Abstract
Vestibular reflexes are critically important for stabilizing gaze and maintaining posture, but comparatively little is known about conscious perceptions of vestibular stimuli and how they may relate to balance function. We used psychophysical methods to determine the ability of normal subjects and a vestibular-deficient subject to discriminate among velocities of earth-vertical sinusoidal rotations. Discrimination thresholds in normal subjects rose from 2.26 deg/s at a peak velocity of 20 deg/s up to 5.16 deg/s at 150 deg/s. The relationship between threshold and peak angular velocity was well described by the power law function ΔI = 0.88I0.37, where I is the magnitude of the stimulus and ΔI is the discrimination threshold. The subject with bilateral vestibular hypofunction had thresholds more than an order of magnitude worse than normals. The performance of normal subjects is much better than that predicted by Weber’s Law, which states that discrimination thresholds increase proportionally with stimulus magnitude (i.e., ΔI/I = C, where C is the “Weber fraction”). This represents a remarkable exception to other sensory systems and may reflect the vestibular system’s ability to stabilize gaze and maintain posture even at high stimulus intensities. Quantifying this relationship may help elucidate the role of higher-level processes in maintaining balance and provide information to diagnose and guide therapy of patients with central causes for imbalance.
Keywords: Semicircular canal, Imbalance, Psychophysics, Human, Vestibular, Discrimination threshold
Introduction
Vestibular input contributes to reflexive functions including gaze stabilization, postural control, and autonomic regulation. It also contributes to many vital non-reflexive, perceptual responses including body orientation (Friedmann 1970) and path integration (Glasauer et al. 2002). The mechanisms of vestibular perception and the relationship of perceptual dysfunction to imbalance and disorientation remain poorly understood.
Rigorous psychophysical methods used to study perception typically rely on quantifying the ability of an observer to discern between two similar stimuli distinguished only by a small difference in magnitude. This discrimination threshold is also known as the just-noticable-difference (JND) or difference limen (Green and Swets 1966). A fundamental law of psychophysics, known as Weber’s Law, states that the ratio between the discrimination threshold at a particular stimulus magnitude and the magnitude of the stimulus itself is constant regardless of stimulus magnitude (i.e., ΔI/I = C, where ΔI represents the discrimination threshold, I is the stimulus magnitude, and C is the “Weber fraction”) (Fechner 1860; Zanker 1995; Dehaene 2003; Brannon et al. 2008; Francisco et al. 2008). This relationship has been widely observed for behavioral responses to stimuli of many sensory systems (Laming 1986), but it has not yet been systematically studied to determine discrimination thresholds of rotational velocity that result from changes in stimulation of the vestibular system.
Vestibular afferents must provide accurate information about head movements over a wide range of velocities, frequencies, and accelerations. This allows the vestibular system to drive the vestibulo-ocular reflex (VOR) and other vestibular reflexes in order to stabilize gaze and maintain balance and posture. Current evidence suggests that the VOR demonstrates essentially constant levels of accuracy across a wide range of angular velocities and accelerations (Pulaski et al. 1981; Weber et al. 2008). If Weber’s Law holds for vestibular perception, it would suggest that central processes above the level of the basic VOR reflex pathway might introduce additional uncertainty into vestibular signals, limiting the performance of the system and increasing discrimination thresholds at higher stimulus magnitudes. It could also indicate that the signals that drive the VOR with constant accuracy are simply not available for higher-level processing or conscious awareness and that perceived rotation is independent of these vestibular afferent signals (Seemungal et al. 2004; Merfeld et al. 2005a, b; Wood et al. 2007; Clement et al. 2008). Alternatively, if psychophysical performance is better than suggested by Weber’s Law, it could indicate that higher-level processes maintain the same degree of certainty present at the level of the VOR. This would represent a remarkable exception to the performance of many other sensory systems.
Establishing the level of uncertainty present at various stages in vestibular information transmission may facilitate explorations of the higher-level operations of the vestibular system and elucidate the mechanisms of postural instability and imbalance caused by dysfunction of central balance-related processes. This is likely to be important with respect to both linear and rotational accelerations. Here, we determine whether the discrimination thresholds to rotational stimuli follow Weber’s Law or whether they surpass it, reflecting the fidelity with which afferents and the VOR operate.
Methods
Subject selection
The Human Studies Committee at Washington University approved this study. Nine right-handed male volunteer test subjects (age range: 16–34 years) participated in the study. Eight healthy subjects (age range: 23–34 years) read and provided a signed consent form and were included in the study as “normals”. None of these eight subjects reported a history of symptoms of vestibular dysfunction, including susceptibility to motion sickness. They showed normal responses to bedside tests including head impulses, post-headshaking nystagmus, and Dix-Hallpike examination. Their vestibular function was also tested using a chair rotating about an earth-vertical axis providing steps of velocity of 100 deg/s and sinusoidal rotations over the frequency range 0.025–0.64 Hz with peak velocities of 60 deg/s. None showed any signs of vestibular dysfunction as would be indicated by decreased gain or time constants in response to steps of velocity, or decreased gain and increased low-frequency phase lead in response to sinusoidal stimuli. One 16-year-old subject with profound hearing loss and vestibular dysfunction due to cytomegalovirus infection was also tested. His hearing loss (auditory thresholds >110 dB HL from 250 to 4,000 Hz) had been previously treated with bilateral cochlear implantation that gave him aided pure-tone thresholds <30 dB HL over the same frequency range. His vestibular loss was confirmed using icewater irrigations, which elicited no response from his right ear and only a 2 deg/s slow-phase nystagmus from his left ear. His response to rotational stimuli, as described above, elicited no measurable response. His other sensory and cognitive abilities were intact. He assented to testing and his parents read and signed a consent form in accordance with the study protocol approved by the Washington University Human Studies Committee.
Psychophysical methods
A two-interval, two-alternative forced-choice (2I, 2AFC) study design was used to determine behavioral discrimination thresholds for peak angular velocity of rotations about an earth-vertical axis. In a 2I, 2AFC paradigm, the subject is tested using multiple trials, each consisting of a pair of sequential stimulus intervals termed the “reference” and the “comparison” intervals (Green and Swets 1966; Macmillan and Creelman 2005), and the subject is asked to compare the stimulus in the first interval to that in the second interval. The 2I, 2AFC paradigm may be used in a “method of constant stimuli” experiment, where many trials are performed in which the reference stimulus is constant but the value of the comparison stimulus varies among trials. The percentage of correct answers at each value of the comparison stimulus determines the psychometric function. The “discrimination threshold” is defined as the difference between the reference and comparison values at which the subject achieves a defined percentage correct. This percentage is chosen by the examiner at a level that is convenient or experimentally relevant, commonly around 75% correct. In the special case where the reference stimulus is zero, the threshold is called the “detection threshold.” In the experiment described here, subjects were presented with two intervals of a rotational stimulus and asked to identify either the first or the second interval as “faster”.
Rotational stimulus
A standard rotational chair (System 2000, Micromedical Technologies, Chatham, IL) was used to deliver the test stimulus. The chair was contained within a lightproof booth that eliminated visual cues. Subjects were restrained using a four-point harness and cushioned with memory foam padding to minimize proprioceptive cues which might otherwise have contributed to perception (Walsh 1961; Gianna et al. 1996; Au Yong et al. 2007; Seidman 2008). External auditory cues were eliminated by using commercial ear muffs (−30 dB noise reduction ratio) in conjunction with a masking white noise generated by Matlab (Natick, MA) delivered to insert earphones. All subjects confirmed that they were unable to hear motor noise during the experiments. Each subject’s head was positioned to bring the horizontal canals into an earth-horizontal orientation by orienting each subject’s Reid’s plane 20 degrees chin-down (Della Santina et al. 2005). Custom-written software in Matlab was used to generate chair trajectories, and a National Instruments Data Acquisition device (BNC-2090, Austin, TX) in conjunction with the Matlab Data Acquisition Toolbox provided input to the chair controller. Chair vibration was monitored by recording the output of a rotational ratemeter (ADXRS150, Analog Devices) and three orthogonal accelerometers (ADXL202, Analog Devices), mounted on the chair adjacent to the head, at a rate of 1,000 samples/s.
Vestibular discrimination is difficult to test in rotational paradigms because of the possible confounding effect of the velocity storage mechanism. Velocity storage prolongs the neural representation of a rotational stimulus so that both reflexive and behavioral responses to a rotation may persist long after the rotation has ceased (Raphan et al. 1979). In a 2I, 2AFC paradigm, the velocity storage mechanism may cause the first stimulus to influence perception of the second stimulus. Although the influence of velocity storage on perceptual function has previously been questioned (Grabherr et al. 2008), other reports (Okada et al. 1999; Sinha et al. 2008) and our own preliminary tests found that it might have a significant effect on our results.
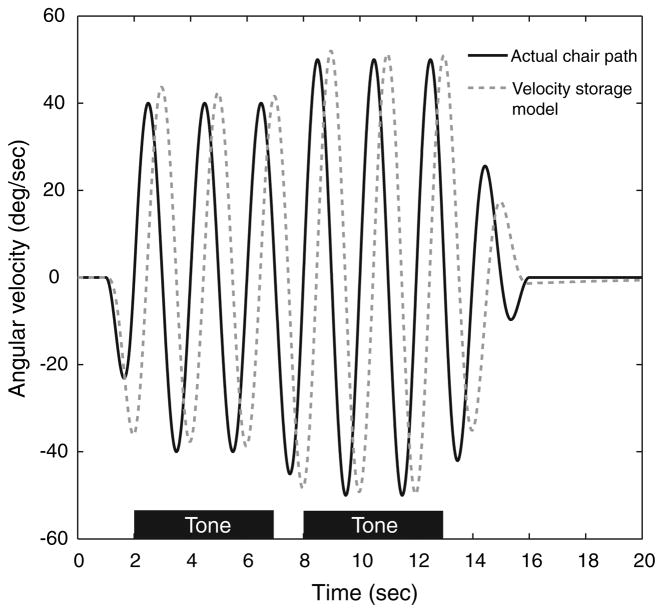
We generated a 2I, 2AFC task with an oscillating stimulus trajectory designed to minimize the effect of velocity storage. The stimulus consisted of a 0.5 Hz-sinusoidal stimulus about the earth-vertical axis. The peak velocity of the sinusoid increased during the initial second of stimulation along a raised cosine envelope from zero to a constant peak velocity lasting 5 s (representing the first interval of the 2I, 2AFC task), then changed along a raised cosine envelope over 1-s to a second constant peak velocity lasting 5 s (the second interval) before decreasing along a raised cosine envelope down to zero over the next 3 s (Fig. 1). The use of a sinusoidal stimulus minimized the effect of velocity storage. The cosine envelope allowed the generation of motion trajectories without discontinuities in velocity or any of its derivatives, which might otherwise have complicated the interpretation of data generated in this motion paradigm (Benson et al. 1986).
Fig. 1.
Stimulus paradigm. The angular velocity of the chair about an earth-vertical axis is shown in black. Two sequential intervals are presented, one at a reference peak velocity (here, 40 deg/s) and one at a comparison velocity somewhat higher than the reference velocity (here, 50 deg/s). The subject is cued with tones (black bars) to indicate the testing intervals and answers which of the two intervals is “faster.” The order of the reference and comparison velocities are randomized. The estimated velocity storage signal is shown as a gray dashed line. The peak amplitude of the estimated velocity storage signal is nearly equal to the peak stimulus velocity and extinguishes rapidly at the end of the stimulus
We implemented a basic model of the combined peripheral vestibular system and velocity storage mechanism to evaluate our stimulus trajectory. Our model used a cupular time constant of 5 s (Fernandez and Goldberg 1971; Dai et al. 1999) and a velocity storage time constant of 12 s (Raphan et al. 1979). The actual stimulus trajectory and the model of the perceived stimulus after accounting for the velocity storage mechanism are shown in Fig. 1. The discrimination threshold for the eight normal subjects was determined at reference peak velocities of 20, 40, 60, 80, and 100 deg/s. Seven of the eight normal subjects were also tested at 150 deg/s, and three were tested at 0 deg/s (to determine the detection threshold). The vestibular-deficient subject was tested at 0, 40, and 100 deg/s. White noise was presented throughout the trial via the earphones, with a superimposed tone signaling each stimulus interval. Subjects responded verbally at any time during the second stimulus or up to 10 s after returning to rest by identifying which of the two intervals was “faster.” There was a 10-s interval between trials during which the chair was stationary.
Adaptive procedure
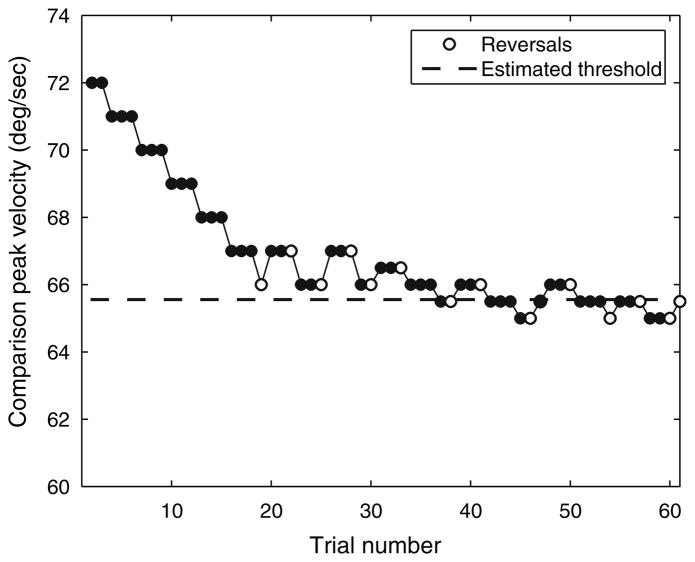
Constructing a full psychometric curve using a relatively long-duration stimulus (15 s) such as that described here would be a laborious and time-consuming process using the method of constant stimuli as described above. Following the example of a previous study (Grabherr et al. 2008), we therefore chose to use an adaptive procedure using a “3-down, 1-up” rule. In this procedure, the comparison stimulus is decreased to a level closer to the reference stimulus only after three consecutive correct responses, while it is increased to a level farther from the reference after just a single incorrect response (Leek 2001). Eventually, the comparison stimulus oscillates around an asymptotic value representing where the subject’s performance is equal to chance. At that point, the probability P of providing a wrong answer (1-up) equals the probability P of providing three correct answers in a row (3-down), so that P = 0.5. In order to achieve the probability of three correct answers in a row equaling 0.5, the chance of a single correct answer must be 0.794 (the cube root of 0.5). Therefore, a “3-down, 1-up” rule targets the 79.4% correct level of the psychometric function.
On a given run, the initial comparison peak velocity was set to exceed the reference peak velocity by at least twice the expected threshold determined by preliminary testing. For reference velocities of 20 deg/s and greater, the step size was initially set at 1 deg/s. After five reversals, the step size was decreased to 0.5 deg/s. The next nine reversals were averaged to calculate the discrimination threshold. For reference velocities of zero deg/s, the step size was held at 0.1 deg/s, and the first nine reversals were averaged to determine the detection threshold. This small step size ensured that the comparison velocity never equaled zero.
We also utilized a maximum-likelihood-estimation paradigm for determining thresholds (Wichmann and Hill 2001). This technique uses the subject’s responses to form an estimate of the entire psychophysical curve rather than simply determining the threshold value, as done by the adaptive paradigm. It allows testing in less time and with less within-subject variability, but has the disadvantage of requiring the general shape of the psychometric curve to be known (Leek et al. 2000). For the data analyzed here, we assumed the psychometric function to be a Weibull distribution, a sigmoidal distribution commonly used in psychophysical research (Wichmann and Hill 2001). We used the same data set for estimating thresholds using the maximum-likelihood-estimation paradigm as we used for estimating thresholds using the adaptive paradigm.
Subject testing
Testing for each subject was divided into two sessions. During the first session, subjects were acclimated to the chair and the experimental task during a training period. Subjects were presented with reference stimuli spanning the range of reference peak velocities and responded as they would during the experiment. Once a subject was comfortable with the task, the first adaptive run was begun, with the reference peak velocity chosen at random from among the test velocities. Discrimination thresholds for two additional randomly chosen peak reference velocities were obtained during the first session. Subjects were allowed a break between each of the three runs. Data for the remaining three test velocities were collected during the second session of testing.
Velocity storage controls
We conducted two experiments to confirm that our stimulus paradigm minimized the effect of velocity storage on our results. In the first, one subject underwent 140 trials at each of the seven reference velocities. The reference and comparison velocities were set to be equal for 20 trials at each reference velocity. These were interspersed randomly with other trials where the intervals were not equal. If velocity storage had been affecting the results of the perceptual task described here, the subjects should have been biased to select one interval or the other. In the second control experiment, we measured the eye movements of one subject by video-oculography following each of several trials at each reference velocity.
Results
To evaluate the possible contribution of vibration to our psychophysical results, we used Matlab to remove the 0.5-Hz signal representing the fundamental frequency of stimulation from the signal recorded from our rotational ratemeter and linear accelerometers and to lowpass filter the signals (cutoff frequency = 80 Hz). We determined the net linear acceleration during each sampling period by taking the vector sum of values recorded from the three orthogonal accelerometers. We considered the resulting rotational and linear signals, sampled at 1-ms periods, to represent vibration in the system.
We used the value for angular velocity in each period to construct a distribution of the values generated when the chair was rotated at reference velocity and another distribution of values generated when the chair was rotated at a comparison velocity equal to the reference velocity plus its corresponding threshold velocity. These distributions represented the rotational vibration during each condition. We compared vibration at the reference velocity to vibration at the reference velocity plus twice the average threshold for that velocity. There was no difference between the two distributions at any reference velocity (t-test; reference velocity = 0 deg/s, P = 0.908; reference velocity = 20 deg/s, P = 0.987; reference velocity = 40 deg/s, P = 0.998; reference velocity = 60 deg/s, P = 0.983; reference velocity = 80 deg/s, P = 0.993; reference velocity = 100 deg/s, P = 0.995; reference velocity = 150 deg/s, P = 0.996). A similar procedure was used to compare the net linear accelerational vibration at reference velocity to the net linear accelerational vibration at reference velocity plus the threshold for that velocity and again to the net linear accelerational vibration at reference velocity plus twice the threshold for that velocity. We compared linear vibration at each reference velocity to linear vibration at the reference velocity plus twice the average threshold for that velocity. There was no difference between the two distributions at any reference velocity (t-test; reference velocity = 0 deg/s, P = 0.966; reference velocity = 20 deg/s, P = 0.998; reference velocity = 40 deg/s, P = 0.976; reference velocity = 60 deg/s, P = 0.964; reference velocity = 8 deg/s, P = 0.927; reference velocity = 100 deg/s, P = 0.974; reference velocity = 150, P = 0.817).
Our first control experiment, designed to test the effect of velocity storage on our results, consisted of one subject undergoing 140 trials at each of the seven reference velocities. The reference and comparison velocities were set to be equal for twenty trials at each reference velocity. The subject showed no preference for one interval or the other at any of the reference velocities (χ2 > 0.05). In the second control experiment, the same subject showed no post-rotational nystagmus (measured by video-oculography) following each of several trials at each reference velocity. While studies (Merfeld et al. 2005a, b) have suggested that vestibuloocular reflexes and perception use qualitatively different mechanisms, the absence of post-rotational nystagmus suggests that our stimulus design was successful in minimizing the effect of velocity storage on perceptual responses. The absence of response bias is another indicator that velocity storage did not have a significant effect on the performance of our subjects.
A typical run for one subject with the reference velocity equal to 60 deg/s is shown in Fig. 2. Each run comprised an average of 78 trials and lasted approximately 35 min. No session needed to be terminated due to subject fatigue or other reasons. Detection thresholds for rotational stimuli have previously been shown to form a normal distribution only after performing a log transformation (Benson et al. 1989). Means, standard deviations, and statistical tests were therefore calculated in the log domain and then converted back for presentation here (Grabherr et al. 2008).
Fig. 2.
Sequence of answers provided by a subject over the course of one run using a “3-down, 1-up” adaptive paradigm. Each trial is indicated by a circle. A subject must answer correctly three times in a row before the comparison stimulus is reduced one step closer to the reference to make the task more difficult. A single error increases the comparison stimulus by an equal amount. Reversals are indicated by open circles. For reference velocities greater than or equal to 20 deg/s, the first five reversals are thrown out and the subsequent nine are averaged to determine the threshold. For reference velocities of 0 deg/s (detection threshold), the first nine reversals are averaged to determine the threshold
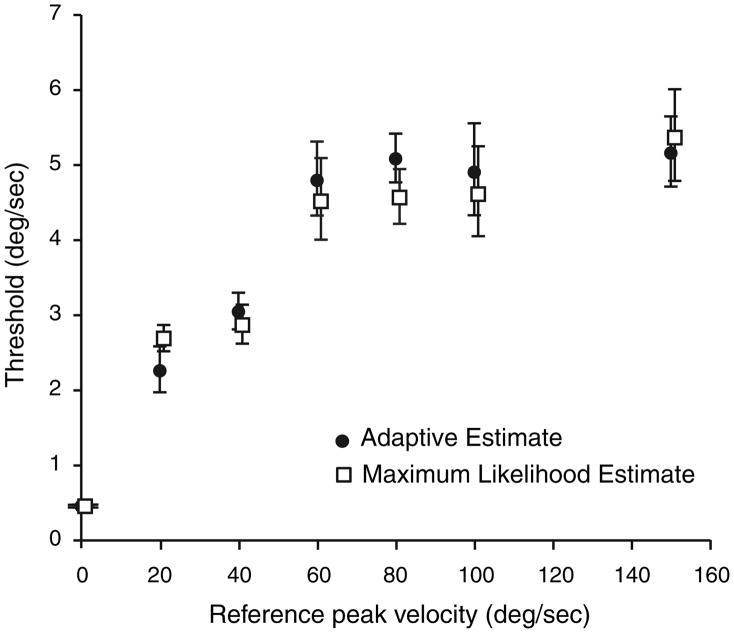
We first calculated detection thresholds using the adaptive paradigm described in the “Methods” section. The mean detection threshold (a reference velocity of 0 deg/s) was determined to be 0.46 deg/s, with discrimination thresholds rising up to a value of 5.16 deg/s at a reference velocity of 150 deg/s (Table 1). We then analyzed our results using a maximum-likelihood-estimation paradigm to determine whether the two paradigms gave similar results (Fig. 3). The mean detection threshold using the maximum-likelihood paradigm was determined to be 0.46 deg/s, increasing to a discrimination threshold of 5.37 deg/s at 150 deg/s. Two-tailed t-test statistics showed that the thresholds determined by the adaptive paradigm and the maximum-likelihood-estimate paradigm were not statistically distinguishable at any of the reference velocities tested. We chose to continue our analysis using data generated by the adaptive paradigm for three reasons. The data had originally been collected using an adaptive paradigm, we did not design our study to determine the shape of the psychometric curve as would be required for using a maximum-likelihood-estimation paradigm, and there was no statistical difference between the results using the two techniques.
Table 1.
Human discrimination thresholds (deg/s) to 0.5-Hz rotations about an earth-vertical axis for eight normal subjects
| Reference angular velocity | Adaptive paradigm |
Maximum likelihood estimate paradigm |
||||
|---|---|---|---|---|---|---|
| Mean discrimination threshold | Standard error (positive) | Standard error (negative) | Mean discrimination threshold | Standard error (positive) | Standard error (negative) | |
| 0 | 0.46 | 0.02 | 0.02 | 0.46 | 0.02 | 0.02 |
| 20 | 2.26 | 0.33 | 0.29 | 2.69 | 0.18 | 0.17 |
| 40 | 3.05 | 0.25 | 0.23 | 2.87 | 0.27 | 0.25 |
| 60 | 4.80 | 0.52 | 0.47 | 4.52 | 0.58 | 0.51 |
| 80 | 5.09 | 0.33 | 0.31 | 4.57 | 0.38 | 0.35 |
| 100 | 4.91 | 0.65 | 0.57 | 4.61 | 0.64 | 0.56 |
| 150 | 5.16 | 0.49 | 0.45 | 5.37 | 0.64 | 0.58 |
Fig. 3.
Discrimination thresholds of rotational velocities about an earth-vertical axis for both adaptive and maximum likelihood estimate paradigms. Thresholds (mean ± SEM as shown in Table 1) are plotted as a function of reference velocity. Filled circles represent estimates from the adaptive paradigm, and open squares represent results of the maximum likelihood estimate paradigm. No statistically significant difference was identified between estimate from the two paradigms at any of the reference velocities
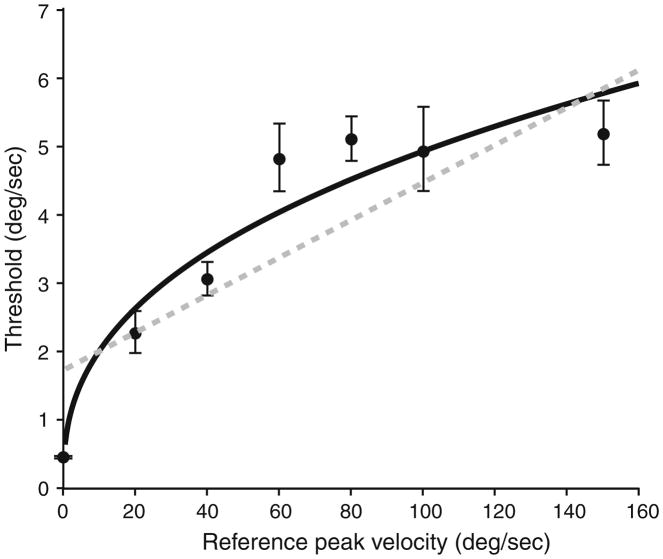
Weber’s Law requires that a linear function should describe the data well. The data presented here could be fit with the linear function ΔI = 0.03I + 1.72 (r2 = 0.69) but were fit substantially better with the power function ΔI = 0.88I0.37 (r2 = 0.88) (Fig. 4). Thresholds were determined at peak reference velocities of 0, 40 and 100 deg/s for the vestibular-deficient subject. His detection threshold at a reference velocity of 0 deg/s was 37.7 deg/s, his discrimination threshold at 40 deg/s was 31.1 deg/s, and his discrimination threshold at 100 deg/s was 34.4 deg/s.
Fig. 4.
Fits to discrimination thresholds plotted as a function of reference velocity (filled circles). Plotted points represent values determined using the adaptive paradigm. A power function (black line) fits the data according to the formula ΔI = 0.88I0.37 (r2 = 0.88). A first-order polynomial (gray dashed line) is a poorer fit to the data according to the formula ΔI = 0.03.I + 1.72 (r2 = 0.69)
Discussion
The mechanisms of vestibular perception and the effect of perceptual dysfunction on balance and orientation remain relatively poorly understood. A critical metric for the perception of a sensory stimulus is the observer’s ability to discriminate among signals that differ in magnitude along a physical dimension. The data presented here represent a critical advance from previous research measuring the psychophysical responses of the vestibular system by describing the discrimination thresholds of the vestibular system to rotations at reference velocities exceeding zero. They show that the vestibular system represents a remarkable exception from the performance of sensory systems as predicted by Weber’s Law. These results may help elucidate the mechanisms of sensory processing within the vestibular system and will form a normative data set for future studies of vestibular perception in normal and imbalanced subjects.
Normal subjects in this study were found to detect rotational motion about an earth-vertical axis with a threshold of 0.46 deg/s at a 79.4% correct level. Many authors have previously reported the detection threshold of normal subjects to angular rotations about an earth-vertical axis (Mach 1875; Guedry 1974; Benson 1982). Most recently, Benson et al. (1989) and Grabherr et al. (2008) used a two-alternative, single-interval forced choice task. In their experiments, subjects were seated in a rotational chair moving along a cosine bell velocity profile either toward the right or the left and were required to identify the correct direction of movement of the chair. Benson et al. found mean detection thresholds (defined as 75% correct) at 0.5 Hz of approximately 1.7 deg/s (Benson et al. 1989). Grabherr et al. found a mean detection threshold (defined as 79.4% correct) at 0.5 Hz of 0.73 deg/s (Grabherr et al. 2008).
Several factors may explain why the detection threshold values reported here are somewhat better than reported in these two earlier studies. The two earlier studies required subjects to identify the direction of movement correctly. This task is somewhat more difficult than simply detecting that motion was occurring as was done here (Benson et al. 1986; Kingma 2005). This difference may be due in part to an illusory reversal of direction occurring during the movement (Benson et al. 1986). The earlier studies also used a single stimulus cycle, which may be more difficult to perceive than paradigms that consisted of several cycles of sinusoidal movement as used here (Kingma 2005). Finally, the participants in the previous studies were up to at least 60 years old, but participants in the current study were a maximum of 34 years old (Benson et al. 1989; Grabherr et al. 2008). Some evidence suggests that perception of motion may deteriorate with age (Seemungal et al. 2004; Kingma 2005). If younger subjects have better thresholds, then the age difference between participants in this study and other studies may help explain the difference in thresholds observed.
The discrimination thresholds reported here were found to relate to stimulus magnitude according to a power law function with an exponent of 0.37. Discrimination thresholds to sensory stimuli generally obey Weber’s Law, which holds that they follow a linear relationship with respect to stimulus magnitude (equivalent to a power law function with an exponent of 1) (Baird 1997). Deviations from Weber’s Law have been observed previously in other sensory systems, although they were much smaller than those described here for the vestibular system. Guilford found that a power law with an exponent of 0.867 fit better than a linear function to data describing the performance of subjects discriminating among lines of different lengths (Kiesow 1926), giving rise to the term “Guilford’s Power Law” to describe this deviation from Weber’s Law (Guilford 1932; Baird 1997). Discrimination of loudness of pure tones has been shown to follow a power law with an exponent of 0.9375, forming what has been termed a “near miss” to Weber’s Law (McGill and Goldberg 1968). Sensation of vibration has also been shown to follow a power law forming a “near miss” to Weber’s Law (Gescheider et al. 1990). The results presented here represent a much more dramatic deviation from Weber’s Law than in these other examples. The vestibular system therefore appears to be uniquely able to maintain low discrimination thresholds even at high stimulus magnitudes.
An observer’s ability to determine the subjective magnitude of a stimulus, rather than discrimination thresholds as reported here, also depends on stimulus magnitude. That relationship has been shown in a wide range of sensory tasks to follow a power function (Stevens 1961). Known as “Stevens’ Power Law”, it is distinct from the power law relating discrimination thresholds to stimulus magnitude that describes the “near miss” to Weber’s Law and the data presented here (McGill and Goldberg 1968; Baird 1997). Stevens’ Law has been shown to apply to the magnitude estimation of rotational accelerations, but estimates of the value of the exponent have varied widely. In one experiment, subjects were exposed to constant rotational accelerations over a range of accelerations from 3 to 24 deg/s2 and stimulus durations up to 100 s (Brown 1966). They were asked to compare their subjective sense of stimulus magnitude to a comparison stimulus of 9 deg/s2. Interpretation of this experiment was complicated by the adaptation of subjects exposed to long-duration accelerations, but the subjective magnitude of acceleration was found to relate to the actual magnitude according to a power law with an exponent of 1.3 (Brown 1966). In another experiment, subjects were rotated sinusoidally about an earth-vertical axis at 0.32 Hz, with angular velocities ranging from 2 to 84 deg/s. They rated their sense of stimulus intensity on a numeric scale. Subjective stimulus intensity was found to relate to the stimulus acceleration by a power law with an exponent of 0.73 (Benson and Brown 1992). The higher values in the prior study were surmised to be due to a stimulus range effect (Benson and Brown 1992).
The data shown here represent performance based on sensory input from the horizontal semicircular canals. The canals and otolith organs act in a coordinated fashion to provide vestibular information to the central nervous system (Day and Fitzpatrick 2005). The psychophysical performance of the otolith organs may be at least as important as the semicircular canals in maintaining posture and equilibrium. Several previous studies have examined psychophysical performance in response to linear accelerations (attributable to the responses of the otolith organs). Detection thresholds for linear motion have been found to be somewhat better in the interaural direction than in the anteroposterior direction, and both were much better than motion along the cranial-caudal axis (Benson et al. 1986; Kingma 2005). Thresholds were found to deteriorate somewhat with age in the anteroposterior direction but not in the interaural direction (Kingma 2005). As in studies of rotation (Benson et al. 1989; Grabherr et al. 2008), studies of linear accelerations have shown that thresholds decrease with higher-frequency accelerations (Benson et al. 1986). Stevens’ power law relationship for magnitude estimation of linear accelerations has been shown to have an exponent greater than one, but the adherence of the perception of linear accelerations to Weber’s Law has not been reported (Golding and Benson 1993).
Previous psychophysical studies of the vestibular system have been interpreted to indicate that the sensitivity of primary vestibular afferents may be critical to determining the psychophysical thresholds of the vestibular system with respect to rotations (Grabherr et al. 2008). Vestibular afferents leading from the semicircular canals have lower sensitivity at lower frequencies of stimulation and higher sensitivity at higher frequencies (Fernandez and Goldberg 1971; Hullar et al. 2005b). This suggests that, if the system were limited by the gain of the signal provided by the afferents, it should have better performance at higher frequencies. This is consistent with the finding that normal human subjects have poorer perceptual detection thresholds at lower frequencies of rotation (approximately 2.8 deg/s at 0.05 Hz) and better thresholds at higher frequencies (approximately 0.6 deg/s at 1.0 Hz) (Benson et al. 1989; Grabherr et al. 2008). A similar argument has been made relating the responses of otolith afferents to the psychophysical responses to linear accelerations (Benson et al. 1986).
The sensitivity of vestibular-nerve afferents remains constant regardless of the peak velocity of sinusoidal stimulation at any particular frequency (Baird et al. 1988; Hullar et al. 2005b). The performance of the VOR, which may serve as an indirect measure of afferent performance, is also independent of velocity even at higher stimulus amplitudes (Pulaski et al. 1981; Weber et al. 2008). In contrast, the perceptual thresholds shown here worsen with increasing peak velocities (although this change becomes relatively minor at higher velocities). A more detailed understanding of the relationship between afferent responses and perceptual performance will require a better understanding of the mechanism by which vestibular afferents encode head rotations (Sadeghi et al. 2007; Hullar 2008).
The exceptional performance of the vestibular system described here could be related to extravestibular sensory information available at higher peak angular velocities. Studies that minimized extravestibular information have found that the perceptual performance of vestibular-deficient subjects was dramatically worse than normals, with thresholds sometimes up to about an order of magnitude worse than normals (Walsh 1961; Okada et al. 1999), However, in one psychometric study of linear accelerations, vestibular-deficient subjects had thresholds quite similar to normal subjects. This finding was thought to relate to the presence of extravestibular information that improved their performance (Gianna et al. 1996). We attempted to minimize non-vestibular cues by utilizing a light-proof chamber, presenting masking noise under noise-reducing earmuffs, and securing subjects with memory foam. Recognizing that vibrational stimuli are known to contribute to perception of self-motion (Au Yong et al. 2007; Seidman 2008), we attempted to minimize motor vibration by carefully balancing the mass of the chair and subjects about the axis of motor rotation and limiting the size of our subjects to less than 175 lb to minimize the torque required by the motor. We verified that there was no significant difference between levels of vibration present at the reference and comparison angular velocities, indicating that vibrational cues should have been unable to contribute meaningfully to the performance recorded here. Other sources, such as air traveling across the skin or truncal gravireceptors, could still have had some effect (Mittelstaedt 1998).
The performance of our vestibular-deficient subject shows that these and other extravestibular inputs were of negligible influence on our results. Other than his vestibular system, his sensory modalities were intact, including proprioception, skin sensation, vibration sensation, and hearing (corrected to <30 dB HL with his cochlear implants). The reason he had any response at all to vestibular stimulation is unclear. He did have a slow-phase nystagmus of 2 deg/s in response to icewater calorics in his left ear, suggesting a minimal level of residual vestibular function that may have contributed to his performance. This residual vestibular signal may explain why his thresholds, like those of subjects with normal vestibular function, did not continue to rise at higher stimulus amplitudes. He reported that he believed his sensation of rotation was due to proprioceptive input from centripetal force on his body provided by the harness and supportive foam during rotations.
We chose to use a stimulus frequency of 0.5 Hz because it falls within the range where vestibular input is important for stabilizing gaze (Leigh and Zee 2006), it encompasses typical head movements (Grossman et al. 1988; MacDougall and Moore 2005), it lies on the flat portion of the Bode plot describing the dynamics of the semicircular canals (Wilson and Jones 1979), the torque of our motion delivery system could attain a wide range of peak velocities at this frequency, and 0.5 Hz stimuli are commonly used by standard clinical rotational chairs (Hullar et al. 2005a). This means that experiments or clinical tests using the same paradigm may be carried out in any of the many clinical and research centers with access to similar equipment.
We recognize that there are multiple remaining questions to be answered surrounding the paradigm described here. While we have presented our data in terms of peak velocity, it is possible that subjects were actually sensing acceleration, jerk, or other motion parameters. Further study will be required to distinguish these possibilities. Vestibular dysfunction can occur asymmetrically, meaning that a bidirectional stimulus such as that described here could elicit different responses in one direction than in the other. While this represents a possible confounding variable, it may actually be helpful in determining the site of a particular vestibular lesion.
We anticipate that the results presented here will help reveal the basic functioning of information transmission in the vestibular system and causes for imbalance in patients who present without clinical signs or symptoms of peripheral vestibular dysfunction. Development of a panel of trajectories, including linear motions using other equipment, may offer a novel means to identify sources of dysfunction such as “retrolabyrinthine” lesions in the vestibular nerve and subcortical and cortical vestibular pathways that affect balance function.
Acknowledgments
We would like to thank Dora Angelaki, Larry Snyder, and Nancy Tye-Murray for their thoughtful contributions to this work. Support provided by NIH/NIDCD grant K08 DC 006869 (TEH) and by the Howard Hughes Medical Institute (RMM and TEH).
Contributor Information
Robert M. Mallery, Department of Otolaryngology-Head and Neck Surgery, Washington University School of Medicine, 660 South Euclid Avenue #8115, Saint Louis, MO 63110, USA
Osarenoma U. Olomu, Department of Otolaryngology-Head and Neck Surgery, Washington University School of Medicine, 660 South Euclid Avenue #8115, Saint Louis, MO 63110, USA
Rosalie M. Uchanski, Department of Otolaryngology-Head and Neck Surgery, Washington University School of Medicine, 660 South Euclid Avenue #8115, Saint Louis, MO 63110, USA, Program in Audiology and Communication Sciences, Washington University School of Medicine, Saint Louis, MO, USA
Valentin A. Militchin, Department of Otolaryngology-Head and Neck Surgery, Washington University School of Medicine, 660 South Euclid Avenue #8115, Saint Louis, MO 63110, USA
Timothy E. Hullar, Email: hullart@ent.wustl.edu, Department of Otolaryngology-Head and Neck Surgery, Washington University School of Medicine, 660 South Euclid Avenue #8115, Saint Louis, MO 63110, USA, Program in Audiology and Communication Sciences, Washington University School of Medicine, Saint Louis, MO, USA, Department of Anatomy and Neurobiology, Washington University School of Medicine, Saint Louis, MO, USA
References
- Au Yong N, Paige GD, Seidman SH. Multiple sensory cues underlying the perception of translation and path. J Neurophysiol. 2007;97:1100–1113. doi: 10.1152/jn.00694.2006. [DOI] [PubMed] [Google Scholar]
- Baird JC. Sensation and judgment. Lawrence Erlbaum Associates; Mahwah, NJ: 1997. [Google Scholar]
- Baird RA, Desmadryl G, Fernandez C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol. 1988;60:182–203. doi: 10.1152/jn.1988.60.1.182. [DOI] [PubMed] [Google Scholar]
- Benson AJ. The vestibular sensory system. In: Barlow HB, Mollon JD, editors. The senses. Cambridge University Press; Cambridge: 1982. pp. 333–368. [Google Scholar]
- Benson AJ, Brown SF. Perception of liminal and supraliminal whole-body angular motion. In: Berthoz A, Graf W, Vidal PP, editors. The head-neck sensory motor system. Oxford University Press; New York: 1992. pp. 483–487. [Google Scholar]
- Benson AJ, Spencer MB, Stott JR. Thresholds for the detection of the direction of whole-body, linear movement in the horizontal plane. Aviat Space Environ Med. 1986;57:1088–1096. [PubMed] [Google Scholar]
- Benson AJ, Hutt EC, Brown SF. Thresholds for the perception of whole body angular movement about a vertical axis. Aviat Space Environ Med. 1989;60:205–213. [PubMed] [Google Scholar]
- Brannon EM, Libertus ME, Meck WH, Woldorff MG. Electrophysiological measures of time processing in infant and adult brains: Weber’s Law holds. J Cogn Neurosci. 2008;20:193–203. doi: 10.1162/jocn.2008.20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JH. Magnitude estimation of angular velocity during passive rotation. J Exp Psychol. 1966;72:169–172. doi: 10.1037/h0023439. [DOI] [PubMed] [Google Scholar]
- Clement G, Tilikete C, Courjon JH. Retention of habituation of vestibulo-ocular reflex and sensation of rotation in humans. Exp Brain Res. 2008;190:307–315. doi: 10.1007/s00221-008-1471-0. [DOI] [PubMed] [Google Scholar]
- Dai M, Klein A, Cohen B, Raphan T. Model-based study of the human cupular time constant. J Vestib Res. 1999;9:293–301. [PubMed] [Google Scholar]
- Day BL, Fitzpatrick RC. The vestibular system. Curr Biol. 2005;15:R583–R586. doi: 10.1016/j.cub.2005.07.053. [DOI] [PubMed] [Google Scholar]
- Dehaene S. The neural basis of the Weber-Fechner law: a logarithmic mental number line. Trends Cogn Sci. 2003;7:145–147. doi: 10.1016/s1364-6613(03)00055-x. [DOI] [PubMed] [Google Scholar]
- Della Santina CC, Potyagaylo V, Migliaccio AA, Minor LB, Carey JP. Orientation of human semicircular canals measured by three-dimensional multiplanar CT reconstruction. J Assoc Res Otolaryngol. 2005;6:1–16. doi: 10.1007/s10162-005-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechner G. Elemente der Psychophysik. Breitkopf & Härtel; Leipzig: 1860. [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophys. 1971;34:661–675. doi: 10.1152/jn.1971.34.4.661. [DOI] [PubMed] [Google Scholar]
- Francisco E, Tannan V, Zhang Z, Holden J, Tommerdahl M. Vibrotactile amplitude discrimination capacity parallels magnitude changes in somatosensory cortex and follows Weber’s Law. Exp Brain Res. 2008;191:49–56. doi: 10.1007/s00221-008-1494-6. [DOI] [PubMed] [Google Scholar]
- Friedmann G. The judgement of the visual vertical and horizontal with peripheral and central vestibular lesions. Brain. 1970;93:313–328. doi: 10.1093/brain/93.2.313. [DOI] [PubMed] [Google Scholar]
- Gescheider GA, Bolanowski SJ, Jr, Verrillo RT, Arpajian DJ, Ryan TF. Vibrotactile intensity discrimination measured by three methods. J Acoust Soc Am. 1990;87:330–338. doi: 10.1121/1.399300. [DOI] [PubMed] [Google Scholar]
- Gianna C, Heimbrand S, Gresty M. Thresholds for detection of motion direction during passive lateral whole-body acceleration in normal subjects and patients with bilateral loss of labyrinthine function. Brain Res Bull. 1996;40:443–449. doi: 10.1016/0361-9230(96)00140-2. [DOI] [PubMed] [Google Scholar]
- Glasauer S, Amorim MA, Viaud-Delmon I, Berthoz A. Differential effects of labyrinthine dysfunction on distance and direction during blindfolded walking of a triangular path. Exp Brain Res. 2002;145:489–497. doi: 10.1007/s00221-002-1146-1. [DOI] [PubMed] [Google Scholar]
- Golding JF, Benson AJ. Perceptual scaling of whole-body low frequency linear oscillatory motion. Aviat Space Environ Med. 1993;64:636–640. [PubMed] [Google Scholar]
- Grabherr L, Nicoucar K, Mast FW, Merfeld DM. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res. 2008;186:677–681. doi: 10.1007/s00221-008-1350-8. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. Wiley; NY: 1966. [Google Scholar]
- Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE. Frequency and velocity of rotational head perturbations during locomotion. Exp Brain Res. 1988;70:470–476. doi: 10.1007/BF00247595. [DOI] [PubMed] [Google Scholar]
- Guedry FEJ. Psychophysics of vestibular sensation. In: Kornhuber HH, editor. Handbook of sensory physiology vestibular system. Springer; New York: 1974. pp. 3–154. [Google Scholar]
- Guilford JP. A generalized psychophysical law. Psychol Rev. 1932;39:73–85. [Google Scholar]
- Hullar T. Neurometric and psychometric thresholds of the semicircular canals. Soc Neurosci Abs. 2008;168:8. [Google Scholar]
- Hullar T, Zee D, Minor L. Evaluation of the patient with dizziness. In: Cummings C, editor. Otolaryngology—head and neck surgery. Elsevier; Philadelphia: 2005a. [Google Scholar]
- Hullar TE, Della Santina CC, Hirvonen TP, Lasker DM, Carey JP, Minor LB. Responses of irregularly discharging chinchilla semicircular canal vestibular-nerve afferents during high-frequency head rotations. J Neurophysiol. 2005b;93:2777–2786. doi: 10.1152/jn.01002.2004. [DOI] [PubMed] [Google Scholar]
- Kiesow E. Über die Vergleichung linearer Strecken und ihre Beziehung zum Weberschen Gesetze. Arch Gesamte Psychol. 1926;56:421–462. [Google Scholar]
- Kingma H. Thresholds for perception of direction of linear acceleration as a possible evaluation of the otolith function. BMC Ear Nose Throat Disord. 2005;5:5. doi: 10.1186/1472-6815-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laming D. Sensory analysis. Academic Press; London: 1986. Weber’s law; pp. 1–17. [Google Scholar]
- Leek MR. Adaptive procedures in psychophysical research. Percept Psychophys. 2001;63:1279–1292. doi: 10.3758/bf03194543. [DOI] [PubMed] [Google Scholar]
- Leek MR, Dubno JR, He N, Ahlstrom JB. Experience with a yes–no single-interval maximum-likelihood procedure. J Acoust Soc Am. 2000;107:2674–2684. doi: 10.1121/1.428653. [DOI] [PubMed] [Google Scholar]
- Leigh R, Zee D. The neurology of eye movements. Oxford University Press; New York: 2006. [Google Scholar]
- MacDougall HG, Moore ST. Marching to the beat of the same drummer: the spontaneous tempo of human locomotion. J Appl Physiol. 2005;99:1164–1173. doi: 10.1152/japplphysiol.00138.2005. [DOI] [PubMed] [Google Scholar]
- Mach E. Grundlinien der Lehre von den Bewegungsempfindungen. Engelman; Leipzig: 1875. [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: a user’s guide. Lawrence Erlbaum Associates; Mahwah: 2005. [Google Scholar]
- McGill WJ, Goldberg JP. Pure-tone intensity discrimination and energy detection. J Acoust Soc Am. 1968;44:576–581. doi: 10.1121/1.1911123. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during Translation and Tilt. J Neurophysiol. 2005a;94:186–198. doi: 10.1152/jn.00904.2004. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. II. VOR and perceptual responses during combined Tilt & Translation. J Neurophysiol. 2005b;94:199–205. doi: 10.1152/jn.00905.2004. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt H. Origin and processing of postural information. Neurosci Biobeh Rev. 1998;22:473–478. doi: 10.1016/s0149-7634(97)00032-8. [DOI] [PubMed] [Google Scholar]
- Okada T, Grunfeld E, Shallo-Hoffmann J, Bronstein AM. Vestibular perception of angular velocity in normal subjects and in patients with congenital nystagmus. Brain. 1999;122:1293–1303. doi: 10.1093/brain/122.7.1293. [DOI] [PubMed] [Google Scholar]
- Pulaski PD, Zee DS, Robinson DA. The behavior of the vestibulo-ocular reflex at high velocities of head rotation. Brain Res. 1981;222:159–165. doi: 10.1016/0006-8993(81)90952-5. [DOI] [PubMed] [Google Scholar]
- Raphan T, Matsuo V, Cohen B. Velocity storage in the vestibulo-ocular reflex arc (VOR) Exp Brain Res. 1979;35:229–248. doi: 10.1007/BF00236613. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Chacron MJ, Taylor MC, Cullen KE. Neural variability, detection thresholds, and information transmission in the vestibular system. J Neurosci. 2007;27:771–781. doi: 10.1523/JNEUROSCI.4690-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemungal BM, Gunaratne IA, Fleming IO, Gresty MA, Bronstein AM. Perceptual and nystagmic thresholds of vestibular function in yaw. J Vestib Res. 2004;14:461–466. [PubMed] [Google Scholar]
- Seidman SH. Translational motion perception and vestibooc-ular responses in the absence of non-inertial cues. Exp Brain Res. 2008;184:13–29. doi: 10.1007/s00221-007-1072-3. [DOI] [PubMed] [Google Scholar]
- Sinha N, Zaher N, Shaikh AG, Lasker AG, Zee DS, Tarnutzer AA. Perception of self motion during and after passive rotation of the body around an earth-vertical axis. Prog Brain Res. 2008;171:277–281. doi: 10.1016/S0079-6123(08)00639-0. [DOI] [PubMed] [Google Scholar]
- Stevens SS. To honor Fechner and repeal his law: a power function, not a log function, describes the operating characteristic of a sensory system. Science. 1961;133:80–86. doi: 10.1126/science.133.3446.80. [DOI] [PubMed] [Google Scholar]
- Walsh EG. Role of the vestibular apparatus in the perception of motion on a parallel swing. J Physiol (Lond) 1961;155:506–513. doi: 10.1113/jphysiol.1961.sp006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KP, Aw ST, Todd MJ, McGarvie LA, Curthoys IS, Halmagyi GM. Head impulse test in unilateral vestibular loss: vestibulo-ocular reflex and catch-up saccades. Neurology. 2008;70:454–463. doi: 10.1212/01.wnl.0000299117.48935.2e. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys. 2001;63:1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Jones GM. Mammalian vestibular physiology. Plenum; London: 1979. [Google Scholar]
- Wood SJ, Reschke MF, Sarmiento LA, Clement G. Tilt and translation motion perception during off-vertical axis rotation. Exp Brain Res. 2007;182:365–377. doi: 10.1007/s00221-007-0994-0. [DOI] [PubMed] [Google Scholar]
- Zanker JM. Does motion perception follow Weber’s law? Perception. 1995;24:363–372. doi: 10.1068/p240363. [DOI] [PubMed] [Google Scholar]