Abstract
Visual appearance depends upon the resolution of ambiguities that arise when 2D retinal images are interpreted as 3D scenes. This resolution may be characterized as a form of Bayesian perceptual inference, whereby retinal sense data combine with prior belief to yield an interpretation. Under this framework, the prior reflects environmental statistics, so an efficient system should learn by changing its prior after exposure to new statistics. We conjectured that a prior would only be modified when sense data contain disambiguating information, such that it is clear what bias is appropriate. This conjecture was tested by using a perceptually bistable stimulus, a rotating wire-frame cube, as a sensitive indicator of changes in the prior for 3D rotation direction, and by carefully matching perceptual experience of ambiguous and unambiguous versions of the stimulus across three groups of observers. We show for the first time that changes in the prior—observed as a change in bias that resists reverse learning the next day—is affected more by ambiguous stimuli than by disambiguated stimuli. Thus, contrary to our conjecture, modification of the prior occurred preferentially when the observer actively resolved ambiguity rather than when the observer was exposed to environmental contingencies. We propose that resolving stimuli that are not easily interpreted by existing visual rules must be a valid method for establishing useful perceptual biases in the natural world.
Keywords: perceptual learning, perceptual bias, Necker cube, Bayesian prior
Introduction
Visual appearance, or the way “things look”, is essential for navigation, manipulation, and comprehension of the immediate environment. However, the retinal images are insufficient to constrain the appearance of objects, most famously for recovering 3D information from scenes. The visual system must therefore employ additional constraints when interpreting input. Current theories of perceptual inference take a Bayesian approach, with the resolution of ambiguity being driven by knowledge of statistics of the encountered environment, as captured by “prior probability” distributions (Geisler & Kersten, 2002a; Kersten, Mamassian & Yuille, 2004; Knill & Richards, 1996; Weiss, Simoncelli & Adelson, 2002a). Such priors are evident as biases towards one perceptual outcome over other alternatives.
Human visual mechanisms retain plasticity into adulthood (e.g. Adams, Banks & van Ee, 2001; Ernst, Banks & Bulthoff, 2000; Jacobs & Fine, 1999; Kohler, 1962; Sinha & Poggio, 1996; Wallach & Austin, 1954), and it has been demonstrated that this plasticity encompasses long term changes in biases (Backus & Haijiang, 2007; Haijiang, Saunders, Stone & Backus, 2006). However, it is not known whether the learning mechanisms that implement long-term perceptual biases require that visual stimuli contain information (e.g. Kersten, O'Toole, Sereno, Knill & Anderson, 1987), or whether they simply resolve ambiguities the same way similar ambiguities were resolved in the past, as they do for short term priming (Knapen, Brascamp, Adams & Graf, 2009). We conjectured that a prior would only be modified when sense data contain disambiguating information, such that it is clear what bias is appropriate.
To measure changes in bias, we used a perceptually bistable stimulus, a wire frame (Necker) cube. An important property of these stimuli is that they are clearly perceived as one of two dichotomous interpretations at stimulus onset. Hence, viewers can easily and confidently report their own perceptual outcome on each trial of the experiment. Perceived rotation direction at stimulus onset can reliably be made contingent on the stimulus’ retinal location (Backus & Haijiang, 2007; Harrison & Backus, 2009). The location-dependent bias is achieved using a classical (Pavlovian) conditioning paradigm: Disambiguating visual cues determine the perceptual outcome on informative (training) trials. The perceptual outcome on inter-leaved ambiguous (test) trials then becomes coincident with that of disambiguated trials. Training of opposite perceptual outcomes at two retinal locations ensures that the measured effects are due to learning by the system rather than pre-existing global biases. The long-lasting component of the learned bias is assessed the following day, using a counter-conditioning procedure: Strong learning on Day 1 causes resistance to relearning from reverse-rotating stimuli on Day 2. In the experiments that follow, we use this known effect to assess whether learning of long-term bias is driven by experience of informative stimulus contingencies, presented in disambiguated trials, or by experience of ambiguous, uninformative, stimuli that have been resolved by the visual system itself.
Methods
Hardware and Software
Experiments were programmed in Python using Vizard platform 3.11 (© WorldViz, Santa Barbara, CA.) on a Dell Precision T3400. Stimuli were rear-projected, using a Christie Mirage S+ 4K projector.
Stimuli
Simulated rotating cube stimuli (Harrison & Backus, 2010) were light against a dark background, and were viewed through red-green glasses at a distance of 1.0 m in an otherwise dark room. They were presented centered 12 degrees above or below a central fixation marker (2 × 2 cm outline square, at screen depth) in a randomly-interleaved sequence. Cube edges were thin rectangular parallelepipeds with length 20.0 cm and width 0.3 cm. Each transparent face of the cube contained 25 randomly-placed spots, 5 mm in diameter, which stabilized appearance on ambiguous trials as a single rigid rotating body.
Cubes rotated about a vertical axis at 45 degrees sec−1, from a starting orientation at stimulus onset such that the images of their front and back edges were vertical and collinear with the center of the screen. The roll and pitch angles of the cube, which determine whether the cube appeared to be viewed from above (top surface towards observer) or below (bottom surface towards observer) at stimulus onset, were ±25 degrees. This parameter was balanced across all trials (including ambiguous trials, which can by definition be perceived at either orientation at any point in time) because “above” and “below” configurations are associated with different motions of the cube edges at stimulus onset, a possible confounding cue.
Each trial contained a single cube, presented at one of two locations. Within a single session, disambiguated cubes had fixed, opposite, rotation at the two locations. Rotation direction was specified by anaglyphic presentation of geometrically-correct binocular disparity, and a central vertical strip (2.0 cm wide, presented stereoscopically at screen depth) around which the cube rotated indicating depth by occlusion. Ambiguous cubes were presented at the same two locations, but contained no cues to depth and were presented monocularly to observers’ right eye; hence their direction of rotation was ambiguous. All cubes were presented using orthographic projection so that perspective cues were could not inform perceptual outcome.
Trial Sequence and Task
A central fixation marker was always present. Subjects were instructed to fixate the marker, then press “0” to initiate presentation of a rotating cube and a probe dot after a 0.5 second delay (Figure 1). The dot repeated cycles of horizontal motion through the fixation marker, with a speed of 16 cm sec−1. It was presented at fixation depth on disambiguated trials and monocularly on ambiguous trials. Subjects’ task was to indicate whether the direction of motion of the comparison dot (leftward or rightward) was the same as the direction of motion of the front of the cube or the back of the cube (keypress ‘2’ and ‘8’ respectively). The cube and probe dot were visible for a minimum of 1.5 seconds and a maximum of 6.0 seconds; the subject’s response terminated the presentation. The ISI between stimuli was typically just under 2 seconds in our self-paced trials (and due to the randomized sequence of presentations, was often much longer for stimuli presented at the same location). Each session contained 480 trials, split into 4 blocks separated by rest intervals of a minimum of 2 minutes.
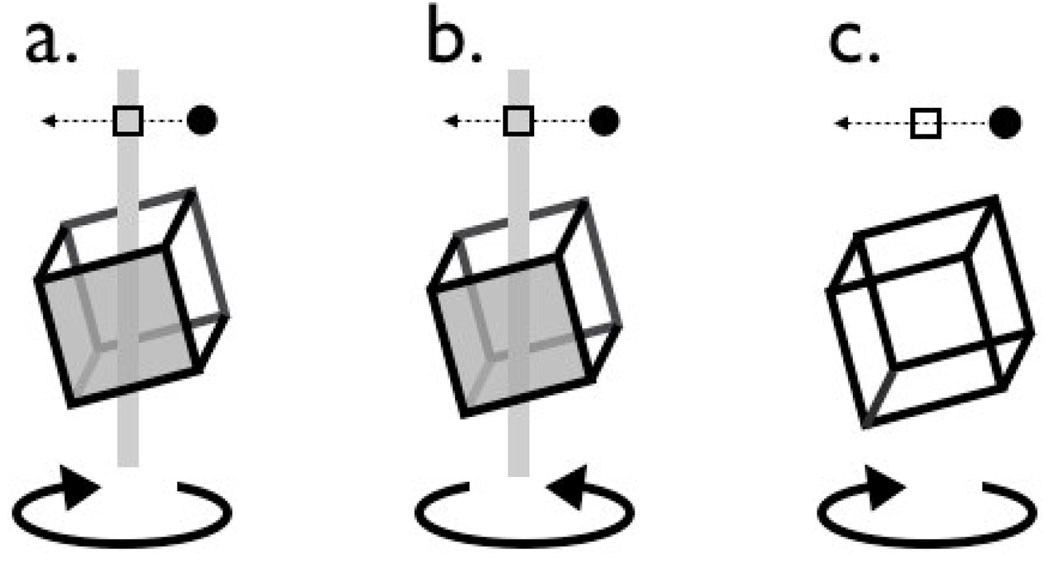
Figure 1.
Schematic representation of rotating cube task. a. The cube is disambiguated (informative). Front (filled) face of cube moves to the left, as does the dot; subject responds “front” (keypress ‘2’). b. The cube is disambiguated (informative). Back (unfilled) face moves left, as does the dot; subject responds “back” (keypress ‘8’). c. The cube is ambiguous (uninformative), but the subject clearly perceives one or other rotation and answers “front” or “back” accordingly. The direction of the probe dot was randomly allocated on each trial, so response was uncorrelated with apparent rotation. Arrows and shaded faces illustrate disambiguation in the figure; in the experiment rotation was disambiguated by binocular disparity and by occlusion (using an axial column similar to the one shown).
Note that we are interested not in the alternation of perceptual experience during continuous uninterrupted viewing (e.g. Long, Toppino & Kostenbauder, 1983) but rather the choice made by the visual system at stimulus onset such as normally occurs during natural vision. Subjects were instructed to strive for accuracy, not speed, in their responses. Subjects typically responded within 2 seconds, and rarely perceived reversals of rotation on ambiguous trials (less than 1 %).
Subjects
Subjects were adult members of the public who were recruited from the local area (New York City) via Craig’s List, and were paid for their time. Subjects’ vision was normal or corrected-to-normal with non-bifocal lenses. Stereoscopic vision was assessed using the TNO Stereoacuity test; subjects were required to have a minimum stereoacuity of 240 seconds of arc. However, stereoacuity in static images is not always an indicator of stereoacuity in dynamic images such as the rotating cube stimulus used here (Rouse, Tittle & Braunstein, 1989). Accordingly, our critical measure of subjects’ suitability for the experiment, in terms of both stereoacuity and task comprehension, was performance on disambiguated trials. Three subjects (one from each of the two groups in Experiment 1 and one from the single group in Experiment 2), who met other criteria but did not have correct performance of 95% or higher on disambiguated trials, were excluded. A total of 24 subjects completed two experimental sessions on consecutive days.
Experiment 1
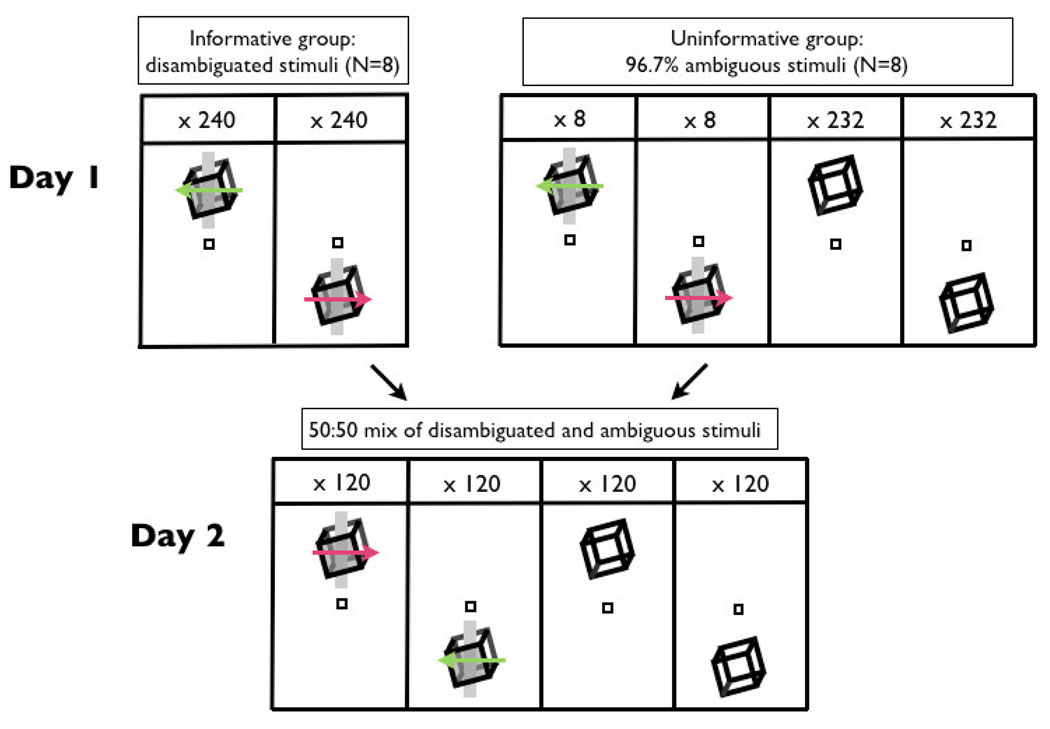
We tested the effect of previous experience on perceptual bias in two groups of eight subjects (Figure 2). On Day 1, half of subjects in each group were presented with disambiguated cubes with “top–clockwise (CW, as viewed from above), bottom–counterclockwise (CCW)” contingency, and half viewed the opposite contingency. The “Informative” group viewed only disambiguated cubes; the “Uninformative” group was so termed because they viewed uninformative, ambiguous cubes on the vast majority (96.7%) of trials. Critical to our experiment design, we anticipated that inclusion of a small number of disambiguated cube trials would be sufficient to prime stable perceived rotation of the ambiguous cubes (Brascamp, Knapen, Kanai, Noest, van Ee & van den Berg, 2008; Klink, van Ee, Nijs, Brouwer, Noest & van Wezel, 2008).
Figure 2.
Schematic representation of experiment design. On Day 1, one group (“Informative”) viewed disambiguated cubes, with rotation direction contingent on location relative to a central fixation marker. A second group (“Uninformative”) viewed predominantly ambiguous cubes, but the percept of rotation was primed to be the same as that of the first group, by inclusion of some disambiguated cubes. On Day 2, all groups viewed a 50:50 mix of ambiguous and disambiguated cubes; disambiguated cubes had a rotation direction opposite to that which each subject had viewed on Day 1.
For the Uninformative group, the first 8 trials were constrained to present all possible combinations of trial type, cube viewpoint and cube location in related disambiguated-ambiguous pairs, and alternating between the two cube locations and two viewpoints. The exact order of presentation was counterbalanced across subjects. This sequence, designed to stabilize opposite perceptual outcome of ambiguous cubes at the two locations, was repeated at the start of each block of 120 trials. All other trials contained ambiguous cubes, randomly drawn from balanced blocks of 8 trials containing two stimuli from each combination of the two cube locations and two viewpoints. The Informative group viewed disambiguated, informative stimuli only, presented in balanced blocks of 8 trials containing two stimuli from each permutation of the two cube locations and two viewpoints. The first 8 trials were constrained as for the Uninformative group, with the exception that all trials were disambiguated.
The long-term influence of perceptual experience on Day 1 was assessed through resistance of the learned bias to counter-conditioning on Day 2 (Backus & Haijiang, 2007; Haijiang et al., 2006). Subjects viewed a 50:50 mix of disambiguated and ambiguous stimuli. Disambiguated stimuli had the opposite rotation-location contingency to that experienced on Day 1. If little long-term learning occurred on Day 1, then subjects should perceive ambiguous, uninformative, stimuli on Day 2 according to the location-rotation contingency of Day 2 disambiguated stimuli. However, if Day 1 experience caused learning, subjects’ perception of ambiguous stimuli on Day 2 should reflect a bias in the direction of the location-rotation contingency experienced on Day 1.
The sequence of the first 8 trials on Day 2 was identical to that of the Day 1 Uninformative group (but with reversed contingency). The remaining trials were randomly drawn from balanced blocks of 8 trials containing all possible permutations of trial type, cube viewpoint, and cube location.
Analysis
The percent of cubes seen as rotating in the direction specified by the Day 1 disambiguated presentations, at each of the two locations, was transformed into a z-score, i.e. we used a probit (inverse-cumulative-normal) transformation (Backus, 2009; Dosher, Sperling & Wurst, 1986). This is a measure of the likelihood of the observations given normally distributed noise in a decision variable. For the purpose of analysis, saturated values (100% or 0%) were replaced with a z-score of ±2.394. This is equivalent to 2 nonconforming responses within 240 observations, or 1 response in 120 observations. For each subject, z-scores for the top and bottom locations were summed, giving a “zDiff” measure of the extent to which perceived rotation differed between the two locations. We use zDiff as our indicator of training-induced bias, which is independent of any global, preexisting bias. All individual zDiffs are reported in Table 1.
Table 1.
Individual zDiffs for all subjects, for all groups and conditions described.
| Day 1 all trails | |||||
|---|---|---|---|---|---|
| Subject | Informative Group | Uninformative Group | 50:50 Group | 50:50 mix additional subjects | |
| 1 | 2.884 | 3.413 | 3.997 | 4.287 | 3.681 |
| 2 | 4.369 | 4.635 | 4.788 | 3.652 | 2.273 |
| 3 | 4.081 | 4.635 | 3.126 | 4.175 | 2.208 |
| 4 | 4.788 | 4.228 | 3.403 | 1.911 | 4.201 |
| 5 | 4.431 | 3.920 | 4.788 | 4.278 | 3.647 |
| 6 | 4.522 | 2.535 | 4.788 | 4.287 | 4.788 |
| 7 | 4.369 | 4.354 | 4.788 | 2.554 | 4.635 |
| 8 | 3.332 | 3.335 | 3.741 | 4.635 | 4.354 |
| Day 2 ambiguous trials | |||||
| 1 | −1.393 | 1.739 | 3.895 | 2.751 | −0.495 |
| 2 | −2.141 | 4.522 | 4.788 | 1.627 | 0.681 |
| 3 | 0.168 | 4.788 | 0.266 | 2.057 | −1.247 |
| 4 | −3.920 | −0.266 | −1.393 | −0.749 | −0.263 |
| 5 | −3.725 | 1.648 | −3.430 | 1.459 | 0.846 |
| 6 | −2.436 | 1.910 | 4.788 | 4.788 | 0.964 |
| 7 | 0.248 | 4.039 | 0.000 | 0.000 | 4.788 |
| 8 | −1.708 | 3.217 | −3.834 | 2.478 | 0.560 |
| Day 1 ambiguous trials for 50:50 group | |||||
| 1 | 4.088 | ||||
| 2 | 4.788 | ||||
| 3 | 3.214 | ||||
| 4 | 3.301 | ||||
| 5 | 4.788 | ||||
| 6 | 4.788 | ||||
| 7 | 4.788 | ||||
| 8 | 3.566 | ||||
We tested for differences in perceptual experience between groups by parametric analysis of zDiffs and use of 95% confidence intervals. (The Welch-Satterthwaite equation was used to estimate degrees of freedom in case of unequal variances or sample sizes, and confidence interval endpoints of 1.25% and 98.75% were used to correct for two comparisons.
Experiment 1 Results
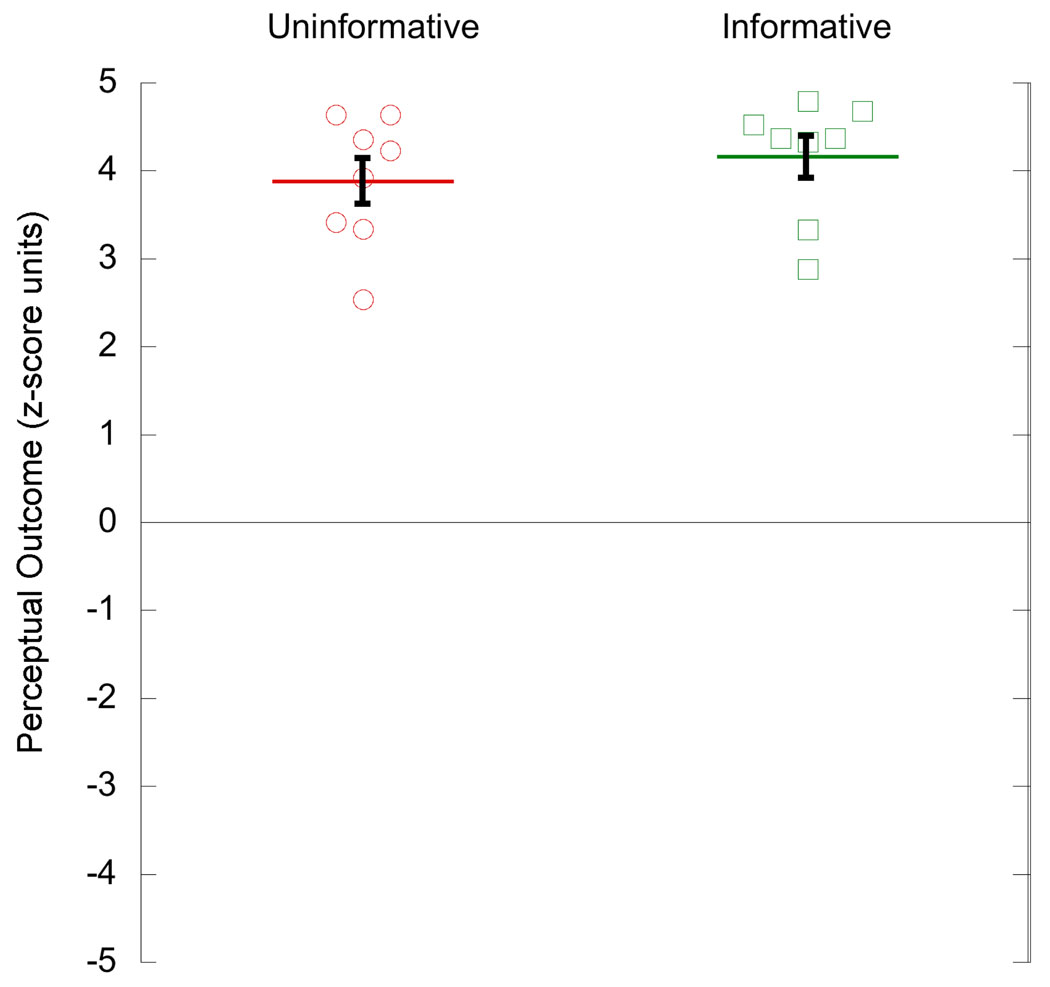
Day 1 zDiffs, over all trials, were not significantly different between the Uninformative and Informative groups (Figure 3; Uninformative group mean = 3.90, s.e.m. = 0.27; Informative group mean = 4.16, s.e.m. = 0.24; t(14) = 0.62, p = 0.55, 95% CI for difference = [−0.53, 0.96]). Critically, this shows that inclusion of only 16 disambiguated cube trials in the Uninformative group was sufficient to prime stable perceived rotation of the ambiguous cubes (Brascamp et al., 2008; Klink et al., 2008). Hence, both groups experienced the same “location-rotation contingency” on Day 1.
Figure 3.
Difference in perceived rotation at the two cube locations on Day 1, assessed over all 480 trials in the session. Data points for 8 subjects in each of two groups. Horizontal lines are mean of group and error bars show s.e.m.
Residual bias on Day 2, as assessed by the perceptual outcome of ambiguous trials during counter-conditioning, is shown in Figure 4. Despite equivalent perceptual experience on Day 1, perceived rotation on Day 2 ambiguous trials was significantly different between the Uninformative and Informative groups (Uninformative group, mean = 2.70, s.e.m. = 0.62; Informative group, mean = −1.86, s.e.m. = 0.55; t(14) = 5.53, p = 0.001). Thus, subjects who saw a vast majority of ambiguous cubes on Day 1 retained a far greater bias than those who saw only informative, disambiguated, cubes. On both Day 1 and Day 2, perceptual outcome was remarkably stable throughout the session, as previously observed and discussed (Harrison & Backus, 2010), with no significant change in zDiff across trials.
Figure 4.
Difference in perceived rotation at the two cube locations on Day 2, assessed over the 240 ambiguous trials in the session. Perceptual outcome is assessed relative to the direction specified by disambiguated trials on Day 1. Data points for 8 subjects in each of two groups. Horizontal lines are mean of group and error bars show s.e.m.
Perceptual experience of ambiguous cubes clearly exerted a greater influence on long-term bias for perceptual outcome than did experience with disambiguated, informative, cubes. It is important to note that Day 1 perceptual outcomes were matched for the 2 groups; differences in the learned bias therefore cannot be attributed to quantitative differences in experience and must instead be due to qualitative differences in the nature of the percept. However, the results of Experiment 1 cannot inform us as to whether there was no learning, or simply reduced learning, in the Informative group.
Experiment 2
To assess whether the Informative group retained any bias from Day 1, we tested a third group of eight subjects who viewed a 50:50 mixture of disambiguated and ambiguous trials on Day 1, as well as on Day 2. This 50:50 group also allows us to test whether seeing a mixture of disambiguated and ambiguous stimuli on Day 1 would produce intermediate learning on Day 2. The trial sequence was as described previously for all subjects on Day 2. As in Experiment 1, subjects viewed opposite location-rotation contingencies on Day 1 and Day 2 disambiguated trials.
Experiment 2: Results
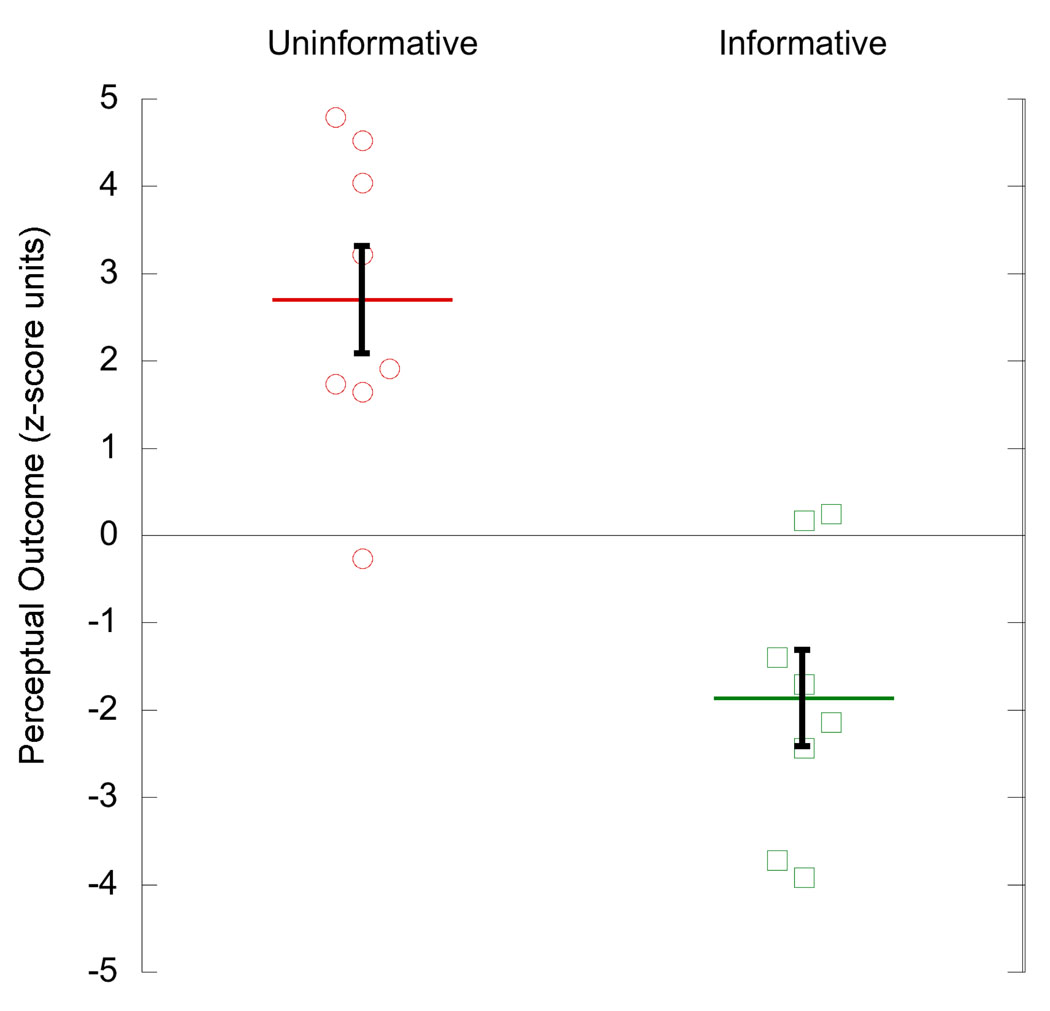
Results from the 50:50 group are shown in Figure 5. As expected, perceived rotation over all trials on Day 1 matched that of the other two groups (red circle data points: mean = 4.18, s.e.m. = 0.25, ANOVA F(21) = 0.36, p = 0.70). As in Experiment 1, because Day 1 perceptual experience was matched to that of the other 2 groups, differences in the learned bias cannot be attributed to quantitative differences in experience.
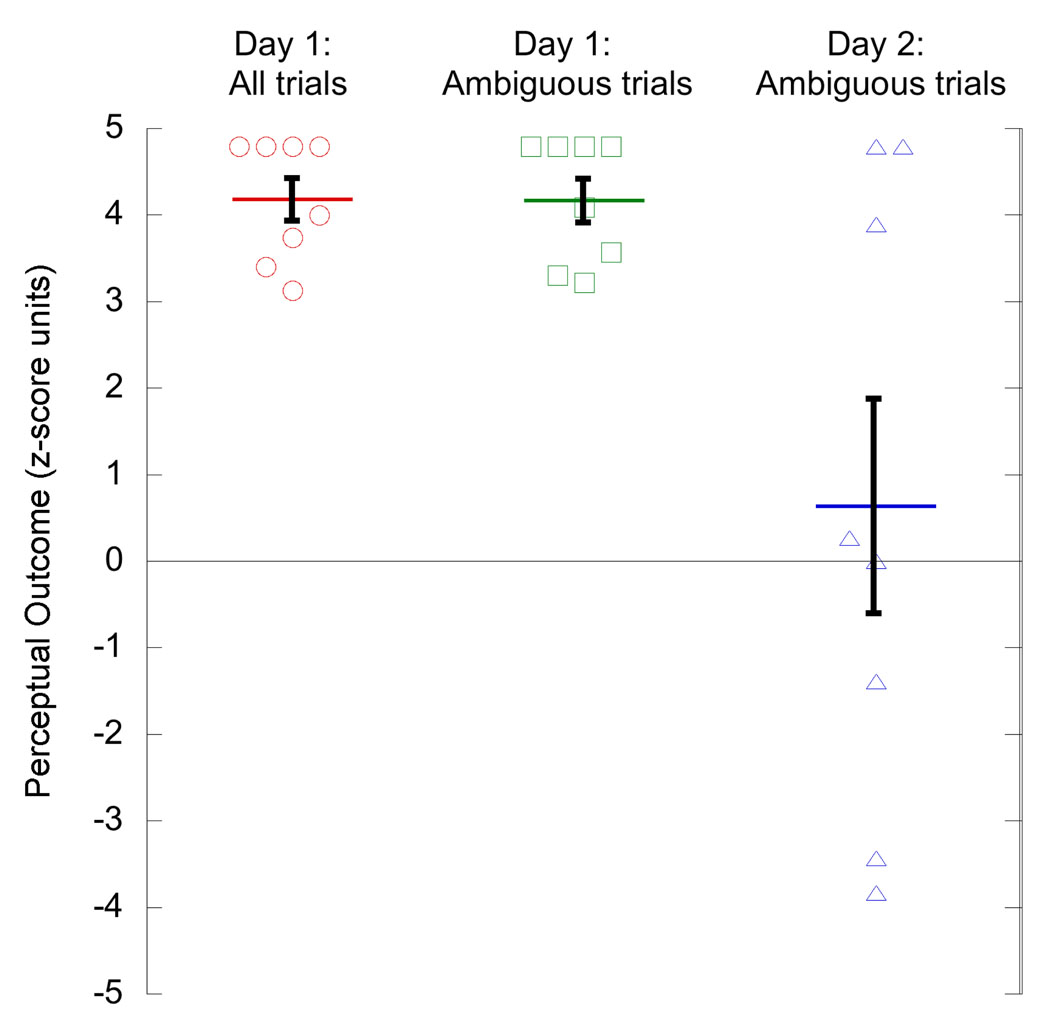
Figure 5.
Results from the 50:50 group; Day 1 perceptual experience over all 480 trials, Day 1 perceptual experience of 240 ambiguous trials only, Day 2 perceptual experience of 240 ambiguous trials only. Data points for 8 subjects. Horizontal lines are mean of group and error bars show s.e.m.
The (sign-reversed) perceptual outcome of ambiguous trials on Day 1 in the 50:50 group differed from that of ambiguous trials on Day 2 in both the Uninformative and Informative groups (green square data points: mean = −4.17, s.e.m. = 0.25; comparison with Uninformative: t(14) = 10.3, p < 0.001, corrected 95% CI [5.2 – 8.5]; comparison with Informative: t(14) = 3.8, p = 0.002, corrected 95% CI [0.8 – 3.8]). Thus, viewing only disambiguated stimuli on Day 1 did cause long term learning, albeit less than when subjects viewed a large majority of ambiguous stimuli.
Average learning in the 50:50 group, as revealed by perceptual outcome on Day 2 ambiguous trials, fell between that of the other two groups (blue triangle data points: mean 0.64, s.e.m. 1.24). However, the variance between subjects was much larger than had been seen in the Uninformative or Informative groups on Day 2, hence pairwise comparisons were not significant. Although inequality in variance across the three groups was not statistically significant (p = 0.064, Bartlett multiple-sample test for equal variances), we recruited data from an additional 16 subjects in a previously-run experiment of the same design (Harrison & Backus, 2010) in order to better assess differences between the groups (Table 1).
First, we confirmed that the combined 50:50 group (N=24) showed no difference from the other two groups on Day 1 (mean = 3.87, s.e.m. = 0.19, F(37) = 0.36, p = 0.70), allowing meaningful comparison of Day 2 perceptual experience. On Day 2, zDiffs for ambiguous trials in the 50:50 group (mean = 1.06, s.e.m = 0.50) were almost significantly lower than the Uninformative group, and significantly higher than the Informative group (comparison with Uniformative: t(16.9) = 2.1, p = 0.053, corrected 95% CI [−0.3 – 3.6]; comparison with Informative: t(19.2) = 3.9, p < 0.001, corrected 95% CI [1.1 – 4.7]).
The Day 2 variance remained larger in the 50:50 group, but again this was not significant (p = 0.30, Bartlett). One possible explanation for a large variance in retained bias in the 50:50 group would be if subjects differ in whether they preferentially process either the disambiguated or ambiguous stimuli on Day 1, leading to lesser or greater retained bias on Day 2 respectively. Another way of framing this possibility is that the visual system adopts either a “sense data” mode or a more computationally expensive “Bayesian inference” mode on Day 1. Although subjects report the same perceptual outcome in both cases, with ceiling performance, the system is learning from either the disambiguated or the ambiguous trial type. The large variance in the bias retained on Day 2 would, in this scenario, reflect individual differences in the processing state of the visual system during Day 1. The smaller variance of the Informative and Uninformative groups would be explained by the more nearly uniform “decisions” made by subjects’ visual systems as to which processing mode was most appropriate.
However a simpler explanation of differences on a trial-by-trial level would also suffice, whereby the variances seen within the Uninformative and Informative groups, reflecting subjects’ different levels of susceptibility to training by each stimulus type, are combined when the two stimulus types are interleaved, i.e., algebraic summation of the two variances results in a larger variance within the 50:50 group.
Discussion
We conjectured that a prior for perceptual outcome would only be modified when sense data contain disambiguating information. Instead, we found the opposite result: Modification of the prior for perceptual outcome in our bistable Necker cubes occurred preferentially with experience of previous uninformative, ambiguous cubes rather than when the observer was exposed to informative, disambiguated stimuli. The large difference in the effectiveness of previous experience on long-term perceptual bias is important because it implies that perceptual learning need not be information-driven. Instead, bias in the way that the visual system interprets ambiguous input at onset may better be understood as resulting from previous resolutions under similar circumstances.
A Bayesian framework is often useful to explain perception, and many current studies relate existing priors to environmental statistics (Geisler & Kersten, 2002b; Kersten, O'Toole, Sereno, Knill & Andersen, 1987; Knill & Saunders, 2003; Weiss, Simoncelli & Adelson, 2002b). Hence, it would not be surprising if changes in priors were likewise effected by changes in external statistics. To the contrary, not only did we find that quantitatively equivalent perceptual experience does not cause equivalent changes in a prior; in fact internal resolution of uninformative, ambiguous stimuli established stronger biases than did experience of informative contingencies. This is not to say that the Bayesian framework is incorrect; however, some account is needed as to why more learning occurred when stimuli were ambiguous.
We suggest that individual stimuli provide the evidence as to whether learning is appropriate on their occurrence (with disambiguated stimuli indicating less that learning should occur). Although one could conjecture a learning mechanism that changes less dynamically, such as outlined above, our preferred interpretation is the former; specifically, that the process of resolving ambiguity itself causes long-term learning. A similar explanation was offered by Braun & Pastukhov (2008) in a study of short-term positive and negative priming effects by disambiguated and ambiguous kinetic depth stimuli, and also by Pearson & Clifford (2005) in relation to short-term priming in binocular rivalry.
Yet another explanation could be that learning of biases is specific to the type of stimulus, with only weak generalization, for instance, from disambiguated stimuli to ambiguous stimuli. Disproving this possibility would require measurement of long-term bias for perceptual outcome in stimuli containing signals that are recognized by the visual system as disambiguating. We consider the specificity explanation to be unlikely, as a previous study has demonstrated generalization of short-term priming effects between ambiguous kinetic depth stimuli and disambiguated stimuli (Nawrot & Blake, 1993). Regardless, such an explanation would not discount our main findings: A prior for resolution of ambiguous stimuli is not predominantly learned from environmental statistics, and, the visual system distinguishes between types of perceptual experience when learning a bias.
Why this long-term mechanism should act in the visual system is open to interpretation. The immediate implication is that the process of resolving stimuli that are not easily resolved by known visual cues must be a valid method for establishing useful perceptual biases in the natural world. However, it is known that both continuous viewing of ambiguous, bistable, stimuli (Babich & Standing, 1981; Leopold, Wilke, Maier & Logothetis, 2002; Orbach, Ehrlich & Heath, 1963) and repetition of ambiguous stimuli with shorter inter-stimulus intervals than used here (Brascamp et al., 2008; Klink et al., 2008; Noest, van Ee, Nijs & van Wezel, 2007) in fact result in percept alternation. Likewise, the influence of prior viewing of a disambiguated stimulus on perceptual outcome for a consequent ambiguous stimulus depends on the stimulus timing parameters in a similar qualitative manner (Long & Moran, 2007; Nawrot & Blake, 1991).
A possible framework within which these findings could be reconciled is that percept alternation or repetition reflects the likelihood of the currently-viewed object being a continuation of the previous occurrence or an entirely new occurrence: For instance, when the ISI is very short in comparison to the stimulus viewing time, this may engage inhibitory mechanisms designed to enhance sensitivity to the onset of new stimuli (Barlow, 1990; Webster, Werner & Field, 2005). In the case of a bistable stimulus, this would result in an increased likelihood of a percept reversal. For longer ISIs as in our experiments, a repetition of the previous perceptual outcome may be beneficial to the visual system, as the “new” event is more likely to be another occurrence of the now-understood ambiguous object. Further validation of this proposal could be provided by study of the temporal statistics of object visibility in the real world.
Conclusion
We show for the first time that changes in the prior—observed as a change in bias that resists reverse learning the next day—are affected more by ambiguous stimuli than by disambiguated stimuli. This has implications for Bayesian models of perceptual inference inasmuch as different stimuli that give rise to the same qualia, or perceptual experience, are not treated as equal, and instead are differentiated by the visual system. Additionally, giving greater weight to previous resolutions of uninformative, ambiguous stimuli than to experience of environmental contingencies runs counter to intuition, as it demonstrates that learning of a prior for resolution of ambiguous stimuli is not driven by experience of external information, but instead by experience of stimulus resolutions within the visual system itself.
Acknowledgements
This research was supported by grants NSF BCS-0810944, NIH R01 EY 013988–07 and HFSP RPG 3/2006.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams WJ, Banks MS, van Ee R. Adaptation to three-dimensional distortions in human vision. Nat Neurosci. 2001;4(11):1063–1064. doi: 10.1038/nn729. [DOI] [PubMed] [Google Scholar]
- Babich S, Standing L. Satiation effects with reversible figures. Percept Mot Skills. 1981;52(1):203–210. doi: 10.2466/pms.1981.52.1.203. [DOI] [PubMed] [Google Scholar]
- Backus BT. The Mixture of Bernoulli Experts: a theory to quantify reliance on cues in dichotomous perceptual decisions. Journal of Vision. 2009;9(1):6, 1–19. doi: 10.1167/9.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus BT, Haijiang Q. Competition between newly recruited and pre-existing visual cues during the construction of visual appearance. Vision Research. 2007;47(7):919–924. doi: 10.1016/j.visres.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. A theory about the functional role and synaptic mechanism of visual aftereffects. In: Blakemore C, editor. Vision: Coding and efficiency. Cambridge, UK: Cambridge University Press; 1990. [Google Scholar]
- Brascamp JW, Knapen TH, Kanai R, Noest AJ, van Ee R, van den Berg AV. Multi-timescale perceptual history resolves visual ambiguity. PLoS ONE. 2008;3(1):e1497. doi: 10.1371/journal.pone.0001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J, Pastukhov A. Unambiguous stimuli have no persistence. Journal of Vision. 2008;7(9) [Google Scholar]
- Dosher BA, Sperling G, Wurst SA. Tradeoffs between stereopsis and proximity luminance covariance as determinants of perceived 3D structure. Vision Research. 1986;26(6):973–990. doi: 10.1016/0042-6989(86)90154-9. [DOI] [PubMed] [Google Scholar]
- Ernst MO, Banks MS, Bulthoff HH. Touch can change visual slant perception. Nat Neurosci. 2000;3(1):69–73. doi: 10.1038/71140. [DOI] [PubMed] [Google Scholar]
- Geisler WS, Kersten D. Illusions, perception and Bayes. Nat Neurosci. 2002a;5(6):508–510. doi: 10.1038/nn0602-508. [DOI] [PubMed] [Google Scholar]
- Geisler WS, Kersten D. Illusions, perception and Bayes. Nature Neuroscience. 2002b;5(6):508–510. doi: 10.1038/nn0602-508. [DOI] [PubMed] [Google Scholar]
- Haijiang Q, Saunders JA, Stone RW, Backus BT. Demonstration of cue recruitment: change in visual appearance by means of Pavlovian conditioning. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(2):483–488. doi: 10.1073/pnas.0506728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SJ, Backus BT. Cue recruitment for the construction of perceptual appearance: World location competes with retinal location in an associative learning paradigm. Journal of Vision. 2009;9(8):881, 881a. [Google Scholar]
- Harrison SJ, Backus BT. Disambiguating Necker cube rotation using a location cue: What types of spatial location signal can the visual system learn? Journal of Vision. 2010 doi: 10.1167/10.6.23. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RA, Fine I. Experience-dependent integration of texture and motion cues to depth. Vision Res. 1999;39(24):4062–4075. doi: 10.1016/s0042-6989(99)00120-0. [DOI] [PubMed] [Google Scholar]
- Kersten D, Mamassian P, Yuille A. Object perception as Bayesian inference. Annu Rev Psychol. 2004;55:271–304. doi: 10.1146/annurev.psych.55.090902.142005. [DOI] [PubMed] [Google Scholar]
- Kersten D, O'Toole AJ, Sereno ME, Knill DC, Andersen JA. Associative learning of scene parameters from images. Applied Optics. 1987;26(23):4999–5006. doi: 10.1364/AO.26.004999. [DOI] [PubMed] [Google Scholar]
- Kersten D, O'Toole AJ, Sereno ME, Knill DC, Anderson JA. Associative learning of scene parameters from images. Applied Optics. 1987;26:4999–5006. doi: 10.1364/AO.26.004999. [DOI] [PubMed] [Google Scholar]
- Klink PC, van Ee R, Nijs MM, Brouwer GJ, Noest AJ, van Wezel RJ. Early interactions between neuronal adaptation and voluntary control determine perceptual choices in bistable vision. Journal of Vision. 2008;8(5):16, 11–18. doi: 10.1167/8.5.16. [DOI] [PubMed] [Google Scholar]
- Knapen T, Brascamp J, Adams WJ, Graf EW. The spatial scale of perceptual memory in ambiguous figure perception. Journal of Vision. 2009;9(13):1–12. doi: 10.1167/9.13.16. (16) [DOI] [PubMed] [Google Scholar]
- Knill D, Richards W. Perception as Bayesian Inference. London: Cambridge University Press; 1996. [Google Scholar]
- Knill DC, Saunders JA. Do humans optimally integrate stereo and texture information for judgments of surface slant? Vision Res. 2003;43(24):2539–2558. doi: 10.1016/s0042-6989(03)00458-9. [DOI] [PubMed] [Google Scholar]
- Kohler I. Experiments with goggles. Sci Am. 1962;206:62–72. doi: 10.1038/scientificamerican0562-62. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Wilke M, Maier A, Logothetis NK. Stable perception of visually ambiguous patterns. Nature Neuroscience. 2002;5(6):605–609. doi: 10.1038/nn0602-851. [DOI] [PubMed] [Google Scholar]
- Long GM, Moran CJ. How to keep a reversible figure from reversing: teasing out top-down and bottom-up processes. Perception. 2007;36(3):431–445. doi: 10.1068/p5630. [DOI] [PubMed] [Google Scholar]
- Long GM, Toppino TC, Kostenbauder JF. As the cube turns: evidence for two processes in the perception of a dynamic reversible figure. Percept Psychophys. 1983;34(1):29–38. doi: 10.3758/bf03205893. [DOI] [PubMed] [Google Scholar]
- Nawrot M, Blake R. The interplay between stereopsis and structure from motion. Percept Psychophys. 1991;49(3):230–244. doi: 10.3758/bf03214308. [DOI] [PubMed] [Google Scholar]
- Nawrot M, Blake R. On the perceptual identity of dynamic stereopsis and kinetic depth. Vision Res. 1993;33(11):1561–1571. doi: 10.1016/0042-6989(93)90149-q. [DOI] [PubMed] [Google Scholar]
- Noest AJ, van Ee R, Nijs MM, van Wezel RJ. Percept-choice sequences driven by interrupted ambiguous stimuli: a low-level neural model. Journal of Vision. 2007;7(8):10. doi: 10.1167/7.8.10. [DOI] [PubMed] [Google Scholar]
- Orbach J, Ehrlich D, Heath HA. Reversibility of the Necker Cube. I. An Examination of the Concept of "Satiation of Orientation". Perceptual and Motor Skills. 1963;17:439–458. doi: 10.2466/pms.1963.17.2.439. [DOI] [PubMed] [Google Scholar]
- Pearson J, Clifford CW. Mechanisms selectively engaged in rivalry: normal vision habituates, rivalrous vision primes. Vision Research. 2005;45(6):707–714. doi: 10.1016/j.visres.2004.09.040. [DOI] [PubMed] [Google Scholar]
- Rouse MW, Tittle JS, Braunstein ML. Stereoscopic depth perception by static stereo-deficient observers in dynamic displays with constant and changing disparity. Optometry and Vision Science. 1989;66(6):355–362. doi: 10.1097/00006324-198906000-00004. [DOI] [PubMed] [Google Scholar]
- Sinha P, Poggio T. Role of learning in three-dimensional form perception. Nature. 1996;384(6608):460–463. doi: 10.1038/384460a0. [DOI] [PubMed] [Google Scholar]
- Wallach H, Austin P. Recognition and the localization of visual traces. American Journal of Psychology. 1954;67:338–340. [PubMed] [Google Scholar]
- Webster MA, Werner JS, Field DJ. Adaptation and the phenomenology of perception. In: Clifford CWG, Rhodes G, editors. Fitting the mind to the world: Adaptation and aftereffects in high level vision. Oxford: Oxford University Press; 2005. pp. 241–277. [Google Scholar]
- Weiss Y, Simoncelli EP, Adelson EH. Motion illusions as optimal percepts. Nat Neurosci. 2002;5(6):598–604. doi: 10.1038/nn0602-858. [DOI] [PubMed] [Google Scholar]