Abstract
Radiofrequency ablation (RFA) has become a valuable therapeutic modality in cancer treatment over the last decade. In orthopedic surgery, RFA is used for the treatment of benign bone tumors and bone metastases. Complications are rare and, to our knowledge, bone fracture as a complication due solely to RFA has not been reported to date. In this report we describe two patients with a fracture in the calcar region of the femur as a complication of RFA treatment for bone malignancies. Since RFA is applied increasingly often, it is important to report this risk of fracture as a complication of treatment of lesions in the femoral calcar.
Keywords: Radiofrequency ablation, Complication, Fracture, Bone neoplasm, Metastasis, Chondrosarcoma
Introduction
Radiofrequency ablation (RFA) has become a valuable therapeutic modality in cancer treatment over the last decade [1, 2]. RFA is a minimally invasive surgical technique in which a high-frequency alternating current heats tissue, resulting in tissue necrosis [3]. RFA is applied in the treatment of both osseous tumors and soft-tissue tumors. In orthopedic surgery, RFA is commonly used for the treatment of osteoid osteoma. More recently, it is also being used as a palliative measure for painful bone metastases [4–7]. RFA provides excellent results, with primary and secondary success rates of 79–96 and 97–100%, respectively, for osteoid osteoma [6, 8–10]. In addition, it has been demonstrated that the use of RFA provides significant pain reduction in cancer patients with bone metastases [11–13]. Complications are rare and are mostly due to heat effects in surrounding tissues, such as burning of the skin or cellulitis [8, 14, 15]. To our knowledge, bone fracture as a complication merely due to RFA has not been reported to date. In this report we describe two patients with a fracture in the calcar region of the femur as a complication of RFA treatment for bone neoplasms. Both patients were informed that data concerning their case would be submitted for publication.
Case reports
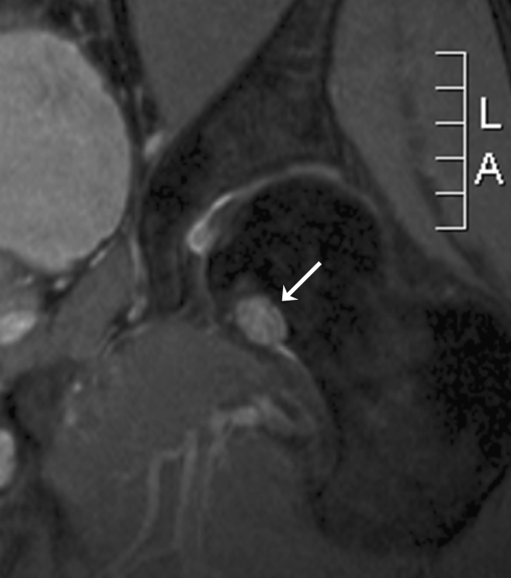
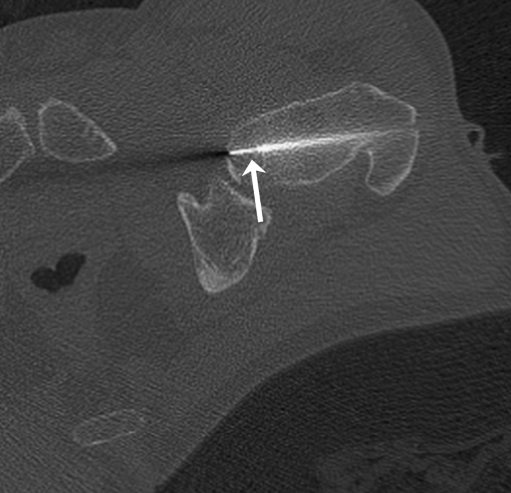
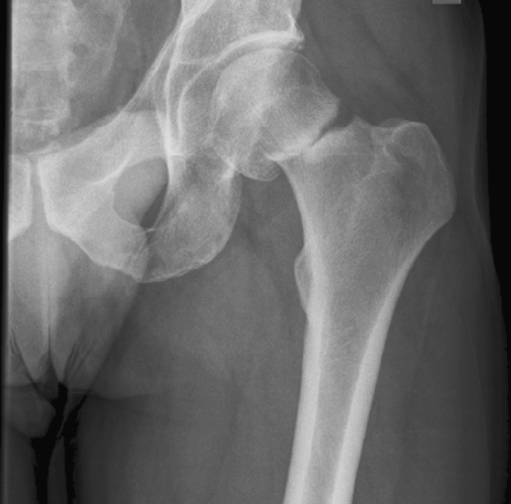
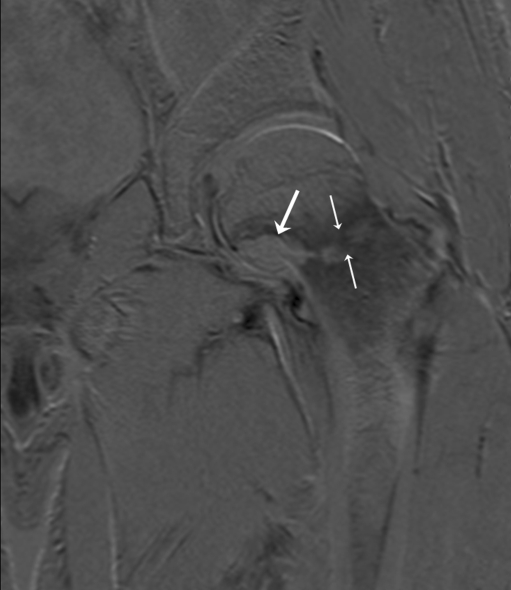
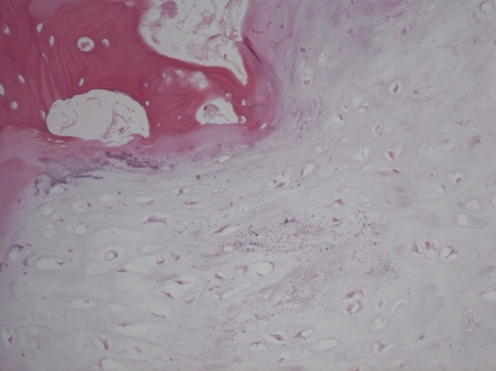
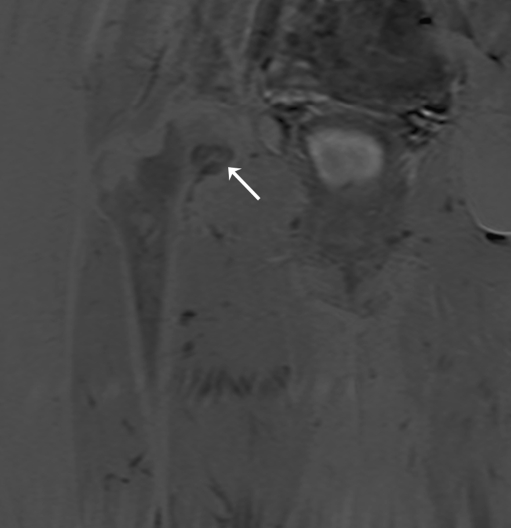
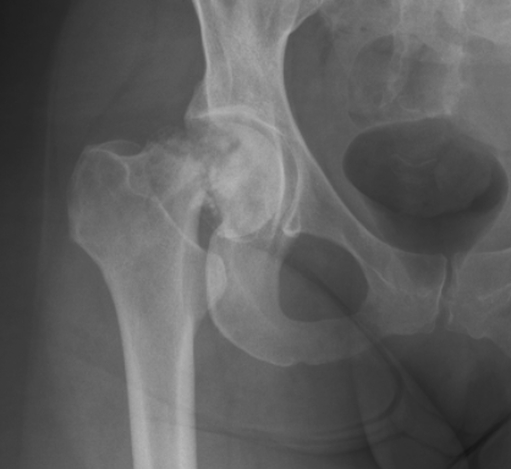
The first patient, a 52-year-old male, had an ill-understood pain in the left groin for 2 years, worsening at night. He had a psychiatric past with recurrent depression. The anesthesiologist and the neurologist could not find any abnormalities and referred him to the department of orthopedic surgery. There were no abnormalities in the left hip upon physical examination. Technetium bone scan did not show hot spots. MRI showed a small cyst-like lesion, which had a benign appearance (Fig. 1). Given the fact that a malignancy was very unlikely, the decision was made to perform a biopsy followed by RFA. Ablation was reached using a Soloisttm single needle electrode (Boston Scientific, Natick, MA, USA) under computed tomography (CT) guidance (Fig. 2). The needle was inserted through a 4.5 mm access hole, and a field of 15–20 mm in diameter was the anticipated size of the heated site. Ablation was reached in three cycles of 5–7 min, starting with 2 W and increasing by 1 W/min. Biopsies taken for histopathological evaluation revealed that the lesion was a chondrosarcoma (CS) grade I, which was a surprising finding. The patient recovered from the procedure and was pain-free after 3 months. Initial weight-bearing was 10 kg for 6 weeks, subsequently followed by 6 weeks of 50% body weight, for a total 3-month period of partial weight-bearing. Although clinical follow-up and MRI seemed unremarkable, the patient developed a pathological fracture while climbing up and down a ladder cleaning windows 3 months after the RFA procedure (Fig. 3). The fracture occurred at the exact location of the ablated lesion and could be seen retrospectively on earlier MR images (Fig. 4). The patient underwent surgery for total hip arthroplasty, with good clinical results over 3 years of follow-up. Follow-up has shown neither local tumor recurrence nor distant metastases in the lungs. Histopathology of tissue that had been retrieved during surgery showed complete necrosis of tumor tissue at the site of the thermal ablation, indicating a successful intervention (Fig. 5).
Fig. 1.
MRI scan of the femoral head of patient 1 prior to surgery. This 52-year-old man had suffered from groin pain for 2 years. Differential diagnosis based on the small, cyst-like lesion (arrow) includes bone cyst, enchondroma, and osteoid osteoma
Fig. 2.
CT-guided radiofrequency ablation (RFA) of the lesion in the collum femoris of patient 1. Following biopsy, the tip of the needle (arrow) is percutaneously inserted in the lesion
Fig. 3.
Plain X-ray of patient 1 3 months after RFA procedure. The fracture occurred while the patient was climbing up and down a ladder, and no trauma was involved
Fig. 4.
MRI subtraction after 3 months showing the fracture of the collum femoris at the site of the ablated lesion (small arrows) and absence of uptake of contrast fluid in the lesion (large arrow), indicating a successful RFA procedure
Fig. 5.
Representative histomorphology of the lesion in patient 1, showing completely necrotic hyaline cartilaginous tumor tissue surrounded by nonvital cancellous bone (magnified 40×)
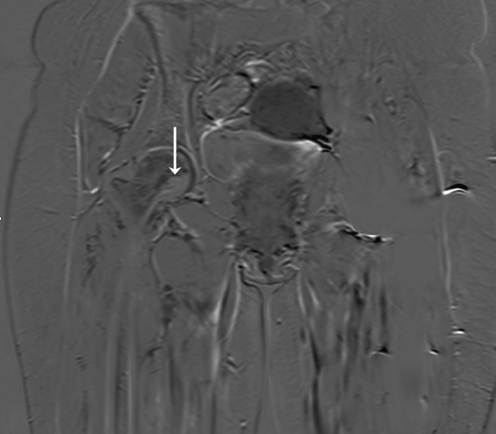
The second patient is a 48-year-old female with a follicular carcinoma of the thyroid with metastases in her left and right femur. A pathological fracture had developed in her left hip, which was initially treated with a gamma nail and subsequently replaced by a Global Modular Resection System (GMRS) tumor prosthesis (Stryker, Limerick, Ireland). Follow-up with MRI showed a lesion at the calcar region of her right femur (Fig. 6). For this lesion, RFA with a LeVeen® umbrella needle (Boston Scientific, Natick, MA, USA) under CT guidance was applied. Dimensions of the access hole and the expected ablated area were 4.5 mm and 20–30 mm in diameter, respectively (Fig. 7). In a single cycle of 21 min, a maximum of 20 W was delivered starting with 2 W and adding 1 W/min. Three months after treatment, the patient was pain-free with 50% weight-bearing, and MRI scans showed no uptake of contrast fluid (Fig. 8). However, a few days later the patient was admitted to our hospital with a pathological fracture of her right hip at the site of the previous ablation (Fig. 9). Retrospectively, no lesions could be seen on the MR images that had been made a few days earlier. Following diagnosis, the patient underwent total hip surgery with good functional results until she died of her primary disease, 2.5 years later. Histopathological analysis of the retrieved femoral neck showed that apart from necrosis, there was still viable tumor tissue in selected slides of the tumor.
Fig. 6.
MRI of patient 2, a 48-year-old woman with a follicular carcinoma of the thyroid. A lesion is shown in the calcar region of the collum femoris (arrow) suspicious for bone metastasis
Fig. 7.
RFA of the bone metastasis in the right collum femoris in patient 2. Ablation was performed with a LeVeen umbrella needle (Boston Scientific) (arrow)
Fig. 8.
MRI subtraction showing absence of contrast fluid in the ablated site (arrow) in patient 2, 3 months after the RFA procedure
Fig. 9.
Pelvic X-ray, showing a subcapital fracture of the right collum of patient 2, 3.5 months after thermal ablation
Discussion
In recent years, surgical excision has been replaced by RFA for the treatment of osteoid osteoma and some skeletal metastases. Because RFA is minimally invasive, precise, inexpensive, and has a low complication rate, this technique is gaining popularity and is being applied in an increasing number of patients [14–17]. At our center, using RFA under CT guidance, we have treated two patients with neoplasms in the subcapital calcar region of the femur who developed pathological fractures of the hip 3 months after treatment.
Potentially, this region is generally more at risk for fracturing, since fracturing after percutaneous cryotherapy has also been reported in the collum femoris [18]. However, heat conduction is very different from cold conduction and there is sufficient experience with RFA in the treatment of osteoid osteomas with only minor complications. We therefore thought it would be safe to perform thermal ablation in the first patient, yet the fracture occurred. Presumably, there is a protective effect from the thick sclerotic bone commonly surrounding an osteoid osteoma that is absent in CS and bone metastases. We therefore think that the risk of fracturing after RFA for malignancies can not be extrapolated to cases of osteoid osteoma. In case of bone metastases, we advocate ablating lesions only in the center of the femoral neck. Lesions located near the supporting cortex should only be ablated if hardware is used for stabilisation.
One may argue the use of thermal ablation in the first case, since RFA is not indicated for treatment of CS grade I. However, RFA was applied in this case under the suspection that the small lesion in the femoral head was benign. In many such cases, biopsy fails to reveal histological evidence and therefore the decision was made to take the biopsy and perform RFA in the same session. Although the size of the entrance, 4.5 mm, was larger than necessary, we preferred to insert the RF needle through the hole created by the biopsy needle.
Subsequent histology of material obtained with needle biopsy during the RFA procedure revealed the presence of a CS grade I, in which cure is intended either with curettage and adjuvant cryosurgery or phenol, or with wide resection. Generally, a CS grade I located at this site should have been treated by femoral head resection with prosthetic reconstruction. However, since the lesion was anticipated to be benign, resection was thought to be too aggressive. Although in retrospect RFA was not the correct option for the treatment of the lesion, final histology of the femoral head showed 100% lesion necrosis. Therefore it was demonstrated that the RFA therapy had been rather successful in terms of tumor ablation.
Considering the presence of a fracture on MR images in patient 1, it was surprising that clinical follow-up was quite unremarkable. The patient had only reported some minor discomfort. However he was on partial weight-bearing, which may have masked the presence of a fracture.
With the fracture of the first patient in mind, the possibility of the second patient developing a fracture was taken into account. The decision to perform thermal ablation was well calculated and the patient was comprehensively informed about the possible risks. The other option, total hip arthroplasty, was not favorable in her palliative setting and could still be performed if RFA treatment failed or fracture occurred.
RFA has proven to be a safe treatment modality for benign bone lesions and selected bone metastases. Since RFA gives excellent clinical results, more challenging sites are being treated, possibly leading to a higher number or severity of complications. The occurrence of a fracture at the calcar region of the femur after ablation in two subsequent patients indicates that this site is at risk for fracture after RFA treatment in malignant cases. Patients should be well informed about this risk and the treating physician should consider prophylactic internal fixation or decreased weight-bearing for more than 3 months. Since RFA is applied increasingly often, it is important to report this risk of fracture as a complication of the treatment of lesions in the femoral calcar.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Dupuy DE, Mayo-Smith WW, Abbott GF, Di Petrillo T. Clinical applications of radio-frequency tumor ablation in the thorax. Radiographics. 2002;22(Spec No):S259–S269. doi: 10.1148/radiographics.22.suppl_1.g02oc03s259. [DOI] [PubMed] [Google Scholar]
- 2.Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217(3):633–646. doi: 10.1148/radiology.217.3.r00dc26633. [DOI] [PubMed] [Google Scholar]
- 3.Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227(4):559–565. doi: 10.1097/00000658-199804000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallazzi MB, Arborio G, Garbagna PG, Perrucchini G, Daolio PA. Percutaneous radio-frequency ablation of osteoid osteoma: technique and preliminary results. Radiol Med (Torino) 2001;102(5–6):329–334. [PubMed] [Google Scholar]
- 5.Lee MH, Ahn JM, Chung HW, Lim HK, Suh JG, Kwag HJ, et al. Osteoid osteoma treated with percutaneous radiofrequency ablation: MR imaging follow-up. Eur J Radiol. 2007;64(2):309–314. doi: 10.1016/j.ejrad.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Lindner NJ, Ozaki T, Roedl R, Gosheger G, Winkelmann W, Wortler K. Percutaneous radiofrequency ablation in osteoid osteoma. J Bone Joint Surg Br. 2001;83(3):391–396. doi: 10.1302/0301-620X.83B3.11679. [DOI] [PubMed] [Google Scholar]
- 7.Monchik JM, Donatini G, Iannuccilli J, Dupuy DE. Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg. 2006;244(2):296–304. doi: 10.1097/01.sla.0000217685.85467.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akhlaghpoor S, Tomasian A, Arjmand Shabestari A, Ebrahimi M, Alinaghizadeh MR. Percutaneous osteoid osteoma treatment with combination of radiofrequency and alcohol ablation. Clin Radiol. 2007;62(3):268–273. doi: 10.1016/j.crad.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Cioni R, Armillotta N, Bargellini I, Zampa V, Cappelli C, Vagli P, et al. CT-guided radiofrequency ablation of osteoid osteoma: long-term results. Eur Radiol. 2004;14(7):1203–1208. doi: 10.1007/s00330-004-2276-6. [DOI] [PubMed] [Google Scholar]
- 10.Woertler K, Vestring T, Boettner F, Winkelmann W, Heindel W, Lindner N. Osteoid osteoma: CT-guided percutaneous radiofrequency ablation and follow-up in 47 patients. J Vasc Interv Radiol. 2001;12(6):717–722. doi: 10.1016/S1051-0443(07)61443-2. [DOI] [PubMed] [Google Scholar]
- 11.Goetz MP, Callstrom MR, Charboneau JW, Farrell MA, Maus TP, Welch TJ, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol. 2004;22(2):300–306. doi: 10.1200/JCO.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 12.Belfiore G, Tedeschi E, Ronza FM, Belfiore MP, Della VT, Zeppetella G, et al. Radiofrequency ablation of bone metastases induces long-lasting palliation in patients with untreatable cancer. Singapore Med J. 2008;49(7):565–570. [PubMed] [Google Scholar]
- 13.Kashima M, Yamakado K, Takaki H, Kaminou T, Tanigawa N, Nakatsuka A, et al. Radiofrequency ablation for the treatment of bone metastases from hepatocellular carcinoma. AJR Am J Roentgenol. 2010;194(2):536–541. doi: 10.2214/AJR.09.2975. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171–175. doi: 10.1148/radiol.2291021053. [DOI] [PubMed] [Google Scholar]
- 15.Finstein JL, Hosalkar HS, Ogilvie CM, Lackman RD. Case reports: an unusual complication of radiofrequency ablation treatment of osteoid osteoma. Clin Orthop Relat Res. 2006;448:248–251. doi: 10.1097/01.blo.0000214412.98840.a1. [DOI] [PubMed] [Google Scholar]
- 16.Callstrom MR, Charboneau JW. Percutaneous ablation: safe, effective treatment of bone tumors. Oncology (Williston Park) 2005;19(11 Suppl 4):22–26. [PubMed] [Google Scholar]
- 17.Jakobs TF, Hoffmann RT, Vick C, Wallnofer A, Reiser MF, Helmberger TK. RFA of bone and soft tissue tumors. Radiologe. 2004;44(4):370–375. doi: 10.1007/s00117-004-1030-z. [DOI] [PubMed] [Google Scholar]
- 18.Tuncali K, Morrison PR, Winalski CS, Carrino JA, Shankar S, Ready JE, et al. MRI-guided percutaneous cryotherapy for soft-tissue and bone metastases: initial experience. AJR Am J Roentgenol. 2007;189(1):232–239. doi: 10.2214/AJR.06.0588. [DOI] [PubMed] [Google Scholar]