Abstract
Systemic administration of nor-binaltorphimine (nor-BNI) produces a long-lasting κ-opioid receptor (κOR) antagonism and has κ1-selectivity in nonhuman primates. The aim of this study was to establish the pharmacological basis of central κOR antagonism in rhesus monkeys (Macaca mulatta). After intracisternal (i.c.) administration of small doses of nor-BNI, the duration and selectivity of nor-BNI antagonism were evaluated against two κOR agonists, (trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]benzeneacetamide (U50,488) and bremazocine. Thermal antinociception was measured in the warm water (50°C) tail-withdrawal assay and sedation was evaluated by observers blind to treatment conditions. Following i.c. pretreatment with 0.32 mg nor-BNI, a 5- to 10-fold right-ward shift of the U50,488 baseline dose-effect curve was observed in antinociception. In contrast, this dose of nor-BNI only produced an insignificant 2-fold shift against bremazocine. Pretreatment with a smaller dose (0.032 mg) of nor-BNI produced a 3-fold shift of U50,488, which lasted for 7 days, but failed to alter the potency of bremazocine. This differential antagonism profile of i.c. nor-BNI also was observed in sedation ratings. In addition, the centrally effective dose of nor-BNI (0.32 mg), when administered s.c. in the back, did not antagonize either U50,488- or bremazocine-induced antinociception and sedation. After i.c. pretreatment with the same dose, nor-BNI also did not antagonize the peripherally mediated effect of U50,488 against capsaicin-induced thermal nociception in the tail. These results indicate that i.c. nor-BNI produces central κOR antagonism and support the notion of two functional κOR subtypes in the central nervous system. Moreover, it provides a valuable pharmacological basis for further characterizing different sources of κOR-mediated effects, namely, from central or peripheral nervous system receptors.
Activation of κ-opioid receptors (κOR) has been implicated in a number of behaviors and functions, including antinociception (Millan 1989; France et al., 1994), diuresis (Leander, 1983; Dykstra et al., 1987), neuroprotective properties (Tortella and DeCoster, 1994; Bausch et al., 1998), hormonal regulation (Krulich et al., 1986; Butelman et al., 1999), and the modulation of reward and aversion (Mello and Negus, 1998; Xi et al., 1998). This receptor population is of particular interest because κOR agonists retain antinociceptive effects but possess several μ-opioid opposing actions. For example, administration of μ-opioid agonists produces euphoria and they can act as reinforcers, whereas κOR agonists produce dysphoria and they do not have reinforcing effects under most conditions (Woods and Winger, 1987; Dykstra et al., 1997). In addition, selective κOR agonists do not have μ-opioid-related side effects such as constipation, pruritus, and respiratory depression. Although there are species differences regarding κOR distribution, binding studies indicate that the κOR population is more abundant than μ- and δ-sites in human and monkey cortex membranes (Kim et al., 1996; Butelman et al., 1998). To what extent this κOR population in the central nervous system (CNS) contributes to particular physiological functions remains to be explored.
Functional studies of κOR in nonhuman primates have raised the possibility of κOR subtypes in modulating antinociceptive actions. Based on the antagonism and binding studies, it has been suggested that κOR agonists such as (trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]benzeneacetamide (U50,488) produce thermal antinociception through κ1-receptors, whereas other κOR agonists such as bremazocine produce effects partially through non-κ1-receptors in rhesus monkeys (Macaca mulatta) (Butelman et al., 1993, 1998; Ko et al., 1998a). Currently, only one type of κOR has been cloned and it appears to resemble the κ1-receptors (Simonin et al., 1995; Zhu et al., 1995). Nevertheless, some studies have reported heterogeneity of κOR in cardiovascular and neurophysiological functions. For instance, in rat neostriatum, activation of κ1-receptors causes inhibition of dopaminergic neurotransmission, whereas activation of κ2-receptors inhibits the activity of cholinergic interneurons (Schoffelmeer et al., 1997). Considering κOR agonists as potential therapeutics, it is important to establish the pharmacological basis in primates for clarifying the site of action of κOR-mediated functions and characterizing newly developed κOR ligands.
The selective κOR antagonist nor-BNI has been widely used to verify κOR-mediated effects (Takemori et al., 1988). This compound produces a long-lasting antagonism against κOR agonists across assays and species in vivo (Endoh et al., 1992; Horan et al., 1992; Butelman et al., 1993; Jewett and Woods, 1995; Picker et al., 1996). More interestingly, nor-BNI was characterized as a functionally selective κ1-antagonist in nonhuman primates (Butelman et al., 1993; Ko et al., 1999). In a thermal nociceptive assay, systemic pretreatment with nor-BNI selectively antagonizes systemic U50,488, but it does not antagonize bremazocine in rhesus monkeys (Butelman et al., 1993). Recently, it also has been reported that local pretreatment with nor-BNI in the monkey’s tail preferentially antagonizes peripherally antinociceptive effects of U50,488, but not bremazocine (Ko et al., 1999). Nevertheless, there are no primate studies to date exploring the selectivity of central nor-BNI antagonism. In particular, behavioral effects of systemic administration of κOR agonists have been evaluated in a variety of assays, including diuresis, antinociception, sedation, and drug discrimination (Dykstra et al., 1987; France et al., 1994). To what extent systemic κOR agonists produce effects that are centrally mediated remains to be determined. Elucidation of this issue may facilitate pharmacological studies of site of action and development of peripherally selective agents.
The aim of this study was therefore to establish the pharmacological basis of central κOR antagonism in nonhuman primates by intracisternal (i.c.) administration of nor-BNI. The duration and selectivity of central nor-BNI antagonism were studied with two κOR agonists, U50,488 and bremazocine, in terms of thermal antinociception and sedation. In addition, the site of action of i.c. nor-BNI was evaluated against peripheral antinociception produced by local administration of U50,488 in an experimental pain model (Ko et al., 1999).
Materials and Methods
Subjects
Seven adult male and female rhesus monkeys with body weights ranging between 6.0 and 12.9 kg were used (their mean weight during this study was 9.5 kg). They were housed individually with free access to water and were fed ~25 to 30 biscuits (Purina Monkey Chow) and fresh fruit daily. All monkeys were previously trained in the tail-withdrawal procedure and did not have exposure to opioids for 1 month before the present study. Animals used in this study were maintained in accordance with the University Committee on the Use and Care of Animals in the University of Michigan, and the Guide for the Care and Use of Laboratory Animals by the Institute of Laboratory Animal Resources.
Procedure
Thermal antinociception was measured by a warm water tail-withdrawal procedure that has been previously described (Ko et al., 1998a). Briefly, the subjects were seated in restraint chairs and the lower part of the shaved tail (~15 cm) was immersed into warm water maintained at temperatures of 40, 50, or 55°C. Tail-withdrawal latencies were recorded by an experimenter who did not know experimental conditions. A maximum cutoff latency (20 s) was used to prevent tissue damage. Each experimental session began with control determinations at each temperature. Then, the agonist was administered s.c. in the back by a cumulative dosing procedure with a 30-min interinjection interval. Subsequent tail-withdrawal latencies were determined starting at 20 min after each injection. In addition, before the measurement of tail-withdrawal latencies, sedation also was estimated by an experimenter blind to drug conditions. The sedation rating was quantified based on an observational scale (Table 1) that was modified from previous studies (Dykstra et al., 1987; Butelman and Woods, 1993).
TABLE 1.
Modified sedation rating scale
| Grade | Sedation |
|---|---|
| 0 | No observable sedation; monkey is alert to environment |
| 1 | Monkey is attentive to ordinary movements of observer |
| 2 | Monkey responds to clapping noise in room |
| 3 | Monkey responds only to noises generated by knocking on its chair |
| 4 | Monkey responds only to clapping noise ~2 feet away from its head |
| 5 | Monkey responds only to touch |
| 6 | Monkey does not respond to touch |
Experimental Design
Most of the observations for this article were obtained from three separate experiments (Table 2) in the same subjects (n = 4). Systemic U50,488 and bremazocine dose-effect curves were determined once in each experiment. After nor-BNI administration, dose-effect curves were redetermined for U50,488 and bremazocine according to the schedule shown in Table 2. The intervals between experiments were 2 to 3 weeks. It needs to be noted that nor-BNI does not have a clear κOR selectivity during the first few hours after injection (Endoh et al., 1992; Horan et al., 1992). To avoid the possible interference of early action of nor-BNI, we used a protocol similar to that of a previous study (Butelman et al., 1993).
TABLE 2.
Experimental schedules for the study of the antagonist effects of nor-BNI against systemic U50,488 and bremazocine
| Experiment 1 | Experiment 2 | Experiment 3 | |
|---|---|---|---|
| Baseline | U50,488 & bremazocine |
U50,488 & bremazocine |
U50,488 & bremazocine |
| Pretreatment conditions |
i.c. nor-BNI 0.32 mg |
i.c. nor-BNI 0.032 mg |
s.c. nor-BNI 0.32 mg in the back |
| Days after nor-BNI injection | |||
| 1 | U50,488 | U50,488 | U50,488 |
| 3 | Bremazocine | Bremazocine | Bremazocine |
| 7 | U50,488 | U50,488 | U50,488 |
| 10 | Bremazocine | Bremazocine | Bremazocine |
| 14 | U50,488 | U50,488 | |
| 21 | U50,488 | U50,488 | |
| 28 | Bremazocine | Bremazocine | |
| 35 | U50,488 | U50,488 | |
| 42 | Bremazocine | ||
| 49 | U50,488 | ||
| 56 | Bremazocine | ||
| 63 | U50,488 | ||
The protocol for capsaicin-induced thermal nociception (Ko et al., 1998b) was used to further evaluate the antagonist effects of i.c. nor-BNI. In this procedure, small, systemically inactive doses of opioid agonists can locally attenuate capsaicin-induced nociceptive responses, which has been demonstrated as a peripheral opioid action (Ko et al., 1998b, 1999). Based on previous studies, 0.1 mg of capsaicin was chosen as a standard noxious stimulus in 46°C water. Capsaicin was injected s.c. in the terminal 1 to 4 cm of the tail, in a constant 0.1-ml volume. U50,488 (0.1 mg) was coadministered with capsaicin in the tail to produce local antinociception against capsaicin. After receiving i.c. nor-BNI (0.32 mg), monkeys were tested again by the above-mentioned procedure to assess whether central nor-BNI could antagonize the peripheral effects of U50,488. A single dosing procedure was used and test sessions were conducted once per week.
Data Analysis
Individual tail-withdrawal latencies were converted to percentage of maximum possible effect (%MPE) by the following formula: %MPE = [(test latency − control latency)/(cutoff latency, 20 s. − control latency)] × 100. Mean ED50 values were obtained from individual ED50 values, which were calculated by least-squares regression with the portion of the dose-effect curves spanning the 50% MPE. The 95% CL also were determined (p < .05). In addition, the dose ratios were calculated by dividing mean ED50 values in the presence of nor-BNI by the baseline ED50 values. The significant shifts of dose-effect curves were analyzed with one-way ANOVA followed by the Newman-Keuls test (p < .05). The sedation rating at the doses that produced 100% MPE in 50°C water was compared. In particular, the sedative effects of κOR agonists with or without nor-BNI pretreatment were evaluated with the Newman-Keuls test (p < .05).
Drugs
U50,488 HCl (Upjohn Company, Kalamazoo, MI) and bremazocine HCl (Research Biochemicals Inc., Natick, MA) were dissolved in sterile water. For systemic administration, all compounds were administered s.c. in the back (i.e., around the scapular region) at a volume of 0.1 ml/kg. Capsaicin (Sigma Chemical Company, St. Louis, MO) was dissolved in a solution of Tween 80/ethanol/saline in a ratio of 1:1:8. For local administration, U50,488 and capsaicin were mixed in a solution and injected in 0.1-ml volume in the tail. For i.c. administration, animals were anesthetized with ketamine HCl (10 mg/kg i.m.) and the dorsal upper neck/lower skull area was shaved and sterilized with Betadine. A spinal needle (22-gauge, 3.8 cm in length; Becton Dickinson & Co., Lincoln Park, NJ) was inserted into the cisterna magna by puncturing the skin and atlanto-occipital membranes. The position of needle was confirmed by free flow of clear cerebrospinal fluid. A 1-ml solution of nor-BNI (provided by Dr. H. I. Mosberg, Division of Medicinal Chemistry, University of Michigan, Ann Arbor) in saline was slowly infused through the spinal needle in 30 s and monkeys were returned to their home cages.
Results
Control Tail-Withdrawal Latencies and Baseline Dose-Effect Curves
The subjects used in this study displayed a consistent profile in tail-withdrawal responses. Normally, they kept their tails in 40°C water for 20 s (cutoff latency) and removed their tails from 50 and 55°C water rapidly (within 1–3 s). After i.c. administration of nor-BNI (0.32 and 0.032 mg), animals gradually recovered from ketamine anesthesia within an hour and they did not have elevated tail-withdrawal latencies in 50 and 55°C water 1 h later (data not shown). Similarly, s.c. administration of nor-BNI (0.32 mg) in the back also did not change the monkey’s baseline latencies from 24 h and beyond.
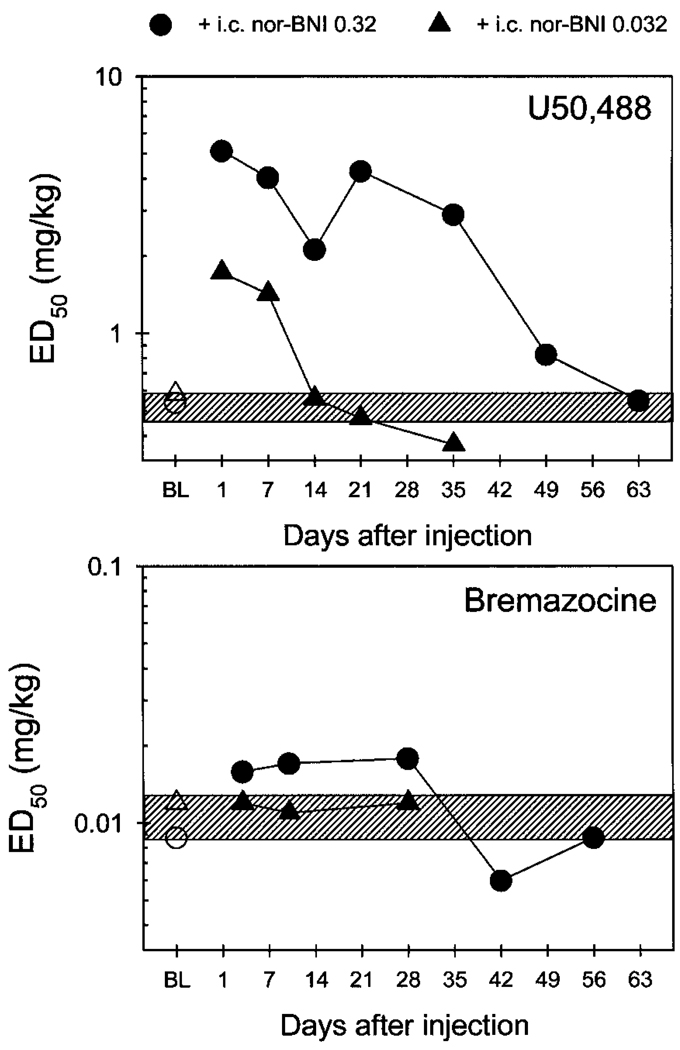
Both U50,488 and bremazocine dose-dependently produced antinociception against 50 and 55°C water. To avoid the convulsant behaviors that occasionally can be observed with high doses of κOR agonists, dosing was only continued until each subject reached 100% MPE in 50°C water. The baseline ED50 values of U50,488 from the three separate experiments were 0.5, 0.6, and 0.4 mg/kg, respectively. Likewise, the baseline ED50 values of bremazocine were 0.009, 0.012, and 0.012 mg/kg, respectively. There was no significant variation among these baseline ED50 values from three separate experiments, indicating that U50,488 or bremazocine dose-effect curves did not change across the entire experimental period. Thus, baseline dose-effect curves for U50,488 and bremazocine were averaged and the ED50 (95% CL) was graphed as a slashed area in Fig. 1 [U50,488: 0.5 (0.47–0.57 mg/kg); bremazocine: 0.011 (0.009–0.013 mg/kg)], to compare the magnitude of i.c. nor-BNI antagonism.
Fig. 1.
ED50 values for systemic U50,488 and bremazocine in antinociception before and after i.c. nor-BNI injection. Each value represents the mean of individual ED50 values (n = 4). Abscissae, days after i.c. injection of nor-BNI; ordinates, ED50 values in mg/kg. Open symbols represent the baseline (BL) ED50 values in each experiment. Filled symbols represent the ED50 values after nor-BNI injection. The slashed area represents the range of 95% CL of averaged baseline ED50 values in three separate experiments. See Figs. 2 and 3 and Experimental Design for other details.
Duration and Selectivity of i.c. Nor-BNI Antagonism
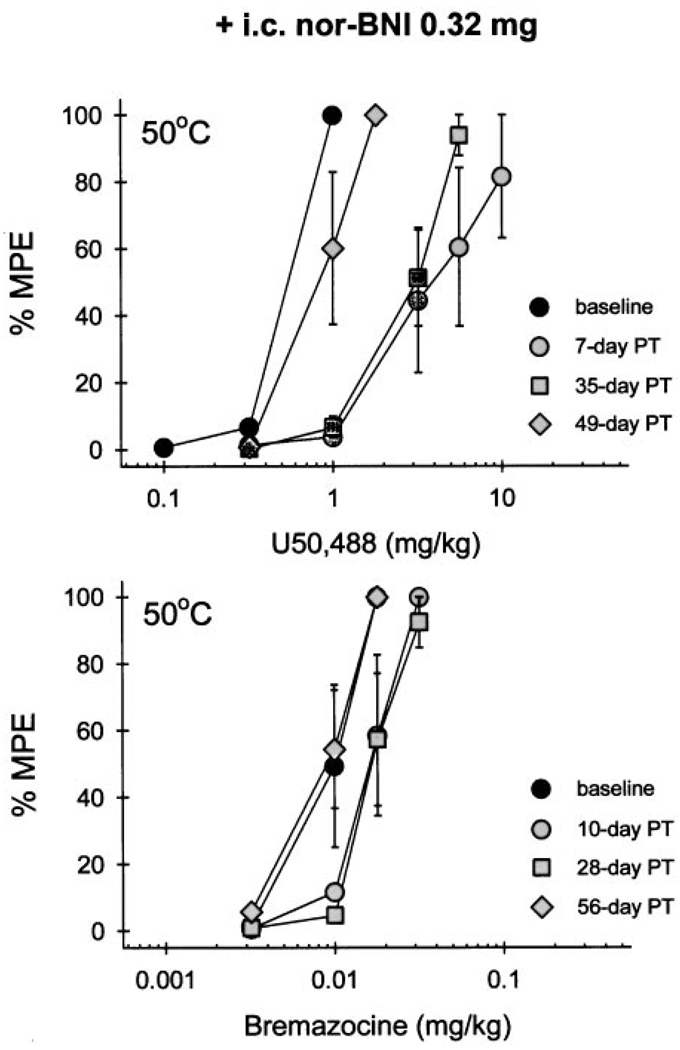
Central pretreatment with nor-BNI produced a different pattern of rightward shifts of dose-effect curves between U50,488 and bremazocine. The i.c. administration of nor-BNI (0.32 mg) caused a very long-lasting antagonism of systemic U50,488-induced antinociception in 50°C water (Fig. 2, top). This significant rightward shift was observed up to 35 days after nor-BNI pretreatment. Then, the U50,488 dose-effect curve gradually returned to baseline levels between 49 and 63 days after nor-BNI injection (Fig. 1 and Table 3). In contrast, the same dose of nor-BNI only produced slight rightward shifts of systemic bremazocine-induced antinociception (Fig. 2, bottom), which did not reach statistical significance. This result continued to be the case up to 56 days after nor-BNI pretreatment (Fig. 1 and Table 3).
Fig. 2.
Cumulative dose-effect curves in 50°C water tail-withdrawal assay for U50,488 (top) and bremazocine (bottom) after i.c. pretreatment (PT) with 0.32 mg of nor-BNI. Each value represents the mean ± S.E. (n = 4). Abscissae, s.c. agonist doses in mg/kg; ordinates, percentage of maximum possible effect (%MPE). Filled symbols represent the dose-effect curve before (baseline) or after nor-BNI injection. Not all of the data are shown for the sake of clarity. See Fig. 1 and Table 3 for complete time course of i.c. nor-BNI antagonism.
TABLE 3.
Magnitude of rightward shift for dose-effect curves of U50,488 and bremazocine in antinociception after nor-BNI pretreatment
| Dose Ratiosa | ||||||
|---|---|---|---|---|---|---|
| Days after nor-BNI |
i.c. nor-BNI 0.32 mg | i.c. nor-BNI 0.032 mg | s.c. nor-BNI 0.32 mg | |||
| versus U50,488 |
versus bremazocine |
versus U50,488 |
versus bremazocine |
versus U50,488 |
versus bremazocine |
|
| 1 | 9.5* | 3.0* | 1.0 | |||
| 3 | 1.8 | 0.9 | 1.0 | |||
| 7 | 7.5* | 2.5* | 1.2 | |||
| 10 | 1.9 | 0.8 | 1.0 | |||
| 14 | 3.9* | 1.0 | ||||
| 21 | 7.9* | 0.8 | ||||
| 28 | 2.0 | 1.0 | ||||
| 35 | 5.4* | 0.7 | ||||
| 42 | 0.7 | |||||
| 49 | 1.5 | |||||
| 56 | 1.0 | |||||
| 63 | 1.0 | |||||
Dose ratios were mean of individual dose ratio values (n = 4).
Significant rightward shift of dose-effect curve *p < .05.
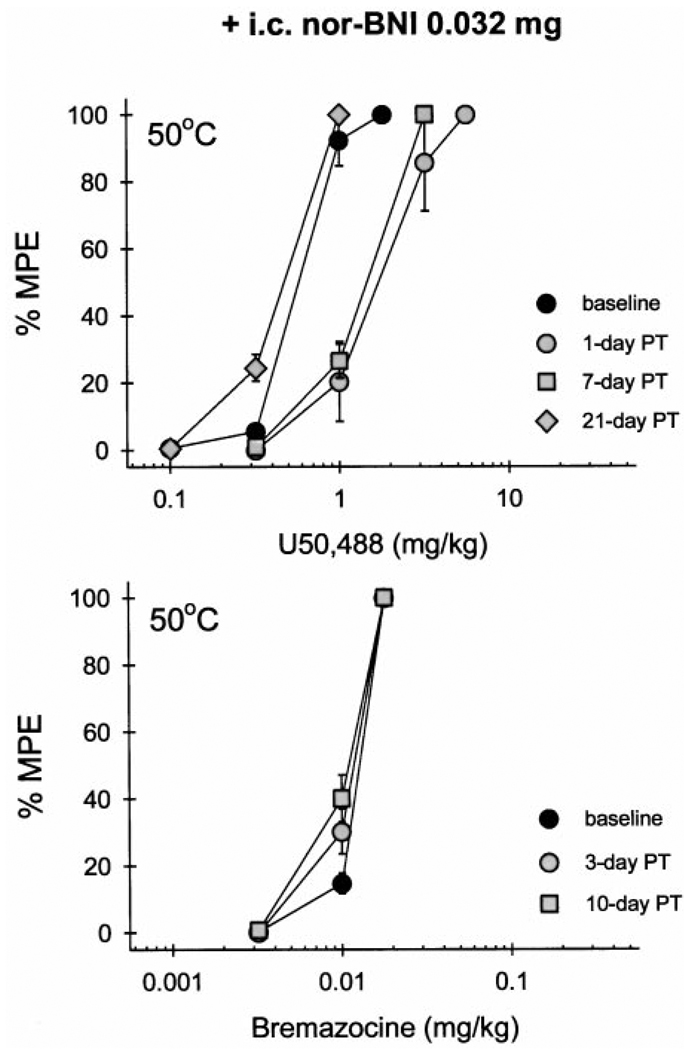
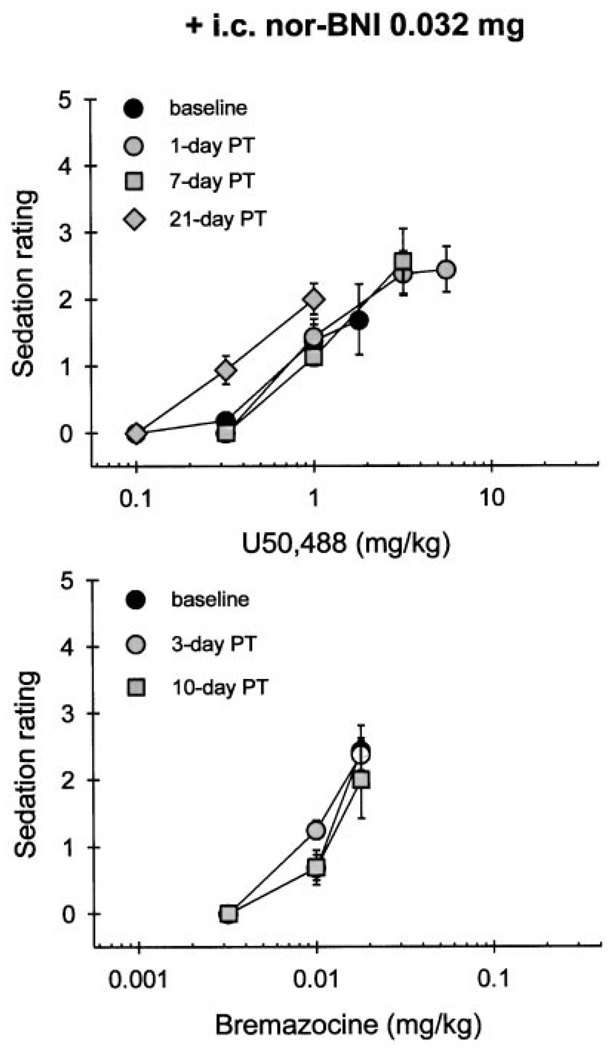
Pretreatment with a smaller dose of nor-BNI (0.032 mg i.c.) also produced rightward shifts of the U50,488 dose-effect curve (Fig. 3, top). However, this significant antagonism was short-lasting and was only observed up to 7 days after nor-BNI injection (Table 3). In contrast, the same dose of nor-BNI caused no change in bremazocine effects (Fig. 3, bottom; Table 3). Taken together, i.c. nor-BNI differentiated systemic U50,488- and bremazocine-induced thermal antinociception in both dose- and time-dependent manners. This profile is displayed in a graphic presentation of the time course of ED50 values of both U50,488 and bremazocine after nor-BNI administration (Fig. 1). However, systemic pretreatment of a centrally effective dose of nor-BNI (0.32 mg s.c. in the back) did not antagonize either U50,488 or bremazocine. It should be noted that ED50 values of both U50,488 and bremazocine did not differ when obtained over a 10-day period after s.c. nor-BNI injection (Table 3). Therefore, the effect of time on any i.c. nor-BNI-induced shift in dose-effect curves probably would be minimal at most.
Fig. 3.
Cumulative dose-effect curves in 50°C water tail-withdrawal assay for U50,488 (top) and bremazocine (bottom) after i.c. pretreatment with a smaller dose of nor-BNI (0.032 mg). Other details are as in Fig. 2.
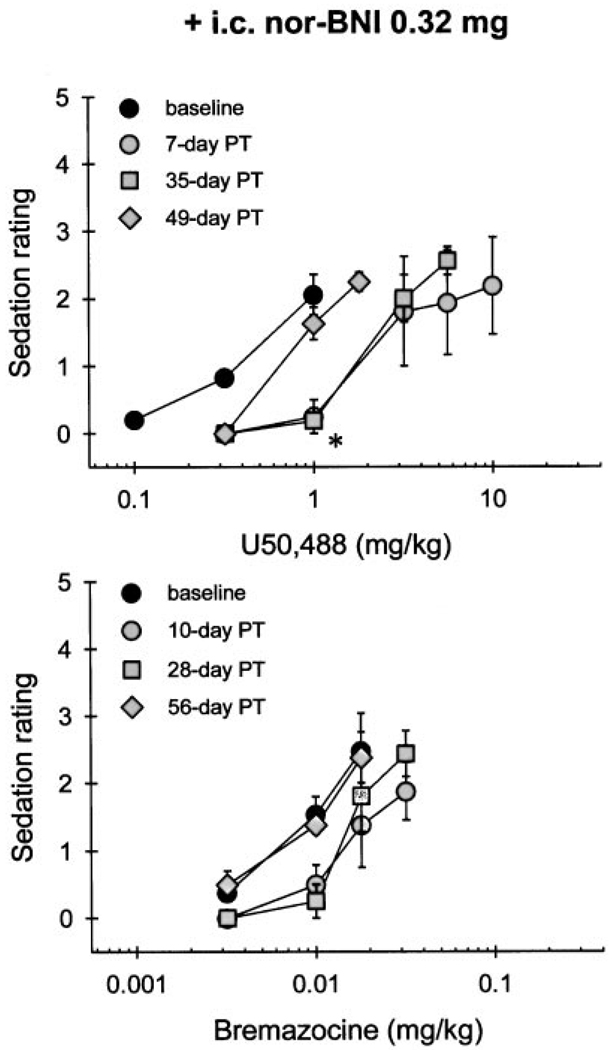
Systemic administration of κOR agonists produced dose-dependent sedation. At the doses of U50,488 (1 mg/kg) and bremazocine (0.018 mg/kg) that produced 100% MPE in 50°C water, animals displayed a mild sedation, typically not responding to ordinary movements of the observer. Figure 4 illustrates that U50,488 (1 mg/kg)-induced sedation was significantly attenuated for 35 days by i.c. pretreatment with 0.32 mg of nor-BNI. This duration of antagonism corresponds with the one that was observed with antinociception measurement. In contrast, i.c. pretreatment with the same dose of nor-BNI did not antagonize bremazocine (0.018 mg/kg)-induced sedation (Fig. 4, bottom). However, after i.c. administration of a smaller dose of nor-BNI (0.032 mg), there was no significant attenuation of either U50,488- or bremazocine-induced sedation at the doses producing full thermal antinociception (Fig. 5). In addition, s.c. pretreatment with 0.32 mg of nor-BNI did not antagonize either U50,488- or bremazocine-induced sedation over the first 2 weeks after injection (data not shown).
Fig. 4.
Cumulative dose-effect curves in sedation for U50,488 (top) and bremazocine (bottom) after i.c. pretreatment with 0.32 mg of nor-BNI. Each value represents the mean ± S.E. (n = 4). Abscissae, s.c. agonist doses in milligrams per kilogram; ordinates, sedation rating. Filled symbols represent the dose-effect curve before (baseline) or after nor-BNI injection. Not all of the data are shown for the sake of clarity. The asterisk represents a significant attenuation compared with baseline at 1 mg/kg of U50,488 (p < .05).
Fig. 5.
Cumulative dose-effect curves in sedation for U50,488 (top) and bremazocine (bottom) after i.c. pretreatment with a smaller dose of nor-BNI (0.032 mg). Other details are as in Fig. 4.
Site of Action of i.c. Nor-BNI Antagonism
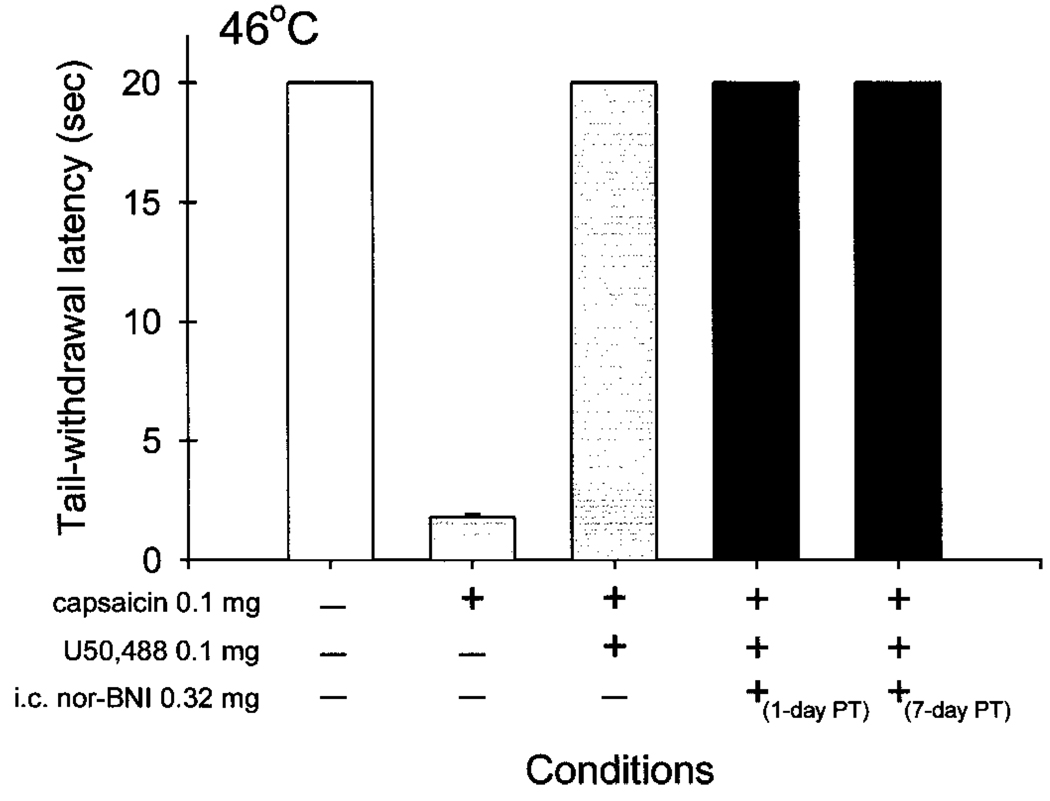
Although the above-mentioned experiments may indicate that i.c. nor-BNI produces central antagonism against systemic κOR agonists, it is not clear whether this central antagonism would interfere with the peripheral actions of κOR agonists following local administration. Figure 6 illustrates that i.c. nor-BNI did not antagonize peripheral antinociception of U50,488 against capsaicin-induced thermal nociception. Each data point was obtained at 15 min after injection because it was the time of peak effects of both capsaicin and κOR agonists (Ko et al., 1998b, 1999). Normally, the monkeys kept their tails in 46°C water until the cutoff time (20 s). When 0.1 mg of capsaicin was injected s.c. into the tail, it evoked a nociceptive response, thermal allodynia, that was shown as reduced tail-withdrawal latencies in 46°C water. Coadministration of U50,488 (0.1 mg) with capsaicin in the tail inhibited capsaicin-induced nociception. The i.c. injection of a centrally effective dose of nor-BNI (0.32 mg) did not antagonize the peripheral antinociception of U50,488. This lack of peripheral antagonism was observed up to 1 week after nor-BNI injection. As noted, local administration of U50,488 (0.1 mg) did not cause any behavioral changes such as sedation in this procedure.
Fig. 6.
Lack of antagonism by i.c. nor-BNI against local antinociception of U50,488 in capsaicin-induced thermal nociception in 46°C water. Each value represents the mean ± S.E. (n = 3). The + indicates that the corresponding conditions were given. The − represents the absence of conditions. Each data point was obtained 15 min after local injection. See Experimental Design for other details.
Discussion
This is the first study to demonstrate that i.c. administration of nor-BNI differentiated κOR agonist-induced behavioral effects in rhesus monkeys. This central nor-BNI antagonism was both dose- and time-dependent. In addition, the centrally effective dose of nor-BNI did not antagonize antinociception mediated by peripheral κOR. These results support the notion that there are at least two functional κOR subtypes in the CNS of nonhuman primates. Systemic administration of U50,488 and bremazocine may produce antinociception and sedation through different central κOR populations.
Duration and Selectivity of i.c. Nor-BNI Antagonism
The present study showed that systemic administration of U50,488 or bremazocine produced thermal antinociception and sedation in a dose-dependent manner in rhesus monkeys. After i.c. pretreatment with nor-BNI (0.32 mg), it caused an ~5- to 10-fold rightward shift of the baseline dose-effect curve of U50,488 for 35 days. This long-lasting κOR antagonism by central administration of nor-BNI has been reported in other species and assays (Horan et al., 1992; Picker et al., 1996). In addition, systemic administration of nor-BNI also has a long-lasting κOR antagonism profile (Endoh et al., 1992; Butelman et al., 1993; Jewett and Woods, 1995). However, the mechanism underlying this unusual long-lasting antagonist effect remains unknown. Whether nor-BNI is resistant to metabolism after central or systemic administration needs to be studied further.
Interestingly, i.c. nor-BNI displays different antagonistic potencies against U50,488 and bremazocine. Both compounds have been characterized as high-efficacy κOR agonists in cell lines stably expressing the human κOR (Zhu et al., 1997; Remmers et al., 1999). In rhesus monkeys, both compounds also have been characterized as selective κOR agonists by a variety of behavioral assays, including antinociception, diuresis, and drug discrimination (Dykstra et al., 1987; Butelman et al., 1993; France et al., 1994; Ko et al., 1998a). In particular, the antinociceptive effects of both U50,488 and bremazocine were not influenced by clocin-namox, a functionally irreversible μ-opioid antagonist. Naltrexone in vivo pA2 analysis also indicated that U50,488 and bremazocine produced antinociception through different κOR populations (Ko et al., 1998a). In this study, differentiation of κOR agonists by i.c. nor-BNI antagonism supports previous findings that U50,488 produces antinociception by acting on κ1 receptors, whereas bremazocine probably on non-κ1 receptors based on pharmacological antagonism studies (Butelman et al., 1993; Ko et al., 1998a).
Nevertheless, there are several issues that need to be addressed regarding the κOR subtypes. The κ1-binding selectivity of nor-BNI in rhesus monkey cortex membranes is small, ~2- to 3- fold, based on κ1- versus κall-receptors in the presence of μ- and δ- receptor blockade (Butelman et al., 1998). However, the κ1-selectivity of nor-BNI in human membranes is ~250-fold, based on κ1- versus κ2-receptors in the presence of μ-, δ-, and κ1-receptor blockade (Kim et al., 1996). It is possible that the κ1-selectivity of nor-BNI in monkey membranes would be increased in the binding condition of further κ1-receptor blockade. Over a certain dose range, nor-BNI appears to be a functionally selective κ1-antagonist in rhesus monkeys, given that a single dose of nor-BNI (3.2 mg/kg s.c. or 0.32 mg i.c.) produced a significant and long-lasting antagonism against U50,488, but not bremazocine (Butelman et al., 1993; present study). However, bremazocine insensitivity to nor-BNI antagonism was only observed in primate studies, and not in rodent or pigeon studies (Horan et al., 1992; Jewett and Woods, 1995). It is possible that bremazocine produces effects through κ1 receptors in rodents because a recent in vitro study reported that bremazocine and U50,488 activate G proteins in guinea pig brain through the same κ1-receptors (Childers et al., 1998).
Bremazocine has been characterized as a κOR agonist across species and assays (Corbett and Kosterlitz, 1986; Dykstra et al., 1987; Horan et al., 1992; Caudle et al., 1994; France et al., 1994). Bremazocine displays decreased susceptibility to nor-BNI antagonism in monkeys and this may suggest that bremazocine is not a selective κ1-agonist, because we can not rule out the possibility of κ1-component involvement in the effects of bremazocine. A recent study has shown that bremazocine and U50,488 have similar magnitude of stimulation in guanosine-5′-O-(3-thio)triphosphate preparation in a cell line expressing the human κ1-receptor (Remmers et al., 1999). It is possible that bremazocine causes antinociception and sedation by activating both κ1- and non-κ1-receptors, and that non-κ1-receptors have an equal or larger contribution in bremazocine actions. To what extent this non-κ1-component contains other proposed κOR subtypes such as κ2 or κ3 is unknown. However, there is a possibility that newly reported κ-δ heterodimers constitute this non-κ1-population. A recent study has illustrated that κ-δ heterodimers do not possess high affinity for selective κOR agonists (e.g., U69,593) or antagonists (nor-BNI), but still maintain high affinity for bremazocine (Jordan and Devi, 1999). This binding profile is similar to that of previously proposed κ2-receptor subtype (Zukin et al. 1988; Kim et al., 1996).
Currently, there is no consensus for κOR subtype populations (Wollemann et al., 1993; Fowler and Fraser, 1994). The interpretation of certain opioid receptors in the presence of μ-, δ-, and κ1-receptor blockade has been complex because there are distinct nomenclatures (e.g., κ1a, b, κ2, κ3, ε) from various researchers (Zukin et al., 1988; Clark et al., 1989; Nock et al., 1990; Rothman et al., 1990; Kim et al., 1996). Due to both the lack of κOR subtype-selective antagonists and the lack of identification of κOR subtype clones, there have been difficulties in resolving these controversial issues. In addition, potential species differences also complicate the interpretation of in vivo studies. For example, naloxone benzoylhydrazone has been characterized as a κ3-analgesic in mice (Paul et al., 1990). However, naloxone benzoylhydrazone does not cause κOR agonist effects in either antinociception or drug discrimination assays in rhesus monkeys (France and Woods, 1992).
Site of Action of i.c. Nor-BNI Antagonism
The i.c. dose of nor-BNI used in this study is a very small dose compared with systemically effective doses in rhesus monkeys (Butelman et al., 1993, 1998). Previous studies have shown that s.c. nor-BNI 3.2 and 10 mg/kg antagonized U50,488 effects with 3- to 8-fold rightward shifts for 21 days. Considering that the mean body weight of the monkeys was 9.5 kg during this study, this indicates that the i.c. dose (0.32 mg) is ~100- to 300-fold smaller than systemically effective doses in this preparation. When this centrally effective dose was injected s.c. in the back, nor-BNI did not antagonize systemic U50,488 or bremazocine in terms of antinociception and sedation. This observation supports the notion that systemic administration of U50,488 and bremazocine produces antinociception and sedation through central κOR populations.
To confirm this central action of i.c. nor-BNI, we used an experimental pain model that evaluates the peripheral antinociceptive effects of U50,488 (Ko et al., 1998b). In this procedure, capsaicin was applied s.c. in the tail to evoke a nociceptive response, thermal allodynia, manifested as a reduced tail-withdrawal latency in normally innocuous water. It has been found that local administration of a small, systemically inactive dose of U50,488 (0.1 mg) can inhibit capsaicin-induced nociception. In particular, local administration of U50,488 produced peripheral antinociception (i.e., antiallodynia) through κ1-receptors (Ko et al., 1999). These observations have been replicated in the present study. Moreover, i.c. pretreatment with nor-BNI (0.32 mg) did not antagonize this peripheral antinociception of U50,488 in this procedure. These findings further support that i.c. nor-BNI mainly produces central κOR antagonism in rhesus monkeys.
It is worth noting that there is a difference in the antagonist duration between central and peripheral actions of nor-BNI. In a previous study, when 0.32 mg of nor-BNI was administered in the tail, it only produced antagonism against local U50,488 for 7 days (Ko et al., 1999). However, when the same dose was administered by the i.c. route, it produced antagonism against systemic U50,488 for 35 days (present study). This difference could be due to the different sites of action of nor-BNI or to different experimental conditions. In particular, local antinociceptive potency of opioid agonists is enhanced in the monkey’s tail pretreated with capsaicin, but not in the tail without capsaicin injection (Ko et al., 1998b, 1999). Under these conditions, opioid agonists may have easier access to neuronal opioid receptors because of perineurial disruption (Antonijevic et al., 1995) or enhanced axonal transport of opioid receptors in the periphery (Hassan et al., 1993). Thus, local administration of nor-BNI in the tail may not have a longer duration of antagonism because the activity of peripheral sensory fibers such as receptor availability is dynamically regulated or due to the differences in degree of nor-BNI distribution.
Collectively, the findings of this study demonstrate that i.c. administration of nor-BNI antagonizes central, but not peripheral κOR antinociception in rhesus monkeys. This central administration of nor-BNI can be used to evaluate peripherally selective κOR agonists in the future. The present study also provides functional evidence of two κOR subtype populations in the CNS, and may be used as the basis of further characterization of other κOR actions such as diuresis and hormone release.
Acknowledgments
We thank Dr. P. F. von Voigtlander for the generous gift of U50,488 and John Busenbark for technical assistance.
ABBREVIATIONS
- κOR
κ-opioid receptors
- nor-BNI
nor-binaltorphimine
- CNS
central nervous system
- U50,488
(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]benzeneacetamide
- i.c.
intracisternal
- MPE
maximum possible effect
Footnotes
This study was supported by U.S. Public Health Service Grant DA00254.
Preliminary results were presented at the 61th annual meeting of the College on Problems of Drug Dependence, Acapulco, Mexico, June 12–17, 1999.
References
- Antonijevic I, Mousa SA, Schäfer M, Stein C. Perineurial defect and peripheral opioid analgesia in inflammation. J Neurosci. 1995;15:165–172. doi: 10.1523/JNEUROSCI.15-01-00165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausch SB, Esteb TM, Terman GW, Chavkin C. Administered and endogenously released kappa opioids decrease pilocarpine-induced seizures and seizure-induced histopathology. J Pharmacol Exp Ther. 1998;284:1147–1155. [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Perez A, Kreek M-J. Effects of systemically administered dynorphin A(1–17) in rhesus monkeys. J Pharmacol Exp Ther. 1999;290:678–686. [PubMed] [Google Scholar]
- Butelman ER, Ko MC, Sobczyk-Kojiko K, Mosberg HI, Van Bemmel B, Zernig G, Woods JH. Kappa-opioid receptor binding populations in rhesus monkey brain: Relationship to an assay of thermal antinociception. J Pharmacol Exp Ther. 1998;285:595–601. [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther. 1993;267:1269–1276. [PubMed] [Google Scholar]
- Butelman ER, Woods JH. Effects of clonidine, dexmedetomidine and xylazine on thermal antinociception in rhesus monkeys. J Pharmacol Exp Ther. 1993;264:762–769. [PubMed] [Google Scholar]
- Caudle RM, Chavkin C, Dubner R. κ2 opioid receptors inhibit NMDA receptor-mediated synaptic currents in guinea pig CA3 pyramidal cells. J Neurosci. 1994;14:5580–5589. doi: 10.1523/JNEUROSCI.14-09-05580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers SR, Xiao R, Vogt L, Sim LJ. Lack of evidence of κ2-selective activation of G-proteins. κ opioid receptor stimulation of [35S]GTPγS binding in guinea pig brain. Biochem Pharmacol. 1998;56:113–120. doi: 10.1016/s0006-2952(98)00123-3. [DOI] [PubMed] [Google Scholar]
- Clark JA, Liu L, Price M, Hersh B, Edelson M, Pasternak GW. Kappa opiate receptor multiplicity: Evidence for two U50,488-sensitive κ1 subtypes and a novel κ3 subtype. J Pharmacol Exp Ther. 1989;251:461–468. [PubMed] [Google Scholar]
- Corbett AD, Kosterlitz HW. Bremazocine is an agonist at κ-opioid receptors and an antagonist at μ-opioid receptors in the guinea-pig myenteric plexus. Br J Pharmacol. 1986;89:245–249. doi: 10.1111/j.1476-5381.1986.tb11141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra LA, Gmerek DE, Winger G, Woods JH. Kappa opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects. J Pharmacol Exp Ther. 1987;242:413–420. [PubMed] [Google Scholar]
- Dykstra LA, Preston KL, Bigelow GE. Discriminative stimulus and subjective effects of opioids with mu and kappa activity: Data from laboratory animals and human subjects. Psychopharmacology. 1997;130:14–27. doi: 10.1007/s002130050208. [DOI] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tanaka C, Nagase H. Nor-binaltorphimine: A potent and selective κ-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn. 1992;316:30–42. [PubMed] [Google Scholar]
- Fowler CJ, Fraser GL. μ-, δ-, κ-Opioid receptors and their subtypes. A critical review with emphasis on radioligand binding experiments. Neurochem Int. 1994;24:401–426. doi: 10.1016/0197-0186(94)90089-2. [DOI] [PubMed] [Google Scholar]
- France CP, Medzihradsky F, Woods JH. Comparison of kappa opioids in rhesus monkeys: Behavioral effects and receptor binding affinities. J Pharmacol Exp Ther. 1994;268:47–58. [PubMed] [Google Scholar]
- France CP, Woods JH. Naloxone benzoylhydrazone is a μ-selective opioid antagonist without κ agonist effects in rhesus monkeys. Behav Pharmacol. 1992;3:133–141. [PubMed] [Google Scholar]
- Hassan AHS, Ableitner A, Stein C, Herz A. Inflammation of the rat paw enhances axonal transport of opioid receptors in the sciatic nerve and increases their density in the inflamed tissue. Neuroscience. 1993;55:185–195. doi: 10.1016/0306-4522(93)90465-r. [DOI] [PubMed] [Google Scholar]
- Horan P, Taylor J, Yamamura HI, Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther. 1992;260:1237–1243. [PubMed] [Google Scholar]
- Jewett DC, Woods JH. Nor-binaltorphimine: An ultra-long acting kappa-opioid antagonist in pigeons. Behav Pharmacol. 1995;6:815–820. [PubMed] [Google Scholar]
- Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature (Lond) 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Eun YA, Soh SM, Eun JS, Cho KP. Ligand binding profiles of U-69,593-sensitive and -insensitive sites in human cerebral cortex membranes: Evidence of kappa opioid receptors heterogeneity. Life Sci. 1996;58:1671–1679. doi: 10.1016/0024-3205(96)00142-7. [DOI] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Traynor JR, Woods JH. Differentiation of kappa opioid agonist-induced antinociception by naltrexone apparent pA2 analysis in rhesus monkeys. J Pharmacol Exp Ther. 1998a;285:518–526. [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Woods JH. The role of peripheral mu opioid receptors in the modulation of capsaicin-induced thermal nociception in rhesus monkeys. J Pharmacol Exp Ther. 1998b;286:150–156. [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Woods JH. Activation of peripheral κ-opioid receptors inhibits capsaicin-induced thermal nociception in rhesus monkeys. J Pharmacol Exp Ther. 1999;289:378–385. [PMC free article] [PubMed] [Google Scholar]
- Krulich L, Koenig JI, Conway S, McCann SM, Mayfield MA. Opioid kappa receptors and the secretion of prolactin (PRL) and growth hormone (GH) in the rat. I. Effects of opioid kappa receptor agonists bremazocine and U-50,488 on secretion of PRL and GH: Comparison with morphine. Neuroendocrinology. 1986;42:75–81. doi: 10.1159/000124252. [DOI] [PubMed] [Google Scholar]
- Leander JD. A kappa opioid effect: Increased urination in the rat. J Pharmacol Exp Ther. 1983;224:89–94. [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of kappa opioid agonists on cocaine- and food-maintained responding by rhesus monkeys. J Pharmacol Exp Ther. 1998;286:812–824. [PubMed] [Google Scholar]
- Millan MJ. Kappa-opioid receptor-mediated antinociception in the rat. I. Comparative actions of mu- and kappa-opioids against noxious thermal, pressure and electrical stimuli. J Pharmacol Exp Ther. 1989;251:334–341. [PubMed] [Google Scholar]
- Nock B, Giordano AL, Cicero TJ, O’Connor LH. Affinity of drugs and peptides for U-69,593-sensitive and -insensitive kappa opiate binding sites: The U-69,593-insensitive site appears to be the beta endorphin-specific epsilon receptor. J Pharmacol Exp Ther. 1990;254:412–419. [PubMed] [Google Scholar]
- Paul D, Levison JA, Howard DH, Pick CG, Hahn EF, Pasternak GW. Naloxone benzoylhydrazone (NalBzoH) analgesia. J Pharmacol Exp Ther. 1990;255:769–774. [PubMed] [Google Scholar]
- Picker MJ, Mathewson C, Allen RM. Opioids and rate of positively reinforced behavior: III. Antagonism by the long-lasting kappa antagonist norbinaltorphimine. Behav Pharmacol. 1996;6:495–504. [PubMed] [Google Scholar]
- Remmers AE, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. Opioid efficacy in a C6 glioma cell line stably expressing the human κ-opioid receptor. J Pharmacol Exp Ther. 1999;288:827–833. [PubMed] [Google Scholar]
- Rothman RB, Bykov V, de Costa BR, Jacobson AE, Rice KC, Brady LS. Interaction of endogenous opioid peptides and other drugs with four κ opioid binding sites in guinea pig brain. Peptides. 1990;11:311–331. doi: 10.1016/0196-9781(90)90088-m. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer ANM, Hogenboom F, Mulder AH. κ1- and κ2-opioid receptors mediating presynaptic inhibition of dopamine and acetylcholine release in rat neostriatum. Br J Pharmacol. 1997;122:520–524. doi: 10.1038/sj.bjp.0701394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F, Gaveriaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, Mattei MG, Charron G, Bloch B, Kieffer B. κ-Opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proc Natl Acad Sci USA. 1995;92:7006–7010. doi: 10.1073/pnas.92.15.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori AE, Ho BY, Naeseth JS, Portoghese PS. Nor-binaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J Pharmacol Exp Ther. 1988;246:255–258. [PubMed] [Google Scholar]
- Tortella FC, DeCoster MA. Kappa opioids: Therapeutic considerations in epilepsy and CNS injury. Clin Neuropharmacol. 1994;17:403–416. [PubMed] [Google Scholar]
- Wollemann M, Benyhe S, Simon J. The kappa-opioid receptor: Evidence for the different subtypes. Life Sci. 1993;52:599–611. doi: 10.1016/0024-3205(93)90451-8. [DOI] [PubMed] [Google Scholar]
- Woods JH, Winger G. Opioids, receptors, and abuse liability. In: Meltzer HY, editor. Psychopharmacology: The Third Generation of Progress. New York: Raven Press; 1987. pp. 1555–1564. [Google Scholar]
- Xi Z-X, Fuller SA, Stein EA. Dopamine release in the nucleus accumbens during heroin self-administration is modulated by kappa opioid receptors: An in vivo fast-cyclic voltammetry study. J Pharmacol Exp Ther. 1998;284:151–161. [PubMed] [Google Scholar]
- Zhu J, Chen C, Xue JC, Kunapuli S, DeRiel JK, Liu-Chen LY. Cloning of a human κ opioid receptor from the brain. Life Sci. 1995;56:PL 201–PL 207. doi: 10.1016/0024-3205(94)00507-o. [DOI] [PubMed] [Google Scholar]
- Zhu J, Luo LY, Li JG, Chen C, Liu-Chen LY. Activation of the cloned human kappa opioid receptor by agonists enhances [35S]GTPγS binding to membranes: Determination of potencies and efficacies of ligands. J Pharmacol Exp Ther. 1997;282:676–684. [PubMed] [Google Scholar]
- Zukin RS, Eghbali M, Olive D, Unterwald EM, Tempel A. Characterization and visualization of rat and guinea pig brain κ opioid receptors: Evidence for κ1 and κ2 opioid receptors. Proc Natl Acad Sci USA. 1988;85:4061–4065. doi: 10.1073/pnas.85.11.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]