Abstract
In this paper, we discuss the interplay between beta-amyloid (Aβ) peptide, Tau fragments, oxidative stress, and mitochondria in the neuronal model of cerebellar granule neurons (CGNs) in which the molecular events reminiscent of AD are activated. The identification of the death route and the cause/effect relationships between the events leading to death could be helpful to manage the progression of apoptosis in neurodegeneration and to define antiapoptotic treatments acting on precocious steps of the death process. Mitochondrial dysfunction is among the earliest events linked to AD and might play a causative role in disease onset and progression. Recent studies on CGNs have shown that adenine nucleotide translocator (ANT) impairment, due to interaction with toxic N-ter Tau fragment, contributes in a significant manner to bioenergetic failure and mitochondrial dysfunction. These findings open a window for new therapeutic strategies aimed at preserving and/or improving mitochondrial function.
1. Introduction
Alzheimer's disease (AD) is a common neurodegenerative disorder characterized by altered processing of specific proteins, formation of neurofibrillary tangles, imbalance of redox homeostasis, and degeneration of synapses and neurons. Although the mechanism of neurodegeneration in AD is not clearly understood, several studies presently indicate that apoptosis might occur and contribute to AD onset and progression [1–5]. Though it remains to be determined whether true apoptosis is a necessary event in neurodegeneration, a growing number of studies support the activation of apoptosis in general, and caspases specifically, as an early event that contributes to neurodegeneration and promote the pathological hallmarks associate with AD [6].
Transgenic animal models have been useful tools to study AD, but currently many of them do not fully replicate the cascade of amyloid deposition, neurofibrillary tangles, and neurodegeneration that characterize the human disease [7]. Thus, as far as the studies about AD are concerned, the lack of an animal model that sufficiently resembles this disease is the reason why research should still proceed along parallel lines: studies carried out in animal models should be integrated and correlated to ad hoc-devised neuronal models in which the identification of single molecular steps is made possible.
Rat cerebellar granule neurons (CGNs) are a neuronal model widely used to study events linking apoptosis and neurodegeneration [8, 9] due to the ease of CGN culture production, their high degree of cellular homogeneity, and the findings revealing that during the onset of apoptosis several molecular events reminiscent of AD are activated [10].
In this paper, the role of key players of the neuronal apoptotic process is discussed with particular attention to the results obtained in CGNs. The production, effect, and interplay of beta-amyloid (Aβ), Tau protein and its fragments are discussed together with the action of these proteins on mitochondria, and this is integrated in the scenario of CGN apoptosis.
2. The Experimental Model of CGNs: A Useful Model to Elucidate Neurodegenerative Mechanism(s)
CGNs survive and differentiate in vitro in the presence of depolarizing concentrations of KCl (25 mM) without additional need for neurotrophic factors [11]. The mechanism of action of KCl is still controversial but, generally, it is believed that the increase in intracellular Ca2+ concentration [12, 13] and the activation of mitogen-activated protein kinase (MAPK) [14] induced by depolarization are involved.
If the serum is removed, and the concentration of KCl is kept below depolarizing levels (K5), the majority of CGNs die by an apoptotic process [12]. Under these conditions, that are equivalent to in vivo deafferentation, neuronal death is initiated and follows a general scheme that has been extensively characterized in recent years (for a review see [15]). The production of reactive oxygen species (ROS) and nitric oxide (NO), the increase in proteasome, antioxidant enzyme, and nitric oxide synthase (NOS) activities, and release of cytochrome c (cyt c) into the cytosol are some of the main events taking place soon after apoptosis induction in CGNs and for which a cause-effect relationship has been defined. In the early phase of apoptosis, ROS, NO, and cGMP production increases as well as the activities of antioxidant enzymes and NOS [16–20], as the cell's attempt to counteract the ongoing oxidative stress [18]. However, due to superoxide production, cyt c is released into the cytosol where it carries out a triple function since it acts (i) as an antioxidant compound and an ROS scavenger, (ii) as a respiratory substrate which can generate the mitochondrial transmembrane potential, and (iii) as the activator of the caspase cascade [21–23]. As a consequence of both NO and superoxide anion production, an increase in the levels of nitrated proteins has been found in the late phase (ranging from 3 to 15 hours after apoptosis induction) [19]. With apoptosis progression, the oxidative damage proceeds, antioxidant enzymes are inactivated by caspases and proteasome [18, 24], and, at the mitochondrial level, the adenine nucleotide translocator (ANT) is progressively impaired thus contributing to the transition pore opening in the late phase of the death process [25, 26].
Furthermore, it has been demonstrated that during the onset of apoptosis of CGNs, several molecular events reminiscent of AD are induced. An amyloidogenic process is activated with an increased production of Aβ which initiates a sort of autocrine toxic loop [27]. Contextually to the increase in Aβ deposition, Tau protein, which is the main constituent of AD neurofibrillary tangles, is cleaved by the concerted action of calpain and caspases with the production of toxic fragments [28, 29]. The mechanism of action of a Tau toxic fragment has been elucidated, and ANT has been identified as the specific mitochondrial target of such fragment [30].
3. Formation of A β and Tau Protein Fragments in AD
One of the central points in the physiopathology of AD is the altered function and/or structure of two “Alzheimer's proteins,” namely the amyloid precursor protein (APP) and Tau.
APP is a membrane glycoprotein, which undergoes complex intracellular trafficking. The biological function of APP is still not fully clear. Roles in cell adhesion, neuronal migration, cell proliferation, neurite outgrowth, axonal transport, neuroprotection, and signal transduction have been proposed [31]. The abnormal cleavage of APP leads to the production of Aβ which is the main component of senile plaques in AD and per se can induce neuronal cell death.
Tau is a neuron-specific microtubule-associated protein and a critical component of the neuronal cytoskeleton which progressively disaggregates during apoptosis. The proper function of Tau depends upon a precise equilibrium between different isoforms and its state of phosphorylation. In AD, as well as in other human dementias, Tau undergoes a series of posttranslational changes including abnormal phosphorylation, glycosylation, glycation, and truncation (see [32]), which may render Tau more prone to form aggregated structures, the neurofibrillary tangles, which constitute a major hallmark of AD. Following such aggregation, the microtubules disintegrate, collapsing the neuron's transport system, with consequent altered communication between neurons, eventually ending in cell death.
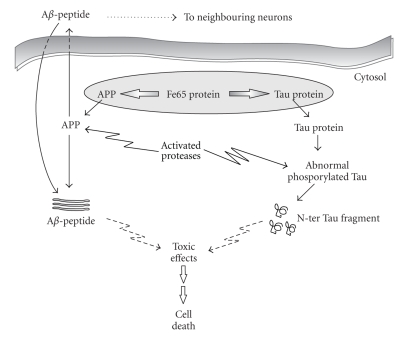
Interestingly, in the experimental model of CGNs, it has been proposed that Tau and APP form a complex in vivo via the adaptor protein Fe65 [33] which is abundantly expressed in the central nervous system of mammals and in particular in the cerebellum and hippocampus [34]. As a consequence, the full-length Tau can play a role in regulating the proper localization of APP and of its partners. During apoptosis, the disruption of the Tau-Fe65 interaction leads to a mislocalization of the APP-Fe65 complex within the cell that in turn could induce a change in the proteolytic fate of both APP and Tau proteins (Figure 1).
Figure 1.
Schematic overview of Aβ peptide and Tau fragments production in CGNs. Aβ is produced intracellularly or taken up from extracellular sources and together with Tau fragments has various pathological effects on cell function.
As far as Aβ production is concerned, it has been reported that in the commitment phase (6 hours) of CGN apoptosis, an amyloidogenic process is activated which rapidly and irreversibly leads to increased production of Aβ [27]. Aβ may be released outside the cell and act as a soluble and diffusible apoptotic death mediator, affecting neighbouring healthy neurons and activating a toxic loop that further accelerates and propagates the process of neurodegeneration. Accordingly, it has been found that coincubation of CGN apoptotic cultures with antibodies directed against Aβ significantly slows down the extension of cell death and quantitatively increases the neuronal survival rate [27]. Studies carried out on CGNs as well as on various cell models indicate that both nonaggregated and, to a greater extent, aggregated Aβ peptides of the short toxic fragment Aβ25–35 can induce apoptosis when externally added to cell cultures [35, 36] and that different Aβ aggregation forms (monomers, protofibrillar intermediate, and mature fibrils) can have diverse effects [37–39].
In the same experimental model (i.e., CGNs), Aβ25–35-induced apoptosis has been found to be associated with the activation of multiple executioner caspases (caspases-2, -3, and -6) [40], and the shorter Aβ fragment (Aβ31–35) is able to induce neurodegeneration with an early increase in bax mRNA level followed by delayed caspase-3 activation [41]. Finally, it has been reported that Aβ may interfere with K+ channel trafficking [42, 43], altering K+ currents and therefore causing an increase in cell death as a result of a decrease in cytoplasmic K+ concentrations. Consistently, the selective upregulation of the expression of two voltage-dependent potassium channel subunits (Kv4.2 and Kv4.3) has been found in CGNs after Aβ25–35 exposure [44].
In CGNs, contextually to the significant increase in amyloidogenic metabolism of APP, Tau also undergoes posttranslational modifications. As soon as 6 hours after apoptosis induction, a change in Tau phosphorylation state occurs in concomitance with caspase and calpain-mediated cleavages (Figure 1). As a consequence, several fragments of Tau protein are produced during apoptosis, the most abundant of which is a 17 kDa residual fragment, probably located at the NH2-terminus of Tau, which is unable to bind to microtubules and is diagnostic for the ongoing apoptotic process [28].
Truncated forms of Tau, besides being produced during apoptosis, can also be effectors of apoptosis by themselves and operate as toxic fragments that further induce cell death so contributing to the progression of neurodegeneration by an “autocatalytic process” [29, 45–47]. Both C-ter and N-ter Tau fragments have been analyzed for their neurotoxicity. While the microtubule-binding capacity of the C-ter fragment is well documented, relatively little is known about the function of the N-terminal domain. Transfection of neuronal cells with C-terminal Tau fragments induces cell death [46, 47] while exogenous overexpression of N-ter Tau fragments in CGNs can be either neuroprotective or neurotoxic depending on its length [29]. The long N-ter Tau fragment (1–230) is antiapoptotic and promotes the prosurvival effect of the AKT pathway. On the other hand, the short N-ter Tau fragment (1–44) exerts a toxic action involving glutamate receptors. Moreover, further analysis performed in the CGN model system further narrowed the extent of the aminoacid stretch which is toxic to the cells, and the N-ter-26–44 Tau fragment was found to be the minimal active moiety which retained a marked neurotoxic effect. On the other hand, the NH2-1–25 Tau fragment was inactive [48].
4. A β and N-ter Tau Fragments Interaction with Mitochondria
Mounting evidence indicates that mitochondrial dysfunction occurs early in AD, worsens with clinical deterioration, and is associated with impairment of energy homeostasis; deficit in the function of complexes of the respiratory chains reduced ATP synthesis as well as altered mitochondrial structure [49–51]. Consistently, a reduced activity of the cytochrome c oxidase (Complex IV of respiratory chain) has been reported in different brain regions [51] as well as in platelets [52] and fibroblasts [53] of AD patients, but the involvement of other mitochondrial oxidative phosphorylation complexes is less documented and more controversial. Cardoso and collaborators [54] found a decreased ATP level in AD cybrids, and other authors report that the activity of Complex IV, but not the activity of F1F0-ATPase (Complex V), decreases in the hippocampus and platelets of AD cases [55, 56]. Because mitochondria are the powerhouse of cells, damage to mitochondria, such as impairment of Complex IV activity, could have functional consequences on energy metabolism [56].
Furthermore, mitochondrial dysfunction has been proposed to be the link between the histopathological hallmarks of AD, caused by Aβ and Tau deposition, and neuronal and synaptic loss [57]. The emerging picture is one in which, at the level of mitochondria, both Alzheimer's proteins exhibit synergistic effects finally leading to the acceleration of neurodegenerative mechanisms (Figure 2).
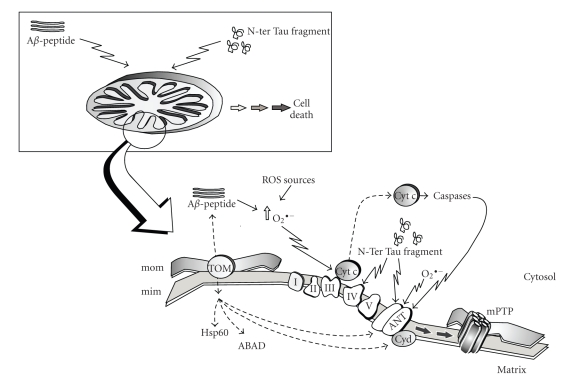
Figure 2.
Proposed mechanism of Aβ peptide and N-ter Tau fragment interaction with mitochondria; for further details see text. mom, mitochondrial outer membrane; mim, mitochondrial inner membrane; TOM, translocase of the outer membrane; I–V, respiratory chain complexes; cyt c, cytochrome c; ANT, adenine nucleotide translocator; CyD, cyclophilin D; mPTP, mitochondrial permeability transition pore.
As far as Aβ is concerned, although the classical view is that Aβ is deposited extracellularly, both cellular and biochemical studies carried out in different models of AD and aging have provided evidence that this peptide can also accumulate inside neurons, target mitochondria, and contribute to disease progression [58–61]. By using in vivo and in vitro approaches, it has been demonstrated that Aβ is transported into rat mitochondria via the translocase of the outer membrane (TOM) [62] and localizes within the mitochondrial cristae. A similar distribution pattern of Aβ in mitochondria has been shown by immunoelectron microscopy in human cortical brain biopsies [62].
Interaction of Aβ with mitochondria could be considered a general route common to different cell types since both in dividing cells (i.e., neuroblastoma cells) and in terminally differentiated neurons (i.e., primary neuronal cultures), either extracellulary applied or secreted A β can be internalized, and it colocalizes with mitochondrial markers [62, 63] (Figure 2). Interaction of Aβ with the matrix protein ABAD (amyloid-binding alcohol dehydrogenase) has been described [64], whereas Caspersen et al. [65] reported that in mouse and human brain samples from AD patients, Aβ colocalizes with the mitochondrial matrix protein Hsp60. Recent biochemical studies imply that the formation of the mitochondrial permeability transition pore (mPTP) is involved in Aβ-mediated mitochondrial dysfunction [66], and by using a computational approach and predictive analysis tools, it has been hypothesized that Aβ can strongly interact in the inner membrane with ANT and Cyclophilin D, two components of the mPTP [67].
A connection between Tau protein and mitochondria has recently been proposed; by overexpressing the N-ter Tau fragment truncated at Asp-421 to mimic caspase cleavage in immortalized neurons, it was possible to induce mitochondrial fragmentation and elevated oxidative stress levels [68].
To the best of our knowledge, the toxicity of N-ter Tau fragments on mitochondria has been deeply investigated only in the CGN model system and has been found to involve a mitochondrial dysfunction with impairment of oxidative phosphorylation [30] (Figure 2). Both Complex IV and ANT proved to be targets of the short NH2-26–44 Tau fragment, but ANT is the only mitochondrial target responsible for the impairment of oxidative phosphorylation. Detailed biochemical studies have revealed that inhibition of ANT is noncompetitive, suggesting that the NH2-26–44 Tau fragment does not interact with the catalytic site but with some other site of the enzyme which could distort the enzyme structure thus also affecting the catalytic binding site.
This finding is consistent with the picture of the apoptotic process in CGN that to date has been built up: in late apoptosis, a noncompetitive-like inhibition of ANT has been found, probably due to caspase activity [26], but it is not dependent on a direct caspase–ANT interaction. However since NH2-26–44 Tau fragment is likely to be generated during apoptosis given that the N-terminal domain of Tau contains consensus sequences suitable for cleavage by caspase(s) [28, 45], which are activated in apoptotic degenerating neurons in AD [69, 70], the possibility exists that caspase(s) gradually inhibit/s ANT as a result of NH2-Tau cleavage and the generation of toxic NH2-26–44 Tau fragment. In this case, NH2-26–44 Tau fragment should directly bind ANT.
5. Nitric Oxide and AD: Interplay between Alzheimer's Proteins, Nitrosative/Oxidative Stress, and Mitochondria
NO produced by NOS, is a molecule endowed with a double role acting as either a prosurvival or a toxic molecule. As a prosurvival molecule, NO plays a role in cell signaling in the nervous system and in synaptic plasticity [71, 72], and it may be involved in diverse biological functions acting through either cGMP-dependent or -independent pathways.
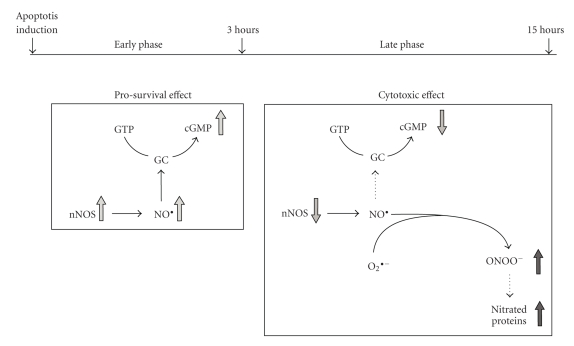
When the role of the NO/NOS system was investigated in CGNs, it was found that NO exerts its dual and opposite effects on the neurodegenerative process, depending on the time after induction of apoptosis (Figure 3). In an early phase, up to 3 h of apoptosis, there is an increase in the expression of the neuronal isoform of NOS (nNOS) as well as in the production of NO, which in turn supports the survival of CGNs through a cGMP-dependent mechanism.
Figure 3.
Schematic representation of the time-dependent, dual role of nitric oxide in CGN apoptosis; see text for details. GC, guanylil cyclase; cGMP, cyclic GMP; nNOS, neuronal nitric oxide synthase; O 2 ∙ −, superoxide anion; O N O O −, peroxynitrite.
Consistently with these results, it has also been reported that: (i) NO may be responsible for neuroprotection during Aβ-induced cell death [73, 74], (ii) low concentration of NO produced by a healthy cerebrovascular endothelium was found to influence the parenchymal brain cells in a protective way [75], and (iii) in cultured human neuroblastoma cells, low concentrations of NO upregulate the expression of alpha-secretase, while downregulating that of beta-secretase, suggesting that, in the relative absence of superoxide, cerebrovascular NO might act to suppress brain production of Aβ [76].
On the other hand, sustained generation of NO has been implicated in the cellular death occurring in different neurodegenerative diseases as well as in AD [77]. As far as the experimental system of CGNs is concerned (Figure 3), it was found that, in the late phase of the apoptotic program, after 3 h, nNOS expression and activity decreased, resulting in the shut down of NO and cGMP production, and the toxic role of nitric oxide prevailed due to the reaction with superoxide anions to produce peroxynitrite (ONOO−) which in turn is able to induce neuronal injury mainly through nitration of tyrosine residues in cellular proteins, whose level increases. These events together with other apoptotic events already described in this cell model [15, 23, 25, 26] would commit these cells irreversibly to death.
Thus, it can be assumed that once accumulated inside the cell, NO can play different roles, depending on its level, cell context, and amount of superoxide anion. In Figure 4, a general picture is shown which takes into account the main findings on the involvement of nitrosative stress in the neurodegenerative process. In brains from AD patients, an early and striking upregulation of all three isoforms of NOS has been reported [78, 79]. This finding is further supported by experimental data obtained in different systems, ranging from in vivo animals to cell lines, which indicates that NO is responsible for Aβ toxicity and highlights a link between NO/NOS level and Aβ-induced brain dysfunction [80, 81]. Activation of the neuronal isoform of NOS (nNOS) [82] and an increased production of NO [83] were also found in rat cerebral cortex and hippocampus after intracerebroventricular administration of Aβ25–30 and in APP-transfected cells, respectively.
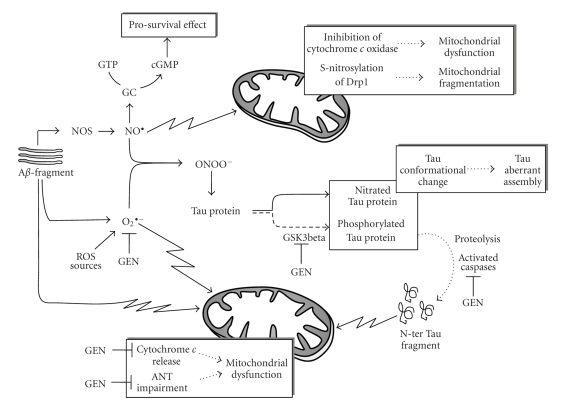
Figure 4.
Schematic overview of the interplay between Aβ, Tau, oxidative/nitrosative stress, and mitochondria; see text for details. GC, guanylil cyclase; cGMP, cyclic GMP; NOS, nitric oxide synthase; O 2 ∙ −, superoxide anion; O N O O −, peroxynitrite; ANT, adenine nucleotide translocator; GSK3beta, glycogen synthase kinase-3beta; GEN, Genistein.
In an early phase, NO could induce a cGMP-mediated prosurvival signaling pathway in an attempt to counteract the ongoing neurodegenerative process [19, 84]. However, NO can also directly trigger mitochondrial dysfunction, a process which is believed to play a causative role in AD onset and progression. Indeed it has been reported that NO both induces a bioenergetic failure, with impairment in the function of Complex IV [85], and triggers mitochondrial fission/fragmentation thus causing cell death in primary culture of cortical neurons [86, 87]. S-nitrosylation, a covalent redox reaction of NO with specific protein thiol groups, could be one mechanism contributing to the NO-induced mitochondrial fragmentation. Accordingly, it has been reported that in AD patients and animal model, NO induces S-nitrosylation of dynamin-related protein 1 (Drp1), a protein specifically involved in mitochondrial fission [88, 89]. On the other hand, Bossy et al. [90] found that NO can also induce Drp1 inactivation by increasing its phosphorylation. Although there are no data on the involvement of Drp1 in the CGN model, it has been recently reported that mitochondrial fragmentation occurs as an early event in response to injury in CGNs, and increased activation of mitofusin 2 (Mfn2), a protein involved in mitochondrial fusion, blocks mitochondrial fragmentation and protects neurons against cell death [91, 92].
In addition to NO, oxidative damage has been reported in aging and age-related neurodegenerative diseases, including AD [93, 94], and superoxide anion production has been induced by Aβ-treatment in neurons [95, 96]. It is known that in the course of neurodegeneration, the superoxide anion can act directly on mitochondria thus inducing cyt c release and precocious impairment of ANT (see [18] and references therein).
On the other hand, NO readily reacts with superoxide anions to form the strong oxidant ONOO− which in turn induces protein nitration. Consistently, an increase in protein nitration has been found in brain tissue from cases of AD which correlates with neurodegeneration [97]. Tau protein can also undergo a ONOO−-mediated process, and nitration of the Tyr29 residue has been proposed as a specific disease-related event [98]. Furthermore, peroxynitrite can also induce AD-like Tau hyperphosphorylation via activation of both glycogen synthase kinase-3beta (GSK3beta) and p38 MAPKs [99].
Nitration, as well as phosphorylation, of Tau protein induces conformational changes that facilitate aberrant Tau assembly. Consistently, it has been reported that nitrated Tau is colocalized with neurofibrillary tangle in AD brain, shows a significantly decreased binding activity to microtubules, and is involved in the formation of filamentous Tau inclusions [100]. In these conditions, Tau fragmentation might occur, and N-ter Tau fragments, together with Aβ and superoxide, can further decrease mitochondrial efficiency thus contributing to mitochondrial dysfunction.
6. Implication of Genistein on Preventing A β and Tau Toxicity
The main goal in AD treatment is focused on a preventive approach. Treatment of declared AD with any compounds may have either a poor effect due to the severe neuronal death occurring in AD or a questionable risk/benefit ratio such as in the case of estrogen. In this regard, estrogen has been shown to block Aβ-induced neuronal cell death in several studies thus suggesting that estradiol replacement therapy should show improvement in patients with AD [101]. However, the efficiency of estradiol in the treatment of AD has been seriously questioned due to its fourth unwanted side effect, that is, proliferative and oncogenic effects on non-neuronal cells [102].
A clear point emerging from the bulk of studies dealing with AD etiopathology is that all factors involved in AD are associated with oxidative stress [103]. In the light of this, natural oxidants have recently received much attention as promising agents for reducing the risk of oxidative stress-related diseases. Among them genistein received a lot of attention.
Genistein (4′.5.7-trihydroxyisoflavone) is the most active compound of soy isoflavones, the one which reaches the highest concentration in human blood [104], possesses an antioxidant activity, shows an affinity to estrogen receptors, thus acting as an estrogen-like compound but without the negative effects of estrogens, and is able to cross the blood-brain barrier (see [105]).
There is considerable literature about the effect of genistein on the progression of neurodegeneration. It has been reported that in the nervous system, isoflavones attenuate primary neuronal apoptosis by activating estrogen receptors [106] and genistein is able both to suppress Aβ25–35-induced ROS overproduction in isolated rat brain synaptosomes [107] and to increase cell viability in cooperation with other trophic factor such as folic acid in cortical neurons [108]. Consistently, Zeng et al. [105] describe the protective effect of genistein on cultured hippocampal neurons against Aβ-induced apoptosis and have demonstrated that genistein inhibits the elevation of intracellular free Ca2+, the production of oxidant free radicals caused by Aβ25–35, the DNA fragmentation, and the activation of caspase-3, thus suggesting that genistein acts upstream of caspase-3 to block apoptosis (Figure 4).
Genistein may also decrease the hyperphosphorylation of Tau protein by inactivating GSK3beta, the kinase involved in Tau phosphorylation in homocysteine-mediated neurodegeneration in SH-SY5Y human neuroblastoma cells [109].
Recently, in CGNs undergoing apoptosis, the effect of genistein was studied at subcellular level and for the first time at mitochondrial level [110]. Genistein and to a lesser extent its analogue daidzein, both used at dietary concentrations, can prevent low potassium-dependent apoptosis in CGNs by reducing the impairment of both aerobic glucose metabolism and mitochondrial uncoupling, two processes occurring in CGN apoptosis [16]. Furthermore, genistein is also able to prevent cyt c release, ANT alteration, and mPTP opening; that is, some steps of the mitochondrial pathway to apoptosis that are somehow related to the ROS production which takes place during apoptosis.
Thus, since both genistein and daidzein have been proved to decrease ROS levels, it has been suggested that the prevention of apoptosis is essentially due to the antioxidant properties of these flavonoids [110]. Nonetheless, the effect of genistein proved to be rather specific since other flavonoids such as catechin and epicatechin failed to prevent CGN death in spite of their shared antioxidant capability.
Consistently, genistein also abolishes neuronal ROS production induced by A β administration to primary culture of cortical neurons [111] and enhances the activities of other antioxidant molecules and enzymes (superoxide dismutase, glutathione peroxidase and reductase) both in vitro and in vivo [112, 113].
7. Conclusions
The etiology of Alzheimer's disease is complex and not fully elucidated. On the other hand, it is important to develop a better understanding of the different biochemical pathways, their role, and their link with the amyloid hypothesis in AD, since it may lead to the development of more effective treatment strategies for this disease. It seems clear then that promising developments as for the prevention and/or delay of the onset of AD can be derived from definition of antiapoptotic treatments acting on the precocious steps of the death process, such as blockade of generation of reactive oxygen species and implementation of the NO prosurvival signaling pathway that, although not able to fully prevent the disease, can at least delay onset or reduce the severity of neurodegeneration. In this regard, genistein and its analogue daidzein may perhaps be of use in neuroprotection. Furthermore, the knowledge emerging from studies conducted on CGNs, that ANT impairment contributes in a significant manner to bioenergetic failure and mitochondrial dysfunction in the course of neurodegeneration, may open a window for new therapeutic strategies aimed at preserving and/or improving mitochondrial function, representing an exciting challenge for biochemists. More studies are required to determine whether phytoestrogens, protease inhibitors and mitochondrial-targeted compounds could fulfill these expectations.
Acknowledgment
The authors thank Mr. Richard Lusardi for linguistic consultation.
References
- 1.Mattson MP. Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxidants & Redox Signaling. 2006;8(11-12):1997–2006. doi: 10.1089/ars.2006.8.1997. [DOI] [PubMed] [Google Scholar]
- 2.Culmsee C, Landshamer S. Molecular insights into mechanisms of the cell death programml: role in the progression of neurodegenerative disorders. Current Alzheimer Research. 2006;3(4):269–283. doi: 10.2174/156720506778249461. [DOI] [PubMed] [Google Scholar]
- 3.Nunomura A, Moreira PI, Lee HG, et al. Neuronal death and survival under oxidative stress in Alzheimer and Parkinson diseases. CNS & Neurological Disorders—Drug Targets. 2007;6(6):411–423. doi: 10.2174/187152707783399201. [DOI] [PubMed] [Google Scholar]
- 4.Honig LS, Rosenberg RN. Apoptosis and neurologic disease. The American Journal of Medicine. 2000;108(4):317–330. doi: 10.1016/s0002-9343(00)00291-6. [DOI] [PubMed] [Google Scholar]
- 5.Eckert A, Marques CA, Keil U, Schüssel K, Müller WE. Increased apoptotic cell death in sporadic and genetic Alzheimer’s Disease. Annals of the New York Academy of Sciences. 2003;1010:604–609. doi: 10.1196/annals.1299.113. [DOI] [PubMed] [Google Scholar]
- 6.Rohn TT, Head E. Caspase activation in Alzheimer’s disease: early to rise and late to bed. Reviews in the Neurosciences. 2008;19(6):383–393. doi: 10.1515/revneuro.2008.19.6.383. [DOI] [PubMed] [Google Scholar]
- 7.Schwab C, Hosokawa M, McGeer PL. Transgenic mice overexpressing amyloid beta protein are an incomplete model of Alzheimer disease. Experimental Neurology. 2004;188(1):52–64. doi: 10.1016/j.expneurol.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Canu N, Calissano P. In vitro cultured neurons for molecular studies correlating apoptosis with events related to Alzheimer disease. Cerebellum. 2003;2(4):270–278. doi: 10.1080/14734220310004289. [DOI] [PubMed] [Google Scholar]
- 9.Calissano P, Matrone C, Amadoro G. Apoptosis and in vitro Alzheimer disease neuronal models. Communitative & Integrative Biology. 2009;2(2):163–169. doi: 10.4161/cib.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cicconi S, Gentile A, Ciotti MT, Parasassi T, Serafino A, Calissano P. Apoptotic death induces Aβ production and fibril formation to a much larger extent than necrotic-like death in CGNs. Journal of Alzheimer’s Disease. 2007;12(3):211–220. doi: 10.3233/jad-2007-12301. [DOI] [PubMed] [Google Scholar]
- 11.Gallo V, Ciotti MT, Aloisi F, Levi G. Developmental features of rat cerebellar neural cells cultured in a chemically defined medium. Journal of Neuroscience Research. 1986;15(3):289–301. doi: 10.1002/jnr.490150302. [DOI] [PubMed] [Google Scholar]
- 12.D’Mello SR, Galli C, Ciotti T, Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassiumml: inhibition of death by insulin-like growth factor I and cAMP. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(23):10989–10993. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galli C, Meucci O, Scorziello A, Werge TM, Calissano P, Schettini G. Apoptosis in cerebellar granule cells is blocked by high KCl, forskolin, and IGF-1 through distinct mechanisms of action: the involvement of intracellular calcium and RNA synthesis. Journal of Neuroscience. 1995;15(2):1172–1179. doi: 10.1523/JNEUROSCI.15-02-01172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen LB, Ginty DD, Weber MJ, Greenberg ME. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron. 1994;12(6):1207–1221. doi: 10.1016/0896-6273(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 15.Bobba A, Atlante A, de Bari L, Passarella S, Marra E. Apoptosis and cytochrome c release in cerebellar granule cells. In Vivo. 2004;18(3):335–344. [PubMed] [Google Scholar]
- 16.Atlante A, Gagliardi S, Marra E, Calissano P. Neuronal apoptosis in rats is accompanied by rapid impairment of cellular respiration and is prevented by scavengers of reactive oxygen species. Neuroscience Letters. 1998;245(3):127–130. doi: 10.1016/s0304-3940(98)00195-5. [DOI] [PubMed] [Google Scholar]
- 17.Atlante A, Passarella S. Detection of reactive oxygen species in primary cultures of cerebellar granule cells. Brain Research Protocols. 1999;4(3):266–270. doi: 10.1016/s1385-299x(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 18.Atlante A, Bobba A, Calissano P, Passarella S, Marra E. The apoptosis/necrosis transition in cerebellar granule cells depends on the mutual relationship of the antioxidant and the proteolytic systems which regulate ROS production and cytochrome c release en route to death. Journal of Neurochemistry. 2003;84(5):960–971. doi: 10.1046/j.1471-4159.2003.01613.x. [DOI] [PubMed] [Google Scholar]
- 19.Bobba A, Atlante A, Moro L, Calissano P, Marra E. Nitric oxide has dual opposite roles during early and late phases of apoptosis in cerebellar granule neurons. Apoptosis. 2007;12(9):1597–1610. doi: 10.1007/s10495-007-0086-4. [DOI] [PubMed] [Google Scholar]
- 20.Bobba A, Atlante A, Petragallo VA, Marra E. Different sources of reactive oxygen species contribute to low potassium-induced apoptosis in cerebellar granule cells. International Journal of Molecular Medicine. 2008;21(6):737–745. [PubMed] [Google Scholar]
- 21.Atlante A, Calissano P, Bobba A, Azzariti A, Marra E, Passarella S. Cytochrome c is released from mitochondria in a reactive oxygen species (ROS)-dependent fashion and can operate as a ROS scavenger and as a respiratory substrate in cerebellar neurons undergoing excitotoxic death. Journal of Biological Chemistry. 2000;275(47):37159–37166. doi: 10.1074/jbc.M002361200. [DOI] [PubMed] [Google Scholar]
- 22.Atlante A, De Bari L, Bobba A, Marra E, Calissano P, Passarella S. Cytochrome c, released from cerebellar granule cells undergoing apoptosis or excytotoxic death, can generate protonmotive force and drive ATP synthesis in isolated mitochondria. Journal of Neurochemistry. 2003;86(3):591–604. doi: 10.1046/j.1471-4159.2003.01863.x. [DOI] [PubMed] [Google Scholar]
- 23.Bobba A, Atlante A, Giannattasio S, Sgaramella G, Calissano P, Marra E. Early release and subsequent caspase-mediated degradation of cytochrome c in apoptotic cerebellar granule cells. FEBS Letters. 1999;457(1):126–130. doi: 10.1016/s0014-5793(99)01018-2. [DOI] [PubMed] [Google Scholar]
- 24.Bobba A, Canu N, Atlante A, Petragallo V, Calissano P, Marra E. Proteasome inhibitors prevent cytochrome c release during apoptosis but not in excitotoxic death of cerebellar granule neurons. FEBS Letters. 2002;515(1–3):8–12. doi: 10.1016/s0014-5793(02)02231-7. [DOI] [PubMed] [Google Scholar]
- 25.Atlante A, Giannattasio S, Bobba A, et al. An increase in the ATP levels occurs in cerebellar granule cells en route to apoptosis in which ATP derives from both oxidative phosphorylation and anaerobic glycolysis. Biochimica et Biophysica Acta. 2005;1708(1):50–62. doi: 10.1016/j.bbabio.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Atlante A, Bobba A, de Bari L, et al. Caspase-dependent alteration of the ADP/ATP translocator triggers the mitochondrial permeability transition which is not required for the low-potassium-dependent apoptosis of cerebellar granule cells. Journal of Neurochemistry. 2006;97(4):1166–1181. doi: 10.1111/j.1471-4159.2006.03820.x. [DOI] [PubMed] [Google Scholar]
- 27.Galli C, Piccini A, Ciotti MT, et al. Increased amyloidogenic secretion in cerebellar granule cells undergoing apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(3):1247–1252. doi: 10.1073/pnas.95.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canu N, Dus L, Barbato C, et al. Tau cleavage and dephosphorylation in cerebellar granule neurons undergoing apoptosis. Journal of Neuroscience. 1998;18(18):7061–7074. doi: 10.1523/JNEUROSCI.18-18-07061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amadoro G, Serafino AL, Barbato C, et al. Role of N-terminal tau domain integrity on the survival of cerebellar granule neurons. Cell Death & Differentiation. 2004;11(2):217–230. doi: 10.1038/sj.cdd.4401314. [DOI] [PubMed] [Google Scholar]
- 30.Atlante A, Amadoro G, Bobba A, et al. A peptide containing residues 26-44 of tau protein impairs mitochondrial oxidative phosphorylation acting at the level of the adenine nucleotide translocator. Biochimica et Biophysica Acta. 2008;1777(10):1289–1300. doi: 10.1016/j.bbabio.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Koo EH. The β-amyloid precursor protein (APP) and alzheimer’s disease: does the tail wag the dog? Traffic. 2002;3(11):763–770. doi: 10.1034/j.1600-0854.2002.31101.x. [DOI] [PubMed] [Google Scholar]
- 32.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 33.Barbato C, Canu N, Zambrano N, et al. Interaction of Tau with Fe65 links tau to APP. Neurobiology of Disease. 2005;18(2):399–408. doi: 10.1016/j.nbd.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Kesavapany S, Banner SJ, Lau K-F, et al. Expression of the Fe65 adapter protein in adult and developing mouse brain. Neuroscience. 2002;115(3):951–960. doi: 10.1016/s0306-4522(02)00422-0. [DOI] [PubMed] [Google Scholar]
- 35.Scorziello A, Meucci O, Florio T, et al. β25-35 alters calcium homeostasis and induces neurotoxicity in cerebellar granule cells. Journal of Neurochemistry. 1996;66(5):1995–2003. doi: 10.1046/j.1471-4159.1996.66051995.x. [DOI] [PubMed] [Google Scholar]
- 36.Yankner BA, Dawes LR, Fisher S, Villa-Komaroff L, Oster-Granite ML, Neve RL. Neurotixicity of a fragment of the amyloid precursor associated with Alzheimer’s disease. Science. 1989;245(4916):417–420. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]
- 37.Hartley DM, Walsh DM, Ye CP, et al. Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. Journal of Neuroscience. 1999;19(20):8876–8884. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44(1):181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. Journal of Neuroscience. 2007;27(11):2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen JW, Eldadah BA, Huang X, et al. Multiple caspases are involved in beta-amyloid-induced neuronal apoptosis. Journal of Neuroscience Research. 2001;65(1):45–53. doi: 10.1002/jnr.1126. [DOI] [PubMed] [Google Scholar]
- 41.Misiti F, Clementi ME, Tringali G, et al. Fragment 31-35 of β-amyloid peptide induces neurodegeneration in rat cerebellar granule cells via bax gene expression and caspase-3 activation. A crucial role for the redox state of methionine-35 residue. Neurochemistry International. 2006;49(5):525–532. doi: 10.1016/j.neuint.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Pannaccione A, Boscia F, Scorziello A, et al. Up-regulation and increased activity of KV3.4 channels and their accessory subunit MinK-related peptide 2 induced by amyloid peptide are involved in apoptotic neuronal death. Molecular Pharmacology. 2007;72(3):665–673. doi: 10.1124/mol.107.034868. [DOI] [PubMed] [Google Scholar]
- 43.Plant LD, Webster NJ, Boyle JP, et al. Amyloid β peptide as a physiological modulator of neuronal ’A’-type K+ current. Neurobiology of Aging. 2006;27(11):1673–1683. doi: 10.1016/j.neurobiolaging.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 44.Pieri M, Amadoro G, Carunchio I, et al. SP protects cerebellar granule cells against β-amyloid-induced apoptosis by down-regulation and reduced activity of Kv4 potassium channels. Neuropharmacology. 2010;58(1):268–276. doi: 10.1016/j.neuropharm.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 45.Rohn TT, Rissman RA, Davis MC, Kim YE, Cotman CW, Head E. Caspase-9 activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiology of Disease. 2002;11(2):341–354. doi: 10.1006/nbdi.2002.0549. [DOI] [PubMed] [Google Scholar]
- 46.Fasulo L, Ugolini G, Visintin M, et al. The neuronal microtubule-associated protein tau is a substrate for caspase-3 and an effector of apoptosis. Journal of Neurochemistry. 2000;75(2):624–633. doi: 10.1046/j.1471-4159.2000.0750624.x. [DOI] [PubMed] [Google Scholar]
- 47.Chung C-W, Song Y-H, Kim I-K, et al. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiology of Disease. 2001;8(1):162–172. doi: 10.1006/nbdi.2000.0335. [DOI] [PubMed] [Google Scholar]
- 48.Amadoro G, Ciotti MT, Costanzi M, Cestari V, Calissano P, Canu N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(8):2892–2897. doi: 10.1073/pnas.0511065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leuner K, Hauptmann S, Abdel-Kader R, et al. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer’s disease? Antioxidants and Redox Signaling. 2007;9(10):1659–1675. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- 50.Rhein V, Baysang G, Rao S, et al. Amyloid-beta leads to impaired cellular respiration, energy production and mitochondrial electron chain complex activities in human neuroblastoma cells. Cellular and Molecular Neurobiology. 2009;29(6-7):1063–1071. doi: 10.1007/s10571-009-9398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mancuso M, Coppedè F, Murri L, Siciliano G. Mitochondrial cascade hypothesis of Alzheimer’s disease: myth or reality? Antioxidants & Redox Signaling. 2007;9(10):1631–1646. doi: 10.1089/ars.2007.1761. [DOI] [PubMed] [Google Scholar]
- 52.Parker WD, Jr., Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 1990;40(8):1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- 53.Tieu K, Perier C, Vila M, et al. L-3-hydroxyacyl-CoA dehydrogenase II protects in a model of Parkinson’s disease. Annals of Neurology. 2004;56(1):51–60. doi: 10.1002/ana.20133. [DOI] [PubMed] [Google Scholar]
- 54.Cardoso SM, Santana I, Swerdlow RH, Oliveira CR. Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Aβ toxicity. Journal of Neurochemistry. 2004;89(6):1417–1426. doi: 10.1111/j.1471-4159.2004.02438.x. [DOI] [PubMed] [Google Scholar]
- 55.Bosetti F, Brizzi F, Barogi S, et al. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer’s disease. Neurobiology of Aging. 2002;23(3):371–376. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 56.Mancuso M, Filosto M, Bosetti F, et al. Decreased platelet cytochrome c oxidase activity is accompanied by increased blood lactate concentration during exercise in patients with Alzheimer disease. Experimental Neurology. 2003;182(2):421–426. doi: 10.1016/s0014-4886(03)00092-x. [DOI] [PubMed] [Google Scholar]
- 57.Eckert A, Schulz KL, Rhein V, et al. Convergence of amyloid-beta and tau pathologies on mitochondria in vivo. Molecular Neurobiology. 2010;41(2-3):107–114. doi: 10.1007/s12035-010-8109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reddy PH. Mitochondrial dysfunction in aging and Alzheimer’s disease: strategies to protect neurons. Antioxidants & Redox Signaling. 2007;9(10):1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- 59.Reddy PH. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Experimental Neurology. 2009;218(2):286–292. doi: 10.1016/j.expneurol.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen X, Yan SD. Mitochondrial Aβ: a potential cause of metabolic dysfunction in Alzheimer’s disease. IUBMB Life. 2006;58(12):686–694. doi: 10.1080/15216540601047767. [DOI] [PubMed] [Google Scholar]
- 61.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-β in Alzheimer’s disease. Nature Reviews Neuroscience. 2007;8(7):499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 62.Hansson Petersen CA, Alikhani N, Behbahani H, et al. The amyloid β-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(35):13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saavedra L, Mohamed A, Ma V, Kar S, De Chaves EP. Internalization of β-amyloid peptide by primary neurons in the absence of apolipoprotein E. Journal of Biological Chemistry. 2007;282(49):35722–35732. doi: 10.1074/jbc.M701823200. [DOI] [PubMed] [Google Scholar]
- 64.Muirhead KE, Borger E, Aitken L, Conway SJ, Gunn-Moore FJ. The consequences of mitochondrial amyloid beta-peptide in Alzheimer's disease. Biochemical Journal. 2010;426(3):255–270. doi: 10.1042/BJ20091941. [DOI] [PubMed] [Google Scholar]
- 65.Caspersen C, Wang N, Yao J, et al. Mitochondrial Aβ: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. The FASEB Journal. 2005;19(14):2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 66.Du H, Yan SS. Mitochondrial permeability transition pore in Alzheimer’s disease: cyclophilin D and amyloid beta. Biochimica et Biophysica Acta. 2010;1802(1):198–204. doi: 10.1016/j.bbadis.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh P, Suman S, Chandna S, et al. Possible role of amyloid-beta, adenine nucleotide translocase and cyclophilin-D interaction in mitochondrial dysfunction of Alzheimer's disease. Bioinformation. 2009;3(10):440–445. doi: 10.6026/97320630003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quintanilla RA, Matthews-Roberson TA, Dolan PJ, Johnsion GVW. Caspase-cleaved tau expression induces mitochondrial dysfunction in immortalized cortical neurons: implications for the pathogenesis of alzheimer disease. Journal of Biological Chemistry. 2009;284(28):18754–18766. doi: 10.1074/jbc.M808908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rohn TT, Head E, Su JH, et al. Correlation between caspase activation and neurofibrillary tangle formation in Alzheimer’s disease. American Journal of Pathology. 2001;158(1):189–198. doi: 10.1016/S0002-9440(10)63957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gastard MC, Troncoso JC, Koliatsos VE. Caspase activation in the limbic cortex of subjects with early Alzheimer’s disease. Annals of Neurology. 2003;54(3):393–398. doi: 10.1002/ana.10680. [DOI] [PubMed] [Google Scholar]
- 71.Dinerman JL, Dawson TM, Schell MJ, Snowman A, Snyder SH. Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: implications for synaptic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(10):4214–4218. doi: 10.1073/pnas.91.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhuo M, Kandel ER, Hawkins RD. Nitric oxide and cGMP can produce either synaptic depression or potentiation depending on the frequency of presynaptic stimulation in the hippocampus. Neuroreport. 1994;5(9):1033–1036. doi: 10.1097/00001756-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 73.Thatcher GRJ, Bennett BM, Reynolds JN. NO chimeras as therapeutic agents in Alzheimer’s disease. Current Alzheimer Research. 2006;3(3):237–245. doi: 10.2174/156720506777632925. [DOI] [PubMed] [Google Scholar]
- 74.Puzzo D, Vitolo O, Trinchese F, Jacob JP, Palmeri A, Arancio O. Amyloid-β peptide inhibits activation of the nitric oxide/cGMP/cAMP-responsive element-binding protein pathway during hippocampal synaptic plasticity. Journal of Neuroscience. 2005;25(29):6887–6897. doi: 10.1523/JNEUROSCI.5291-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCarty MF. Toward prevention of alzheimers disease—potential nutraceutical strategies for suppressing the production of amyloid beta peptides. Medical Hypotheses. 2006;67(4):682–697. doi: 10.1016/j.mehy.2006.04.067. [DOI] [PubMed] [Google Scholar]
- 76.Pak T, Cadet P, Mantione KJ, Stefano GB. Morphine via nitric oxide modulates β-amyloid metabolismml: a novel protective mechanism for Alzheimer’s disease. Medical Science Monitor. 2005;11(10):BR357–BR366. [PubMed] [Google Scholar]
- 77.Seyidova D, Aliyev A, Rzayev N, et al. The role of nitric oxide in the pathogenesis of brain lesions during the development of Alzheimer’s disease. In Vivo. 2004;18(3):325–334. [PubMed] [Google Scholar]
- 78.Lüth HJ, Holzer M, Gertz H-J, Arendt TH. Aberrant expression of nNOS in pyramidal neurons in Alzheimer’s disease is highly co-localized with p21(ras) and p16(INK4a) Brain Research. 2000;852(1):45–55. doi: 10.1016/s0006-8993(99)02178-2. [DOI] [PubMed] [Google Scholar]
- 79.de la Monte SM, Lu B-X, Sohn Y-K, et al. Aberrant expression of nitric oxide synthase III in Alzheimer’s disease: relevance to cerebral vasculopathy and neurodegeneration. Neurobiology of Aging. 2000;21(2):309–319. doi: 10.1016/s0197-4580(99)00108-6. [DOI] [PubMed] [Google Scholar]
- 80.Tran MH, Yamada K, Olariu A, Mizuno M, Ren XH, Nabeshima T. Amyloid β-peptide induces nitric oxide production in rat hippocampus: association with cholinergic dysfunction and amelioration by inducible nitric oxide synthase inhibitors. The FASEB Journal. 2001;15(8):1407–1409. doi: 10.1096/fj.00-0719fje. [DOI] [PubMed] [Google Scholar]
- 81.Haas J, Storch-Hagenlocher B, Biessmann A, Wildemann B. Inducible nitric oxide synthase and argininosuccinate synthetase: co-induction in brain tissue of patients with Alzheimer’s dementia and following stimulation with β-amyloid 1–42 in vitro. Neuroscience Letters. 2002;322(2):121–125. doi: 10.1016/s0304-3940(02)00095-2. [DOI] [PubMed] [Google Scholar]
- 82.Stepanichev MYU, Onufriev MV, Yakovlev AA, et al. Amyloid-β (25–35) increases activity of neuronal NO-synthase in rat brain. Neurochemistry International. 2008;52(6):1114–1124. doi: 10.1016/j.neuint.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 83.Keil U, Bonert A, Marques CA, et al. Amyloid β-induced changes in nitric oxide production and mitochondrial activity lead to apoptosis. Journal of Biological Chemistry. 2004;279(48):50310–50320. doi: 10.1074/jbc.M405600200. [DOI] [PubMed] [Google Scholar]
- 84.Ha K-S, Kim K-M, Kwon Y-G, et al. Nitric oxide prevents 6-hydroxydopamine-induced apoptosis in PC12 cells through cGMP-dependent PI3 kinase/Akt activation. The FASEB Journal. 2003;17(9):1036–1047. doi: 10.1096/fj.02-0738com. [DOI] [PubMed] [Google Scholar]
- 85.de la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer’s disease. Journal of Alzheimer’s Disease. 2006;9(2):167–181. doi: 10.3233/jad-2006-9209. [DOI] [PubMed] [Google Scholar]
- 86.Yuan H, Gerencser AA, Liot G, et al. Mitochondrial fission is an upstream and required event for bax foci formation in response to nitric oxide in cortical neurons. Cell Death and Differentiation. 2007;14(3):462–471. doi: 10.1038/sj.cdd.4402046. [DOI] [PubMed] [Google Scholar]
- 87.Barsoum MJ, Yuan H, Gerencser AA, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. The EMBO Journal. 2006;25(16):3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cho D-H, Nakamura T, Fang J, et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324(5923):102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gu Z, Nakamura T, Lipton SA. Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Molecular Neurobiology. 2010;41(2-3):55–72. doi: 10.1007/s12035-010-8113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bossy B, Petrilli A, Klinglmayr E, et al. S-Nitrosylation of DRP1 does not affect enzymatic activity and is not specific to Alzheimer’s disease. Journal of Alzheimer’s Disease. 2010;20(2):513–526. doi: 10.3233/JAD-2010-100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jahani-Asl A, Cheung ECC, Neuspiel M, et al. Mitofusin 2 protects cerebellar granule neurons against injury-induced cell death. Journal of Biological Chemistry. 2007;282(33):23788–23798. doi: 10.1074/jbc.M703812200. [DOI] [PubMed] [Google Scholar]
- 92.Su B, Wang X, Zheng L, Perry G, Smith MA, Zhu X. Abnormal mitochondrial dynamics and neurodegenerative diseases. Biochimica et Biophysica Acta. 2010;1802(1):135–142. doi: 10.1016/j.bbadis.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA. Involvement of oxidative stress in Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 2006;65(7):631–641. doi: 10.1097/01.jnen.0000228136.58062.bf. [DOI] [PubMed] [Google Scholar]
- 94.Su B, Wang X, Nunomura A, et al. Oxidative stress signaling in Alzheimer’s disease. Current Alzheimer Research. 2008;5(6):525–532. doi: 10.2174/156720508786898451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jana A, Pahan K. Fibrillar amyloid-β peptides kill human primary neurons via NADPH oxidase-mediated activation of neutral sphingomyelinase:implications for Alzheimer’s disease. Journal of Biological Chemistry. 2004;279(49):51451–51459. doi: 10.1074/jbc.M404635200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shelat PB, Chalimoniuk M, Wang J-H, et al. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. Journal of Neurochemistry. 2008;106(1):45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- 97.Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer’s disease. Journal of Neuroscience. 1997;17(8):2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reynolds MR, Reyes JF, Fu Y, et al. Tau nitration occurs at tyrosine 29 in the fibrillar lesions of Alzheimer’s disease and other tauopathies. Journal of Neuroscience. 2006;26(42):10636–10645. doi: 10.1523/JNEUROSCI.2143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y-J, Xu Y-F, Liu Y-H, et al. Peroxynitrite induces Alzheimer-like tau modifications and accumulation in rat brain and its underlying mechanisms. The FASEB Journal. 2006;20(9):1431–1442. doi: 10.1096/fj.05-5223com. [DOI] [PubMed] [Google Scholar]
- 100.Horiguchi T, Uryu K, Giasson BI, et al. Nitration of tau protein is linked to neurodegeneration in tauopathies. American Journal of Pathology. 2003;163(3):1021–1031. doi: 10.1016/S0002-9440(10)63462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Frontiers in Neuroendocrinology. 2009;30(2):239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. Journal of the American Medical Association. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 103.Perry G, Cash AD, Smith MA. Alzheimer disease and oxidative stress. Journal of Biomedicine and Biotechnology. 2002;2002(3):120–123. doi: 10.1155/S1110724302203010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bang OY, Hong HS, Kim DH, et al. Neuroprotective effect of genistein against beta amyloid-induced neurotoxicity. Neurobiology of Disease. 2004;16(1):21–28. doi: 10.1016/j.nbd.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 105.Zeng H, Chen Q, Zhao B. Genistein ameliorates β-amyloid peptide (25-35)-induced hippocampal neuronal apoptosis. Free Radical Biology and Medicine. 2004;36(2):180–188. doi: 10.1016/j.freeradbiomed.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 106.Linford NJ, Dorsa DM. 17β-Estradiol and the phytoestrogen genistein attenuate neuronal apoptosis induced by the endoplasmic reticulum calcium-ATPase inhibitor thapsigargin. Steroids. 2002;67(13-14):1029–1040. doi: 10.1016/s0039-128x(02)00062-4. [DOI] [PubMed] [Google Scholar]
- 107.Andersen JM, Myhre O, Fonnum F. Discussion of the role of the extracellular signal-regulated kinase-phospholipase A2 pathway in production of reactive oxygen species in Alzheimer’s disease. Neurochemical Research. 2003;28(2):319–326. doi: 10.1023/a:1022389503105. [DOI] [PubMed] [Google Scholar]
- 108.Yu H-L, Li L, Zhang X-H, et al. Neuroprotective effects of genistein and folic acid on apoptosis of rat cultured cortical neurons induced by beta-amyloid 31-35. British Journal of Nutrition. 2009;102(5):655–662. doi: 10.1017/S0007114509243042. [DOI] [PubMed] [Google Scholar]
- 109.Park Y-J, Jang Y-M, Kwon YH. Isoflavones prevent endoplasmic reticulum stress-mediated neuronal degeneration by inhibiting tau hyperphosphorylation in SH-SY5Y Cells. Journal of Medicinal Food. 2009;12(3):528–535. doi: 10.1089/jmf.2008.1069. [DOI] [PubMed] [Google Scholar]
- 110.Atlante A, Bobba A, Paventi G, Pizzuto R, Passarella S. Genistein and daidzein prevent low potassium-dependent apoptosis of cerebellar granule cells. Biochemical Pharmacology. 2010;79(5):758–767. doi: 10.1016/j.bcp.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 111.Vallés SL, Borrás C, Gambini J, et al. Oestradiol or genistein rescues neurons from amyloid beta-induced cell death by inhibiting activation of p38. Aging Cell. 2008;7(1):112–118. doi: 10.1111/j.1474-9726.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- 112.Cai Q, Wei H. Effect of dietary genistein on antioxidant enzyme activities in SENCAR mice. Nutrition and Cancer. 1996;25(1):1–7. doi: 10.1080/01635589609514423. [DOI] [PubMed] [Google Scholar]
- 113.Foti P, Erba D, Riso P, Spadafranca A, Criscuoli F, Testolin G. Comparison between daidzein and genistein antioxidant activity in primary and cancer lymphocytes. Archives of Biochemistry and Biophysics. 2005;433(2):421–427. doi: 10.1016/j.abb.2004.10.008. [DOI] [PubMed] [Google Scholar]