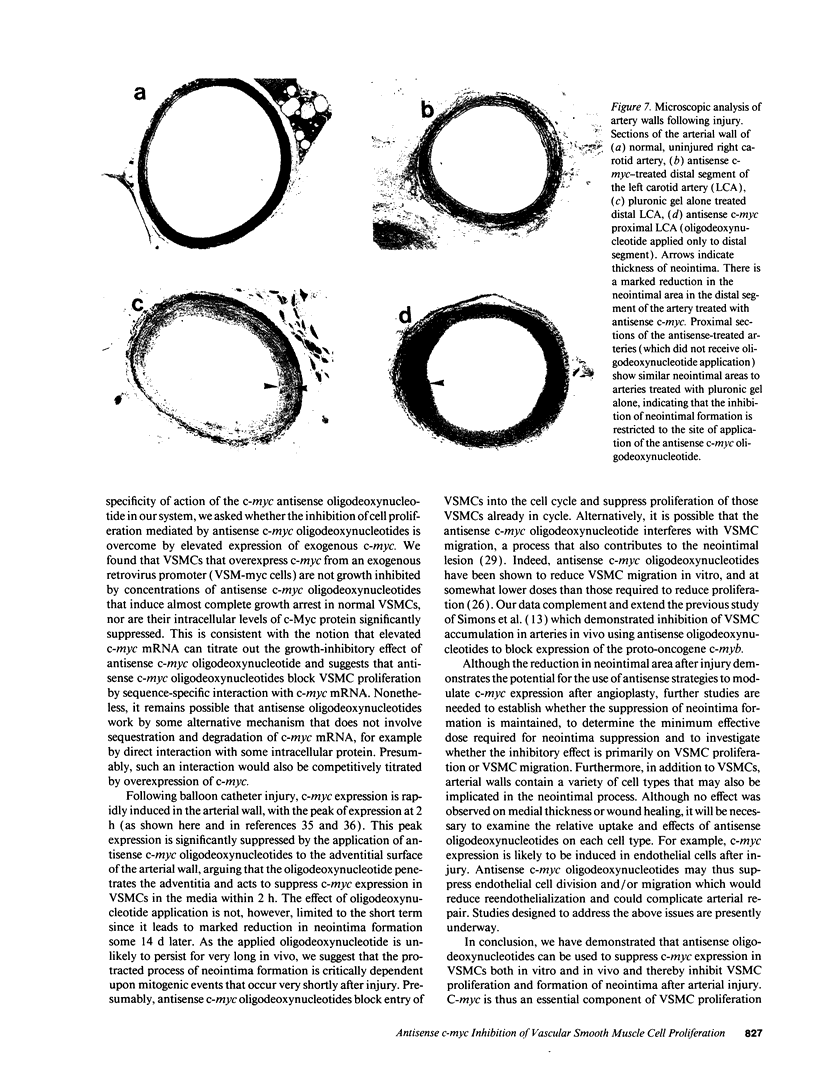
Abstract
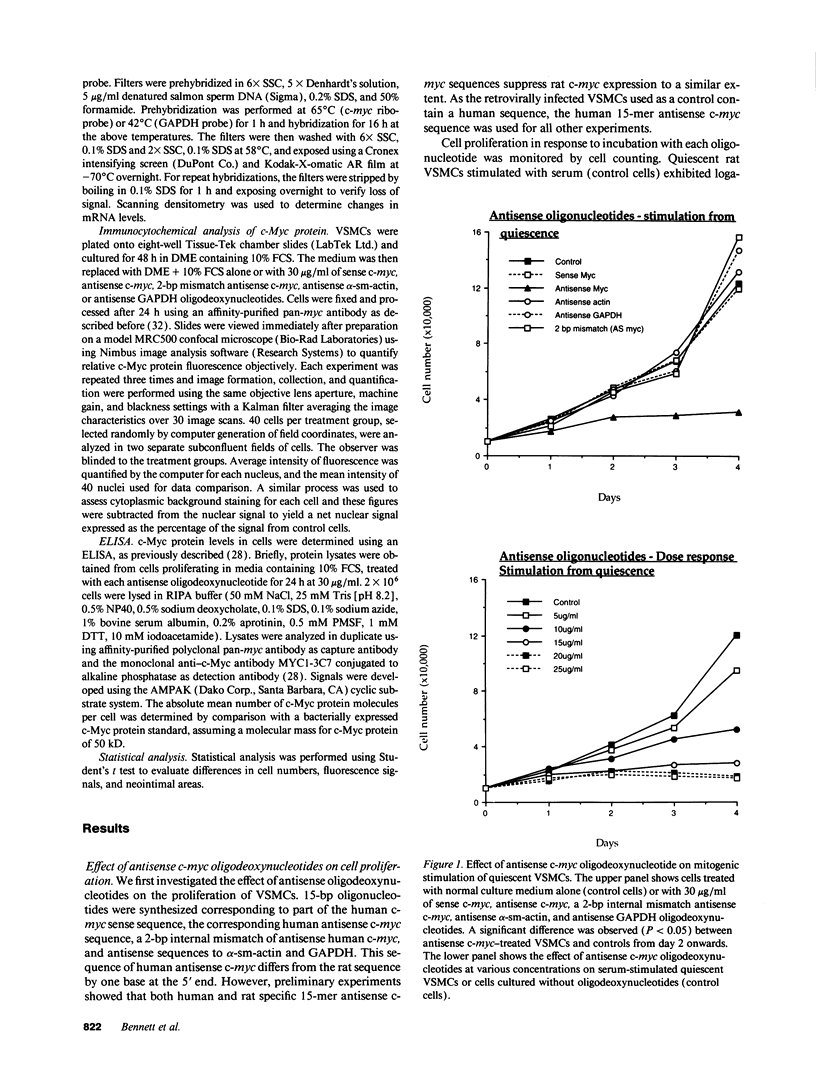
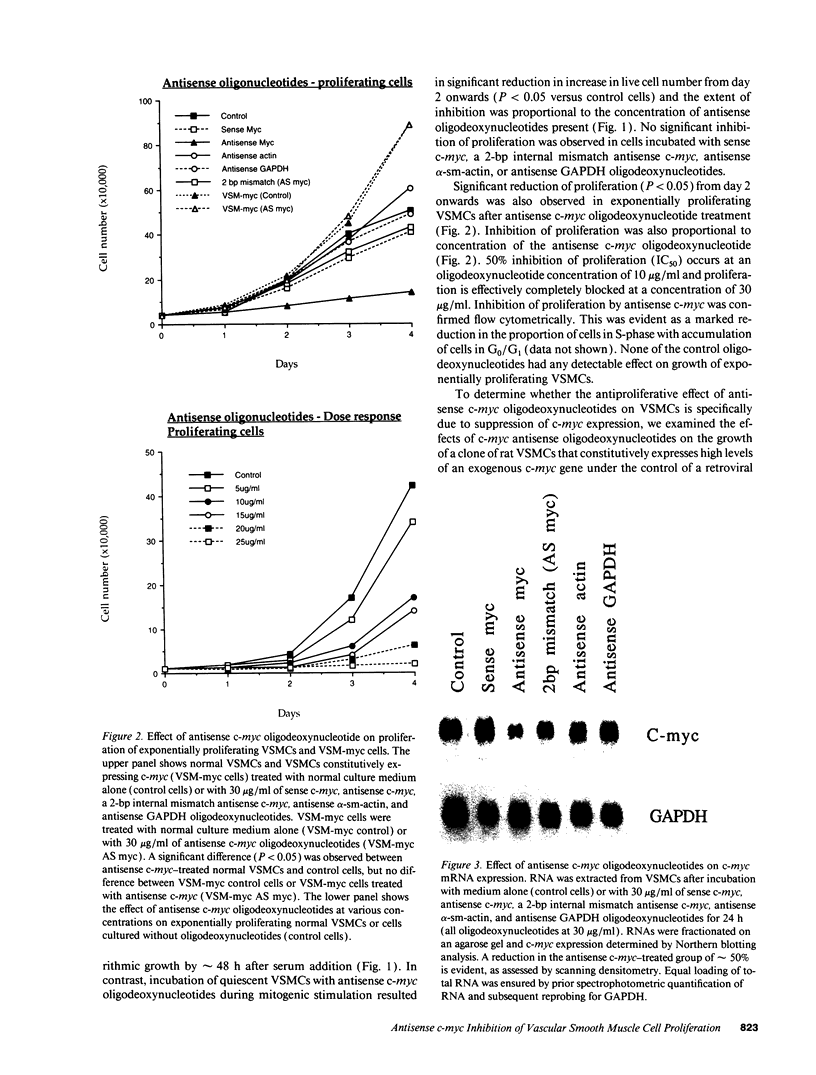
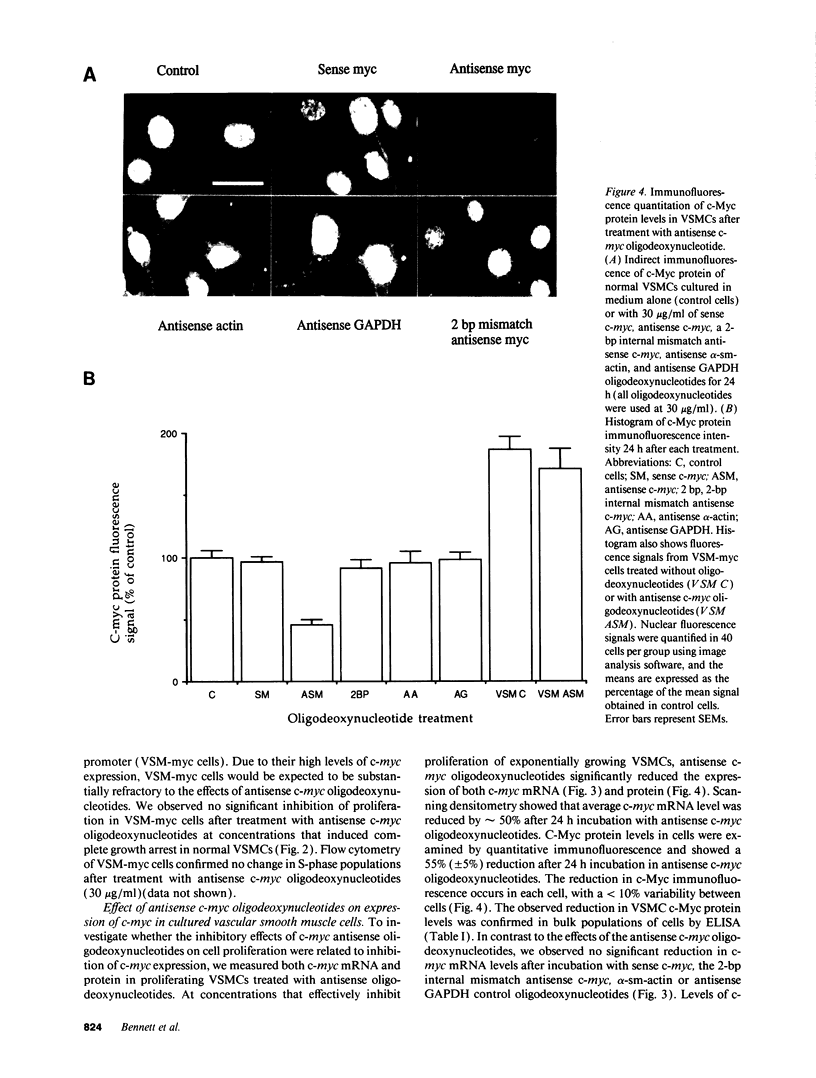
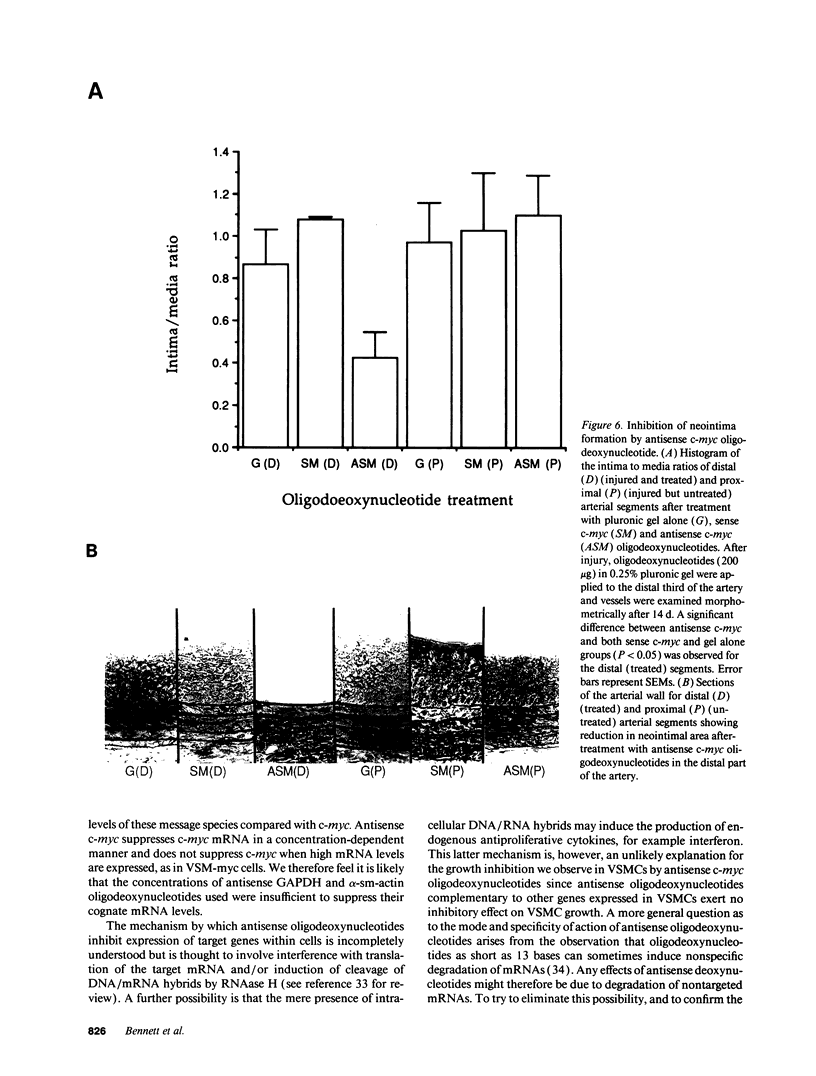
Restenosis after angioplasty is due predominantly to accumulation of vascular smooth muscle cells (VSMCs). The resistance of restenosis to pharmacological treatment has prompted investigation of genes involved in VSMC proliferation. We have examined the effect on VSMC proliferation of blocking expression of the c-myc proto-oncogene with antisense oligodeoxynucleotides, both in vitro and in a rat carotid artery injury model of angioplasty restenosis. Antisense c-myc oligodeoxynucleotides reduced average cell levels of c-myc mRNA and protein by 50-55% and inhibited proliferation of VSMCs when mitogenically stimulated from quiescence or when proliferating logarithmically (IC50 = 10 micrograms/ml). Corresponding sense c-myc, two-base-pair mismatch antisense c-myc, antisense alpha-actin or glyceraldehyde phosphate dehydrogenase oligodeoxynucleotides did not suppress c-myc expression or inhibit VSMC proliferation. Antisense c-myc inhibition was relieved by overexpression of an exogenous c-myc gene. After balloon catheter injury, peak c-myc mRNA expression occurred at 2 h. Antisense c-myc applied in a pluronic gel to the arterial adventitia reduced peak c-myc expression by 75% and significantly reduced neointimal formation at 14 d, compared with sense c-myc and gel application alone. We conclude that c-myc expression is required for VSMC proliferation in vitro and in the vessel wall. C-myc is a therefore a potential target for adjunctive therapy to reduce angioplasty restenosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin G. E., Ratliff N. B., Hollman J., Tabei S., Phillips D. F. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985 Aug;6(2):369–375. doi: 10.1016/s0735-1097(85)80174-1. [DOI] [PubMed] [Google Scholar]
- Bauters C., de Groote P., Adamantidis M., Delcayre C., Hamon M., Lablanche J. M., Bertrand M. E., Dupuis B., Swynghedauw B. Proto-oncogene expression in rabbit aorta after wall injury. First marker of the cellular process leading to restenosis after angioplasty? Eur Heart J. 1992 Apr;13(4):556–559. doi: 10.1093/oxfordjournals.eurheartj.a060213. [DOI] [PubMed] [Google Scholar]
- Biro S., Fu Y. M., Yu Z. X., Epstein S. E. Inhibitory effects of antisense oligodeoxynucleotides targeting c-myc mRNA on smooth muscle cell proliferation and migration. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):654–658. doi: 10.1073/pnas.90.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campan M., Desgranges C., Gadeau A. P., Millet D., Belloc F. Cell cycle dependent gene expression in quiescent stimulated and asynchronously cycling arterial smooth muscle cells in culture. J Cell Physiol. 1992 Mar;150(3):493–500. doi: 10.1002/jcp.1041500309. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M. Kinetics of cellular proliferation after arterial injury. II. Inhibition of smooth muscle growth by heparin. Lab Invest. 1985 Jun;52(6):611–616. [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Colman A. Antisense strategies in cell and developmental biology. J Cell Sci. 1990 Nov;97(Pt 3):399–409. doi: 10.1242/jcs.97.3.399. [DOI] [PubMed] [Google Scholar]
- Ebbecke M., Unterberg C., Buchwald A., Stöhr S., Wiegand V. Antiproliferative effects of a c-myc antisense oligonucleotide on human arterial smooth muscle cells. Basic Res Cardiol. 1992 Nov-Dec;87(6):585–591. doi: 10.1007/BF00788668. [DOI] [PubMed] [Google Scholar]
- Freytag S. O. Enforced expression of the c-myc oncogene inhibits cell differentiation by precluding entry into a distinct predifferentiation state in G0/G1. Mol Cell Biol. 1988 Apr;8(4):1614–1624. doi: 10.1128/mcb.8.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadeau A. P., Campan M., Desgranges C. Induction of cell cycle-dependent genes during cell cycle progression of arterial smooth muscle cells in culture. J Cell Physiol. 1991 Mar;146(3):356–361. doi: 10.1002/jcp.1041460304. [DOI] [PubMed] [Google Scholar]
- Griep A. E., Westphal H. Antisense Myc sequences induce differentiation of F9 cells. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6806–6810. doi: 10.1073/pnas.85.18.6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton J. R., Rosenberg R. D., Clowes A. W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. In vivo studies with anticoagulant and nonanticoagulant heparin. Circ Res. 1980 May;46(5):625–634. doi: 10.1161/01.res.46.5.625. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Holm J. Interferon-gamma inhibits arterial stenosis after injury. Circulation. 1991 Sep;84(3):1266–1272. doi: 10.1161/01.cir.84.3.1266. [DOI] [PubMed] [Google Scholar]
- Heikkila R., Schwab G., Wickstrom E., Loke S. L., Pluznik D. H., Watt R., Neckers L. M. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. 1987 Jul 30-Aug 5Nature. 328(6129):445–449. doi: 10.1038/328445a0. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Redner R. L., Nienhuis A. W. An oligomer complementary to c-myc mRNA inhibits proliferation of HL-60 promyelocytic cells and induces differentiation. Mol Cell Biol. 1988 Feb;8(2):963–973. doi: 10.1128/mcb.8.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindy M. S., Sonenshein G. E. Regulation of oncogene expression in cultured aortic smooth muscle cells. Post-transcriptional control of c-myc mRNA. J Biol Chem. 1986 Sep 25;261(27):12865–12868. [PubMed] [Google Scholar]
- McBride W., Lange R. A., Hillis L. D. Restenosis after successful coronary angioplasty. Pathophysiology and prevention. N Engl J Med. 1988 Jun 30;318(26):1734–1737. doi: 10.1056/NEJM198806303182606. [DOI] [PubMed] [Google Scholar]
- Miano J. M., Tota R. R., Vlasic N., Danishefsky K. J., Stemerman M. B. Early proto-oncogene expression in rat aortic smooth muscle cells following endothelial removal. Am J Pathol. 1990 Oct;137(4):761–765. [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., Hancock D. C., Littlewood T. D., Evan G. I. A sensitive and quantitative enzyme-linked immunosorbence assay for the c-myc and N-myc oncoproteins. Oncogene Res. 1987;2(1):65–80. [PubMed] [Google Scholar]
- Penn L. J., Brooks M. W., Laufer E. M., Littlewood T. D., Morgenstern J. P., Evan G. I., Lee W. M., Land H. Domains of human c-myc protein required for autosuppression and cooperation with ras oncogenes are overlapping. Mol Cell Biol. 1990 Sep;10(9):4961–4966. doi: 10.1128/mcb.10.9.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietenpol J. A., Holt J. T., Stein R. W., Moses H. L. Transforming growth factor beta 1 suppression of c-myc gene transcription: role in inhibition of keratinocyte proliferation. Proc Natl Acad Sci U S A. 1990 May;87(10):3758–3762. doi: 10.1073/pnas.87.10.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. S., Clozel J. P., Müller R. K., Kuhn H., Hefti F., Hosang M., Baumgartner H. R. Inhibitors of angiotensin-converting enzyme prevent myointimal proliferation after vascular injury. Science. 1989 Jul 14;245(4914):186–188. doi: 10.1126/science.2526370. [DOI] [PubMed] [Google Scholar]
- Powell J. S., Müller R. K., Rouge M., Kuhn H., Hefti F., Baumgartner H. R. The proliferative response to vascular injury is suppressed by angiotensin-converting enzyme inhibition. J Cardiovasc Pharmacol. 1990;16 (Suppl 4):S42–S49. doi: 10.1097/00005344-199016004-00010. [DOI] [PubMed] [Google Scholar]
- Prochownik E. V., Kukowska J., Rodgers C. c-myc antisense transcripts accelerate differentiation and inhibit G1 progression in murine erythroleukemia cells. Mol Cell Biol. 1988 Sep;8(9):3683–3695. doi: 10.1128/mcb.8.9.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. S., Holmes D. R., Jr, Topol E. J. The restenosis paradigm revisited: an alternative proposal for cellular mechanisms. J Am Coll Cardiol. 1992 Nov 1;20(5):1284–1293. doi: 10.1016/0735-1097(92)90389-5. [DOI] [PubMed] [Google Scholar]
- Simons M., Edelman E. R., DeKeyser J. L., Langer R., Rosenberg R. D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992 Sep 3;359(6390):67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Simons M., Rosenberg R. D. Antisense nonmuscle myosin heavy chain and c-myb oligonucleotides suppress smooth muscle cell proliferation in vitro. Circ Res. 1992 Apr;70(4):835–843. doi: 10.1161/01.res.70.4.835. [DOI] [PubMed] [Google Scholar]
- Speir E., Epstein S. E. Inhibition of smooth muscle cell proliferation by an antisense oligodeoxynucleotide targeting the messenger RNA encoding proliferating cell nuclear antigen. Circulation. 1992 Aug;86(2):538–547. doi: 10.1161/01.cir.86.2.538. [DOI] [PubMed] [Google Scholar]
- Spencer C. A., Groudine M. Control of c-myc regulation in normal and neoplastic cells. Adv Cancer Res. 1991;56:1–48. doi: 10.1016/s0065-230x(08)60476-5. [DOI] [PubMed] [Google Scholar]
- Stone J., de Lange T., Ramsay G., Jakobovits E., Bishop J. M., Varmus H., Lee W. Definition of regions in human c-myc that are involved in transformation and nuclear localization. Mol Cell Biol. 1987 May;7(5):1697–1709. doi: 10.1128/mcb.7.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters C. M., Littlewood T. D., Hancock D. C., Moore J. P., Evan G. I. c-myc protein expression in untransformed fibroblasts. Oncogene. 1991 May;6(5):797–805. [PubMed] [Google Scholar]
- Woolf T. M., Melton D. A., Jennings C. G. Specificity of antisense oligonucleotides in vivo. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7305–7309. doi: 10.1073/pnas.89.16.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]