Abstract
Tolfenamic acid (TA) is a non-steroidal anti-inflammatory drug that inhibits pancreatic cancer cell and tumor growth THROUGH decreasing expression of specificity protein (Sp) transcription factors. TA also inhibits growth of erbB2-overexpressing BT474 and SKBR3 breast cancer cells; however, in contrast to pancreatic cancer cells, TA induced downregulation of erbB2 but not Sp proteins. TA-induced erbB2 downregulation was accompanied by decreased erbB2-dependent kinase activities, induction of p27, and decreased expression of cyclin D1. TA also decreased erbB2 mRNA expression and promoter activity, and this was due to decreased mRNA stability in BT474 cells and, in both cell lines, TA decreased expression of the YY1 and AP-2 transcription factors required for basal erbB2 expression. In addition, TA also inhibited tumor growth in athymic nude mice in which BT474 cells were injected into the mammary fat pad. TA represents a novel and promising new anticancer drug that targets erbB2 by decreasing transcription of this oncogene.
Keywords: erbB2, breast cancer, downregulation, tolfenamic acid, anticancer
INTRODUCTION
Breast cancer is one of the major causes of premature death in women; however, the combination of early detection coupled with improved treatment has significantly improved survival from this disease (1–3). Antiestrogens and aromatase inhibitors are highly effective endocrine therapies used for treating early stage estrogen receptor (ER)- positive breast cancer. Compounds that include tamoxifen, raloxifene, fulvestrant, and their combinations or sequential use provide successful outcomes for patients with hormone-responsive tumors (2–7). Later stage or less differentiated ER-negative breast cancers are more aggressive; patient survival is relatively low; and various therapeutic regimes are less effective (8–11). Improvements in the effectiveness of chemotherapies have been obtained using drug combinations and differences in the timing of drug delivery (11). In addition, newer mechanism-based anticancer drugs that target critical kinase, survival, growth promoting and angiogenic pathways are also promising new chemotherapies for treating breast and other tumor types (10, 11).
Epidermal growth factor receptors (EGFRs) are receptor tyrosine kinases overexpressed in many cancers and erbB2/HER2/neu is an oncogene overexpressed in 20–30% of all breast cancers. ErbB2-positive tumors tend to be aggressive with a poor prognosis for patient survival, and the recombinant monoclonal antibody trastuzumab (Herceptin) has been used as a single agent and in combination therapy for successfully treating patients with breast tumors overexpressing erbB2 (12–15). Since Herceptin targets the extracellular domain of erbB2, there is a decrease in receptor tyrosine kinase activity and various downstream targets that are important for erbB2-dependent tumor growth and survival. For example, treatment of breast cancer cells overexpressing erbB2 with Herceptin decreased erbB2 phosphorylation and also mitogen-activated protein kinase (MAPK)- and phosphatidylinositol-3-kinase (PI3K)-dependent phosphorylation of MAPK and Akt, respectively (16).
Tolfenamic acid (TA) is a non-steroidal anti-inflammatory drug (NSAID) used for treatment of migraine headaches and alcohol-induced hangovers (17); however, recent studies have demonstrated the efficacy of this drug for cancer chemotherapy (18, 19). TA inhibits pancreatic cancer cell growth in vitro and tumor growth in vivo through inducing proteasome-dependent degradation of Sp1, Sp3 and Sp4 proteins which are overexpressed in these cells and tumors (18–20). The effectiveness of TA is associated with repression of Sp proteins and Sp-dependent genes such as vascular endothelial growth factor (VEGF) and VEGF receptor 1 (VEGFR1). The antiangiogenic activity of TA correlated with the inhibition of liver metastasis in an orthotopic model for pancreatic cancer (17). In this study, we show that TA also inhibits growth of erbB2-overexpressing BT474 and SKBR3 breast cancer cells; however, this is not accompanied by a coordinate repression of Sp proteins. Inhibition of erbB2-overexpressing breast cancer cell and tumor growth by TA is associated with downregulation of erbB2. This novel observation highlights the possibility that erbB2-overexpressing breast tumors and tumors derived from other tissues may be targeted by TA and structurally-related NSAIDs that exhibit relatively low toxicity.
MATERIALS AND METHODS
Chemicals, antibodies, plasmids, and reagents
Tolfenamic acid, mefenamic acid, flufenamic acid, N, flumic acid and diclofenac were purchased from LKT Laboratories, Inc. (St. Paul, MN). Lactacystin, cycloheximide and β-actin antibody were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies against erbB2 (C-18), Sp1 (PEP2), Sp3 (D-20), Sp4 (V-20), Akt (H-136), p-Akt (Ser473), MAPK(C-14), p-MAPK (E- 4), cyclin D1 (M-20), p27 (C-19), PEA3 (16), AP-2α (C-18 and 3B5) and YY1 (H-10) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); the erbB2 (Ab-3) antibody was obtained from Calbiochem (San Diego, CA) and the EEA1 antibody was purchased from Upstate (Lake Placid, NY). The perbB2-500 construct was kindly provided by Dr. Christopher C. Benz (University of California, San Francisco, CA) and the full length AP-2 cDNA construct TFAP2A was purchased from Open Biosystem (Huntsville, AL). Reporter lysis buffer and luciferase reagent for luciferase studies were purchased from Promega (Madison, WI). β-Galactosidase (β-gal) reagent was obtained from Tropix (Bedford, MA). LipofectAMINE reagent was supplied by Invitrogen (Carlsbad, CA). Western lightning chemiluminescence reagent was from Perkin-Elmer Life Sciences (Boston, MA).
Cell lines
Human mammary carcinoma cell lines MDA-MB-231, MCF-7, BT474 and SKBR3 were obtained from the American Type Culture Collection (Manassas, VA). Cell lines were cultured with 10% fetal bovine serum (FBS) in DMEM (BT474, MDA-MB-231 and MCF-7) or McCoy’s 5A medium (SKBR3). Cells were maintained at 37°C in the presence of 5% CO2.
Cell proliferation assay
Cells (2–3 × 104 per well) were plated in 12-well plates and allowed to attach for 24 hr. The medium was then changed to DMEM/Ham's F-12 medium containing 2.5% charcoal-stripped FBS, and either vehicle (DMSO) or different concentrations of TA were added. Fresh medium and compounds were added every 48 hr, and cells were then trypsinized and counted at the indicated time points using a Coulter Z1 cell counter. Each experiment was done in triplicate, and results are expressed as means ± SE for each set of experiments.
Western blotting
Cells were rinsed with PBS and collected by scraping cells from the culture plate in 200 µL of lysis buffer. The cell lysates were incubated on ice for 1 hr with intermittent vortex mixing and then centrifuged at 40,000 g for 10 min at 4°C. Equal amounts of protein were separated on SDS-polyacrylamide gels. Proteins were transferred to Immobilon P membranes (Millipore, Bedford, MA) using a Bio-Rad Trans-blot apparatus and transfer buffer (48 mM Tris, 39 mM glycine, 0.0375% SDS, 20% methanol). After blocking in TBST-Blotto [10 mmol/L Tris-HCl, 150 mmol/L NaCl (pH 8), 0.05% Triton X-100, 5% nonfat dry milk] for 30 min, the membranes were incubated with primary antibodies overnight at 4°C and then with horseradish peroxidase-conjugated secondary antibody for 2 hr at room temperature. Proteins were visualized using the chemiluminescence substrate (Perkin-Elmer Life Sciences) for 1 min and exposed to Kodak X-OMAT AR autoradiography film (Eastman Kodak, Rochester, NY). For protein quantitation, band intensities were normalized to β-actin (loading control) and compared to band intensities for the DMSO (control) set at 1.0 or 100%
Quantitative real-time PCR
Total RNA was purified using RNeasy Mini Kit (Qiagen, Germantown, MD) and cDNA was prepared using Reverse Transcription System (Promega, Madison, WI). Each PCR was carried out in triplicate in a 30 µL volume using SYBR Green Mastermix (Applied Biosystems, Foster City, CA) for 15 min at 95°C for initial denaturing, followed by 40 cycles of 95°C for 30 s and 60°C for 1 min in the Applied Biosystems 7900HT Fast Real-time PCR System. The ABI Dissociation Curves software was used following a brief thermal protocol (95°C for 15 s and 60°C for 15 s, followed by a slow ramp to 95°C) to control for multiple species in each PCR amplification. Values for each gene were normalized to expression levels of TATA-binding protein (TBP). Primers were obtained from Integrated DNA Technologies. The following primers were used:
hNeu(F): 5'-ACC GGC ACA GAC ATG AAG CT-3'.
hNeu(R): 5'-AGG AAG GAC AGG CTG GCA TT-3'.
TBP (F): 5'-TGC ACA GGA GCC AAG AGT GAA-3'.
TBP (R): 5'-CAC ATC ACA GCT CCC CAC CA-3'.
DNA transfection and luciferase assays
Cells were plated in 12-well plates at 1 × 105 per well and cultured as described above. After growth for 16 to 20 hr, transfections were carried out by using LipofectAMINE (Invitrogen) according to the manufacturer's protocol. After 5 hr of transfection, the transfection mix was replaced with complete media containing either vehicle (DMSO) or TA for 20 to 22 hr. Cells were then lysed with 100 µL of 1x reporter lysis buffer, and 30 µL of cell extract were used for luciferase and β-gal assays. Lumicount was used to quantitate luciferase and β-gal activities, and the luciferase activities were normalized to β-gal activity.
Immunofluorescence microscopy
Cells were fixed immediately in 4% paraformaldehyde, added with 0.3% Triton X-100 (Roche Molecular Biochemicals, Indianapolis, IN) for 10 min, permeabilized in PBS with 0.3% Triton X-100 for 10 min, and preincubated for 1 hr with 10% normal goat serum (Vector Laboratories, Burlingame, CA). Cells were incubated with anti-erbB2 antibody (1:80) or anti-EEA1 antibody (1:200) overnight and incubated with FITC-conjugated or Cy5-conjugated secondary antibody (1:200; Chemicon, Temecula, CA) for 1 hr. The two-well chambers were mounted with mounting medium (Vector Laboratories). The slides were viewed using an LSM 510 Meta confocal microscope (Carl Zeiss, Jena, Germany) equipped with 40× and 63× objectives. Images were analyzed and processed using the LSM software v. 3.2 (Carl Zeiss) and occasionally Adobe Photoshop 7.0.
Animals and orthotopic implantation of breast tumor cells
Female ovariectomized athymic nu/nu mice (5–7 wk old) were purchased from the Animal Production Area of the National Cancer Institute Frederick Cancer Research and Development Center (Frederick, MD). The mice were housed and maintained under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the United States Department of Agriculture, United States Department of Health and Human Services, and the NIH. Under anesthetic condition, a 0.72-mg 60-day release 17β-estradiol pellet (Innovative Research, Sarasota, FL) was implanted into the interscapular region of each mouse. One day later, BT474 cells (3×106 cells) were injected s.c. under the mammary fat pad area of each mouse. The tumor sites were monitored twice a week and when palpable (12 days), mice were randomized into two groups of 5 mice per group and dosed by oral gavage with corn oil or 25 mg/kg/d tolfenamic acid for 27 days. The mice were weighed, and tumor size was measured at the indicated time with calipers to permit calculation of tumor volumes: V = LW2/2, where L and W were length and width, respectively. Final body and tumor weights were determined at the end of the dosing regimen, and tumor blocks were obtained for histopathologic analysis.
Immunohistochemistry
Tissue sections (4–5 µM thick) mounted on poly-Llysine-coated slide were deparaffinized by standard methods. Endogenous peroxidase was blocked by the use of 2% hydrogen peroxide in PBS for 1 min. Antigen retrieval for erbB2 and p-MAPK staining was done for 10 min in 10 mmol/L sodium citrate buffer (pH 6) heated at 95°C in a steamer followed by cooling at room temperature for 15 min. The slides were washed with PBS and incubated for 30 min at room temperature with a protein blocking solution (VECTASTAIN Elite ABC kit, Vector Laboratories, Burlingame, CA). Excess blocking solution was drained, and the samples were incubated overnight at 4°C with one of the following: a 1:60 dilution of erbB2 antibody or a 1:80 dilution of p-MAPK, AP-2 and YY1 antibodies. Sections were then incubated with biotinylated secondary antibody followed by streptavidin (VECTASTAIN Elite ABC kit). The color was developed by exposing the peroxidase to diaminobenzidine reagent (Vector Laboratories), which forms a brown reaction product. The sections were then counterstained with Gill's hematoxylin. ErbB2, AP-2, YY-1 and p-MAPK expression were identified by the brown cytoplasmic staining. H&E staining was determined as previously described (18–20).
RESULTS
1. TA inhibits proliferation of BT474 and SKBR3 cells
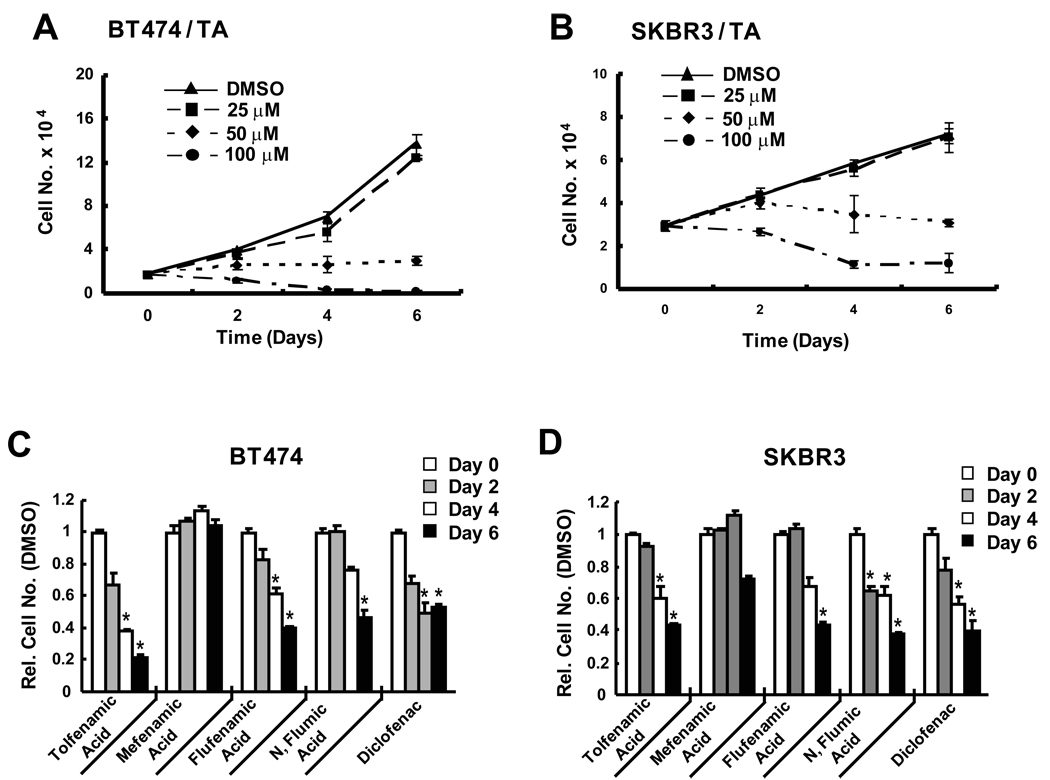
Figures 1A and 1B illustrate the effects of TA on proliferation of erbB2-overexpressing BT474 and SKBR3 breast cancer cell lines. The lowest concentration of TA (25 µM) had minimal effects, whereas 50 and 100 µM TA inhibited growth of BT474 and SKBR3 cells and IC50 values were 41.5 and 52.5 µM, respectively. These results are similar to those previously reported in pancreatic cancer cells (18) and in ongoing studies in other cancer cell lines. The growth inhibitory effects of TA were also observed in other breast cancer cell lines, and Supplement Figure 1 shows that TA inhibits proliferation of MCF-7 and MDA-MB-231 cells. The effects of TA and other substituted biphenylamine-1-carboxylic acids on proliferation of BT474 and SKBT3 cell was also determined (Figs. 1C and 1D). A comparison of the growth inhibitory effects of 50 µM TA, and structurally-related mefanamic acid, flufenamic acid, N-flumic acid, and diclofenac indicated that mefanamic acid was the least active among these 5 structurally related analogs. Differences among the other 4 substituted biphenylamine-1-carboxylic acid NSAIDs were not large; however, TA was the most active compound in BT474 cells as previously observed for these compounds in and was used as the prototype for the remaining studies. We also investigated the effects of mefanamic acid on BT474 cell proliferation (Supplement Fig. 2) and the results show that 50 µM mefanamic acid did not inhibit cell proliferation. Higher concentrations (≥ 100 µM) were growth inhibitory (data not shown).
Figure 1.
Effects of TA and related compounds on cell proliferation. TA-mediated inhibition of BT474 (A) and SKBR3 (B) cell growth. Cells were treated with different concentrations of TA for up to 6 days and the number of cells in each treatment group were determined as described in the Materials and Methods. Significant (p < 0.05) inhibition of cell growth was observed for 50 and 100 µM TA. Inhibition of BT474 (C) and SKBR3 (D) cell proliferation by TA and related compounds. Cells were treated with 50 µM TA and related compounds as described above and significant (p < 0.05) growth inhibition is indicated (*). Results are expressed as means ± SE for at least 3 replicate determinations for each treatment group.
2. TA downregulates erbB2 and erbB2-dependent responses
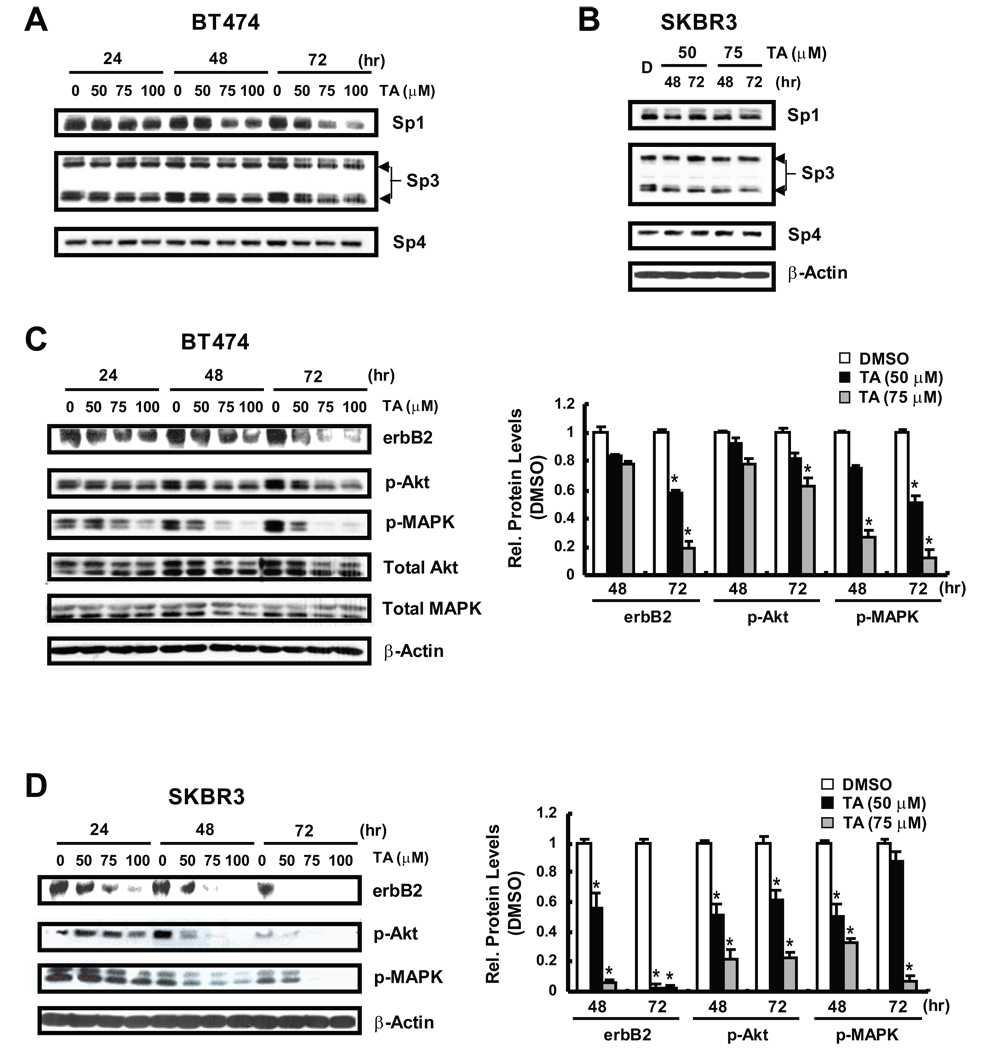
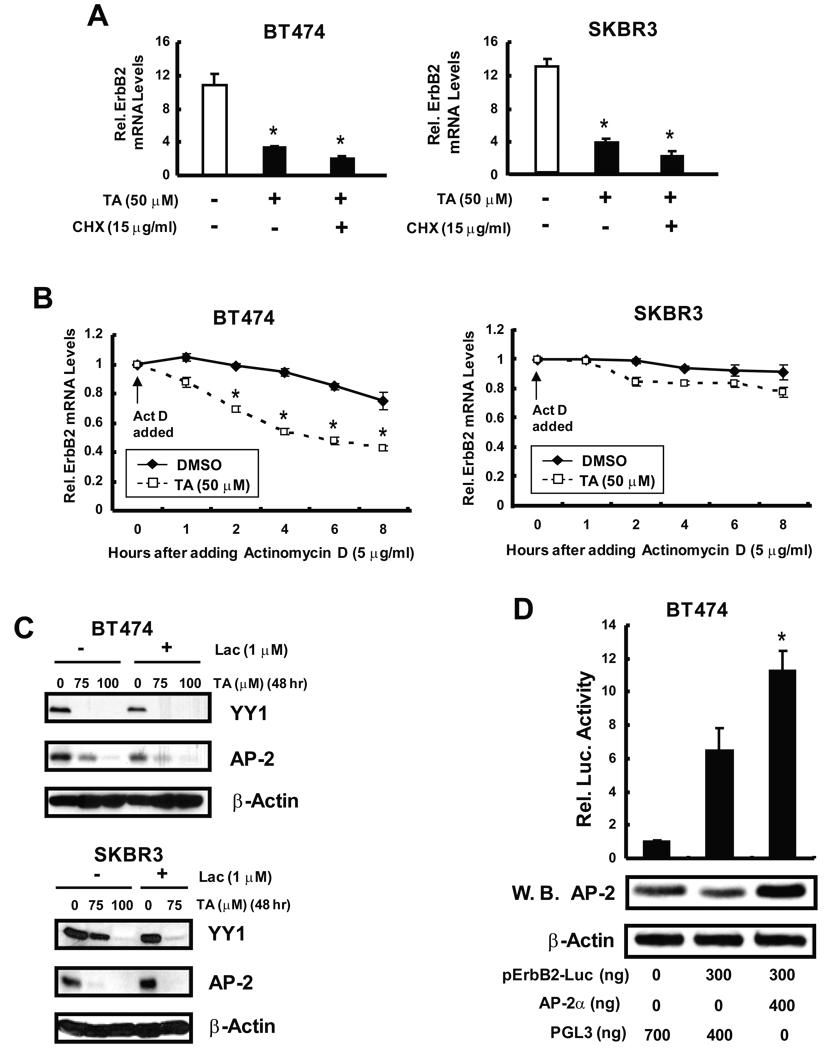
The growth inhibitory activity of TA and related compounds in cancer cells has been correlated with downregulation of Sp1, Sp3 and Sp4 proteins (18–20); however, results in Figures 2A and 2B show that up to 100 µM TA did not appreciably affect Sp3 or Sp4 expression in BT474 or SKBR3 cells, and 75–100 µM TA decreased Sp1 protein only after treatment of BT474 cells for 72 hr. Since erbB2 is a major driving force for the growth and survival of both cell lines, we also examined the effects of TA on erbB2 protein expression (Figs. 2C and 2D). TA induced a time-and concentration-dependent decrease in erbB2 protein in BT474 cells and this was accompanied by decreased phosphorylation of MAPK and Akt. As a control for this experiment, we also observed that mefanamic acid decreased erbB2 and phosphorylation of MAPK and Akt but at higher concentrations than required for TA (Supplement Fig. 2). Thus, TA specifically decreased expression of erbB2 protein and erbB2-dependent phosphorylation pathways in BT474 and SKBR3 cells. We also observed some treatment-related changes in MAPK and Akt proteins (Supplement Fig. 3) in BT474 cells and this was particularly evident for MAPK (but not Akt) after treatment for 72 hr. Cyclin D1 and p27 are two proteins up- and downregulated by erbB2-dependent kinases, respectively (16), and results in Figure 3A show that TA also decreased cyclin D1 and increased p27 expression in BT474 and SKBR3 cells. Previous reports indicate that geldanamycin and ansamycins decrease erbB2 protein through destabilizing interactions with chaperones resulting in enhanced proteasome-dependent degradation of erbB2 (21–23). Initial studies with the proteasome inhibitor MG132 gave conflicting results since MG132 alone decreased erbB2 protein in BT474 and SKBR3 cells (data not shown). Studies with lactacystin, another proteasome inhibitor showed that TA-induced downregulation of erbB2 protein was not inhibited after cotreatment with lactacystin (Fig. 3B). Treatment of BT474 and SKBR3 with 50 µM TA for 2, 6 and 12 hr resulted in a time-dependent decrease in erbB2 mRNA levels (Fig. 3C) and in cells transfected with perbB2, a construct containing the −0.5 kB region from the erbB2 promoter, TA also decreased luciferase activity in both cell lines (Fig. 3D). The results demonstrate that TA acts, in part, by decreasing erbB2 transcription in BT474 and SKBR3 cells.
Figure 2.
Effects of TA on Sp, erbB2 and erbB2-dependent proteins. Sp protein expression in BT474 (A) and SKBR3 (B) cells treated with TA. Cells were treated with different concentrations of TA for up to 72 hr and whole cell lysates were analyzed by western blots as described in the Materials and Methods. ErbB2 and erbB2-dependent protein expression in BT474 (C) and SKBR3 (D) cells treated with TA. Cells were treated as described in (A)/(B) and whole cell lysates were analyzed by western blots as described in the Materials and Methods. Bar graphs compare protein expression (normalized to β-actin) relative to DMSO (set at 1.0) and results are means ± SE for 3 replicate determinations for each treatment group. Significant (p < 0.05) decreases in protein expression are indicated (*).
Figure 3.
TA decreases erbB2-dependent genes and erbB2 gene expression. Effects of TA on cyclin D1/p27 (A) and erbB2 (B) protein levels. BT474 and SKBR3 cells were treated with TA alone or TA in combination with 2 µM lactacystin for 48 or 72 hr as indicated, and whole cell lysates were analyzed by western blots as described in the Materials and Methods. (C) TA decreases erbB2 mRNA levels. BT474 and SKBR3 cells were treated with DMSO or 50 µM TA for different times and erbB2 mRNA levels were determined by RT-PCR as described in the Materials and Methods. Results are expressed as means ± SE for three replicate determinations for each treatment group and significantly (p < 0.05) decreased mRNA levels are indicated. (D) TA decreases luciferase activity. Cells were transfected with perbB2, treated with DMSO, or 50 µM TA, and luciferase activity (relative to β-gal) was determined as described in the Materials and Methods. Results are expressed as means ± SE for 3 replicate determinations for each treatment group and significant (p < 0.05) decreases in activity are indicated (*).
3. Mechanisms of erbB2 downregulation by TA
The mechanisms of TA-dependent inhibition of erbB2 transcription were investigated in BT474 and SKBR3 cells treated with TA alone or in combination with the protein synthesis inhibitor cycloheximide (Fig. 4A). Cycloheximide did not affect TA-dependent erbB2 mRNA downregulation in either cell line, suggesting that TA does not induce an inhibitory protein that acts on erbB2 transcription. However, in studies on erbB2 mRNA stability carried out in the presence or absence of the transcriptional inhibitor actinomycin D, TA significantly decreased erbB2 mRNA stability in BT474 cells over the 8 hr duration of this experiment (Fig. 4B). In contrast, only minimal effects were observed in SKBR3 cells, demonstrating cell context-dependent effects of TA on erbB2 mRNA stability. Previous studies show that the transcription factor PEA3 suppresses erbB2 expression (24, 25). Therefore, we investigated the effects of TA on PEA3 expression in BT474 and SKBR3 cells (Supplemental Fig. 4) and did not observe any changes in expression of this transcription factor. YY1 and AP-2 cooperatively regulate erbB2 expression in erbB2 overexpressing cells (26), and Figure 4C summarizes the effects of TA on expression of these transcription factors in BT474 and SKBR3 cells. TA decreased YY1 and AP-2 protein levels in BT474 and SKBR3 cells and, in cells cotreated with TA plus the proteasome inhibitor lactacystin, TA-dependent downregulation of YY1 and AP-2 proteins was not reversed. These results indicate that downregulation of these transcription factors was proteasome-independent.
Figure 4.
Effects of TA on erbB2 expression and transcriptional regulatory proteins. Effects of cycloheximide (A) and actinomycin D (B) on erbB2 mRNA levels and stability in cells treated with TA. BT474 and SKBR3 cells were treated with 50 µM TA alone or in combination with cycloheximide or actinomycin D and erbB2 mRNA levels were determined at various time points as described in the Materials and Methods. Results are expressed as means ± SE for 3 replicate determinations for each treatment group and significant (p < 0.05) decreases are indicated (*). (C) Effects of TA on YY1/AP-2 protein levels. BT474 and SKBR3 cells were treated with TA for the indicated times and whole cell lysates were analyzed by western blot analysis as indicated in the Materials and Methods. (D) AP-2 activates the erbB2 promoter. BT474 cells were transfected with empty vector (PGL3) or the perbB2-luc construct, and one treatment group was cotransfected with AP-2 expression plasmid. Luciferase activity was determined as described in the Materials and Methods. Results are means ± SE for 3 separate determinations and significant (p < 0.05) induction of luciferase activity by AP-2 is indicated (*). Western blot analysis of lysates demonstrates that the AP-2 expression plasmid increases AP-2 protein.
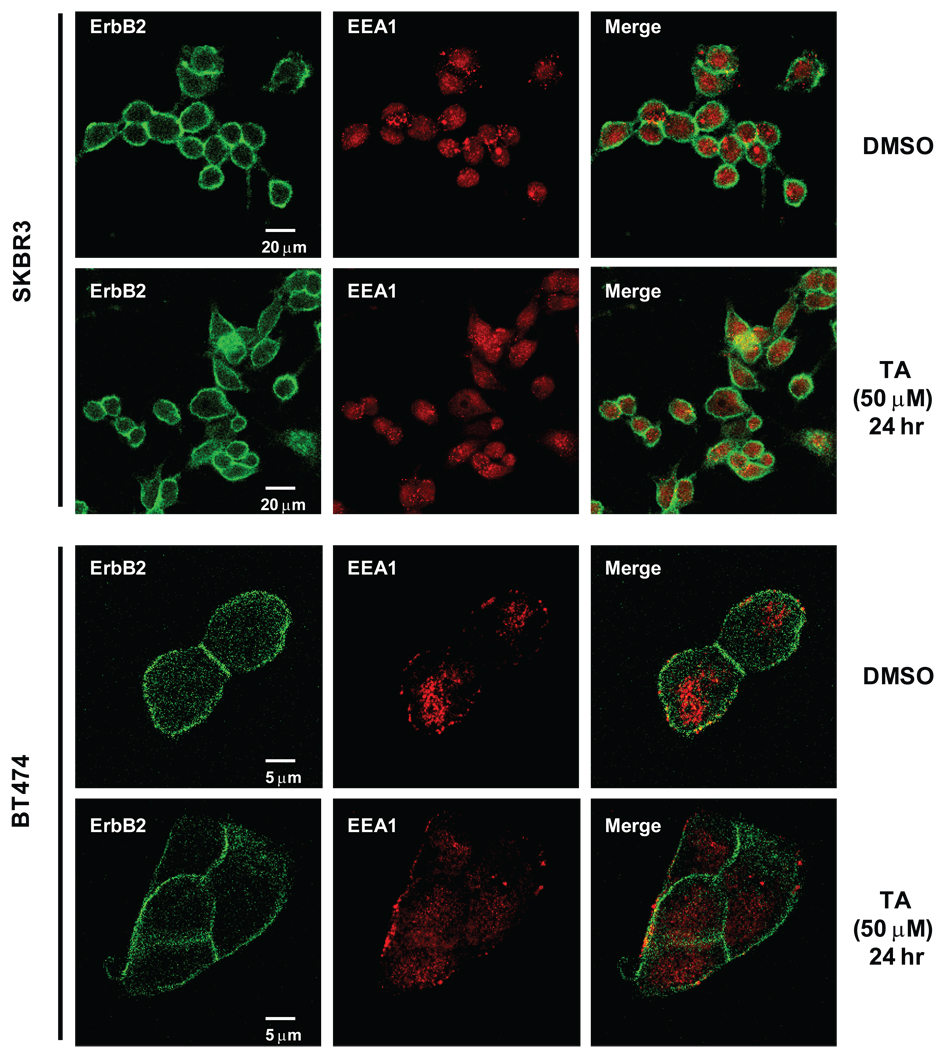
We also investigated the effects of TA on subcellular localization of erbB2 since many drugs that decrease erbB2 protein induce erbB2 delocalization from the plasma membrane into the cytoplasm (27–29). In solvent (DMSO)-treated SKBR3 cells, erbB2 staining was primarily on the plasma membrane (Fig. 5), whereas the endosome marker, early endosome antigen 1 (EEA1), staining was on the endosome and did not colocalize with erbB2. After treatment with 50 µM TA for 24 hr, the erbB2 plasma membrane staining was observed and, in the merge of both erbB2 and EEA1, it was evident that TA did not significantly induce internalization of erbB2 as reported for other agents (27–29). Similar results were observed in BT474 cells demonstrating that TA did not induce internalization and subsequent degradation of erbB2 in the erbB2 overexpressing breast cancer cell lines. BT474 cells were transfected with PGL3 empty vector or the perbB2-luc construct (Fig. 4D). In cells, cotransfected with a AP-2 expression plasmid, there was a significant induction of luciferase activity demonstrating that AP-2 expression activates the erbB2 promoter and demonstrates the importance of AP-2 for erbB2 expression.
Figure 5.
Immunostaining of erbB2. SKBR3 and BT474 cells were treated with 50 µM TA for 24 hr and cells were fixed, immunostained with erbB2 or EEA1 antibodies and analyzed by confocal microscope and softwares as described in the Materials and Methods.
4. TA inhibits tumor growth in athymic nude mice bearing BT474 xenografts
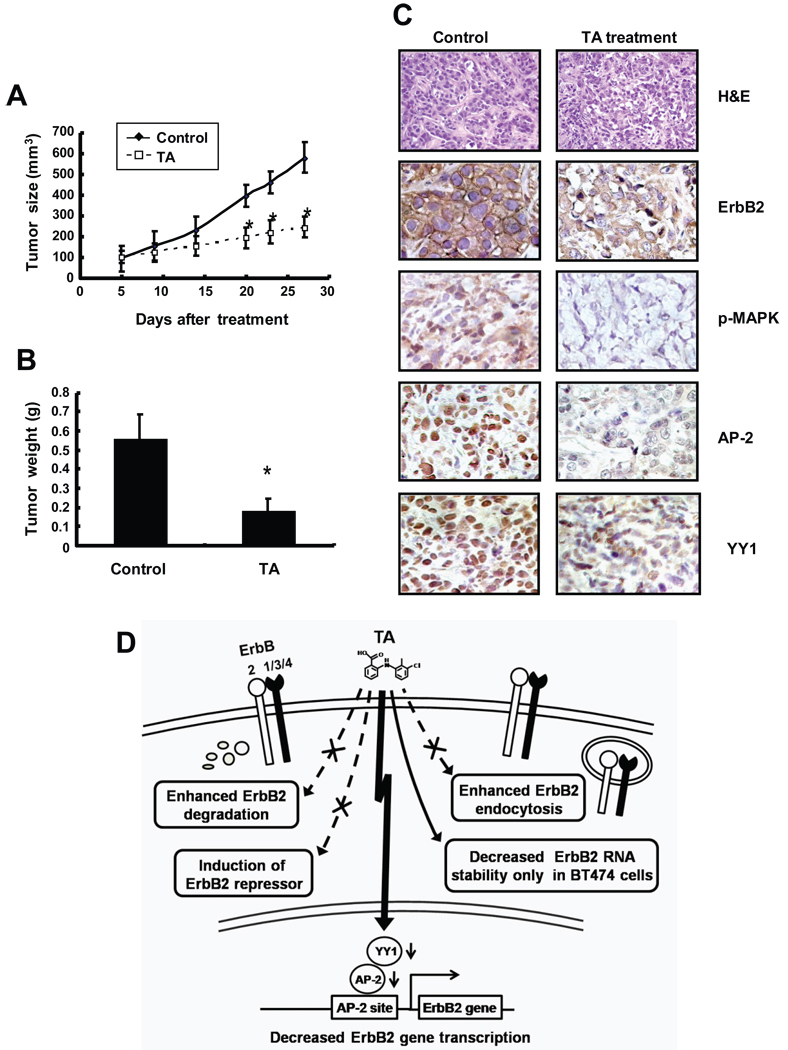
We also investigated the in vivo antitumorigenic activity of TA (20 mg/kg/d) which was administered orally by gavage to female athymic nude mice bearing BT474 cells injected into the mammary fat pad. Tumor size was determine over the treatment period (Fig. 6A) and there was a significant decrease in mice treated with TA compared to those treated with the solvent alone. In addition, TA also decreased mammary tumor weight compared to solvent treated animals (Fig. 6B). H & E staining (Fig. 6C) shows that tumors from untreated mice consisted of nests of cells in a semi-organized fashion with nuclear molding and high nuclear to cytoplasmic ratio. In addition, cells with marked atypical features such as anisocytosis, anisokaryosis, and multiple variably sized nucleoli were also noted. Tumors from tolfenamic acid-treated mice consisted of neoplastic cells similar to that noted from the untreated mice. However, the nests of tumor cells were highly disorganized with multiple nuclear fragmentations and condensations; in addition, epithelial atypia was decreased. Treatment of mice with TA also decreased expression of erbB2, phospho-MAPK, AP-2 and YY-1 in tumors compared to levels in tumors from mice treated with corn oil (Fig. 6C). Thus, results of both in vivo and in vitro data show that TA inhibits tumor and cancer cell growth through downregulating erbB2 expression, suggesting that this relatively non-toxic NSAID may represent a novel clinical approach for treatment of cancers that overexpress this oncogene.
Figure 6.
TA inhibits tumor (BT474 xenografts) growth. Inhibition of tumor volume (A) and weight (B). Athymic nude mice bearing BT474 cells as xenografts were treated with TA (25 mg/kg/d) and tumor volumes and weights were determined as described in the Materials and Methods. Significantly (p < 0.05) decreased tumor weights are indicated (*). (C) H&E staining and immunostaining. Tumors from vehicle control (corn oil)- and TA-treated mice were fixed and stained (H&E) and immunostaining for erbB2, phospho-MAPK, YY1 and AP-2 as described in the Materials and Methods. (D) A schematic model summarizing the effects of TA on erbB2 in BT474 and SKBR3 cells.
DISCUSSION
The development of Herceptin as a biotherapy for erbB2-overexpressing breast cancer patients has been an important innovation for treating this subset of individuals (12–15). Moreover, combination therapy of Herceptin plus other drugs including paclitaxel are also being used as adjuvant therapy for breast cancer. Herceptin is not without side effects, and cardiotoxicity has been reported in a small number of patients (15). Based on the success of targeting erbB2 for cancer chemotherapy, other chemotherapeutic agents have been developed for blocking activity of this receptor, and these include both selective and non-selective tyrosine kinase inhibitors, geldanamycin and compounds that interfere with chaperones such as heat shock protein 90, fatty acid synthase (FAS) inhibitors, and orlistat, a drug used in weight loss (14–16, 21–23, 27–36). These compounds all block activation of erbB2 and erbB2-dependent downstream responses, although their overall mechanisms of action are highly variable. TA is a relatively non-toxic NSAID used for treatment of migraine headaches in humans, and TA has multiple applications in veterinary medicine. Development of this drug for cancer chemotherapy is promising due to the relatively low toxicity of TA and related compounds. Previous studies in this laboratory reported that the anticancer activity of TA in pancreatic cancer cell lines is associated with their repression of Sp proteins and Sp-dependent genes (18–20), and we hypothesized that TA may be effective in treatment of erbB2-positive breast cancer through a comparable mechanism.
Sp proteins are overexpressed in ER-positive and ER-negative breast cancer cell lines including SKBR3 and BT474 cells (37), and Figure 1 shows that TA inhibits growth of both cell lines with potencies similar to that observed for this compound in pancreatic 16 cancer cells (18–20). However, treatment of SKBR3 and BT474 cells with TA did not appreciably affect Sp1, Sp3 and Sp4 protein levels, although we did observe a consistent 20–30% decrease in Sp1 in BT474 cells treated with 75–100 µM TA for 3 days. These results contrast to ongoing studies in pancreatic and other cancer cell lines where TA decreases Sp1, Sp3 and Sp4 proteins (data not shown). However, further analysis of protein expression in BT474 or SKBR3 cells treated with TA showed that erbB2 protein expression was decreased (Fig. 2) and these results are consistent with the growth inhibitory effects of TA in cells, where their growth and survival are erbB2-dependent. Herceptin and other classes of drugs that block phospho-erbB2 formation/activation or degrade erbB2 exhibit similar effects on erbB2-dependent downstream responses including decreased phosphorylation of Akt and MAPK, downregulation of cyclin D1 and induction of p27 (16, 27–36). Figures 2C, 2D and Figure 3A illustrate that TA also exhibits an identical pattern of responses in BT474 and SKBR3 cells which is consistent with TA-dependent downregulation of erbB2 protein.
We also compared the effects of TA with other agents that block erbB2 signaling. Tyrosine kinase inhibitors such as ZD1839 may or may not affect erbB2 expression but, in the short term, their effects are primarily on decreased erbB2 phosphorylation (16, 30–33). In contrast, ansamycins, proteasome inhibitors such as bortezomib, and FAS synthase inhibitors all decrease erbB2 protein expression in BT474 and/or SKBR3 cells (21–23, 27–29, 34–36), and similar responses were observed for TA (Figs. 2C and 2D). However, in contrast to FAS inhibitors (36), TA did not induce PEA3 which inhibits erbB2 expression at the transcriptional level (Fig. 4C). Ansamycins such as geldanamycin induce proteasome dependent degradation of erbB2 (21–23, 27), whereas TA-induced repression of erbB2 protein was proteasome-independent (Fig. 3A). Interestingly, geldanamycin, the proteasome inhibitor bortezomib (Valcade), and the reversible tyrosine kinase inhibitor CI-1033 decrease erbB2 expression and this is associated, in part, with intracellular localization of erbB2 from the cell membrane and this process is related to the subsequent decrease in erbB2 protein (27, 28). In contrast, treatment with TA did not induce translocation of cell membrane erbB2 into the cell (Fig. 5), indicating that the mechanism of TA-dependent downregulation of erbB2 is different from these classes of drugs.
TA clearly affected erbB2 transcription and decreased erbB2 mRNA levels (Fig. 3C) and promoter activity in BT474 and SKBR3 cells transfected with the perbB2 constructs that contained a −0.5 kB promoter insert (Fig. 3D). The protein synthesis inhibitor cycloheximide did not affect TA-induced repression of erbB2 mRNA levels (Fig. 4A), suggesting that an induced "inhibitory" protein was not involved. These results were consistent with the failure of TA to induce PEA3 (Supplemental Fig. 4) which inhibits erbB2 expression (24, 25) and plays a role in the reported downregulation of erbB2 gene expression by inhibitors of FAS (34–36). A previous study showed that YY1 and AP-2 transcription factors cooperatively regulate erbB2 expression in BT474 and other cancer cell lines (26). Figure 4C illustrates that TA decreased expression of YY1 and AP-2 transcription factors in both BT474 and SKBR 3 cells after treatment for 48 hr, and this response was not reversed by the proteasome inhibitor lactacystin. The role of AP-2 in basal expression of erbB2 in BT474 cells is illustrated in Figure 4D showing that overexpression of AP-2 activates the erbB2 promoter. Thus, TA-induced downregulation of erbB2 protein and mRNA levels was due to the effects of this compound on AP-2 and YY1 expression in both cell lines, and decreased erbB2 mRNA stability (Fig. 4B) also contributed to these effects in BT474 cells. It is also possible that TA affects expression of other factors in BT474 and SKBR3 cells that decrease erbB2 transcription and these are currently being investigated.
The effects of TA on SKBR3 and BT474 cell growth and on erbB2 in vitro were also observed in athymic nude mice injected with BT474 cells into mammary fat pads (Fig. 6). TA decreased tumor growth and weight and downregulated erbB2 protein and erbB2-dependent responses (phospho-MAPK). TA also decreased immunostaining of both AP-2 and YY-1 in TA-treated tumors (compared to corn oil-treated) and these in vivo results complemented the cell culture studies. Thus, the anticarcinogenic activity of TA is associated with downregulation of Sp transcription factors in some cell lines (18– 20) and repression of the oncogene erbB2 in breast cancer cell lines overexpressing this oncogene. The mechanisms of action of TA are, in part, cell context-dependent since this NSAID decreases erbB2 mRNA stability in BT474 and not SKBR3 cells and this may involve differential effects on factors that control mRNA stability (Fig. 6D). However, the critical TA-dependent effects in both cell lines involves downregulation of YY1 and AP-2 (which regulate erbB2 expression), whereas proteasome-dependent degradation of erbB2, induction of a repressor such as PEA3 or enhanced erbB2 endocytosis are not involved in downregulation of erbB2 by TA (Fig. 6D). The mechanisms of TA-induced repression of YY-1 and AP-2 and the potential clinical applications for TA in treatment of erbB2 overexpressing cancers are currently being investigated.
Supplementary Material
Acknowledgments
Financial Support: This research was supported by the National Institutes of Health (ES09106) and the Texas Agricultural Experiment Station.
REFERENCES
- 1.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Glass AG, Lacey JV, Jr, Carreon JD, Hoover RN. Breast cancer incidence, 1980–2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst. 2007;99:1152–1161. doi: 10.1093/jnci/djm059. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 5.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 6.Semiglazov VF, Semiglazov VV, Dashyan GA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110:244–254. doi: 10.1002/cncr.22789. [DOI] [PubMed] [Google Scholar]
- 7.Howell A, Pippen J, Elledge RM, et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma: a prospectively planned combined survival analysis of two multicenter trials. Cancer. 2005;104:236–239. doi: 10.1002/cncr.21163. [DOI] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: patient-level metaanalysis of randomised trials. Lancet. 2008;371:29–40. doi: 10.1016/S0140-6736(08)60069-0. [DOI] [PubMed] [Google Scholar]
- 9.Buzdar AU. Advances in endocrine treatments for postmenopausal women with metastatic and early breast cancer. Oncologist. 2003;8:335–341. doi: 10.1634/theoncologist.8-4-335. [DOI] [PubMed] [Google Scholar]
- 10.Hobday TJ, Perez EA. Molecularly targeted therapies for breast cancer. Cancer Control. 2005;12:73–81. doi: 10.1177/107327480501200202. [DOI] [PubMed] [Google Scholar]
- 11.Moulder S, Hortobagyi GN. Advances in the treatment of breast cancer. Clin Pharmacol Ther. 2008;83:26–36. doi: 10.1038/sj.clpt.6100449. [DOI] [PubMed] [Google Scholar]
- 12.Demonty G, Bernard-Marty C, Puglisi F, Mancini I, Piccart M. Progress and new standards of care in the management of HER-2 positive breast cancer. Eur J Cancer. 2007;43:497–509. doi: 10.1016/j.ejca.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Harari D, Yarden Y. Molecular mechanisms underlying erbB2/HER2 action in breast cancer. Oncogene. 2000;19:6102–6114. doi: 10.1038/sj.onc.1203973. [DOI] [PubMed] [Google Scholar]
- 14.Nunes RA, Harris L. HER-2: Therapeutic implications for breast cancer. J Women's Cancer. 2002;4:159–170. [Google Scholar]
- 15.Amar S, Moreno-Aspitia A, Perez EA. Issues and controversies in the treatment of HER2 positive metastatic breast cancer. Breast Cancer Res Treat. 2008;109:1–7. doi: 10.1007/s10549-007-9636-2. [DOI] [PubMed] [Google Scholar]
- 16.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- 17.Tfelt-Hansen P, McEwen J. Nonsteroidal inflammatory drugs in the acute treatment of migraine. In: Olesen J, Tfelt-Hansen P, Welch KMA, editors. The Headaches 2. Philadelphia, PA: Lippincott-Raven; 2000. pp. 391–397. [Google Scholar]
- 18.Abdelrahim M, Baker CH, Abbruzzese JL, Safe S. Tolfenamic acid and pancreatic cancer growth, angiogenesis, and Sp protein degradation. J Natl Cancer Inst. 2006;98:855–868. doi: 10.1093/jnci/djj232. [DOI] [PubMed] [Google Scholar]
- 19.Abdelrahim M, Baker CH, Abbruzzese JL, et al. Regulation of vascular endothelial growth factor receptor-1 (VEGFR1) expression by specificity proteins 1, 3 and 4 in pancreatic cancer cells. Cancer Res. 2007;67:3286–3294. doi: 10.1158/0008-5472.CAN-06-3831. [DOI] [PubMed] [Google Scholar]
- 20.Abdelrahim M, Smith R, III, Burghardt R, Safe S. Role of Sp proteins in regulation of vascular endothelial growth factor expression and proliferation of pancreatic cancer cells. Cancer Res. 2004;64:6740–6749. doi: 10.1158/0008-5472.CAN-04-0713. [DOI] [PubMed] [Google Scholar]
- 21.Miller P, DiOrio C, Moyer M, et al. Depletion of the erbB-2 gene product p185 by benzoquinoid ansamycins. Cancer Res. 1994;54:2724–2730. [PubMed] [Google Scholar]
- 22.Mimnaugh EG, Chavany C, Neckers L. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J Biol Chem. 1996;271:22796–22801. doi: 10.1074/jbc.271.37.22796. [DOI] [PubMed] [Google Scholar]
- 23.Munster PN, Marchion DC, Basso AD, Rosen N. Degradation of HER2 by ansamycins induces growth arrest and apoptosis in cells with HER2 overexpression via a HER3, phosphatidylinositol 3'-kinase-AKT-dependent pathway. Cancer Res. 2002;62:3132–3137. [PubMed] [Google Scholar]
- 24.Xing X, Wang SC, Xia W, et al. The ets protein PEA3 suppresses HER-2/neu overexpression and inhibits tumorigenesis. Nat Med. 2000;6:189–195. doi: 10.1038/72294. [DOI] [PubMed] [Google Scholar]
- 25.Shepherd TG, Kockeritz L, Szrajber MR, Muller WJ, Hassell JA. The pea3 subfamily ets genes are required for HER2/Neu-mediated mammary oncogenesis. Curr Biol. 2001;11:1739–1748. doi: 10.1016/s0960-9822(01)00536-x. [DOI] [PubMed] [Google Scholar]
- 26.Begon DY, Delacroix L, Vernimmen D, Jackers P, Winkler R. Yin Yang 1 cooperates with activator protein 2 to stimulate ERBB2 gene expression in mammary cancer cells. J Biol Chem. 2005;280:24428–24434. doi: 10.1074/jbc.M503790200. [DOI] [PubMed] [Google Scholar]
- 27.Citri A, Alroy I, Lavi S, et al. Drug-induced ubiquitylation and degradation of ErbB receptor tyrosine kinases: implications for cancer therapy. EMBO J. 2002;21:2407–2417. doi: 10.1093/emboj/21.10.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marx C, Yau C, Banwait S, et al. Proteasome-regulated ERBB2 and estrogen receptor pathways in breast cancer. Mol Pharmacol. 2007;71:1525–1534. doi: 10.1124/mol.107.034090. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen NM, Madshus IH, Haslekas C, Stang E. Geldanamycin-induced down-regulation of erbB2 from the plasma membrane is clathrin dependent but proteasomal activity independent. Mol Cancer Res. 2008;6:491–500. doi: 10.1158/1541-7786.MCR-07-0191. [DOI] [PubMed] [Google Scholar]
- 30.Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61:8887–8895. [PubMed] [Google Scholar]
- 31.Moasser MM, Basso A, Averbuch SD, Rosen N. The tyrosine kinase inhibitor ZD1839 ("Iressa") inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 2001;61:7184–7188. [PubMed] [Google Scholar]
- 32.Barbacci EG, Pustilnik LR, Rossi AM, et al. The biological and biochemical effects of CP-654577, a selective erbB2 kinase inhibitor, on human breast cancer cells. Cancer Res. 2003;63:4450–4459. [PubMed] [Google Scholar]
- 33.Jani JP, Finn RS, Campbell M, et al. Discovery and pharmacologic characterization of CP-724,714, a selective erbB2 tyrosine kinase inhibitor. Cancer Res. 2007;67:9887–9893. doi: 10.1158/0008-5472.CAN-06-3559. [DOI] [PubMed] [Google Scholar]
- 34.Menendez JA, Mehmi I, Verma VA, Teng PK, Lupu R. Pharmacological inhibition of fatty acid synthase (FAS): a novel therapeutic approach for breast cancer chemoprevention through its ability to suppress Her-2/neu (erbB-2) oncogene-induced malignant transformation. Mol Carcinog. 2004;41:164–178. doi: 10.1002/mc.20054. [DOI] [PubMed] [Google Scholar]
- 35.Menendez JA, Vellon L, Mehmi I, et al. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc Natl Acad Sci U S A. 2004;101:10715–10720. doi: 10.1073/pnas.0403390101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menendez JA, Vellon L, Lupu R. Antitumoral actions of the anti-obesity drug orlistat (Xenical™) in breast cancer cells: blockade of cell cycle progression, promotion of apoptotic cell death and PEA3-mediated transcriptional repression of Her2/neu (erbB-2) oncogene. Ann Oncol. 2005;16:1253–1267. doi: 10.1093/annonc/mdi239. [DOI] [PubMed] [Google Scholar]
- 37.Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein (Sp) transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.