in adults with recurrent malignant glioma. Cancer. 2005b;104(7):1478–1486. doi: 10.1002/cncr.21316. [DOI] [PubMed] [Google Scholar]
- Reardon DA, Quinn JA, Rich JN, Gururangan S, Vredenburgh J, Sampson JH, et al. Phase 2 trial of BCNU plus irinotecan in adults with malignant glioma. Neuro Oncol. 2004;6(2):134–144. doi: 10.1215/S1152851703000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DA, Quinn JA, Vredenburgh J, Rich JN, Gururangan S, Badruddoja M, et al. Phase II trial of irinotecan plus celecoxib in adults with recurrent malignant glioma. Cancer. 2005c;103(2):329–338. doi: 10.1002/cncr.20776. [DOI] [PubMed] [Google Scholar]
- Reardon DA, Quinn JA, Vredenburgh JJ, Gururangan S, Friedman AH, Desjardins A, et al. Phase 1 trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin Cancer Res. 2006;12(3 Pt 1):860–868. doi: 10.1158/1078-0432.CCR-05-2215. [DOI] [PubMed] [Google Scholar]
- Reardon DA, Zalutsky MR, Akabani G, Coleman RE, Friedman AH, Herndon JE, et al. A pilot study: 131I-antitenascin monoclonal antibody 81c6 to deliver a 44-Gy resection cavity boost. Neuro Oncol. 2008c;10(2):182–189. doi: 10.1215/15228517-2007-053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht L, Torres CO, Smith TW, Raso V, Griffin TW. Transferrin receptor in normal and neoplastic brain tissue: implications for brain-tumor immunotherapy. J Neurosurg. 1990;72(6):941–945. doi: 10.3171/jns.1990.72.6.0941. [DOI] [PubMed] [Google Scholar]
- Reszka R, Jacobs A, Voges J. Liposome-Mediated Suicide Gene Therapy in Humans. Methods Enzymol. 2005;391:200–208. doi: 10.1016/S0076-6879(05)91012-4. [DOI] [PubMed] [Google Scholar]
- Rich JN, Bigner DD. Development of novel targeted therapies in the treatment of malignant glioma. Nat Rev Drug Discov. 2004;3(5):430–446. doi: 10.1038/nrd1380. [DOI] [PubMed] [Google Scholar]
- Rich JN, Reardon DA, Peery T, Dowell JM, Quinn JA, Penne KL, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22(1):133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- Rich JN, Sathornsumetee S, Keir ST, Kieran MW, Laforme A, Kaipainen A, et al. ZD6474, a novel tyrosine kinase inhibitor of vascular endothelial growth factor receptor and epidermal growth factor receptor, inhibits tumor growth of multiple nervous system tumors. Clin Cancer Res. 2005;11(22):8145–8157. doi: 10.1158/1078-0432.CCR-05-0319. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- Robarge KD, Brunton SA, Castanedo GM, Cui Y, Dina MS, Goldsmith R, et al. GDC-0449—A potent inhibitor of the hedgehog pathway. Bioorg Med Chem Lett. 2009;19(19):5576–5581. doi: 10.1016/j.bmcl.2009.08.049. [DOI] [PubMed] [Google Scholar]
- Rodeck U, Herlyn M, Herlyn D, Molthoff C, Atkinson B, Varello M, et al. Tumor Growth Modulation by a Monoclonal Antibody to the Epidermal Growth Factor Receptor: Immunologically Mediated and Effector Cell-independent Effects. Cancer Res. 1987;47(14):3692–3696. [PubMed] [Google Scholar]
- Rook AH, Kehrl JH, Wakefield LM, Roberts AB, Sporn MB, Burlington DB, et al. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986;136(10):3916–3920. [PubMed] [Google Scholar]
- Rosenblum MG, Yung WK, Kelleher PJ, Ruzicka F, Steck PA, Borden EC. Growth inhibitory effects of interferon-beta but not interferon-alpha on human glioma cells: correlation of receptor binding, 2′,5′-oligoadenylate synthetase and protein kinase activity. J Interferon Res. 1990;10(2):141–151. doi: 10.1089/jir.1990.10.141. [DOI] [PubMed] [Google Scholar]
- Rubsam LZ, Boucher PD, Murphy PJ, KuKuruga M, Shewach DS. Cytotoxicity and accumulation of ganciclovir triphosphate in bystander cells cocultured with herpes simplex virus type 1 thymidine kinase-expressing human glioblastoma cells. Cancer Res. 1999;59(3):669–675. [PubMed] [Google Scholar]
- Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361(12):1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JS, Brady K, Burgan WE, Cerra MA, Oswald KA, Camphausen K, et al. Gleevec-mediated inhibition of Rad51 expression and enhancement of tumor cell radiosensitivity. Cancer Res. 2003;63(21):7377–7383. [PubMed] [Google Scholar]
- Salgia R, Hong DS, Camacho LH, Ng CS, Janisch L, Ratain MJ, et al. A phase I dose-escalation study of the safety and pharmacokinetics (PK) of XL184, a VEGFR and MET kinase inhibitor, administered orally to patients (pts) with advanced malignancies. J Clin Oncol (Meeting Abstracts) 2007;25(18_suppl):14031. [Google Scholar]
- Sampson JH, Akabani G, Archer GE, Berger MS, Coleman RE, Friedman AH, et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10(3):320–329. doi: 10.1215/15228517-2008-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JH, Akabani G, Archer GE, Bigner DD, Berger MS, Friedman AH, et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol. 2003;65(1):27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]
- Sampson JH, Archer G, Pedain C, Wembacher-Schroder E, Westphal M, Kunwar S, et al. Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg. 2009 doi: 10.3171/2009.11.JNS091052. (in press) [DOI] [PubMed] [Google Scholar]
- Sandstrom M, Johansson M, Andersson U, Bergh A, Bergenheim AT, Henriksson R. The tyrosine kinase inhibitor ZD6474 inhibits tumour growth in an intracerebral rat glioma model. Br J Cancer. 2004;91(6):1174–1180. doi: 10.1038/sj.bjc.6602108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom M, Johansson M, Bergstrom P, Bergenheim AT, Henriksson R. Effects of the VEGFR inhibitor ZD6474 in combination with radiotherapy and temozolomide in an orthotopic glioma model. J Neurooncol. 2008;88(1):1–9. doi: 10.1007/s11060-008-9527-3. [DOI] [PubMed] [Google Scholar]
- Sathornsumetee S, Desjardins A, Vredenburgh JJ, Rich JN, Gururangan S, Friedman AH, et al. Phase II study of bevacizumab plus erlotinib for recurrent malignant gliomas. J Clin Oncol (Meeting Abstracts) 2009;27(15S):2045. [Google Scholar]
- Scheurer ME, Bondy ML, Aldape KD, Albrecht T, El-Zein R. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neurochir Suppl. 2008;116(1):79–86. doi: 10.1007/s00401-008-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Wolf GD, Negrin RS, Schmidt-Wolf IG. Activated T cells and cytokine-induced CD3+CD56+ killer cells. Ann Hematol. 1997;74(2):51–56. doi: 10.1007/s002770050257. [DOI] [PubMed] [Google Scholar]
- Schneider T, Gerhards R, Kirches E, Firsching R. Preliminary results of active specific immunization with modified tumor cell vaccine in glioblastoma multiforme. J Neurooncol. 2001;53(1):39–46. doi: 10.1023/a:1011856406683. [DOI] [PubMed] [Google Scholar]
- Schonthal AH. Antitumor properties of dimethyl-celecoxib, a derivative of celecoxib that does not inhibit cyclooxygenase-2: implications for glioma therapy. Neurosurg Focus. 2006;20(4):E21. doi: 10.3171/foc.2006.20.4.14. [DOI] [PubMed] [Google Scholar]
- Scott EN, Meinhardt G, Jacques C, Laurent D, Thomas AL. Vatalanib: the clinical development of a tyrosine kinase inhibitor of angiogenesis in solid tumours. Expert Opin Investig Drugs. 2007;16(3):367–379. doi: 10.1517/13543784.16.3.367. [DOI] [PubMed] [Google Scholar]
- Shapiro WR, Carpenter SP, Roberts K, Shan JS. (131)I-chTNT-1/B mAb: tumour necrosis therapy for malignant astrocytic glioma. Expert Opin Biol Ther. 2006;6(5):539–545. doi: 10.1517/14712598.6.5.539. [DOI] [PubMed] [Google Scholar]
- Sharif TR, Sharif M. Overexpression of protein kinase C epsilon in astroglial brain tumor derived cell lines and primary tumor samples. Int J Oncol. 1999;15(2):237–243. [PubMed] [Google Scholar]
- Shono T, Tofilon PJ, Bruner JM, Owolabi O, Lang FF. Cyclooxygenase-2 Expression in Human Gliomas: Prognostic Significance and Molecular Correlations. Cancer Res. 2001;61(11):4375–4381. [PubMed] [Google Scholar]
- Short MP, Choi BC, Lee JK, Malick A, Breakefield XO, Martuza RL. Gene delivery to glioma cells in rat brain by grafting of a retrovirus packaging cell line. J Neurosci Res. 1990;27(3):427–439. doi: 10.1002/jnr.490270322. [DOI] [PubMed] [Google Scholar]
- Short SC, Traish D, Dowe A, Hines F, Gore M, Brada M. Thalidomide as an anti-angiogenic agent in relapsed gliomas. J Neurooncol. 2001;51(1):41–45. doi: 10.1023/a:1006414804835. [DOI] [PubMed] [Google Scholar]
- Slichenmyer WJ, Rowinsky EK, Grochow LB, Kaufmann SH, Donehower RC. Camptothecin analogues: studies from The Johns Hopkins Oncology Center. Cancer Chemother Pharmacol. 1994;34(S1):S53–S57. doi: 10.1007/BF00684864. [DOI] [PubMed] [Google Scholar]
- Slupianek A, Schmutte C, Tombline G, Nieborowska-Skorska M, Hoser G, Nowicki MO, et al. BCR/ABL regulates mammalian RecA homologs, resulting in drug resistance. Mol Cell. 2001;8(4):795–806. doi: 10.1016/s1097-2765(01)00357-4. [DOI] [PubMed] [Google Scholar]
- Stark-Vance V. Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma. Neuro Oncol. 2005;7(3):369. [Google Scholar]
- Stea B, Falsey R, Kislin K, Patel J, Glanzberg H, Carey S, et al. Time and dose-dependent radiosensitization of the glioblastoma multiforme U251 cells by the EGF receptor tyrosine kinase inhibitor ZD1839 (‘Iressa’) Cancer Lett. 2003;202(1):43–51. doi: 10.1016/j.canlet.2003.07.006. [doi: DOI: 10.1016/j.canlet.2003.07.006] [DOI] [PubMed] [Google Scholar]
- Stefanik DF, Fellows WK, Rizkalla LR, Rizkalla WM, Stefanik PP, Deleo AB, et al. Monoclonal antibodies to vascular endothelial growth factor (VEGF) and the VEGF receptor, FLT-1, inhibit the growth of C6 glioma in a mouse xenograft. J Neurooncol. 2001;55(2):91–100. doi: 10.1023/a:1013329832067. [DOI] [PubMed] [Google Scholar]
- Steinbok P, Thomas JP, Grossman L, Dolman CL. Intratumoral autologous mononuclear cells in the treatment of recurrent glioblastoma multiforme. A phase 1 (toxicity) study. J Neurooncol. 1984;2(2):147–151. doi: 10.1007/BF00177901. [DOI] [PubMed] [Google Scholar]
- Steiner HH, Bonsanto MM, Beckhove P, Brysch M, Geletneky K, Ahmadi R, et al. Antitumor Vaccination of Patients With Glioblastoma Multiforme: A Pilot Study to Assess Feasibility, Safety, and Clinical Benefit. J Clin Oncol. 2004;22(21):4272–4281. doi: 10.1200/JCO.2004.09.038. [DOI] [PubMed] [Google Scholar]
- Stoker M, Perryman M. An epithelial scatter factor released by embryo fibroblasts. J Cell Sci. 1985;77:209–223. doi: 10.1242/jcs.77.1.209. [DOI] [PubMed] [Google Scholar]
- Stupp R, Goldbrunner R, Neyns B, Schlegel U, Clement P, Grabenbauer GG, et al. Phase I/IIa trial of cilengitide (EMD121974) and temozolomide with concomitant radiotherapy, followed by temozolomide and cilengitide maintenance therapy in patients (pts) with newly diagnosed glioblastoma (GBM) J Clin Oncol (Meeting Abstracts) 2007;25(18_suppl):2000. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Styczynski J, Olszewska-Slonina D, Kolodziej B, Napieraj M, Wysocki M. Activity of bortezomib in glioblastoma. Anticancer Res. 2006;26(6B):4499–4503. [PubMed] [Google Scholar]
- Supko JG, Grossman SA, Peereboom DM, Chowdhary S, Lesser GJ, Nabors LB, et al. Feasibility and phase I trial of tandutinib in patients with recurrent glioblastoma. J Clin Oncol (Meeting Abstracts) 2009;27(15S):2039. [Google Scholar]
- Swanson DA, Quesada JR. Interferon therapy for metastatic renal cell carcinoma. Semin Surg Oncol. 1988;4(3):174–177. doi: 10.1002/ssu.2980040307. [DOI] [PubMed] [Google Scholar]
- Taga T, Suzuki A, Gonzalez-Gomez I, Gilles FH, Stins M, Shimada H, et al. alpha v-Integrin antagonist EMD 121974 induces apoptosis in brain tumor cells growing on vitronectin and tenascin. Int J Cancer. 2002;98(5):690–697. doi: 10.1002/ijc.10265. [DOI] [PubMed] [Google Scholar]
- Takayama N, Sato N, O’Brien SG, Ikeda Y, Okamoto SI. Imatinib mesylate has limited activity against the central nervous system involvement of Philadelphia chromosome-positive acute lymphoblastic leukaemia due to poor penetration into cerebrospinal fluid. British Journal of Haematology. 2002;119(1):106–108. doi: 10.1046/j.1365-2141.2002.03881.x. [DOI] [PubMed] [Google Scholar]
- Tenan M, Fulci G, Albertoni M, Diserens AC, Hamou MF, El Atifi-Borel M, et al. Thrombospondin-1 Is Downregulated by Anoxia and Suppresses Tumorigenicity of Human Glioblastoma Cells. J Exp Med. 2000;191(10):1789–1798. doi: 10.1084/jem.191.10.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiessen B, Stewart C, Tsao M, Kamel-Reid S, Schaiquevich P, Mason W, et al. A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: clinical outcomes, pharmacokinetics and molecular correlation. [10.1007/s00280–009–1041–6] Cancer Chemother Pharmacol. 2010;65(2):353–361. doi: 10.1007/s00280-009-1041-6. [DOI] [PubMed] [Google Scholar]
- Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001;98(11):6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres LA, Coca MA, Batista JF, Casaco A, Lopez G, García I, et al. Biodistribution and internal dosimetry of the 188Re-labelled humanized monoclonal antibody anti-epidemal growth factor receptor, nimotuzumab, in the locoregional treatment of malignant gliomas. Nucl Med Commun. 2008;29(1):66–75. doi: 10.1097/MNM.0b013e3282f1bbce. [DOI] [PubMed] [Google Scholar]
- Tremblay MR, Lescarbeau A, Grogan MJ, Tan E, Lin G, Austad BC, et al. Discovery of a potent and orally active hedgehog pathway antagonist (IPI-926) J Med Chem. 2009;52(14):4400–4418. doi: 10.1021/jm900305z. [DOI] [PubMed] [Google Scholar]
- Trifan OC, Durham WF, Salazar VS, Horton J, Levine BD, Zweifel BS, et al. Cyclooxygenase-2 inhibition with celecoxib enhances antitumor efficacy and reduces diarrhea side effect of CPT-11. Cancer Res. 2002;62(20):5778–5784. [PubMed] [Google Scholar]
- Tuettenberg J, Grobholz R, Korn T, Wenz F, Erber R, Vajkoczy P. Continuous low-dose chemotherapy plus inhibition of cyclooxygenase-2 as an antiangiogenic therapy of glioblastoma multiforme. J Cancer Res Clin Oncol. 2004;131(1):31–40. doi: 10.1007/s00432-004-0620-5. [DOI] [PubMed] [Google Scholar]
- van den Bent MJ, Brandes AA, Rampling R, Kouwenhoven MC, Kros JM, Carpentier AF, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27(8):1268–1274. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16(4):525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- Virasch N, Kruse CA. Strategies using the immune system for therapy of brain tumors. Hematol Oncol Clin North Am. 2001;15(6):1053–1071. doi: 10.1016/s0889-8588(05)70267-7. [DOI] [PubMed] [Google Scholar]
- Volarevic S, Thomas G. Role of S6 phosphorylation and S6 kinase in cell growth. Prog Nucleic Acid Res Mol Biol. 2001;65:101–127. doi: 10.1016/s0079-6603(00)65003-1. [DOI] [PubMed] [Google Scholar]
- Vredenburgh JJ, Desjardins A, Herndon JE, Dowell JM, Reardon DA, Quinn JA, et al. Phase II Trial of Bevacizumab and Irinotecan in Recurrent Malignant Glioma. Clin Cancer Res. 2007a;13(4):1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- Vredenburgh JJ, Desjardins A, Herndon JE, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab Plus Irinotecan in Recurrent Glioblastoma Multiforme. J Clin Oncol. 2007b;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- Wachsberger P, Burd R, Cardi C, Thakur M, Daskalakis C, Holash J, et al. VEGF Trap in Combination With Radiotherapy Improves Tumor Control in U87 Glioblastoma. Int J Radiat Oncol Biol Phys. 2007;67(5):1526–1537. doi: 10.1016/j.ijrobp.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Wagner SA, Desjardins A, Reardon DA, Marcello J, Herndon JE, Quinn JA, et al. Update on survival from the original phase II trial of bevacizumab and irinotecan in recurrent malignant gliomas. J Clin Oncol (Meeting Abstracts) 2008;26(15S):2021. [Google Scholar]
- Walker MD, Green SB, Byar DP, Alexander E, Jr, Batzdorf U, Brooks WH, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303(23):1323–1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA. Plant Antitumor Agents. I. The Isolation and Structure of Camptothecin, a Novel Alkaloidal Leukemia and Tumor Inhibitor from Camptotheca acuminata1,2. J Am Chem Soc. 1966;88(16):3888–3890. [Google Scholar]
- Wang CC, Liao YP, Mischel PS, Iwamoto KS, Cacalano NA, McBride WH. HDJ-2 as a target for radiosensitization of glioblastoma multiforme cells by the farnesyltransferase inhibitor R115777 and the role of the p53/p21 pathway. Cancer Res. 2006;66(13):6756–6762. doi: 10.1158/0008-5472.CAN-06-0185. [DOI] [PubMed] [Google Scholar]
- Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol. 2002;3(6):430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- Wang S, Jiang F, Bai L, Long JJ, Qiu S, Chen J, et al. Effect of (−)-gossypol (AT-101) on transcriptional regulation of Noxa and Puma and on Mcl-1-mediated cancer cell resistance to apoptosis. ASCO Meeting Abstracts. 2009;27(15S):e14611–e14611. [Google Scholar]
- Weaver M, Laske DW. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J Neurooncol. 2003;65(1):3–13. doi: 10.1023/a:1026246500788. [DOI] [PubMed] [Google Scholar]
- Weber F, Asher A, Bucholz R, Berger M, Prados M, Chang S, et al. Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J Neurooncol. 2003;64(1–2):125–137. doi: 10.1007/BF02700027. [DOI] [PubMed] [Google Scholar]
- Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65(10):4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- Wen PY, Cloughesy T, Kuhn J, Lamborn K, Abrey LE, Lieberman F, et al. Phase I/II study of sorafenib and temsirolimus for patients with recurrent glioblastoma (GBM) (NABTC 05–02) J Clin Oncol (Meeting Abstracts) 2009;27(15S):2006. [Google Scholar]
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- Wen PY, Yung WK, Lamborn KR, Dahia PL, Wang Y, Peng B, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99–08. Clin Cancer Res. 2006;12(16):4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick W, Puduvalli VK, Chamberlain MC, van den Bent MJ, Carpentier AF, Cher LM, et al. Phase III Study of Enzastaurin Compared With Lomustine in the Treatment of Recurrent Intracranial Glioblastoma. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.23.2595. JCO.2009.2023.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox ME. Reovirus as an Oncolytic Agent Against Experimental Human Malignant Gliomas. J Natl Cancer Inst. 2001;93(12):903–912. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A. 1987;84(19):6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CG, Mulders P. Vitespen: a preclinical and clinical review. Future Oncol. 2009;5(6):763–774. doi: 10.2217/fon.09.46. [DOI] [PubMed] [Google Scholar]
- Wrann M, Bodmer S, de Martin R, Siepl C, Hofer-Warbinek R, Frei K, et al. T cell suppressor factor from human glioblastoma cells is a 12.5-kd protein closely related to transforming growth factor-beta. EMBO J. 1987;6(6):1633–1636. doi: 10.1002/j.1460-2075.1987.tb02411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima N, Yamanaka R, Mine T, Tsuchiya N, Homma J, Sano M, et al. Immunologic Evaluation of Personalized Peptide Vaccination for Patients with Advanced Malignant Glioma. Clin Cancer Res. 2005;11(16):5900–5911. doi: 10.1158/1078-0432.CCR-05-0559. [DOI] [PubMed] [Google Scholar]
- Yamada S, Bu X-Y, Khankaldyyan V, Gonzales-Gomez I, McComb JG, Laug WE. Effect of the angiogenesis inhibitor Cilengitide (EMD 121974) on glioblastoma growth in nude mice. Neurosurgery. 2006;59(6):1304–1312. doi: 10.1227/01.NEU.0000245622.70344.BE. discussion 1312–1304–1312; discussion 1312. [DOI] [PubMed] [Google Scholar]
- Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T, et al. Clinical Evaluation of Dendritic Cell Vaccination for Patients with Recurrent Glioma: Results of a Clinical Phase I/II Trial. Clin Cancer Res. 2005;11(11):4160–4167. doi: 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- Yin D, Ong JM, Hu J, Desmond JC, Kawamata N, Konda BM, et al. Suberoylanilide Hydroxamic Acid, a Histone Deacetylase Inhibitor: Effects on Gene Expression and Growth of Glioma Cells In vitro and In vivo. Clin Cancer Res. 2007;13(3):1045–1052. doi: 10.1158/1078-0432.CCR-06-1261. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Tanaka R, Takai N, Ono K. Local Administration of Autologous Lymphokine-activated Killer Cells and Recombinant Interleukin 2 to Patients with Malignant Brain Tumors. Cancer Res. 1988;48(17):5011–5016. [PubMed] [Google Scholar]
- Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with Tumor Lysate-Pulsed Dendritic Cells Elicits Antigen-Specific, Cytotoxic T-Cells in Patients with Malignant Glioma. Cancer Res. 2004;64(14):4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, et al. Vaccination of Malignant Glioma Patients with Peptide-pulsed Dendritic Cells Elicits Systemic Cytotoxicity and Intracranial T-cell Infiltration. Cancer Res. 2001;61(3):842–847. [PubMed] [Google Scholar]
- Yuan R, Kay A, Berg W, Lebwohl D. Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. J Hematol Oncol. 2009;2(1):45. doi: 10.1186/1756-8722-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung WK, Prados M, Levin VA, Fetell MR, Bennett J, Mahaley MS, et al. Intravenous recombinant interferon beta in patients with recurrent malignant gliomas: a phase I/II study. J Clin Oncol. 1991;9(11):1945–1949. doi: 10.1200/JCO.1991.9.11.1945. [DOI] [PubMed] [Google Scholar]
- Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88(11):2606–2618. [PubMed] [Google Scholar]
- Zalutsky MR, Moseley RP, Coakham HB, Coleman RE, Bigner DD. Pharmacokinetics and Tumor Localization of 131I-Labeled Anti-Tenascin Monoclonal Antibody 81C6 in Patients with Gliomas and Other Intracranial Malignancies. Cancer Res. 1989;49(10):2807–2813. [PubMed] [Google Scholar]
- Zhang Y, Guessous F, Kofman A, Schiff D, Abounader R. XL-184, a MET, VEGFR-2 and RET kinase inhibitor for the treatment of thyroid cancer, glioblastoma multiforme and NSCLC. Idrugs. 2010;13(2):112–121. [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Gallo JM. Differential effect of sunitinib on the distribution of temozolomide in an orthotopic glioma model. Neuro Oncol. 2009;11(3):301–310. doi: 10.1215/15228517-2008-088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Guo P, Gallo JM. Impact of angiogenesis inhibition by sunitinib on tumor distribution of temozolomide. Clin Cancer Res. 2008;14(5):1540–1549. doi: 10.1158/1078-0432.CCR-07-4544. [DOI] [PubMed] [Google Scholar]
- Zhou YH, Tan F, Hess KR, Yung WKA. The Expression of PAX6, PTEN, Vascular Endothelial Growth Factor, and Epidermal Growth Factor Receptor in Gliomas. Clin Cancer Res. 2003;9(9):3369–3375. [PubMed] [Google Scholar]