Abstract
Bone morphogenetic proteins (BMPs) were first studied as growth factors or morphogens of the transforming growth factor-beta super family. These growth molecules, originally associated with bone and cartilage development, are now known to play important roles in morphogenesis and homeostasis in many other tissues. More recently, significant contributions of BMPs, their receptors, and interacting molecules have been linked to carcinogenesis and tumor progression. On the other hand, BMPs can sometimes play a role as a tumor suppressor. Our report highlights these new roles in the pathogenesis of cancer that may suggest novel targets for therapeutic intervention.
Keywords: Bone Morphogenetic Proteins, Bone morphogenetic receptors, TGF- β, Cancer, Metastasis
1.0. Introduction
1.1. BMP introduction, signaling cascades, and interacting molecules
Bone Morphogenetic Proteins (BMPs) are a family of evolutionarily conserved growth factors and morphogens most of which belong to the transforming growth factor-β (TGF-β) super-family. BMPs were discovered by Marshall Urist in 1965, who found that decalcified bone matrix fragments have bone induction activity when transplanted into rats and rabbits [1]. Wozney et al., (1988) isolated and identified molecules from bone extracts capable of inducing bone and cartilage formation and named them BMPs [2]. Further studies reveal that BMPs not only regulate bone and cartilage, but exert a wide range of morphogenetic activity that is both tissue and context dependent [3, 4, 5].
BMPs exist as dimeric pro-protein complexes in the cytoplasm that are cleaved by proteases before their intended action. After the BMP molecules are secreted, they are further processed by another layer of regulators, Noggin and Chordin, and then bind to their specific receptors on the plasma membrane of their target cells [6, 7]. BMPs exert their activities by way of serine-threonine kinase receptors of which there are three type-I and three type-II [8]. Different BMPs show preference in the combination of receptors, but in general utilize one receptor of each type [9, 10]. As most BMPs are TGF- β family members, they tend to use the same signaling pathways, principally MAPK and SMAD, although Notch and WNT are also used [11–13]. Binding of the BMPs to their specific receptors triggers cross phosphorylation of the type-I receptor by the type-II receptor [14]. The type-I receptor then releases R-SMADs that oligomerize with SMAD-4 to form a complex that translocates from the cytoplasm to the nucleus where it exerts transcriptional activity for the activation or repression of BMP-specific genes [15]. Some BMPs are under the control of tissue specific cis-regulatory elements [16].
Recent literature suggests that BMPs may be involved in human cancers in addition to their roles as tissue morphogens. This review focuses on the role of BMPs in oncogenic cellular processes including proliferation, metastasis, angiogenesis, differentiation, and epigenetic regulation. In preparing this review, we found that BMPs exert both pro- and anti-carcinogenic activities.
2. Evidence of BMP involvement in tumorigenesis
2.1. Differential BMP expression and copy number alteration are associated with human tumor progression
A large compilation of expression studies shows the attempt to understand the molecular mechanism and involvement of different BMPs and their complex interactions in both normal and cancer cells [Table 1, Figure 1]. Bentley et al., (1992) correlates interaction among prostate tumors, their bone metastasis and various BMPs with their differential expression [17]. This study suggests that BMPs can play a role in bone stimulation and skeletal metastases in prostate cancer. The study uses low sample numbers but still provides initial insight into the role of BMPs in prostate and possibly other cancers. Similarly other BMPs could possibly be a determining factor for the fate and progression of prostate cancer cells via SMAD activation [18, 19, Table 1].
Table 1.
Bone morphogenetic proteins and their involvement in various human cancers
| Serial No. | Cancer type | Biological material used for the study/BMPs and their receptors involved [Note: (▾) represents lower expression and (▴) higher expression] | Type of alteration during cancer/mechanism of involvement in human cancer | References |
|---|---|---|---|---|
| 1. | Prostate cancer |
Tissue: prostate adenocarcinoma, Benign prostatic hyperplasia and ocular melanoma, Cell lines: DU145; and PC3 BMPs involved: BMP1–6 |
Role in osteoinductive activities and pathogenesis of osteoblastic metastases | [17] |
|
Cell line: PC3 (Prostatic adenocarcinoma) BMPs involved: BMP1–4, and -6 |
Expression of BMPs | [18] | ||
|
Tissue: prostate cancer tissues Cell lines: LNCaP BMs involved: BMPRIB (▴) |
Higher expression of BMPRIB | [45] | ||
|
Cell lines: LNCaP (androgen sensitive) BMPs involved: BMPRIA, -IB, BMP2 |
BMPR-IA elicits growth stimulation and BMPR-IB conveys a regulatory signal in response to BMP2 | [46] | ||
|
Tissue: Benign and malignant prostatic cancer BMPs involved: BMP6 |
BMP6 expression in metastases and localized cancer | [100] | ||
|
Tissue: prostate cancer sample BMPs involved: BMPRII, -IA, -IB |
Loss of expression of BMPRs and role in cancer progression | [50] | ||
|
Cell lines: PC-3 and DU-145 BMPs involved: BMP7 |
Anti-proliferatory and growth inhibitory effects | [57] | ||
|
Cell lines: PC-3M BMPs involved: BMP-IA, -IB, and –II |
Loss of expression of BMPRs | [50] | ||
|
Cell lines: LNCaP, PC-3 BMPs involved: BMP2 and -4 |
Growth inhibitory effects of BMP2 and -4 | [69] | ||
|
Cell lines: MLC and HTS-40C (non-neoplastic prostatic epithelial cell lines); PC-3, LAPC-4, DU145, LNCaP, C4-2B, and CWR22R; and HSP-40F and HTS-40C (stromal cell lines) BMPs involved: BMP2 and -7 |
Positive expression and association with BMP2 and -7 | [19] | ||
|
Tissue: Primary prostate tumor BMPs involved: BMP2 -7 |
Increased expression of BMP2, -4 and -7 | [20] | ||
|
Cell lines: LAPC-4, DU145, LNCAP, CWR22R, HPS-40F, HTS-40C, and MLC BMPs involved: BMPRIA, -IB, -II, ActRI, -RII, and -RIIB |
Consistent expression of BMP type I and -II receptors | [19] | ||
|
Tissue: prostate cancer sample BMPs involved: BMP2, -5, and -7 |
Gain of gene copy number | [42] | ||
|
Cell lines: DU-145, PC3, CAHPV10, LNCaP, PZHPV7, PNT1A, PNT2C2 BMPs involved: BMP9 and -10 (▾) |
BMP9 and -10 as tumor suppressors | [151, 64] | ||
| 2. | Breast cancer |
Tissue: Breast tumor BMPs involved: BMP2 |
Anti-proliferative effects | [54] |
|
Cell lines: MCF-7 BMPs involved: BMP2, -3; BMP receptors -1A, -1B, and –II |
Growth inhibitory effects | [48] | ||
|
Tissue: Breast cancer (invasiveductal, invasive-lobular, invasive-medullar, medullar, mucous, and tubulo-lobular carcinoma) Cell lines: hormone sensitive: MCF-7, ZR-75-1, BT-20; hormone insensitive: MDA-MB-453, SK-BR-3 BMPs involved: BMP6 |
Positive regulation of BMP6 through epidermal growth factor receptor | [47] | ||
|
Cell lines: MCF-7 BMPs involved: BMP2 and -7 |
Growth stimulatory and inhibitory effects in cell and differentiation specific states of cells | [55] | ||
| Tissue: Breast cancer patient BMPs involved: BMP7 | Higher expression | [20] | ||
|
Cell lines: MCF-7 BMPs involved: BMP2 |
BMP2 acts as a chemo attractant for cancerous cells and increases cell migration and invasion | [110] | ||
|
Tissue: Breast tumor Cell lines: MCF7, ZR-75-1, and BT-474 BMPs involved: BMP7 |
Amplification of DNA copy number | [43] | ||
|
Tissue: Breast cancer tissue BMPs involved: BMP15 and GDF-9a |
Inhibitory effect on the progression of human breast cancer | [62] | ||
|
Cell lines: MDA-231 -B/Luc (+) BMPs involved: BMP7 |
Inverse relation of BMP7 expression with tumorigenicity and invasive behavior | [98] | ||
|
Cell lines: MCF-7 (ER+ve), MDA-MB-231 (ER-ve) BMPs involved: BMP6 |
Suppression of breast cancer metastasis | [101] | ||
|
Tissue: Breast cancer tissue BMPs involved: BMP1–7; BMP4 (▴) |
Inconsistent variation in expression of BMPs | [21] | ||
|
Tissue: Breast tumor Cell lines: MDA-MB-231 BMPs involved: BMP6 |
Anti-metastasis role | [63] | ||
|
Tissue: Breast cancer tissue BMPs involved: BMPRIB |
Pathogenesis of breast cancer | [134] | ||
|
Tissue: Primary tumor Cell lines: 22 breast cancer cell lines BMPs involved: BMP4 and -7 |
Wide spread expression of BMP4 and -7 | [22] | ||
| 3. | Pancreatic cancer |
Tissue: Human pancreatic carcinoma BMP involved: BMP2 |
Regulation of BMP2 and its interaction with SMAD4 in pancreatic cancer | [75] |
|
Tissue: Human pancreatic cancer Cell lines: ASPC-1, CAPAN-1, PANC-1, COLO-357, MiaPaCa-2, and T3M4 BMPs involved: BMP2 (▴), BMPR-IA(▴), BMPR-1B(▴) |
Growth stimulatory role | [31] | ||
| 4. | Non small cell lung carcinoma |
Tissue: NSCLC BMPs involved: BMP2 |
Anti-proliferative effects | [54] |
|
Tissue: NSCLC Cell lines: A549 BMPs involved: BMP3b and BMP6 |
Down regulation of BMP3b | [141] | ||
|
Cell lines: A549 BMPs involved: BMP4 |
Induction of senescence | [59] | ||
|
Tissue: NSCLC BMP involved: BMP8 (▴) |
Over expression | [35] | ||
|
Tissue: Lung cancer patients Cell lines: Shp77 (small cell carcinoma), H322M and A549 (non small cell carcinoma), DU145, and IMR90 (lung fibroblast), HT and WTK1 (lymphoblastic cell lines) BMPs involved: BMP6, BMP3b |
Epigenetic regulation of BMP6 and BMP3b | [37] | ||
|
Tissue: Lung tumor BMP involved: BMP2 (▴), BMP type 1A, 1B and II receptors |
Role in tumor cell growth, migration and invasion | [33,76] | ||
|
Tissue: NSCLC and SCLC BMPs involved: BMP2 and -4(▴) |
Over expression | [34] | ||
| 5. | Gastric cancer |
Cell lines: MKN28, MKN45 BMPs involved: BMP1, -2, -4, and -7 |
Expression study | [18] |
|
Tissue: Gastric cancer Cell lines: MKKN74 BMPs involved: BMP2 |
Growth inhibitory effects and epigenetic regulation | [142] | ||
| 6. | Ovarian cancer |
Tissue: Ovarian cancer BMPs involved: BMP2 (▴) |
BMP2 up-regulation leads to differentiation | [28] |
|
Cell lines: IOSE397 and EOC BMPs involved: BMP9 |
Role in proliferation | [52] | ||
|
Cell lines: HepG2 BMPs involved: BMP9 |
rhBMP9 stimulates HepG2 cell proliferation | [51] | ||
| 7. | Bladder cancer |
Tissue: Bladder cancer BMPs involved: BMP2 |
BMP2 recruitment from tumor cells | [96] |
|
Cell lines: C3 and C8 BMPs involved: BMP2 (▴) |
Over expression | [111] | ||
| 8. | Colorectal cancer |
Tissue: Human samples BMP involved: the SMAD7 gene |
SMAD7 association with colorectal cancer | [137, 138] |
|
Cell lines: HCT116 BMPs involved: BMP4 |
Migration and invasion | [115] | ||
|
Tissue: Human samples BMPs involved: BMP2 and -4 |
Association with colorectal cancer | [136] | ||
| 9. | Kidney and Colon cancer |
Tissue: Colon cancer BMPs involved: BMP2, BMP4–6 |
Prominent expression of BMP5 and -6 | [24] |
|
Cell lines: PC/AAC1, SW48, SW480, and HCT8/S11, HT29 BMPs involved: BMP7 |
Cell scattering and pro- invasive response | [107] | ||
| 10. | Medullobl astoma |
Tissue: Medulloblastoma cancer BMPs involved: BMP2 |
Retinoid activity and induction of apoptosis | [68] |
| Glioblastoma |
Tissue: Brain tissue of glioma BMPs involved: BMP4 |
BMP4 reduces tumor initiating cell | [61] | |
| Glioma |
Tissue: Brain tissue of glioma BMPs involved: BMP2 |
BMP2 as a prognosis markers in glioma | [26] | |
| 11. | Adenocortical carcinoma |
Tissue: Adrenocortical carcinoma BMPs involved: BMP2 and -5 (▾) |
Anti-proliferatory effects | [66] |
| 12. | Myeloma |
Cell lines: ANBL-6, IH-1, INA-6, OH-2, and RPMI-8226, ANBL-6, IH-1 BMPs involved: BMP4–7 |
Growth and proliferation inhibitory effects | [58] |
| Malignant melanoma |
Cell lines: Mel Ei, Mel Wei, Mel Ho, and Mel Juso, Mel Im, Mel Ju, SK Mel 28, SK Mel3, and HTZI9d BMPs involved: BMP2, -4 (▴), and -7 (▴) |
Role in tube formation and migratory | [23, 124] | |
| 13. | Esophage al squamous cell carcinoma |
Cell lines: TE-2 (esophageal squamous cell carcinoma) BMPs involved: BMP1 –7 |
Expression study | [18] |
|
Tissue: Tumor from esophageal squamous cell carcinoma patients Cell lines: COLO-680N and FaDu BMPs involved: BMP6 |
BMP6 protein is inversely correlated with tumor differentiation | [36] | ||
| Esophage al adenocarc inoma |
Cell lines: TE7 BMPs involved: BMP7 |
EMT changes reversed by the treatment of BMP7 | [98] | |
| 14. | Oral epithelial cancer |
Tissue: normal oral mucosa, non specific chronic inflammation, hyperkeratinosis, squamous cell papilloma, and squamous cell carcinoma BMPs involved: BMP-2, -4, and -5 |
Role in metastasis of oral carcinoma | [41] |
| 15. | Osteosarc orna |
Cell lines: NOS1, NOS-2, Saos-2 and HuO9 BMPs involved: BMP1–7; BMP2 and -4 (▴) |
Expression study | [18] |
|
Tissue: Osteosarcoma and malignant fibrous histiocytoma patients BMPs involved: BMP2, -4, and BMPRs (-IA, -IB, and -II) |
Differential expression of BMPs and BMPRs | [44] | ||
| 16. | Renal cell carcinoma |
Cell lines: OS-RC-2(Renal cell carcinoma), HepG2 (hepatoma cell line) BMPs involved: BMP1, -2, -4 |
Expression study | [18] |
| 17. | Pleomorphic adenomas |
Tissue: Pleomorphic adenomas of the salivary gland BMPs involved: BMP2 (▴) |
Over expression and role in ectopic chondrogenesis | [38] |
|
Tissue: Benign neoplastic tumor of the salivary gland BMPs involved: BMP6 (▴) |
Role in pleomorphic adenomas, chondroid formation, and tubulo-glandular differentiation | [39] | ||
| 18. | Mesothelioma, Serous adenocarcinoma; Mucinous adenocarcinoma and Fibrosarcoma |
Cell lines: (NK-211, SHIN-3, KF-1, A2780, KK-92, KOC-2S); (OMC-3); (KEN-3) BMPs involved: BMP2 |
Expression study | [27] |
| 19. | Salivary adenocarc inoma | Cell lines: HSG-S8 BMPs involved: BMP2 |
Expression study | [29] |
| 20. | Multiple adenoma |
Tissue: Multiple adenoma BMPs involved: BMPR1A |
Alteration in BMPR1A gene | [128] |
| 21. | Juvenile polyposis |
Tissue: FJP families samples BMPs involved: BMPR1A |
Germline mutation in FJP families | [127] |
| 22. | Anaplastic thyroid carcinoma |
Cell lines: HTh7, HTh74, C643 and SW1736 BMPs involved: BMP7 |
Growth inhibitory effects | [56] |
| 23. | Hepatocell ular carcinoma |
Tissue: Carcinoma tissue Cell lines: Hepatocellular carcinoma BMPs involved: BMP4 |
Hepatocellular carcinoma progression | [112] |
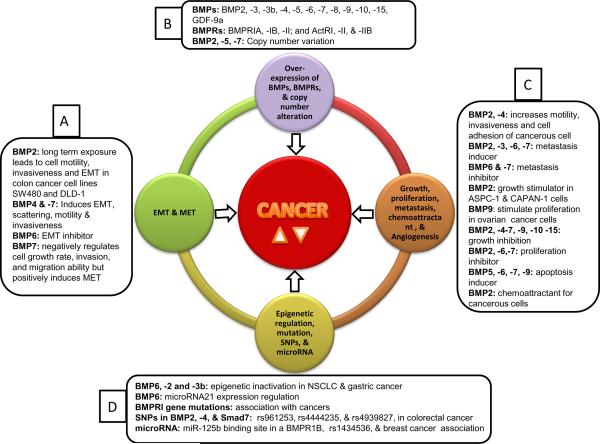
Figure 1.
Figure showing the involvement of various BMP molecules and their receptors in complex and multi-step molecular process of human cancer. The four outer circles are representing the events reported to be associated with progression of cancer. Outermost text boxes are showing a snapshot about the particular BMPs, BMPR, and the way these are involved in various cancerous processes either protectively or anti-protectively. These cancerous processes decides the fate of a living cell in its micro environment. Text box A, showing role of BMPs in EMT and MET; text box B, showing expression, over expression and copy number alteration in BMPs and BMPRs during cancer; text box C, showing BMPs during growth, proliferation, metastasis and angiogenesis; and text box D, showing the association of mutations, SNPs, microRNA with BMPs and their receptors and relation with epigenetic regulation during various human cancer. In the center of the figure the text CANCER and two symbol upward ( ) and downward triangle ( ), means these different BMPs may increase and decrease the incidence of human cancer in various cell, tissue and cancer type.
Prostate is not the only tissue type in which BMPs have an effect. BMP2, -4, and -7 are generally expressed in breast and prostate cancers, and particularly BMP7 in breast cancer [20]. However, BMP4 is expressed equally in both normal and breast cancer [21]. Another group observed in the investigation that BMP4 and -7 are expressed most frequently in breast cancer [22] and up-regulated in melanoma and metastases of malignant melanoma [23]. Other cell line specific differential expression of BMPs is observed in gastric [18] and colon adenocarcinoma [24, Table 1].
In various human cancers including glioma [25, 26], ovarian cancer [27, 28], salivary adenocarcinoma [29], mesothelioma [30], serous adenocarcinoma, mucinous adenocarcinoma, fibrosarcoma, and human pancreatic cancer, BMP2 is expressed as sensitive marker [31]. However, cell line studies show that mature BMP2 transcript can have increases up to 17-fold in non small cell lung carcinoma (NSCLC) [32, 33] and 25.6-fold in small cell lung carcinoma (SCLC) [34] along with other BMPs [35]. Over-expression of BMP6 suggests a role in esophageal squamous cell carcinoma [36], small cell carcinoma, non small cell carcinoma, prostate carcinoma [37], whereas BMP2 and -6 in cancers of salivary gland [38, 39].
Culture conditions, compact tissue architecture and cancer tissue grades can change BMP expression significantly as evident in giant cell tumor, oral carcinoma [40, 41], and other cancer related to kidney, esophagus and liver [18, Table 1].
In addition to relative gene over-expression, gene copy number may also be altered in various cancer cell lines. A gain of gene copy number signal (Fluorescent in situ hybridization or FISH signal) is seen for BMP2, -5, and -7 in 50–58% of tumor loci in tissue sections of benign cutaneous epithelium, high grade prostatic intraepithelial neoplasm, and prostate carcinoma [42, Table 1]. Extremely high copy number amplification of BMP7 is seen in several breast cancer cell lines [43]. The aberrant expression of a gene in either normal or pathological conditions often relates to the variation in copy number of the gene. Additional copies can arise from various molecular rearrangements within the DNA. The additional copies could be functional or non-functional or even have abnormal/unknown functions. Hence, additional copies can be either active or inactive in the genome. The identification of the functional copy number of a gene and its consequences in the normal and cancer genome is a critical problem needing resolution. Moreover, BMPs show highly variable expression in cell, tissue, and cultural conditions. We note that most of the study to date report examples of BMP over-expression. BMP2, -4 and -7 appears to be most frequently over expressed in various cancers and cell lines.
2.2. BMP receptors show aberrant expression in a number of human cancers
BMPs signal through receptors located on plasma membrane of the cell. When BMP receptors are over expressed in any cancer type, the BMP receptor may allow more ligand molecules to bind with the receptor inducing some abnormal cellular function to occur, as is evident in various cancers [Table 1, 2 and 3]. Interestingly, BMPs and their receptors are expressed differently in osteosarcoma and malignant fibrous histiocytoma (MFH), where BMP2 and -4 are expressed in both osteosarcoma and MFH. However, immunoreactivity in BMPRs is absent in MFH cases. The presence of BMPs and absence of BMPRs can be used to differentiate MFH and fibroblastic type of osteosarcoma [44]. Therefore, the presence of BMP2 and -4 with the absence of BMPRs may be a key to why MFH tumors cells do not ossify or it may be a another layer of complexity to cancer heterogeneity or behavior of BMPs.
Table 2.
BMPs and their involvement during the process of metastasis, cell migration, invasion and angiogenesis
| Serial No. | Cancer type | BMPs involved | Cancer processes metastasis, angiogenesis, motility, invasiveness, adhesion | References |
|---|---|---|---|---|
| 1. | Prostate cancer | BMP4 | Increases adhesion of PC-3 cells to the endothelium of bone marrow | [89, 91] |
| BMP7 | An inducer for EMT with changes in the morphology, motility, invasiveness, and molecular markers | [19] | ||
| Prostate cancer | BMP2 | Enhances the invasivess of C4-2B cells | [92] | |
| Metastatic bone lesions of prostate cancer | BMP7 | Metastasis | [118] | |
| Prostatic adenocarcinomas both in primary tumor and in skeletal metastases | BMP6 | Metastasis | [83] | |
| 2. | Breast cancer cells | BMP2 and BMP3 | Metastasis | [48] |
| Breast cancer | BMP7 | Metastasis to bone | [95] | |
| Breast cancer | BMP7 | Cell migration and invasion | [53] | |
| Breast cancer cells | BMP6 | Inhibits metastasis and EMT | [102] | |
| Breast cancer | BMP4 | Cell migration and invasion | [79] | |
| 3. | Malignant melanoma cells | BMP2 | Metastasis | [23] |
| Melanoma | BMP4 and BMP7 | Promotion of melanoma cell invasion, migration, and progression | [23] | |
| BMP2 and BMP4 | Tube formation as well as migration in the micro-vascular network of melanoma | [124] | ||
| BMP7 | Potential metastasis inhibitor | [117] | ||
| 4. | Oral carcinoma | BMP2, -4, and -5 | Metastasis | [41] |
| 5. | Gastric cancer | BMP2 | Motility and invasiveness | [84] |
| BMP2 | Metastasis | [97] | ||
| 6. | Giant cell tumor | BMP2–6 | Metastasis | [40] |
| 7. | Osteogenic sarcoma-derived Saos-2 and HOS cell lines | BMP2 | Regulator of cell adhesion | [109] |
| 8. | Primary human epithelial ovarian cancerous cells | BMP4 signaling | Changes in cellular morphology adhesion, motility, invasion, and alteration of EMT markers Snail and Slug | [125] |
| 9. | Malignant epithelial cells | BMP6 | Metastasis | [100] |
| 10. | Human colorectal cells | BMP4 | Changes in cellular morphology, expression of vimentin a EMT marker, migration and invasion | [115, 116] |
| 11. | NSCLC | BMP2 | Enhances migration and invasion in vivo | [32] |
| 12. | Bladder carcinoma | BMP2 | Heterotopic ossification in metastatic lesions from urothelial bladder carcinoma | [96] |
| 13. | Colon and kidney cancer cells | BMP7 | Role in proinvasive activities like scattering | [107] |
Table 3.
BMPs and their expression in various cancerous cell lines
| Cancer type | Cell lines | BMPs and their expression | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMP1 | BMP2 | BMP3 | BMP4 | BMP5 | BMP6 | BMP7 | BMP9 | BMP10 | |||
| Prostatic adenocarcinoma | PC3, DU145 | + | + | [17] | |||||||
| Prostatic adenocarcinoma | PC3 | + | + | + | + | + | [18] | ||||
| Non-neoplastic prostatic epithelial | MLC and HTS-40C | + | + | [19] | |||||||
| Prostate cancer | PC-3, LAPC-4, DU145, LNCaP, C4-2B, CWR22R | + | + | [19] | |||||||
| Prostatic stromal | HSP-40F, HTS-40C | + | + | [19] | |||||||
| Primary cutaneous melanoma | Mel Ei, Mel Wei, Mel Ho, and Mel Juso | + | + | [23] | |||||||
| Metastases of malignant melanoma | Mel Im, Mel Ju, SK Mel 28, SK Mel3, and HTZI9d | + | + | [23] | |||||||
| Melanoma | SK Mel28, Mel Ei | + | [23] | ||||||||
| Gastric adenocarcinomas | MKN28 | + | + | + | [18] | ||||||
| Gastric adenocarcinomas | MNK45 | + | + | + | + | [18] | |||||
| Pancreatic cancer | ASPC-1, CAPAN-1, PANC-1, COLO-357, MiaPaCa-2, and T3M4 | + | [31] | ||||||||
| Fibrosarcoma | NK-211,SHIN-3, KF-1, A2780, KK-92, KOC-2S, OMC-3, KEN-3 | + | [27] | ||||||||
| Esophageal squamous cell carcinoma | COLO-680N and FaDu | + | [36] | ||||||||
| Small cell carcinoma | Shp77, H322M, A549, DU145, IMR90 | + | [37] | ||||||||
| Osteosarcoma | NOS1, NOS-2, Saos-2, HuO9 | + | + | + | + | + | + | + | [18] | ||
| Osteogenic Sarcoma derived | OS-RC-2 | + | + | + | [18] | ||||||
| Esophageal squamous cell carcinoma | TE-2 | + | + | + | + | + | + | + | [18] | ||
| Hepatoma | HepG2 | + | + | + | + | [18] | |||||
| Breast cancer (Hormone sensitive) | MCF-7, ZR-75-1, BT-20 | + | [47] | ||||||||
| Breast cancer | MCF-7 | + | + | [48] | |||||||
| Breast cancer (Hormone insensitive) | MDA-MB-453, SK-BR-3 | + | [47] | ||||||||
| Salivary adenocarcinoma | HSG-S8 | + | [29] | ||||||||
| Hepatocellular carcinoma | HCC | + | [112] | ||||||||
| Anaplastic thyroid carcinoma | HTh7, HTh74, C643 and SW1736 | + | [56] | ||||||||
| Bladder cancer | C3 and C8 sublines of BLI7/2 human bladder cancer cell lines | + | [111] | ||||||||
| Non small cell lung carcinoma | A549 | + | [59] | ||||||||
| Colon cancer | PC/AAC1, SW48, SW480, HT29, HCT8/S11 | + | [107] | ||||||||
| Gastric cancer | MKKN74 (LE=Loss of expression) | LE | [142] | ||||||||
| Gastric cancer | SNU-5, SNU-16, SNU-216, MKN-45, SNU-638, MKN-28, MKN-74, AGS | + | + | [108] | |||||||
| Prostate cancer | PC3, DU-145, CA-HPV-10 | + | [64] | ||||||||
| Prostate cancer | DU-145 | + ▾ | [74] | ||||||||
| Prostate cancer | DU-145 | + | + | + | +▴ | [57] | |||||
| Prostate cancer | PC-3 and LNCaP | + | + | [57] | |||||||
| Colorectal cancer | HCT116 | + | [115] | ||||||||
| Breast cancer | MCF7, ZR-75-1, and BT-474 (CN=Copy number variation) | + CN | [43] | ||||||||
| Breast cancer | MCF7, MDA-231, MDA-134 | + | + | [22] | |||||||
Consistent expression of BMPRs (type I and type II) is observed in various prostate cancer cell lines [19, Table 4], and tissues [45]. The differential expression of the BMPRs may be due to their hormone sensitive behavior. For example, the expression of BMPR-IB is regulated in androgen sensitive human prostate cancer cell lines LNCaP by androgen, whereas the expression of BMPR-IA and BMPR-II is not regulated by androgen [46]. Addition of rh-BMP2 protein to LNCaP cells in presence of androgen, cell growth is inhibited, and the growth rate is increased by the BMP2 in the absence of androgen and the amounts of BMPR-IB mRNA decreased significantly. These observations show that BMPR-IB is regulated by androgen (negative regulatory signal in response to BMP2) in LNCaP cells. Thus, BMPR-IA and BMPR-IB differentially modulate the prostate cancer cell growth in response to BMP2 under different hormonal conditions [46].
Table 4.
BMPRs and their expression in various cancerous cell lines
| Cancer type | Cell lines | BMPR1A | BMPR1B | Act RI | BMPRII | ActRII | ActRII B | References |
|---|---|---|---|---|---|---|---|---|
| Prostate cancer | LAPC-4, DU145, LNCaP, CWR22 R, HPS-40F, HTS-40C, and MLC | + | + | + | + | + | + | [19] |
| Prostate cancer | LNCaP and PC-3 | + | [46] | |||||
| Breast cancer | MCF-7, ZR-75-1,BT-20 | [47] | ||||||
| MCF-7 | + | + | + | [48] | ||||
| Pancreatic cancer | ASPC-1, CAPAN-1, PANC-1, COLO-357, and T3M4 | + | [31] | |||||
| Prostate cancer | PC3M | loss of expression | loss of expression | loss of expression | [50] | |||
| PC3, DU-145, CA-HPV-10, LNCaP, PZHPV | + | + | [64] | |||||
| Myeloma | ANBL-6, INA-6, OH-2, IH-1, | + | [58] | |||||
| Gastric cancer | SNU-16, SNU-216, SNU-638, MKN-28, MKN-74, AGS | + | + | [108] | ||||
| SNU-5, MKN-45 | + | [108] |
Similarly, another study suggests a coordinated relationship among epidermal growth factor receptor (EGFR), BMP6, and breast cancer. BMP6 is positively regulated by EGFR, and is expressed in hormone sensitive (MCF-7, ZR-75-1, BT-20) and hormone insensitive (MDA-MB-453, SK-BR-3) cells, and in breast cancer tissues [47]. The hormone sensitive breast cancer cells also express BMP2, -3, and BMPRs -1A, -1B, and -II [48].
Cell line specific expression of BMPR-IA is seen in human pancreatic cancer cell lines ASPC-1, CAPAN-1, PANC-1, COLO-357, and T3M4, but not in MiaPaCa-2. These cell lines over express BMPR-1A (2 fold), BMPR-II (8 fold) and variable BMP2 receptor [31], whereas an inverse correlation is observed between abnormal BMPR expression [49], and loss of receptor expressions in prostate cancer [50].
Other BMPRs such as BMP type 1A, 1B and II are also expressed in lung cancer and osteosarcoma [32, 42]. However, ligand and receptor binding studies, as well as hormone induced behavior studies of BMPRs are required in order to provide mechanistic and functional progress in this area.
2.3. BMPs can modulate cancer progression as well as suppression activities
BMPs are described as both growth stimulators [31, 32, 51–53, Table 1] and anti-proliferative or anti-growth molecules [48, 54–66, Table 1]. As demonstrated by the following examples, BMPs can act as growth and proliferation inhibitor and in some cases they can enhance the growth and proliferation of the cancer cell. Their mechanism of action may be different at molecular level in different cancer environments. BMP2 and -5 can inhibit proliferation, and modulate steroidogenesis of human adrenocortical tumor cells in vitro through a BMP dependent pathway. These features are modified by demethylation and over-expression of GATA6 regulator, an important transcription factor in adrenocortical tumor biology [66]. However, in some cases growth retardation is observed due to BMP2 in colon and breast tumor cell lines, when BMP2 is under the control of carcinoembryonic antigen (CEA) promoter in a viral vector transfected to tumor cells for BMP2 production. These BMP2-CEA expressing positive cells drive the differentiation of mesenchymal stem cells to bone lineages [65]. Additionally, BMP2 decreases the proliferation of MCF-7 breast cancer cells suggesting a crucial role of BMP2 in repressing the activity of growth inhibitory pathway such as TGF-β [48]. Proliferation inhibitory effects of BMP2, -6 and -7 are observed in breast cancer [54, 63]. BMP2 and -7 elicit both growth stimulatory and inhibitory effects in cell-specific and differentiation-specific states of breast cancer cells. Growth inhibitory effects of BMP2 on breast carcinoma cells are mediated by up regulation of the p21CIP1/WAF1 protein, inhibition of Cdk2 kinase activity, and hypo-phosphorylation of pRb [55]. However, in gastric cancer, BMP2 act as growth inhibitor in a dose dependent manner by cell cycle arrest in the G1-phase mediated by p21/WAF1/CIP1, and enhances the pepsinogen II gene a differentiation marker of glandular cells of stomach, suggesting role in gastric cancer cell modulation [67]. Retinoid activity (regulation of cell growth in cancer) and apoptosis inducing property of BMP2 is observed in medulloblastoma through its paracrine effect [68].
Growth inhibitory effects of BMP2 and -4 are observed in LNCaP prostate cancer cell line, while PC3 was unaffected. Growth inhibition due to BMP2 is observed during the arrest of the G1 phase of cell cycle in LNCaP cell lines. Treatment with BMP2 and -4 in prostate cancer activates downstream signaling pathway involving SMAD-1, up regulation of p21CIP1/WAF1 (CDK inhibitor), and changes in phosphorylation of the retinoblastoma gene [69]. However, BMP7 can inhibit the proliferation and growth of androgen-insensitive prostate cancer cell lines via induction of cyclin dependent kinase 1 (CDK1) [57]. In contrast, in normal mammary epithelial cells, BMP4 can also potentiate growth factor induced cell proliferation [70].
Dose dependent growth inhibitory effects of BMP5-7 are observed in melanoma due to apoptosis [58] but not in other cancers [19, Table 2] where JNK pathway is active [71]. Additionally, most potent growth inhibitory effects of BMP7 are observed in human anaplastic thyroid carcinoma cell lines via inhibition of Cdk activity and hypophosphorylated state of Rb protein [56]. In addition BMP7 inhibits proliferation of the androgen dependent, androgen receptor expressing LNCaP cells but not the castration resistant PC3 cells this may be due to alteration of androgen receptor signaling [72]. Similarly, BMP4 reduces tumor initiating cell pools of human glioblastomas via BMP-BMPR signaling system and can be used to prevent growth and recurrence of glioblastomas in human brain tissue [61]. Similar growth inhibitory effects of the growth and differentiation factor-9a (GDF-9a) and BMP15 (or GDF-9b) are observed in breast cancer [62]. The role and behavior of BMP4 in lung cancer development is not yet understood. But it is shown that BMP4 can induce senescence and thus negatively regulates the growth of lung cancer [59]. Such process of senescence may be a physiological mechanism for thwarting the proliferation cycle of tumor cell.
BMP genes BMP9 and -10 with 40.5% similar in their amino acid sequence, function as putative suppressors for prostate cancer [73]. It is evident by decrease or null expression of BMP9 in higher pathological grade prostate cancer. However, forced expression of BMP9 and -10 prevents in vitro growth, cell matrix adhesion, invasion, and migration of prostate cancer cells [64]. Similarly, the BMP10 transcript is detected in DU-145 cell line but not in other prostate cancer and prostate epithelial cells [74]. BMP10 is detected in normal prostate tissue, but not in higher grade or disrupted gland structure of prostate cancer [74]. BMP9 induces apoptosis in prostate cancer via up-regulation of prostate apoptosis response-4 through a SMAD dependent pathway [64], and BMP10 activates X-chromosome linked IAP (XIAP/ILP) and ERK1/2 through a SMAD independent pathway [74]. These studies conclude that both BMP9 and -10 may function as tumor suppressors.
Most of the BMPs are implicated in anti cancer activity but a few of them also contribute to tumor progression, such as BMP2, acting as a growth stimulator in ASPC-1 and CAPAN-1 pancreatic cells due to MAPK activation and is blocked by MAPK inhibitor PD98059 in CAPAN-1, but not in ASPC-1 cells. These results show that BMP2 can act as a mitogen when SMAD4 (a common mediator of BMP pathway) is mutated suggesting a role of BMP2 in the pathobiology of pancreatic cancer. In ASPC-1 and CAPAN-1, expression of wild type SMAD4 abolishes the BMP2 mediated growth stimulation [31], whereas the SMAD4 is frequently mutated in pancreatic cancer [75], suggesting regulation of BMP2 and its interaction with SMAD4 in pancreatic cancerous environments. Enrichment of BMP2 in NSCLC tumor cell enhances tumor growth in vivo [32], due to activation of SMAD-1/5 [76]. Similarly, BMP7 increases cell growth in certain breast cancer [53]; rhBMP-9 stimulates HepG2 cell proliferation [51]; and autocrine BMP9 act as a proliferative factor for immortalized ovarian surface epithelial cells and ovarian cell lines (signaling via ALK2/SMAD1/SMAD4 pathway) [52]. In a number of cases, the growth inhibitory effects of BMPs were mediated by G1 cell cycle arrest [55, 69, 77–79]. The involvement of various BMPs in tumor progression or suppression is primarily affected by BMP dosage, micro-environment and genetic background of the cell under investigation. Role of BMP2, -4-7, -9 and -10 appears to be anti-tumorigenic, whereas BMP2 shows its dual behaviors and contributes in tumor progression along with BMP7 and –9 (Table 1).
2.4. BMPs are involved in metastasis, epithelial to mesenchymal transformation, altered cellular behavior, and angiogenesis in human cancer
An important attribute acquired by cancer cells during development is the ability to move from one organ system to other via blood stream or lymphatic system by metastasis after attaining a certain size, generally 1–2 mm3 [80], due to lack of appropriate oxygen supply as well as other limiting factors. In addition, tumors adopt angiogenesis as an additional survival mechanism, where the tumor secretes growth factors to induce growth of blood vessel. Angiogenesis is an active process during embryonic development, oestrous cycle and placental development but it becomes quiescent during adulthood except for healing and some pathological conditions, including cancer [81]. Onset of angiogenesis can occur at any stage of tumor progression but depends on the tumor type and micro environment. Angiogenesis is orchestrated by a diverse set of activators and inhibitors [82]. Recently, there have been major advances in understanding in the role of BMPs in metastasis and angiogenesis [Table 2].
It is common in advanced phases of prostate cancer to find BMPs associated with bone metastasis [85, 86]. In prostate cancer 65 to 75% of men are at high risk for metastases in advanced phases that will result into significant skeletal morbidity [86, 87]. Bone metastasis disrupts the normal process of bone remodeling via increased bone metabolism processes, leading to facilitation of metastasis. Induction of bone resorption causes the release of bone-derived growth factors that enhance tumor-cell survival, proliferation, chemotaxis, and adhesion to the bone marrow endothelium in new environments [88]. For example, several factors such as IGF-I, TGF-β, and TGF-II, collagen Type I peptides, and osteonectin in bone matrix are chemoattractants for prostate cancer cells in vitro [89, 90]. In particular, BMP4 increases adhesion of prostate cancer cells to the endothelium of bone marrow [90, 91]. Such intricate interactions between bone environment and human prostate cancer cells promote colonization process in bone. In bone metastases of prostate cancer, phosphatidylinositol 3-kinase (PI3K)/Akt pathway is used for the activation of BMP2 signaling by nuclear factor (NF)-kappaB [92]. The enhanced metastatic potential of these cancer cells is associated with transcriptional up-regulation of osteopontin, osteocalcin, and collagen IA1 in osteotropic prostate cancer cells, suggesting the connection between prostate cancer metastasis and bone. This study suggests a role of complex interactions among BMP2 and various transcriptional factors in bone [92].
Bone is also frequent metastasis site for breast cancer cells suggesting that some breast cancers express specific proteins to facilitate metastasis. Favorable factors for the cancer cells to metastasize to bones require the interaction among cells, growth factors, cytokines, receptors, and bone anatomy [93, 94]. A number of studies demonstrate the involvement of several BMPs and their receptors in the highly metastatic breast cancer cells. BMP7 is one of them associated with accelerated bone metastasis in breast cancer [22, 95]. Irradiated cells have a significant reduction of BMP2 and -6 in metastatic breast cancer [48] whereas BMPs activities are also present in metastasis of aggressive giant cell tumors which can metastasize to bone [40].
BMP2 produced by tumor cells is involved in heterotopic ossification in metastatic lesions from several types of cancerous cells such as urothelial bladder carcinoma [96]. BMP2 is over-expressed in metastases of malignant melanoma, while BMP4 and -7 are strongly associated with metastasis in other melanoma [23] and gastric cancer [96]. In human breast cancer cells and esophageal adenocarcinoma, an inverse relation is observed between BMP7, tumorigenicity, EMT and invasive behavior [98, 99]. Such relations bring more complexity in BMP functioning.
BMP6 is detected exclusively in malignant epithelial prostate cells in patient samples with metastases [100], and in isolated skeletal metastases from prostate carcinomas [83] suggesting the BMP6 involvement during metastasis. In contrast, BMP6 also plays an indirect role in suppression of breast cancer metastasis through repression of δEF1 gene, as BMP6 is an inhibitor of breast cancer EMT through rescuing E-cadherin expression. Repression of δEF1 mediates BMP6 induced transcriptional activation of E-cadherin in ER+ve and ER-ve breast cancer. The higher level of δEF1 expression is associated with invasive breast cancer phenotypes. Thus the finding suggests that δEF1 is a regulatory switch for BMP6 by restoring E-Cadherin mediated cell- to -cell adhesion and preventing metastasis of breast cancer [101]. Additionally, microRNA-21 gene is expressed and regulated by BMP6 during metastasis in breast cancer. BMP6 induces inhibition of microRNA-21 and suggest that BMP6 may function as an anti-metastasis factor by a mechanism involving transcriptional repression of microRNA-21 in breast cancer [102].
BMPs behave differently at different doses, cell types, and cellular environments. This affects cellular attributes such as cell movement, adhesion, invasiveness, epithelial to mesenchymal transformation (EMT) and mesenchymal to epithelial transformation (MET). The processes of motility and invasiveness are closely related to normal EMT and MET. Some BMPs have been shown to stimulate the migration of normal [103–106] and cancer cells [23, 107, 108]. Increased concentrations of BMP2 in culture enhance motility and invasiveness in gastric cancer via phosphorylation of Akt and activation of phosphatidylinositol 3-kinase (PI3K)/Akt pathway, whereas no increase in motility and invasiveness was observed in cells treated with Noggin, a BMP2 inhibitor [84]. Cell line specific behavior of BMP2 enhances invasion of some cells, but not of others. This effect is abrogated by Noggin [92]. Simultaneously, during tumor progression, the adhesion of cancer cells to the appropriate surface is crucial as the cell needs to proliferate for its own survival. BMP2 can act as a regulator of cell adhesion by modifying expression of integrin type adhesion receptors and attachment of the cells to a family of connective tissues i.e., laminin in osteogenic sarcoma-derived cells [109].
BMP2 can positively and negatively affect MET and EMT depending on the dosage in colon cancer cells. When these cells were exposed to BMP2 for 24 hr, their growth was inhibited while the other remaining cells (> 24 hr) became resistant to growth inhibition, and led to EMT, enhanced motility, and invasiveness. In a highly metastatic mesenchymal colon carcinoma cells, inhibition of BMP2 signaling by siRNA prevented EMT, motility, and invasiveness; hence a blockade of BMP2 signaling caused MET. Further inhibition of the PI3 kinase/Akt pathway using the kinase inhibitor (LY294002) is correlated with the development of BMP2 resistance and invasion in BMP2 induced EMT in colon cancer [84, Table 2].
Chemoattractant activity of BMP2 was observed for breast cancer cell culture at range of 12.5 to 50 nM. Addition of rh-BMP2 increased migration of cells towards a BMP2 source in comparison with untreated cells, whereas BMP2 over-expression enhanced migratory and invasive properties in vitro, and ultimately induced tumor growth [110]. Similarly, BMP2 is up-regulated (8.9 fold) in invasive human bladder cancer [111], and enhanced migration and invasion in vivo in lung cancer [32] via activation of SMAD-1/5 [76].
In hepatocellular carcinoma BMP4 plays a role in migration, invasiveness, and anchorage independent growth. These properties are strongly suppressed using BMP4 specific siRNA [112]. In addition, BMP4 supports lumen formation, outgrowth of multiple invasive cord like structures, individual cell scattering, disrupted cell-cell adhesion in mammary epithelial cells [113] and induces EMT in pancreatic cancer [114]. BMP4 also promotes invasion and migration various cancers including melanoma under the control of transcription factor Ets-1 [23], colorectal [115], SMAD4-deficient human colorectal [116], and breast [79].
BMP7 induces scattering and proinvasive responses in kidney and colon cancer cell lines via SMAD4 and src independent pathways as well as signaling cascades using FAK phosphorylation at Y925, and activation of ERK1/2, RacI, and JNK [107]. Scattering of cells, motility, and proinvasiveness might be the role of BMP7 via its potential autocrine and paracrine mode of action during the progression from premalignant colon adenomatous lesions to carcinoma cells including inflammation of the sigmoid flexure in colon or sigmoiditis [107]. BMP7 also acts as an inducer for EMT with changes in the morphology, motility, invasiveness, and molecular markers [19]. However, BMP7 in a melanoma cell line appears to be a negative regulator of cell growth rate, invasion, and migration ability, but positively induced apparent MET. Conceivably, BMP7 could be used as a potential metastasis inhibitor in human melanoma cells [117]. So, BMP7 appears to be a positive regulator of EMT and MET. The expression of BMP7 in metastatic bone lesions of prostate cancer suggests an origin of BMP7 expressing cells in bone metastasis from prostate cancer cell [118]. BMP7 induction shows various cell line specific behaviors in breast cancer such as reduced cell growth in some cell lines, and enhanced cell migration and invasion in other cell line under suitable environment [53, Table 1].
BMPs are known to be associated with angiogenesis, particularly during embryogenesis [119]. However, BMPs and their role in the process of angiogenesis in cancer remain to be determined [120–123]. BMP2 and -4 are associated with tube formation as well as migration in the micro-vascular network of melanoma cells, therefore these molecules seem to act as angiogenic factors as well as chemoattractants [124]. BMP2 may act as an angiogenic factor along with VEGF in lung cancer [34]. Additionally, BMP4 signaling affects cellular behaviors including changes in morphology, adhesion, motility, and invasion in primary human epithelial ovarian cancerous cells. In an exogenous response to BMP4, the EMT markers SNAIL and SLUG proteins are up-regulated, E-cadherin protein down regulated and the network of alpha smooth muscle actin changes to resemble a mesenchymal cell. These changes in cellular morphology and alteration of EMT markers suggest a role for BMP4 in aggressiveness of ovarian cancer [125].
A number of studies [Please see Table 2] show an association among BMPs, metastasis, angiogenesis, and EMT in cancer on the basis of BMP expression, and their contribution in cellular motility, invasiveness, tube formation, and migration. Since the processes of metastasis and angiogenesis are very complex and poorly understood, there may be other contributing factors, aside from the involvement of BMP6 [126]. The role of BMP6 in metastases, and BMP7 in EMT and MET are conceivably relevant because these are well established morphogenetic molecules in mammalian bones. Furthermore, in vitro and in vivo investigations are required to prove the definitive roles of BMPs in the process of metastases and angiogenesis in human cancer.
2.5. BMPs and their connection with cancer via mutations, microRNAs, and SNPs
Mutations, SNP variants and microRNAs can alter BMP regulations in cancer. One disease worth mentioning is Familial Juvenile Polyposis (FJP), an autosomal dominant condition characterized by multiple polyps in the gastrointestinal tract. In this disease, nonsense and missense germline mutations have been reported in the BMPR1A gene in four FJP families, 44–47delTGTT (exon1), 715C>T (exon7), 812G>A (eon7), and 961delC (exon8), affecting the exons [127]. A three base deletion (nucleotide position 1079–1081), in exon 10 (codon 360) in the BMPR1A gene is reported which results in the loss of histidine residue. The histidine residue is highly conserved and lies within the kinase domain of the BMPR1A protein, and is essential for the activation of the downstream signaling targets SMAD1 and SMAD5. This suggests role in the “multiple” adenoma phenotype [128]. Additionally, there are many reports of patients with juvenile polyposis developing gastrointestinal malignancy, including cancer of the colon [129, 130], stomach [129–131], and pancreas [129, 132]. Whether these mutations may directly contribute to the preceding cancers or whether they create a favorable environment that makes a person more susceptible to stomach and colon cancer is not known at this time.
Determining the expression pattern of micro RNAs in disease including human cancers is a relatively new area of research [133]. Recently, a risk variant in an miR-125b binding site in the BMPR1B gene is found to be associated with pathogenesis of breast cancer [134]. In the Cancer Genetic Markers of Susceptibility study, two SNPs, rs1970801 and rs11097457, presented in strong linkage disequilibrium (LD) with rs1434536, an SNP that resides within a miR-125b target site in the 3'untranslated region (UTR) of the BMPR1B gene. Positive association of the BMPR1B SNP (rs1434536) with breast cancer is also validated in an independent cohort of admixture-corrected cases from families with multiple case histories. RT-PCR and an over expression study supports the conclusion that the allele specific regulation of the miR-125 binding site in the BMPR1B gene correlates with breast cancer risk [134]. This study highlights a need for determining the disease regulatory networks of micro RNAs in BMPs and their receptors. BMPs and their association with breast cancer have been previously reviewed [74].
A large number of Genome Wide Association Studies (GWAS) have been performed following the sequencing of the human genome, with the advent of new less costly genotyping technologies, and large collaboration of scientific groups for access to appropriate sample size to improve the power and significance of the data collected. These GWAS studies are very powerful in terms of the sample size, number of relevant SNP markers across the genome, and their enormous statistical power and ability to dissect the disease variants to determine their association with complex diseases (including complicated cancers of various origin) [135]. Recently, in a GWA study, a few SNPs in BMPs and their signaling molecules are found to be involved in colorectal cancer. One of the SNPs, rs4444235, (major allele T and minor allele C) in BMP4 gene is found strongly associated (Odds ratio: 1.11, confidence interval or CI 1.08 to 1.15) with colorectal cancer in a population from the United Kingdom (1952 cases and 1977 control) with the significance (p= 2.0 × 10−10). This suggests that BMP4 may be a risk locus in colorectal cancer [136]. Another SNPs rs961253, in the BMP2 gene, is found to be associated (Odds ratio: 1.12, CI: 1.08 to1.16) with colorectal cancer in the UK population as well (1952 cases and 1977 control) [136]. Similarly, SNP rs4939827, in intron 3 of the SMAD7 gene (an important component of TGFβ signaling pathway) is also found to be associated with colorectal cancer in a GWAS in population of England (total 8413 cases and 6949 control) and Scotland (14500 cases and 13294 control) with Odds ratio and CI, 1.88 (1.12–1.23) and 1.20 (1.16–1.24) respectively [137, 138]. The presence of an association between an intronic SNP and colorectal cancer samples suggests the presence of hidden cis and trans gene regulatory networks within the genome. The associations suggest important information may be embedded in the non-coding regions of genome, making dissection of complex disease mechanisms ever more complicated.
2.6. Evidence that BMPs are epigenetically regulated in caner
Methylation of DNA is an important process for inactivation of different genes during the process of cancer. DNA methylation pattern differs among various differentiation stages, cell types and cancers. Aberrant DNA methylation is reported in 10% of human malignancies in genome wide scanning studies [139], as well as scanning studies for methylation in promoter regions [140]. There are several reports supporting epigenetic regulation of BMPs in cancer. Epigenetic regulation of BMP6 and epigenetic inactivation of BMP3b is suggested in NSCLC [37]. BMP6 is expressed in various cancer cell lines including small cell carcinoma, non small cell carcinoma, prostate carcinoma, and lung fibroblasts, but not in lymphoblastic cell lines, due to methylation of cDNA. Treatment of HT and WTK1 cells with 5-aza-deoxycytidine (an inhibitor of methylatransferase) restores the BMP6 expression, suggesting loss of transcription of BMP6 may be due to the epigenetic inactivation. The BMP6 gene promoter methylation status is found to be different in methylated and unmethylated conditions in cell line specific manner. Furthermore, the concurrent methylation of both BMP3b and BMP6 are strongly associated with k-ras mutation (p<0.003) [37]. Aberrant DNA hypermethylation in the BMP3b promoter is associated with down regulation of BMP3b transcription in primary human lung cancer, and lung cancer cell line due to increased promoter methylation, and loss of heterozygosity in microsatellite markers [141]. These studies provide evidence for epigenetic regulation of BMP3b and BMP6, resulting in gene silencing, and present a possible epigenetic mechanism promoting lung tumor development [140, 37]. Additionally, BMP2 promoter methylation promotes loss of BMP2 expression in diffuse type primary gastric carcinoma [142], heterozygous loss of SMAD5 in human leukemia [143], and SMAD8 silencing by hyper-mythelation in breast and colon cancer [144]. In addition to these properties, BMP2 and -4 are also involved in retinoic acid induced differentiation of F9 embryonal carcinoma cells into parietal endoderm [145].
3.0. BMPs and small molecule inhibitors
A large number of strategies are available to modify, modulate, or block BMP signaling such as recombinant endogenous antagonists, neutralizing antibodies, gene transfer of ligands, receptors, and antagonists, etc. There are a few reports of small molecule inhibitors known for their ability to perturb the BMP signaling cascades. One such molecule is Dorsomorphin, a BMP type I receptor inhibitor, discovered using embryonic zebra fish screening assay [146]. Additionally, Dorsomorphin derivatives (LDN-193189, 4-quinolyinyl substituted Dorsomorphin etc.) are also available for this purpose; however the specificity of these agents has been questioned [147]. Similarly, TNF-α is a known repressor for BMP4 activity [148], DMH1, a potent BMP inhibitor [149], and IN-1130 inhibitor of ALK-4/5/7 that ultimately inhibits TGF-β, activin, and nodal signaling [150]. Further refinement is needed for specifically modifying specific BMP signaling in vivo, as these available small molecules also have other non-specific targets.
4.0. Conclusions
In light of ongoing research in our laboratory, we have reviewed the literature on the role of BMPs in human oncogenic processes. We found that BMPs and their receptors are associated with variety of human cancers through their differential expression, association with specific mutations/SNPs, microRNA, and epigenetics, as well as in complex carcinogenic processes such as metastasis and angiogenesis [Fig. 1, 2]. As in the case with TGF-beta family members, BMPs are a double-edged sword in tumor biology: they function both as tumor promoters and tumor suppressors depending on the type of cell or tissue and the BMP dosage in the micro-environment [Table 1]. This behavior suggests that in tumors, as in morphogenesis, there should be some type of cellular homeostatic mechanism through a receptor or some as yet known regulatory molecules. Additionally, we found no clear-cut pro- or anti-carcinogenic BMPs in the oncogenic process, although as shown in Figure 1 and 2, some BMPs appear more often on the side of tumor promotion than others and vice-versa. Second, we found no clear-cut mechanism of BMP de-regulation in carcinogenesis. However, BMP2, -4 and -7 appears to be involved mostly in metastasis, EMT and envasion in most of the cancers, whereas BMP6 and -7 acts as inhibitor of metastasis in breast cancer and melanoma respectively. Changes in gene amplification, increased gene expression as well as epigenetic alterations in BMP activity appear to be a function of the type of cell or tissue, the hormonal environment, or possibly the tumor microenvironment. However, it is not yet clear about specific regulatory factors responsible for dual behavior of BMP in cancer and non cancer environment.
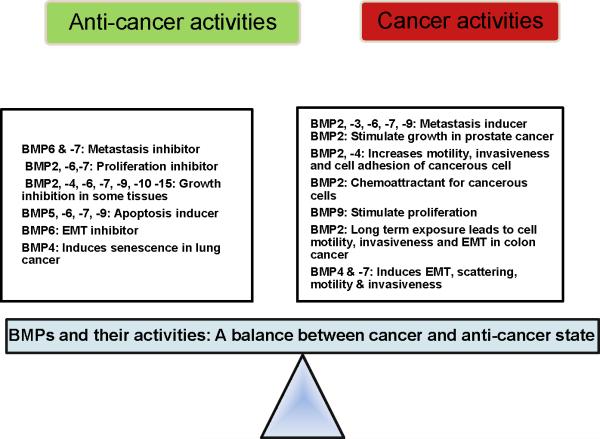
Figure 2.
BMPs and their cancer and anti-cancer activities are described in a cancer cell. We hypothesize here that there is a delicate balance between the cancer and anti-cancer environment of a cells. BMP molecules, depending on their environmental stimuli can switch the delicate balance in either direction. Such balance is necessary for the survival, normal growth, and development of a cell. [Table 1 and 2]
The foregoing conclusions suggest several avenues of research that may move the field forward. In complex oncogenic processes such as angiogenesis or metastasis, the role of individual BMPs may be direct or indirect; however, further in vitro and in vivo studies are required to determine a mechanism in a specific type of cell, tissue or cancer. Further investigations are needed to determine the combined effects of multiple BMPs on normal or cancer tissue, as the synergistic effects on cells may be different compared to individual effects. Such experiments would also provide support (or lack thereof) for the use of certain BMPs in cancer therapy. Moreover, the senescence effect of BMP4, might find clinical application. Furthermore, development of small molecule inhibitors of specific BMPs or their receptors such as Dorsomorphin and its derivatives, should be useful adjuncts to RNAi and kinase inhibitor strategies for dissection of cancer signaling pathways or therapeutics.
Finally, a note of caution regarding roles for BMPs and their receptors in cancer most of the work performed so far has used cancer cell lines. We submit that one of the field's greatest needs is careful study with fresh cells and tissue of known cancer type and grade. We submit further that longitudinal investigation of fresh cancer cells and tissues, particularly identification of intracellular components of BMP signaling pathways. Additionally, crystallographic studies on BMP-BMPRs complexes should facilitate a clearer picture of BMPs in terms of therapeutic opportunities and development of new drug entities. Finally, although we see the greater value in working with human material, significant mechanistic and functional understanding of BMPs and cancer could be made with the use of induced or naturally occurring mouse mutations in the various BMPs and their receptors with the context of oncogenic processes.
Acknowledgements
This work was supported by NIAMS, NIH grant RO1 AR052713 (RJM). We would like to thanks Anupama Singh, PhD and Nyssa Readio for their helpful suggestions and careful reading of the manuscript.
List of abbreviations
- BMPs
- BMPRs
- TGF- β
- SMAD
Biographies

Rebecca J. Morris received her Ph.D. in 1981 in Biology from Syracuse University. After working as a Research Associate with Thomas Slaga's group at M.D. Andersons' Science Park, she accepted a position at the Lankenau Institute for Medical Research in Wynnewood, PA. In 2001, Dr. Morris moved her program to the Departments of Dermatology and Pathology at Columbia University Medical Center in Manhattan, New York. She is currently Leader of the Laboratory of Stem Cells and Cancer at the Hormel Institute/University of Minnesota directed by Dr. Zigang Dong. Dr. Morris has maintained an interest in keratinocyte stem cells and cancer from her graduate work, and throughout this time, has been funded by grants from the ACS, NIAMS, and NCI. Her interest in BMPs and cancer, particularly BMP5, grew out of a genetic approach to identify stem cell regulatory genes in the epidermis.

Ashok Singh was born in 1978 in Pauri Garhwal, India. He passed his M.Sc. in Zoology from Allahabad University. After qualifying a National Eligibility Test he received his Ph.D. in 2008 in Population Genetics from Dr. Srikanta Kumar Rath's Laboratory, Central Drug Research Institute, Lucknow, where he was registered from Jawaharlal Nehru University, New Delhi, India. He is a member of Indian Genome Variation Consortium and trained in cell culture, molecular biology, and genomics. In 2008, he was a research fellow in the department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic College of Medicine, Scottsdale, AZ. Currently he is working as Hormel Fellow in Hormel Institute, Univ. Minnesota, USA. His research interest includes cancer biology, gene mapping and identification of keratinocyte stem cell regulatory genes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Urist M. Bone: Formation by autoinduction. Science. 1965;150(3698):893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- [2].Wozney VR John M., Celeste Anthony J., Mitsock Lisa M., Whitters Matthew J., Kriz Ronald W., Hewick Rodney M., Wang Elizabeth a. Novel regulator of bone formation: Molecular clones and activities. Science. 1988;242:1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- [3].Hopkins DR, Keles S, Greenspan DS. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol. 2007;26(7):508–23. doi: 10.1016/j.matbio.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Katagiri T, Suda T, Miyazono K. In: The bone morphogenetic proteins in The TGF-β Family. Derynck R, Miyazono K, editors. 2008. pp. 121–49. [Google Scholar]
- [5].Senta H, Park H, Bergeron E, Drevelle O, Fong D, Leblanc E, et al. Cell responses to bone morphogenetic proteins and peptides derived from them: biomedical applications and limitations. Cytokine Growth Factor Rev. 2009;20(3):213–22. doi: 10.1016/j.cytogfr.2009.05.006. [DOI] [PubMed] [Google Scholar]
- [6].Holley SA, Neul JL, Attisano L, Wrana JL, Sasai Y, O'Connor MB, et al. The Xenopus dorsalizing factor noggin ventralizes Drosophila embryos by preventing DPP from activating its receptor. Cell. 1996;86(4):607–17. doi: 10.1016/s0092-8674(00)80134-8. [DOI] [PubMed] [Google Scholar]
- [7].Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86(4):589–98. doi: 10.1016/s0092-8674(00)80132-4. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yamashita H, Ten Dijke P, Heldin CH, Miyazono K. Bone morphogenetic protein receptors. Bone. 1996;19(6):569–74. doi: 10.1016/s8756-3282(96)00259-1. [DOI] [PubMed] [Google Scholar]
- [9].Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, et al. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci U S A. 1995;92(17):7632–6. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, et al. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269(25):16985–8. [PubMed] [Google Scholar]
- [11].Zhang J, Li L. BMP signaling and stem cell regulation. Dev Biol. 2005;284(1):1–11. doi: 10.1016/j.ydbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- [12].Herpin A, Cunningham C. Cross-talk between the bone morphogenetic protein pathway and other major signaling pathways results in tightly regulated cell-specific outcomes. FEBS J. 2007;274(12):2977–85. doi: 10.1111/j.1742-4658.2007.05840.x. [DOI] [PubMed] [Google Scholar]
- [13].Marquis ME, Lord E, Bergeron E, Drevelle O, Park H, Cabana F, et al. Bone cells-biomaterials interactions. Front Biosci. 2009;14:1023–67. doi: 10.2741/3293. [DOI] [PubMed] [Google Scholar]
- [14].Liu F, Ventura F, Doody J, Massague J. Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol Cell Biol. 1995;15(7):3479–86. doi: 10.1128/mcb.15.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16(3):251–63. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- [16].Pregizer S, Mortlock DP. Control of BMP gene expression by long-range regulatory elements. Cytokine Growth Factor Rev. 2009;20(5–6):509–15. doi: 10.1016/j.cytogfr.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bentley H, Hamdy FC, Hart KA, Seid JM, Williams JL, Johnstone D, et al. Expression of bone morphogenetic proteins in human prostatic adenocarcinoma and benign prostatic hyperplasia. Br J Cancer. 1992;66(6):1159–63. doi: 10.1038/bjc.1992.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ogose A, Motoyama T, Hotta T, Watanabe H. Expression of bone morphogenetic proteins in human osteogenic and epithelial tumor cells. Pathol Int. 1996;46(1):9–14. doi: 10.1111/j.1440-1827.1996.tb03527.x. [DOI] [PubMed] [Google Scholar]
- [19].Yang S, Zhong C, Frenkel B, Reddi AH, Roy-Burman P. Diverse biological effect and Smad signaling of bone morphogenetic protein 7 in prostate tumor cells. Cancer Res. 2005;65(13):5769–77. doi: 10.1158/0008-5472.CAN-05-0289. [DOI] [PubMed] [Google Scholar]
- [20].Bobinac D, Maric I, Zoricic S, Spanjol J, Dordevic G, Mustac E, et al. Expression of bone morphogenetic proteins in human metastatic prostate and breast cancer. Croat Med J. 2005;46(3):389–96. [PubMed] [Google Scholar]
- [21].Davies SR, Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. Bone morphogenetic proteins 1 to 7 in human breast cancer, expression pattern and clinical/prognostic relevance. J Exp Ther Oncol. 2008;7(4):327–38. [PubMed] [Google Scholar]
- [22].Alarmo EL, Kuukasjarvi T, Karhu R, Kallioniemi A. A comprehensive expression survey of bone morphogenetic proteins in breast cancer highlights the importance of BMP4 and BMP7. Breast Cancer Res Treat. 2007;103(2):239–46. doi: 10.1007/s10549-006-9362-1. [DOI] [PubMed] [Google Scholar]
- [23].Rothhammer T, Poser I, Soncin F, Bataille F, Moser M, Bosserhoff AK. Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res. 2005;65(2):448–56. [PubMed] [Google Scholar]
- [24].Imai N, Iwai A, Hatakeyama S, Matsuzaki K, Kitagawa Y, Kato S, et al. Expression of bone morphogenetic proteins in colon carcinoma with heterotopic ossification. Pathol Int. 2001;51(8):643–8. doi: 10.1046/j.1440-1827.2001.01243.x. [DOI] [PubMed] [Google Scholar]
- [25].Liu C, Tian G, Tu Y, Fu J, Lan C, Wu N. Expression pattern and clinical prognostic relevance of bone morphogenetic protein-2 in human gliomas. Jpn J Clin Oncol. 2009;39(10):625–31. doi: 10.1093/jjco/hyp094. [DOI] [PubMed] [Google Scholar]
- [26].Zhang MY, Wu J, Gong X, Chen RK, Fang JS. Bao Xi, Fen Yu, Mian Zi, Xue Yi, Zhi Za., editors. Clinical significance of BMP-2 protein and mRNA expression in human glioma. 2009;25(7):637–9. [PubMed] [Google Scholar]
- [27].Kiyozuka Y, Nakagawa H, Senzaki H, Uemura Y, Adachi S, Teramoto Y, et al. Bone morphogenetic protein-2 and type IV collagen expression in psammoma body forming ovarian cancer. Anticancer Res. 2001;21(3B):1723–30. [PubMed] [Google Scholar]
- [28].Le Page C, Ouellet V, Madore J, Ren F, Hudson TJ, Tonin PN, et al. Gene expression profiling of primary cultures of ovarian epithelial cells identifies novel molecular classifiers of ovarian cancer. Br J Cancer. 2006;94(3):436–45. doi: 10.1038/sj.bjc.6602933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hatakeyama S, Ohara-Nemoto Y, Kyakumoto S, Satoh M. Expression of bone morphogenetic protein in human adenocarcinoma cell line. Biochem Biophys Res Commun. 1993;190(3):695–701. doi: 10.1006/bbrc.1993.1105. [DOI] [PubMed] [Google Scholar]
- [30].Kiyozuka Y, Miyazaki H, Yoshizawa K, Senzaki H, Yamamoto D, Inoue K, et al. An autopsy case of malignant mesothelioma with osseous and cartilaginous differentiation: bone morphogenetic protein-2 in mesothelial cells and its tumor. Dig Dis Sci. 1999;44(8):1626–31. doi: 10.1023/a:1026627413715. [DOI] [PubMed] [Google Scholar]
- [31].Kleeff J, Maruyama H, Ishiwata T, Sawhney H, Friess H, Buchler MW, et al. Bone morphogenetic protein 2 exerts diverse effects on cell growth in vitro and is expressed in human pancreatic cancer in vivo. Gastroenterology. 1999;116(5):1202–16. doi: 10.1016/s0016-5085(99)70024-7. [DOI] [PubMed] [Google Scholar]
- [32].Langenfeld EM, Calvano SE, Abou-Nukta F, Lowry SF, Amenta P, Langenfeld J. The mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cells. Carcinogenesis. 2003;24(9):1445–54. doi: 10.1093/carcin/bgg100. [DOI] [PubMed] [Google Scholar]
- [33].Langenfeld EM, Bojnowski J, Perone J, Langenfeld J. Expression of bone morphogenetic proteins in human lung carcinomas. Ann Thorac Surg. 2005;80(3):1028–32. doi: 10.1016/j.athoracsur.2005.03.094. [DOI] [PubMed] [Google Scholar]
- [34].Bieniasz M, Oszajca K, Eusebio M, Kordiak J, Bartkowiak J, Szemraj J. The positive correlation between gene expression of the two angiogenic factors: VEGF and BMP-2 in lung cancer patients. Lung Cancer. 2009;66(3):319–26. doi: 10.1016/j.lungcan.2009.02.020. [DOI] [PubMed] [Google Scholar]
- [35].Henderson LJ, Coe BP, Lee EH, Girard L, Gazdar AF, Minna JD, et al. Genomic and gene expression profiling of minute alterations of chromosome arm 1p in small-cell lung carcinoma cells. Br J Cancer. 2005;92(8):1553–60. doi: 10.1038/sj.bjc.6602452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Raida M, Sarbia M, Clement JH, Adam S, Gabbert HE, Hoffken K. Expression, regulation and clinical significance of bone morphogenetic protein 6 in esophageal squamous-cell carcinoma. Int J Cancer. 1999;83(1):38–44. doi: 10.1002/(sici)1097-0215(19990924)83:1<38::aid-ijc8>3.0.co;2-b. 24. [DOI] [PubMed] [Google Scholar]
- [37].Kraunz KS, Nelson HH, Liu M, Wiencke JK, Kelsey KT. Interaction between the bone morphogenetic proteins and Ras/MAP-kinase signalling pathways in lung cancer. Br J Cancer. 2005;93(8):949–52. doi: 10.1038/sj.bjc.6602790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kusafuka K, Yamaguchi A, Kayano T, Fujiwara M, Takemura T. Expression of bone morphogenetic proteins in salivary pleomorphic adenomas. Virchows Arch. 1998;432(3):247–53. doi: 10.1007/s004280050162. [DOI] [PubMed] [Google Scholar]
- [39].Kusafuka K, Yamaguchi A, Kayano T, Takemura T. Immunohistochemical localization of the bone morphogenetic protein-6 in salivary pleomorphic adenomas. Pathol Int. 1999;49(12):1023–7. doi: 10.1046/j.1440-1827.1999.00991.x. [DOI] [PubMed] [Google Scholar]
- [40].Kudo N, Ogose A, Ariizumi T, Kawashima H, Hotta T, Hatano H, et al. Expression of bone morphogenetic proteins in giant cell tumor of bone. Anticancer Res. 2009;29(6):2219–25. [PubMed] [Google Scholar]
- [41].Jin Y, Tipoe GL, Liong EC, Lau TY, Fung PC, Leung KM. Overexpression of BMP-2/4, -5 and BMPR-IA associated with malignancy of oral epithelium. Oral Oncol. 2001;37(3):225–33. doi: 10.1016/s1368-8375(00)00087-7. [DOI] [PubMed] [Google Scholar]
- [42].Doak SH, Jenkins SA, Hurle RA, Varma M, Hawizy A, Kynaston HG, et al. Bone morphogenic factor gene dosage abnormalities in prostatic intraepithelial neoplasia and prostate cancer. Cancer Genet Cytogenet. 2007;176(2):161–5. doi: 10.1016/j.cancergencyto.2007.03.011. [DOI] [PubMed] [Google Scholar]
- [43].Alarmo EL, Rauta J, Kauraniemi P, Karhu R, Kuukasjarvi T, Kallioniemi A. Bone morphogenetic protein 7 is widely overexpressed in primary breast cancer. Genes Chromosomes Cancer. 2006;45(4):411–9. doi: 10.1002/gcc.20307. [DOI] [PubMed] [Google Scholar]
- [44].Mehdi R, Shimizu T, Yoshimura Y, Gomyo H, Takaoka K. Expression of bone morphogenetic protein and its receptors in osteosarcoma and malignant fibrous histiocytoma. Jpn J Clin Oncol. 2000;30(6):272–5. doi: 10.1093/jjco/hyd073. [DOI] [PubMed] [Google Scholar]
- [45].Ide H, Katoh M, Sasaki H, Yoshida T, Aoki K, Nawa Y, et al. Cloning of human bone morphogenetic protein type IB receptor (BMPR-IB) and its expression in prostate cancer in comparison with other BMPRs. Oncogene. 1997a;14(11):1377–82. doi: 10.1038/sj.onc.1200964. [DOI] [PubMed] [Google Scholar]
- [46].Ide H, Yoshida T, Matsumoto N, Aoki K, Osada Y, Sugimura T, et al. Growth regulation of human prostate cancer cells by bone morphogenetic protein-2. Cancer Res. 1997b;57(22):5022–7. [PubMed] [Google Scholar]
- [47].Clement JH, Sanger J, Hoffken K. Expression of bone morphogenetic protein 6 in normal mammary tissue and breast cancer cell lines and its regulation by epidermal growth factor. Int J Cancer. 1999;80(2):250–6. doi: 10.1002/(sici)1097-0215(19990118)80:2<250::aid-ijc14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- [48].Arnold SF, Tims E, McGrath BE. Identification of bone morphogenetic proteins and their receptors in human breast cancer cell lines: importance of BMP2. Cytokine. 1999;11(12):1031–7. doi: 10.1006/cyto.1999.0508. [DOI] [PubMed] [Google Scholar]
- [49].Kim IY, Lee DH, Ahn HJ, Tokunaga H, Song W, Devereaux LM, et al. Expression of bone morphogenetic protein receptors type-IA, -IB and -II correlates with tumor grade in human prostate cancer tissues. Cancer Res. 2000;60(11):2840–4. [PubMed] [Google Scholar]
- [50].Kim IY, Lee DH, Lee DK, Ahn HJ, Kim MM, Kim SJ, et al. Loss of expression of bone morphogenetic protein receptor type II in human prostate cancer cells. Oncogene. 2004;23(46):7651–9. doi: 10.1038/sj.onc.1207924. [DOI] [PubMed] [Google Scholar]
- [51].Song JJ, Celeste AJ, Kong FM, Jirtle RL, Rosen V, Thies RS. Bone morphogenetic protein-9 binds to liver cells and stimulates proliferation. Endocrinology. 1995;136(10):4293–7. doi: 10.1210/endo.136.10.7664647. [DOI] [PubMed] [Google Scholar]
- [52].Herrera B, van Dinther M, Ten Dijke P, Inman GJ. Autocrine bone morphogenetic protein-9 signals through activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian cancer cell proliferation. Cancer Res. 2009;69(24):9254–62. doi: 10.1158/0008-5472.CAN-09-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Alarmo EL, Parssinen J, Ketolainen JM, Savinainen K, Karhu R, Kallioniemi A. BMP7 influences proliferation, migration, and invasion of breast cancer cells. Cancer Lett. 2009;275(1):35–43. doi: 10.1016/j.canlet.2008.09.028. [DOI] [PubMed] [Google Scholar]
- [54].Soda H, Raymond E, Sharma S, Lawrence R, Cerna C, Gomez L, et al. Antiproliferative effects of recombinant human bone morphogenetic protein-2 on human tumor colony-forming units. Anticancer Drugs. 1998;9(4):327–31. doi: 10.1097/00001813-199804000-00006. [DOI] [PubMed] [Google Scholar]
- [55].Ghosh-Choudhury N, Ghosh-Choudhury G, Celeste A, Ghosh PM, Moyer M, Abboud SL, et al. Bone morphogenetic protein-2 induces cyclin kinase inhibitor p21 and hypophosphorylation of retinoblastoma protein in estradiol-treated MCF-7 human breast cancer cells. Biochim Biophys Acta. 2000;1497(2):186–96. doi: 10.1016/s0167-4889(00)00060-4. [DOI] [PubMed] [Google Scholar]
- [56].Franzen A, Heldin NE. BMP-7-induced cell cycle arrest of anaplastic thyroid carcinoma cells via p21(CIP1) and p27(KIP1) Biochem Biophys Res Commun. 2001;285(3):773–81. doi: 10.1006/bbrc.2001.5212. [DOI] [PubMed] [Google Scholar]
- [57].Miyazaki Y, Oshima K, Fogo A, Ichikawa I. Evidence that bone morphogenetic protein 4 has multiple biological functions during kidney and urinary tract development. Kidney Int. 2003;63(3):835–44. doi: 10.1046/j.1523-1755.2003.00834.x. [DOI] [PubMed] [Google Scholar]
- [58].Ro TB, Holt RU, Brenne AT, Hjorth-Hansen H, Waage A, Hjertner O, et al. Bone morphogenetic protein-5, -6 and -7 inhibit growth and induce apoptosis in human myeloma cells. Oncogene. 2004;23(17):3024–32. doi: 10.1038/sj.onc.1207386. [DOI] [PubMed] [Google Scholar]
- [59].Buckley S, Shi W, Driscoll B, Ferrario A, Anderson K, Warburton D. BMP4 signaling induces senescence and modulates the oncogenic phenotype of A549 lung adenocarcinoma cells. Am J Physiol Lung Cell Mol Physiol. 2004;286(1):L81–6. doi: 10.1152/ajplung.00160.2003. [DOI] [PubMed] [Google Scholar]
- [60].Nishanian TG, Kim JS, Foxworth A, Waldman T. Suppression of tumorigenesis and activation of Wnt signaling by bone morphogenetic protein 4 in human cancer cells. Cancer Biol Ther. 2004;3(7):667–75. doi: 10.4161/cbt.3.7.965. [DOI] [PubMed] [Google Scholar]
- [61].Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444(7120):761–5. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- [62].Hanavadi S, Martin TA, Watkins G, Mansel RE, Jiang WG. The role of growth differentiation factor-9 (GDF-9) and its analog, GDF-9b/BMP-15, in human breast cancer. Ann Surg Oncol. 2007;14(7):2159–66. doi: 10.1245/s10434-007-9397-5. [DOI] [PubMed] [Google Scholar]
- [63].Du J, Yang S, Wang Z, Zhai C, Yuan W, Lei R, et al. Bone morphogenetic protein 6 inhibit stress-induced breast cancer cells apoptosis via both Smad and p38 pathways. J Cell Biochem. 2008;103(5):1584–97. doi: 10.1002/jcb.21547. [DOI] [PubMed] [Google Scholar]
- [64].Ye L, Kynaston H, Jiang WG. Bone morphogenetic protein-10 suppresses the growth and aggressiveness of prostate cancer cells through a Smad independent pathway. J Urol. 2009;181(6):2749–59. doi: 10.1016/j.juro.2009.01.098. [DOI] [PubMed] [Google Scholar]
- [65].Fong S, Chan MK, Fong A, Bowers WJ, Kelly KJ. Viral vector-induced expression of bone morphogenetic protein 2 produces inhibition of tumor growth and bone differentiation of stem cells. Cancer Gene Ther. 2010;17(2):80–5. doi: 10.1038/cgt.2009.56. [DOI] [PubMed] [Google Scholar]
- [66].Johnsen IK, Kappler R, Auernhammer CJ, Beuschlein F. Bone morphogenetic proteins 2 and 5 are down-regulated in adrenocortical carcinoma and modulate adrenal cell proliferation and steroidogenesis. Cancer Res. 2009;69(14):5784–92. doi: 10.1158/0008-5472.CAN-08-4428. [DOI] [PubMed] [Google Scholar]
- [67].Wen XZ, Miyake S, Akiyama Y, Yuasa Y. BMP-2 modulates the proliferation and differentiation of normal and cancerous gastric cells. Biochem Biophys Res Commun. 2004;316(1):100–6. doi: 10.1016/j.bbrc.2004.02.016. [DOI] [PubMed] [Google Scholar]
- [68].Hallahan AR, Pritchard JI, Chandraratna RA, Ellenbogen RG, Geyer JR, Overland RP, et al. BMP-2 mediates retinoid-induced apoptosis in medulloblastoma cells through a paracrine effect. Nat Med. 2003;9(8):1033–8. doi: 10.1038/nm904. [DOI] [PubMed] [Google Scholar]
- [69].Brubaker KD, Corey E, Brown LG, Vessella RL. Bone morphogenetic protein signaling in prostate cancer cell lines. J Cell Biochem. 2004;91(1):151–60. doi: 10.1002/jcb.10679. [DOI] [PubMed] [Google Scholar]
- [70].Montesano R, Sarkozi R, Schramek H. Bone morphogenetic protein-4 strongly potentiates growth factor-induced proliferation of mammary epithelial cells. Biochem Biophys Res Commun. 2008;374(1):164–8. doi: 10.1016/j.bbrc.2008.07.007. [DOI] [PubMed] [Google Scholar]
- [71].Yang S, Lim M, Pham LK, Kendall SE, Reddi AH, Altieri DC, et al. Bone morphogenetic protein 7 protects prostate cancer cells from stress-induced apoptosis via both Smad and c-Jun NH2-terminal kinase pathways. Cancer Res. 2006;66(8):4285–90. doi: 10.1158/0008-5472.CAN-05-4456. [DOI] [PubMed] [Google Scholar]
- [72].Morrissey C, Brown LG, Pitts TE, Vessella RL, Corey E. Bone morphogenetic protein 7 is expressed in prostate cancer metastases and its effects on prostate tumor cells depend on cell phenotype and the tumor microenvironment. Neoplasia. 2010;12(2):192–205. doi: 10.1593/neo.91836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chen H, Shi S, Acosta L, Li W, Lu J, Bao S, et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131(9):2219–31. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ye L, Bokobza SM, Jiang WG. Bone morphogenetic proteins in development and progression of breast cancer and therapeutic potential (review) Int J Mol Med. 2009;24(5):591–7. doi: 10.3892/ijmm_00000269. [DOI] [PubMed] [Google Scholar]
- [75].Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271(5247):350–3. doi: 10.1126/science.271.5247.350. 19. [DOI] [PubMed] [Google Scholar]
- [76].Langenfeld EM, Kong Y, Langenfeld J. Bone morphogenetic protein 2 stimulation of tumor growth involves the activation of Smad-1/5. Oncogene. 2006;25(5):685–92. doi: 10.1038/sj.onc.1209110. [DOI] [PubMed] [Google Scholar]
- [77].Miyazaki H, Watabe T, Kitamura T, Miyazono K. BMP signals inhibit proliferation and in vivo tumor growth of androgen-insensitive prostate carcinoma cells. Oncogene. 2004;23(58):9326–35. doi: 10.1038/sj.onc.1208127. [DOI] [PubMed] [Google Scholar]
- [78].Beck SE, Jung BH, Fiorino A, Gomez J, Rosario ED, Cabrera BL, et al. Bone morphogenetic protein signaling and growth suppression in colon cancer. Am J Physiol Gastrointest Liver Physiol. 2006;291(1):G135–45. doi: 10.1152/ajpgi.00482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ketolainen JM, Alarmo EL, Tuominen VJ, Kallioniemi A. Parallel inhibition of cell growth and induction of cell migration and invasion in breast cancer cells by bone morphogenetic protein 4. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-0808-0. Epub/In press. [DOI] [PubMed] [Google Scholar]
- [80].McDougall SR, Anderson AR, Chaplain MA. Mathematical modelling of dynamic adaptive tumour-induced angiogenesis: clinical implications and therapeutic targeting strategies. J Theor Biol. 2006;241(3):564–89. doi: 10.1016/j.jtbi.2005.12.022. [DOI] [PubMed] [Google Scholar]
- [81].Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- [82].Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- [83].Autzen P, Robson CN, Bjartell A, Malcolm AJ, Johnson MI, Neal DE, et al. Bone morphogenetic protein 6 in skeletal metastases from prostate cancer and other common human malignancies. Br J Cancer. 1998;78(9):1219–23. doi: 10.1038/bjc.1998.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kang MH, Kang HN, Kim JL, Kim JS, Oh SC, Yoo YA. Inhibition of PI3 kinase/Akt pathway is required for BMP2-induced EMT and invasion. Oncol Rep. 2009;22(3):525–34. doi: 10.3892/or_00000467. [DOI] [PubMed] [Google Scholar]
- [85].Orr FW, Sanchez-Sweatman OH, Kostenuik P, Singh G. Tumor-bone interactions in skeletal metastasis. Clin Orthop Relat Res. 1995;(312):19–33. [PubMed] [Google Scholar]
- [86].Mundy G. Mechanisms of bone metastasis. Cancer [Supplementry] 1997;80:1546–56. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1546::aid-cncr4>3.3.co;2-r. [DOI] [PubMed] [Google Scholar]
- [87].McMurtry CT, McMurtry JM. Metastatic prostate cancer: complications and treatment. J Am Geriatr Soc. 2003;51(8):1136–42. doi: 10.1046/j.1532-5415.2003.51367.x. [DOI] [PubMed] [Google Scholar]
- [88].Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- [89].Cooper CR, Pienta KJ. Cell adhesion and chemotaxis in prostate cancer metastasis to bone: a minireview. Prostate Cancer Prostatic Dis. 2000;3(1):6–12. doi: 10.1038/sj.pcan.4500387. [DOI] [PubMed] [Google Scholar]
- [90].Cooper CR, Chay CH, Gendernalik JD, Lee HL, Bhatia J, Taichman RS, et al. Stromal factors involved in prostate carcinoma metastasis to bone. Cancer. 2003;97(3 Suppl):739–47. doi: 10.1002/cncr.11181. [DOI] [PubMed] [Google Scholar]
- [91].Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62(6):1832–7. [PubMed] [Google Scholar]
- [92].Graham TR, Agrawal KC, Abdel-Mageed AB. Independent and cooperative roles of tumor necrosis factor-alpha, nuclear factor-kappaB, and bone morphogenetic protein-2 in regulation of metastasis and osteomimicry of prostate cancer cells and differentiation and mineralization of MC3T3-E1 osteoblast-like cells. Cancer Sci. 2010;101(1):103–11. doi: 10.1111/j.1349-7006.2009.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Mastro AM, Gay CV, Welch DR. The skeleton as a unique environment for breast cancer cells. Clin Exp Metastasis. 2003;20(3):275–84. doi: 10.1023/a:1022995403081. [DOI] [PubMed] [Google Scholar]
- [94].Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, Pienta KJ, et al. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 2005;146(4):1727–36. doi: 10.1210/en.2004-1211. [DOI] [PubMed] [Google Scholar]
- [95].Alarmo EL, Korhonen T, Kuukasjarvi T, Huhtala H, Holli K, Kallioniemi A. Bone morphogenetic protein 7 expression associates with bone metastasis in breast carcinomas. Ann Oncol. 2008;19(2):308–14. doi: 10.1093/annonc/mdm453. [DOI] [PubMed] [Google Scholar]
- [96].Komai Y, Morimoto S, Saito K, Urushibara M, Sakai K, Ikeda S. Possible involvement of bone morphogenetic protein 2 in heterotopic ossification in metastatic lesion from urothelial carcinoma of bladder. Int J Urol. 2006;13(8):1126–8. doi: 10.1111/j.1442-2042.2006.01488.x. [DOI] [PubMed] [Google Scholar]
- [97].Park Y, Kim JW, Kim DS, Kim EB, Park SJ, Park JY, et al. The Bone Morphogenesis Protein-2 (BMP-2) is Associated with Progression to Metastatic Disease in Gastric Cancer. Cancer Res Treat. 2008;40(3):127–32. doi: 10.4143/crt.2008.40.3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Rees JR, Onwuegbusi BA, Save VE, Alderson D, Fitzgerald RC. In vivo and in vitro evidence for transforming growth factor-beta1-mediated epithelial to mesenchymal transition in esophageal adenocarcinoma. Cancer Res. 2006;66(19):9583–90. doi: 10.1158/0008-5472.CAN-06-1842. [DOI] [PubMed] [Google Scholar]
- [99].Buijs JT, Henriquez NV, van Overveld PG, van der Horst G, Que I, Schwaninger R, et al. Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res. 2007;67(18):8742–51. doi: 10.1158/0008-5472.CAN-06-2490. [DOI] [PubMed] [Google Scholar]
- [100].Hamdy FC, Autzen P, Robinson MC, Horne CH, Neal DE, Robson CN. Immunolocalization and messenger RNA expression of bone morphogenetic protein-6 in human benign and malignant prostatic tissue. Cancer Res. 1997;57(19):4427–31. [PubMed] [Google Scholar]
- [101].Yang S, Du J, Wang Z, Yuan W, Qiao Y, Zhang M, et al. BMP-6 promotes E-cadherin expression through repressing deltaEF1 in breast cancer cells. BMC Cancer. 2007;7:211. doi: 10.1186/1471-2407-7-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Du J, Yang S, An D, Hu F, Yuan W, Zhai C, et al. BMP-6 inhibits microRNA-21 expression in breast cancer through repressing deltaEF1 and AP-1. Cell Res. 2009;19(4):487–96. doi: 10.1038/cr.2009.34. [DOI] [PubMed] [Google Scholar]
- [103].Cunningham NS, Paralkar V, Reddi AH. Osteogenin and recombinant bone morphogenetic protein 2B are chemotactic for human monocytes and stimulate transforming growth factor beta 1 mRNA expression. Proc Natl Acad Sci U S A. 1992;89(24):11740–4. doi: 10.1073/pnas.89.24.11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Willette RN, Gu JL, Lysko PG, Anderson KM, Minehart H, Yue T. BMP-2 gene expression and effects on human vascular smooth muscle cells. J Vasc Res. 1999;36(2):120–5. doi: 10.1159/000025634. [DOI] [PubMed] [Google Scholar]
- [105].King GN, Hughes FJ. Bone morphogenetic protein-2 stimulates cell recruitment and cementogenesis during early wound healing. J Clin Periodontol. 2001;28(5):465–75. doi: 10.1034/j.1600-051x.2001.028005465.x. [DOI] [PubMed] [Google Scholar]
- [106].Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002;87(3):305–12. doi: 10.1002/jcb.10309. [DOI] [PubMed] [Google Scholar]
- [107].Grijelmo C, Rodrigue C, Svrcek M, Bruyneel E, Hendrix A, de Wever O, et al. Proinvasive activity of BMP-7 through SMAD4/src-independent and ERK/Rac/JNK-dependent signaling pathways in colon cancer cells. Cell Signal. 2007;19(8):1722–32. doi: 10.1016/j.cellsig.2007.03.008. [DOI] [PubMed] [Google Scholar]
- [108].Kang MH, Kim JS, Seo JE, Oh SC. BMP2 accelerates the motility and invasiveness of gastric cancer cells via activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Exp Cell Res. 2010;316(1):24–37. doi: 10.1016/j.yexcr.2009.10.010. [DOI] [PubMed] [Google Scholar]
- [109].Nissinen L, Pirila L, Heino J. Bone morphogenetic protein-2 is a regulator of cell adhesion. Exp Cell Res. 1997;230(2):377–85. doi: 10.1006/excr.1996.3438. [DOI] [PubMed] [Google Scholar]
- [110].Clement JH, Raida M, Sanger J, Bicknell R, Liu J, Naumann A, et al. Bone morphogenetic protein 2 (BMP-2) induces in vitro invasion and in vivo hormone independent growth of breast carcinoma cells. Int J Oncol. 2005;27(2):401–7. [PubMed] [Google Scholar]
- [111].Hung TT, Wang H, Kingsley EA, Risbridger GP, Russell PJ. Molecular profiling of bladder cancer: involvement of the TGF-beta pathway in bladder cancer progression. Cancer Lett. 2008;265(1):27–38. doi: 10.1016/j.canlet.2008.02.034. [DOI] [PubMed] [Google Scholar]
- [112].Maegdefrau U, Amann T, Winklmeier A, Braig S, Schubert T, Weiss TS, et al. Bone morphogenetic protein 4 is induced in hepatocellular carcinoma by hypoxia and promotes tumour progression. J Pathol. 2009;218(4):520–9. doi: 10.1002/path.2563. [DOI] [PubMed] [Google Scholar]
- [113].Montesano R. Bone morphogenetic protein-4 abrogates lumen formation by mammary epithelial cells and promotes invasive growth. Biochem Biophys Res Commun. 2007;353(3):817–22. doi: 10.1016/j.bbrc.2006.12.109. [DOI] [PubMed] [Google Scholar]
- [114].Hamada S, Satoh K, Hirota M, Kimura K, Kanno A, Masamune A, et al. Bone morphogenetic protein 4 induces epithelial-mesenchymal transition through MSX2 induction on pancreatic cancer cell line. J Cell Physiol. 2007;213(3):768–74. doi: 10.1002/jcp.21148. [DOI] [PubMed] [Google Scholar]
- [115].Deng H, Makizumi R, Ravikumar TS, Dong H, Yang W, Yang WL. Bone morphogenetic protein-4 is overexpressed in colonic adenocarcinomas and promotes migration and invasion of HCT116 cells. Exp Cell Res. 2007;313(5):1033–44. doi: 10.1016/j.yexcr.2006.12.020. [DOI] [PubMed] [Google Scholar]
- [116].Deng H, Ravikumar TS, Yang WL. Overexpression of bone morphogenetic protein 4 enhances the invasiveness of Smad4-deficient human colorectal cancer cells. Cancer Lett. 2009;281(2):220–31. doi: 10.1016/j.canlet.2009.02.046. [DOI] [PubMed] [Google Scholar]
- [117].Na YR, Seok SH, Kim DJ, Han JH, Kim TH, Jung H, et al. Bone morphogenetic protein 7 induces mesenchymal-to-epithelial transition in melanoma cells, leading to inhibition of metastasis. Cancer Sci. 2009;100(11):2218–25. doi: 10.1111/j.1349-7006.2009.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Masuda H, Fukabori Y, Nakano K, Takezawa Y, T CS, Yamanaka H. Increased expression of bone morphogenetic protein-7 in bone metastatic prostate cancer. Prostate. 2003;54(4):268–74. doi: 10.1002/pros.10193. [DOI] [PubMed] [Google Scholar]
- [119].David L, Feige J-J, Bailly S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine & Growth Factor Reviews. 2009;20(3):203–12. doi: 10.1016/j.cytogfr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- [120].Langenfeld EM, Langenfeld J. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res. 2004;2(3):141–9. [PubMed] [Google Scholar]
- [121].Valdimarsdottir G, Goumans MJ, Rosendahl A, Brugman M, Itoh S, Lebrin F, et al. Stimulation of Id1 expression by bone morphogenetic protein is sufficient and necessary for bone morphogenetic protein-induced activation of endothelial cells. Circulation. 2002;106(17):2263–70. doi: 10.1161/01.cir.0000033830.36431.46. [DOI] [PubMed] [Google Scholar]
- [122].Raida M, Clement JH, Leek RD, Ameri K, Bicknell R, Niederwieser D, et al. Bone morphogenetic protein 2 (BMP-2) and induction of tumor angiogenesis. J Cancer Res Clin Oncol. 2005;131(11):741–50. doi: 10.1007/s00432-005-0024-1. [DOI] [PubMed] [Google Scholar]
- [123].Peng H, Usas A, Olshanski A, Ho AM, Gearhart B, Cooper GM, et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20(11):2017–27. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- [124].Rothhammer T, Bataille F, Spruss T, Eissner G, Bosserhoff AK. Functional implication of BMP4 expression on angiogenesis in malignant melanoma. Oncogene. 2007;26(28):4158–70. doi: 10.1038/sj.onc.1210182. [DOI] [PubMed] [Google Scholar]
- [125].Theriault BL, Shepherd TG, Mujoomdar ML, Nachtigal MW. BMP4 induces EMT and Rho GTPase activation in human ovarian cancer cells. Carcinogenesis. 2007;28(6):1153–62. doi: 10.1093/carcin/bgm015. [DOI] [PubMed] [Google Scholar]
- [126].Thomas BG, Hamdy FC. Bone morphogenetic protein-6: potential mediator of osteoblastic metastases in prostate cancer. Prostate Cancer Prostatic Dis. 2000;3(4):283–5. doi: 10.1038/sj.pcan.4500482. [DOI] [PubMed] [Google Scholar]
- [127].Howe JR, Bair JL, Sayed MG, Anderson ME, Mitros FA, Petersen GM, et al. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat Genet. 2001;28(2):184–7. doi: 10.1038/88919. [DOI] [PubMed] [Google Scholar]
- [128].Lipton L, Sieber OM, Thomas HJ, Hodgson SV, Tomlinson IP, Woodford-Richens K. Germline mutations in the TGF-beta and Wnt signalling pathways are a rare cause of the “multiple” adenoma phenotype. J Med Genet. 2003;40(4):e35. doi: 10.1136/jmg.40.4.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Stemper TJ, Kent TH, Summers RW. Juvenile polyposis and gastrointestinal carcinoma. A study of a kindred. Ann Intern Med. 1975;83(5):639–46. doi: 10.7326/0003-4819-83-5-639. [DOI] [PubMed] [Google Scholar]
- [130].Scott-Conner CE, Hausmann M, Hall TJ, Skelton DS, Anglin BL, Subramony C. Familial juvenile polyposis: patterns of recurrence and implications for surgical management. J Am Coll Surg. 1995;181(5):407–13. [PubMed] [Google Scholar]
- [131].Yoshida T, Haraguchi Y, Tanaka A, Higa A, Daimon Y, Mizuta Y, et al. A case of generalized juvenile gastrointestinal polyposis associated with gastric carcinoma. Endoscop. 1988;20(1):33–5. doi: 10.1055/s-2007-1018122. [DOI] [PubMed] [Google Scholar]
- [132].Walpole IR, Cullity G. Juvenile polyposis: a case with early presentation and death attributable to adenocarcinoma of the pancreas. Am J Med Genet. 1989;32(1):1–8. doi: 10.1002/ajmg.1320320102. [DOI] [PubMed] [Google Scholar]
- [133].Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- [134].Saetrom P, Biesinger J, Li SM, Smith D, Thomas LF, Majzoub K, et al. A risk variant in an miR-125b binding site in BMPR1B is associated with breast cancer pathogenesis. Cancer Res. 2009;69(18):7459–65. doi: 10.1158/0008-5472.CAN-09-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Hardy J, Singleton A. Genomewide association studies and human disease. N Engl J Med. 2009;360(17):1759–68. doi: 10.1056/NEJMra0808700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Houlston RS, Webb E, Broderick P, Pittman AM, Di Bernardo MC, Lubbe S, et al. Meta-analysis of genome-wide association data identifies four new susceptibility loci for colorectal cancer. Nat Genet. 2008;40(12):1426–35. doi: 10.1038/ng.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Broderick P, Carvajal-Carmona L, Pittman AM, Webb E, Howarth K, Rowan A, et al. A genome-wide association study shows that common alleles of SMAD7 influence colorectal cancer risk. Nat Genet. 2007;39(11):1315–7. doi: 10.1038/ng.2007.18. [DOI] [PubMed] [Google Scholar]
- [138].Tenesa AFS, Prendergast JG, Porteous ME, Walker M, Haq N, Barnetson RA, et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on 11q23 and replicates risk loci at 8q24 and 18q21. Nat Genet. 2008;40(5):631–7. doi: 10.1038/ng.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Costello JF, Fruhwald MC, Smiraglia DJ, Rush LJ, Robertson GP, Gao X, et al. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nat Genet. 2000;24(2):132–8. doi: 10.1038/72785. [DOI] [PubMed] [Google Scholar]
- [140].Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–96. [PubMed] [Google Scholar]
- [141].Dai Z, Popkie AP, Zhu WG, Timmers CD, Raval A, Tannehill-Gregg S, et al. Bone morphogenetic protein 3B silencing in non-small-cell lung cancer. Oncogene. 2004;23(20):3521–9. doi: 10.1038/sj.onc.1207441. [DOI] [PubMed] [Google Scholar]
- [142].Wen XZ, Akiyama Y, Baylin SB, Yuasa Y. Frequent epigenetic silencing of the bone morphogenetic protein 2 gene through methylation in gastric carcinomas. Oncogene. 2006;25(18):2666–73. doi: 10.1038/sj.onc.1209297. [DOI] [PubMed] [Google Scholar]
- [143].Zavadil J, Brezinova J, Svoboda P, Zemanova Z, Michalova K. Smad5, a tumor suppressor candidate at 5q31.1, is hemizygously lost and not mutated in the retained allele in human leukemia cell line HL60. Leukemia. 1997;11(8):1187–92. doi: 10.1038/sj.leu.2400750. [DOI] [PubMed] [Google Scholar]
- [144].Cheng KH, Ponte JF, Thiagalingam S. Elucidation of epigenetic inactivation of SMAD8 in cancer using targeted expressed gene display. Cancer Res. 2004;64(5):1639–46. doi: 10.1158/0008-5472.can-03-2688. [DOI] [PubMed] [Google Scholar]
- [145].Rogers MB, Rosen V, Wozney JM, Gudas LJ. Bone morphogenetic proteins-2 and -4 are involved in the retinoic acid-induced differentiation of embryonal carcinoma cells. Mol Biol Cell. 1992;3(2):189–96. doi: 10.1091/mbc.3.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Hao J, Daleo MA, Murphy CK, Yu PB, Ho JN, Hu J, et al. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS One. 2008;3(8):e2904. doi: 10.1371/journal.pone.0002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Hong CC, Yu PB. Applications of small molecule BMP inhibitors in physiology and disease. Cytokine Growth Factor Rev. 2009;20(5–6):409–18. doi: 10.1016/j.cytogfr.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Zhu NL, Li C, Huang HH, Sebald M, Londhe VA, Heisterkamp N, et al. TNF-alpha represses transcription of human Bone Morphogenetic Protein-4 in lung epithelial cells. Gene. 2007;393(1–2):70–80. doi: 10.1016/j.gene.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, et al. In Vivo Structure-Activity Relationship Study of Dorsomorphin Analogues Identifies Selective VEGF and BMP Inhibitors. ACS Chem Biol. 2010;5(2):245–53. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Lee GT, Hong JH, Mueller TJ, Watson JA, Kwak C, Sheen YY, et al. Effect of IN-1130, a small molecule inhibitor of transforming growth factor-beta type I receptor/activin receptor-like kinase-5, on prostate cancer cells. J Urol. 2008;180(6):2660–7. doi: 10.1016/j.juro.2008.08.008. [DOI] [PubMed] [Google Scholar]
- [151].Ye L, Kynaston H, Jiang WG. Bone morphogenetic protein-9 induces apoptosis in prostate cancer cells, the role of prostate apoptosis response-4. Mol Cancer Res. 2008;6(10):1594–606. doi: 10.1158/1541-7786.MCR-08-0171. [DOI] [PubMed] [Google Scholar]