Summary
The role of fatty acids (FA) in prostate carcinogenesis is unclear. Interest in the interrelationship among different types of FA has resulted in new analytic approaches to FA and their role in cancer development.
We evaluated the association between erythrocyte FA and prostate cancer in 127 prostate cancer patients and 183 screen negative controls. We present three approaches to analyses of the FA and prostate cancer association; 1) individual or common groups of FA, 2) biologically meaningful FA ratios and 3) principal components analysis.
Monounsaturated FA and the alpha-linolenic:eicosapentaenoic ratio were associated with reduced risk of prostate cancer. However, Factor 1, which was strongly correlated with some long chain saturated FA, was associated with an increased risk of prostate cancer.
We provide an example of modeling FA and their interrelationships on the risk of prostate cancer. Comparing three approaches suggests the importance of considering the impact of the entire fatty acid profile in disease prevention.
Introduction
The role of dietary intake of fats and fatty acids and their relative concentrations in the body has been an evolving area of research in prostate chemoprevention. The understanding of fatty acids as key players in control of gene expression and regulation has developed over the past decades and resulted in a shift in research from simply measuring total fat intake to a consideration of “types” of fats, based on number and location of double bonds, and individual fatty acids [1-3]. Correlational data supports an inverse association between some omega-3 fatty acids and monounsaturated fatty acids and prostate cancer risk, and a direct association between omega-6 fatty acids and prostate cancer risk [4, 5]. This epidemiological evidence has been supported by in vitro and animal studies, which have described multiple biologic mechanisms for fatty acids in chemoprevention including altering inflammatory response [6], lipid metabolism [7] and oxidative stress response [8]. Despite this strong initial evidence, however, results from case-control and cohort studies of types of fats, individual fatty acids, and ratios of fatty acids have been largely inconclusive [9-12]. Reasons for the inconsistencies across studies may range from variability in methods used for estimating fatty acid exposure (questionnaire, blood, adipose tissue) to inadequate variability in intake of key fatty acids (specifically the long-chain omega-3 fatty acids) in the populations under study. However, inconsistencies may also be the result of a lack of consideration for the inter-relationship among fatty acids and how this may impact fatty acid metabolism, storage and activity.
Fatty acid metabolism and the resultant prostanoids are affected by the mix of fatty acids in the cell and circulation. For example, omega-3 fatty acids and omega-6 fatty acids compete for desaturases and acyl-transferases, such that high levels of omega-6 fatty acids in the diet (as is common in most Western countries) results in higher cell membrane omega-6 fatty acids, lower omega-3 fatty acids and the more pro-inflammatory, pro-proliferation prostanoid synthesis reflective of omega-6 fatty acid metabolism [13-15]. Further, many of the monounsaturated fatty acids, in addition to being present in the diet, are synthesized by the desaturation of dietary saturated fatty acids. Polyunsaturated fatty acids regulate the desaturase enzymes and hence may impact levels of the monounsaturated fatty acids in the cell. To address the interactive nature of fatty acids, some investigators have evaluated the association between ratios of fatty acids and prostate cancer risk. Newcomer et al reported no association between the omega-3:omega-6 fatty acid ratio and prostate cancer incidence [16]. However, Kositsawat et al reported a direct association between the ratio of oleic to stearic acid, an indirect marker of stearoyl-coenzyme A desaturase activity and prostate cancer biochemical recurrence [17]. While considering fatty acid ratios is an improvement over considering each fatty acid separately, even ratios may not adequately represent the complex interactions between multiple fatty acids. Bougnoux et al and others have suggested a more “global” assessment of fatty acids using a data driven principal components approach and lipid profile arrays (lipidome) to develop groupings of fatty acids that may relate to the biologic interactions of these compounds [18-20].
In the current case-control analyses we demonstrate the use of three approaches to evaluate the potential associations between fatty acids and prostate cancer risk. First, we used the more traditional approach to evaluate the independent association between individual erythrocyte levels of several common saturated, mono-unsaturated and poly-unsaturated fatty acids and prostate cancer risk. Second we developed ratios of several of these fatty acids that indirectly reflect the activity of key lipid metabolizing enzymes, including stearoyl co-enzyme A desaturase (n-7 and n-9 saturation indexes), elongase-6 (palmitic: stearic), delta-6 desaturase (LA:GLA and ALA:EPA) and delta-5 desaturase (d-GLA:AA), or in the case of omega-3 to omega-6 and EPA+DHA:AA, the impact of competitive metabolism by phospholipase A2 [21-25]. Third we used a principal components analysis to develop data driven groupings of fatty acids to predict prostate cancer risk.
Patients and Methods
Subjects in this case-control study were recruited through the Portland Veterans Affairs Medical Center (PVAMC) as a part of the Diet and Prostate Cancer Risk study [26]. Prostate cancer cases were identified from among men referred to the PVAMC urology clinic for a prostate biopsy. Of the men referred for biopsy, 832 met our eligibility criteria; no previous diagnosis of cancer or dementia, no participation in another research study and no medical conditions that in the view of the urologist would make study participation an undue burden and 644 provided permission to be contacted. Of the eligible men, 424 agreed to participate and provided a blood specimen. Of these, prostate cancer was histologically confirmed in 127 (30.0%) subjects, 46 had prostatic intraepithelial neoplasia, and 251 were biopsy negative.
To identify screen negative clinic controls, information was obtained on all men seen at the PVAMC primary care clinic who had a PSA test result < 4ng/mL within the past 12 months. From this list, 1 potential control subject, frequency age-matched to each biopsy subject was randomly selected. If this control was deemed ineligible a second frequency age-matched control was randomly selected. If the second selected subject was deemed ineligible he was not replaced in the dataset. In this fashion we identified 428 eligible subjects, frequency age matched to biopsy subjects, with a normal (<4ng/mL) PSA in the past 12 months. Of these, 74 refused permission to be contacted, and 235 (66.4 percent of those contacted or 54.9 percent of total eligible) subjects agreed to participate of whom 183 provided an adequate blood specimen for erythrocyte fatty acid analyses. Thus our final case-control sample consisted of 127 men with biopsy confirmed prostate cancer and 183, PSA normal, clinic controls.
Information on dietary intake was obtained using an adapted version of the National Cancer Institute Diet History Questionnaire (DHQ) [27]. A separate risk factor questionnaire requested information on prostate cancer risk factors. Subjects were interviewed prior to their scheduled prostate biopsy procedure to avoid potential recall bias associated with a cancer diagnosis. Statin use data were recorded from an electronic pharmacy database at the PVAMC as described previously [28].
Blood was collected in a 10ml EDTA tube and immediately processed to obtain plasma and washed erythrocytes. Samples were aliquoted into 0.5ml cryovials and stored at -80C for analyses. All patients provided written informed consent according to both the PVAMC and Oregon Health & Science University (OHSU) Institutional Review Boards' requirements. This protocol, consent forms, and Health Insurance Portability and Accountability Act (HIPAA) authorization forms were reviewed and approved by the PVAMC and OHSU Institutional Review Boards.
Two hundred and fifty ul of red cells were mixed with an equivalent volume of distilled water and lipids were extracted with 2-propanol and chloroform according to Rose [29]. Erythrocyte fatty acids levels were determined in transesterified fatty acid methyl esters using gas chromatography as previously described [30, 31]. The case/control status was unknown to the laboratory personnel. Fatty acid composition is reported as a weight percent of the total RBC fatty acids.
Statistical Analyses
All statistical analyses were performed using the Statistical Analysis System (SAS)/PC program, version 9.2(SAS Institute, Inc., Cary, North Carolina). Spearman and Pearson correlations between any pair of fatty acids including the omega-3 (EPA and DHA), omega-6 (linoleic acid (LA) and arachidonic acid (AA)) fatty acids, and the other fatty acids, were examined for any obvious fatty acid groupings. Differences in covariate distribution by case status were summarized and compared using a chi-square test or Fisher's exact test depending on the expected cell counts. Tests were considered statistically significant at a p value (two sided) of less than 0.05.
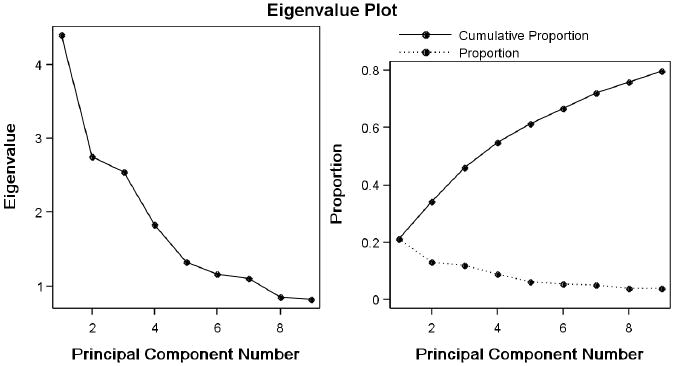
We used principal components analysis of the erythrocyte fatty acid composition from all 607 subjects (127 cases, 183 clinic controls, 251 biopsy negative controls, 46 prostatic intraepithelial neoplasia) recruited for the study to extract factors from the 21 fatty acids present at 0.2% or more by weight in red blood cells or that were of primary interest in our analyses (docosadienoic acid and ALA) (Noted as included in Table 1). The fatty acid weight % values were standardized (converted to Z scores) by subtracting the mean for the fatty acid and dividing by the standard deviation. Examination of the distributions of the fatty acids indicated that a normal distribution could not be assumed for all fatty acids, and that no transformation could be applied uniformly to all the fatty acids to make all of their distributions compatible with a normal distribution. As the assumption of normality is not required for Principal Components Analysis when the purpose is to summarize relationships between variables, and a consistent approach to variable handling is important, we used the untransformed fatty acid z scores to generate the Factor Scores. Varimax rotation was used to increase the interpretability of the factors. As shown in Figure 1 the slope of the scree plot flattens after 6 factors and these first 6 factors explain 62.5% of the observed variance in the fatty acids of study participants. Factor scores for the first 6 factors were computed from the rotated loading factors and were modeled using logistic regression as described below. Relationships between the unstandardized FA rbc weight % composition and the Factor scores were examined using Spearman rank correlations.
TABLE 1. FA Used in Extraction of Principal Component Factors.
| FA | Mean RBC weight% | St. Dev. | Use in PCA |
|---|---|---|---|
| 14:0 | 0.25 | 0.09 | Included |
| 15:0 | 0.11 | 0.03 | Not Used |
| 16:0 | 18.95 | 0.98 | Included |
| 17:0 | 0.32 | 0.05 | Included |
| 18:0 | 14.09 | 1.04 | Included |
| 20:0 | 0.35 | 0.06 | Included |
| 22:0 | 1.62 | 0.25 | Included |
| 14:1n5 | 0.01 | 0.01 | Not Used |
| 16:1n7t | 0.11 | 0.02 | Not Used |
| 16:1n9c | 0.05 | 0.02 | Not Used |
| 161n7c | 0.33 | 0.21 | Included |
| 17:1n9c | 0.91 | 0.14 | Included |
| 18:1n9t | 0.30 | 0.15 | Included |
| 18:1n9c | 11.59 | 1.16 | Included |
| 18:1n7c | 0.93 | 0.14 | Included |
| 20:1n9 | 0.20 | 0.04 | Included |
| 22:1n9 | 0.05 | 0.03 | Not Used |
| 18:2n6 | 9.66 | 1.76 | Included |
| 18:3n6 | 0.06 | 0.03 | Not Used |
| 20:2n6 | 0.23 | 0.04 | Included |
| 20:3n6 | 1.44 | 0.31 | Included |
| 20:4n6 | 14.07 | 1.24 | Included |
| 22:2n6 | 0.05 | 0.02 | Included |
| 18:3n3 | 0.13 | 0.06 | Included |
| 20:5n3 | 0.49 | 0.29 | Included |
| 22:5n3 | 1.99 | 0.36 | Included |
| 22:6n3 | 3.48 | 1.04 | Included |
Yellow shading indicates minor FA omitted from the extraction of Factors through principle components analysis.
Figure 1. Proportion of total sample variability explained by the Principal Component Factors.
Logistic regression was used to estimate the odds ratio (OR) and 95% confidence interval (95% CI) for the association between fatty acids, fatty acid ratios and Principal component factor scores and prostate cancer risk. Fatty acids levels, ratios and factor scores were categorized into tertiles based on their distributions in the clinic control subjects. All models were adjusted for age. Potential confounders, including race, body mass index (BMI), total calorie intake, education, smoking status, alcohol consumption, use of non-steroidal anti-inflammatory medications, total cholesterol intake, total fat intake, dietary intake of lycopene and vitamin E and statin use were considered for inclusion in the multivariate models. Confounders were entered into the regression model independently and were maintained in the final adjusted model if they 1) altered the OR of the primary predictor variable by greater than 10%, 2) were considered established prostate cancer risk factors, or 3) were independently associated with prostate cancer risk in this study population. Age, BMI, race and family history of prostate cancer were maintained in the final adjusted model. Tests were considered statistically significant at a p-value for the trend OR of less than 0.05. All models were also evaluated stratified by disease grade (low grade, Gleason <= 6 vs. high grade, Gleason >=7).
Results
As shown in Table 2, PSA normal clinic controls had a higher BMI than prostate cancer cases and were less likely to report having a family history of prostate cancer. There were no significant differences with regards to other demographic and lifestyle factors, including race/ ethnicity, smoking history, education, marital status, and use of non-steroidal anti-inflammatory medications.
TABLE 2. Frequencies of selected characteristics of men in the Diet and Prostate Cancer Risk study, 2001-2006.
| Characteristic | Prostate cancer cases (n=127) |
PSA Normal Controls (n=183) |
||
|---|---|---|---|---|
| Age, in years | ||||
| Mean Age | 65.5 | 64.7 | ||
| Range | 52-86 | 50-85 | ||
| N | % | N | % | |
| Ethnic Origin | ||||
| White | 112 | 88.2 | 165 | 90.2 |
| Black | 8 | 6.3 | 4 | 2.2 |
| Hispanic | 2 | 1.6 | 3 | 1.6 |
| Missing/Other | 5 | 3.9 | 11 | 6.0 |
| BMI | ||||
| Less Than 25 | 22 | 16.5 | 27 | 14.8 |
| 25-29 | 49 | 38.6 | 60 | 32.8 |
| 30-34 | 42 | 33.1 | 52 | 28.4 |
| ≥ 35 | 14 | 11.0 | 44 | 24.0* |
| Missing | 1 | 0.8 | 0 | |
| Family history of prostate cancer | ||||
| Yes | 16 | 12.6 | 11 | 6.0* |
| Smoking | ||||
| Never | 16 | 12.6 | 32 | 17.5 |
| Former | 74 | 58.3 | 99 | 54.1 |
| Current | 34 | 26.8 | 45 | 24.6 |
| Missing/ Unknown | 3 | 2.4 | 7 | 3.8 |
| Education | ||||
| ≤ 12 years | 52 | 40.9 | 60 | 32.8 |
| Some | 43 | 33.9 | 70 | 38.3 |
| College/Tech. | ||||
| ≥ College graduate | 30 | 23.6 | 49 | 26.8 |
| Missing/Other | 2 | 1.6 | 4 | 2.2 |
| Marital status | ||||
| Single/ Divorced/ Widowed | 37 | 29.3 | 58 | 31.7 |
| Married/ Partner | 87 | 68.5 | 121 | 66.1 |
| Missing | 3 | 2.3 | 4 | 2.2 |
| NSAID use | ||||
| Yes | 45 | 35.4 | 76 | 41.5 |
| No | 80 | 63.0 | 103 | 56.3 |
| Missing | 2 | 1.6 | 4 | 2.2 |
P-value for chi-square difference between cases and control group
p<0.05
In the analyses of individual or traditionally grouped fatty acids, total monounsaturated fatty acids were significantly inversely associated with prostate cancer risk. There was no significant association between any of the other individual fatty acids or traditional groupings of fatty acids and risk of total prostate cancer (Table 3). When these data were stratified by disease grade, palmitic acid levels were significantly inversely associated with risk of high grade disease, while total monounsaturated fatty acid levels were only associated with a significant reduction in low grade (Gleason 6) disease (Table 3).
Table 3. Erythrocyte fatty acid concentrations among veterans and risk of prostate cancer, Gleason =6 prostate cancer and Gleason >= 7 prostate cancer as compared to PSA normal controls*. (Odds Ratio (OR) and 95% Confidence Interval (95% CI)).
| Tertiles of Erythrocyte FA (weight % of total) | 1 | 2 | 3 | P value trend |
|---|---|---|---|---|
| Total SUFAs | 0 < n ≤ 35.10 | 35.10 < n ≤ 36.07 | 36.07< n | |
| All Cancer Vs. Control | 1.00 | 1.31 (0.75-2.31) | 0.97 (0.53-1.75) | 0.50 |
| Gleason =6 Vs. Control | 2.35 (1.08-5.11) | 1.66 (0.73-3.78) | 0.10 | |
| Gleason >=7 Vs. Control | 0.85 (0.42-1.72) | 0.63 (0.29-1.35) | 0.49 | |
| Palmitic (16:0) | 0 < n ≤ 18.56 | 18.56 < n ≤19.18 | 19.18< n | |
| All Cancer Vs. Control | 1.00 | 0.59 (0.33-1.07) | 0.76 (0.43-1.36) | 0.23 |
| Gleason =6 Vs. Control | 0.61 (0.28-1.33) | 1.20 (0.59-2.45) | 0.81 | |
| Gleason >=7 Vs. Control | 0.58 (0.28-1.20) | 0.36 (0.16-0.82) | 0.02 | |
| Stearic (18:0) | 0 < n ≤ 13.65 | 13.65 < n ≤14.45 | 14.45 < n | |
| All Cancer Vs. Control | 1.00 | 1.65 (0.93-2.92) | 1.14 (0.62-2.09) | 0.80 |
| Gleason =6 Vs. Control | 1.71 (0.82-3.53) | 1.10 (0.50-2.44) | 0.84 | |
| Gleason >=7 Vs. Control | 1.79 (0.85-3.79) | 1.15 (0.52-2.56) | 0.78 | |
| Total MUFAs | 0 < n ≤ 14.26 | 14.26 < n ≤ 15.41 | 15.41< n | |
| All Cancer Vs. Control | 1.00 | 0.67 (0.38-1.18) | 0.54 (0.30-0.98) | 0.03 |
| Gleason =6 Vs. Control | 0.63 (0.31-1.28) | 0.39 (0.18-0.85) | 0.02 | |
| Gleason >=7 Vs. Control | 0.70 (0.33-1.48) | 0.72 (0.34-1.53) | 0.24 | |
| Heptadecenoic acid (17:1 n9) | 0 < n ≤0.843 | 0.843 < n ≤ 0.978 | 0.978< n | |
| All Cancer Vs. Control | 1.00 | 1.26 (0.71-2.22) | 0.89 (0.49-1.60) | 0.48 |
| Gleason =6 Vs. Control | 1.50 (0.75-2.99) | 0.60 (0.26-1.36) | 0.07 | |
| Gleason >=7 Vs. Control | 1.05 (0.49-2.26) | 1.15 (0.56-2.39) | 0.93 | |
| Elaidic acid (18:1 n9) | 0 < n ≤0.256 | 0.256 < n ≤ 0.362 | 0.362< n | |
| All Cancer Vs. Control | 1.00 | 0.48 (0.26-0.90) | 0.54 (0.31-0.92) | 0.03 |
| Gleason =6 Vs. Control | 0.47 (0.21-1.03) | 0.42 (0.21-0.85) | 0.03 | |
| Gleason >=7 Vs. Control | 0.40 (0.17-0.96) | 0.69 (0.35-1.37) | 0.12 | |
| Palmitoleic acid (16:1 n-7) | 0 < n ≤ 0.23 | 0.23 < n ≤ 0.34 | 0.34< n | |
| All Cancer Vs. Control | 1.00 | 1.29 (0.73-2.28) | 0.90 (0.49-1.65) | 0.48 |
| Gleason =6 Vs. Control | 1.48 (0.71-3.11) | 1.01 (0.46-2.22) | 0.85 | |
| Gleason >=7 Vs. Control | 1.13 (0.55-2.34) | 0.70 (0.32-1.55) | 0.29 | |
| Oleic acid (18:1 n-9c) | 0 < n≤ 11.13 | 11.13 < n ≤ 12.16 | 12.16< n | |
| All Cancer Vs. Control | 1.00 | 0.88 (0.50-1.55) | 0.59 (0.33-1.07) | 0.07 |
| Gleason =6 Vs. Control | 0.99 (0.49-2.00) | 0.49 (0.22-1.09) | 0.09 | |
| Gleason >=7 Vs. Control | 0.68 (0.32-1.45) | 0.68 (0.32-1.44) | 0.22 | |
| Total PUFAs | 0 < n ≤ 34.30 | 34.30 < n ≤ 35.61 | 35.61< n | |
| All Cancer Vs. Control | 1.00 | 1.50 (0.83-2.71 | 1.43 (0.78-2.61) | 0.36 |
| Gleason =6 Vs. Control | 1.00 | 1.54 (0.75-3.17) | 1.05 (0.48-2.33) | 0.42 |
| Gleason >=7 Vs. Control | 1.00 | 1.60 (0.71-3.58) | 1.95 (0.88-4.34) | 0.26 |
| n-6 PUFAs2 | 0 < n ≤28.05 | 28.05 < n ≤ 29.68 | 29.68< n | |
| All Cancer Vs. Control | 1.00 | 1.19 (0.67-2.12) | 0.87 (0.47-1.60) | 0.61 |
| Gleason =6 Vs. Control | 0.97 (0.48-1.99) | 0.73 (0.34-1.57) | 0.32 | |
| Gleason >=7 Vs. Control | 1.36 (0.63-2.93) | 1.05 (0.47-2.93) | 0.95 | |
| Linoleic acid (18:2) | 0 < n ≤8.69 | 8.69 < n ≤ 10.07 | 10.07< n | |
| All Cancer Vs. Control | 1.00 | 0.92 (0.51-1.67) | 1.04 (0.58-1.86) | 0.99 |
| Gleason =6 Vs. Control | 0.74 (0.35-1.54) | 0.63 (0.30-1.32) | 0.19 | |
| Gleason >=7 Vs. Control | 1.31 (0.59-2.95) | 1.78 (0.80-3.96) | 0.24 | |
| γ-linolenic acid (18:3 n-6) | 0 < n ≤0.05 | 0.05 < n ≤ 0.06 | 0.06< n | |
| All Cancer Vs. Control | 1.00 | 0.61 (0.30-1.22) | 1.10 (0.65-1.84) | 0.87 |
| Gleason =6 Vs. Control | 0.70 (0.28-1.75) | 1.35 (0.71-2.58) | 0.38 | |
| Gleason >=7 Vs. Control | 0.49 (0.20-1.20) | 0.67 (0.34-1.34) | 0.24 | |
| Arachidonic acid (20:4) | 0 < n ≤13.56 | 13.56 < n ≤ 14.56 | 14.56< n | |
| All Cancer Vs. Control | 1.00 | 1.33 (0.74-2.32) | 1.35 (0.75-2.44) | 0.29 |
| Gleason =6 Vs. Control | 1.10 (0.52-2.33) | 1.28 (0.61-2.69) | 0.55 | |
| Gleason >=7 Vs. Control | 1.89 (0.86-4.18) | 1.53 (0.68-3.45) | 0.30 | |
| n-3 PUFAs1 | 0 < n≤5.37 | 5.37 < n ≤ 6.19 | 6.19< n | |
| All Cancer Vs. Control | 1.00 | 1.14 (0.62-2.11) | 1.31 (0.72-2.39) | 0.28 |
| Gleason =6 Vs. Control | 1.35 (0.61-3.02) | 1.64 (0.75-3.59) | 0.14 | |
| Gleason >=7 Vs. Control | 0.91 (0.40-2.08) | 1.06 (0.49-2.30) | 0.80 | |
| α-linoleic acid (18:3 n-3) | 0 < n ≤0.107 | 0.107 < n ≤ 0.135 | 0.135< n | |
| All Cancer Vs. Control | 1.00 | 0.85 (0.48-1.48) | 0.72 (0.41-1.28) | 0.54 |
| Gleason =6 Vs. Control | 1.07 (0.54-2.14) | 0.64 (0.30-1.36) | 0.37 | |
| Gleason >=7 Vs. Control | 0.71 (0.34-1.49) | 0.72 (0.35-1.52) | 0.58 | |
| Eicosapentaenoic acid (20:5) | 0 < n≤ 0.35 | 0.35 < n ≤ 0.47 | 0.47< n | |
| All Cancer Vs. Control | 1.00 | 0.70 (0.37-1.30) | 1.12 (0.64-1.96) | 0.65 |
| Gleason =6 Vs. Control | 0.78 (0.35-1.73) | 1.27 (0.62-2.59) | 0.31 | |
| Gleason >=7 Vs. Control | 0.59 (0.26-1.33) | 0.83 (0.39-1.75) | 0.65 | |
| Docosahexaenoic acid (22:6) | 0 < n ≤2.93 | 2.93 < n ≤ 3.67 | 3.67< n | |
| All Cancer Vs. Control | 1.00 | 0.91 (0.49-1.68) | 1.14 (0.62-2.09) | 0.48 |
| Gleason =6 Vs. Control | 0.90 (0.41-1.97) | 1.23 (0.57-2.65) | 0.50 | |
| Gleason >=7 Vs. Control | 0.82 (0.36-1.88) | 1.06 (0.48-2.32) | 0.65 |
Models adjusted for age, BMI, race, and family history of prostate cancer.
126 Prostate cancer, 61 Gleason =6, 63 Gleason >=7, 182 clinic controls.
18:3n-3 + 20:3n-3 + 20:5n-3 + 22:5n-3 + 22:6n-3
18:2n-6cc + 18:3n-6 + 20:2n-6 + 20:3n-6 + 20:4n-6 + 22:2n-6 + 22:4n-6
In table 4 we present results for ratios of fatty acids that may indirectly reflect the activity of enzymes involved in fatty acid metabolism. When all cases were considered together, the ratio of ALA to EPA was inversely associated with all prostate cancer risk as compared to PSA normal clinic controls, (OR for highest vs. lowest tertile = 0.62, 95% CI (0.35 – 1.12) though the association appears somewhat U-shaped (Table 4). When stratified by disease grade, a higher n-7 saturation index was significantly associated with an increase in low grade disease (OR for highest vs. lowest tertile = 2.33, 95% CI (1.10 – 4.93) and ALA to EPA ratio was associated with a significant reduction in risk of low grade disease (OR for highest vs. lowest tertile = 0.53, 95% CI (0.25-1.11).
Table 4.
Ratios of erythrocyte fatty acid concentrations among veterans and risk of all prostate cancer, Gleason =6 prostate cancer and Gleason >= 7 prostate cancer as compared to PSA normal controls*. (Odds Ratio (OR) and 95% Confidence Interval (95% CI).
| Tertiles of Erythrocyte FA levels | 1 | 2 | 3 | P value trend |
|---|---|---|---|---|
| n-7 Saturation Index (16:0/16:1 n7) | 0 < n ≤ 53.18 | 53.18 < n ≤79.22 | 79.22 < n | |
| All Cancer Vs. Control | 1.00 | 1.38 (0.77-2.49) | 0.99 (0.54-1.81) | 0.77 |
| Gleason =6 Vs. Control | 1.36 (0.65-2.83) | 0.89 (0.41-1.93) | 0.95 | |
| Gleason >=7 Vs. Control | 1.61 (0.73-3.54) | 1.25 (0.56-2.76) | 0.48 | |
| n-9 Saturation Index (18:0/18:1 n9) | 0 < n ≤ 1.12 | 1.12 < n ≤1.23 | 1.23 < n | |
| All Cancer Vs. Control | 1.00 | 1.67 (0.60-2.28) | 1.41 (0.78-2.55) | 0.20 |
| Gleason =6 Vs. Control | 1.14 (0.51-2.71) | 2.33 (1.10-4.93) | 0.02 | |
| Gleason >=7 Vs. Control | 0.86 (0.40-1.86) | 0.90 (0.44-1.87) | 0.92 | |
| Palmitic : Stearic (16:0/18:0) | 0 < n ≤ 1.28 | 1.28 < n ≤ 1.40 | 1.40 < n | |
| All Cancer Vs. Control | 1.00 | 1.41 (0.80-2.49) | 0.87 (0.47-1.61) | 0.65 |
| Gleason =6 Vs. Control | 1.01 (0.49-2.08) | 0.81 (0.38-1.74) | 0.58 | |
| Gleason >=7 Vs. Control | 1.95 (0.92-4.15) | 0.92 (0.40-2.15) | 0.81 | |
| Total n-31 : Total n-62 | 0 < n≤ 0.18 | 0.18 ≤ 0.22 | 0.22 < n | |
| All Cancer Vs. Control | 1.00 | 0.98 (0.54-1.78) | 1.22 (0.66-2.25) | 0.38 |
| Gleason =6 Vs. Control | 1.17 (0.54-2.55) | 1.43 (0.64-3.21) | 0.26 | |
| Gleason >=7 Vs. Control | 0.75 (0.34-1.64) | 1.01 (0.46-2.20) | 0.78 | |
| ALA:EPA (18:3n3/ 20:5n3) | 0 < n ≤0.25 | 0.25 < n ≤ 0.35 | 0.35 < n | |
| All Cancer Vs. Control | 1.00 | 0.54 (0.30-0.97) | 0.62 (0.35-1.12) | 0.05 |
| Gleason =6 Vs. Control | 0.38 (0.17-0.82) | 0.53 (0.25-1.11) | 0.03 | |
| Gleason >=7 Vs. Control | 0.57 (0.26-1.26) | 0.83 (0.39-1.77) | 0.41 | |
| EPA+DHA:AA ((20:5n-3 + 22:6n-3)/ 20:4n-6) | 0 < n ≤0.15 | 0.15 < n ≤ 0.18 | 0.18 < n | |
| All Cancer Vs. Control | 1.00 | 0.92 (0.50-1.68) | 1.07 (0.58-1.97) | 0.83 |
| Gleason =6 Vs. Control | 1.48 (0.66-3.34) | 1.54 (0.67-3.53) | 0.23 | |
| Gleason >=7 Vs. Control | 0.54 (0.24-1.22) | 0.78 (0.36-1.70) | 0.45 | |
| LA:GLA (18:2/18:3n-6) | 0 < n ≤6.17 | 6.17 < n ≤ 7.61 | 7.61< n | |
| All Cancer Vs. Control | 1.00 | 1.02 (0.56-1.84) | 0.96 (0.54-1.70) | 0.99 |
| Gleason =6 Vs. Control | 0.77 (0.37-1.63) | 0.76 (0.37-1.53) | 0.46 | |
| Gleason >=7 Vs. Control | 1.93 (0.85-4.36) | 1.74 (0.79-3.85) | 0.23 | |
| dGLA:AA (20:3n6/ 20:4n6) | 0 < n ≤0.09 | 0.09 < n ≤ 0.11 | 0.11 < n | |
| All Cancer Vs. Control | 1.00 | 1.20 (0.67-2.12) | 1.28 (0.71-2.31) | 0.99 |
| Gleason =6 Vs. Control | 1.33 (0.63-2.81) | 1.30 (0.61-2.80) | 0.43 | |
| Gleason >=7 Vs. Control | 1.10 (0.53-2.30) | 0.99 (0.46-2.14) | 0.97 |
126 Prostate cancer, 61 Gleason =6, 63 Gleason >=7, 182 clinic controls. Models adjusted for age, BMI, race, and family history of prostate cancer.
18:3n-3 + 20:3n-3 + 20:5n-3 + 22:5n-3 + 22:6n-3
18:2n-6cc + 18:3n-6 + 20:2n-6 + 20:3n-6 + 20:4n-6 + 22:2n-6 + 22:4n-6
Principal components analysis resulted in six factors that explained 62.5% of the variance in the full set of fatty acids. As shown in Table 5, factor 1 was positively correlated with the saturated fatty acids arachidic, behenic, heptadecanoic and stearic, but negatively correlated with palmitic acid and palmitoleic acid. Factor 2 was positively correlated with the omega-6 fatty acid LA and the and omega-3 fatty acid ALA and the saturated fatty acid myristic, but negatively correlated with the longer chain omega-3 and omega-6 fatty acids (aracadonic, docodienoic, DPA and DHA) and the saturated fatty acid stearic. Factor 3 was strongly positively correlated with the monounsaturated fatty acid eicosenoic, and the omega-6 fatty acids eicosadienoic and docosedienoic, but negatively correlated with the saturated fatty acids myristic and the monounsaturated fatty acid palmitoleic. Factor 4 was strongly positively correlated with most of the omega-3 fatty acids (EPA, DPA and DHA) and negatively correlated with the omega-6 fatty acid, AA. Factor 5 was positively correlated with two minor mono-unsaturated fatty acids, heptadecenoic acid and elaidic acid, both found primarily in butter (or ruminant) fat. Factor 6 was negatively correlated with a single omega-6 fatty acid, dihomo-gamma-linolenic acid, the intermediate fatty acid in the desaturation and elongation of LA to AA.
Table 5. Factor Patterns of Principal Components Analysis.
| Loading Coefficients for Standardized Fatty Acids | |||||||
|---|---|---|---|---|---|---|---|
| RBC Fatty Acid | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | |
| 14:0 | myristic | -0.14 | 0.58 | -0.42 | -0.08 | -0.37 | 0.01 |
| 16:0 | palmitic | -0.56 | 0.12 | -0.26 | 0.18 | -0.29 | 0.07 |
| 17:0 | heptadecanoic | 0.55 | 0.04 | 0.016 | -0.03 | 0.35 | -0.23 |
| 18:0 | stearic | 0.41 | -0.49 | 0.01 | 0.08 | 0.03 | 0.05 |
| 20:0 | arachidic | 0.74 | 0.04 | -0.21 | -0.02 | 0.01 | -0.22 |
| 22:0 | behenic | 0.78 | -0.17 | -0.11 | -0.06 | -0.15 | -0.03 |
| 16:1n7 | palmitoleic | -0.66 | 0.37 | -0.41 | -0.14 | -0.27 | -0.05 |
| 17:1n9 | heptadecenoic | 0.04 | -0.30 | 0.36 | 0.28 | 0.66 | -0.01 |
| 18:1n9t | elaidic | 0.07 | 0.14 | -0.36 | -0.26 | 0.69 | 0.24 |
| 18:1n9c | oleic | -0.39 | 0.58 | -0.16 | -0.14 | 0.09 | -0.29 |
| 18:1n7 | vaccenic | -0.66 | 0.21 | -0.02 | -0.10 | 0.07 | -0.33 |
| 20:1n9 | eicosenoic | -0.04 | 0.18 | 0.67 | -0.11 | 0.16 | -0.26 |
| 18:2n6 | linoleic | 0.02 | 0.75 | 0.27 | -0.23 | 0.01 | 0.22 |
| 20:2n6 | eicosadienoic | -0.07 | 0.19 | 0.81 | -0.04 | 0.06 | 0.17 |
| 20:3n6 | dihomo-g-linolenic | -0.13 | 0.11 | 0.05 | -0.12 | 0.09 | 0.79 |
| 20:4n6 | arachidonic | 0.02 | -0.64 | -0.06 | -0.46 | -0.10 | -0.27 |
| 22:2n6 | docosadienoic | 0.04 | -0.13 | 0.83 | -0.003 | -0.21 | 0.13 |
| 18:3n3 | α-linolenic | -0.13 | 0.78 | 0.09 | 0.07 | -0.07 | -0.02 |
| 20:5n3 | eicosapentaenoic | -0.07 | 0.14 | -0.11 | 0.91 | -0.03 | -0.10 |
| 22:5n3 | docosapentaenoic | -0.04 | -0.16 | 0.02 | 0.76 | -0.14 | 0.04 |
| 22:6n3 | docosahexaenoic | 0.08 | -0.07 | -0.03 | 0.82 | 0.14 | -0.09 |
As shown in Table 6, when each factor was modeled as an independent predictor of prostate cancer risk, Factor 1 was associated with a significant increase in risk as compared to controls (OR for highest vs. lowest tertile = 1.92, 95% CI (1.03 -3.57)). Factor 5 was associated with a significant reduction in risk (p =0.05), though the confidence interval for the third versus first tertile included 1.00, limiting the confidence in this finding (OR for highest vs. lowest tertile = 0.59, 95% CI (0.33 – 1.06)). In analyses stratified by disease grade, the increase in risk with Factor 1 was seen only for risk of high grade (Gleason >=7) disease while the protective effect of Factor 5 was greater for risk of low grade disease.
Table 6. Principal components of erythrocyte fatty acid concentrations among veterans and risk of prostate cancer as compared to PSA normal controls. Odds Ratio (OR) and 95% Confidence Interval (95% CI) a.
| Tertiles of Factor Scores | 1 | 2 | 3 | P value Type III effect |
|---|---|---|---|---|
| Factor 1 | ||||
| All Cancer Vs. Control | 1.00 | 1.58 (0.85 - 2.94) | 1.92 (1.03 - 3.57) | 0.03 |
| Gleason =6 Vs. Control | 1.38 (0.63 – 3.02) | 1.77 (0.82 – 3.80) | 0.14 | |
| Gleason >=7 Vs. Control | 1.85 (0.80 - 4.31) | 2.04 (0.88 - 4.73) | 0.05 | |
| Factor 2 | ||||
| All Cancer Vs. Control | 1.00 | 1.30 (0.73 – 2.32) | 0.84 (0.46 – 1.52) | 0.52 |
| Gleason =6 Vs. Control | 1.06 (0.52 – 2.16) | 0.63 (0.29 – 1.36) | 0.22 | |
| Gleason >=7 Vs. Control | 1.82 (0.83 – 4.00) | 1.01 (0.45 – 2.27) | 0.90 | |
| Factor 3 | ||||
| All Cancer Vs. Control | 1.00 | 1.76 (0.97 – 3.19) | 0.87 (0.47 -1.62) | 0.59 |
| Gleason =6 Vs. Control | 1.09 (0.53 – 2.26) | 0.61 (0.28 – 1.33) | 0.27 | |
| Gleason >=7 Vs. Control | 3.87 (1.63 – 9.17) | 1.62 (0.67 – 3.96) | 0.60 | |
| Factor 4 | ||||
| All Cancer Vs. Control | 1.00 | 0.79 (0.43 – 1.44) | 1.16 (0.64 – 2.10) | 0.49 |
| Gleason =6 Vs. Control | 0.96 (0.44 – 2.13) | 1.48 (0.68 – 3.23) | 0.17 | |
| Gleason >=7 Vs. Control | 0.63 (0.28 – 1.39) | 0.92 (0.43 – 1.99) | 0.92 | |
| Factor 5 | ||||
| All Cancer Vs. Control | 1.00 | 0.62 (0.35 – 1.11) | 0.59 (0.33 – 1.06) | 0.05 |
| Gleason =6 Vs. Control | 0.82 (0.39 – 1.70) | 0.46 (0.21 – 0.99) | 0.04 | |
| Gleason >=7 Vs. Control | 0.39 (0.17 – 0.88) | 0.79 (0.38 – 1.64) | 0.34 | |
| Factor 6 | ||||
| All Cancer Vs. Control | 1.00 | 0.98 (0.54 – 1.75) | 1.13 (0.63 – 2.04) | 0.89 |
| Gleason =6 Vs. Control | 1.23 (0.57 – 2.67) | 1.55 (0.73 – 3.31) | 0.24 | |
| Gleason >=7 Vs. Control | 0.81 (0.39 – 1.69) | 0.72 (0.33 – 1.58) | 0.28 |
Models adjusted for age, BMI, race, and family history of prostate cancer.
126 Prostate cancer, 61 Gleason =6, 63 Gleason >=7, 182 clinic controls.
Discussion and Conclusions
In our analyses we investigated the association between erythrocyte fatty acid compositions and prostate cancer risk using three approaches, traditional individual or grouped fatty acids, fatty acid ratios, and principal components analysis. For the first two approaches, fatty acids were considered in our analyses individually and as logical ratios created to indirectly reflect the activity of several enzymes involved in lipid metabolism that have also been identified as dysregulated in carcinogenesis [4, 13, 32, 33]. In the third approach, principal components analysis was utilized to allow us to evaluate the effect of all fatty acids simultaneously by transforming the data from a set of highly correlated variables (fatty acids) into uncorrelated “components” or factors. Each factor explains a portion of the variability in the 21 fatty acids analyzed, with the first factor explaining most of the variability and each subsequent factor explaining less.
Though there were several suggestive trends in the traditional analyses, the only significant associations were a reduction in risk of high grade disease with increasing tertiles of palmitic acid and a reduction in risk of total and low grade disease with increasing tertiles of total monounsaturated fatty acid compositions. When the data was analyzed using biologically meaningful fatty acid ratios, the ratio of ALA:EPA was associated with a significant reduction in risk of total and low grade prostate cancer, while there was a significant increase in risk of low grade disease with an increasing n-9 saturation index (stearic:oleic). When implementing a data driven approach that allows for concurrent consideration of all fatty acids, two of the six factors extracted were determined to be significantly associated with risk of prostate cancer, Factor 1 and Factor 5. Factor 1 was negatively correlated with palmitic acid, so the finding of a positive association of this factor with advanced disease status agrees with the approach utilizing individual fatty acids. However, utilizing the Factor approach allowed the detection of a significant association of the fatty acid pattern with disease status in all cancer cases, which was not observed when palmitic acid was considered in isolation. These findings suggest that consideration of a single or even a group or ratio of fatty acids may not capture the full effect of the interactions between fatty acids and their effects on prostate cancer risk.
Despite consistent in vitro and animal evidence for the role of fatty acids, particularly the omega-3 and omega-6 polyunsaturated fatty acids, in cell proliferation and apoptosis in prostate cancer, human evidence of these effects is much less consistent [34]. This lack of consistency was highlighted in our own analyses, where the hypothesized inverse association between omega-3 fatty acids and prostate cancer risk and direct association between omega-6 fatty acids and prostate cancer risk was not found. However, unexpected associations with palmitic acid, monounsaturated fatty acids, the n-9 saturation index and the ratio of ALA: EPA, were identified. These types of findings raise the possibility that the function of fatty acids may be dramatically impacted by metabolic enzyme activity or by the interaction between all fatty acids present in the cell membrane.
The potential importance of considering biologically meaningful fatty acid ratios is noted in our finding of an inverse association between the ALA:EPA ratio and prostate cancer risk. There is a great deal of inconsistency in studies of the association between dietary and blood ALA and prostate cancer, with a meta-analysis and a nested case-cohort study suggesting higher levels are associated with increased risk [35, 36], while a large cohort study and more recent meta-analysis have reported no to very minimal direct association with risk [12, 37]. However, to our knowledge, none of these analyses have taken into consideration the potential role of metabolic processes and possible genetic variation on this association. The erythrocyte ratio of ALA to EPA attempts to do this as it is an indirect assessment of the activity of delta-6 desaturase and elongase 5, both enzymes that are regulated by dietary fat quality as well as genetic variation.
In an attempt to further address the more complex inter-relationships between the fatty acids and their metabolism we also evaluated our data using an approach which evaluates the association of complex groupings of fatty acids identified through a data driven principal components analysis. The concept of an individual fatty acid profile being used as a predictor of cancer risk has been described by Bougnoux and others as a method to account for the complex interactions between fatty acids [18, 19]. Fatty acid metabolism often makes use of shared pathways, with the concentration of one fatty acid potentially altering the metabolism or synthesis of another. This lack of independence may be accounted for in the principal components and allow interactions of fatty acids, such as those that may be driving the protective effect seen with increasing tertiles of Factor 5, to surface.
In our analyses, individuals with higher levels of Factor 1 had a significant increase in risk of prostate cancer, and more specifically higher grade disease. Factor 1 reflects (as determined by a loading coefficient of greater than +/-0.4) higher levels of many of the saturated fatty acids (heptadecanoic, stearic, arachidic, behenic) and lower levels of palmitic, palmitoleic and vaccenic acids. This pattern may be indicative of metabolic processes associated with reduced risk for diabetes. In recent analyses, Krechler et al reported that high levels of heptadecanoic acid and lower levels of palmitoleic acid were associated with reduced risk of diabetes [38]. This is of interest, as there have been several reports of an inverse association between diabetes and prostate cancer risk. The biologic rationale for this finding has not been elucidated but may reflect the variations in fatty acid metabolism that are identified in Factor 1. Factor 5 had a 50% lower risk of prostate cancer, and more specifically less severe disease. Interestingly, this factor is most reflective of a fatty acid profile that is high in the trans fatty acid elaidic acid and the minor monounsaturated fatty acid heptadecenoic acid, both found in bovine and caprine milk fat, though margarine is the primary source of the trans-fatty acid, elaidic acid, and lower in myristic acid, found in coconut oil. While most previous studies of trans-fatty acids report a positive association with prostate cancer, few if any consider levels of these fatty acids in relation to other fatty acids in the blood. It is possible that this factor simply represents individuals with greater intake of dairy fats, though this hypothesis is not strongly supported in the literature, where most evidence supports increased prostate cancer risk with increasing dairy consumption; though one group has reported an inverse association specifically for dairy fats and prostate cancer risk among heavy smokers [39].
To our knowledge, only one other group has published on the association between the fatty acid groupings based on a principal components approach and cancer risk in humans [18, 19] and none have attempted to evaluate the association fatty acids with risk of prostate cancer using the principal components approach or compared findings from three different approaches. The comparison of these three approaches allows us to identify how analysis of single fatty acids or even pre-determined ratios may result in missing potential complex interactions between fatty acids that may have a more dramatic impact on risk then either an individual fatty acid or the ratio between two fatty acids. In the work by Bougnoux, principal components analysis was used to identify principal components that explained the variability in white adipose tissue fatty acid concentrations in women with and without breast cancer. Bougnoux et al report a significant increase in risk of breast cancer with lower levels of the omega-6/omega-3 and cis-monounsaturated fatty acids present primarily in their second component. While we were able to conduct a very similar analysis using erythrocyte fatty acid concentrations, the factors we identified were different from those presented by Bougnoux et al [18, 19].
In interpreting the results from our principal components analysis it is important to remember that this methodology is limited at several points due to the necessity for investigator judgment [40]. This includes decisions regarding the number of factors to extract, the choice of method of rotation and the emphasis placed on naming or describing the different factors. As described we chose to use a scree test to determine our cut off of six factors. This was a visual test but was also very similar to the result we would obtain with a simple cut-off of eigenvalue < 1.0. As our goal was to identify uncorrelated factors, we chose an orthogonal rotation method. Finally we have provided all factor loadings for each fatty acid, thus allowing for the reader to develop their own interpretation of the meaning of each factor.
There are a number of strengths to the current analyses, including the availability of complete data on a number of potential confounding variables and the use of erythrocyte fatty acids measures. Individuals in these analyses completed a detailed diet history questionnaire and risk factor questionnaire, providing the capacity to consider adjustment for other dietary factors and demographic characteristics potentially associated with prostate cancer. Further, fatty acid levels were determined in the erythrocyte membrane, considered an intermediate marker of fatty acid intake, representing approximately the past three months of intake. While this measure does not provide the long-term assessment available from adipose tissue samples, the acquisition of blood samples is more feasible in an epidemiologic study, provides more precision and avoids concerns of recall bias common to the use of food frequency questionnaires. It is notable though that the erythrocytes still remain only an estimate of the fatty acid exposure in the prostate. Work by Christensen et al [14] demonstrated that leukocyte levels of EPA and DHA were highly correlated with prostate tissue levels (r=0.80 and 0.53 respectively, p<0.001) in both men with benign hyperplasia and in men with prostate cancer. However leukocyte levels of ALA were not correlated with prostate tissue levels in the men with cancer, suggesting that there may be differential incorporation of fatty acids into prostate tissue, particularly among men with cancer.
Despite these strengths, our analyses are limited by our small sample size and retrospective case-control design. Hence, while intriguing, our results with regards to the association between fatty acids and prostate cancer must be viewed conservatively and require confirmation in larger and ideally prospective studies. While sample size is a clear limitation in interpreting the strength of these findings with relation to prostate cancer risk, and particularly as stratified by disease severity, the results are meaningful as an exemplar of the various methods by which one may evaluate the independent or interactive effect of fatty acids on cancer risk.
Acknowledgments
We would like to acknowledge Ms. Laura Peters, RN without whose assistance we would not have been able to conduct this study.
Work Supported by: United States Public Health Service grants (5 M01 RR000334), (1 UL1 RR024120-01) and (K22CA94973) and was supported by the resources and facilities of the Portland Veterans Affairs Medical Center. Biostatistics support was provided through the Knight Cancer Institute Biostatistics Shared Resource (P30 CA069533-09) and the Oregon Clinical and Translational Research Institute (UL1 RR024140).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jump DB. Dietary polyunsaturated fatty acids and regulation of gene transcription. Curr Opin Lipidol. 2002;13:155–164. doi: 10.1097/00041433-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Jump DB. Fatty acid regulation of gene transcription. Crit Rev Clin Lab Sci. 2004;41:41–78. doi: 10.1080/10408360490278341. [DOI] [PubMed] [Google Scholar]
- 3.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Bio. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 4.Ritch CR, Wan RL, Stephens LB, et al. Dietary fatty acids correlate with prostate cancer biopsy grade and volume in Jamaican men. J Urol. 2007;177:97–101. doi: 10.1016/j.juro.2006.08.105. discussion 101. [DOI] [PubMed] [Google Scholar]
- 5.Yang YJ, Lee SH, Hong SJ, et al. Comparison of fatty acid profiles in the serum of patients with prostate cancer and benign prostatic hyperplasia. Clin Biochem. 1999;32:405–409. doi: 10.1016/s0009-9120(99)00036-3. [DOI] [PubMed] [Google Scholar]
- 6.Hyde CA, Missailidis S. Inhibition of arachidonic acid metabolism and its implication on cell proliferation and tumour-angiogenesis. Int Immunopharmacol. 2009;9:701–715. doi: 10.1016/j.intimp.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Clarke SD. The multi-dimensional regulation of gene expression by fatty acids: polyunsaturated fats as nutrient sensors. Curr Opin Lipidol. 2004;15:13–18. doi: 10.1097/00041433-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Covey TM, Edes K, Fitzpatrick FA. Akt activation by arachidonic acid metabolism occurs via oxidation and inactivation of PTEN tumor suppressor. Oncogene. 2007;26:5784–5792. doi: 10.1038/sj.onc.1210391. [DOI] [PubMed] [Google Scholar]
- 9.MacLean CH, Newberry SJ, Mojica WA, et al. Effects of omega-3 fatty acids on cancer risk: a systematic review. Jama. 2006;295:403–415. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- 10.Chan J, Holick C, Leitzmann M, et al. Diet After Diagnosis and the Risk of Prostate Cancer Progression, Recurrence, and Death (United States) Cancer Causes and Control. 2006;17:199. doi: 10.1007/s10552-005-0413-4. [DOI] [PubMed] [Google Scholar]
- 11.Chavarro JE, Stampfer MJ, Hall MN, et al. A 22-y prospective study of fish intake in relation to prostate cancer incidence and mortality. Am J Clin Nutr. 2008;88:1297–1303. doi: 10.3945/ajcn.2008.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavarro JE, Stampfer MJ, Li H, et al. A Prospective Study of Polyunsaturated Fatty Acid Levels in Blood and Prostate Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2007;16:1364–1370. doi: 10.1158/1055-9965.EPI-06-1033. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi N, Barnard RJ, Henning SM, et al. Effect of Altering Dietary {omega}-6/{omega}-3 Fatty Acid Ratios on Prostate Cancer Membrane Composition, Cyclooxygenase-2, and Prostaglandin E2. Clin Cancer Res. 2006;12:4662–4670. doi: 10.1158/1078-0432.CCR-06-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen JH, Fabrin K, Borup K, et al. Prostate tissue and leukocyte levels of n-3 polyunsaturated fatty acids in men with benign prostate hyperplasia or prostate cancer. BJU International. 2006;97:270–273. doi: 10.1111/j.1464-410X.2006.05951.x. [DOI] [PubMed] [Google Scholar]
- 15.Raederstorff D, Moser U. Influence of an increased intake of linoleic acid on the incorporation of dietary (n-3) fatty acids in phospholipids and on prostanoid synthesis in rat tissues. Biochim Biophys Acta. 1992;1165:194–200. doi: 10.1016/0005-2760(92)90187-z. [DOI] [PubMed] [Google Scholar]
- 16.Newcomer LM, King IB, Wicklund KG, et al. The association of fatty acids with prostate cancer risk. Prostate. 2001;47:262–268. doi: 10.1002/pros.1070. [DOI] [PubMed] [Google Scholar]
- 17.Kositsawat J, Flanigan RC, Meydani M, et al. The Ratio of Oleic-to-Stearic Acid in the Prostate Predicts Biochemical Failure After Radical Prostatectomy for Localized Prostate Cancer. The Journal of Urology. 2007;178:2391. doi: 10.1016/j.juro.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Bougnoux P, Giraudeau B, Couet C. Diet, Cancer, and the Lipidome. Cancer Epidemiol Biomarkers Prev. 2006;15:416–421. doi: 10.1158/1055-9965.EPI-05-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bougnoux P, Hajjaji N, Couet C. The lipidome as a composite biomarker of the modifiable part of the risk of breast cancer. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2008;79:93. doi: 10.1016/j.plefa.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Seppanen-Laakso T, Oresic M. How to study lipidomes. J Mol Endocrinol. 2009;42:185–190. doi: 10.1677/JME-08-0150. [DOI] [PubMed] [Google Scholar]
- 21.Flowers MT. The {Delta}9 Fatty Acid Desaturation Index as a Predictor of Metabolic Disease. Clin Chem. 2009;55:2071–2073. doi: 10.1373/clinchem.2009.135152. [DOI] [PubMed] [Google Scholar]
- 22.Peter A, Cegan A, Wagner S, et al. Hepatic Lipid Composition and Stearoyl-Coenzyme A Desaturase 1 mRNA Expression Can Be Estimated from Plasma VLDL Fatty Acid Ratios. Clin Chem. 2009;55:2113–2120. doi: 10.1373/clinchem.2009.127274. [DOI] [PubMed] [Google Scholar]
- 23.Sjögren P, Sierra-Johnson J, Gertow K, et al. Fatty acid desaturases in human adipose tissue: relationships between gene expression, desaturation indexes and insulin resistance. Diabetologia. 2008;51:328. doi: 10.1007/s00125-007-0876-9. [DOI] [PubMed] [Google Scholar]
- 24.Wahl HGn, Kausch C, Machicao F, et al. Troglitazone Downregulates î”-6 Desaturase Gene Expression in Human Skeletal Muscle Cell Cultures. Diabetes. 2002;51:1060–1065. doi: 10.2337/diabetes.51.4.1060. [DOI] [PubMed] [Google Scholar]
- 25.Warensjo E, Rosell M, Hellenius ML, et al. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: links to obesity and insulin resistance. Lipids in Health and Disease. 2009;8:37. doi: 10.1186/1476-511X-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shannon J, Phoutrides E, Palma A, et al. Folate intake and prostate cancer risk: a case-control study. Nutr Cancer. 2009;61:617–628. doi: 10.1080/01635580902846593. [DOI] [PubMed] [Google Scholar]
- 27.Subar AF, Midthune D, Kulldorff M, et al. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol. 2000;152:279–286. doi: 10.1093/aje/152.3.279. [DOI] [PubMed] [Google Scholar]
- 28.Shannon J, Tewoderos S, Garzotto M, et al. Statins and prostate cancer risk: a case-control study. Am J Epidemiol. 2005;162:318–325. doi: 10.1093/aje/kwi203. [DOI] [PubMed] [Google Scholar]
- 29.Rose HG, Oklander M. Improved Procedure For The Extraction Of Lipids From Human Erythrocytes. J Lipid Res. 1965;63:428–431. [PubMed] [Google Scholar]
- 30.Shannon J, King IB, Lampe JW, et al. Erythrocyte fatty acids and risk of proliferative and nonproliferative fibrocystic disease in women in Shanghai, China. Am J Clin Nutr. 2009;89:265–276. doi: 10.3945/ajcn.2008.26077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shannon J, King IB, Moshofsky R, et al. Erythrocyte fatty acids and breast cancer risk: a case-control study in Shanghai, China. Am J Clin Nutr. 2007;85:1090–1097. doi: 10.1093/ajcn/85.4.1090. [DOI] [PubMed] [Google Scholar]
- 32.Kelavkar U, Lin Y, Landsittel D, et al. The yin and yang of 15-lipoxygenase-1 and delta-desaturases: dietary omega-6 linoleic acid metabolic pathway in prostate. J Carcinog. 2006;5:9. doi: 10.1186/1477-3163-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore S, Knudsen B, True LD, et al. Loss of stearoyl-CoA desaturase expression is a frequent event in prostate carcinoma. Int J Cancer. 2005;114:563–571. doi: 10.1002/ijc.20773. [DOI] [PubMed] [Google Scholar]
- 34.Astorg P. Dietary n – 6 and n – 3 Polyunsaturated Fatty Acids and Prostate Cancer Risk: A Review of Epidemiological and Experimental Evidence. Cancer Causes and Control. 2004;15:367. doi: 10.1023/B:CACO.0000027498.94238.a3. [DOI] [PubMed] [Google Scholar]
- 35.Brouwer IA, Katan MB, Zock PL. Dietary alpha-linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: a meta-analysis. J Nutr. 2004;134:919–922. doi: 10.1093/jn/134.4.919. [DOI] [PubMed] [Google Scholar]
- 36.Crowe FL, Allen NE, Appleby PN, et al. Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;88:1353–1363. doi: 10.3945/ajcn.2008.26369. [DOI] [PubMed] [Google Scholar]
- 37.Simon JA, Chen YH, Bent S. The relation of {alpha}-linolenic acid to the risk of prostate cancer: a systematic review and meta-analysis. Am J Clin Nutr. 2009;89:1558S–1564. doi: 10.3945/ajcn.2009.26736E. [DOI] [PubMed] [Google Scholar]
- 38.Krachler B, Norberg M, Eriksson JW, et al. Fatty acid profile of the erythrocyte membrane preceding development of Type 2 diabetes mellitus. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2008;18:503. doi: 10.1016/j.numecd.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Neuhouser ML, Barnett MJ, Kristal AR, et al. (n-6) PUFA increase and dairy foods decrease prostate cancer risk in heavy smokers. J Nutr. 2007;137:1821–1827. doi: 10.1093/jn/137.7.1821. [DOI] [PubMed] [Google Scholar]
- 40.Martinez ME, Marshall JR, Sechrest L. Invited commentary: Factor analysis and the search for objectivity. Am J Epidemiol. 1998;148:17–19. doi: 10.1093/oxfordjournals.aje.a009552. [DOI] [PubMed] [Google Scholar]


