Abstract
Dopamine depletion in Parkinson's disease (PD) alters the neuronal activity in basal ganglia circuits. Characterizing these changes in network activity is an important step in understanding the disease and how therapies mitigate symptoms. Non-linear analysis methods can complement the traditional description of neuronal firing characteristics. Here we examine the entropy of subthalamic neurons in PD patients undergoing stereotactic surgery for deep brain stimulation (DBS). The activity of 8 neurons was recorded prior to, during, and following systemic administration of the dopamine agonist apomorphine at clinically effective doses. Apomorphine induced a decrease in entropy measured in the inter-spike intervals of subthalamic neurons in 6 of the 8 neurons. This is the first report that anti-parkinsonian drugs affect non-linear features of neuronal firing in the basal ganglia of parkinsonian patients.
Keywords: nonlinear, firing pattern, interspike interval
The motor signs and symptoms of Parkinson's disease (PD) are in large part caused by the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc), and the resultant loss of dopamine throughout the basal ganglia. As a consequence, neuronal activity is altered throughout the basal ganglia in general, including the subthalamic nucleus (STN).
Changes in STN neuron activity have been described in both rodents and non-human primates after dopamine depletion. There is a large body of evidence suggesting that dopamine depletion increases firing rates in the STN (Albin et al., 1995; DeLong, 1990; Bergman et al., 1994; Kreiss et al., 1997; Wichmann et al., 1994). This is supported by clinical data showing that systemic administration of the dopamine receptor agonist apomorphine leads to a decrease in mean firing rates in the STN of PD patients (Levy et al., 2001). Systemic apomorphine administration also inhibited firing in most STN neurons tested in the 6-hydroxydopamine (6-OHDA) rat model of PD (Kreiss et al., 1997). In addition to time domain analyses that are based on the probability distribution of interspike intervals (ISIs), other studies have characterized the firing activity of STN neurons in PD patients or in animal models of PD using spectral analyses and demonstrated that dopamine depletion leads to the occurrence of periodic patterns in the STN neurons (Bevan, et al., 2002; Brown, 2007; Gatev et al., 2006). Linear analyses look at linear combinations of independent patterns in the data stream, as in, for example, the characterization of the ISI series by the summation of several probability distributions or several frequencies. In addition to these linear analyses, recent studies have also pointed to the presence of random aperiodic fluctuations in the spike trains of basal ganglia neurons (Rodriguez et al., 2003; Darbin et al., 2006; Dorval et al., 2008; Cruz et al., 2009). These patterns are quantified by non-linear analyses such as the approximate entropy (ApEn), a measure of statistical irregularity (Pincus et al. 1991; Pincus and Goldberger, 1994; Pincus and Minkin, 1998; Darbin et al., 2006; Dorval et al., 2008). As explained originally by Pincus (1991), the ApEn gives a measure of the regularity in the pattern of a series. The lower the ApEn, the more regular (and thus predictable) the series is. The higher the ApEn, the more random the series is such that knowing a value at any given time does not help predict the next value in the series. It has since been shown that dopamine depletion decreases entropy in the ISI series of rat globus pallidus neurons (Cruz et al., 2009), and that therapeutic deep brain stimulation (DBS) reduces the entropy in the STN neurons of MPTP-treated monkeys (Dorval et al., 2008).
Although the clinical relevance of these features of neuronal discharge is not yet clear, they may provide new insights into basal ganglia circuitry dysfunction. The aim of the present study was to investigate the effects of apomorphine on the non-linear features of the temporal organization of STN neuronal firing in PD patients. To our knowledge, this is the first report on the effects of therapeutic drug administration on the entropy of basal ganglia neurons in human patients. We report that systemic administration of apomorphine reduces the approximate entropy of the fluctuations in ISI trains recorded from STN neurons.
The data presented in this study were obtained from 8 STN neurons that were recorded in 7 awake patients who underwent surgery for implantation of DBS electrodes in the STN for treatment of advanced PD. All procedures were approved by the University Health Network Ethical Review Board at the University of Toronto. Patients gave written informed consent. Details of the surgical procedures have been described elsewhere (Levy et al., 2007; Lozano et al., 1996). The surgeries were performed, under local anesthesia, following withdrawal of dopaminergic medication for 12 hours. Patients were challenged preoperatively with apomorphine to determine the dose required to produce an ON state without dyskinetic movements (average dosage:4.7± 0.8 mg), using the same procedures that were used in a previous study from our group (Levy et al., 2001). Patients were also premedicated with a peripherally acting dopamine receptor antagonist (domperidone, Motilium, Janssen) to minimize unwanted side effects of the systemic apomorphine administration. A partial Unified Parkinson's Disease Rating Score (UPDRS) motor assessment was performed both pre and intraoperatively by a neurologist as reported elsewhere (Levy et al., 2007).
Recordings were obtained using gold and platinum-plated tungsten microelectrodes (15-25 μm exposed tip, Microprobes for Lifescience, Gaithersburg, MD), amplified 5,000–10,000 times, filtered at 10 to 5,000 Hz (analog Butterworth filters: high-pass, one pole; low-pass, two poles) and digitized with the Guideline 3000 system amplifiers (Axon Instruments, Foster City, CA) and captured digitally using Spike2 software (Cambridge Electronic Devices, Cambridge, UK). As in our previous studies, the neurons in the STN were recorded using an anterior parasagittal approach. The borders of the STN along a given electrode track were defined as outlined in Hutchison et al. (1998), with the dorsal border as the electrode depth at which the baseline cellular noise increased, and the ventral border as the location of the last irregularly firing neuron. Single-units were discriminated using a dual window discriminator and a storage oscilloscope. In each patient, a single well-isolated STN neuron was recorded for 2-3 minutes at rest, and then during and after subcutaneous apomorphine administration for as long as possible. In one patient, it was possible to discriminate two units throughout the recording. Because ApEn is dependent on the number of ISIs in the input sequence, segments must contain a fixed number of ISIs. In the present study, the longest segment we could extract for all conditions contained 2683 ISIs and thus, this length was selected. We compared the 2683 ISIs occurring immediately prior to the apomorphine injection (pre-APO) to those occurring at the end of the recording. There was therefore a varying delay between the pre-APO and post-APO periods ranging between 2.5 and 26.1 minutes (12.5 ± 8.4 min (mean ± SD). The rationale for analyzing the firing activity at the very end of the recording was to observe the effects of apomorphine at a time where they may be maximal (compared to earlier post-injection). There was no significant difference between the length of the pre-APO segments (1.1±0.5 min) and that of the post-APO segments (1.8±1.1 min) (paired t-test, ptwo-tailed=0.191).
Statistical irregularity was measured following the method of Pincus to calculate ApEn (Pincus, 1995). It is generally accepted that ApEn quantifies a degree of complexity in the temporal organization of the data stream. Low ApEn values are indicative of low irregularity, while high ApEn values denote greater complexity (or higher irregularity) (Darbin et al., 2006; Dorval et al., 2008; Pincus, 1995) in the time series. Three parameters were used to compute the ApEn value: the number of spikes in the input sequence (N), the embedding dimension (m), and the vector comparison length (r). Because ApEn is dependent on N, the number of ISIs included must be fixed. In the present study, segments of 2683 ISIs (duration, 68 ± 30 s) were extracted as this was the largest segment that we had available across all recordings. The embedding dimension m was empirically set to 2 and the parameter r was calculated for each cell as 15% of the standard deviation of ISIs (Pincus, 1995). As discussed by Pincus (1995), the use of small m and moderate r ensures the reliability of the ApEn estimate and provides better accuracy for comparisons between samples. As the first step in the calculation of the ApEn value, the ‘correlation integral’ was computed as the number of vectors whose distance from the vector under study was less than r, using a lag of 1. The natural logarithm of the correlation integral was averaged over the N points. This process was repeated m+1 times, and the ApEn value finally computed as the difference between the values at m and m+1 (Pincus, 1995).
Statistical comparisons prior and after apomorphine administration were performed using one-tailed paired t-tests. Data are expressed in average (avg) and standard deviation (SD). In addition to statistical irregularity, other characteristics of firing were measured such as the firing rate and the percent of spikes in bursts. Neurons were also classified according to the criteria of Kaneoke and Vitek (1996) as regular firing, bursty firing, or with no dominant period (also arguably called ‘random’ in the original study).
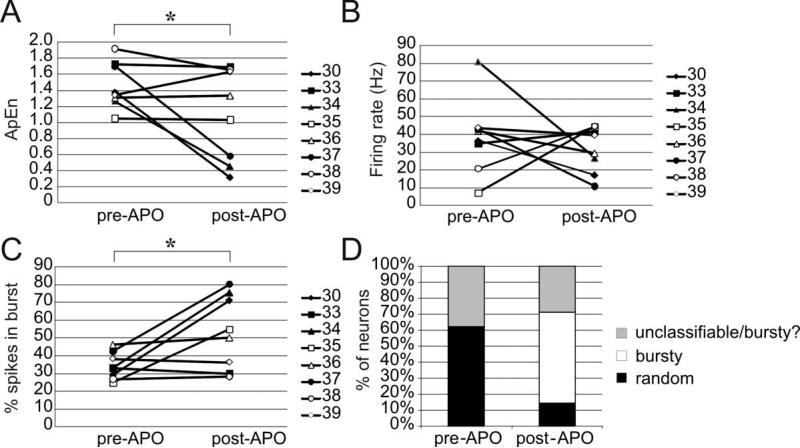
Segments of 2683 ISIs (duration, 68 ± 30 s) were extracted immediately prior to injection. The last 2683 ISIs of each recording were also extracted (duration, 107 ± 67 s) and taken as the post-injection segment The average firing rate after the injection (31.2 ± 12.9 spikes/s) was not significantly different than that before the injection (38.4 ±28.3 spikes/s, Figure 1B). The distribution of firing patterns was, however, different following injection compared to before the injection (Figure 1D). Before the injection, 5 neurons were classified as randomly firing, while the other 3 were unclassifiable. Following the injection, however, only 2 cells were classified as randomly firing while 4 were classified as bursty and the remaining 2 were unclassifiable (one of the neurons that was previously unclassifiable prior to apomorphine became frankly bursty following it). ApEn values for ISIs measured prior to apomorphine injection ranged from 1.05 to 1.92 and the median value was 1.36 (1.28-1.71 for the 25th and 75th percentiles, respectively). Following injection, ApEn values ranged from 0.31 to 1.69 (median=1.18, 0.52-1.64 for the 25th and 75th percentiles, respectively). Following apomorphine administration, 6 of 8 neurons (75 %) exhibited a decrease in ApEn value while the other two showed increases in ApEn (Figure 1A). This decrease of ApEn following apomorphine injection was statistically significant (paired t-test, pone-tailed=0.046). Interestingly, the 3 neurons which exhibited the most striking decrease in the ApEn value also showed a switch in their Kaneoke and Vitek (1996) classification from a random firing pattern to a bursty firing pattern. In contrast, the two neurons whose entropy increased fired with a random pattern both before and after apomorphine. The percentage of spikes in bursts, detected using the Poisson surprise method described by Legendy and Salcman (1985), was also significantly increased following apomorphine (paired t-test, ptwo-tailed = 0.036) (Figure 1C).
Figure 1.
Changes in firing rates and patterns prior to and following administration of apomorphine. Following apomorphine administration, the ApEn (A) decreased significantly (one-tailed paired t-test, p= 0.046) while the percentage of spikes in bursts (C)increased significantly two-tailed paired t-test, p=0.036. The firing rate (B) did not change significantly in this sample of neurons. As expected due to the increased percentage of spikes in bursts, the prevalence of bursty neurons (using the classification of Kaneoke and Vitek (1996)) increased following apomorphine.
Ethical and technical considerations limit the number of units that can be studied in human patients for long periods following drug injection. However, we felt that the advantage of following the same units over time compared to studying populations of units with or without treatment warranted this approach since entropy levels may fluctuate across cells in ways that could obscure a treatment effect. The lower ApEn values observed in 6/8 neurons after apomorphine suggest that the irregularities in the ISI series of STN neurons may lower under medication than in the practically defined OFF state. In line with previous experimental investigations (Cruz et al., 2009; Darbin et al., 2006; Dorval et al., 2008; Rodriguez et al., 2003), our study supports the hypothesis that the timing of neuronal discharge in the STN shows non-linear features, and that entropy in STN discharge is modified by dopamine receptor activation. In other words, it appears that, in the dopamine-depleted human brain, systemic administration of apomorphine reduces the complexity of the temporal organization in the ISI series.
Paradoxically, while Cruz et al. (2009) have recently reported a significant decrease in GPe network entropy following chronic dopamine depletion in rats, Dorval and colleagues (2008) reported a decrease in the entropy of GPe neurons following STN-DBS in parkinsonian monkeys (Dorval et al., 2008). Accordingly, both dopamine depletion and dopaminergic treatment would be expected to lower the entropy since they both represent functional alterations of the normal state.
Our data obtained in the STN of PD patients supports the hypothesis that the beneficial effects of anti-parkinsonian treatments are associated with a decrease in the complexity of neuronal activity in the basal ganglia. Clearly, the treatment with apomorphine does not result in a true restoration of the non-linear properties of STN firing, for instance, because the dopamine agonist treatment is applied in the chronically dopamine-depleted state, that is characterized not only by the absence of dopamine, but also by secondary morphological changes such as a redistribution of dopamine receptors, or changes in spine morphology at the striatal level (reviewed in Smith et al. 2009). Furthermore, the non-linear properties of basal ganglia firing may strongly depend on the temporally and spatially precise delivery of dopamine in the striatum. Systemic apomorphine treatment is neither temporally nor spatially as precise. Of course, it can also not be ruled out that dopamine-induced changes in entropy may differ between species. Given the discrepancy between the results of the dopamine-depletion experiments in rodents and the current apomorphine experiments in humans, it appears that there is no simple relationship between the improvement in motor performance and the change in the ApEn.
Drug-induced changes in nonlinear features of neuronal firing may result from their direct effects on neurons by affecting intrinsic membrane or channel properties (Komendantov and Kononenko 1996; Leao et al. 2005). In addition, drug effects on network interactions between the basal ganglia nuclei may also contribute to the nonlinear features detected in the STN. Since D1 dopamine receptors are expressed on STN neurons and both D1/D2 are ubiquitous in the basal ganglia circuitry, the apomorphine-induced entropy decrease may result either from direct or indirect effects, or a combination of both (Allers et al., 2000; Cragg et al., 2004; Loucif et al., 2005). Concurrent changes in firing rates and entropy as well as the complexity behind interpolating entropy changes at the single neuron level to the network level (i.e. as measured by cross-entropy between neurons) could help explain the mechanisms by which dopamine regulates neuronal entropy in the circuitry of the basal ganglia.
In conclusion, our study suggests that dopamine receptor agonist therapy decreases entropy of firing activity in most STN neurons of PD patients. This finding highlights the importance of dopamine in modulating non-linear features of basal ganglia activity. An important future extension of this work will be to understand the causal relations between decreases in neuronal entropy, functions of the basal ganglia and treatment benefits for movement disorders.
Acknowledgements
The authors wish to acknowledge Dr. Ron Levy for participating in the original data recordings and for commenting on the manuscript. This work was supported by NIH grants RR-000165 (Yerkes Center grant), R01-NS054976 (TW), and by a grant from the CIHR MOP 42505 (JD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Albin RL, Young AB, Penney JB. The functional anatomy of disorders of the basal ganglia. Trends Neurosci. 1995;18:63–64. [PubMed] [Google Scholar]
- Allers KA, Kreiss DS, Walters JR. Multisecond oscillations in the subthalamic nucleus: effects of apomorphine and dopamine cell lesion. Synapse. 2000;38(1):38–50. doi: 10.1002/1098-2396(200010)38:1<38::AID-SYN5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Baufreton J, Xue Y, Bolam JP, Bevan MD. Synaptic release of dopamine in the subthalamic nucleus. Eur J Neurosci. 2004;20(7):1788–802. doi: 10.1111/j.1460-9568.2004.03629.x. [DOI] [PubMed] [Google Scholar]
- Cruz AV, Mallet N, Magill PJ, Brown P, Averbeck BB. Effects of dopamine depletion on network entropy in the external globus pallidus. J Neurophysiol. 2009;102:1092–1102. doi: 10.1152/jn.00344.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbin O, Casebeer DJ, Naritoku DK. Cardiac dysrhythmia associated with the immediate postictal state after maximal electroshock in freely moving rat. Epilepsia. 2002;43:336–341. doi: 10.1046/j.1528-1157.2002.34801.x. [DOI] [PubMed] [Google Scholar]
- Darbin O, Soares J, Wichmann T. Nonlinear analysis of discharge patterns in monkey basal ganglia. Brain Res. 2006;1118:84–93. doi: 10.1016/j.brainres.2006.08.027. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Dorval AD, Russo GS, Hashimoto T, Xu W, Grill WM, Vitek JL. Deep brain stimulation reduces neuronal entropy in the MPTP-primate model of Parkinson's disease. J Neurophysiol. 2008;100:2807–2818. doi: 10.1152/jn.90763.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison WD, Allan RJ, Opitz H, Levy R, Dostrovsky JO, Lang AE, Lozano AM. Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson's disease. Ann Neurol. 1998;44:622–628. doi: 10.1002/ana.410440407. [DOI] [PubMed] [Google Scholar]
- Kaneoke Y, Vitek JL. Burst and oscillation as disparate neuronal properties. J Neurosci Methods. 1996;68(2):211–23. doi: 10.1016/0165-0270(96)00081-7. [DOI] [PubMed] [Google Scholar]
- Komendantov AO, Kononenko NI. Deterministic chaos in mathematical model of pacemaker activity in bursting neurons of snail, Helix pomatia. J Theor Biol. 1996;183:219–230. doi: 10.1006/jtbi.1996.0215. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Anderson LA, Walters JR. Apomorphine and dopamine D(1) receptor agonists increase the firing rates of subthalamic nucleus neurons. Neuroscience. 1996;72:863–876. doi: 10.1016/0306-4522(95)00583-8. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Mastropietro CW, Rawji SS, Walters JR. The response of subthalamic nucleus neurons to dopamine receptor stimulation in a rodent model of Parkinson's disease. J Neurosci. 1997;17:6807–6819. doi: 10.1523/JNEUROSCI.17-17-06807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RN, Leao FN, Walmsley B. Non-random nature of spontaneous mIPSCs in mouse auditory brainstem neurons revealed by recurrence quantification analysis. Proc Biol Sci. 2005;272:2551–2559. doi: 10.1098/rspb.2005.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Dostrovsky JO, Lang AE, Sime E, Hutchison WD, Lozano AM. Effects of apomorphine on subthalamic nucleus and globus pallidus internus neurons in patients with Parkinson's disease. J Neurophysiol. 2001;86:249–260. doi: 10.1152/jn.2001.86.1.249. [DOI] [PubMed] [Google Scholar]
- Levy R, Lozano AM, Hutchison WD, Dostrovsky JO. Dual microelectrode technique for deep brain stereotactic surgery in humans. Neurosurgery. 2007;60:277–283. doi: 10.1227/01.NEU.0000255389.85161.03. [DOI] [PubMed] [Google Scholar]
- Loucif AJ, Woodhall GL, Sehirli US, Stanford IM. Depolarisation and suppression of burst firing activity in the mouse subthalamic nucleus by dopamine D1/D5 receptor activation of a cyclic-nucleotide gated non-specific cation conductance. Neuropharmacology. 2008;55(1):94–105. doi: 10.1016/j.neuropharm.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Lozano A, Hutchison W, Kiss Z, Tasker R, Davis K, Dostrovsky J. Methods for microelectrode-guided posteroventral pallidotomy. J Neurosurg. 1996;84:194–202. doi: 10.3171/jns.1996.84.2.0194. [DOI] [PubMed] [Google Scholar]
- Naritoku DK, Casebeer DJ, Darbin O. Effects of seizure repetition on postictal and interictal neurocardiac regulation in the rat. Epilepsia. 2003;44:912–916. doi: 10.1046/j.1528-1157.2003.48302.x. [DOI] [PubMed] [Google Scholar]
- Pincus S. Approximate entropy (ApEn) as a complexity measure. Chaos. 1995;5:110–117. doi: 10.1063/1.166092. [DOI] [PubMed] [Google Scholar]
- Pincus SM, Cummins TR, Haddad GG. Heart rate control in normal and aborted-SIDS infants. Am J Physiol. 1993;264:R638–R646. doi: 10.1152/ajpregu.1993.264.3.R638. [DOI] [PubMed] [Google Scholar]
- Pincus SM, Gladstone IM, Ehrenkranz RA. A regularity statistic for medical data analysis. J Clin Monit. 1991;7:335–345. doi: 10.1007/BF01619355. [DOI] [PubMed] [Google Scholar]
- Pincus SM, Goldberger AL. Physiological time-series analysis: what does regularity quantify? Am J Physiol. 1994;266:H1643–H1656. doi: 10.1152/ajpheart.1994.266.4.H1643. [DOI] [PubMed] [Google Scholar]
- Pincus SM, Minkin MJ. Assessing sequential irregularity of both endocrine and heart rate rhythms. Curr Opin Obstet Gynecol. 1998;10:281–291. doi: 10.1097/00001703-199808000-00001. [DOI] [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278:H2039–H2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Pereda E, Gonzalez J, Abdala P, Obeso JA. Neuronal activity in the substantia nigra in the anaesthetized rat has fractal characteristics. Evidence for firing-code patterns in the basal ganglia. Exp Brain Res. 2003;151:167–172. doi: 10.1007/s00221-003-1442-4. [DOI] [PubMed] [Google Scholar]
- Smith Y, Villalba RM, Raju DV. Striatal spine plasticity in Parkinson's disease: pathological or not? Parkinsonism Relat Disord. 2009;15(Suppl 3):S156–S161. doi: 10.1016/S1353-8020(09)70805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. I. Functional properties in intact animals. J Neurophysiol. 1994;72:494–506. doi: 10.1152/jn.1994.72.2.494. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Boston: p. 1998. [Google Scholar]