Abstract
PURPOSE Participatory decision making (PDM) is associated with improved diabetes control. We examine a causal model linking PDM to improved clinical outcomes that included patient activation and medication adherence.
METHODS This observational study was conducted in 5 family physician offices. Diabetic patients were recruited by mail and by completing a study interest card at the conclusion of their office visit. Two survey questionnaires, administered 12 months apart, elicited patients’ ratings of their physician’s PDM style at baseline and their level of activation and medication adherence both at baseline and at follow-up. Measures of glycated hemoglobin (hemoglobin A1c), systolic blood pressure, and low-density lipoprotein (LDL) cholesterol were abstracted from the medical record starting 12 months before the baseline survey to 12 months after the follow-up survey. A path analysis using a structural equation model was used to test hypotheses.
RESULTS We mailed questionnaires to 236 participants; 166 (70%) returned the baseline questionnaire, and 141 (80%) returned the follow-up questionnaire. Hemoglobin A1c levels, systolic blood pressure, and LDL cholesterol values all declined significantly, and patient activation and medication adherence improved. PDM at baseline was associated with patient activation at follow-up. Patient activation at follow-up was associated with medication adherence at follow-up, and medication adherence at follow-up was associated with change in hemoglobin A1c levels and LDL cholesterol values but not with systolic blood pressure.
CONCLUSIONS Participatory decision making during primary care encounters by patients with type 2 diabetes resulted in improvements in hemoglobin A1c levels and LDL cholesterol values by improving patient activation, which in turn improved medication adherence.
Keywords: Patient-centered care; physician-patient relations; diabetes mellitus, type 2
INTRODUCTION
A key feature of the widely promoted patient-centered medical home is found in its title: the care provided is centered on the patient. Although descriptions of patient-centered care differ, the Institute of Medicine definition describes shared or participatory decision making between patient and clinician as a critical component of “a partnership among practitioners, patients and their families…to ensure that decisions respect patients wants, needs and preferences and solicit patients’ input on the education and support they need to make decisions and participate in their own care.”1 Participatory decision making (PDM) is associated with better outcomes in patients with a chronic illness such as diabetes.2,3
Suboptimal control of intermediate clinical outcomes among patients with type 2 diabetes—glycated hemoglobin (hemoglobin A1c) level, systolic blood pressure, and low-density lipoprotein (LDL) cholesterol level—has been a persistent problem, and medication adherence is frequently cited as an explanation.4,5 Rates of adherence for oral hypoglycemic medications have been estimated to range from 67% to 85% in prospective electronic monitoring studies,6 and between 30% to 90% of patients with hypertension do not take their medications as prescribed.7 In a study of adherence to lipid-lowering agents, 50% of patients were not taking their statin 6 months after the initial prescription.8
Prior research suggests that patients who actively participate in the medical encounter have improved medication adherence.9–11 High levels of patient activation, combined with a more balanced partnership within the medical encounter, represent 2 important conceptual dimensions of a healthy therapeutic alliance.12 The patient-physician relationship has been repeatedly documented as a significant factor influencing appropriate medication adherence, subsequently resulting in improved outcomes.13–15 In addition, specific efforts to improve both patient activation and the patient-centeredness of the medical encounter have been shown to result in better glucose control.2,16
Given that some studies found a participatory decision-making style on the part of the physician is associated with patients who participate more actively in the encounter, and that separate studies found active participation is associated with medication adherence, the purpose of this study was to assess a potential causal pathway among these relationships, including changes in hemoglobin A1c levels, systolic blood pressure, and LDL cholesterol levels over a 1-year period among a patients with type 2 diabetes in primary care settings. Our hypotheses were as follows:
Change in hemoglobin A1c level, blood pressure, and lipid control will be positively associated with medication adherence.
Medication adherence will be better among patients who participate more actively in the encounter.
Active patient participation in the encounter will be associated with higher levels of patient-physician participatory decision making.
METHODS
Study Population and Sample
We conducted an observational prospective study in 5 independent primary care practices that were members of the South Texas Ambulatory Research Network, a practice-based research network. We chose these practices because of their proximity to the University of Texas Health Science Center and because each site used an electronic medical record. One practice had 2 family physicians, the other 4 practices had 1 physician each; 2 of those practices also had a nurse practitioner or physician assistant. Each practice was owned by the physicians and run as a small business. This scenario is not unusual, as approximately 20% of primary care practices in the United States are solo physician practices. None of the physicians had any financial or other incentive tied to a quality-of-care or performance measurement.
Patients were recruited for participation by 2 methods. First, a list of all patients with a diagnosis of type 2 diabetes in the past 12 months was generated from the billing system in each physician’s office. From this list, letters from the physician were mailed to all patients asking them to contact the study coordinator if they were interested in participating. Patients who contacted the study coordinator were mailed an enrollment packet that included a baseline survey questionnaire and a consent form to return. Second, patients who were seen in each practice during a 2-month period were given an information sheet about the study and asked to complete a study interest card with their contact information for the study coordinator. The target enrollment for each practice was 50 patients.
Data Collection and Measures
All respondents who enrolled in the study were administered a questionnaire to collect baseline information and again 12 months later to collect follow-up information. Participants also had their medical records abstracted to obtain values for hemoglobin A1c, systolic blood pressure, and LDL cholesterol.
Physician Participatory Decision Making
Patients were asked about their physician’s participation in decision-making style at baseline using the scale developed by Kaplan: “How often does your regular doctor or primary care provider who takes care of you: (1) discuss pros and cons of each choice with you; (2) ask you which choices or options you would prefer; (3) take your preferences into account when making treatment decisions?”17 This scale, plus the additional item of “offer choices in medical care,” has been used in previous studies of diabetes self-management.3
Patient Activation
The extent to which patients took an active role in their own treatment was examined in both the baseline and follow-up questionnaires with the Lorig communication scale: “When you visit your regular or primary care provider, how often do you do the following: (1) prepare a list of questions for your doctor; (2) ask questions about the things you want to know and things you don’t understand about your treatment; (3) discuss any personal problems that may be related to your illness.” This scale has been validated by the Stanford Patient Education Research Center and used in a variety of studies to measure the degree to which patients use techniques to engage their physicians in making decisions about their care.18
Medication Adherence
Medication adherence at baseline and at the 1-year follow-up was assessed with the Morisky scale,19 a 4-item yes-or-no instrument frequently used for adherence research across a variety of chronic medical and psychiatric conditions.19–21 Patients responded yes or no to each of the following 4 self-reported obstacles to good adherence: “Have you ever: (1) stopped taking medications because you were feeling better; (2) stopped taking your medications because you were feeling worse; (3) been careless at times about when you should take your medication; or (4) forgotten to take medications.” For purposes of the analysis, the scale was scored as a continuous variable between 0 and 4, with higher values representing more problems with medication adherence. The scale shows good test-retest reliability and has been correlated with appropriate control of hemoglobin A1c levels.22
Control of Diabetes Complication Risk Factors
We measured 3 intermediate clinical outcomes: hemoglobin A1c level, systolic blood pressure, and LDL cholesterol level. All values for hemoglobin A1c, systolic blood pressure, and LDL cholesterol for a 36-month period from 1 year before the first questionnaire was administered to 1 year after the second questionnaire was administered were obtained from chart review. We recorded and included for analysis patient-level data for up to 9 most recent separate values of each outcome. After the 9 most recent values, additional values did not contribute pertinent information to the latent growth curve analysis described below.
Analysis
Using SAS software, preliminary analysis of the out-come variables (hemoglobin A1c level, systolic blood pressure, and LDL cholesterol level) involved visual inspection and assessment of the distribution for outliers (eg, responses of greater than 3 standard deviations from the mean) and normality (eg, skewness and kurtosis). It was determined that transforming the data for use in the modeling was not necessary, and all biologically plausible variables were included in the analysis.
We assessed changes in hemoglobin A1c level, systolic blood pressure, and LDL cholesterol level over time using separate latent growth curve (LGC) models for each measure. The advantage in using a LGC model is that the full trajectory of change across each individual’s measurement points can be estimated.23,24 The LGC model provides parameter estimates for 2 latent growth variables: (1) a latent intercept (baseline value), and (2) a latent slope (eg, an estimate of the rate of change over time). Two parameters of each of these latent variables are of specific interest: the fixed effects (group averages) and the random effects (individual variability). The fixed effects for the latent slope represents the average rate of change in hemoglobin A1c level, systolic blood pressure, or LDL cholesterol level, whereas the random effect component is an estimate of the variation around the group’s average rate of change.
In the current analysis, the fixed effect for the latent intercept represents the average lipid or blood pressure value at baseline, and the random effect represents the variation around that average baseline value. Likewise, the fixed effect for the latent slope represents the average rate of change in lipid level or systolic blood pressure, whereas the random effect component is an estimate of the variation around the group’s average rate of change. Variability around the averages is of interest because it indicates whether individual heterogeneity is present in the sample. Significant variation in the rate of change in a latent growth variable indicates that there are statistically significant individual differences in the rates of change. Nonsignificant variation would indicate homogeneity or that all participants follow a similar change trajectory. These analyses were conducted using AMOS software.25
A separate structural equation model using path analysis was constructed for each outcome: 1 model with the rate of change in the hemoglobin A1c level obtained from the LGC model as the outcome, 1 model for systolic blood pressure, and 1 model for the LDL cholesterol level. The principal advantage of such a path analysis is that it allows one to estimate the relative influence of variables within a hypothesized causal network. Causal sequences cannot be validated by such a model, however, and the validity of such models is dependent on creating a path model that is based on a strong theoretical framework.
Missing data were addressed using the full information maximum likelihood method, which is currently recommended as a state-of-the art method.26 This study was approved by the Institutional Review Board at the University of Texas Health Science Center at San Antonio.
RESULTS
A total of 680 potential participants were sent a letter, and 186 patients completed a study interest card at the end of their clinic visit. From the letters, 139 patients called the study coordinator and enrolled in the study resulting in a participation rate of 20.4%. Of those who returned a study interest card, 98 were contacted and enrolled in the study, resulting in a participation rate of 70.5%. Thus, a total of 237 questionnaires were mailed to eligible participants. Of those, 166 (70%) returned a questionnaire, and 1 year later 141 (88%) returned the follow-up questionnaire.
The mean age of the participants was 57.7 years (SD = 10.7); 61% were female, and 51% Hispanic. Regarding socioeconomic status, 20% had a college degree or higher, 35.5% had either a high school degree or a high school degree and some college, and 47% had a household income of $30,000 or less in the past year. Participants were offered the questionnaire in either Spanish or English; only 2.4% requested a Spanish version.
Patient participation/activation and medication adherence improved between the baseline and follow-up surveys (Table 1▶). Table 2▶ displays the estimates of the LGC models. The hemoglobin A1c baseline value is an estimated 7.05%, with a small but significant average decline of 0.03% between each measurement during the 36-month period. The baseline systolic blood pressure value is estimated to be 130.4 mm Hg, with an estimated small but statistically significant 0.3-mm Hg rate of decline per clinic visit. The LDL cholesterol baseline value was estimated at 102.9 mg/dL, with an average rate of decrease at 2.89 mg/dL per measurement. For these types of models, different indices evaluate different ways of adequate model fit.27 A χ2 test is commonly used, but it is inflated when the sample size increases or when the normality assumption does not hold. A second method of determining adequate model fit is to calculate the root mean square error of approximation (RMSEA). This value normalizes the χ2 index when normality is a problem. The comparative fit index (CFI) measures the improvement in fit from the hypothesized model over using a model with independence assumed between the variables. Based on these indicators (RMSEA = 0.08; CFI >0.90, and χ2/df ratio <3.0), all 3 models were judged to have adequate model fit.27
Table 1.
Summary Description of Scales Used in Analyses
|
Baseline Score |
Follow-up Score |
|||||
|---|---|---|---|---|---|---|
| Variable | Meaning of High Score | n | Mean (SD); Range | n | Mean (SD) | P Value |
| Participatory decision making | Greater participatory decision making | 60 | 2.40 (1.14); 1–5 | NA | NA | NA |
| Patient activation | Higher level of patient activation | 162 | 12.48 (3.53); 3–18 | 143 | 13.31 (3.61) | .014 |
| Medication adherence | More problems with medication adherence | 156 | 1.23 (0.97); 0–4 | 145 | 1.10 (0.93) | .017 |
NA=not applicable.
aDetermined by paired t test.
Table 2.
Parameters of the Latent Growth Curve Model for Hemoglobin A1c, Systolic Blood Pressure, and LDL Cholesterol
|
Model Parameters |
|||||
|---|---|---|---|---|---|
| Measure | Mean (SE) | Sign | Variance (SE) | P Value | Model Fit χ2 (df); CFI; RMSEA |
| Hemoglobin A1c | 26.9 (30); 0.99; 0.030 | ||||
| Baseline | 7.05 (0.97) | <.001 | 2.05 (.275) | <.001 | |
| Rate of change | −0.03 (0.015) | <.10 | 0.10 (.017) | <.001 | |
| Systolic blood pressure | 57.0 (30); 0.97; 0.044 | ||||
| Baseline | 130.41 (.98) | <.001 | 177.38 (21.88) | <.001 | |
| Rate of change | −0.30 (0.15) | <.05 | 2.52 (0.58) | <.001 | |
| LDL cholesterol | 60.6 (30); 0.93; 0.059 | ||||
| Baseline | 102.89 (2.30) | <.001 | 847.00 (116.20) | <.001 | |
| Rate of change | −2.89 (0.48) | <.001 | 11.14 ( 5.80) | <.01 | |
Hemoglobin A1c=glycated hemoglobin; CFI=Comparative Fit Index; df = degree of freedom; LDL = low-density lipoprotein; RMSEA = root mean square error of approximation.
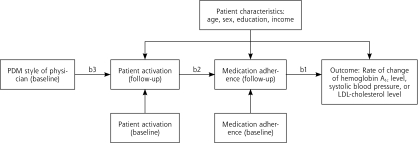
Formal results of the tests for each of the 3 hypotheses are found in an examination of regression coefficients for the full path model depicted in Figure 1▶ and are shown in Table 3▶. The results in each model were adjusted for age, sex, education, and income.
Figure 1.
Path analysis depicting the associations between PDM, activation, medication adherence, and clinical outcome.
Hemoglobin A1c=glycated hemoglobin; LDL=low-density lipoprotein; PDM=participatory decision making.
Note: refer to Table 3▶ for specific coefficients for each of the labeled paths: b1, b2, and b3.
Table 3.
Regression Weights of Study Variables on Outcome Rates of Change From Structural Equation Models
|
Hemoglobin A1c |
Systolic Blood Pressure |
LDL Cholesterol |
||||||
|---|---|---|---|---|---|---|---|---|
| Path | Independent Variable | Dependent Variable | Regression Coefficient (SE) | P Value | Regression Coefficient (SE) | P Value | Regression Coefficient (SE) | P Value |
| b3 | PDMa | Patient activation follow-upb | 0.44 (0.21); −0.14c | .03 | 0.43 (0.21); 0.14c | 0.04 | 0.42 (0.21); 0.14c | .04 |
| b2 | Patient activation follow-up | Medication adherence follow-upd | −0.04 (0.02); −0.16c | .02 | −0.004 (0.02); −0.15c | 0.02 | −0.04 (0.02); −0.16c | .02 |
| b1 | Medication adherence follow-up | Rate of change in outcome | 0.04 (0.02); 0.26c | .05 | 0.04 (0.17); 0.05c | 0.80 | 1.08 (0.53); 0.40c | .04 |
A1 = glycated hemoglobin; LDL = low-density lipoprotein; PDM = participatory decision making.
Note: See Figure 1▶ for path descriptions.
aMeasured by Kaplan-Greenfield scale.
bMeasured by Lorig communication scale.
cStandardized regression coefficient.
dMeasured by Morisky scale.
The full path model for each structural equation model is shown in Figure 1▶. Hypothesis 1 is tested by coefficient b1, hypothesis 2 by coefficient b2, and hypothesis 3 by coefficient b3. The results in each model were adjusted for age, sex, education, and income.
Hypothesis 1: Change in Hemoglobin A1c Level, Blood Pressure, and Lipid Control Will Be Positively Associated With Medication Adherence
As shown in the bottom row of Table 3▶, the coefficient for path b1 was significant for changes in both hemoglobin A1c and LDL cholesterol values, but not for systolic blood pressure. That is, medication adherence was associated with a significant change in both hemoglobin A1c and LDL values over time, but not with a change in systolic blood pressure. Since higher scores on the Morisky scale reflect more problems with medication adherence, the regression coefficient indicates that more adherence problems are associated with undesirable changes in hemoglobin A1c and LDL cholesterol values over time—eg, a smaller rate of decline over time. A closer examination of the standardized regression coefficients shows that the relationship between the clinical outcome and medication adherence is strongest for change in LDL cholesterol level.
Hypothesis 2: Medication Adherence Will be Better Among Patients Who Are More Active Participants in the Encounter
This hypothesis is tested with path b2 in the model, and results are found in the second row in Table 3▶. For all 3 outcomes—hemoglobin A1c, systolic blood pressure, and LDL cholesterol—the level of patient activation is inversely associated with medication adherence. More specifically, after controlling for both baseline levels of medication adherence and patient activation, patient activation at follow-up is a significant predictor of medication adherence at follow-up: the higher the patient activation, the lower the Morisky score, reflective of fewer problems with medication adherence. The strength of this relationship as reflected in the standardized regression coefficients, which are similar across all 3 outcomes.
Hypothesis 3: Active Patient Participation in the Encounter Will Be Associated With Higher Levels of Patient-Physician Participatory Decision Making
This hypothesis is tested in path b3 in the model, and results for this path are found in the first row of Table 3▶. For all 3 models of outcomes—hemoglobin A1c, systolic blood pressure, and LDL cholesterol—the participatory decision making between the patient and clinician is a significant predictor of the level of patient activation at follow-up, controlling for baseline level of patient activation. Of interest is the finding that there was no direct relationship between participatory decision making and medication adherence. As hypothesized, however, PDM predicts patient activation and patient activation predicts adherence.
Because it is possible that those who returned a baseline questionnaire were those who had higher levels of patient activation and medication adherence at baseline, we examined the data to determine whether the level of PDM, activation, or medication adherence was significantly different at baseline between those who did and did not return a follow-up survey and found no significant differences.
DISCUSSION
These results suggest that PDM may contribute to such improved clinical outcomes as control of hemoglobin A1c level, systolic blood pressure, and LDL cholesterol level through its actions on patient activation and medication adherence. The results supported all 3 hypotheses: (1) patients who report a higher level of participatory decision making are more likely to actively participate in the medical encounter; (2) active participation in the encounter is associated with medication adherence; and (3) medication adherence was associated with improvement in control of glucose and lipids, but not with blood pressure.
Multiple studies have documented the benefit of shared or participatory decision making, including better recovery from discomfort, better emotional health, and fewer diagnostic tests and referrals, better self-care activities, and improved clinical outcomes.3,28–30 In at least 1 study, this collaborative or participatory decision-making style appears to be independent of a more global conceptualization of good physician communication.9 The results of our study take the research on this area one step further and show a more complete pathway between participatory decision making and improvements in the clinical intermediate outcomes for hemoglobin A1c, systolic blood pressure, and LDL cholesterol values over time.
PDM was not directly associated with adherence. Instead, we found that PDM is associated with the level of patient activation, which in turn is associated with medication adherence. By setting up the path analysis in this manner, we hypothesized that patient activation was a mediating or intermediate variable between PDM and medication adherence.31 By definition, a mediator must occur after that which it mediates and before the outcome.32 As noted in Figure 1▶, the mediator, patient activation, is measured at the 1-year follow-up, whereas PDM, the variable it mediates, was measured at baseline. In addition, the model dictated by the path analysis controls for the baseline level of patient activation when examining the association between PDM and activation. In addition, the model examining the association between activation and adherence controls for the baseline measure of adherence. Thus PDM cannot lead to adherence without patient activation.
One exception to our findings was systolic blood pressure. In the model for systolic blood pressure, PDM was related to more-active participation, which in turn predicted medication adherence. Medication adherence was not related to improvement in systolic blood pressure control, however. Although patient adherence is important to achieve adequate blood pressure control, it may not be sufficient. The other explanation for the lack of association is that of clinical inertia on the part of the physician.33 Indeed, it is possible that in visits with higher levels of PDM, patients are resistant to intensification of therapy for hypertension and provide soft excuses for why their blood pressure therapy should not be intensified.
A critical question raised by this and prior studies about the importance of PDM between patients and primary care physicians is whether any effective intervention exists that would improve this attribute in a manner that would result in improved medication adherence. A recent review of the evidence assessing interventions to improve medication adherence among patients with type 2 diabetes concluded that the question about effective interventions to improve adherence remains unanswered.34 At least one study, however, found that a patient-level intervention to improve patient participation in decision making during the encounter resulted in improved diabetes control, but there was no measure of medication adherence in this study.17 Unfortunately, the intervention was highly resource and time intensive and thus not feasible in the typical primary care setting.
A limitation of this study is that it was conducted in a small sample of primary care practices with physicians who may not reflect the full range of PDM styles. The physicians in the study were not randomly selected and may not be typical in their degree of patient-centeredness. The mean (2.40), and SD (1.14) of a possible range between 1 and 5 for the PDM scores suggests, however, that patients report experiencing a full range of PDM experiences.
In addition, the method of participant recruitment may have resulted in biasing the sample toward patients who are healthier and more actively involved in their care, because recruitment model required patients to proactively contact the study coordinator. If so, then it is possible that patients who enrolled in this study would have better control of their hemoglobin A1c levels, systolic blood pressure, and LDL cholesterol levels than patients who did not. A comparison of these values for patients who enrolled in this study was done with data from a study done 2 years earlier in these same 5 clinics with a consecutive sample of patients who sought for care of their diabetes.35 We found that the mean hemoglobin A1c level in the earlier study was 7.5%, almost 0.5% higher than for patients who enrolled in the present study. Systolic blood pressure and LDL cholesterol levels for these patients, however, were nearly identical to baseline levels in the present study: 130.6 mm Hg and 100.2 mg/dL respectively. In the earlier study, the consecutive patient sampling method may have biased the sample toward those with worse overall control who return for follow-up more often. If we did enroll patients with higher levels of activation, then the bias should have been toward not detecting a relationship between the observed or measured variables, as it would have decreased the amount of variance resulting in a decrease in overall power with this small sample size. We also compared our baseline outcomes with those reported in the TRIAD study. When we compared our mean outcome values in 2004 with VA TRIAD data collected in 2001–2002, we found similar control of hemoglobin A1c level at <8.5% (84% in our participants, 83% in the VA TRIAD) and LDL cholesterol level at <100 mg/dL (61% in our participants, 52% in the VA TRIAD) but better overall blood pressure control among our participants: blood pressure <130/85 (48% in our data, 29% in VA TRIAD).36
Finally, it is possible that participants in this study who have a higher level of activation may have selected physicians with a more PDM style to fit their preferences. Even so, in this study we found that after controlling for baseline patient activation, the level of activation at follow-up was higher if the PDM style of the physician was also higher. One strength of this study is the high response rate to the questionnaires administered compared with the response rates of earlier studies.3,9
A pointed out by others, in addition to the requisite knowledge, skills, and attitudes on the part of the physician required to encourage participatory decision making, one barrier to incorporating a PDM approach and encouraging more active patient participation is the current structure and resources available in most primary care settings.37 This type of care requires sufficient encounter time to discuss treatment options and to elicit patient preferences. Unfortunately, recent studies have documented high levels of competing demands during encounters with patients who have type 2 diabetes.35,38 In one study the encounter lasted 17 minutes, and during that encounter 17 different topics, questions and problems were discussed.35 Although it might be ideal to have other primary health care team members trained to provide support and patient activation activities in primary care settings, the reality is that many of these settings do not have the resources to provide this support.39
If patients with complex chronic illnesses, such as type 2 diabetes, are to have a patient-centered medical home that results in improved outcomes, such as control of hemoglobin A1c, blood pressure, and lipid levels, it will require much more than the implementation of currently described components of enhanced access and electronic health records. There must also be adequate time and resources to involve patients in active decision making about their care. As pointed out by others, doing so will require a fundamental restructuring and transformation of many, if not most, primary care settings.39,40
Acknowledgments
We would like to express our appreciation to the physicians and offices staff in the South Texas Ambulatory Research Network (STARNet) for their participation in this study: Nancy Hinitt, MD; Kenneth Lyssy, MD; Jane Lyssy, MD; Michael Mann, MD; Ramon Reyes, MD; and Liem Du, MD; and the staff at the Atascosa Community Health Center in Pleasanton, Texas.
Conflicts of interest: none reported
Funding support: This research was supported by the National Institutes of Health, National Institute for Diabetes, Digestive and Kidney Disorders (DK067300-02); and the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
REFERENCES
- 1.Institute of Medicine. Envisioning the National Health Care Quality Report. Bethesda, MD: Institute of Medicine; 2000.
- 2.Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care. 1998;21(10):1644–1651. [DOI] [PubMed] [Google Scholar]
- 3.Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med. 2002;17(4):243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saaddine JB, Cadwell B, Gregg EW, et al. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988–2002. Ann Intern Med. 2006;144(7):465–474. [DOI] [PubMed] [Google Scholar]
- 5.Grant R, Adams AS, Trinacty CM, et al. Relationship between patient medication adherence and subsequent clinical inertia in type 2 diabetes glycemic management. Diabetes Care. 2007;30(4):807–812. [DOI] [PubMed] [Google Scholar]
- 6.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218–1224. [DOI] [PubMed] [Google Scholar]
- 7.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. [DOI] [PubMed] [Google Scholar]
- 8.Mann DM, Allegrante JP, Natarajan S, Halm EA, Charlson M. Predictors of adherence to statins for primary prevention. Cardiovasc Drugs Ther. 2007;21(4):311–316. [DOI] [PubMed] [Google Scholar]
- 9.Naik AD, Kallen MA, Walder A, Street RL Jr. Improving hypertension control in diabetes mellitus: the effects of collaborative and proactive health communication. Circulation. 2008;117(11):1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heisler M, Vijan S, Anderson RM, Ubel PA, Bernstein SJ, Hofer TP. When do patients and their physicians agree on diabetes treatment goals and strategies, and what difference does it make? J Gen Intern Med. 2003;18(11):893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMatteo MR, Sherbourne CD, Hays RD, et al. Physicians’ characteristics influence patients’ adherence to medical treatment: results from the Medical Outcomes Study. Health Psychol. 1993;12(2):93–102. [DOI] [PubMed] [Google Scholar]
- 12.Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc Sci Med. 2000;51(7):1087–1110. [DOI] [PubMed] [Google Scholar]
- 13.Becker ER, Roblin DW. Translating primary care practice climate into patient activation: the role of patient trust in physician. Med Care. 2008;46(8):795–805. [DOI] [PubMed] [Google Scholar]
- 14.Fuertes JN, Mislowack A, Bennett J, et al. The physician-patient working alliance. Patient Educ Couns. 2007;66(1):29–36. [DOI] [PubMed] [Google Scholar]
- 15.Ciechanowski P, Russo J, Katon W, et al. Influence of patient attachment style on self-care and outcomes in diabetes. Psychosom Med. 2004;66(5):720–728. [DOI] [PubMed] [Google Scholar]
- 16.Williams GC, McGregor H, Zeldman A, Freedman ZR, Deci EL, Elder D. Promoting glycemic control through diabetes self-management: evaluating a patient activation intervention. Patient Educ Couns. 2005;56(1):28–34. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan SH, Greenfield S, Ware JE Jr. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27(3)(Suppl):S110–S127. [DOI] [PubMed] [Google Scholar]
- 18.Stanford Chronic Disease Self-Management Study. Psychometrics reported in: Lorig K, Stewart A, Ritter P, González V, Laurent D, Lynch J. Outcome Measures for Health Education and other HealthCare Interventions. Thousand Oaks CA: Sage Publications; 1996:24,40.
- 19.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. [DOI] [PubMed] [Google Scholar]
- 20.Zeber JE, Copeland LA, Good CB, Fine MJ, Bauer MS, Kilbourne AM. Therapeutic alliance perceptions and medication adherence in patients with bipolar disorder. J Affect Disord. 2008;107(1–3):53–62. [DOI] [PubMed] [Google Scholar]
- 21.George CF, Peveler RC, Heiliger S, Thompson C. Compliance with tricyclic antidepressants: the value of four different methods of assessment. Br J Clin Pharmacol. 2000;50(2):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krapek K, King K, Warren SS, et al. Medication adherence and associated hemoglobin A1c in type 2 diabetes. Ann Pharmacother. 2004;38(9):1357–1362. [DOI] [PubMed] [Google Scholar]
- 23.McArdle J, Bell R. An introduction to latent growth models for developmental data analysis. In: Little T, Schnabel K, Baumert J, eds. Modeling Longitudinal and Multilevel Data: Practical Issues, Applied Approaches, and Specific Examples. Mahwah, NJ: Lawrence Erlbaum Associates; 2000.
- 24.Willet J, Sayer A. Using covariance structure analysis to detect correlates and predictors of individual change over time. Psychol Bull. 1994;116:363–381. [Google Scholar]
- 25.Arbuckle JL. Amos 5.0 Update to the Amos User’s Guide. Chicago, IL: Smallwaters Corporation; 2003.
- 26.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- 27.Bollen KA, Long JS. Testing Structural Equation Models. Thousand Oaks, CA: Sage Publishing; 1993.
- 28.Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423–1433. [PMC free article] [PubMed] [Google Scholar]
- 29.Little P, Everitt H, Williamson I, et al. Preferences of patients for patient centred approach to consultation in primary care: observational study. BMJ. 2001;322(7284):468–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenfield S, Kaplan SH, Ware JE Jr, Yano EM, Frank HJ. Patients’ participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med. 1988;3(5):448–457. [DOI] [PubMed] [Google Scholar]
- 31.Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 1998.
- 32.Last JM. A Dictionary of Epidemiology. New York, NY: Oxford University Press; 1995.
- 33.Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–834. [DOI] [PubMed] [Google Scholar]
- 34.Vermeire E, Wens J, Van Royen P, Biot Y, Hearnshaw H, Linden-meyer A. Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(2):CD003638. [DOI] [PMC free article] [PubMed]
- 35.Parchman ML, Romero RL, Pugh JA. Encounters by patients with type 2 diabetes—complex and demanding: an observational study. Ann Fam Med. 2006;4(1):40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerr EA, Gerzoff RB, Krein SL, et al. Diabetes care quality in the Veterans Affairs Health Care System and commercial managed care: the TRIAD study. Ann Intern Med. 2004;141(4):272–281. [DOI] [PubMed] [Google Scholar]
- 37.Heisler M. Actively engaging patients in treatment decision making and monitoring as a strategy to improve hypertension outcomes in diabetes mellitus. Circulation. 2008;117(11):1355–1357. [DOI] [PubMed] [Google Scholar]
- 38.Parchman ML, Pugh JA, Romero RL, Bowers KW. Competing demands or clinical inertia: the case of elevated glycosylated hemoglobin. Ann Fam Med. 2007;5(3):196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rittenhouse DR, Shortell SM. The patient-centered medical home: will it stand the test of health reform? JAMA. 2009;301(19): 2038–2040. [DOI] [PubMed] [Google Scholar]
- 40.Stange KC. Transformation to the patient-centered medical home. Ann Fam Med. 2009;7(3):370–373. [DOI] [PMC free article] [PubMed] [Google Scholar]