Abstract
Much evidence suggests that targeting the neurotensin (NT) system may provide a novel and promising treatment for schizophrenia. Our recent work shows that: NTS1 knockout (NTS1−/−) mice may provide a potential animal model for studying schizophrenia by investigating the effect of deletion NTS1 receptor on amphetamine-induced hyperactivity and neurochemical changes. The data indicate a hyper-dopaminergic state similar to the excessive striatal DA activity reported in schizophrenia. The present study was done to determine if NTS1−/− mice also have similar changes in behavior, in prefrontal neurotransmitters, and in protein expression, as observed in wild type (WT) mice treated with the psychotomimetic phencylclidine (PCP), an animal model for schizophrenia. Our results showed many similarities between untreated NTS1−/− mice and WT mice chronically treated with PCP (as compared with untreated WT mice): 1) lower PCP-induced locomotor activity; 2) similar avolition-like behavior in forced-swim test and tail suspension test; 3) lower prefrontal glutamate levels; 4) less PCP-induced dopamine release in medial prefrontal cortex (mPFC); and 5) down-regulation of mRNA and protein for DA D1, DA D2, and NMDAR2A in mPFC. Therefore, these data strengthen the hypothesis that the NTS1−/− mouse is an animal model of schizophrenia, particularly for dysfunction of the prefrontal cortex. In addition, after chronic PCP administration, the DA D1 receptor was up-regulated in NTS1−/− mice, results which suggest a possible interaction of NTS1/DA D1 in mPFC contributing to chronic PCP-induced schizophrenia-like signs.
Keywords: neurotensin receptor, schizophrenia, phencyclidine, dopamine, glutamate, prefrontal cortex
1. Introduction
The neuropeptide neurotensin (NT) has long been implicated in the pathophysiology of schizophrenia. Antipsychotic drugs increase CSF NT levels in a subset of patients with schizophrenia (Garver et al., 1991; Sherman et al., 1991) and endogenous NT may mediate the effects of antipsychotic drugs through the regulation of dopaminergic, glutamatergic, GABAergic, and 5-HT-mediated circuits in areas associated with the pathophysiology of schizophrenia (Binder et al., 2001). Therefore, targeting the NT system may provide a novel and promising treatment for schizophrenia (Boules et al., 2003; Breslin et al., 1994; Garver et al., 1991). NT mediates its effects through its receptors: the high affinity, NT receptor (NTS1), the low affinity NT receptor (NTS2), and NTS3. NTS1 and NTS2 are G-protein coupled, 7-transmembrane spanning proteins, while NTS3 is not so coupled and spans the membrane once. NTS1, the most studied of the NT receptors, modulates dopamine receptors (Fuxe et al., 1992a; Fuxe et al., 1992b), mediates several of the central and peripheral effects of NT, and plays a major role in the peptide’s modulation of neurotransmitter systems (Antonelli et al., 2007; Leonetti et al., 2004; Pettibone et al., 2002).
Phencyclidine (PCP), as a noncompetitive antagonist of the N-methyl-D-aspartate (NMDA)/glutamate receptor, induces schizophrenia-like cognitive dysfunction and psychotic behavior in otherwise normal individuals (Javitt and Zukin, 1991; Snyder, 1980). Thus, PCP administration to rodents has proven useful as an animal model for studying schizophrenia (Jentsch and Roth, 1999). NT69L, an analog of NT(8–13) and an agonist at NTS1 (Tyler-McMahon et al., 2000) and NTS2 (Boules, et al 2010) receptors, blocks behavior and neurochemical changes induced by PCP administration (Li et al., 2010a). These results suggest that NTS1 is involved in the modulation of PCP-induced schizophrenia-like signs, except that NT69L also affects NTS2. Repeated use of PCP by humans induces more persistent schizophrenic-like signs and symptoms including psychosis, hallucinations, flattened affect, delusions, formal thought disorder, cognitive dysfunction, and social withdrawal (Allen and Young, 1978; Cosgrove and Newell, 1991; Javitt and Zukin, 1991; Rainey and Crowder, 1975). Therefore, the effects of chronic, rather than acute, exposure to PCP may better represent a model for some facets of schizophrenia.
In recent investigations of NTS1 knockout (NTS1−/−) mice from our group (Li et al., 2010b; Liang et al., 2010), we found that NTS1−/− mice: 1) were more sensitive to d-amphetamine-induced hyperactivity; 2) had higher DA levels in striatum than did wild type (WT) mice; 3) had a significantly higher DA D2/DA D1 mRNA ratio in the striatum than that for WT mice; and 4) had lower d-amphetamine-induced striatal glutamate and GABA release, possibly through an interaction between NTS1 and DA receptors. These results support the idea that NTS1 receptors are involved in the pathogenesis of schizophrenia. Previous study indicates that dysregulation of DA function revealed by amphetamine challenge in schizophrenic patients stems from a deficit in prefrontal glutamate transmission (Laruelle et al., 2005). To further characterize NTS1−/− mouse as a potential animal model for studying schizophrenia, we focused on the prefrontal cortex and investigated the effect of acute and chronic PCP on the behavioral, neurotransmitter, and molecular changes in NTS1−/− and WT mice. We hypothesized that NTS1−/− mice would show similar schizophrenia-like signs to the PCP-treated WT mice. Additionally, the deletion of NTS1 would decrease the neurotransmitter effects of acute PCP administration.
2. Materials and Methods
2.1 Animals and housing
Male wild type C57BL/6J and male NTS1−/− mice were approximately 2 months of age and weighing 20~25 g at the beginning of the study. NTS1−/− was established at Roche (Palo Alto CA, USA) and characterized as described by Mechanic et al. (Mechanic et al., 2009). Briefly, NT receptor null allele was originally created in the Bruce-4ES cell line, which was derived from a C57BL/6 mouse strain. Cells were injected into BALB/c blastocysts to generate chimeras. Male chimera mated with female C57BL/6 to give rise to the F1 or N1 heterozygotes (+/−). To ensure a pure genetic background, one additional backcross was performed (male F1 heterozygote x female C57BL/6) to generate the N2 heterozygotic mice. Homozygotic KO (−/−) and WT (+/+) mice (F2) were from N2 heterozygote X heterozygote intercrosses. These KO and WT mice were used to establish the NTS1 KO and WT colonies, respectively. All mice were housed in a temperature-controlled room (23±2 °C) with a 12-h light/dark cycle and free access to food and water throughout the study. Animal use was approved by Mayo Foundation Institutional Animal Use and Care Committee and was consistent with NIH Guide for the Care and Use of Laboratory Animals.
2.2 Drugs
[3H]raclopride (62.2 Ci/mmol) and [3H]CGP 39653 (40.5 Ci/mmol) were purchased from PerkinElmer (Waltham, MA, USA). Raclopride (catalog # 98185-20-7) and phencyclidine (catalog #956-90-1, 100%, EQP level-Premium) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.3 Behavior tests
Locomotor activity
Test chambers were equipped with infrared photocell emitters and detectors were situated along the long axis (Opto-Varimex; Columbus Instruments, Columbus, OH, USA). In acute studies, WT or NTS1−/− mice was placed individually in a chamber and allowed 2 h for acclimation. After acclimation, activity was recorded for 2 h for baseline, 2 h following 0.9% saline injection (intraperitoneally, i.p.), and 2 h following either PCP (5 mg/kg, i.p.) or saline injection (Table 1––1). For chronic studies, mice were injected daily for 21 d with the same dose of PCP (chronic PCP or “CP”, 5 mg/kg, i.p.) or equal volume of saline (chronic saline or “CS”, i.p.) as shown in Table 1––1. Activity was recorded on day 22 with the use of the same procedure as described for the acute studies.
Table 1.
Experiment design.
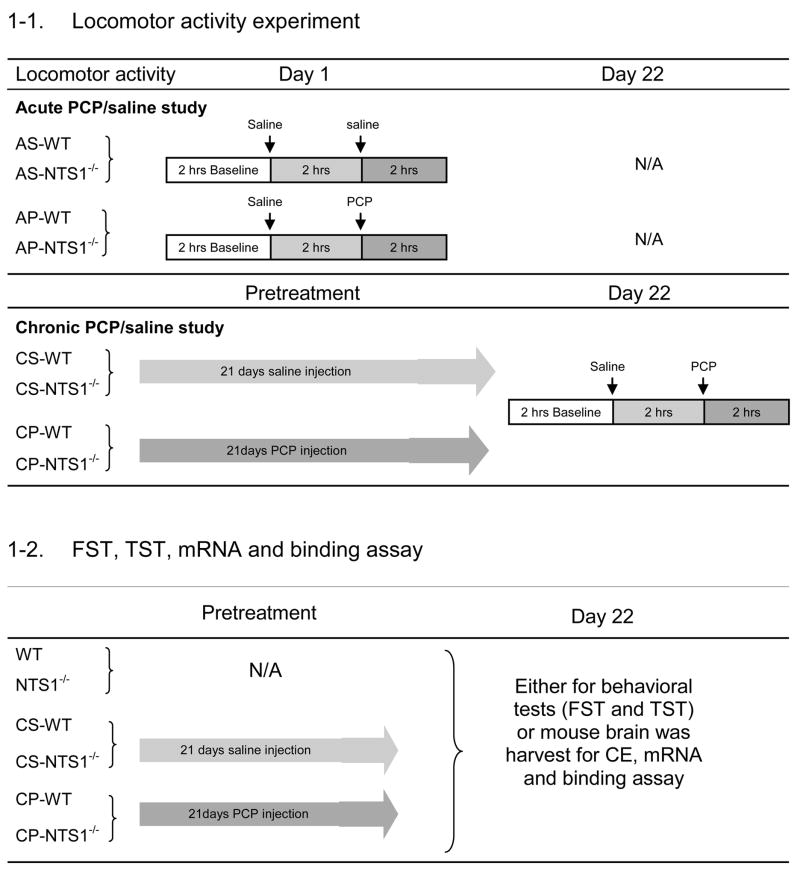 
|
Acute PCP (“AP,” 5 mg/kg PCP, i.p.) administration; Acute Saline (“AS,” equal volume 0.9% saline, i.p.) administration; Chronic PCP (“CP,” 21-d 5 mg/kg PCP daily, i.p.) administration; Chronic Saline (“CS,” 21-d equal volume 0.9% saline daily, i.p.) administration (N=4–7 for each group, each group constitutes from mice with the same treatment). Different sets of mice have been used in each experiment and there is no repeat use of any set of mice in the whole experiments.
Forced-swim test
The forced-swim test has been used to measure avolition, which is one of the negative symptoms of schizophrenia (Noda et al., 2000; Noda et al., 1997; Noda et al., 1995). The procedure was conducted as originally described by Abdel-Naby Sayed et al. (Abdel-Naby Sayed et al., 2001). WT, NTS1−/−, CS-WT, CS- NTS1−/−, CP-WT and CP- NTS1−/− mice were tested (see Table 1–2). Mice was individually placed in vertical cylinders (height, 40 cm; internal diameter, 19 cm) containing water (22–23°C) to a level of 15 cm. The procedure involved a pretest and a 180 s test separated by 24 h. During the pretest mice, which had been adapted to the experimental room for at least 1 h, were placed in the cylinder for 180 s. The duration of swimming was recorded and the immobility time was calculated as follows: 180(s)-swimming time (s) = immobility time (s). Then, CS-WT, CP-WT, CS-NTS1−/−, and CP-NTS1−/− mice received their 21st injection of either PCP (5 mg/kg, i.p.) or 0.9% saline (equal volume, i.p.) and returned to their home cages. The following day (day 22), each mouse was placed in water again for 180 s after 1 h habituation to the experimental room, and the immobility time was calculated.
Tail-Suspension Test
The tail-suspension test in mice (Steru et al., 1985) has been used as a corroboration of the forced-swim test. WT, NTS1−/−, CS-WT, CS- NTS1−/−, CP-WT, and CP- NTS1−/− mice were tested. The treatment design was the same as that for the forced-swim test. (see Table 1–2). Thus, on day 22 mice were individually suspended by their tails 35 cm above the tabletop with the use of an adhesive tape placed 1 cm from the tip of the tail. Behavior was scored every 5 s throughout the 6-min test as either mobile or immobile. Mice were considered immobile only when hanging passively and completely motionless.
2.4 Microdialysis Procedure and Capillary Electrophoresis Detection
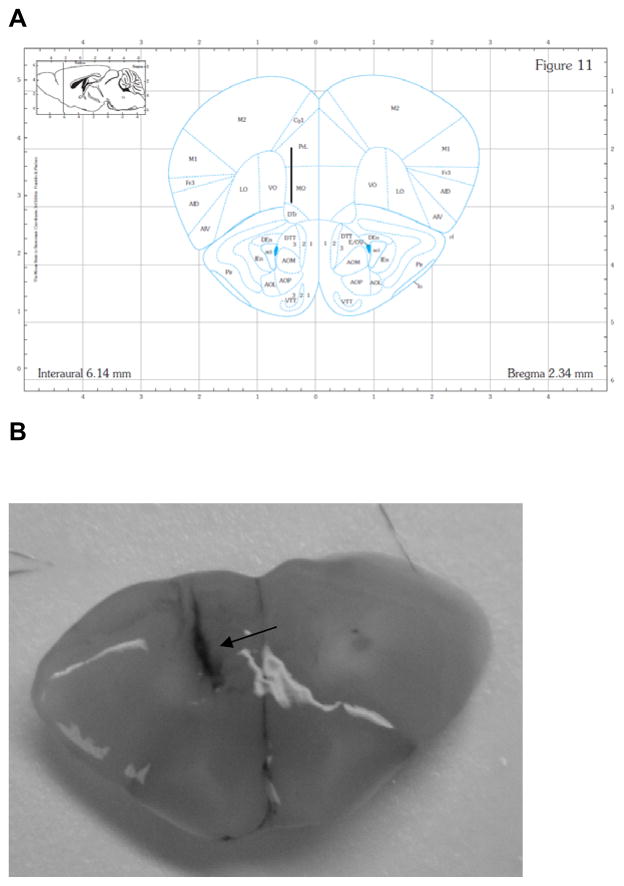
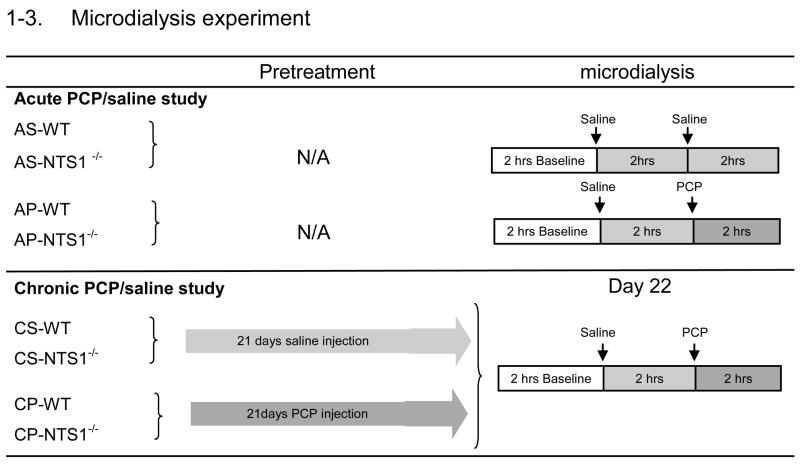
Each mouse was cannulated in the mPFC (anterior 2.3, lateral 0.5, ventral 2.0) relative to bregma and skull (Paxinos and Franklin, 2001). After 3 to 5 days for recovery from surgery, microdialysis experiments were carried out on conscious, freely moving mice. The microdialysis probe (CMA/7 with 2 mm membrane for mPFC, CMA Microdialysis Inc., Acton, MA, USA) was perfused at 2 μl/min with artificial cerebrospinal fluid. After at least a 2-h equilibration, dialysate samples were automatically collected every 20 min into vials containing 2 μl of perchloric acid (0.5 M) to retard oxidation of dopamine. Six baseline fractions were collected before saline injection (Table 1–3). Dopamine and amino acids in the samples were measured by capillary electrophoresis (Agilent 3DCE, Agilent Technologies, Inc., Wilmington, DE, USA.) as described in our previous study (Li et al., 2010b). The detection limits of glutamate, GABA, glycine, aspartate, and dopamine were respectively: 84 nM, 45 nM,. 29 nM, 92 nM, and 23 pM. For chronic PCP administration (Table 1–3), mice received PCP (5 mg/kg, i.p.) daily for 21 d and the microdialysis experiments were performed on day 22 (3–5 days after cannulation surgeries). Results are reported as % baseline and the area under the curve (AUC) during baseline, following the saline injection and following PCP injection. The position of the probe was verified by visual inspection at the end of each experiment (Figure 1).
Figure 1.
Coronal sections showing microdialysis probe placement within mPFC for all animals. Panel A: the placement of the probe in mPFC. Numbers below the figure represent the position of the slice relative to bregma. The figure was adapted from Paxinos and Franklin (Paxinos and Franklin, 2001). Panel B: A picture of real brain coronal section. After each microdialysis, the brains were removed and sectioned to verify the correct position of the probe by injecting trypan blue through probe. Black arrow points out probe location.
2.5. Determination of DA and amino acids in tissue with the use of CE-LIF
PFC tissue from WT, NTS1−/−, CP-WT, CP-NTS1−/−, CS-WT, and CS-NTS1−/− mice (Table 1–2) were harvested on ice, homogenized in buffer (0.01 N perchloric acid containing 10 mM EDTA), centrifuged (10 min, 26,000 × g, 4°C), and the supernatant applied to CE as described in our previous study (Li et al., 2010b).
2.6 Real-time PCR analysis
Animal treatment and RNA extraction: mice from WT, NTS1−/−, CP-WT, CP-NTS1−/−, CS-WT, and CS-NTS1−/− groups (Table 1–2) were decapitated and the brains were dissected on ice. Total RNA was extracted from brain tissue (prefrontal cortex) with the use of TRIZOL reagent (Life Technologies, Frederick, MD, USA) and purified by RNeasy cleanup/DNase set procedure (Qiagen, Valencia, CA, USA, cat. #74103/79254). Total RNA contents were determined by OD260 with the use of a NanoDrop ND-1000 (Thermo Scientific, Wilmington, DE, USA). Total RNA was converted to single stranded cDNA with the use of the high capacity cDNA archive kit (Applied Biosystems, Inc., Foster City, CA, USA) according to the manufacturer’s instructions. Real-time RT-PCR was then performed to determine relative mRNA expression of dopamine receptors D1 (DA D1) and D2 (DA D2), tyrosine 3-hydroxylase (TH), NMDA receptor subtype 1 (NMDAR1), and NMDA receptor 2A subunit (NMDAR2A) in the PFC. The real-time RT-PCR reactions were performed with the use of predesigned primers and probes from Applied Biosystems, which use TaqMan DNA minor groove binding probes that are fluorescently labeled with FAM™ dye for detection. The probe sets were based on the following sequences for mouse: DA D1, Mm02620146_s1; DA D2, Mm00438545_m1; TH, Mm00447557_m1; NMDAR1, Mm00433800_m1; and NMDAR2A, Mm00433802_m1. Murine18s ribosomal RNA, Mm02601776_g1 was used for the internal control. The total reaction volume was 20 μl containing TaqMan universal PCR master mix, TaqMan primers and probes, and cDNA. Reactions were run in triplicate in optical grade 384-well plates on an ABI Prism 7900HT PCR machine with the following thermal cycling conditions: 2 min @ 50°C, 10 min @ 95°C, 40 cycles of 15 sec at 95°C and 1 min at 60°C. Expression levels of each gene were calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001) with 18s RNA as an internal control.
2.7 DA D2 and NMDAR2A Binding Assay
DA D2 and NMDAR2A binding were done in brain homogenates from WT, NTS1−/−, CP-WT, CP-NTS1−/−, CS-WT, and CS-NTS1−/− groups (Table 1–2) as previously described (Hall et al., 1988) with the use of [3H]raclopride as the radioligand for DA D2 and [3H]CGP 39653 [D,L-(E)-2-amino-4-propyl-5-phosphono-3-pentenoic acid] as the radioligand for NMDAR2A. The detail procedure of DA D2 binding has been described in our previously study (Liang et al., 2010). For NMDAR2A binding, PFC tissue was homogenized in ice cold solution of EDTA (10mM), phenylmethylsulphonyl fluoride (1 μg/ml), E-64 (1 μM, Cat.# E3132, Sigma-Aldrich, St. Louis, MO, USA)and centrifuged at 1,000 × g for 10 min at 4°C. The pellets were re-suspended in cold Tris acetate (10 mM) pH7.4, with EDTA (10 mM). The mixture was then centrifuged at 30,000 × g for 15 min at 4°C. The pellets were re-suspended in the same buffer and the homogenate was frozen to −80°C at least overnight. Before the binding experiment, the membranal suspension was washed twice with the Tris acetate solution, and finally resuspended in the same buffer. The final protein concentration was determined by using the BCA assay. Varying concentrations (10 nM to 100 μM) of glutamate were added to 96-well plates with radio-labeled CGP 39653 (final concentration in the assay was 20 nM) and crude membranal homogenates (100 μg/well). To achieve equilibrium conditions for the antagonists, membranal homogenates were incubated for 60 min with drugs at 4°C. The reaction was stopped by rapid filtration through a Whatman GF/B glass fiber filter (presoaked in 0.1% polyethyleneimine) in a 48-place Brandel cell harvester (Brandel, Gaithersburg, MD). The filter was dried at least overnight and placed in a scintillation vial containing 5 ml of scintillation cocktail, and counted in a scintillation counter. Specific binding was calculated as the difference between the total binding and nonspecific binding. Data were analyzed by LIGAND (Munson and Rodbard, 1980) and presented as geometric means, with the standard error of the geometric mean being calculated as described by De Lean et al (De Lean et al., 1982).
2.8 Statistical analyses
One Way Analysis of Variance (ANOVA) was performed for the behavioral studies followed by Tukey’s test for multiple comparisons with the use of Sigma Stat software (SPSS, Inc., Chicago, IL, USA). For the microdialysis experiments, two-way repeated measures ANOVA followed by Tukey’s test was used to compare the percentage baseline between groups, time, and treatment as independent factors, and time as the repeated factor. Difference in AUC between groups was analyzed by one-way ANOVA with the use of the same software. P< 0.05 was considered significant. Student’s t-test was used to compare the binding assay results and the mRNA expression data.
3. Results
3.1 Effect of acute and chronic PCP administration on Locomotor Activity
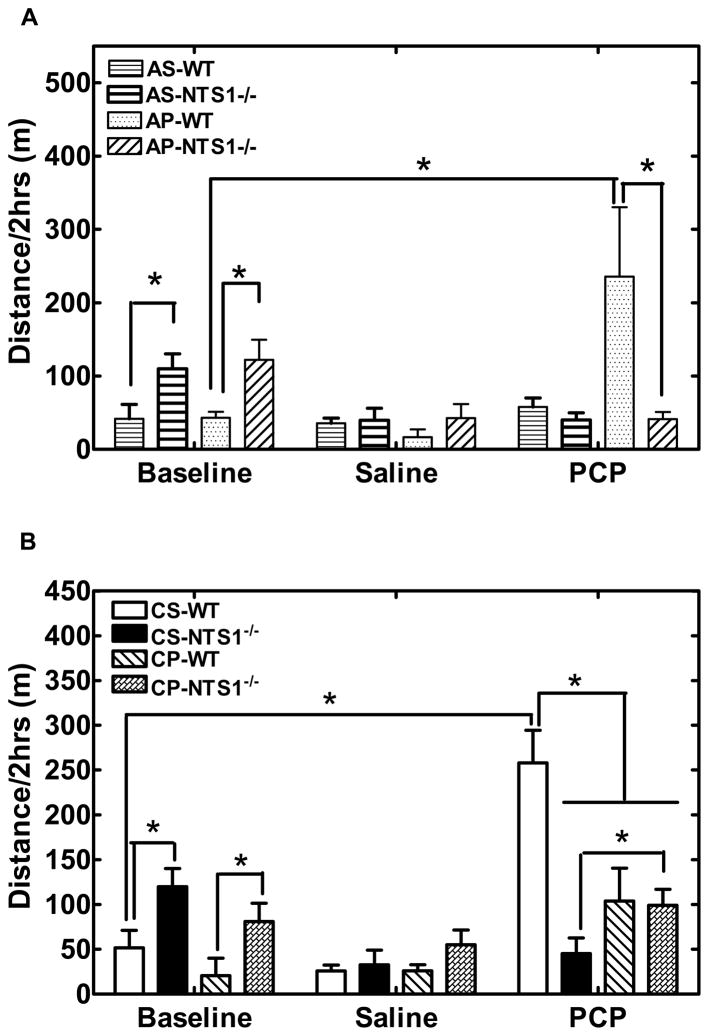
NTS1−/− mice had significantly higher basal locomotor activity than did WT mice (P=0.01) (Fig. 2). Acute saline injection had no effect on locomotor activity for WT and NTS1−/− mice (Fig. 2A). Acute PCP injection induced hyperactivity in WT but not in NTS1−/− mice (P=0.02) (Fig. 2A). In the chronic study, repeated saline injections had no effect on the basal locomotor activity and the locomotor effects of an acute injection of PCP as compared with acute PCP groups (Fig. 2B). Chronic PCP injections significantly decreased activity for WT mice (P=0.04) and increased activity (sensitization) in NTS1−/− mice (P=0.04) (Fig. 2B) induced by a single PCP challenge injection. Repeated PCP or saline injections had no effect on body weight for WT and NTS1−/− mice (data not shown).
Figure 2.
Effect of acute and chronic PCP or saline administration on locomotor activity in WT and NTS1−/− mice. Panel A. Effect of acute PCP or saline administration on locomotor activity in both WT and NTS1−/− mice. Panel B. Effect of chronic PCP or saline administration on locomotor activity in both WT and NTS1−/− mice. All data are shown as mean ± S.E.M (N=5–7 for each group). *, P<0.05.
3.2 Effect of acute and chronic PCP administration in the Forced-Swim Test
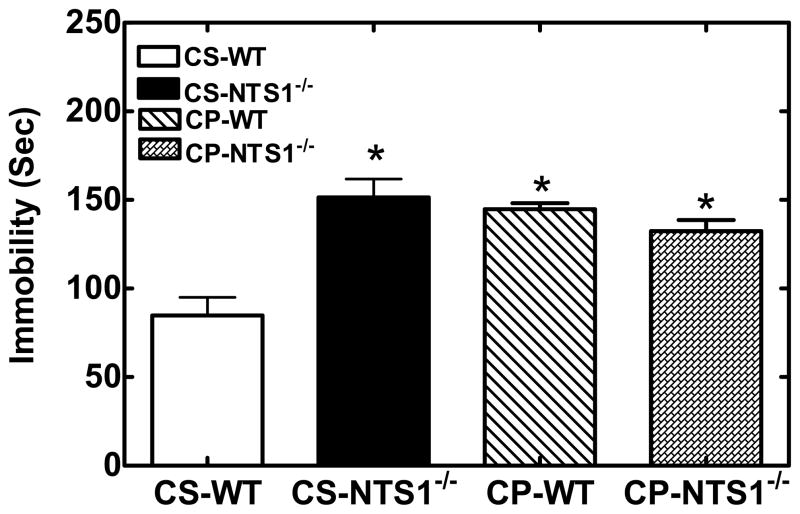
NTS1−/− mice had significantly higher immobility in the FST as compared with WT mice (WT: 100±10 s; NTS1−/−: 170±10 s, P<0.001). Chronic saline injection had no effect on the immobility time in the forced-swim test as compared with non-treated mice (CS-WT: 80±20 s; CS- NTS1−/−: 150±20 s). Chronic treatment with PCP resulted in an increase in immobility time for WT mice (P<0.001) as compared to saline-injected controls and no change in NTS1−/− mice (Fig. 3).
Figure 3.
Effect of chronic PCP or saline administration on forced swim-induced immobility in WT and NTS1−/− mice. Results are expressed as the mean±S.E.M. (N=5–7 rats). *, P < 0.05 versus WT mice with chronic saline administration.
3.3 Effect of acute and chronic PCP administration in the Tail-Suspension Test
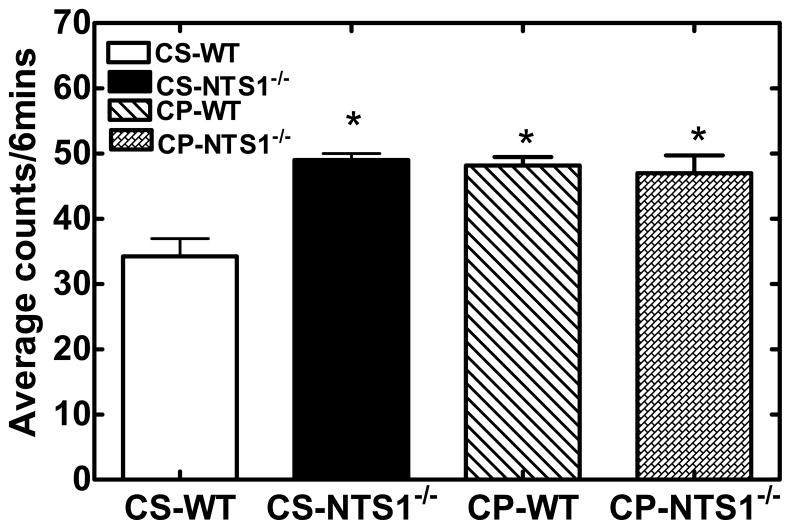
NTS1−/− mice had significantly higher immobility as compared with WT mice in the tail suspension test (Immobility WT: 31±2; NTS1−/−: 56±5, P<0.001). No significant effect was shown following chronic saline injection in WT and in NTS1−/− mice (CS-WT: 34±3; CS- NTS1−/−: 49±2). Chronic treatment of PCP resulted in an increase in immobility (P<0.001) for WT mice as compared to saline-injected controls and again no change in NTS1−/− mice (Fig. 4).
Figure 4.
Effect of chronic PCP or saline administration on the amount of immobility in WT and NTS1−/− mice during the tail-suspension test. Behavior was observed every 5 s during the 6-min test period and scored as mobile or immobile. Results are expressed as the mean number of counts over the 6-min period (±S.E.M.) (N=5 mice for each group). *, P < 0.05 versus WT mice with chronic saline administration.
3.4 In vivo microdialysis
Acute PCP administration
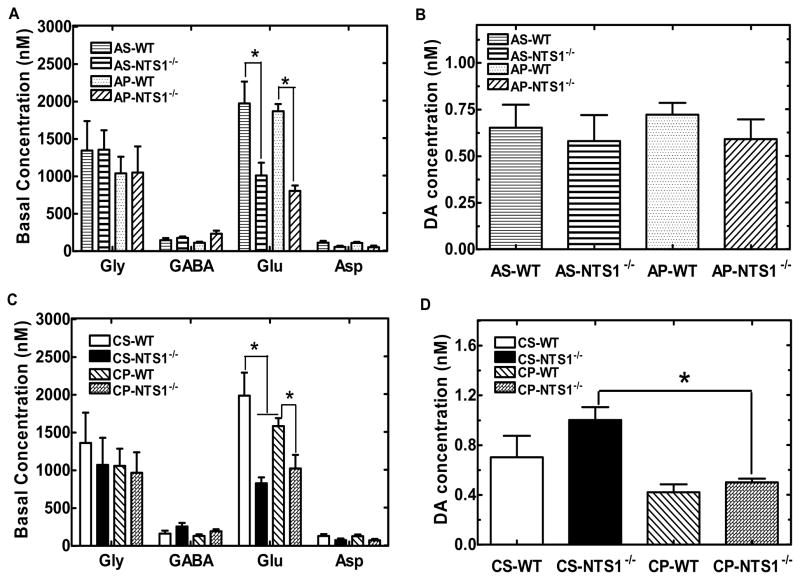
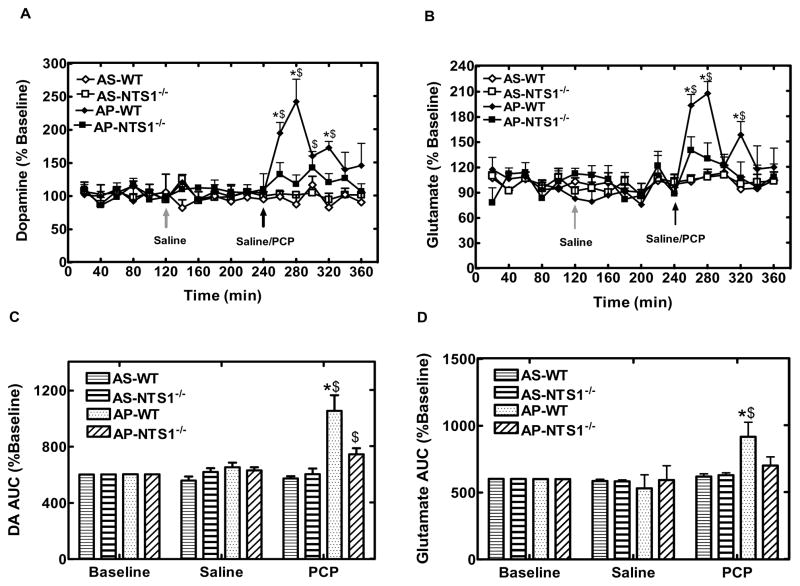
In acute PCP microdialysis experiment, microdialysate samples were tested for 2-hr basline, 2-hr following saline injection and 2-hr following PCP/saline injection (5 mg/kg, i.p.) as showed in table 1–3. There was no difference in the basal levels of glycine, GABA, aspartate, and DA in mPFC between WT and NTS1−/− mice (Fig 5A, 5B). Notably, basal concentrations of glutamate were significantly lower in NTS1−/− mice than in WT mice (P<0.001) (Fig. 5A). Also, acute PCP injections significantly increased extracellular concentrations of DA by 140% in WT and 40% in NTS1−/− mice in mPFC. The results were supported by a significant treatment effect at time 260–320 min for WT (all P<0.01). There was a significant difference for PCP-induced DA release between WT and NTS1−/− mice (Fig. 6A and 6C). This observation is supported by a significant time X group interaction, which was mainly due to a group effect at 260 min (P<0.001), 280 min (P<0.001), and 320 min (P=0.005). Additionally, acute PCP administration significantly increased extracellular concentrations of glutamate in mPFC of WT mice by 110% and by 40% in NTS1−/− mice (P=0.06) (Fig. 6B and 6D). The results were supported by a significant treatment effect at time 260 min, 280 min, 320 min for WT (all P<0.02). There was a significant difference for PCP-induced glutamate release between WT and NTS1−/− mice (Fig. 6B and 6D). This observation is supported by a significant time X group interaction, which was mainly due to a group effect at 260 min (P=0.007), 280 min (P<0.001), and 320 min (P=0.04). Saline injection by itself did not affect DA and glutamate release in both WT and NTS1−/− mice (Fig 6A–D). Overall, a significant interaction of treatment X group was observed in both DA (P=0.005) and glutamate transmission (P=0.01).
Figure 5.
The basal concentration of neurotransmitters in mPFC in both WT and NTS1−/− mice in the acute and chronic PCP studies. The samples were measured by microdialysis coupled with CE. Panel A and B: The basal level of amino acids and DA in mPFC of WT and NTS1−/− mice. Panel C and D: Effect of chronic PCP or saline administration on basal level of amino acids and dopamine in mPFC. All data are shown as mean ± S.E.M (N=4–7 for each group). *, P<0.05.
Figure 6.
Effect of acute PCP or saline injection on DA and glutamate release in mPFC in both WT and NTS1−/− mice. Panel A and C, Effect of acute PCP or saline injection on DA release in mPFC. The data are shown as % of baseline (± S.E.M) (N=4–7 for each group). Panel B and D, Effect of acute PCP or saline injection on glutamate release in mPFC. The data are shown as AUC (± S.E.M) (N=4–7 for each group). $, P<0.05, significantly different from saline injection within the group; *, P<0.05, increase vs. AS- NTS1−/−, AP-WT and AP- NTS1−/−.
Chronic PCP administration
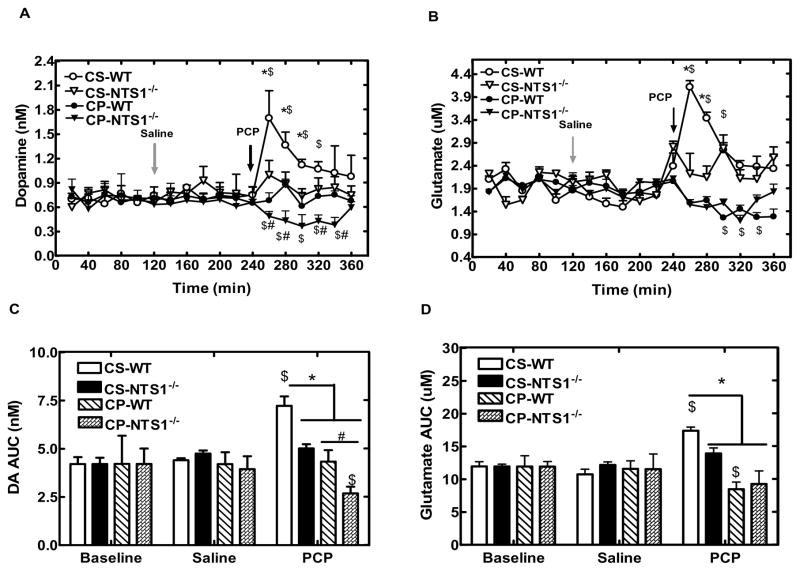
Repeated PCP injections had no effect on the basal levels of glycine, GABA, and aspartate, but resulted in a decreased basal release of glutamate in mPFC of WT mice (P=0.04) as compared with chronic saline administration (Fig. 5C). Basal concentrations of DA were significantly decreased in CP-NTS1−/− mice (P=0.005), but not in CP-WT mice (Fig. 5D). Chronic saline injections had no effect on either the basal levels of neurotransmitters or PCP-induced effect on neurotransmitter release in both WT and NTS1−/− mice. A single PCP challenge injection decreased extracellular concentrations of DA by 50% in CP-NTS1−/− mice with no change in CP-WT mice in mPFC (Fig. 7A and 7C). The results were supported by a significant treatment effect at time 260–340 min for CP-WT (all P<0.01). There was a significant difference for PCP-induced DA release between CP-WT and CP-NTS1−/− mice (Fig. 7A and 7C). This observation is supported by a significant time X group interaction, which was mainly due to a group effect at 260 min (P=0.03), 280 min (P<0.001), 320 min (P=0.002), and 340min (P<0.001). In addition, chronic PCP injections decreased extracellular concentrations of glutamate by 25% in WT mice and 35% in NTS1−/− mice in mPFC without a difference between groups (Fig. 7B and 7D). The results were supported by a significant treatment effect at time 300 min and 340 min for CP-WT (all P<0.05), and at time 320 min for CP-NTS1−/− (P=0.04). Overall, a significant interaction of treatment X group was observed in both DA (P=0.009) and glutamate transmission (P=0.02). Table 2 summarizes the microdialysis results of DA and glutamate in WT and in NTS1−/− mice in either acute or chronic PCP administration study.
Figure 7.
Effect of chronic PCP or saline injection on DA and glutamate release in mPFC in both WT and NTS1−/− mice. Panel A and C, Effect of chronic PCP or saline injection on DA release in mPFC. The data are shown as real concentration (nM) (± S.E.M) (N=4–7 for each group). Panel B and D, Effect of chronic PCP or saline injection on glutamate release in mPFC. The data are shown as AUC (± S.E.M) (N=4–7 for each group). $, P<0.05, significantly different from saline injection within the group; *, P<0.05, increase vs CS- NTS1−/−, CP-WT and CP- NTS1−/−. #, P<0.05, decrease vs. CP-WT.
Table 2.
Qualitative summary of the experimental results.
| Baseline | Acute PCP study | Chronic PCP study | |||||
|---|---|---|---|---|---|---|---|
| WT | NTS1 −/− | AP-WT | AP-NTS1−/− | CP-WT | CP-NTS1−/− | ||
| Behavioral tests | Locomotor activity | +++ | +++ | ++ | ++ | ||
| FST | +++ | N/A | N/A | +++ | +++ | ||
| TST | +++ | N/A | N/A | +++ | +++ | ||
| Microdialysis | DA | +++ | − − − | ||||
| Glu | − − − | +++ | − − | − − | |||
| mRNA | DA D1 | − − − | N/A | N/A | − − − | − − | |
| DA D2 | − − − | N/A | N/A | − − − | − − − | ||
| TH | N/A | N/A | |||||
| NMDAR1 | N/A | N/A | ++ | ||||
| NMDAR2A | − − − | N/A | N/A | − − − | − − − | ||
| Binding assay | DA D2 | − − − | N/A | N/A | − − − | − − − | |
| NMDAR2A | − − − | N/A | N/A | − − − | − − − | ||
“Grayed-out areas” = No change as compared with WT baseline
“+++” = large increase
“++” = moderate increase
“−−−” = large decrease
“−−” = moderate decrease N/A= not tested
3.5 DA and amino acid levels in mPFC tissue
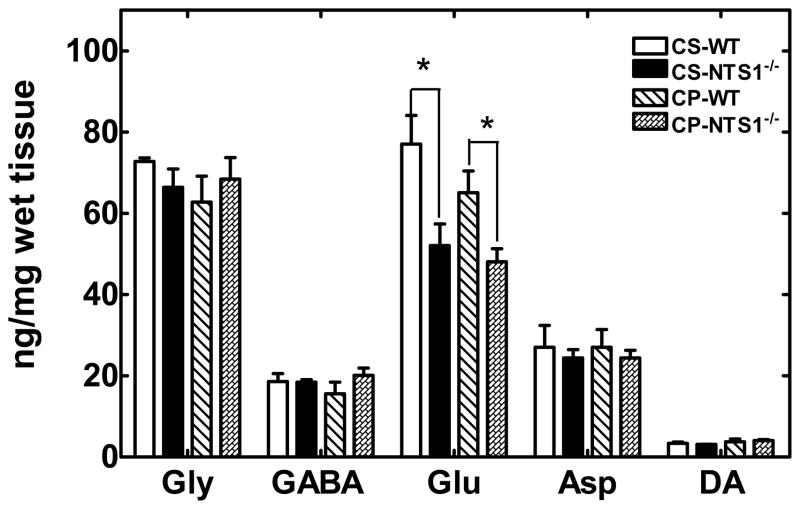
NTS1−/− mice had significantly lower glutamate (55 ±9 pg/mg tissue) levels in PFC homogenates than did WT mice (82±11 pg/mg tissue) as measured by capillary electrophoresis (P=0.02). These data support the results observed in the microdialysis experiments (Figure 5A, 5C). However, levels for DA in the CP-NTS1−/− mice were not significantly different from the CS- NTS1−/− mice (Fig. 8). The reason that there was no DA decrease in CP-NTS1−/− tissue, as would be expected based on the microdialysis studies (Fig 5D), is not known. No difference was observed following either repeated PCP or repeated saline injections (Fig. 8) as compared with that in the non-treated mice.
Figure 8.
DA, glutamate glycine, GABA, and aspartate levels in mPFC homogenates of CS-WT, CS-NTS1−/−, CP-WT and CP- NTS1−/− mice as measured by CE-LIFD. Mouse brains were harvested, dissected on ice, and frozen at −80°C until assayed. Brain tissue was homogenized as described in Materials and Methods and applied to CE-LIFD for detection of neurotransmitter levels (pg/mg tissue). The data were shown as mean (pg/mg wet tissue) ± S.E.M (N=5~7 for each group). P<0.05 is considered significant. *, significantly different from WT.
3.6 DA D1, DA D2, TH, NMDAR1, and NMDAR2A mRNA expression
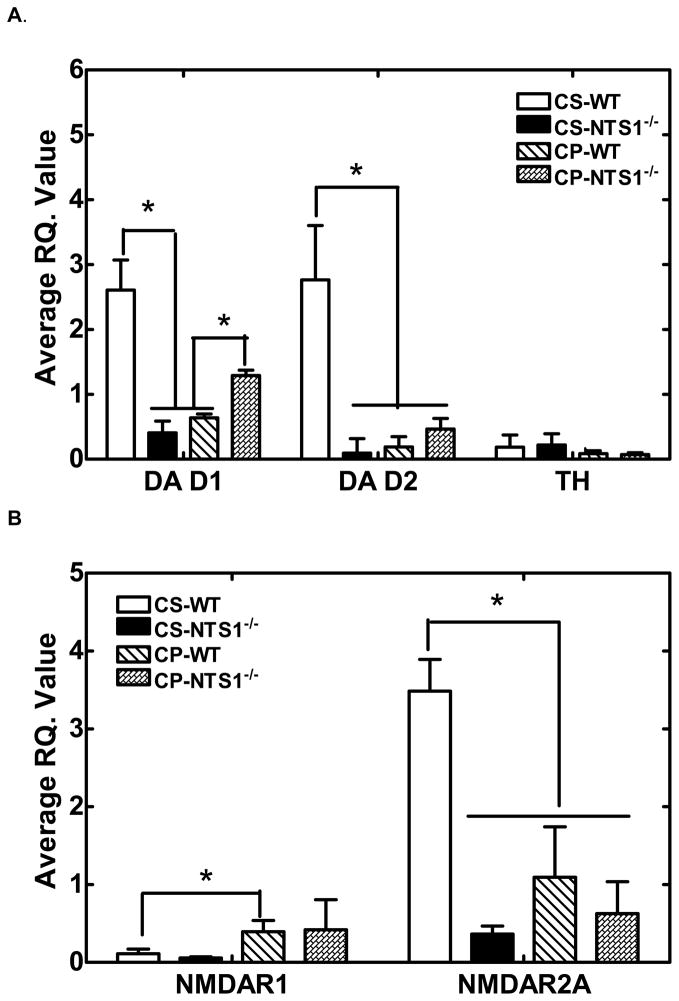
CS-NTS1−/− mice and CP-WT mice had significantly lower DA D1 (P<0.001), DA D2 (P=0.002), and NMDAR2A (P=0.01) mRNA expression in mPFC homogenates relative to CS-WT mice (Fig. 9). DA D1 was up-regulated in CP-NTS1−/− mice (P=0.009) (Fig. 9A) and NMDAR1 was up-regulated in CP-WT mice (P=0.04) (Fig. 9B). Chronic saline injection had no effect on the mRNA expression of DA receptors, TH, and NMDA receptor as compared with non-treated mice. Table 2 summarizes qualitatively these mRNA expression changes relative to controls for both WT and NTS1−/− mice.
Figure 9.
Average R.Q. ratio of DA D1 and DA D2 receptors, and TH (Panel A); NMDAR1 and NMDAR2A (Panel B) for WT and NTS1−/− mice following chronic PCP administration relative to saline treated mice. Mouse brains were harvested, dissected on ice, and frozen at −80°C until assayed. Brain tissue was homogenized and assayed as described in Materials and Methods. Results are presented as mean ± S.E.M (N=5~7 for each group). P<0.05 is considered significant. *, P<0.05.
3.7 DA D2 and NMDAR2A binding
DA D2 and NMDAR2A binding was done in brain homogenates from WT, NTS1−/−, CP-WT, CP-NTS1−/−, CS-WT, and CS-NTS1−/− mice (Table 1–2). Consistent with the mRNA data, both DA D2 and NMDAR2A binding were significantly lower in CP-WT, NTS1−/−, CS-NTS1−/−and CP-NTS1−/−mice as compared with WT mice (P=0.03 for DA D2; P=0.008 for NMDAR2A) (Table 3). Chronic saline injection had no effect on the levels of binding of DA D2 and NMDAR2A receptors.
Table 3.
DA D2 and NMDAR2A receptor binding in WT, NTS1−/−, CP-WT and CP- NTS1−/−.
| DA D2 (fmol/mg protein) | NMDAR2A (fmol/mg protein) | |
|---|---|---|
| WT | 10±1 | 70±10 |
| NTS1−/− | 5±2 * | 35±4* |
| CS-WT | 11±1 | 70±10 |
| CS-NTS1−/− | 5±1 * | 34±5* |
| CP-WT | 5.9±0.8 * | 30±7* |
| CP- NTS1−/− | 6.8±0.4 * | 37±3* |
significantly lower than WT. N=4~6 for each group.
4. Discussion
4.1 Difference between NTS1−/− and WT at baseline
Many previous studies have indicated that NT receptors play important roles in animal models related to schizophrenia. Recent studies using mice lacking NTS1 have shown differences in behavior and in response to drugs between NTS1−/− mice and WT mice (Feifel et al., 2010; Kim et al., 2008; Kinkead et al., 2005; Liang et al., 2010; Mechanic et al., 2009). The current study confirmed our recent finding (Liang et al., 2010) that NTS1−/− mice have significantly higher basal locomotor activity as compared to that for WT mice, a difference that is in association with higher striatal DA concentrations in NTS1−/− mice than in WT mice (Liang et al., 2010). Additionally, our data showed that NTS1−/− mice had significantly higher immobility in both the forced swim test and the tail suspension test. These phenomena have been postulated to be similar to avolition (Noda et al., 2000; Noda et al., 1997; Noda et al., 1995), one of negative symptoms of schizophrenia.
As mentioned in the Introduction, our group has reported that NTS1−/− mice mimic some aspects of schizophrenic patients by having excessive DA activity (Liang et al., 2010) and lower D-serine level in striatum (Li et al., 2010b). The present study showed that basal levels of glutamate and the density of both NMDAR2A subunit and DA D1 receptors were significantly lower in the mPFC of NTS1−/− mice as compared with those for WT mice. These changes have also been reported in schizophrenic patients (Kim et al., 1980; Okubo et al., 1997a; Okubo et al., 1997b). These observations support the idea that dysregulation of striatal DA function in schizophrenia results from a deficit in frontal cortical glutamate transmission (van Berckel et al., 2006). The dysregulation of the DA system in the striatum might further weaken NMDA transmission in corticostriatal glutamate projections (Laruelle et al., 2005). Therefore, the results suggest that NTS1 receptors play an important role in the pathophysiology of schizophrenia, especially frontal cortical dysfunction. Further more, the NTS1−/− mouse may provide a good model for investigating the treatment and etiology of schizophrenia.
NTS2 mRNA expression in brains of NTS1−/− mice is elevated (Liang et al., 2010), a result which could contribute to all the observed changes in NTS1−/− mice. However, the previous work was in whole brain and we found that the mRNA expression level of NTS2 in the PFC of NTS1−/− mice did not significantly differ from the WT mice (average RQ. Value: 1.1±0.2 for WT and 1.5±0.3 for NTS1−/−, unpublished observations). Therefore, over-expression of NTS2 receptors appears not to be the case in PFC.
4.2 The effect of acute and chronic PCP administration on NTS1−/− and WT mice
In the present study, acute PCP injection induced hyperactivity in WT mice as expected, but not in NTS1−/− mice. Microdialysis experiments showed that acute PCP administration caused a significant increase of DA release in mPFC of WT and NTS1−/− mice, results which are consistent with previous studies (Hertel et al., 1995; Jentsch et al., 1997a; Jentsch et al., 1997b; Verma and Moghaddam, 1996). However, PCP-induced DA release was much higher in WT than that in NTS1−/− mice.
Additionally, chronic PCP administration caused decreased locomotor activity in WT mice, a result that is similar to one previous study reporting a slight decrease in locomotion following chronic administration of MK-801, which like PCP is another non-competitive antagonist of the NMDA receptor (Zuo et al., 2006). However, several studies have reported that chronic or subchronic PCP induces sensitization to the locomotor activity in rats (Scalzo and Holson, 1992; Xu and Domino, 1994b) and one study shows this to occur in C57BL/6J mice (Xu and Domino, 1994a). The discrepancy between those studies and the current study may be due to the different dosage and regimen used, or to the animal species.
Unlike the results with WT mice, our data showed that chronic PCP administration to NTS−/− mice induced a sensitization of locomotor activity. A decrease of DA basal levels and a decrease of PCP-induced DA release in mPFC of CP-NTS1−/− mice were simultaneously observed. One interpretation of the behavioral and neurochemical changes is that of an inverse relationship between the mesocortical and mesolimbic DA systems. Thus, hypoactivity of the cortical DA system propagates subcortical DA hyperactivity (Deutch, 1992; Deutch et al., 1990; Grace, 1991; Meyer-Lindenberg et al., 2002; Pycock et al., 1980; Roberts et al., 1994). Therefore, the reduction of both basal and evoked DA release in mPFC may have lead to the sensitization of locomotor activity in CP-NTS1−/− mice.
Abdel-Naby Sayed’s group (Abdel-Naby Sayed et al., 2001) has shown that repeated treatment of mice with PCP (10 mg/kg, s.c. for 14 d) decreases spontaneous locomotor activity and enhances the forced swim-induced immobility. This latter behavioral change is similar to avolition (Noda et al., 2000; Noda et al., 1997; Noda et al., 1995), one of negative symptoms of schizophrenia. Our results from the FST and the TST confirmed that repeated PCP injections increase immobility in WT mice, results that are similar to that for untreated NTS1−/− mice. It has been reported that repeated PCP treatment can induce hyperfunction and hypofunction of serotonergic and dopaminergic systems, respectively, in the PFC of mice that show enhancement of immobility with this treatment (Noda and Nabeshima, 2000). Therefore, significantly less PCP-evoked DA release and down- regulation of both DA D1 and DA D2 receptors in the mPFC of both CP-WT and NTS1−/− mice may be related to the enhancement of immobility in FST and TST.
In an attempt to understand the behavioral and neurotransmitter changes following acute or chronic PCP administration in WT and NTS1−/− mice, we measured the mRNA expression of DA D1, DA D2, TH, NMDAR2A, and NMDAR1. Our molecular experiments showed a down-regulation of mRNA expression for NMDAR2A, the subunit binding site for glutamate, in CP-WT mice, a result that suggests a desensitization of NMDAR2A in mPFC of WT mice following chronic PCP injections. Similar to CP-WT mice, both NTS1−/− mice and CP- NTS1−/− mice had significantly lower NMDAR2A mRNA expression in mPFC. These results may suggest that the reduced effect of PCP on locomotion and the increased immobility in FST and TST in CP-WT, NTS1−/−, and CP-NTS1−/− mice vs. WT mice are due to the desensitization of NMDAR2A.
A down-regulation of DA D1 in mPFC of both CP-WT and NTS1−/− mice was observed, results that are consistent with previous findings of: 1) reduced DA D1 receptor mRNA levels in the monkey PFC after subchronic PCP treatment (Jentsch and Roth, 1999); 2) down- regulation of DA D1 receptors in schizophrenic patients, related to negative symptoms of schizophrenia and poor performance in the Wisconsin Card Sorting Test (WCST)(Okubo et al., 1997a; Okubo et al., 1997b); and 3) reduced function of DA D1 receptors playing an important role in dysfunction of the dopaminergic system in mPFC of patients with schizophrenia (Sawaguchi and Goldman-Rakic, 1991). Therefore, down-regulation of DA D1 receptors in mPFC may be related to avolition-like signs in CP-WT and in untreated NTS1−/− mice.
Additionally, chronic PCP injections induced a large increase of mRNA expression of DA D1 in mPFC of NTS1−/− mice. These results suggest that there is an NTS1/DA D1 receptor interaction in mPFC and that this interaction plays an important role in the chronic PCP-induced schizophrenia-like signs. Several studies have suggested that there is an antagonistic NTS1/DA D2 receptor interaction (Antonelli et al., 2007; Ferraro et al., 2008), but no antagonistic post-junctional NTS1/DA D1 receptor interactions (Diaz-Cabiale et al., 2002; Fuxe et al., 1992a; Fuxe et al., 1992b). However, all of the studies were preformed in striatum, not mPFC. On the other hand, there was a decrease of DA D2 receptor mRNA expression in CP-WT, NTS1−/−, and CP- NTS1−/− mice, which has been found in caudate-putamen (Tomita et al., 1995), but not previously in mPFC. This result was confirmed by binding assay. Further studies are still needed to investigate the possible mechanisms involved.
Previous research with dopamine-deficient mice (Chartoff et al., 2005) demonstrates that glutamate and DA act cooperatively to mediate the locomotor and molecular effects of NMDA receptor antagonists. In the current study, acute PCP injections induced a significant increase of glutamate in the mPFC in NTS1−/− mice and in WT mice, results that are in agreement with the well-established acute effects of PCP on glutamate (Adams and Moghaddam, 1998; Moghaddam and Adams, 1998). Significantly lower glutamate basal levels in NTS1−/− mice and decreased glutamate levels in CP-WT were observed in the present study. These results are consistent with the finding that the concentration of glutamate is decreased in the PFC of patients with chronic schizophrenia as compared to that for controls (Sherman et al., 1991; Tsai et al., 1995).
After chronic PCP administration, a PCP-challenge injection resulted in a slight decrease of glutamate release in mPFC of both WT and NTS1−/− mice, results consistent with previous work (Zuo et al., 2006). All of the neurochemical changes of glutamate in CP-WT may be related to up- regulation of NMDA receptors. The current study showed an up-regulation of NMDAR1 mRNA in CP-WT mice, results that confirms previous findings that chronic (but not acute) exposure to PCP increases expression of NMDAR1 mRNA levels in rat prefrontal cortex after (Tomita et al., 1995).
4.3 The similarities between NTS1−/− mice and CP- WT mice
Table 2 summarizes in a qualitative fashion the present results from behavioral, neurotransmitter, and molecular studies. There are many similarities between the findings for NTS1−/− mice not treated with PCP as compared to those for WT mice chronically treated with PCP (CP-WT). Specifically, we found: 1) lower PCP-induced locomotor activity (Fig 2); 2) similar avolition-like behavior in both FST and TST (Fig 3, 4), 3) lower basal levels of glutamate in mPFC (Fig 5A, 5C), 4) less PCP-induced dopamine release in mPFC (Fig 6, 7); 5) lower mRNA expression of DA D1, DA D2, and NMDAR2A in mPFC (Fig 9); and 6) lower binding of DA D2 and NMDAR2A receptors in mPFC (Table 3). Taken together these results suggest that NTS1−/− mice represent a useful animal model for studying schizophrenic frontal cortical dysfunction, because of its similarities to animals repeatedly injected with PCP. Thus, deletion of NTS1 produced a phenotype similar to chronic administration of PCP to wild type mice, an animal model mimicking some aspects of schizophrenia, and involved both dopaminergic and glutamatergic systems. Furthermore, there may be an interaction between NTS1 and DA D1 receptors in mPFC and this interaction may contribute to chronic PCP-induced schizophrenia-like signs.
Acknowledgments
This work was supported by NIMH grant MH71241.
Footnotes
Financial Disclosure
All the authors have no disclosures. Dr. Richelson has received grant support from NIMH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Naby Sayed M, et al. Enhancement of immobility induced by repeated phencyclidine injection: association with c-Fos protein in the mouse brain. Behav Brain Res. 2001;124:71–6. doi: 10.1016/s0166-4328(01)00235-2. [DOI] [PubMed] [Google Scholar]
- Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–54. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RM, Young SJ. Phencyclidine-induced psychosis. Am J Psychiatry. 1978;135:1081–4. doi: 10.1176/ajp.135.9.1081. [DOI] [PubMed] [Google Scholar]
- Antonelli T, et al. Neurotensin receptor mechanisms and its modulation of glutamate transmission in the brain: relevance for neurodegenerative diseases and their treatment. Prog Neurobiol. 2007;83:92–109. doi: 10.1016/j.pneurobio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Binder EB, et al. Enhanced neurotensin neurotransmission is involved in the clinically relevant behavioral effects of antipsychotic drugs: evidence from animal models of sensorimotor gating. J Neurosci. 2001;21:601–8. doi: 10.1523/JNEUROSCI.21-02-00601.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boules M, et al. Current topics: brain penetrating neurotensin analog. Life Sci. 2003;73:2785–92. doi: 10.1016/s0024-3205(03)00674-x. [DOI] [PubMed] [Google Scholar]
- Breslin NA, et al. CSF concentrations of neurotensin in schizophrenia: an investigation of clinical and biochemical correlates. Schizophr Res. 1994;12:35–41. doi: 10.1016/0920-9964(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, et al. Dopamine is not required for the hyperlocomotor response to NMDA receptor antagonists. Neuropsychopharmacology. 2005;30:1324–33. doi: 10.1038/sj.npp.1300678. [DOI] [PubMed] [Google Scholar]
- Cosgrove J, Newell TG. Recovery of neuropsychological functions during reduction in use of phencyclidine. J Clin Psychol. 1991;47:159–69. doi: 10.1002/1097-4679(199101)47:1<159::aid-jclp2270470125>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- De Lean A, et al. Validation and statistical analysis of a computer modeling method for quantitative analysis of radioligand binding data for mixtures of pharmacological receptor subtypes. Mol Pharmacol. 1982;21:5–16. [PubMed] [Google Scholar]
- Deutch AY. The regulation of subcortical dopamine systems by the prefrontal cortex: interactions of central dopamine systems and the pathogenesis of schizophrenia. J Neural Transm Suppl. 1992;36:61–89. doi: 10.1007/978-3-7091-9211-5_5. [DOI] [PubMed] [Google Scholar]
- Deutch AY, et al. Prefrontal cortical dopamine depletion enhances the responsiveness of mesolimbic dopamine neurons to stress. Brain Res. 1990;521:311–5. doi: 10.1016/0006-8993(90)91557-w. [DOI] [PubMed] [Google Scholar]
- Diaz-Cabiale Z, et al. Neurotensin-induced modulation of dopamine D2 receptors and their function in rat striatum: counteraction by a NTR1-like receptor antagonist. Neuroreport. 2002;13:763–6. doi: 10.1097/00001756-200205070-00006. [DOI] [PubMed] [Google Scholar]
- Feifel D, et al. Sensorimotor gating in neurotensin-1 receptor null mice. Neuropharmacology. 2010;58:173–8. doi: 10.1016/j.neuropharm.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, et al. Neurotensin receptors as modulators of glutamatergic transmission. Brain Res Rev. 2008;58:365–73. doi: 10.1016/j.brainresrev.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Fuxe K, et al. Evidence for a substrate of neuronal plasticity based on pre- and postsynaptic neurotensin-dopamine receptor interactions in the neostriatum. Proc Natl Acad Sci U S A. 1992a;89:5591–5. doi: 10.1073/pnas.89.12.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, et al. Intramembrane interactions between neurotensin receptors and dopamine D2 receptors as a major mechanism for the neuroleptic-like action of neurotensin. Ann N Y Acad Sci. 1992b;668:186–204. doi: 10.1111/j.1749-6632.1992.tb27350.x. [DOI] [PubMed] [Google Scholar]
- Garver DL, et al. Relation of CSF neurotensin concentrations to symptoms and drug response of psychotic patients. Am J Psychiatry. 1991;148:484–8. doi: 10.1176/ajp.148.4.484. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Hall H, et al. Raclopride, a new selective ligand for the dopamine-D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:559–68. doi: 10.1016/0278-5846(88)90001-2. [DOI] [PubMed] [Google Scholar]
- Hertel P, et al. Effects of D-amphetamine and phencyclidine on behavior and extracellular concentrations of neurotensin and dopamine in the ventral striatum and the medial prefrontal cortex of the rat. Behav Brain Res. 1995;72:103–14. doi: 10.1016/0166-4328(96)00138-6. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–8. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, et al. Phencyclidine increases forebrain monoamine metabolism in rats and monkeys: modulation by the isomers of HA966. J Neurosci. 1997a;17:1769–75. doi: 10.1523/JNEUROSCI.17-05-01769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–25. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, et al. Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology. 1997b;17:92–9. doi: 10.1016/S0893-133X(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Kim ER, et al. Impaired anorectic effect of leptin in neurotensin receptor 1-deficient mice. Behav Brain Res. 2008;194:66–71. doi: 10.1016/j.bbr.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Kim JS, et al. Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci Lett. 1980;20:379–82. doi: 10.1016/0304-3940(80)90178-0. [DOI] [PubMed] [Google Scholar]
- Kinkead B, et al. Neurotensin-deficient mice have deficits in prepulse inhibition: restoration by clozapine but not haloperidol, olanzapine, or quetiapine. J Pharmacol Exp Ther. 2005;315:256–64. doi: 10.1124/jpet.105.087437. [DOI] [PubMed] [Google Scholar]
- Laruelle M, et al. Mechanism of action of antipsychotic drugs: from dopamine D(2) receptor antagonism to glutamate NMDA facilitation. Clin Ther. 2005;27(Suppl A):S16–24. doi: 10.1016/j.clinthera.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Leonetti M, et al. Specific involvement of neurotensin type 1 receptor in the neurotensin-mediated in vivo dopamine efflux using knock-out mice. J Neurochem. 2004;89:1–6. doi: 10.1046/j.1471-4159.2003.02231.x. [DOI] [PubMed] [Google Scholar]
- Li Z, et al. The novel neurotensin analog NT69L blocks phencyclidine (PCP)-induced increases in locomotor activity and PCP-induced increases in monoamine and amino acids levels in the medial prefrontal cortex. Brain Res. 2010a;1311:28–36. doi: 10.1016/j.brainres.2009.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, et al. Effect of amphetamine on extracellular concentrations of amino acids in striatum in neurotensin subtype 1 and 2 receptor null mice: a possible interaction between neurotensin receptors and amino acid systems for study of schizophrenia. Neuropharmacology. 2010b;58:1174–8. doi: 10.1016/j.neuropharm.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, et al. Hyperactivity of the dopaminergic system in NTS1 and NTS2 null mice. Neuropharmacology. 2010;58:1199–205. doi: 10.1016/j.neuropharm.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mechanic JA, et al. Involvement of the neurotensin receptor 1 in the behavioral effects of two neurotensin agonists, NT-2 and NT69L: lack of hypothermic, antinociceptive and antipsychotic actions in receptor knockout mice. Eur Neuropsychopharmacol. 2009;19:466–75. doi: 10.1016/j.euroneuro.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–71. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–52. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Munson PJ, Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–39. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Noda Y, et al. Repeated phencyclidine treatment induces negative symptom-like behavior in forced swimming test in mice: imbalance of prefrontal serotonergic and dopaminergic functions. Neuropsychopharmacology. 2000;23:375–87. doi: 10.1016/S0893-133X(00)00138-X. [DOI] [PubMed] [Google Scholar]
- Noda Y, et al. Effects of antidepressants on phencyclidine-induced enhancement of immobility in a forced swimming test in mice. Eur J Pharmacol. 1997;324:135–40. doi: 10.1016/s0014-2999(97)00067-8. [DOI] [PubMed] [Google Scholar]
- Noda Y, Nabeshima T. [Neuropsychopharmacological study on an animal model for negative symptom of schizophrenia induced by repeated phencyclidine treatment] Yakugaku Zasshi. 2000;120:677–82. [PubMed] [Google Scholar]
- Noda Y, et al. Enhancement of immobility in a forced swimming test by subacute or repeated treatment with phencyclidine: a new model of schizophrenia. Br J Pharmacol. 1995;116:2531–7. doi: 10.1111/j.1476-5381.1995.tb15106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo Y, et al. Possible role of dopamine D1 receptors in schizophrenia. Mol Psychiatry. 1997a;2:291–2. doi: 10.1038/sj.mp.4000281. [DOI] [PubMed] [Google Scholar]
- Okubo Y, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997b;385:634–6. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. Academic Press; NY: 2001. [Google Scholar]
- Pettibone DJ, et al. The effects of deleting the mouse neurotensin receptor NTR1 on central and peripheral responses to neurotensin. J Pharmacol Exp Ther. 2002;300:305–13. doi: 10.1124/jpet.300.1.305. [DOI] [PubMed] [Google Scholar]
- Pycock CJ, et al. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on neurotransmitter systems in subcortical sites in the rat. J Neurochem. 1980;34:91–9. doi: 10.1111/j.1471-4159.1980.tb04625.x. [DOI] [PubMed] [Google Scholar]
- Rainey JM, Jr, Crowder MK. Prolonged psychosis attributed to phencyclidine: report of three cases. Am J Psychiatry. 1975;132:1076–8. doi: 10.1176/ajp.132.10.1076. [DOI] [PubMed] [Google Scholar]
- Roberts AC, et al. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. J Neurosci. 1994;14:2531–44. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–50. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Scalzo FM, Holson RR. The ontogeny of behavioral sensitization to phencyclidine. Neurotoxicol Teratol. 1992;14:7–14. doi: 10.1016/0892-0362(92)90023-4. [DOI] [PubMed] [Google Scholar]
- Sherman AD, et al. Deficient NMDA-mediated glutamate release from synaptosomes of schizophrenics. Biol Psychiatry. 1991;30:1191–8. doi: 10.1016/0006-3223(91)90155-f. [DOI] [PubMed] [Google Scholar]
- Snyder SH. Phencyclidine. Nature. 1980;285:355–6. doi: 10.1038/285355a0. [DOI] [PubMed] [Google Scholar]
- Steru L, et al. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Tomita H, et al. Changes in dopamine D2 and GluR-1 glutamate receptor mRNAs in the rat brain after treatment with phencyclidine. Acta Med Okayama. 1995;49:61–8. doi: 10.18926/AMO/30393. [DOI] [PubMed] [Google Scholar]
- Tsai G, et al. Abnormal excitatory neurotransmitter metabolism in schizophrenic brains. Arch Gen Psychiatry. 1995;52:829–36. doi: 10.1001/archpsyc.1995.03950220039008. [DOI] [PubMed] [Google Scholar]
- Tyler-McMahon BM, et al. Highly potent neurotensin analog that causes hypothermia and antinociception. European Journal of Pharmacology. 2000;390:107–11. doi: 10.1016/s0014-2999(99)00877-8. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–9. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Domino EF. Genetic differences in the locomotor response to single and daily doses of phencyclidine in inbred mouse strains. Behav Pharmacol. 1994a;5:623–629. doi: 10.1097/00008877-199410000-00008. [DOI] [PubMed] [Google Scholar]
- Xu X, Domino EF. Phencyclidine-induced behavioral sensitization. Pharmacol Biochem Behav. 1994b;47:603–8. doi: 10.1016/0091-3057(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Zuo DY, et al. Effect of acute and chronic MK-801 administration on extracellular glutamate and ascorbic acid release in the prefrontal cortex of freely moving mice on line with open-field behavior. Life Sci. 2006;78:2172–8. doi: 10.1016/j.lfs.2005.09.022. [DOI] [PubMed] [Google Scholar]