Abstract
Objective
Extend evidence suggesting that essential hypertension influences neuropsychological performance and that brain function prior to treatment is related to the success of pharmacological lowering of blood pressure (BP).
Methods
A voxel-based examination of the whole brain was conducted among forty-three hypertensive patients treated for one year with assessment pre and post treatment using positron emission tomography (PET) and neuropsychological testing.
Results
Neuropsychological performance improved over the year of treatment, but was unrelated to change in regional cerebral blood flow (rCBF). Neither mean resting rCBF nor responsivity to a working memory task changed significantly with treatment. However, patients with greater lowering of systolic BP during treatment showed increased rCBF responsivity to a working memory task in medial and orbital frontal areas, and decreased rCBF responsivity in mid frontal, parietal, thalamus, and pons (as well as lower thalamic rCBF pre-treatment). Improved working memory performance over the treatment period was related to decreased responsivity in medial frontal, medullary, and parietal areas. Patients showing greater lowering of BP with treatment appeared to reduce excitatory and enhance inhibitory coupling between memory processing and BP more than those with less treatment success.
Conclusion
Degree of treatment success for both blood pressure and cognitive performance among hypertensives is related to differing patterns of rCBF. Overall, the results emphasize the relevance of brain function to the treatment of hypertension.
Keywords: Positron emission tomography (PET), hypertension, pharmacological treatment, working memory
An interest in brain function in association with hypertension was initiated by findings that hypertension, particularly if present in middle age and untreated, is associated with mild neuropsychological deficits (1). Pharmacologic treatment for hypertension generally improves or maintains neuropsychological function although this result has been less consistent (1–5) and it remains difficult to separate pharmacologic effects on the brain from effects mediated directly through systemic BP reduction (4) (2) (1).
Understanding changes in cognitive function with hypertension and its treatment should be advanced by examining brain function more directly than in neuropsychological testing. Our prior work comparing normotensive and hypertensive participants showed comparable resting regional cerebral blood flow (rCBF) whereas during working memory tasks, untreated hypertensive participants manifested lower but more widespread rCBF responsivity (6). Although the effects of hypertension on the brain are typically thought to occur only late in the disease progression, early stage hypertension has been related physiologically to processes in brain areas such as the hypothalamus and medulla and functionally to increases in the cardiovascular response to psychological stress (7). If altered brain function is implicated in early hypertension (8), then structural and functional brain indices might indicate the severity of the disease, and through this relate to treatment success. Substantial and increasing evidence suggests significant representation and control of the vasculature within the brain (9). For example, baroreceptor control of BP is well recognized and functions via brain stem areas that adjust neural control to maintain a relatively constant BP. Hypertensive disease and its treatment alter BP levels and as such would seem to necessarily interact with the intrinsic central regulation of BP. Indeed both pre-treatment indices of brain aging (e.g., ventricular and sulcal volumes, white matter hyperintensities) as well as the thalamic response to a working memory task have been found to relate to degree of treatment success with a year of standard pharmacological treatment of BP (10). This report from our laboratory was based on a limited sample with quantitative PET findings derived from a small set of specific regions of interest.
The current aim is to extend our prior report by examining a somewhat expanded sample using relative measures of blood flow and coverage of the whole brain. A voxel based approach using Statistical Parametric Mapping (SPM) was applied with an analysis focused on regions of interest that were drawn from regions previously found to show differences between hypertensives and normotensives (6, 11) and/or known to be brain regions related to control of autonomic function (9).
We had two sets of hypotheses. The first set was based on the assumption that treatment with an angiotensin converting enzyme inhibitor (ACE-I) was expected to alter rCBF more than treatment with a beta blocker based on the former’s efficacy in reversing the vascular remodeling that occurs with hypertension (12–17). We suggested that the ACE-I would improve both rCBF and performance more than the beta blocker. Greater improvement in rCBF response to working memory with the ACE-I was anticipated to occur in areas previously shown to separate hypertensive and normotensive participants: posterior parietal, thalamic, and prefrontal areas (6). Successful treatment with ACE-I should also show greater relative improvement in neuropsychological performance, particularly working memory, in conjunction with improvements in rCBF induced by medication.
The second set of hypotheses focused on individual differences in a) BP response to treatment, b) changes in rCBF responsivity, and c) changes in neuropsychological performance. First, degree of BP reduction should be related to rCBF such that greater BP reduction would associate with pressor areas showing initially low rCBF and pressor areas that respond less to the demands of working memory performance after treatment. Conversely, successful hypertension treatment should correlate with enhanced rCBF responsivity in areas related to the inhibition of pressor areas. Finally, participants showing increases over the course of treatment in rCBF response in areas related to working memory (parietal, frontal, and thalamic) should have improved cognitive performance.
Methods
Participants
Participants were a community sample recruited from the Pittsburgh area via radio, television, newspaper, and health fairs. Participants were between 35 and 65 years of age, and had an average diastolic (5th phase) BP of 90–109 mm Hg, systolic BP of 140–179 mm Hg, or both. Seated BP was assessed after at least 5 minutes rest using the ausculatory technique with a mercury manometer. Baseline BP was calculated from the average of the last 2 of 3 readings done on two occasions. Participants were required to have either no prior pharmacologic treatment for hypertension or no more than 6 months of BP medication within the past 5 years with no BP medication taken in the 6 months preceding enrollment. Detailed inclusion/exclusion criteria for this study and screening outcomes have been previously presented (10). Screening was designed to exclude secondary hypertension, use of drugs/substances interfering with accurate and safe treatment/assessment, and presence of other serious disease, notably coronary or cerebrovascular disease. Participant characteristics were assessed with the Beck Depression Inventory (18), the PANAS (19), and the NEO-FFI (20, 21). The University of Pittsburgh Institutional Review Board approved all procedures as consistent with ethical principles and subjects provided informed consent.
Design
Initial BP assessment, medical screening and history, physical examination, administration of self-report questionnaires, and detailed consent followed phone screening. The next session included the second BP assessment and neuropsychological examination. Separate sessions followed for brachial artery ultrasound, MRI, and PET examinations. All examinations were repeated after 1 year except for the screening and self-report instruments. Data were collected between October 2001 and April 2006. This report focuses on the 43 of the 45 participants who completed the entire study. Thirty-six other participants signed consent forms, but then withdrew due to a failure to pass medical screening, a change in interest in the study, or claustrophobia (see 17). Participants were similar to non-completing individuals in age, education, and personality factors; they differed in that continuing participants were significantly (Chi-square p<.05) more likely to be male, white, and married.
Medication procedures
Patients were treated for 1 year within a randomized, double-blind design employing either lisinopril or atenolol—a choice of drugs based on the initial hypothesis that lisinopril would favorably affect cerebral vasculature relative to atenolol (6). During a 6-week titration phase, drug dosage was gradually increased across 4 dosage levels: 10, 20, 30, and 40 mg for lisinopril and 25, 50, 75, and 100 mg for atenolol. Upward titration stopped if a participant’s BP had fallen to 135/85 mm Hg or less, or if resting pulse fell below 50 beats per minute. If a participant’s BP remained greater than 140/90 mm Hg on the full dose treatment, 12.5 mg hydrochlorothiazide was added (4 in lisinopril group and 7 in beta-blocker group). Participants were withdrawn from the study if BP averaged > 160/95 on two consecutive visits during the maintenance phase (Week 6 through the final visit at Week 52). During visits, physical symptoms and impact of hypertension on quality of life were assessed with the Bulpitt Hypertension Questionnaire (22, 23) and adherence was estimated based upon returned pill counts. The hypertension treatment effect was calculated from standardized BP assessment performed during the last regular clinical visit (approximately week 40).
Structural MRI Measures
All subjects underwent a structural MRI using a GE Signa 1.5 Tesla scanner (Milwaukee, WI) to provide a detailed image for mapping PET results. A brief scout T1-weighted image was followed by an axial series oriented to the plane along anterior and posterior commissures: fast spin echo T2 weighted (effective echo time [TE]/repetition time [TR]= 102/2,500 milliseconds, 1 excitation), proton density weighted (effective TE/TR = 17/2,000 milliseconds, 1 excitation), and fast fluid-attenuated inversion recovery, FLAIR (effective TE/TR = 56/9,002 milliseconds, time to inversion. 2,200 milliseconds, 1 excitation) (24). Section thickness was 5 mm with a 1-mm intersection gap. A field of view of 24 cm and image matrix of 256 by 192 pixels were used for all axial MR series. A volumetric spoiled gradient-recalled (SPGR) sequence with parameters optimized for maximal contrast among gray matter, white matter, and spinal fluid was acquired for purposes of ROI placement in the coronal plane (TE/TR = 5/25 milliseconds, flip angle 40°, 1 excitation, slice thickness 1.5 mm, 0-mm interslice). Participants showing evidence of significant lacunar or other infarcts (n=2) were excluded from participation.
Functional PET Measures
Nine PET scans were performed that tested rCBF responses during information processing (visuomotor and working memory tasks), a rest period, and following administration of acetazolamide. This report focuses on the 6 scans related to working memory. Each task was administered twice and used the same display and two button responses. The Control task required the subject to respond with the thumb to any letter appearing on the left of the screen and with the index finger for a letter appearing on the right. The One-back Memory task required the subject to respond with the thumb to occurrence of a letter on the current presentation that was in the same spatial position as a letter on the immediately preceding presentation and respond with the index finger if it did not. The Two-back Memory task required the subject to respond with the thumb if spatial position of the letter currently appearing matched the spatial position which appeared two presentations earlier and the index finger if it did not. Each task lasted 5 minutes (approximately 150 presentations) and started 2 minutes prior to tracer injection. Task order was randomized with approximately 5 minute inter-task intervals during which the subjects rested and the next tracer injection was prepared for delivery (see prior paper for further description (10)). PET scans were acquired in three-dimensional imaging mode using a Siemens/CTI ECAT HR+ PET scanner (63 transaxial planes, 2.4-mm slice thickness). PET data were corrected for radioactive decay, photon attenuation, and scatter (25). Data were reconstructed using filtered back projection and the final image resolution was 6 mm (transverse and axial). Head motion was minimized using a thermoplastic mask. A peripheral vein catheter was inserted for tracer injections and a radial artery catheter for arterial blood sampling. An initial 10-minute transmission scan was performed using rotating 68Ge/68Ga rods (attenuation correction). Nine millicuries of [15O]water was injected as a rapid bolus using an automated injector system. A 180-second emission scan was acquired in 20 sequential frames (10×3 sec, 3×10 sec, 4×15 sec, and 3×20 sec), followed by a 5-minute rest period for a total of 8 minutes between injections. All PET images were corrected for small head movements using Automated Image Registration (26) which was also used in a modified form to align and center the PET data (27).
Assessing rCBF
The [15O]water 1 minute radiation uptake data were analyzed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/). Individual data were aligned between scans and registered to their SPGR anatomical MRI. Results were then co-registered to the Montreal Neurological Institute T1 standard image and images were smoothed with a 12 mm full width half height filter. Individual data were then submitted to a first level analysis which identified voxels showing activation responsive to a working memory load vector that weighted the control task -1, the one-back memory task 1 and the two-back task 2 and the difference in response to this vector at post as compared to pre-treatment testing. Data were expressed as a proportion of total radiation assessed, i.e., relative blood flow measures of rCBF. Results of the level 1 comparison were then tested at a second level comparing individuals differing in treatment condition, degree of success in lowering BP or performance on the memory tasks. Results were examined using a statistical significance of p<.001, uncorrected, but statistical significance was accepted using a corrected (p<.05) significance based on the correction from random field theory (see discussion and references, http://www.fil.ion.ucl.ac.uk/spm/doc/biblio/Keyword/RFT.html). Results were tested first using a whole brain analysis followed by regions of interests (ROIs) including: posterior parietal and thalamus, previously shown to differentiate between hypertensive participants and normotensive controls (28); medial frontal, orbital frontal, insula, anterior cingulate, mid brain, and limbic lobe, areas drawn from the central autonomic network (9), and; dorsolateral prefrontal and posterior parietal cortices, areas related to working memory. The Wake Forest Pick Atlas was used to identify these ROI’s (29, 30).
Analyses of the rCBF responses employing SPM at the second level assessed the impact of medication, change in systolic BP, and change in neuropsychological performance on the change in rCBF response to 2 back working memory over the 1 year treatment period. Whole brain analyses were followed up with tests confined to the pre-defined ROI’s detailed above. These analyses were performed for resting rCBF prior to treatment, rCBF response during working memory prior to treatment, and change in rCBF response from pre to post treatment.
Assessing Performance
A 1.5 to 2 hour battery of neuropsychological tests was administered both prior to after treatment. The battery was designed to emphasize memory and executive functions previously related to hypertension (31–33). Standard tests with known and acceptable reliability and validity were drawn from the Wechsler Adult Intelligence Scale, III (Object Assembly, Block Design, Digit Symbol, Grooved Pegboard, and Verbal Fluency) and the Wechsler Memory Scale III (Story Recall, Faces, Verbal Paired Associates, Family Pictures, Letter-Number Sequencing, Spatial Span). In addition, the Trail Making test, Four Word Short Term Memory, and National Adjusted Reading Tests were administered. Neuropsychological data were reduced to four factors in order to control the number of comparisons required. Varimax rotated factor analysis was performed with pre-treatment data, which had previously been corrected for any differences between the two neuropsychology examiners via regression. Summary neuropsychological measures from pre-treatment testing, 22 variables, were correlated for 79 participants (the number available prior to participant withdrawals at time of initial brain imaging). Four factors had eigen values over 1 and together accounted for 68% of the variance. Variables loading >=.7 (absolute value) were transformed to z-scores and combined with unit weighting. The factors were labeled: Verbal Memory (Logical Memory, 1st recall; Auditory Immediate Memory, Auditory Delayed Memory; 4-Word Short Term Memory, and Verbal Paired Associates), Visuospatial Memory (Family Pictures immediate and delayed, Visual Immediate Memory and delayed, and General Memory Index,), Psychomotor Speed (Grooved Pegboard preferred and non-preferred hands), and Spatial Construction (Object Assembly and Block Design). Working memory performance on the n-back tasks tested both in the scanner and in the neuropsychological testing was also examined. Working memory was assessed using an A’ signal detection index derived from the proportion of hit and false alarms observed in the responses of the participants (34, 35).
These cognitive measures were analyzed with a general linear model that included age and estimated IQ as covariates and assessed the within subject effect of pre vs. post treatment and the between subject effect of medication (ACE-Inhibitor vs. Beta Blocker). Factors testing the effect of gender and adding or not adding hydrochlorothiazide were also examined. Due to the small n in specific cells for these latter comparisons, higher order interactions were not examined.
Results
Sample characteristics
Table 1 presents the characteristics of the 43 participants that completed the study. The table depicts a largely white, well-educated, middle-aged sample. Although gender participation was reasonably similar at the time of consent, the table shows that a preponderance of males completed the study. Participants reported low levels of depression and personality characteristics appropriate for their age cohort. The table also documents the success of the medication treatment for these participants.
Table 1.
Characteristics of participants (n=43).
| Mean or N | Standard Deviation or Percent |
|
|---|---|---|
| Age | 52.5 | 6.7 |
| Male Gender | 33 | 77% |
| Body Mass Index | 30.0 | 4.8 |
| Race, white | 38 | 88% |
| Non-smoker | 37 | 86% |
| Less than 1 alcoholic drink per week | 21 | 49% |
| Initial Blood pressure: SBP mmHG |
147.4 | 10.5 |
| DBP mmHG | 95.5 | 8.3 |
| Final Blood pressure: SBP mmHG |
125.7 | 11.5 |
| DBP mmHG | 79.7 | 9.1 |
| Beck Depression Inventory | 4.1 | 4.1 |
| PANUS positive (trait) | 34.0 | 7.8 |
| PANUS negative (trait) | 15.7 | 6.1 |
| NEO Neuroticism | 28.0 | 7.4 |
| NEO Extraversion | 40.7 | 6.4 |
| NEO Openess | 39.4 | 5.6 |
| NEO Agreeableness | 44.0 | 5.30 |
| NEO Conscientiousness | 45.6 | 6.7 |
Direct effects of treatment on rCBF response to working memory
Increasing working memory load elicited the expected rCBF response with maximal change in parietal and prefrontal regions (see Figure 1); however, our initial hypothesis was questioned as no statistically significant change in this response pattern was observed between pre- and post-treatment scans and no even marginally significant effects related to medication were observed (see 17, 36).
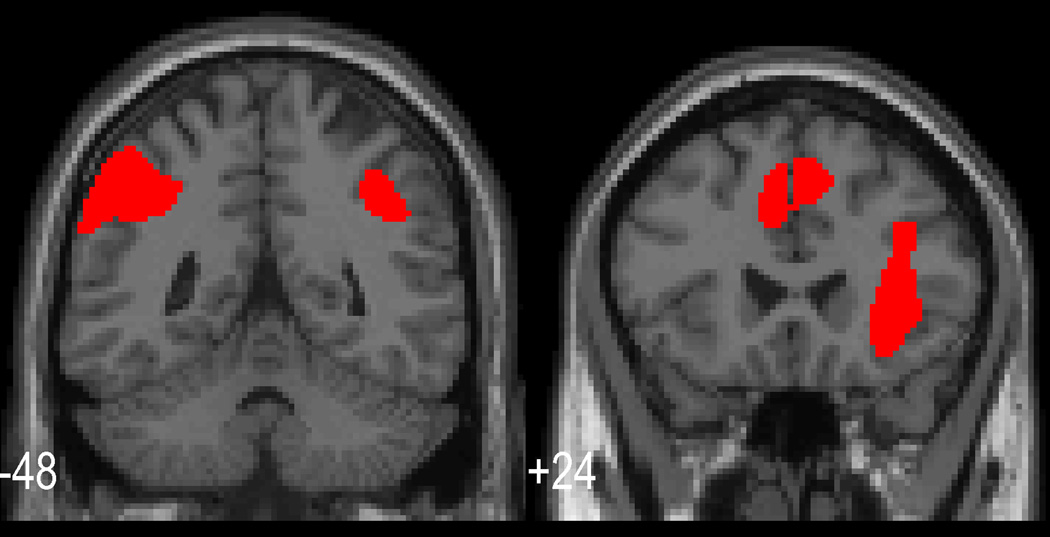
Figure 1.
Cortical response to the vector of increasing working memory load requirements. Coronal slices are shown which illustrate the typical rCBF activations for this task in posterior parietal and mid frontal regions. Additional cingulate activation is also shown. (All area activations, p<.01 fwe corrected.)
Direct effects of treatment on neuropsychological performance
Performance on the neuropsychological tests improved from pre to post test. The overall effect of treatment was significant for the scores of the factors for Verbal Memory (F (1,36) =10.8, p<.01), Visuospatial Memory (F (1,36) = 5.54, p=.02), and Spatial Construction (F (1,36) = 12.8, p<.01). No significant effects were present for the Psychomotor factor. Table 2 presents the overall treatment effects for the four factors. No significant changes due to treatment were observed in the working memory performance scores from the working memory tasks performed in the scanner (pre-treatment for 2-back memory, a’=.89(standard error, .02); post-treatment a’=.91 (standard error, .02)).
Table 2.
Pre and post treatment scores for the neuropsychological factors. Means are presented in z-score units accompanied by the standard error of the mean. Positive scores indicate better performance.
| Variable | Pre | Post |
|---|---|---|
| Verbal Memorya | −.17 (.13) | .10 (.12) |
| Visuospatial Memorya | −.06 (.15) | .23(.15) |
| Psychomotor (time scores) | −.11(.10) | −.18 (.12) |
| Spatial Constructiona | .00 (.16) | .25 (.16) |
p< .05 or smaller, see text. Effect size (Cohen’s D) as derived from the t-test value for significant effects range from .64 to 1.07 (56).
The results, therefore, also cast doubt on the second hypothesis. Although a general increase in neuropsychological performance occurred, working memory performance did not improve and the mean performance changes that were observed were not accompanied by mean changes in rCBF. The four neuropsychological composite/factor scores failed to relate strongly to mean pre-treatment or post-pre treatment resting or responsive rCBF. No areas met whole brain levels of significance. In addition, change in cognitive performance was unrelated to decline of BP during treatment or to changes in BP reactivity during task performance.
Individual differences in response to hypertension treatment in relation to rCBF
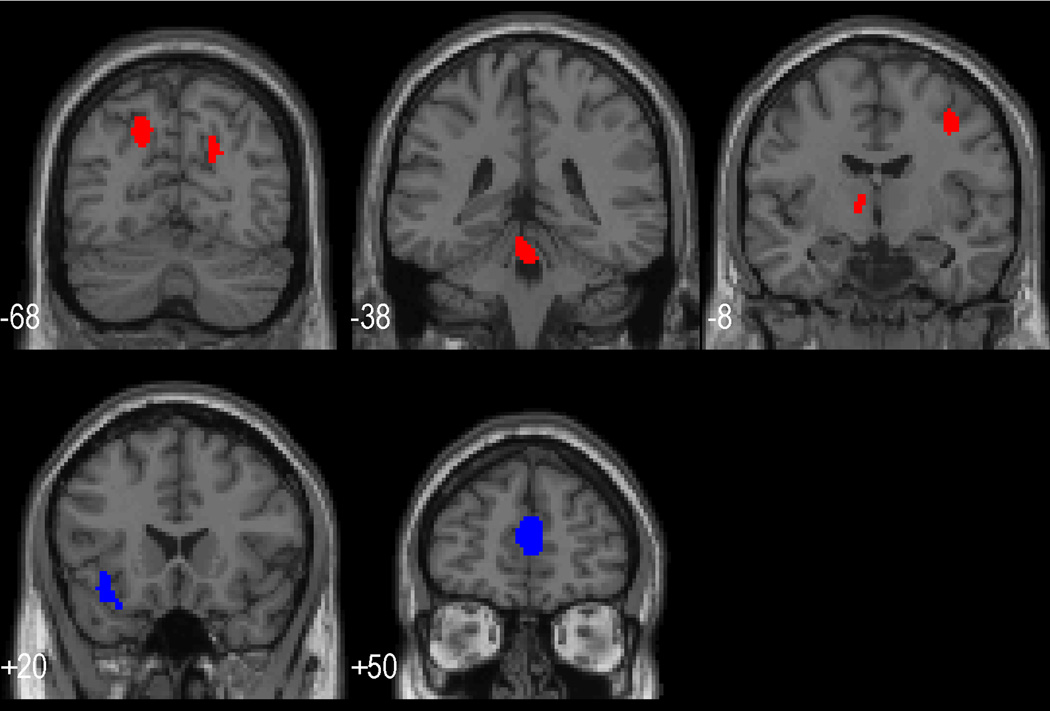
Our second set of hypotheses asked whether differences between individuals in brain indices and their response to treatment related to the success of blood pressure treatment or change in cognitive function with treatment. Table 3 presents areas in which a greater response to working memory post treatment, relative to pre-treatment, related to a better BP response to antihypertensive therapy (i.e. a greater drop in systolic BP). Table 3 indicates that this relationship was present in areas related to autonomic control: anterior cingulate, medial frontal, and orbital frontal areas. Resting rCBF prior to treatment showed no significant relationships with improvements in BP with treatment. Table 4 presents areas in which a greater response to working memory post treatment, relative to pre treatment, or greater pre-treatment rCBF was related to a poorer response to antihypertensive therapy (i.e., a lesser drop in systolic BP). Both greater thalamic resting rCBF pre treatment and greater thalamic rCBF increase after treatment in the response to working memory were related to a relatively smaller decrease in clinical BP with treatment. Similarly, a lesser BP decrease was related to a greater responsivity after treatment to working memory in middle frontal and posterior parietal regions, areas robustly related to working memory performance, as well as in the pons. Figure 2 presents saggital slices of the brain which illustrate the areas related to greater BP decrease (indicated in blue) and areas related to a smaller BP decrease (indicated in red). Note that pre-treatment responses during working memory were not associated with BP response to treatment.
Table 3.
Areas in which increases in the rCBF response to working memory after relative to before treatment related to greater BP reduction with treatment.
| Area | MNI coordinates | Extent (voxels) | t-value | p (fwe-corrected) |
|---|---|---|---|---|
| Right and Left | 2 50 12 | 308 | 5.43 | .03 (whole brain) |
| Medial Frontal (BA10,32) |
||||
| Left Orbital | −30 20 −24 | 265 | 4.40 | .04 (ROI) |
| Frontal (BA47) | −38 30 −10 |
Note. BA refers to Brodman’s Area, MNI is Montreal Neurological Institute and refers to the location in x, y, and z coordinates on the reference brain from that institute. fwe refers to the correction for multiple testing based on random field theory (see description and references, http://www.fil.ion.ucl.ac.uk/spm/doc/biblio/Keyword/RFT.html). p values are corrected based on the number of whole brain voxels or if not significant for the whole brain within predefined regions of interest (ROI). Effect sizes (Cohen’s D) derived from t-value range from 1.36 to 1.67 (56).
Table 4.
Areas in which greater rCBF response to working memory post relative to pre treatment or resting rCBF related to smaller Hypertension treatment response.
| Resting rCBF pre-Treatment | ||||
|---|---|---|---|---|
| Area | MNI coordinates | Extent (voxels) | t-value | p (fwe-corrected) |
| Thalamus | 6 −4 −4 | 247 | 4.53 | p<.01 (ROI) |
| Change in rCBF Response to Working Memory with pharmacological treatment | ||||
|---|---|---|---|---|
| Area | MNI coordinates | Extent (voxels) | t-value | p (fwe-corrected) |
| Right Middle | 38 −6 46 | 108 | 5.52 | .03 (whole brain) |
| Frontal Gyrus (BA6) |
||||
| Pons | 0 −38 −20 | 226 | 5.43 | .03 (whole brain) |
| Left and Right | −16 −68 42 | 195 | 4.81 | .02 (ROI) |
| Posterior Parietal, Precuneus (BA7) |
20 −68 30 | 73 | ||
| Thalamus | −10 −8 2 | 24 | 3.89 | .03 (ROI) |
Figure 2.
Coronal slices with superimposed areas of significant rCBF activation. Areas shown in blue indicate that greater activation post relative to pre treatment was related to a relatively better BP response to treatment (see Table 3); while areas shown in red indicate areas in which lesser activation post relative to pre treatment were related to better Hypertension treatment success (see Table 4).
Individual differences in performance changes with treatment in relation to rCBF
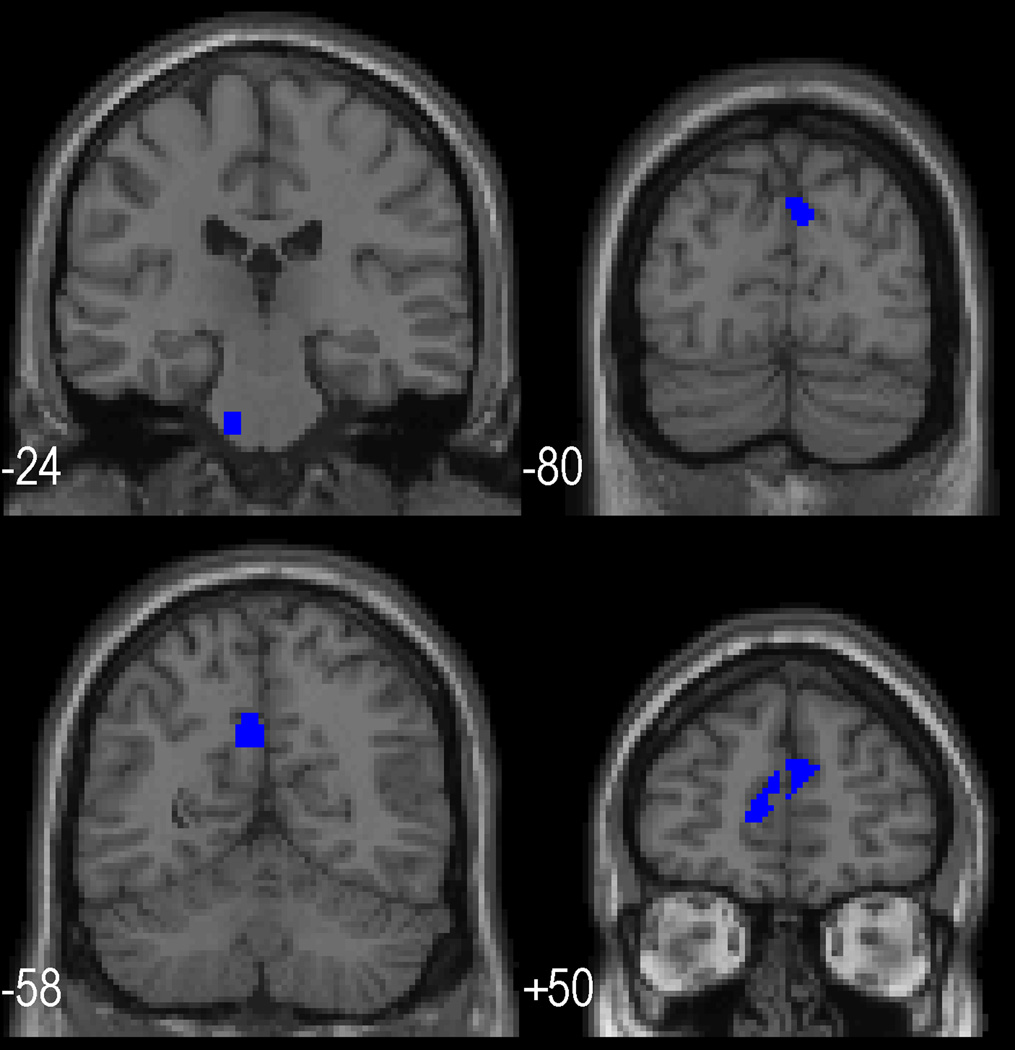
The hypothesis of positively correlated change in cognitive function and rCBF was not supported, but specific regions did show an inverse relationship between performance and change in rCBF. Table 5 shows regions in which an increase in the rCBF response to working memory in post relative to pre treatment was related to a decrease in performance on the task during scanning. No regions were identified in which baseline rCBF, pre-treatment rCBF response or change in response to the working memory task related to improved performance. Relatively poorer performance on the working memory task post treatment was related to a relatively greater rCBF response post treatment in both anterior brain areas (medial frontal gyrus) and posterior areas (precuneus, cuneus) as well as in the medulla. Figure 3 illustrates (indicated in blue) on saggital slices these areas related to relatively poorer performance.
Table 5.
Regions in which increases in the rCBF response to working memory pre to post treatment were associated with a decline in working memory performance during scanning
| Area | MNI coordinates | Extent (voxels) | t-value | p (fwe-corrected) |
|---|---|---|---|---|
| Right and Left | 4 50 20 | 240 | 4.90 | .01 (ROI) |
| Medial Frontal Gyrus (BA9, 10) |
−6 50 10 | 11 | ||
| Left Medulla | −12 −22 −40 | 44 | 5.18 | .003 (ROI) |
| Right and Left | −4 −58 34 | 108 | 4.58 | .04 (ROI) |
| Precuneus (BA7), Cuneus (BA19) |
6 −80 34 | 40 |
Note. Effect sizes (Cohen’s D) derived from t-values range from 1.42 to 1.51 (56).
Figure 3.
Coronal slices with superimposed areas of significant rCBF activation. Areas shown in blue were those in which relatively decreased activation post relative to pre was associated with an improvement over treatment in performance on the working memory task (see table 5).
Joint relationship with performance and treatment response for CBF response in medial frontal cortex
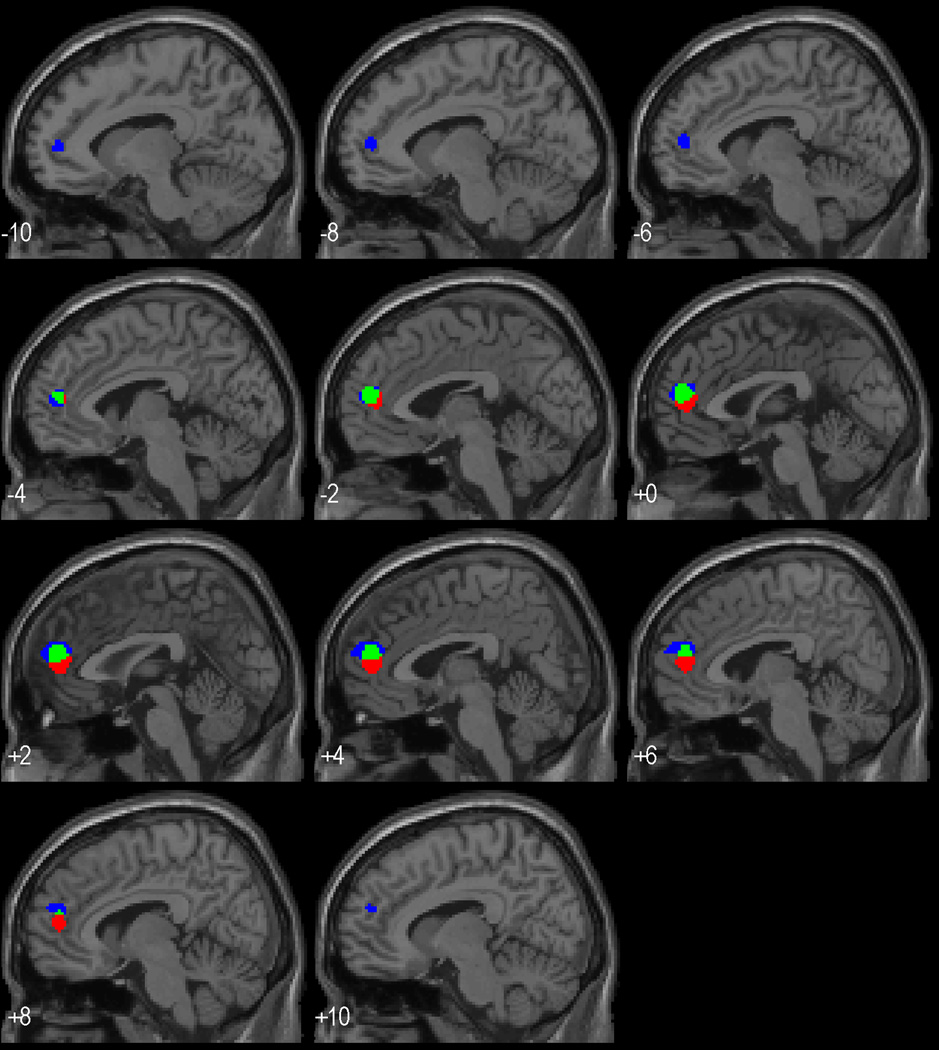
As noted, pre- to post-treatment change in the medial frontal cortex response to working memory related both to the success of hypertension treatment and the change in performance on the working memory task. We examined this more closely by repeating each analysis excluding the significant voxels from the other analysis. This approach identified voxels which related to both BP decline and working memory performance and voxels specific to either. Figure 4 shows that the area of overlap is relatively medial and central to the rCBF change related to BP decline, which is itself concentrated more medially than the rCBF change related to performance. The analysis masking out the changes related to treatment success showed a cluster of 236 voxels (relative to the prior cluster of 394 voxels in the unmasked analysis) that remained significant at p<.001 (uncorrected, t=4.4) and were somewhat more left lateralized and rostral to the cluster of overlapping activation. The analysis masking out the changes related to the change in performance showed a cluster of 150 voxels (relative to 308 in the unmasked analysis) that remained significant at p<.001 (uncorrected t=5.3) and were slightly caudal relative to the area showing overlapping activation. Masking either analysis with the rCBF change to working memory prior to treatment showed no overlap of medial prefrontal activation, i.e. the associations with BP lowering and change in performance were not based on voxels that significantly activated during memory processing pre-treatment.
Figure 4.
Saggital slices with superimposed areas activated solely in predicted a better response to BP medication (red areas), activated solely in predicting a poorer improvement in working memory performance over the year of treatment (blue areas), and activated and jointly related to treatment success and less improvement in working memory (green areas).
Treatment course
Compliance with treatment was high (greater than 97% report of compliance with medication) and reported side effects were minimal with the median number of side effects reported over the course of treatment being 1 (sd=1.9). Four patients reported a change in diet, primarily a reduction in salt and 2 reported weight gains of 5 pounds or less. Levels of glucose, sodium, and potassium did not differ pre to post treatment. Six patients did report some depressive mood not requiring a visit to a physician at least at one point during the course of treatment. These reports corresponded to the higher average Beck Depression scores in these participants prior to the start of treatment (mean =7.6 relative to the mean of the other participants mean=3.7, t=2.1, p<.05). Over the course of treatment momentary PANAS negative affect scores declined significantly from a mean of 13 to a mean of 5 (F(1,41)=356.9, p<.001). Overall, these treatment indicators were consistent with successful blood pressure treatment with minimal adverse side effects.
Discussion
Our voxel-based whole brain analysis failed to support our hypotheses concerning direct effects of rCBF and concomitant changes in rCBF and performance, although some support was found for our hypotheses regarding individual differences in BP responses to treatment. Our initial hypotheses were not supported in that treatment did not appear to shift hypertensive brain function toward that observed in normotensive samples. Neuropsychological performance did improve after treatment, but performance on the working memory task performed in the MR scanner did not significantly change. More importantly, we failed to see any increase in the rCBF response to working memory after treatment. Thus, those hypotheses stipulating a concomitant effect of treatment on brain function and performance cannot be accepted. Note that this result is relevant only to the particular treatment employed; neither study medication crossed the blood-brain barrier. Centrally acting medications might yield different results.
The current results did extend our prior results showing that rCBF prior to and during treatment is related to the success of pharmacological lowering of BP among hypertensive patients. We previously reported on quantitative blood flow assessed only in a priori specified ROI’s and found that the magnitude of BP decline with treatment was related to resting rCBF in the thalamus pre-treatment and the change in thalamic responsivity to the working memory task over 1 year of pharmacological treatment (8, 36). The current results analyzed the whole brain with voxel-based, relative measures of rCBF in an expanded sample. We expected that low rCBF and low responsiveness in midbrain and medullary areas known to have pressor effects would relate to the success of hypertension treatment, see reviews (37, 38). This expectation held true for the thalamus and pons, areas known in humans to increase BP when stimulated (39). The thalamus result verified and extended our prior work with quantitative blood flow (36) , and the current whole brain analysis extended the findings to the pons. We also supported the related hypothesis that high responsiveness in areas showing inhibitory influences on pressor increases would relate positively to the success of hypertension treatment. Relatively better response to hypertension treatment was observed among those whose rCBF response during working memory increased after treatment in medial frontal and left orbital frontal rCBF areas. The inhibitory role of these structures on midbrain and medullary circuits has been well documented in electrophysiological and anatomical studies in animals (37, 40, 41), although in humans some areas within these same structures appear to have excitatory effects (38). Note that the sensitivity and spatial resolution of our current PET techniques pose a limit to the extent and specificity of areas localized.
Parietal and middle frontal areas, not typically associated with pressor effects, also showed a relationship between treatment success and relatively lower rCBF responsivity post treatment. These areas are strongly and consistently activated during working memory performance, e.g., (42, 43) and were similarly activated by the working memory task in this data (see Figures 1 and 2, as confirmed by masking analysis eliminating overlap of areas). This finding may relate to the observation that young participants doing well on a task often show relatively smaller activations relative to those doing poorly or older (44, 45). Speculatively, participants with less task activation may then have less advanced effects of hypertension on the brain, change less over treatment, and benefit more from treatment.
Correlations with level of working memory performance during scanning implicated other areas; specifically, improved performance on the working memory task in the scanner was associated with decreases in rCBF response in medial frontal and medullary areas implicated in central autonomic control (37, 38). This may indicate that coordinated control of autonomic activation was less strongly engaged during successful working memory performance post-treatment relative to pre-treatment. Reduced activation in the precuneus was also associated with enhanced performance. This area is significantly activated by working memory task performance (42, 43) and thus, the observed association directly contradicts our hypothesis. An alternative conceptualization, however, considers other observations that suggest that the precuneus is active during rest (i.e., acts as a portion of the so-called default network) and inhibited during performance of a working memory task (46–49). With this conceptualization decreased precuneus activation would be expected to relate to enhanced working memory performance. Clearly though, one must argue for functional or anatomical differences within the precuneus in order to account for observations that areas within the precuneus both increase and decrease during working memory performance.
A striking concordance among our set of findings was that greater responses to working memory after relative to before treatment in the medial frontal cortex were related to better hypertension treatment outcomes but relatively poorer working memory performance. Analyses masking the two analyses with the results of the other suggested a core rCBF area that changed over treatment in response to the working memory task and related to both better treatment response and poorer performance. Relatively more lateral medial frontal cortex areas showed a unique relationship to poorer performance; relatively more rostral rCBF related uniquely to improved hypertension treatment response. The precise role of the overlapping and non-overlapping areas is difficult to interpret at this time given the variability of findings and paradigms for this area.
A thorough quantitative review of the literature with a focus on emotion does seem relevant, however. Kober and colleagues (50) analyzed 162 studies related to affect and used quantitative techniques to assess areas commonly activated as well as areas concomitantly active within the studies. The areas we have observed that relate to treatment success and performance change are clearly encompassed in the area termed dorsomedial prefrontal cortex (dmPFC) in their review. Their mediation analyses based on imaging studies of emotion identify co-activation of dmPFC separately with two areas critical for autonomic control, the hypothalamus and periaqueductal grey/thalamus. Functionally, they interpret the dmPFC as evaluating context and appropriately modulating lower structures related to the expression of affect/motivation. Although the current study did not directly vary affect, the relevance of this dmPFC area and its interpretation is justified first by the close anatomical association with the dmPFC area identified by Kobor et al. (50). Based on 67 activations they center a region of 496 voxels on coordinates −2 51 29), essentially overlapping our area of rCBF activation. Second, the relationships shown to autonomic control are clearly relevant to our focus on adjustment in BP with treatment. Pharmacological decreases in BP might reasonably be assumed to alter how motivational/affective aspects of a task are linked to BP. Based on this assumption, an integrated interpretation may be made to form a working hypothesis for future work. Performance of routine cognitive tasks, such as maintenance of items in working memory, is presumed to induce both rCBF changes required for such maintenance as well as autonomic changes configuring the cardiovascular system to appropriately support the central processing. Relative to baseline activity, heart rate and BP are known to show small increases during such tasks (for review see (51)). Individual differences in the degree to which such changes occur are known to relate prospectively to hypertension (52–54). As suggested in the Kober et al. (50) review, dorsal medial prefrontal cortex as well as orbital frontal cortex evaluate the degree of autonomic support appropriate to the task at hand. Thalamic, hypothalamic and medullary areas then organize the autonomic efferent response. Our findings suggest that treatment alters these task linked pressor responses by increasing prefrontal inhibitory activity and decreasing thalamic/medullary excitatory effects—at least among those showing a relatively better response to treatment. This may concomitantly reduce the motivational commitment to the task—accounting for the relationship with performance scores and possibly, the decreased response in prefrontal and parietal memory related area.
Our interpretation of this study is clearly limited. The initial design compared medications over a year of treatment, but did not include an untreated group. As a result, we are unable to examine the effect of repetition per se of our neuropsychological and PET testing after one year. Within this and most imaging designs, we are only able to observe parallel changes in function and brain state, i.e. such correlational results are not sufficient to infer causation. The two medications that we did examine failed to provide any clear differential influences on performance or rCBF, but other effective antihypertensive medication were not studied and we cannot generalize to their effects. Finally, the arduous experimental protocol led to substantial self-selection of participants, most clearly a bias toward males in participation. This again limits the generalizability of the current results. The reasonably small sample size limited our ability to detect small effects; larger samples could potentially find some support for our initial hypotheses.
Overall, the current results provide further evidence of the relevance of brain function in the disease course and treatment of essential hypertension as recently reviewed (55). Ultimately, therapeutic approaches should be informed by recognition of the linkage between central aspects autonomic nervous system regulation of the vasculature, cognitive function, and hypertension.
Acknowledgments
We acknowledge our support from training and research grants from the National Heart, Lung, and Blood Institutes grants HL057529 (Jennings/Muldoon/Ryan/Meltzer); HL076852/076858 (Jennings); HL040962 (Muldoon/Jennings); and HL07560 (Christie)
Abbreviations
- BP
blood pressure
- PET
positron emission tomography
- rCBF
regional cerebral blood flow
- MRI
magnetic resonance imaging
- SPGR
spoiled gradient-recalled sequence
- ROI
region of interest
- BA
Brodman’s Area
- MNI
Montreal Neurological Institute
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Staessen JA, Richart T, Birkenhager WH. Less atherosclerosis and lower blood pressure for a meaningful life perspective with more brain. Hypertension. 2007;49:389–400. doi: 10.1161/01.HYP.0000258151.00728.d8. [DOI] [PubMed] [Google Scholar]
- 2.Muldoon MF, Waldstein SR, Ryan CM, Jennings JR, Polefrone JM, Shapiro AP, Manuck SB. Effects of six anti-hypertensive medications on cognitive performance. J Hypertens. 2002;20:1643–1652. doi: 10.1097/00004872-200208000-00028. [DOI] [PubMed] [Google Scholar]
- 3.Waldstein SR, Jennings JR, Ryan CM, Muldoon MF, Shapiro AP, Polefrone JM, Fazzari TV, Manuck SB. Hypertension and neuropsychological performance in men: interactive effects of age. Health Psychol. 1996;15:102–109. doi: 10.1037//0278-6133.15.2.102. [DOI] [PubMed] [Google Scholar]
- 4.Muldoon MF, Waldstein SR, Jennings JR. Neuropsychological consequences of antihypertensive medication use. Exp Aging Res. 1995;21:353–368. doi: 10.1080/03610739508253990. [DOI] [PubMed] [Google Scholar]
- 5.Feigin V, Ratnasabapathy Y, Anderson C. Does blood pressure lowering treatment prevents dementia or cognitive decline in patients with cardiovascular and cerebrovascular disease? J Neurol Sci. 2005;229–230:151–155. doi: 10.1016/j.jns.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Gamalo MA, Ombao H, Jennings JR. Comparing extent of activation: a robust permutation approach. Neuroimage. 2005;24:715–722. doi: 10.1016/j.neuroimage.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Joint National Committee on Prevention Detection, Evaluation, and Treatment of High Blood Pressure, National Heart, Lung, and Blood Institute, National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 8.Jennings JR, Christie I, Muldoon M, Price J, Meltzer CC. Thalamic blood flow during rest and working memory differentially predict success of pharmacological treatment for hypertension. Neuroimage. 2009;47:s90. [Google Scholar]
- 9.Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- 10.Esler M, Eikelis N, Schlaich M, Lambert G, Alvarenga M, Dawood T, Kaye D, Barton D, Pier C, Guo L, Brenchley C, Jennings G, Lambert E. Chronic mental stress is a cause of essential hypertension: presence of biological markers of stress. Clin Exp Pharmacol Physiol. 2008;35:498–502. doi: 10.1111/j.1440-1681.2008.04904.x. [DOI] [PubMed] [Google Scholar]
- 11.Jennings JR, Muldoon MF, Ryan CM, Mintun MA, Meltzer CC, Townsend DW, Sutton-Tyrrell K, Shapiro AP, Manuck SB. Cerebral blood flow in hypertensive patients: an initial report of reduced and compensatory blood flow responses during performance of two cognitive tasks. Hypertension. 1998;31:1216–1222. doi: 10.1161/01.hyp.31.6.1216. [DOI] [PubMed] [Google Scholar]
- 12.Baumbach GL, Chillon JM. Effects of angiotensin-converting enzyme inhibitors on cerebral vascular structure in chronic hypertension. J Hypertens - Suppl. 2000;18:S7–S11. [PubMed] [Google Scholar]
- 13.Baumbach GL, Heistad DD. Adaptive changes in cerebral blood vessels during chronic hypertension. J Hypertens. 1991;9:987–991. doi: 10.1097/00004872-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Chillon JM, Baumbach GL. Effects of an angiotensin-converting enzyme inhibitor and a beta-blocker on cerebral arterioles in rats. Hypertension. 1999;33:856–861. doi: 10.1161/01.hyp.33.3.856. [DOI] [PubMed] [Google Scholar]
- 15.Chillon JM, Baumbach GL. Effects of an angiotensin-converting enzyme inhibitor and a beta-blocker on cerebral arteriolar dilatation in hypertensive rats. Hypertension. 2001;37:1388–1393. doi: 10.1161/01.hyp.37.6.1388. [DOI] [PubMed] [Google Scholar]
- 16.Fu CH, Yang CC, Kuo TB. Effects of different classes of antihypertensive drugs on cerebral hemodynamics in elderly hypertensive patients. Am J Hypertens. 2005;18:1621–1625. doi: 10.1016/j.amjhyper.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Jennings JR, Muldoon MF, Whyte EM, Scanlon J, Price J, Meltzer CC. Brain imaging findings predict blood pressure response to pharmacological treatment. Hypertension. 2008;52:1113–1119. doi: 10.1161/HYPERTENSIONAHA.108.120196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 19.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 20.Costa PT, Jr, McCrae RR. Normal personality assessment in clinical practice: The NEO personality inventory. Psychol Assess. 1992;4:5–13. [Google Scholar]
- 21.McCrae RR. A note on some measures of profile agreement. J Pers Assess. 2008;90:105–109. doi: 10.1080/00223890701845104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croog SH, Levine S, Testa MA, Brown B, Bulpitt CJ, Jenkins CD, Klerman GL, Williams GH. The effects of antihypertensive therapy on the quality of life. N Engl J Med. 1986;314:1657–1664. doi: 10.1056/NEJM198606263142602. [DOI] [PubMed] [Google Scholar]
- 23.Bulpitt CJ, Dollery CT. Side effects of hypotensive agents evaluated by a self-administered questionnaire. Br Med J. 1973;3:485–490. doi: 10.1136/bmj.3.5878.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastianello S, Bozzao A, Paolillo A, Giugni E, Gasperini C, Koudriavtseva T, Millefiorini E, Horsfield MA, Colonnese C, Toni D, Fiorelli M, Pozzilli C, Bozzao L. Fast spin-echo and fast fluid-attenuated inversion-recovery versus conventional spin-echo sequences for MR quantification of multiple sclerosis lesions. Am J Neuroradiol. 1997;18:699–704. [PMC free article] [PubMed] [Google Scholar]
- 25.Watson CC, Newport D, Casey ME, DeKemp RA, Beanlands RS. Evaluation of simulation-based scatter correction for 3D PET cardiac imaging. IEEE Trans Nucl Sci. 1997;44 [Google Scholar]
- 26.Woods RP, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Minoshima S, Berger KL, Mintun MA. An automated method for rotational correction and centering of three-dimensional functional brain images. J Nucl Med. 1992;33:1579–1585. [PubMed] [Google Scholar]
- 28.Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, van der Veen FM, Meltzer CC. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005;64:1358–1365. doi: 10.1212/01.WNL.0000158283.28251.3C. [DOI] [PubMed] [Google Scholar]
- 29.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 31.Robbins MA, Elias MF, Elias PK, Budge MM. Blood pressure and cognitive function in an African-American and a Caucasian-American sample: the Maine-Syracuse Study. Psychosom Med. 2005;67:707–714. doi: 10.1097/01.psy.0000171164.50990.80. [DOI] [PubMed] [Google Scholar]
- 32.Waldstein SR, Manuck SB, Ryan CM, Muldoon MF. Neuropsychological correlates of hypertension: review and methodologic considerations. Psychol Bull. 1991;110:451–468. doi: 10.1037/0033-2909.110.3.451. [DOI] [PubMed] [Google Scholar]
- 33.Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension. 2005;45:374–379. doi: 10.1161/01.HYP.0000156744.44218.74. [DOI] [PubMed] [Google Scholar]
- 34.Aaronson D, Watts B. Extensions of Grier's computational formulas for A' and B" to below-chance performance. Psychol Bull. 1987;102:439–442. [PubMed] [Google Scholar]
- 35.Grier J. Nonparametric indexes for sensitivity and bias: Computing formulas. Psychol Bull. 1971;75:424–429. doi: 10.1037/h0031246. [DOI] [PubMed] [Google Scholar]
- 36.Jennings JR, Muldoon MF, Whyte EM, Scanlon J, Price J, Meltzer CC. Brain imaging findings predict blood pressure response to pharmacological treatment. Hypertension. 2008;52:1113–1119. doi: 10.1161/HYPERTENSIONAHA.108.120196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cechetto DF, Shoemaker JK. Functional neuroanatomy of autonomic regulation. Neuroimage. 2009;47:795–803. doi: 10.1016/j.neuroimage.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 38.Gianaros PJ, Sheu LK. A review of neurimaging studies of stressor-evoked blood pressure reactivity: Emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage. 2009;47:922–936. doi: 10.1016/j.neuroimage.2009.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thornton JM, Aziz T, Schlugman D, Paterson DJ. Electrical stimulation of the midbrain increases heart rate and arterial blood pressure in awake humans. J Physiol (Lond) 2002;539:615–621. doi: 10.1113/jphysiol.2001.014621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chalmers J. Volhard Lecture: Brain, blood pressure, and stroke. J Hypertens. 1998;16:1849–1858. doi: 10.1097/00004872-199816121-00002. [DOI] [PubMed] [Google Scholar]
- 41.Resstel LBM, Correa FMA. Involvement of the medial prefrontal cortex in central cardiovascular modulation in the rat. Auton Neurosci: Basic and Clin. 2006;126:130–138. doi: 10.1016/j.autneu.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 42.Gianaros PJ, Greer PJ, Ryan CM, Jennings JR. Higher blood pressure predicts lower regional grey matter volume: Consequences on short-term information processing. Neuroimage. 2006;31:754–765. doi: 10.1016/j.neuroimage.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 44.Gazzaley AH, D'Esposito MD. BOLD functional MRI and cognitive aging. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. New York: Oxford University Press; 2005. pp. 107–131. [Google Scholar]
- 45.Rypma B, D'Esposito MD. Isolating the neural mechanisms of age-related changes in human working memory. Nature Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- 46.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 47.Dohnel K, Sommer M, Ibach B, Rothmayr C, Meinhardt J, Hajak G. Neural correlates of emotional working memory in patients with mild cognitive impairment. Neuropsychologia. 2008;46:37–48. doi: 10.1016/j.neuropsychologia.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Luber B, Kinnunen LH, Rakitin BC, Ellsasser R, Stern Y, Lisanby SH. Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: frequency- and time-dependent effects. Brain Res. 2007;1128:120–129. doi: 10.1016/j.brainres.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 50.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jennings JR, Coles MGH, editors. Handbook of cognitive psychophysiology: Central and autonomic nervous system approaches. Oxford, England: John Wiley & Sons; 1991. [Google Scholar]
- 52.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- 53.Raikkonen K, Matthews KA, Kuller LH. Trajectory of psychological risk and incident hypertension in middle-aged women. Hypertension. 2001;38:798–802. [PubMed] [Google Scholar]
- 54.Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Jennings JR, Zanstra Y. Is the brain the essential in hypertension? Neuroimage. 2009;47:914–921. doi: 10.1016/j.neuroimage.2009.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenthal R. Meta-analytic procedures for social research. Rev ed. Newbury Park: Sage Publications; 1991. [Google Scholar]