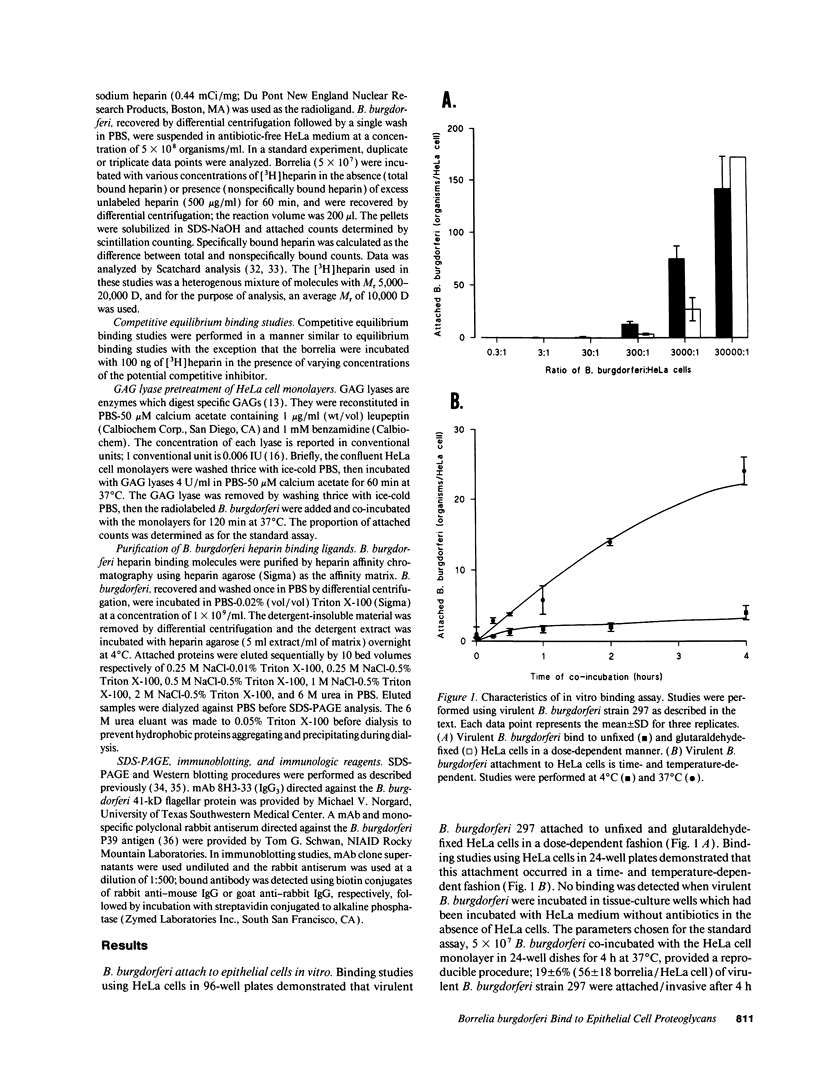
Abstract
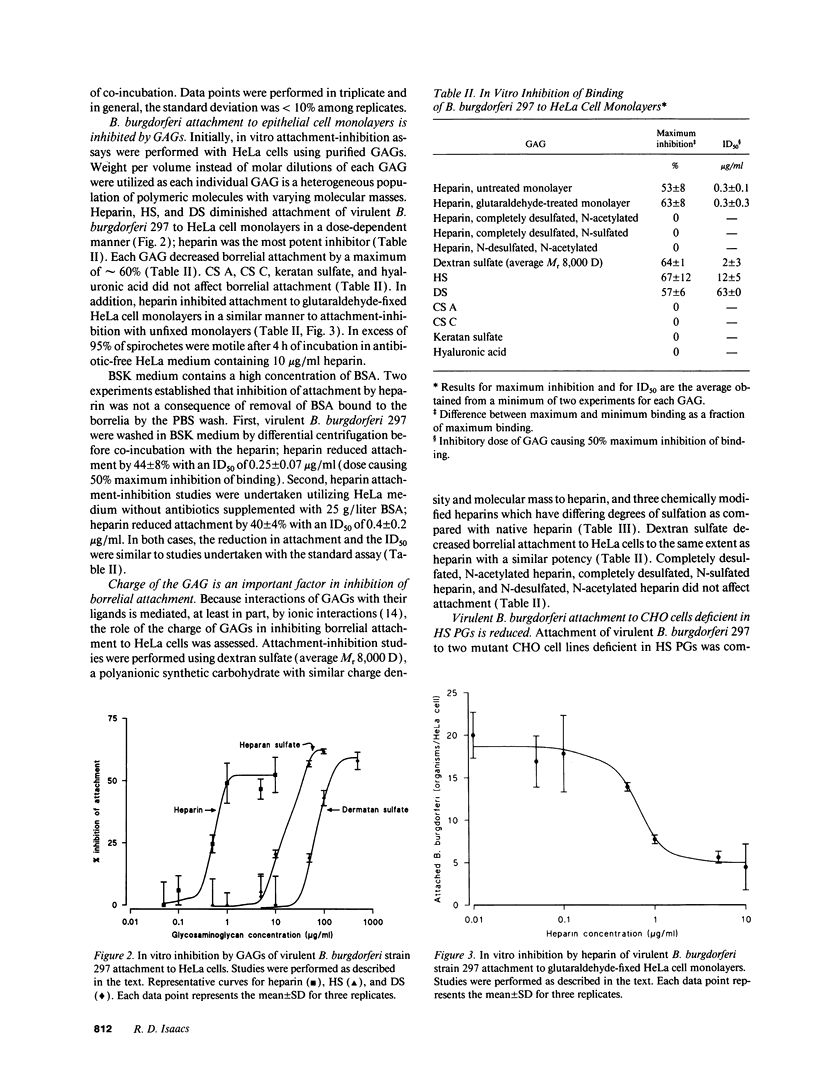
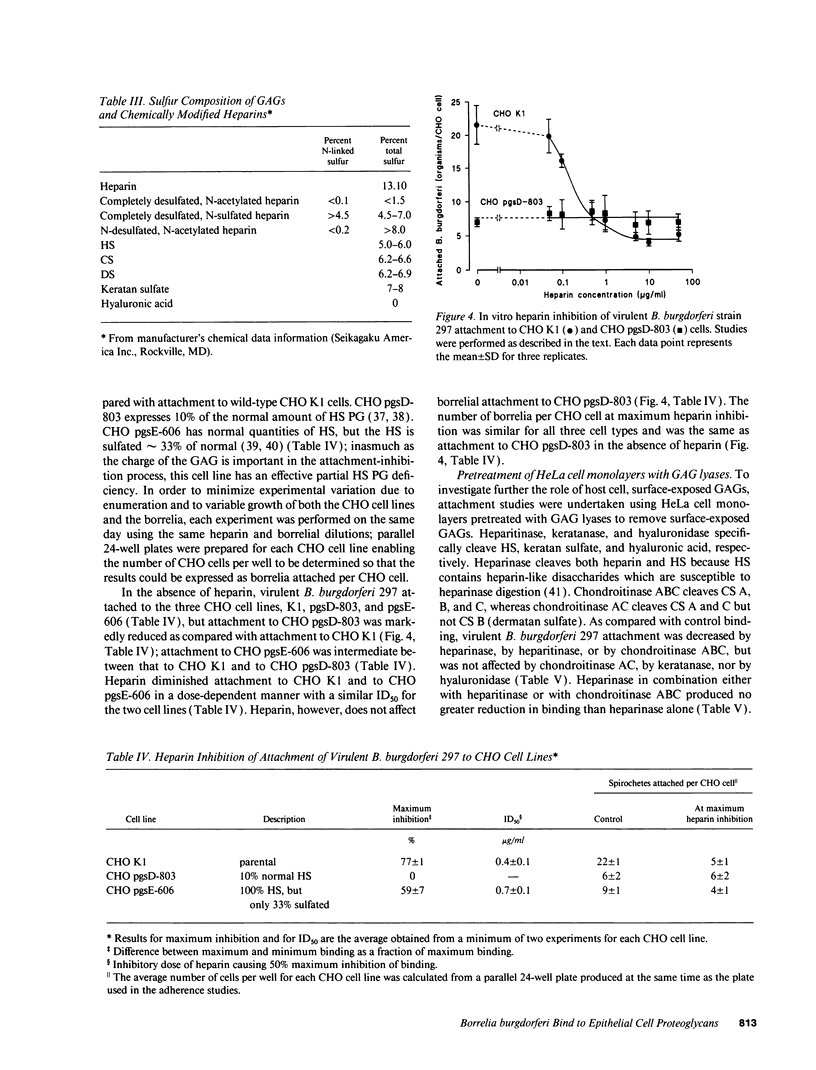
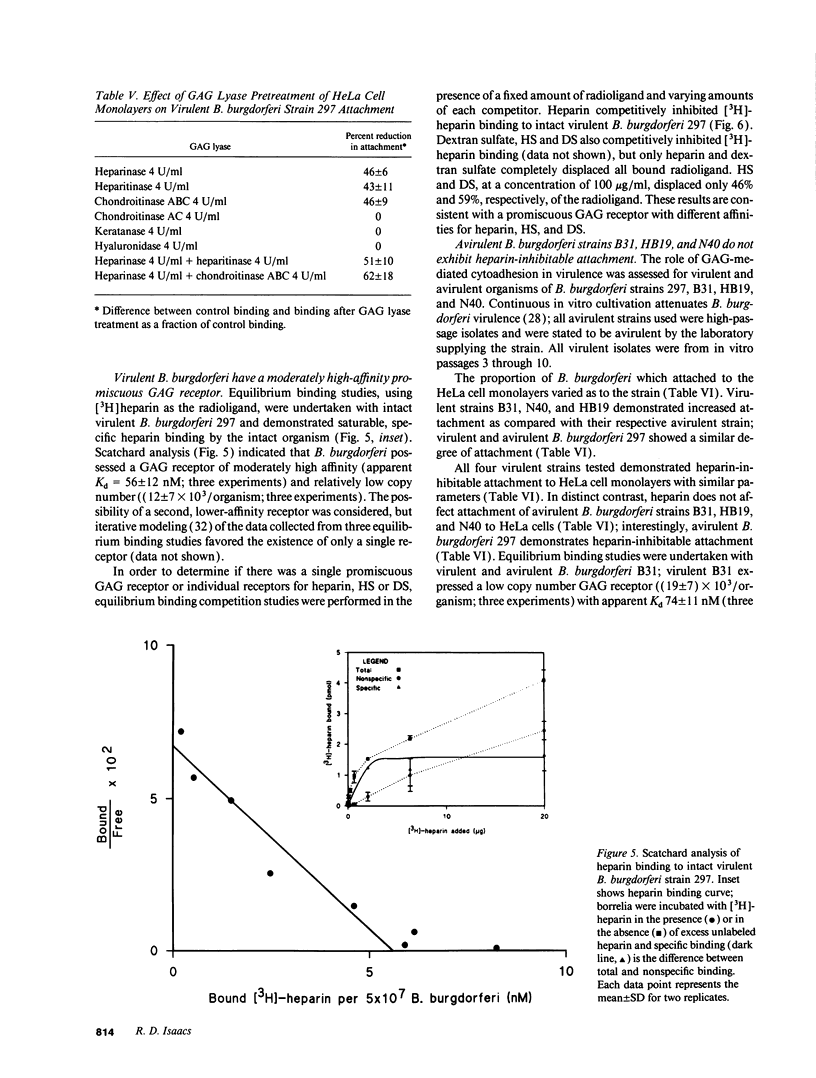
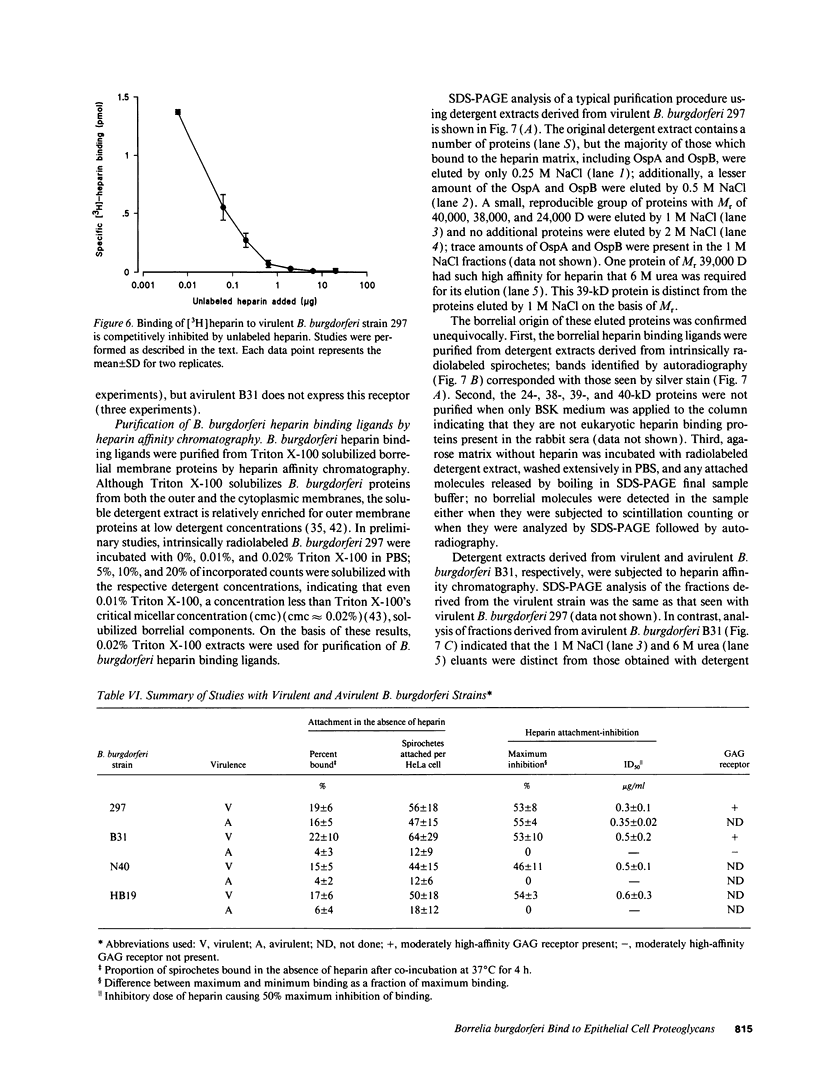
Borrelia burgdorferi adhere to mammalian cells in vitro but neither the ligand(s) nor the receptor(s) has (have) been clearly established. Using an in vitro attachment-inhibition assay, a B. burgdorferi attachment mechanism has been identified. Heparin, heparan sulfate, and dermatan sulfate reduced the attachment of virulent B. burgdorferi strain 297 to HeLa cells by approximately 60%. In addition, virulent, but not avirulent, B. burgdorferi strains B31, N40, and HB19 demonstrated heparin attachment-inhibition. Attachment to Chinese hamster ovary cells deficient in heparan sulfate proteoglycans was reduced by 68% compared to attachment to wild-type cells and was identical to attachment at maximum heparin inhibition to the wild-type cells. Pretreatment of HeLa cell monolayers with heparitinase, heparinase, and chondroitinase ABC, but not with chondroitinase AC, reduced borrelial attachment by approximately 50%. A moderately high affinity, low copy number, promiscuous B. burgdorferi glycosaminoglycan receptor was demonstrated by equilibrium binding studies. A 39-kD polypeptide, purified by heparin affinity chromatography from Triton X-100 extracts derived from virulent borrelia, was a candidate for this receptor. These studies indicate that one mode of B. burgdorferi attachment to eukaryotic cells is mediated by a borrelial glycosaminoglycan receptor attaching to surface-exposed proteoglycans on mammalian cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bame K. J., Esko J. D. Undersulfated heparan sulfate in a Chinese hamster ovary cell mutant defective in heparan sulfate N-sulfotransferase. J Biol Chem. 1989 May 15;264(14):8059–8065. [PubMed] [Google Scholar]
- Bame K. J., Lidholt K., Lindahl U., Esko J. D. Biosynthesis of heparan sulfate. Coordination of polymer-modification reactions in a Chinese hamster ovary cell mutant defective in N-sulfotransferase. J Biol Chem. 1991 Jun 5;266(16):10287–10293. [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Persing D. H., Armstrong A. L., Peeples R. A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991 Aug;139(2):263–273. [PMC free article] [PubMed] [Google Scholar]
- Brusca J. S., McDowall A. W., Norgard M. V., Radolf J. D. Localization of outer surface proteins A and B in both the outer membrane and intracellular compartments of Borrelia burgdorferi. J Bacteriol. 1991 Dec;173(24):8004–8008. doi: 10.1128/jb.173.24.8004-8008.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W. Lyme borreliosis: ten years after discovery of the etiologic agent, Borrelia burgdorferi. Infection. 1991 Jul-Aug;19(4):257–262. doi: 10.1007/BF01644963. [DOI] [PubMed] [Google Scholar]
- Butcher B. A., Shome K., Estes L. W., Choay J., Petitou M., Sie P., Glew R. H. Leishmania donovani: cell-surface heparin receptors of promastigotes are recruited from an internal pool after trypsinization. Exp Parasitol. 1990 Jul;71(1):49–59. doi: 10.1016/0014-4894(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Butcher B. A., Sklar L. A., Seamer L. C., Glew R. H. Heparin enhances the interaction of infective Leishmania donovani promastigotes with mouse peritoneal macrophages. A fluorescence flow cytometric analysis. J Immunol. 1992 May 1;148(9):2879–2886. [PubMed] [Google Scholar]
- Esko J. D., Rostand K. S., Weinke J. L. Tumor formation dependent on proteoglycan biosynthesis. Science. 1988 Aug 26;241(4869):1092–1096. doi: 10.1126/science.3137658. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Stewart T. E., Taylor W. H. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci U S A. 1985 May;82(10):3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S. Bacterial entry into eukaryotic cells. Cell. 1991 Jun 28;65(7):1099–1102. doi: 10.1016/0092-8674(91)90003-h. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Monco J. C., Fernandez-Villar B., Benach J. L. Adherence of the Lyme disease spirochete to glial cells and cells of glial origin. J Infect Dis. 1989 Sep;160(3):497–506. doi: 10.1093/infdis/160.3.497. [DOI] [PubMed] [Google Scholar]
- Georgilis K., Steere A. C., Klempner M. S. Infectivity of Borrelia burgdorferi correlates with resistance to elimination by phagocytic cells. J Infect Dis. 1991 Jan;163(1):150–155. doi: 10.1093/infdis/163.1.150. [DOI] [PubMed] [Google Scholar]
- Hechemy K. E., Samsonoff W. A., Harris H. L., McKee M. Adherence and entry of Borrelia burgdorferi in Vero cells. J Med Microbiol. 1992 Apr;36(4):229–238. doi: 10.1099/00222615-36-4-229. [DOI] [PubMed] [Google Scholar]
- Hechemy K. E., Samsonoff W. A., McKee M., Guttman J. M. Borrelia burgdorferi attachment to mammalian cells. J Infect Dis. 1989 Apr;159(4):805–806. doi: 10.1093/infdis/159.4.805. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V. Proteoglycans: structure, function, and role in neoplasia. Lab Invest. 1985 Oct;53(4):373–396. [PubMed] [Google Scholar]
- Jackson R. L., Busch S. J., Cardin A. D. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991 Apr;71(2):481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liang O. D., Ascencio F., Fransson L. A., Wadström T. Binding of heparan sulfate to Staphylococcus aureus. Infect Immun. 1992 Mar;60(3):899–906. doi: 10.1128/iai.60.3.899-906.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Sturrock A., Weis J. J. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect Immun. 1991 Feb;59(2):671–678. doi: 10.1128/iai.59.2.671-678.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menozzi F. D., Gantiez C., Locht C. Interaction of the Bordetella pertussis filamentous hemagglutinin with heparin. FEMS Microbiol Lett. 1991 Feb;62(1):59–64. doi: 10.1111/j.1574-6968.1991.tb04417.x. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C., Zsak L., Zuckermann F., Sugg N., Kern H., Ben-Porat T. Interaction of glycoprotein gIII with a cellular heparinlike substance mediates adsorption of pseudorabies virus. J Virol. 1990 Jan;64(1):278–286. doi: 10.1128/jvi.64.1.278-286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody K. D., Barthold S. W., Terwilliger G. A. Lyme borreliosis in laboratory animals: effect of host species and in vitro passage of Borrelia burgdorferi. Am J Trop Med Hyg. 1990 Jul;43(1):87–92. doi: 10.4269/ajtmh.1990.43.87. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay N. K., Shome K., Saha A. K., Hassell J. R., Glew R. H. Heparin binds to Leishmania donovani promastigotes and inhibits protein phosphorylation. Biochem J. 1989 Dec 1;264(2):517–525. doi: 10.1042/bj2640517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nader H. B., Dietrich C. P., Buonassisi V., Colburn P. Heparin sequences in the heparan sulfate chains of an endothelial cell proteoglycan. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3565–3569. doi: 10.1073/pnas.84.11.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer J. M. Detergents: an overview. Methods Enzymol. 1990;182:239–253. doi: 10.1016/0076-6879(90)82020-3. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Matsuzaki T., Sugahara Y., Okada J., Hasebe M., Iwamura Y., Ohnishi M., Kanno T., Shimizu M., Honda E. BHV-1 adsorption is mediated by the interaction of glycoprotein gIII with heparinlike moiety on the cell surface. Virology. 1991 Apr;181(2):666–670. doi: 10.1016/0042-6822(91)90900-v. [DOI] [PubMed] [Google Scholar]
- Ortega-Barria E., Pereira M. E. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell. 1991 Oct 18;67(2):411–421. doi: 10.1016/0092-8674(91)90192-2. [DOI] [PubMed] [Google Scholar]
- Radolf J. D., Chamberlain N. R., Clausell A., Norgard M. V. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent triton X-114. Infect Immun. 1988 Feb;56(2):490–498. doi: 10.1128/iai.56.2.490-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn D. W. Lyme disease: clinical manifestations, diagnosis, and treatment. Semin Arthritis Rheum. 1991 Feb;20(4):201–218. doi: 10.1016/0049-0172(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Rahn D. W., Malawista S. E. Lyme disease: recommendations for diagnosis and treatment. Ann Intern Med. 1991 Mar 15;114(6):472–481. doi: 10.7326/0003-4819-114-6-472. [DOI] [PubMed] [Google Scholar]
- Riley B. S., Oppenheimer-Marks N., Hansen E. J., Radolf J. D., Norgard M. V. Virulent Treponema pallidum activates human vascular endothelial cells. J Infect Dis. 1992 Mar;165(3):484–493. doi: 10.1093/infdis/165.3.484. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pekkala A., Engvall E. Effect of dextran sulfate on fibronectin-collagen interaction. FEBS Lett. 1979 Nov 1;107(1):51–54. doi: 10.1016/0014-5793(79)80461-5. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Diaz C., Nussenzweig V. Attachment of Trypanosoma cruzi trypomastigotes to receptors at restricted cell surface domains. Exp Parasitol. 1991 Jan;72(1):76–86. doi: 10.1016/0014-4894(91)90123-e. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh M. T., WuDunn D., Montgomery R. I., Esko J. D., Spear P. G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992 Mar;116(5):1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. J., Schrumpf M. E., Schwan T. G. Reactivity of human Lyme borreliosis sera with a 39-kilodalton antigen specific to Borrelia burgdorferi. J Clin Microbiol. 1990 Jun;28(6):1329–1337. doi: 10.1128/jcm.28.6.1329-1337.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski A., Benach J. L. Lyme borreliosis: host responses to Borrelia burgdorferi. Microbiol Rev. 1991 Mar;55(1):21–34. doi: 10.1128/mr.55.1.21-34.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski A., Furie M. B., Benach J. L., Lane B. P., Fleit H. B. Interaction between Borrelia burgdorferi and endothelium in vitro. J Clin Invest. 1990 May;85(5):1637–1647. doi: 10.1172/JCI114615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Comstock L. E. Interaction of Lyme disease spirochetes with cultured eucaryotic cells. Infect Immun. 1989 Apr;57(4):1324–1326. doi: 10.1128/iai.57.4.1324-1326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight T. N., Kinsella M. G., Lark M. W., Potter-Perigo S. Vascular cell proteoglycans: evidence for metabolic modulation. Ciba Found Symp. 1986;124:241–259. doi: 10.1002/9780470513385.ch13. [DOI] [PubMed] [Google Scholar]
- WuDunn D., Spear P. G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989 Jan;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. P., Stephens R. S. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell. 1992 May 29;69(5):861–869. doi: 10.1016/0092-8674(92)90296-o. [DOI] [PubMed] [Google Scholar]