Abstract
The Cry1Ia12 entomotoxin from a Brazilian Bacillus thuringiensis strain is currently being expressed in cotton cultivars to confer resistance to insect-pests. The present study aimed to assess the effects of a diet containing Cry1Ia12 protein on growing rats. A test diet containing egg white and Cry1Ia12 (0.1% of total protein) as a protein source was offered to rats for ten days. In addition, an acute toxicity bioassay was performed in rats with a single oral dose of the entomotoxin (12 mg/animal). No adverse effects were observed in the animals receiving the test diet when compared to those receiving a control diet (egg white). The analysed parameters included relative dry weight of internal organs, duodenum histology, blood biochemistry, and nutritional parameters. The results of the acute toxicity test showed no mortality or behaviour alteration. Thus, Cry1Ia12 toxin at the tested concentration does not cause deleterious effects on growing rats when incorporated in the diet for 10 days.
1. Introduction
Cotton, Gossypium hirsutum L., is grown worldwide primarily as a source of fibre for textile manufacturing and Brazil is one of the largest producers in the world. However, the production of this commodity is highly affected by large losses caused mainly by insect-pests, such as Anthonomus grandis (Coleoptera: Curculionidae) and Spodoptera frugiperda (Lepidoptera: Noctuidae) [1]. In general, the control of these insects requires heavy chemical pesticides, which are very expensive. In addition, the use of pesticides causes considerable risks to human health, the environment, nontarget organisms, and can promote the development of resistance in insects [2]. The most promising cost-effective and sustainable method for controlling the cotton boll weevil (A. grandis) is the development of genetically resistant plants [1].
Genetic engineering has been widely used as an important tool in cotton production to control target insect-pests. Among the cotton cultivars available on the market, those genetically modified to express insecticidal genes (Cry genes) from Bacillus thuringiensis (Bt) stand out among the most commercialised. However, these genes confer resistance to major lepidopteran insect-pests [3] but are inefficient regarding the cotton boll weevil. In this context, a toxin belonging to the Cry1 class (termed Cry1Ia12) exhibiting entomotoxic activity against A. grandis and S. frugiperda has recently been isolated from B. thuringiensis S811 and characterised [1].
Numerous data from toxicity studies have not shown any significant adverse effects of Cry proteins on mammals, including humans [4, 5]. Nevertheless, it is mandatory that a rigorous assessment be performed of each newly isolated Cry protein for the generation of GM cotton. To assess the potential risks of transgenic organisms, the International Food Biotechnology Council (IFBC) has reported the safety evaluation of GMOs. Similarly, groups like the Organisation for Economic Cooperation and Development (OECD), the Food and Agriculture Organisation of the United Nations (FAO), the World Health Organisation (WHO), and the International Life Science Institute (ILSI) have established safety assessment guidelines to assure that foods produced from biotechnology-derived products are as safe as those produced from conventionally bred crops [6]. The risk assessment of a biotechnology-derived crop is a comparative safety assessment using conventional food as reference, and such analyses are conducted in accordance with the concept of substantial equivalence [7]. The assessment process considers two main categories of potential risk: those related to the properties and functions of the introduced protein(s) and those resulting from insertion of the introduced gene(s) into the plant genome that might theoretically cause unintended (pleiotropic) effects [8].
The generation of a GM plant normally requires a significant investment of time, and consequently, it is very important to evaluate the properties of the transgenic protein with regard to toxicity, allergenicity, and antinutritional potential before its introduction into the target plant [9]. This procedure ensures that the protein inserted in the genetically modified plant is safe for human health. It also aims to answer whether possible nutritional or pathological alterations are insignificant and whether they are related to the Cry toxin or to the changes in the genome of the transgenic cotton [5]. Thus, the present work aimed to assess the effects of a Cry1Ia12-containing diet (100 mg/Kg or 0.1% total protein) on growing rats, as well as to analyse the relative wet and dry weight of internal organs, duodenum histology, and blood biochemistry. In addition, an acute toxicity bioassay was performed in rats with a single oral dose of the entomotoxin to assess the mortality rate and behaviour alteration of the animals.
2. Materials and Methods
2.1. Construction of E. Coli Expression Vector pET101-Cry1Ia12
The E. coli expression vector pET101-Cry1Ia12 was constructed as described previously [1]. The Cry1Ia12 gene was selected based on its insecticidal specificity and activity against the cotton insect-pests A. grandis and S. frugiperda. This gene is currently being introduced into cotton cultivars with the goal of generating resistance against insect attack.
2.2. Recombinant Cry1Ia12 Toxin Expression and Purification
E. coli BL21 Star (DE3) cells transformed with pET101-Cry1Ia12 were grown at 37°C in 500 mL of Luria-Bertani broth at 200 rpm agitation with 200 μg/mL ampicillin until O.D.600 = 0.6–0.8. The expression of Cry1Ia12 was induced by addition of 1 mM isopropyl-beta-D-thiogalactopyranoside (IPTG) when the culture reached O.D.600 = 0.7. Sixteen hours after induction, the cells were harvested by centrifugation at 4,000 × g. The pellet containing the His-tagged Cry1Ia12 protein was then resuspended in the lysis buffer (50 mM sodium phosphate buffer, 300 mM NaCl, and 0.5% Triton X-100, pH 7.0). The crude extract was sonicated three times for 5 min (large tip, Virsonic Cell Disrupter—Model 16-850), centrifuged at 10,000 × g for 20 min at 4°C, and the supernatant was analysed by 12% SDS-PAGE [10]. The supernatant was also used for the purification of recombinant Cry1Ia12 using Ni2+ nitrilotriacetic acid affinity resin (Ni-NTA, QIAGEN) treated with equilibration buffer (50 mM sodium phosphate buffer, 300 mM NaCl, 10 mM imidazole pH 7.0). The supernatant containing E. coli expressing recombinant His-tagged Cry1Ia12 protein was incubated for 30 min within the equilibrated column. The column was then first washed with 100 mL of equilibration buffer and subsequently with 100 mL of the same buffer containing 20 mM imidazole. The recombinant His-tagged Cry1Ia12 protein was eluted with 6 mL of equilibration buffer containing 250 mM imidazole. All the steps were run at a flow rate of 2 mL/min. The eluted protein was dialysed against water at 4°C. The purified protein was quantified according to Lowry's method [11].
2.3. Animals
Eighteen male Wistar rats were obtained from the Animal Facilities of the Universidade Federal do Ceará (UFC). The rat weights were in the range of 55–60 g at the beginning of the treatments, which followed the procedures approved by the Commission of Ethics in Animal Research of the Universidade Federal do Ceará—CEPA/UFC.
2.4. Diets
For this assay, three diets were prepared: (1) a control diet containing egg white (EW) as reference protein due to its high biological value [12]; (2) a test diet containing Cry1Ia12 protein (99.9 g of egg-white protein and 0.1 g of Cry1Ia12); (3) a nonprotein diet (NPC). Diets 1 and 2 were prepared to contain the equivalent of 100-g protein/kg diet. The Cry1Ia12 protein comprised 0.1% of total protein. The NPC diet was fed to allow the determination of various nutritional parameters (Table 1).
Table 1.
Composition (g/kg) of the NPC, EW, and experimental diets*.
| Ingredients | Diets | ||
|---|---|---|---|
| NPC | EW | Cry1Ia12 | |
| Maize starch | 475 | 342.55 | 342.55 |
| Glucose | 150 | 150 | 150 |
| Maize oil | 150 | 150 | 150 |
| Vitamin mix** | 50 | 50 | 50 |
| Mineral mix*** | 50 | 50 | 50 |
| Neofibre*** | 125 | 125 | 125 |
| Egg White (75.5%) | — | 132.45 | 132.35 |
| Cry1Ia12 | — | — | 0.1 |
*Cry1Ia12: Cry1Ia12 protein; NPC: nonprotein control; EW: egg-white protein.
**Vitamin mix (g/kg): vitamin B12 (100%), 0.02; folic acid, 0.04; biotin (1%), 4.0; pyridoxine HCl, 0.04; thiamine HCl, 0.06; riboflavin (99%), 0.21; Ca-pantothenate (45%), 1.2; nicotinic acid, 4.0; inositol, 4.0; p-amino-benzoic acid, 12.0; choline chloride (50%), 24.0; maize starch, 950.43.
***Mineral mix (g/kg): calcium citrate, 296.1; calcium carbonate (40%), 65.8; copper carbonate, 1.1; magnesium carbonate, 34.3; zinc carbonate, 0.48; ferric citrate, 9.1; magnesium chloride · 6H2O, 5.82; sodium chloride, 74.0; potassium chloride, 119.5; monobasic calcium phosphate, 108.2; dibasic potassium phosphate, 210.1; sodium fluoride, 0.48; potassium iodate, 0.1; magnesium sulphate, 75.4.
****Neofibre (Nuteral, 80% fibre, Fortaleza, Brazil).
2.5. Animal Feeding Trial
Wistar male rats were weaned at 21 days of age and given a commercial stock diet until their weights reached 55–60 g. They were then fed the control diet (egg white as the sole source of protein) ad libitum for three days as a period of adaptation to pulverised diet. Then, the animals were selected according to body weight. It was allowed for weight variation of up to 10 g within the group and up to 5 g among groups.
The animals were divided into three groups of six rats each, individually housed in screen-bottomed cages and fed the control (egg-white), nonprotein containing (NPC), or test diet (0.1% of Cry1Ia12 toxin) for ten days. Food and water were supplied ad libitum. Rats total weight, diet spillage, and refused diet were recorded daily. Faeces were collected during the last five days of the experimental period, bulked, freeze-dried, weighed, and ground in a coffee grinder. At the end of the trial, the rats were euthanised by cervical dislocation and the internal organs were dissected. The organs were, then, freeze-dried whereas the carcasses were dehydrated in an oven at 100°C for 24 h. Dry weights were recorded before incorporating the organs into their original carcasses, which were then ground and kept in a desiccator until subsequent analyses.
2.6. Chemical Analyses
Diets, carcasses, and ground fecal samples were analysed for moisture and total nitrogen content [13]. The data were used to calculate apparent protein digestibility, net protein utilization (NPU), and biological value, according to Miller and Bender [14]. All the results were calculated for each rat and the mean was calculated within each group.
2.7. Blood Biochemistry
Blood samples were collected and serum was separated from cells very rapidly. Blood biochemistry determinations were performed manually with a spectrophotometer (Thermo Spectronic, model Genesys 10, Rochester, USA). Parameters were total protein (biuret method), albumin (bromocresol green method), alkaline phosphatase (modified Roy's method), blood urea nitrogen (colorimetric enzymatic method), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) (Reitman and Frankel methods). All methodologies are well described in the manufacturer instructions (Bioclin kits, QUIBASA Basic Chemical Ltda, Belo Horizonte, Brazil).
2.8. Histopathological Analyses
The stomach, intestine, caecum and colon, liver, pancreas, kidneys, heart, thymus, lungs, and spleen were dissected and weighed immediately. The relative organ dry weights (ratios of organ weight to final body weight) were calculated. Samples of the small intestine (duodenum) were collected and immediately fixed in 10% formaldehyde for 24 h. Following a routine procedure, the fixed tissues were embedded in paraffin and 5 μm thick tissue sections were stained with haematoxylin and eosin (H&E) and analysed by light microscopy.
2.9. Statistical Analyses
The data were subjected to a one-way analysis of variance (ANOVA) and the significance among means was determined by Tukey's test.
3. Results
3.1. Recombinant Cry1Ia12: E. Coli Expression and Purification
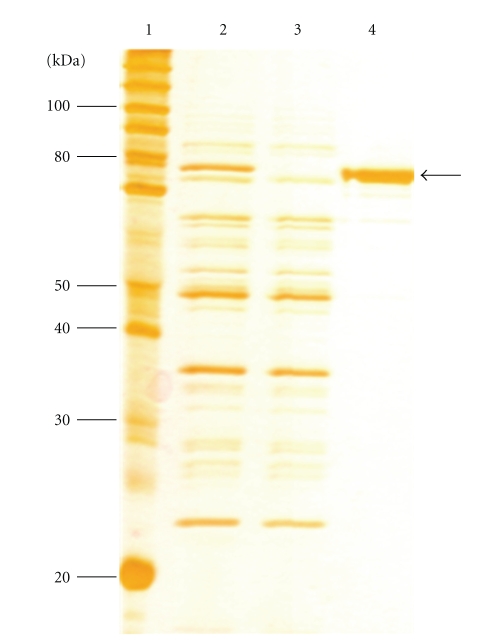
E. coli strain BL21 Star (DE3) harbouring the pET101-Cry1Ia12 construct was induced with 1 mM IPTG to express recombinant His-tagged Cry1Ia12 protein. SDS-PAGE analysis of E. coli extracts showed a differential band corresponding to the expected 74-kDa recombinant His-tagged Cry1Ia12 protein (Figure 1). No additional bands were identified in the noninduced cells with empty pET101 vector. Around 2 mg/L of His-tagged protein was purified using Ni2+-NTA affinity chromatography from sonicated E. coli crude extract. Using these conditions, the Cry1Ia12 protein was produced in large scale (150 mg) to be used in the biological assays.
Figure 1.
SDS-PAGE (12%) profile showing the expressed and purified recombinant Cry1Ia12 protein. Lane 1: Marker BenchMark Protein Ladder (INVITROGEN). Lane 2: E. coli crude extract induced with 1 mM IPTG after 16 h expressing recombinant Cry1Ia12 protein, sonicated and centrifuged. Arrows indicate the expressed 74-kDa recombinant Cry1Ia12. Lane 3: Noninduced E. coli strain BL21Star (DE) containing the expression vector without insert was used as a negative control of the expression. Lane 4: Recombinant Cry1Ia12 protein purified by using Ni-NTA column. Arrow shows the expected purified 74-kDa recombinant Cry1Ia12 protein.
3.2. Nutritional Parameters
Cry1Ia12 toxin was included in the diet of growing Wistar rats representing 0.1% of the total protein (Table 1). Body weight gain and diet intake were measured daily and the following nutritional parameters: NPU, protein digestibility, and biological value, were calculated (Table 2). The total weight gain of rats fed Cry1Ia12 toxin was not significantly different from that of rats fed the control diet (egg white as the sole source of protein). On the other hand, total food intake for both diets was 50% higher than that of rats fed the NPC diet. The low growth rate observed in rats fed the NPC diet may be related to food intake since these rats consumed much less food than those on the control diet. However, the rats fed the Cry1Ia12 toxin diet did not exhibit a significant difference when compared to rats fed the control diet (P > .05) (Table 2). No significant difference was observed in NPU, in vivo protein digestibility, or protein biological value (P > .5) in rats fed the Cry1Ia12 toxin diet when compared to those on the egg white diet. Similarly, no difference in N balance was detected between these groups (Table 3).
Table 2.
Nutritional parameters of rats fed the Cry1Ia12 toxin diet compared to those of rats fed the egg white (EW) and nonprotein (NPC) diets.
| Diet* | |||
|---|---|---|---|
| NPC | EW | Cry1Ia12 | |
| Initial body weightδ (g) | 71.96 ± 3.95b | 70.89 ± 5.72a | 70.98 ± 5.83a |
| Final body weightδ (g) | 56.7 ± 1.67b | 110.10 ± 7.66a | 101.31 ± 6.42a |
| Daily diet intakeδ (g) | 5.11 ± 1.00b | 11.8 ± 1.17a | 11.29 ± 0.84a |
| Net Protein Utilization (NPU)¶ % | — | 91.05 ± 3.20a | 90.76 ± 4.81a |
| Protein Digestibilityd % | — | 91.27 ± 9.71a | 99.98 ± 9.94a |
| Biological Valued % | — | 90.67 ± 9.41a | 99.99 ± 9.77a |
Values are means ± standard deviation (n = 6). Values in a row with different letters differ significantly (P < .05).
*For key to diets see Section 2.
δPer rat.
¶Per group of 6 rats.
Table 3.
Relative faecal dry matter (g) and nitrogen output (g) of rats fed the egg white control (EW) and test diets (Cry1Ia12) calculated for test days 6 to 10.
| Parameters¶ | Diets | |
|---|---|---|
| EW | Cry1Ia12 | |
| Diet intake (D) | 70.64 ± 7.53a | 62.32 ± 6.47a |
| N intake (N) | 1.12 ± 0.09a | 0.99 ± 0.17a |
| Faecal output (F) | 3.98 ± 0.67a | 3.17 ± 0.69a |
| Faecal N (FN) | 0.16 ± 0.04a | 0.14 ± 0.05a |
| F/D × 100 | 5.27 ± 1.01a | 5.58 ± 0.44a |
| FN/N × 100 | 13.22 ± 3.27a | 15.05 ± 4.12a |
Values are means ± standard deviation (n = 6).
aValues in a row with different letters differ significantly (P < .05).
¶g per rat.
3.3. Blood Biochemistry Analysis
The results of blood biochemical parameters analysis (Table 4) revealed no significant difference between values (total protein, albumin, alkaline phosphatase, blood urea nitrogen, alanine aminotransferase, and aspartate aminotransferase) for rats fed the Cry1Ia12 toxin diet and those of rats fed the egg white control diet.
Table 4.
Blood biochemical parameters of rats fed the nonprotein (NPC), egg white (EW), and test (Cry1Ia12) diets.
| Parameter | Diet | ||
|---|---|---|---|
| NPC | EW | Cry1Ia12 | |
| Protein (g/dL) | 6.18 ± 0.26b | 8.10 ± 0.66a | 7.98 ± 0.36a |
| Albumin (g/dL) | 4.24 ± 0.22b | 5.40 ± 0.16a | 5.60 ± 0.22a |
| Urea blood nitrogen (mg/dL) | 15.90 ± 0.70b | 4.80 ± 0.50a | 5.20 ± 1.00a |
| Alkaline phosphatase (U/mL) | 107.57 ± 10.98b | 139.00 ± 11.20a | 133.06 ± 12.89a |
| ALT (U/mL) | 16.70 ± 1.60a | 15.67 ± 0.60a | 15.03 ± 1.00a |
| AST (U/mL) | 18.96 ± 1.90a | 17.30 ± 1.20a | 16.95 ± 1.32a |
Values are means ± standard deviation (n = 6). Values in a row with different letters differ significantly (P < .05).
3.4. Organ Weights and Histopathology
The absolute and relative organ wet and dry weights (Table 5) were not significantly different between the rats fed the Cry1Ia12 diet and those on the control diet. On the other hand, the small intestine, heart, kidneys, and lungs of rats fed the NPC diet were significantly larger than those of standard control diet. Furthermore, the NPC diet induced atrophy of the thymus when compared to the control diet. Details regarding organ wet and dry weights are summarised in Table 5.
Table 5.
Relative organ wet and dry weights (g/100 g wet or dry body weight) of rats fed the nonprotein (NPC), egg white (EW), and test (Cry1Ia12) diets.
| Organ | Diet | |||
|---|---|---|---|---|
| NPC | EW | Cry1Ia12 | ||
| Stomach | wet | 1.28 ± 0.19a | 0.99 ± 0.07a | 0.98 ± 0.07a |
| dry | 0.75 ± 0.05a | 0.64 ± 0.10a | 0.57 ± 0.04a | |
| Intestine | wet | 5.84 ± 0.51b | 4.06 ± 0.44a | 4.49 ± 0.51a |
| dry | 2.96 ± 0.19a | 2.32 ± 0.30b | 2.36 ± 0.27b | |
| Caecum and colon | wet | 2.14 ± 0.33a | 1.97 ± 0.36a | 1.98 ± 0.18a |
| dry | 1.12 ± 0.20a | 1.04 ± 0.10a | 1.04 ± 0.08a | |
| Liver | wet | 5.13 ± 0.66a | 5.43 ± 0.58a | 4.95 ± 0.40a |
| dry | 4.70 ± 0.40a | 4.49 ± 0.40a | 4.41 ± 0.40a | |
| Pancreas | wet | 0.29 ± 0.07a | 0.42 ± 0.12a | 0.36 ± 0.07a |
| dry | 0.30 ± 0.05a | 0.35 ± 0.06a | 0.33 ± 0.08a | |
| Heart | wet | 0.55 ± 0.04a | 0.50 ± 0.05a | 0.52 ± 0.03a |
| dry | 0.39 ± 0.03a | 0.31 ± 0.04b | 0.34 ± 0.02b | |
| Kidneys | wet | 1.26 ± 0.08a | 1.02 ± 0.07b | 1.09 ± 0.16ab |
| dry | 0.95 ± 0.08a | 0.69 ± 0.07b | 0.76 ± 0.11b | |
| Lungs | wet | 0.86 ± 0.10a | 0.90 ± 0.22a | 0.89 ± 0.16a |
| dry | 0.58 ± 0.06a | 0.47 ± 0.05b | 0.50 ± 0.07ab | |
| Thymus | wet | 0.23 ± 0.08a | 0.42 ± 0.04b | 0.40 ± 0.06b |
| dry | 0.18 ± 0.05a | 0.28 ± 0.05b | 0.29 ± 0.04b | |
| Spleen | wet | 0.21 ± 0.02a | 0.36 ± 0.06b | 0.31 ± 0.02b |
| dry | 0.20 ± 0.02a | 0.23 ± 0.03a | 0.22 ± 0.02a |
Values in a row with different letters differ significantly (P < .05); n = 6.
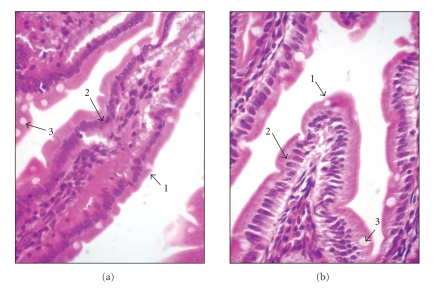
The histopathological analysis (Figure 2) revealed no alteration in duodenum of rats fed Cry1Ia12 toxin. The villi and microvilli were continuous and no alteration in the nuclei of enterocytes or increase in defence cell number was noted.
Figure 2.
Histological duodenum sections of rats fed standard (based on egg white) diet (a) and CryIa12 toxin-containing diet (b) (600x amplification). Arrow 1 shows that the edge formed by microvilli remains intact for all treatments. Arrow 2 shows the nucleus of enterocytes, normal in all treatments. Arrow 3 shows the goblet cells with normal size for all treatments. The tissues were stained with hematoxylin-eosin.
4. Discussion
The Cry toxins of Bacillus thuringiensis have been the object of many recent studies to assess their safety to nontarget organisms [15]. In this context, it is important the carrying out of laborious animal feeding trials with diets containing a high percentage of recombinant entomotoxin or diets containing transgenic seeds meal [16]. In the present paper, Cry1Ia12 toxin was included in a high biological value diet at 100 mg/Kg diet, accounting for 0.1% total protein. The rationale for this concentration is that the expression level in a genetically modified plant varies from 0.01% to 0.1% of total protein content [17]. The proportion of recombinant protein used in this study was greater than that described for the test diets with transgenic rice Cry1Ab toxin, which was approximately 10 mg/Kg diet [5], and greater than that used in the test diets for transgenic tomato [18], which consisted of 10% lyophilised transgenic tomato extract (40 ng Cry1Ab/mg total protein), and well above that in the test diets for cotton seed bran, which had 0.28 mg Cry1F/Kg diet and 0.096 mg Cry1Ac/Kg diet [19]. Presumably, the higher content of Cry1Ia12 used in the present study allows a safer assessment considering the short duration of the experiment which investigated the effects of this toxin on the nutritional performance and other health aspects of growing rats.
The protein Cry1Ia12 shows high identity (99%) and high similarity (99%) with other Cry1Ia toxins deposited in databases. There are substitutions only at positions 476 (a for g) and 1719 (c for t) resulting in a single substitution of Arg for Lys159 in domain I (localised at the second alpha-helix). This shows that the sequences of Cry1Ia type proteins do not differ much from one another [1]. This similarity among Cry1Ia toxins is consistent with the lack of deleterious effects of the entomotoxin evaluated in this study since several of Cry1 toxins have been studied with regard to food safety in mammals [5, 19].
The results of the feeding trials with rats fed a diet containing 100 mg Cry1Ia12/Kg diet showed that the growth rate of the rats was similar to those fed the control diet (egg white as the sole source of protein). This might be explained by the fact that there was no difference in body weight gain, food intake, or alimentary conversion among the different treatments involving test and control diets. In fact, these results are comparable to those described by Schrøder et al. [5] who reported similar growth rates between rats fed transgenic rice expressing the Cry1Ab toxin and rats fed conventional rice. Noteborn et al. [18] and Dryzga et al. [19] have also reported the same trend in rats receiving the toxin Cry1Ab and Cry1Ac, respectively, for at least 90 days.
The lack of deleterious effects of Cry entomotoxins in mammals has been explained by stomach acidification and by the absence of specific binding sites for Cry toxins in the intestine [4]. In this sense, the rapid degradation of these proteins in gastric fluid (about 90% in 2 min) has been verified. Although Cry protein degradation is slower in the intestine, this does not have much importance for the organism since after ingestion these proteins are hydrolysed almost completely by pepsin in the stomach [20, 21]. Thus, these proteins would have a much more significant role in supplying amino acids than in inducing other effects. Another parameter considered in the present paper was nitrogen balance (Table 4). The ratio of dietary intake and faecal excretion and the relative values of nitrogen excretion of rats fed the test diet were similar to those fed the control diet. Therefore, Cry1Ia12 at the concentration of 100 mg/Kg diet was not able to interfere with egg white protein utilization. There was good nitrogen retention, which can be explained by the excellent amino acid profile, the high protein digestibility and especially the lack of antinutritional and/or toxic effects of the Cry toxin included in the diet.
In order to substantiate the lack of deleterious effects of Cry protein upon dietary protein absorption, other nutritional parameters, including digestibility, NPU, and biological value were investigated (Table 2). All these parameters for the group on the diet containing Cry1Ia12 toxin were similar to those of rats on the control diet. Almost all the parameters were greater than or very close to 90%, confirming the lack of toxic/antinutritional effects of the Cry toxin, at least for rats. Poor digestibility is one of the main factors that compromise the net efficiency of a protein, and consequently its biological value. When some peptide bonds are not hydrolysed, part of the protein is excreted in faeces or transformed into metabolites by large intestine microorganisms [22]. Thus, the rationale for the assessment of this parameter was the concern that undigested proteins might bind to intestinal mucosa, causing changes related to those shown by invertebrates susceptible to Cry proteins, such as inactivation of the system that maintains the pH gradient [23] and osmotic cytolysis [24]. This binding could also result in the internalisation of the protein and other subsequent systemic effects, such as changes in internal organs reported for several lectins [22]. No difference was detected in the relative wet or dry weight of the internal organs of rats on the Cry1Ia12 diet when compared to those on the control diet (Table 5). This was also reported for rats fed on transgenic cotton expressing the entomotoxic proteins Cry1F and Cry1Ac [19].
In contrast to what has been reported for larvae of susceptible insects, rats on the diet containing Cry1Ia12 did not show any changes in intestinal epithelium which would be indicative of injury to intestinal mucosa [25]. As a matter of fact, it has been reported that Cry toxins do not interact with any region of the digestive tract [18].
As for the blood biochemistry analysis, which could have indicated signs of toxicity, no significant change was detected between control rats and those fed the Cry1Ia12 diet. Only those rats on the nonprotein containing diet showed changes in blood parameters, which is related to protein malnutrition [12].
Cry1Ia12 toxin was also administered as a single oral dose (12 mg) to rats, but no animal died or showed any apparent change in behaviour. The amount of Cry1Ia12 delivered was greater than that of other Cry proteins expressed in genetically modified plants. For instance, the highest levels of Cry1Ab protein in the transgenic corn MON 810 were detected in leaves, from 4.91 to 12.5 mg/kg fresh weight. In the whole plant, the values have been reported to be in the range of 3.34 to 4.88 mg/Kg, and in grains, from 0.31 to 0.57 mg/Kg fresh weight [26]. These figures are much lower when related to total protein: 10.34 ng/mg total protein in leaves, 4.65 ng/mg total protein in the whole plant, and from 0.19 to 0.39 ng/mg total protein in grains. On the other hand, the Cry1Ac protein, present in thirteen commercial varieties of cotton, has shown an average level of 1.5 mg/Kg protein [27]. Therefore, the data of the present paper seem to point to the safety of Cry1Ia12 protein when administered per os to rats, corroborating several other studies that have shown the lack of toxicity or any other antinutritional effects of Cry proteins in mammals [28].
5. Conclusion
In summary, Cry1Ia12 entomotoxic protein of B. thuringiensis when included in diets for growing rats at 100 mg/kg diet for ten days did not interfere with their nutritional performance. When the toxin was administered as a single oral dose of 12 mg per animal, the rats did not show any sign of toxicity. The results of the present study indicate the biotechnological potential of Cry1Ia12 protein and may contribute with relevant information that makes it an effective and safe tool for the control of important pests of cotton crops. Nevertheless, further studies on the structural homology of the toxin with other toxic or allergenic proteins and on the potential toxicity of higher doses of this entomotoxin must be conducted to ensure the safety of its use.
Acknowledgment
This work was supported by grants from the Brazilian government EMBRAPA, CNPq, and CAPES.
References
- 1.Grossi-de-Sa MF, De Magalhães MQ, Silva MS, et al. Susceptibility of Anthonomus grandis (cotton boll weevil) and Spodoptera frugiperda (fall armyworm) to a Cry1Ia-type toxin from a Brazilian Bacillus thuringiensis strain. Journal of Biochemistry and Molecular Biology. 2007;40(5):773–782. doi: 10.5483/bmbrep.2007.40.5.773. [DOI] [PubMed] [Google Scholar]
- 2.Hossain F, Pray CE, Lu Y, Huang J, Fan C, Hu R. Genetically modified cotton and farmers’ health in China. International Journal of Occupational and Environmental Health. 2004;10(3):296–303. doi: 10.1179/oeh.2004.10.3.296. [DOI] [PubMed] [Google Scholar]
- 3.Shelton AM, Zhao J-Z, Roush RT. Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annual Review of Entomology. 2002;47:845–881. doi: 10.1146/annurev.ento.47.091201.145309. [DOI] [PubMed] [Google Scholar]
- 4.McClintock JT, Schaffer CR, Sjoblad RD. A comparative review of the mammalian toxicity of Bacillus thuringiensis-based pesticides. Pesticide Science. 1995;45(2):95–105. [Google Scholar]
- 5.Schrøder M, Poulsen M, Wilcks A, et al. A 90-day safety study of genetically modified rice expressing Cry1Ab protein (Bacillus thuringiensis toxin) in Wistar rats. Food and Chemical Toxicology. 2007;45(3):339–349. doi: 10.1016/j.fct.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 6. Bt cotton India. A Status Report, http://www.parc.gov.pk/bt_cotton.pdf.
- 7.Peng D, Chen S, Ruan L, Li L, Yu Z, Sun M. Safety assessment of transgenic Bacillus thuringiensis with VIP insecticidal protein gene by feeding studies. Food and Chemical Toxicology. 2007;45(7):1179–1185. doi: 10.1016/j.fct.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Hammond BG, Dudek R, Lemen JK, Nemeth MA. Results of a 90-day safety assurance study with rats fed grain from corn borer-protected corn. Food and Chemical Toxicology. 2006;44(7):1092–1099. doi: 10.1016/j.fct.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Delaney B, Astwood JD, Cunny H, et al. Evaluation of protein safety in the context of agricultural biotechnology. Food and Chemical Toxicology. 2008;46(2):S71–S97. doi: 10.1016/j.fct.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 12.Vasconcelos IM, Maia AAB, Siebra EA, et al. Nutritional study of two Brazilian soybean (Glycine max) cultivars differing in the contents of antinutritional and toxic proteins. Journal of Nutritional Biochemistry. 2001;12(1):55–62. doi: 10.1016/s0955-2863(00)00148-0. [DOI] [PubMed] [Google Scholar]
- 13.Baethgen WE, Alley MM. A manual colorimetric procedure for measuring ammonium nitrogen in soil and plant kjeldahl digests. Communications in Soil Science and Plant Analysis. 1989;20:961–969. [Google Scholar]
- 14.Miller DS, Bender AC. The determination of net utilisation of protein by shostened method. British Journal of Nutrition. 1955;9:382–383. doi: 10.1079/bjn19550055. [DOI] [PubMed] [Google Scholar]
- 15.Constable A, Jonas D, Cockburn A, et al. History of safe use as applied to the safety assessment of novel foods and foods derived from genetically modified organisms. Food and Chemical Toxicology. 2007;45(12):2513–2525. doi: 10.1016/j.fct.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 16.EFSA GMO Panel Working Group on Animal Feeding Trials. Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials. Food and Chemical Toxicology. 2008;46:S2–S70. doi: 10.1016/j.fct.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Kiliç A, Akay MT. A three generation study with genetically modified Bt corn in rats: biochemical and histopathological investigation. Food and Chemical Toxicology. 2008;46(3):1164–1170. doi: 10.1016/j.fct.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Noteborn H, BienenmannPloum ME, VandenBerg JHJ. Safety assessment of the Bacillus thuringiensis insecticidal crystal protein CRYIA(b) expressed in transgenic tomatoes. In: Engel KH, Takeoka GR, Teranishi R, editors. Genetically Modified Foods: Safety Issues. Washington, DC, USA: ACS; 1995. pp. 134–147. (ACS Symposium Series). [Google Scholar]
- 19.Dryzga MD, Yano BL, Andrus AK, Mattsson JL. Evaluation of the safety and nutritional equivalence of a genetically modified cottonseed meal in a 90-day dietary toxicity study in rats. Food and Chemical Toxicology. 2007;45(10):1994–2004. doi: 10.1016/j.fct.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 20.English L, Slatin SL. Mode of action of delta-endotoxins from Bacillus thuringiensis: a comparison with other bacterial toxins. Insect Biochemistry and Molecular Biology. 1992;22(1):1–7. [Google Scholar]
- 21.Gill SS, Cowles EA, Pietrantonio PV. The mode of action of Bacillus thuringiensis endotoxins. Annual Review of Entomology. 1992;37(1):615–636. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- 22.Vasconcelos IM, Oliveira JTA. Antinutritional properties of plant lectins. Toxicon. 2004;44(4):385–403. doi: 10.1016/j.toxicon.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Wolfersberger MG. V-ATPase-energized epithelia and biological insect control. Journal of Experimental Biology. 1992;172:377–386. doi: 10.1242/jeb.172.1.377. [DOI] [PubMed] [Google Scholar]
- 24.Knowles BH, Ellar DJ. Colloid-osmotic lysis is a general feature of the mechanism of action of Bacillus thuringiensisδ-endotoxins with different insect specificity. Biochimica et Biophysica Acta. 1987;924(3):509–518. [Google Scholar]
- 25.Garcia-Robles I, Sánchez J, Gruppe A, et al. Mode of action of Bacillus thuringiensis PS86Q3 strain in hymenopteran forest pests. Insect Biochemistry and Molecular Biology. 2001;31(9):849–856. doi: 10.1016/s0965-1748(01)00030-3. [DOI] [PubMed] [Google Scholar]
- 26.Sanders PR, Lee TC, Groth ME, Astwood JD, Fuchs RL. Safety assessment of insect-protected corn. In: Thomas JA, editor. Biotechnology and Safety Assessment. London, UK: Taylor and Francis; 1998. pp. 241–256. [Google Scholar]
- 27.Adamczyk JJ, Jr., Sumerford DV. Potential factors impacting season-long expression of Cry1Ac in 13 commercial varieties of Bollgard® cotton. Journal of Insect Science. 2001;6:1–13. [PMC free article] [PubMed] [Google Scholar]
- 28.Doull J, Gaylor D, Greim HA, Lovell DP, Lynch B, Munro IC. Report of an expert panel on the reanalysis by Séralini et al. (2007) of a 90-day study conducted by Monsanto in support of the safety of a genetically modified corn variety (MON 863) Food and Chemical Toxicology. 2007;45(11):2073–2085. doi: 10.1016/j.fct.2007.08.033. [DOI] [PubMed] [Google Scholar]