Abstract
Importance of the field
HIV/TB coinfection is common and associated with high mortality. Simultaneous highly active antiretroviral therapy (HAART) during TB treatment is associated with substantial survival benefit but drug-drug interactions complicate NNRTI dosing.
Areas covered in this review
We reviewed the impact of rifampicin-containing TB therapy on the NNRTIs pharmacokinetics and clinical outcome. Pub Med database was searched from 1966 to July 2009, using the terms efavirenz, rifampicin, nevirapine, pharmacokinetics, pharmacogenetics, HIV, TB, CYP2B6, CYP3A4 and metabolism. References from identified articles and abstracts from meetings were also reviewed.
What the reader will gain
A comprehensive review of the literature on this subject including pharmacokinetic and clinical studies. Most studies were small, observational or underpowered to detect the true effect of rifampicin on NNRTI-based therapy. None of the studies controlled for genetic factors and there was limited data on children.
Take home message
There was insufficient data to make definitive recommendations about dose adjustment of the NNRTIs during rifampin-containing therapy. Current data suggest that standard dose of efavirenz or nevirapine is adequate in most HIV/TB co-infected adults. However, more research is needed in pediatric populations as well as to define role of drug-gene interactions.
Keywords: Drug interactions, Efavirenz, HIV, Nevirapine, Rifampicin, Tuberculosis
1. Introduction
Tuberculosis (TB) remains a major cause of morbidity and mortality in persons with human immunodeficiency virus (HIV) infection. Of the estimated 9.27 million incident TB cases in 2007, an estimated 1.37 million (15%) were HIV-infected.1 During the period, 456,000 deaths occurred among HIV-infected with TB, which represented 33% of the HIV-positive cases of TB and 23% of the estimated 1.8 million TB deaths in 2007.1 Several observational studies have found that simultaneous highly active antiretroviral therapy (HAART) during TB treatment is associated with substantially reduction in mortality.2–4 However, challenges to simultaneous therapy including high pill burden, overlapping drug toxicities, drug-drug interactions, and concerns about immune reconstitution inflammatory syndrome have often been cited as reasons to delay or defer HAART during TB treatment.5, 6
Antiretroviral therapy (ART) programmes have been rapidly scaled up in resource-limited countries and WHO estimates that 4 million persons were on treatment at the end of 2007 (UNAIDS). Tuberculosis is the most common associated infection and is often the entry point for a significant proportion of HIV-infected patients into care and treatment.7 While TB can occur at any stage of HIV disease, the incidence increases as immunodeficiency advances. Thus, a significant proportion of HIV-infected patients eligible for HAART also require concomitant TB requiring treatment.8 Although, early initiation of HAART is associated with reduced mortality in patients with concurrent HIV/TB coinfection,9 immune reconstitution due to HAART does not eliminate the higher risk of developing TB in HIV-infected individuals compared to the general population.10 Thus, it is evident that the treatment of HIV/TB co-infected patients is a major challenge facing many programs, especially those with a limited repertoire of antiretroviral drugs.
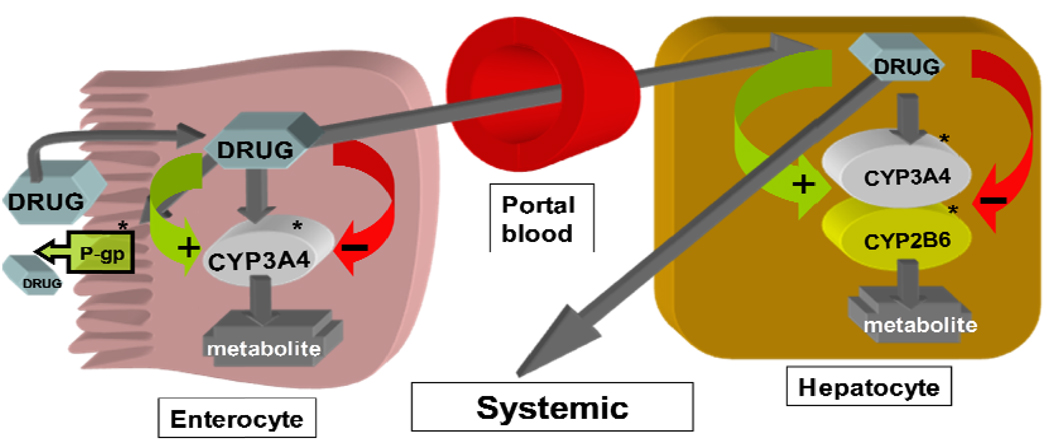
Rifampicin, a key component of short-course chemotherapy for TB, is a potent inducer of the cytochrome P450 (CYP) enzyme system that metabolizes several drugs including nonnucleoside reverse transcriptase inhibitor (NNRTI) antiretroviral agents. The interactions between rifampicin and the antiretroviral drugs can be complex and occur at different sites including the intestine and the liver (Figure 1). Prototypical inducers such as rifampicin increases the expression of CYP3A4 and CYP2B6 by interacting with the nuclear receptors, pregnane X receptor (PXR) or constitutive androstane receptor (CAR), which forms a heterodimer with the retinoid X receptor (RXR). The heterodimer then binds to the regulatory region of CYP3A4 or CYP2B6 resulting in up-regulation of enzyme synthesis and increased metabolic activity.11, 12 The nuclear receptors PXR and CAR have been linked to the function and activity of drug transporters in the liver, intestines and kidney and play a vital role in drug absorption, distribution and excretion.11, 12 The HIV protease inhibitors and the NNTRIs are themselves potent inhibitors and/or inducers of CYP enzymes, which may complicate prediction of the net effect of drug-drug interactions. The NNRTIs are recommended as components of initial combination HAART regimens in the public health approach recommended by WHO.13 Among the NNRTIs, nevirapine is widely used in resource-constrained countries because of its availability as generic fixed-drug combination tablets and lower cost. Unlike efavirenz, the preferred NNRTI in the setting of TB treatment, nevirapine is suitable for women of child bearing potential, because it is not a known teratogen. In many countries, it is the only available NNRTI.
Figure 1.
Disposition of cytochrome P450 substrates such the protease inhibitors (PIs) or the nonnucleoside reverse transcriptase inhibitors (NNRTIs) and potential influence of induction or inhibition of enzymes or transporters on systemic drug exposure. *Rifampin is a potent inducer and the NNRTIs and PIs are themselves inducers and/or inhibitors of CYP enzymes or P-glycoprotein transporter. Modulation of these systems may cause altered metabolism and drug concentrations when inducers and/or inhibitors are used concurrently with enzyme substrates.
There are currently more than 20 approved antiretroviral drugs in six different classes from which combination HAART regimens can be constructed. However, in sub-Saharan Africa or Asia where over 90% of the HIV/TB co-infected patients in the world live, treatment options are severely limited. The recommended first-line HAART regimen in resource-limited settings is either efavirenz or nevirapine with two nucleoside/nucleotide reverse transcriptase inhibitors, though in the setting of concurrent TB therapy, efavirenz is preferred.13 The implications of potential pharmacokinetic drug-drug interactions between rifampin and the NNRTIs, efavirenz and nevirapine are not well understood. Consequently, there is no consensus about the appropriate doses of efavirenz or nevirapine when used in the setting of rifampicin-containing TB treatment. Further, pharmacokinetic variability can be due to differences in drug absorption, distribution, metabolism, protein binding and drug-drug interactions. Increased hepatic clearance found in patients of Caucasian origin versus African, Asian or Hispanic patients, and reports of differences in treatment response and adverse effects among various ethnic groups suggest that genetic polymorphisms which alter the expression of drug membrane transporter proteins or metabolizing enzymes could influence drug pharmacokinetics.
We performed a literature search to find published articles that evaluated drug-drug interactions between rifampicin or rifampicin-containing TB treatment and the NNRTIs or NNRTI-based HAART. A Pub Med database was searched from 1966 to July 2009, using the terms efavirenz, rifampicin, nevirapine, pharmacokinetics, pharmacogenetics, HIV, TB, CYP2B6, CYP3A4 and metabolism. References from identified articles were also reviewed and abstract from recent meetings were included. Studies were included if they evaluated the influence of rifampicin-containing therapy on pharmacokinetics or clinical effects of efavirenz or nevirapine. Studies were also included if they evaluated the pharmacogenetics of efavirenz or nevirapine metabolism in the presence or absence of concurrent TB therapy. The primary objective of this review is to address the influence of drug-drug interactions as well as biological or genetic factors on the pharmacokinetics and treatment responses to efavirenz and nevirapine-based HAART in the setting of rifampicin-containing TB treatment. Our opinions on the factors that should be considered in deciding appropriate dose adjustment of the NNRTIs and the direction of future research are discussed.
2. Efavirenz
Efavirenz is, an NNRTI is a key component of one of the preferred regimens in the initial treatment of HIV infection. It is also available in generic form in developing countries, making efavirenz-based therapy a major option for HIV-infected person requiring HAART in resource poor settings. The standard dose is 600 mg daily and often taken at night to reduce the effects of central nervous system side effects. Efavirenz was approved the United States Food and Drug Administration (FDA) for HIV treatment in 1998 and in July 2006 a fixed-dose combination tablet containing efavirenz 600 mg, tenofovir 300 mg and emtricitabine 200 mg was approved by the FDA under the brand name Atripla®.
2.1 Metabolism
Efavirenz is metabolized primarily by hydroxylation through hepatic cytochrome P450 (CYP) 2B6 (CYP2B6), with minor contributions from CYP3A4/5 and CYP2A6 to form inactive metabolites 8-hydroxy efavirenz and 7-hydroxy efavirenz.14, 15 The main metabolite, 8-hydroxy efavirenz is further hydroxylated primarily by CYP2B6 to form 8,14-hydroxy efavirenz. The oxidative metabolites undergo conjugation by UDP-glucuronyltransferase (UGT) pathway and are excreted in the urine as glucuronides.15 Efavirenz also undergoes direct conjugation by UGT to form N-glucuronide, but until recently the enzyme isoform was unknown.15, 16 UGT2B7 has recently been implicated in the direct glucuronidation of efavirenz.17 The 8-hydroxylation pathway represents the major route of metabolism of efavirenz accounting for over 90% of efavirenz oxidation,15 and alternate pathways such as 7–hydroxylation and N-glucuronidation may be important in individuals with loss-of-function of CYP2B6.18
2.2 Influence of TB therapy on efavirenz pharmacokinetics
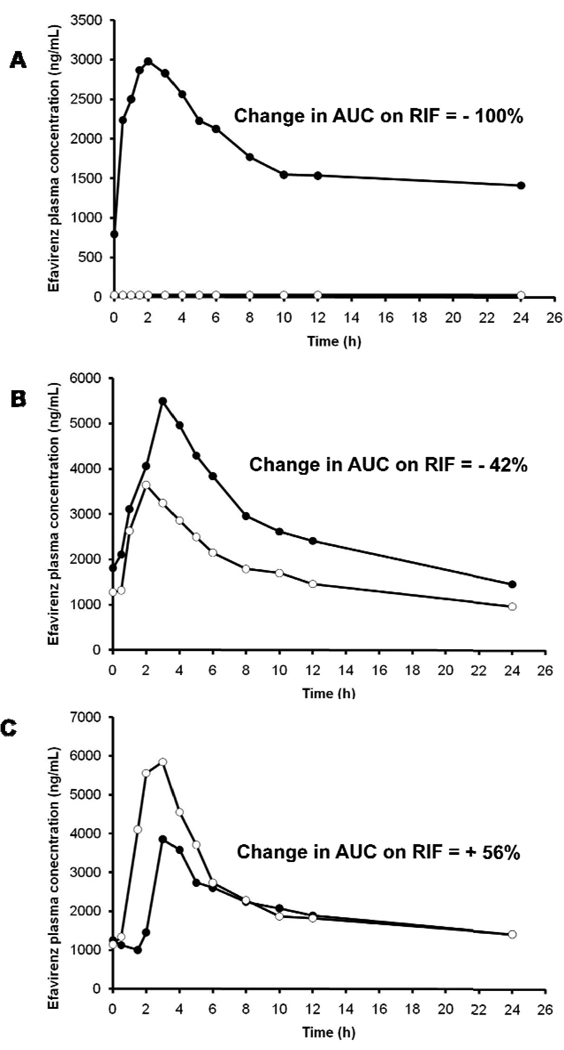
Rifampicin induces the function and activity of CYP2B6, the main metabolic enzyme for efavirenz. In primary human hepatocytes, the increase in CYP2B6 activity due to rifampicin varies widely from 2.5 fold to 13-fold.19–21 However, in vivo, co-administration of efavirenz with rifampicin led to only a modest (22 – 26%) reduction in efavirenz plasma exposure.22, 23 In one of these pharmacokinetic studies, the change in efavirenz exposure with concomitant rifampicin ranged from a decrease of 65% to an increase of 37%,23 suggesting inter-individual differences in the inducibility of the drug metabolizing enzymes. Another example of wide inter-individual variability in rifampin effect is illustrated in Figure 2, in which healthy volunteers treated with efavirenz in the presence or absence of rifampicin showed a change in efavirenz exposure that ranged from a decrease of 100% to an increase of 56% on compared to off rifampin (unpublished data). The variability in efavirenz concentrations was found to be greater in the presence of rifampicin than without rifampicin,24, 25, which is probably a manifestation of inherent differences in the inducibility of CYP2B6 variants. The possible inter-individual differences in enzyme induction on the clearance of efavirenz when co-administered with rifampicin further complicate decisions about dose adjustments of efavirenz in the setting of concurrent rifampin-containing TB therapy.
Figure 2.
Efavirenz concentration-time profile in three healthy volunteers in the absence (close circles) and presence of rifampin (open circles). The effect of rifampin co-administration varied from a reduction in efavirenz area under the curve by 100% (A) to an intermediate of a reduction by 42% (B) to an increase by 56% (C).
2.3 Clinical studies of concurrent efavirenz and rifampicin-containing therapy
The concern about the drug-drug interactions between rifampicin and efavirenz is that reduction in efavirenz concentrations due to induction of metabolism by rifampin could lead to HIV treatment failure and development of drug resistance. Concurrent HIV and TB therapy is necessary as it is associated with reduced mortality but clinicians are often faced with the challenges of managing the drug-drug interactions when multiple drugs are used in a regimen. The 22–26% reduction in mean efavirenz plasma exposure due to induction effect of rifampicin on efavirenz clearance has led some experts to recommend an increased efavirenz dose to 800 mg/day when co-administered with rifampin.26–28 The fixed daily dose of 600 mg for adults is known to be associated with significant inter-individual variability in plasma concentrations as well as some clinical effects.29–31 Mid-dose or trough efavirenz plasma concentrations below 1000 ng/mL has been associated with increased risk of virologic failure in HIV-infected patients not receiving concurrent rifampicin-containing therapy,30–32 while concentrations above 4000 ng/mL have been associated with risk of central nervous system side effects.30, 31
Thus, the goal of efavirenz dose adjustment when co-administered with rifampicin is to avoid sub-therapeutic concentrations. However, it must be balance with the need to avoid supra-therapeutic efavirenz plasma concentrations in individuals who are genetically predisposed to impaired enzyme activity. Recent studies have shown that individuals with CYP2B6 516 TT genotypes are at risk of high efavirenz plasma exposures even in the presence of rifampicin-containing therapy.33, 34 Thus, increase in efavirenz dose during rifampin-containing therapy may not be necessary in individuals with slow metabolizing phenotype. Clinically, efavirenz 800 mg/day has been used in some patients with TB/HIV co-infection on rifampicin-containing therapy but the increased dose has not been shown to result in superior virologic suppression rates.35–37 Rather, the increased dose was associated with a high frequency of central nervous system and hepatic toxicities associated with high efavirenz plasma concentrations in one study that predominantly enrolled native Africans.35 In contrast, another study in which 50% of the participants were Caucasian did not report a higher frequency of supra-therapeutic concentrations and/or increased toxicity in individuals treated with efavirenz 800 mg daily.25 There are case reports of the need for higher efavirenz doses up to 1600 mg daily to achieve desired plasma concentrations, as well as virologic suppression in two patients with no identifiable slow-metabolizing phenotype mutation who were also treated rifampin.38 However, in most published studies, efavirenz 600 mg/day appears to be adequate in the setting of TB therapy in most patients.24, 37, 39 In addition, the only randomised study of efavirenz 600 or 800 mg daily was conducted in Thai patients and found no difference in virological outcome between the two groups.37 However, this study was limited by small size of the study population, which could have missed small but clinically meaningful effect of the increased dose. Overall, a review of available literature of clinical studies that used efavirenz 600 or 800 mg daily in HIV/TB co-infected patients undertaken by the Food and Drug Administration did not find sufficient evidence to support an increased in dose to 800 mg/day.40
2.4 Pediatric studies of efavirenz and rifampin interactions
There is very limited data on the pharmacokinetic interactions between rifampin-containing TB treatment and efavirenz in children. Our literature review revealed only one published study to date.41 Among 15 HIV/TB co-infected children treated with standard efavirenz-based HAART and rifampin-containing TB treatment, a wide inter-patient variability in efavirenz concentration as well as a bimodal distribution of efavirenz trough concentrations was observed.41 Overall, TB therapy had no significant influence on mean change in efavirenz concentration in the children but 60% and 53% of them had efavirenz trough concentration < 1000 ng/mL during and after antitubercular therapy, respectively. Contrary to expectations, four children with slow metabolizing phenotype had higher efavirenz concentrations during antitubercular therapy than when they were off the rifampin-containing TB treatment.41 Viral load data was available in 13 of the 15 children, 11 of whom had full suppression of HIV RNA at 6 months of HAART. Of the two children who had detectable viral load, both had efavirenz concentration < 1000 ng/mL. These data suggest that current dosing of efavirenz may be suboptimal in most children irrespective of antitubercular therapy.
2.5 Pharmacogenetics of efavirenz therapy
There is substantial inter-individual variability in the pharmacokinetics of efavirenz. Population pharmacokinetic studies have found the coefficient variation (CV) in apparent oral clearance of efavirenz to range from 40 – 55%.29, 42, 43 The variability in plasma concentrations in response to the fixed adult dose of 600 mg is up to 120%.30 The variability in efavirenz pharmacokinetics is likely due to combination of factors including biologic, exogenous and genetic factors. Unlike the strong and consistent association between CYP2B6 516G>T single nucleotide polymorphism (SNP) and efavirenz exposure,44, 45 there is no conclusive evidence to suggest that biological factors such as gender, and body weight significantly influences efavirenz plasma concentrations significantly. While some authors found higher efavirenz concentrations in women compared to men,46 others have found no sex-related differences in efavirenz concentrations.29, 47–49 Likewise, some studies found body weight to be associated with efavirenz plasma concentrations or clearance,44, 49 whiles other have not.34, 46, 50 On the other hand, ethnicity or race (probably a reflection of host genetics) has been consistently associated with efavirenz concentrations such that Blacks or Asians tend to have higher efavirenz concentrations than Whites.29, 34, 46, 48, 49, 51
CYP2B6, the main metabolic enzyme for efavirenz is highly polymorphic and is subject to pronounced inter-individual variability in expression and activity,52 and genotyping for functional SNPs has proven to be useful in the prediction of efavirenz concentrations in pharmacokinetic studies.53, 54 In particular, the CYP2B6 c.516G>T is a common polymorphism (21 to 38% allele frequency)55 that has been consistently associated with reduced enzyme activity and higher efavirenz exposure in studies of different populations with varied racial and ethnic backgrounds.51, 53, 56–58 The influence of CYP2B6 516G>T SNP on efavirenz disposition has also been observed in children,59, 60 as well as during co-administration with rifampicin-containing TB therapy.33, 34 The more recently described CYP2B6 c.983T>C variant with up to 10% allele frequency is also associated with lower enzyme activity and higher efavirenz concentrations but appears to be exclusively found in populations of African descent.54, 61, 62 Other CYP2B6 polymorphisms that have been identified have either minimal impact on efavirenz metabolism, or are relatively rare (i.e. <5% allele frequency),55 and recently, CYP2A6 genetic polymorphisms,18, 44, 50 CYP3A4*1B and CYP3A4_rs464643744 have been also found to influence efavirenz plasma concentrations or clearance.
3. Nevirapine
Nevirapine is the other NNRTI that is widely available for HIV treatment in resource-limited settings. Nevirapine was one of the earlier drugs to be developed and introduced into treatment regimens for HIV-infected patients in 1996.
3.1 Metabolism
Like efavirenz, nevirapine is extensively biotransformed via oxidative metabolism by the CYP pathway to form several hydroxylated metabolites in vivo and in vitro. In vitro studies with human liver microsomes suggest that oxidative metabolism of nevirapine is mediated primarily by CYP3A and CYP2B6 enzymes. Hence, substances that induce or inhibit the CYP enzyme system could have a profound effect on the metabolism of nevirapine, by decreasing or increasing blood levels of nevirapine respectively. For example, rifampicin and corticosteroids are inducers and would decrease blood levels while fluconazole is an inhibitor of the CYP enzyme system and could thus increase plasma levels of nevirapine during concurrent administration.21
Nevirapine biotransformation involves extensive hydroxylation and glucuronidation of hydroxylated metabolites. The metabolites are then largely excreted into the urine, where 2-hydroxy, 3-hydroxy and 12-hydroxy nevirapine glucuronides account for 68% of the total. Thus, CYP metabolism, glucuronide conjugation and urinary excretion of glucuronidated metabolites represent the primary route of nevirapine biotransformation and elimination in humans.63 Formation of 2- and 12-hydroxy nevirapine is mediated by CYP3A4/5, while that of 3- and 8-hydroxy nevirapine by CYP2B6.
In clinical studies, nevirapine is readily (>90%) absorbed after oral administration in healthy volunteers and in patients with HIV infection.64 After a single 200-mg dose, plasma nevirapine concentrations reach a maximum of 2µg/ml by 4 h post-dose and decline log linearly thereafter, resulting in a terminal phase half-life of ~45 h; steady state plasma concentrations of nevirapine would be higher. Nevirapine is an inducer of CYP metabolism, thereby auto inducing its own metabolism and reducing its half-life from 45 to 30 h after two weeks of dosing with 200 mg per day compared with a single dose.65 In adults, nevirapine metabolism does not change substantially with age (range 18 – 68 years) and a review of the literature failed to find a significant association between sex and nevirapine pharmacokinetics.66
3.2 Influence of TB therapy on NVP pharmacokinetics
Rifampicin induces the expression and activity of the CYP metabolic enzymes in the liver,67, 68 thereby greatly reducing plasma concentration and exposure to nevirapine during concomitant treatment with both drugs.69 Cohen et al observed a significant decrease in the ratio between the exposure to nevirapine and its inactive 12-hydroxy metabolite (produced primarily by CYP3A4) in the presence of rifampin-containing TB treatment indicating enhanced metabolism of nevirapine by CYP3A4.70 The therapeutic range of nevirapine is generally considered to be 3.4 to 12.0 µg/ml and several studies (in patients without TB) have found an association between low nevirapine trough levels and suboptimal response to treatment.71–74 Hence, some treatment guidelines recommend therapeutic drug monitoring and maintaining nevirapine levels within this range by appropriate dose adjustment.75 The interaction between nevirapine and rifampicin has been studied using three different approaches (pharmacokinetic studies, observational cohort studies and clinical trials), all providing different perspectives on the issue.
3.3 Pharmacokinetic Studies
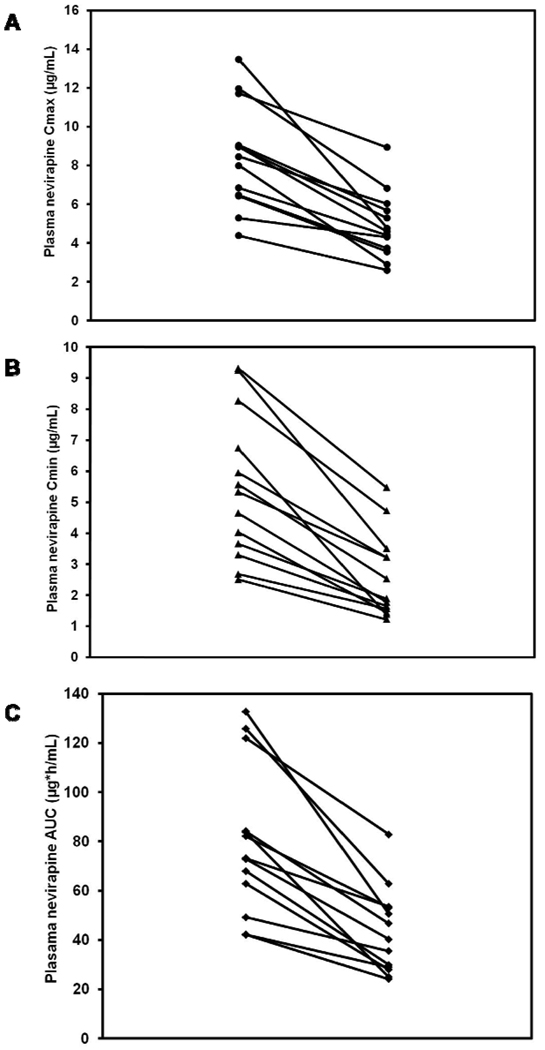
Pharmacokinetic studies in patients on concurrent rifampicin treatment have shown variable reductions in nevirapine blood concentrations ranging from 10% to 68%.49, 70, 76–79 Studies done in Indian78 and African70 HIV co-infected TB patients found a significant proportion with sub-therapeutic plasma nevirapine concentrations. Ramachandran et al found that 8 of 13 patients studied had nevirapine Cmin < 3 µg/ml.78 Unlike the interaction with efavirenz where some patients paradoxically have increases in efavirenz concentrations during co-administration with rifampin,24, 41 as shown in Figure 3, all 13 patients who received nevirapine in the presence of rifampin showed significant reductions in Cmax (A), Cmin (B) and AUC (C) in the presence of rifampicin compared to values in the absence of rifampicin. An attempt was made to overcome this interaction by increasing the dose of nevirapine from 200 mg to 300 mg twice daily in patients with trough nevirapine levels < 3.0 µg /mL.78 While this resulted in therapeutic blood levels in the 7 patients who had sub-therapeutic concentrations and no adverse events, the study was limited by a small sample size and the short (2 weeks) duration of treatment with the higher dose. In a population pharmacokinetic study of nevirapine in South African patients, the simulations suggested that an increased dose of 300mg twice daily would achieve adequate nevirapine concentrations in most patients during rifampicin-containing anti-TB treatment.77
Figure 3.
Nevirapine Cmax (A), Cmin (B) and AUC (C) in 13 patients with HIV and tuberculosis in the absence and presence of rifampicin. Nevirapine and rifampicin were administered at standard dosage and patients were in steady state. Rifampicin co-administration caused a mean reduction in Cmax by 42%, Cmin by 53% and AUC by 46%.
3.4 Observational Studies
In a retrospective analysis of a large cohort of HIV-infected patients in south Africa, Boulle et al showed that the probability of virological failure was higher when patients initiated nevirapine-based HAART while on rifampicin but not when they initiated efavirenz-based HAART (adjusted OR 1.7, 95% CI 1.2 to 2.6) (36).80 However, if tuberculosis developed while patients were stable on nevirapine-based treatment, the failure rates were similar to those on efavirenz.
Several studies have shown that despite expected reductions in serum levels of nevirapine, values remained above the inhibitory concentrations of most wild type strains with satisfactory immunological and virological responses.76, 79, 81, 82 Among 74 Thai patients, Autar et al reported that 86% had nevirapine plasma concentrations within the therapeutic range during rifampicin co-administration.82 A retrospective Spanish study of 32 patients reported that 74% of patients with concomitant nevirapine and rifampicin attained undetectable viral loads.81 A Thai cohort study of 70 patients on concomitant nevirapine and rifampicin found that virological suppression at 60 weeks was similar to a control group without rifampicin treatment.83, 84 Although these data are reassuring and suggest that the majority of patients respond well to treatment in spite of the drug-drug interaction, it is difficult to generalize these findings to other populations due to differences in nutritional status, genetics and the retrospective nature of the studies.
3.5 Clinical Trials
The key question of whether nevirapine (in the standard or higher dose) when administered along with rifampicin results in an increased risk of virological failure can only be answered through clinical trials with simultaneous therapeutic drug monitoring and determination of virological outcomes at set time points. In a small clinical trial conducted in Thailand in HIV co-infected TB patients, it was observed that the 48-week efficacy of antiretroviral treatment (based on immunologic and virologic responses) was similar in patients treated with 400 or 600mg/day of nevirapine.85 A high percentage of suboptimal levels were found during the 200 mg/day lead-in period whereas the 200 mg twice a day lead-in was associated with more drug hypersensitivity. The authors concluded that NVP 200 mg twice a day should be sufficient for most Thai HIV-infected patients receiving rifampicin. Manosuthi et al conducted another randomized clinical trial (the N2R study) comparing the efficacy and plasma drug concentrations of nevirapine and efavirenz in combination with TB treatment. Blood levels of the NNRTIs were correlated with the proportion of patients with undetectable plasma viral load at 48 weeks of ART. This study showed that low drug exposure and low body weight were important predictors for treatment failure and also found a significant correlation between treatment failure and reduced nevirapine trough levels.86 Swaminathan et al compared the efficacy of once-daily nevirapine (400mg) vs. efavirenz (600mg) in combination with a dual NRTI backbone among HIV-infected TB patients on antitubercular therapy and found that virological suppression was significantly worse with the nevirapine regimen; 50 of 59 in the efavirenz arm and 38 of 57 patients in the nevirapine arm had VL< 400 copies/mL at 24 weeks‥87 In this study and many others, patients were already on rifampicin when ART was initiated and nevirapine was given at a lead-in dose of 200mg for the first two weeks of therapy, potentially resulting in low blood levels and the development of resistance mutations during that period leading to poor outcomes.
3.6 Pediatric Studies
Nevirapine is the backbone of first-line HAART, usually in combination with two NRTIs as part of a 3-drug fixed-dose combination (FDC) in resource-limited settings. Pharmacokinetics of many antiretroviral drugs are highly variable in children with absorption, distribution, hepatic metabolism and renal function all changing with age.88 Given that maturation of the hepatic CYP enzymes is generally not complete till 2–5 years of age, younger children require higher doses/kg body weight of drugs metabolized by this system.89 Several generic pediatric FDCs are now available that contain nevirapine in a higher ratio to the other drugs (stavudine and lamivudine) and simplified dosing recommendations have been made by the WHO for use by health care personnel in the field. Studies performed in Thailand, Africa and India using these pediatric FDCs have generally shown adequate nevirapine exposure and satisfactory short-term clinical and immunological outcomes.90–92 However, long-term data is lacking and in view of the sub-therapeutic blood levels observed in a significant proportion of children, especially younger ones, more studies are required.
Apart from age, the factors known to influence nevirapine drug levels include co-administered drugs and pharmacogenetic variability, as in adults.93, 94 Saitoh et al studied HIV-infected children who received nevirapine as a component of HAART and examined the association between CYP2B6 (G516T) and ABCB1 (C3435T) gene polymorphisms on the one hand and plasma concentrations of nevirapine and clinical responses to antiretroviral therapy on the other.94 This study demonstrated that children with the CYP2B6 516TT genotype may have a better response to therapy due to a favorable pharmacokinetic profile. In a study in Indian children on nevirapine-based HAART, this polymorphism was found to be one of the factors significantly influencing drug levels (Swaminathan, personal communication, unpublished data).
One factor that has not been given much attention is the impact of malnutrition on nevirapine levels. A study conducted in Malawi and Zambia suggested that stunting (low height for age, suggestive of chronic malnutrition) may be associated with lower blood levels of nevirapine, while wasting (low weight for height) was associated with higher levels.91 Findings in Indian children are very similar - factors significantly impacting (lowering) nevirapine levels included age <3 years and stunting (Swaminathan, unpublished data). The impact of malnutrition on antiretroviral drug levels and its role in drug metabolism, pharmacokinetics and response to treatment deserves further study, since the majority of children initiating treatment in resource-poor settings, especially those with tuberculosis, are malnourished.
Data on the influence of concomitant rifampicin on nevirapine blood levels in HIV-infected children are limited. Kamateeka et al observed that the clinical, immunological and virological outcomes in 26 Ugandan children receiving Triomune® (NVP/3TC/d4T)-based HAART with concomitant anti-TB therapy was similar to 101 children without TB receiving the same HAART regimen.95 Oudijk et al conducted a pharmacokinetic study in 21 Zambian children < 3 years co-treated with Triomune® FDC and rifampicin-based TB therapy and reported substantial reductions in nevirapine concentrations in young children receiving concurrent rifampicin which led them to suggest that an increase in dose may be required.96
3.7 Genetic determinants of nevirapine pharmacokinetics
The majority of pharmacogenetic studies to date have focused on efavirenz; however, a few recent studies have investigated the influence of genetic polymorphisms on nevirapine. Patients with the 516TT polymorphism in the CYP2B6 enzyme were observed to have a 1.7 fold increase in plasma AUC of nevirapine compared with 516GG patients.58 Similar findings from Uganda97 and India98 confirmed the prominent role of CYP2B6 in nevirapine elimination; however the difference in blood levels between patients with the homozygous mutant and wild forms of the allele were less pronounced than in the case of efavirenz. Other CYP polymorphisms such as CYP2B6 C1459T and CYP3A4 A392G as well as polymorphisms in the MDR gene (ABCB1 C3435T) have not been shown to have much impact on NVP pharmacokinetics.94, 99 Overall, these findings suggest that pharmacogenetics has the potential to be used as a useful tool in the management of HIV-infected patients and could help design regimens and drug dosages with minimal toxicity and maximum effectiveness.
4. Conclusions
The NNRTIs, efavirenz and nevirapine are essential components of life-saving regimens for the treatment of HIV in resource-limited settings. These NNRTIs in combination with two nucleoside reverse transcriptase inhibitors are often the only options available to patients with HIV/TB coinfection requiring rifampicin-containing therapy in areas devastated by the HIV and Tb epidemics as rifabutin is not available to allow the use of PIs in alternate regimens. However, one unresolved issue for the effective use of these NNRTIs in the setting of rifampicin-containing therapy is the appropriate effective dose. Most of the published pharmacokinetic studies that evaluated drug-drug interactions between these drugs and rifampicin were small, underpowered and often did not control for genetic factors that influence efavirenz and nevirapine metabolism. A majority of the clinical studies were observational in nature and were also underpowered to detect the true effect of rifampicin on NNRTI-based therapy. In addition, there were very limited pharmacokinetic and clinical data in children. Overall, we did not find sufficient data in the literature that could be used to support definitive recommendations on dose adjustment of the NNRTIs during rifampicin-containing TB therapy. However, it does appear that one dose adjustment will not be appropriate for all persons because of wide inter-individual differences in the disposition of these NNRTIs in the presence of rifampin-containing TB therapy.
5. Expert Opinion
5.1 Efavirenz
While the minimum effective efavirenz plasma concentration and the degree of the effect of rifampin-containing TB therapy on efavirenz pharmacokinetics and clinical effect is debatable, the well-known substantial inter-individual variability (>100% coefficient of variation) in efavirenz plasma concentrations after fixed standard dosing has the potential to place some individuals at risk of supra-therapeutic or sub-therapeutic concentrations. The ultimate goal of efavirenz dose adjustment during rifampicin-containing therapy is to avoid sub-therapeutic concentrations while minimizing unnecessary increase in efvairenz plasma exposure that will lead to increase frequency of treatment side effects in some individuals. Therefore one would expect that increasing efavirenz dose by 200 mg/day will likely not be appropriate for all patients, as it does not take into consideration the variability due to genetic factors. The frequency of the slow metabolizing genotype, CYP2B6 516TT genotype is about 25% of African and Indian populations.33, 34, 56 Consequently, dosage adjustment of efavirenz during co-administration with rifampin based on a Spanish study may not be applicable to other populations such as Africans or Indians. To our knowledge, no published studies in which efavirenz concentration was measured on and off rifampin-containing TB treatment in the same patient at different times has shown a statistically significant difference with p < 0.05.23, 24 Since efavirenz itself induces CYP2B6 (i.e. autoinduction) it is possible that rifampicin cannot increase CYP2B6 expression beyond that resulting from chronic efavirenz exposure. Efavirenz dose adjustment during rifampicin-containing TB therapy will need to be individualized based genetic polymorphisms of CYP2B6 enzyme, the one single important predictor of efavirenz disposition to date or based on a combination of clinical and genetic factors. Increased understanding of the interactions between rifampicin and functional variants of efavirenz metabolizing enzymes is urgently needed to guide the management of efavirenz-rifampin pharmacokinetic interactions. In addition, future pharmacokinetic and clinical trials must include children as there is a dearth of data in this population.
5.2 Nevirapine
Current evidence suggests that nevirapine is inferior to efavirenz when given to patients on rifampicin-containing TB therapy, using standard methods of administration and in standard doses. The difference in rates of virological suppression between nevirapine and efavirenz when used along with rifampicin have ranged from 0 to 18% in different studies.87,88,89 Most studies have used nevirapine in the conventional dose of 200mg for the first two weeks before dose escalation. When administered in this fashion in the presence of enzyme induction, the levels achieved in the first two weeks have been very low, probably overcoming the low genetic barrier to resistance of HIV-1 and leading to the development of resistance mutations. The one small trial that did use nevirapine during the lead-in phase in the higher dose of 200 mg BID did not report better efficacy; on the other hand, adverse events were more frequent.88 The safety and efficacy of initiating nevirapine at the higher lead-in dose is currently being tested in the CARINE 12146 trial in Mozambique.100 Hence, there is insufficient evidence at this time to recommend a higher dose of nevirapine for patients on rifampicin, though there is a much better rationale to start with the full dose, under close monitoring. It is possible that host genetics may influence the decision to dose adjust nevirapine during co-administration with rifampicin and this needs to be investigated in future studies.
While nevirapine may be inferior to efavirenz for patients taking rifampicin, it should still be considered the alternate drug in situations where efavirenz cannot be administered (e.g. pregnancy, adverse reactions or intolerance to efavirenz). It would be effective in a majority of patients (>75%) and hence a life-saving intervention in the absence of other alternatives. An important point to consider is the sequence of the two treatments. For patients who are stable on nevirapine-based therapy (presumably with low or undetectable viral loads), the addition of rifampicin-containing TB treatment does not appear to result in any therapeutic penalty. Future research should examine these different scenarios in which patients get treated in order to recommend appropriate case management strategies. Studies in young children with TB and HIV should address the question of whether higher doses of nevirapine would be required in order to overcome the effect of both age and rifampicin on its metabolism. As efavirenz is contra-indicated in children under 3 years, nevirapine is the only NNRTI that can be used and safety and efficacy in this setting need to be established.
Highlights box
Concurrent antiretroviral therapy during TB treatment is associated with substantial survival benefit but the appropriate dose of the NNRTI components is unresolved.
Rifampin induces the metabolism of efavirenz resulting in decrease plasma concentrations but the influence of rifampicin appears to be highly variable from one individual to another.
CYP2B6 516G>T genotype is strongly associated with efavirenz disposition irrespective of rifampicin-containing therapy.
Most pharmacokinetic and clinical studies that evaluated the interactions between efavirenz and rifampicin-containing TB therapy are small and inconclusive and do not take into account the highly inter-individual variability in the rifampin effect.
A marked decrease in nevirapine concentrations during co-administration with rifampicin is consistently observed in most pharmacokinetics studies but the clinical significance is unclear.
The drug to drug interactions between nevirapine and rifampicin may also be confounded by host genetics.
There is very limited data on the interactions between the NNRTIs and rifampicin containing TB therapy in pediatric populations.
Acknowledgements
Dr. Kwara was supported by NIH K23 developmental award (NIH K23 AI071760). Drs. Ramachandran and Swaminathan received support under the Fogarty AIDS International Training and Research Program 5D437W000237, Lifespan/Tufts/Brown Center for AIDS Research (Dr. Kenneth Mayer, Program Director).
Footnotes
Declaration of interest
Dr Kwara has previously received a research grant not related to this work and had been on the speaker’s bureau of Bristol Myer-Squibb. Drs Ramachandran and Swaminathan declare no conflict of interest.
References
- 1.WHO. Global tuberculosis control - epidemiology, strategy, financing. [Last accessed August 13, 2009];WHO Report 2009. WHO/HTM/TB/2009411. 2009 Available at http://www.who.int/tb/publications/global_report/2009/pdf/full_report.pdf.
- 2.Dheda K, Lampe FC, Johnson MA, Lipman MC. Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J Infect Dis. 2004 Nov 1;190(9):1670–1676. doi: 10.1086/424676. [DOI] [PubMed] [Google Scholar]
- 3.Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr. 2006 Sep;43(1):42–46. doi: 10.1097/01.qai.0000230521.86964.86. [DOI] [PubMed] [Google Scholar]
- 4.Velasco M, Castilla V, Sanz J, Gaspar G, Condes E, Barros C, et al. Effect of simultaneous use of highly active antiretroviral therapy on survival of HIV patients with tuberculosis. J Acquir Immune Defic Syndr. 2009 Feb 1;50(2):148–152. doi: 10.1097/QAI.0b013e31819367e7. [DOI] [PubMed] [Google Scholar]
- 5.Burman WJ. Issues in the management of HIV-related tuberculosis. Clin Chest Med. 2005 Jun;26(2):283–294. vi–vii. doi: 10.1016/j.ccm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Kwara A, Flanigan TP, Carter EJ. Highly active antiretroviral therapy (HAART) in adults with tuberculosis: current status. Int J Tuberc Lung Dis. 2005 Mar;9(3):248–257. [PubMed] [Google Scholar]
- 7.Brinkhof MW, Egger M, Boulle A, May M, Hosseinipour M, Sprinz E, et al. Tuberculosis after initiation of antiretroviral therapy in low-income and high-income countries. Clin Infect Dis. 2007 Dec 1;45(11):1518–1521. doi: 10.1086/522986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006 Aug 1;20(12):1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 9.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008 Oct 1;22(15):1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawn SD, Lipman MC, Easterbrook PJ. Immune reconstitution disease associated with mycobacterial infections. Curr Opin HIV AIDS. 2008 Jul;3(4):425–431. doi: 10.1097/COH.0b013e3282fe99dc. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005 May 26;352(21):2211–2221. doi: 10.1056/NEJMra032424. [DOI] [PubMed] [Google Scholar]
- 12.Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005 Mar;28(3):249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Geneva, Switzerland: World Health Organization; Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings:towards universal access. 2006
- 14. Desta Z, Saussele T, Ward B, Blievernicht J, Li L, Klein K, et al. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics. 2007 Jun;8(6):547–558. doi: 10.2217/14622416.8.6.547. • The authors show in vitro evidence that CYP2A6 is involved in the metabolism of efavirenz.
- 15. Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther. 2003 Jul;306(1):287–300. doi: 10.1124/jpet.103.049601. •• The first study to identify CYP2B6 as the main catalyst of efavirenz metabolism.
- 16.Mutlib AE, Chen H, Nemeth GA, Markwalder JA, Seitz SP, Gan LS, et al. Identification and characterization of efavirenz metabolites by liquid chromatography/mass spectrometry and high field NMR: species differences in the metabolism of efavirenz. Drug Metab Dispos. 1999 Nov;27(11):1319–1333. [PubMed] [Google Scholar]
- 17. Belanger AS, Caron P, Harvey M, Zimmerman PA, Mehlotra RK, Guillemette C. Glucuronidation of the antiretroviral drug efavirenz by UGT2B7 and an in vitro investigation of drug-drug interaction with zidovudine. Drug Metab Dispos. 2009 Sep;37(9):1793–1796. doi: 10.1124/dmd.109.027706. • The authors provide in vitro data that identified UGT2B7 as the main enzyme for glucuronidation of efavirenz.
- 18.di Iulio J, Fayet A, Arab-Alameddine M, Rotger M, Lubomirov R, Cavassini M, et al. In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet Genomics. 2009 Apr;19(4):300–309. doi: 10.1097/FPC.0b013e328328d577. [DOI] [PubMed] [Google Scholar]
- 19.Faucette SR, Wang H, Hamilton GA, Jolley SL, Gilbert D, Lindley C, et al. Regulation of CYP2B6 in primary human hepatocytes by prototypical inducers. Drug Metab Dispos. 2004 Mar;32(3):348–358. doi: 10.1124/dmd.32.3.348. [DOI] [PubMed] [Google Scholar]
- 20.Hesse LM, Sakai Y, Vishnuvardhan D, Li AP, von Moltke LL, Greenblatt DJ. Effect of bupropion on CYP2B6 and CYP3A4 catalytic activity, immunoreactive protein and mRNA levels in primary human hepatocytes: comparison with rifampicin. J Pharm Pharmacol. 2003 Sep;55(9):1229–1239. doi: 10.1211/0022357021657. [DOI] [PubMed] [Google Scholar]
- 21.Madan A, Graham RA, Carroll KM, Mudra DR, Burton LA, Krueger LA, et al. Effects of prototypical microsomal enzyme inducers on cytochrome P450 expression in cultured human hepatocytes. Drug Metab Dispos. 2003 Apr;31(4):421–431. doi: 10.1124/dmd.31.4.421. [DOI] [PubMed] [Google Scholar]
- 22.Benedeck IHJA, Fiske WD, et al. Pharmacokinetic interaction between efavirenz and rifampin in healthy volunteers. 12th World AIDS Conference; June 28 – July 3 1998; Geneva, Switzerland. [Google Scholar]
- 23. Lopez-Cortes LF, Ruiz-Valderas R, Viciana P, Alarcon-Gonzalez A, Gomez-Mateos J, Leon-Jimenez E, et al. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin Pharmacokinet. 2002;41(9):681–690. doi: 10.2165/00003088-200241090-00004. •• This small pharmacokinetic study of Spanish patients is often cited to support an increased in efavirenz dose to 800 mg daily during co-administration with rifampicin-containing TB therapy.
- 24. Friedland G, Khoo S, Jack C, Lalloo U. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J Antimicrob Chemother. 2006 Dec;58(6):1299–1302. doi: 10.1093/jac/dkl399. • This study showed excellent virologic outcome despite wide variability in efavirenz concentration during co-administration with rifampicin-containing TB therapy in native African patients.
- 25.Matteelli A, Regazzi M, Villani P, De Iaco G, Cusato M, Carvalho AC, et al. Multiple-dose pharmacokinetics of efavirenz with and without the use of rifampicin in HIV-positive patients. Curr HIV Res. 2007 May;5(3):349–353. doi: 10.2174/157016207780636588. [DOI] [PubMed] [Google Scholar]
- 26.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003 Feb 15;167(4):603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 27.CDC. [Last accessed July 10, 2009];Managing drug interactions in the treatment of HIV-related tuberculosis. 2007 [online] Available from URL: http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm.
- 28.Pozniak AL, Miller RF, Lipman MC, Freedman AR, Ormerod LP, Johnson MA, et al. BHIVA treatment guidelines for tuberculosis (TB)/HIV infection 2005. HIV Med. 2005 Jul;6 Suppl 2:62–83. doi: 10.1111/j.1468-1293.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 29.Csajka C, Marzolini C, Fattinger K, Decosterd LA, Fellay J, Telenti A, et al. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin Pharmacol Ther. 2003 Jan;73(1):20–30. doi: 10.1067/mcp.2003.22. [DOI] [PubMed] [Google Scholar]
- 30. Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. Aids. 2001 Jan 5;15(1):71–75. doi: 10.1097/00002030-200101050-00011. •• The authors show that efavirenz mid-dose concentration predicts clinical outcome.
- 31.Stahle L, Moberg L, Svensson JO, Sonnerborg A. Efavirenz plasma concentrations in HIV-infected patients: inter- and intraindividual variability and clinical effects. Ther Drug Monit. 2004 Jun;26(3):267–270. doi: 10.1097/00007691-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Langmann P, Weissbrich B, Desch S, Vath T, Schirmer D, Zilly M, et al. Efavirenz plasma levels for the prediction of treatment failure in heavily pretreated HIV-1 infected patients. Eur J Med Res. 2002 Jul 24;7(7):309–314. [PubMed] [Google Scholar]
- 33. Kwara A, Lartey M, Sagoe KW, Xexemeku F, Kenu E, Oliver-Commey J, et al. Pharmacokinetics of efavirenz when co-administered with rifampin in TB/HIV co-infected patients: pharmacogenetic effect of CYP2B6 variation. J Clin Pharmacol. 2008 Sep;48(9):1032–1040. doi: 10.1177/0091270008321790. • The authors showed the well-recognised relationship between CYP2B6 516TT genotype and impaired efavirenz clearance is observed even in the induced state.
- 34. Ramachandran G, Hemanth Kumar AK, Rajasekaran S, Kumar P, Ramesh K, Anitha S, et al. CYP2B6 G516T polymorphism but not rifampin coadministration influences steady-state pharmacokinetics of efavirenz in human immunodeficiency virus-infected patients in South India. Antimicrob Agents Chemother. 2009 Mar;53(3):863–868. doi: 10.1128/AAC.00899-08. • The authors showed that CYP2B6 G516TT genotype but not rifampin coadministration influenced steady-state pharmacokinetics of efavirenz during co-administration.
- 35.Brennan-Benson P, Lyus R, Harrison T, Pakianathan M, Macallan D. Pharmacokinetic interactions between efavirenz and rifampicin in the treatment of HIV and tuberculosis: one size does not fit all. AIDS. 2005 Sep 23;19(14):1541–1543. doi: 10.1097/01.aids.0000183519.45137.a6. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Cortes LF, Ruiz-Valderas R, Ruiz-Morales J, Leon E, de Campos AV, Marin-Niebla A, et al. Efavirenz trough levels are not associated with virological failure throughout therapy with 800 mg daily and a rifampicin-containing antituberculosis regimen. J Antimicrob Chemother. 2006 Nov;58(5):1017–1023. doi: 10.1093/jac/dkl357. [DOI] [PubMed] [Google Scholar]
- 37. Manosuthi W, Kiertiburanakul S, Sungkanuparph S, Ruxrungtham K, Vibhagool A, Rattanasiri S, et al. Efavirenz 600 mg/day versus efavirenz 800 mg/day in HIV-infected patients with tuberculosis receiving rifampicin: 48 weeks results. AIDS. 2006 Jan 2;20(1):131–132. doi: 10.1097/01.aids.0000196181.18916.9b. •• The authors show that efavirenz mid-dose concentration and virological outcome is not different in patients treated with efavirenz 600 mg/day versus 800 mg/day.
- 38.Cabrera SE, Cordero M, Iglesias A, Valverde MP, Dominguez-Gil A, Garcia MJ. Efavirenz-rifampicin interaction: therapeutic drug monitoring to efavirenz dosage optimization in HIV/TBC patients. Aids. 2008 Nov 30;22(18):2549–2551. doi: 10.1097/QAD.0b013e3283189c07. [DOI] [PubMed] [Google Scholar]
- 39.Patel A, Patel K, Patel J, Shah N, Patel B, Rani S. Safety and antiretroviral effectiveness of concomitant use of rifampicin and efavirenz for antiretroviral-naive patients in India who are coinfected with tuberculosis and HIV-1. J Acquir Immune Defic Syndr. 2004 Sep 1;37(1):1166–1169. doi: 10.1097/01.qai.0000135956.96166.f0. [DOI] [PubMed] [Google Scholar]
- 40. DiGiacinto JL, Chan-Tack KM, Robertson SM, Reynolds KS, Struble KA. Are literature references sufficient for dose recommendations? An FDA case study of efavirenz and rifampin. J Clin Pharmacol. 2008 Apr;48(4):518–523. doi: 10.1177/0091270008315308. •• This reviewed failed to find adequate evidence to support increasing efavirenz dose in the presence of rifampicin-containing therapy.
- 41. Ren Y, Nuttall JJ, Eley BS, Meyers TM, Smith PJ, Maartens G, et al. Effect of rifampicin on efavirenz pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr. 2009 Apr 15;50(5):439–443. doi: 10.1097/QAI.0b013e31819c33a3. •• The authors found a high prevalence of low efavirenz concentrations in children irrespective of rifampicin-containing therapy.
- 42.Cabrera SE, Santos D, Valverde MP, Dominguez-Gil A, Gonzalez F, Luna G, et al. Influence of the cytochrome P450 2B6 genotype on population pharmacokinetics of efavirenz in human immunodeficiency virus patients. Antimicrob Agents Chemother. 2009 Jul;53(7):2791–2798. doi: 10.1128/AAC.01537-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kappelhoff BS, Huitema AD, Yalvac Z, Prins JM, Mulder JW, Meenhorst PL, et al. Population pharmacokinetics of efavirenz in an unselected cohort of HIV-1-infected individuals. Clin Pharmacokinet. 2005;44(8):849–861. doi: 10.2165/00003088-200544080-00006. [DOI] [PubMed] [Google Scholar]
- 44.Arab-Alameddine M, Di Iulio J, Buclin T, Rotger M, Lubomirov R, Cavassini M, et al. Pharmacogenetics-based population pharmacokinetic analysis of efavirenz in HIV-1-infected individuals. Clin Pharmacol Ther. 2009 May;85(5):485–494. doi: 10.1038/clpt.2008.271. [DOI] [PubMed] [Google Scholar]
- 45.Motsinger AA, Ritchie MD, Shafer RW, Robbins GK, Morse GD, Labbe L, et al. Multilocus genetic interactions and response to efavirenz-containing regimens: an adult AIDS clinical trials group study. Pharmacogenet Genomics. 2006 Nov;16(11):837–845. doi: 10.1097/01.fpc.0000230413.97596.fa. [DOI] [PubMed] [Google Scholar]
- 46.Burger D, van der Heiden I, la Porte C, van der Ende M, Groeneveld P, Richter C, et al. Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race, and CYP2B6 polymorphism. Br J Clin Pharmacol. 2006 Feb;61(2):148–154. doi: 10.1111/j.1365-2125.2005.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kappelhoff BS, van Leth F, MacGregor TR, Lange J, Beijnen JH, Huitema AD. Nevirapine and efavirenz pharmacokinetics and covariate analysis in the 2NN study. Antivir Ther. 2005;10(1):145–155. [PubMed] [Google Scholar]
- 48.Pfister M, Labbe L, Hammer SM, Mellors J, Bennett KK, Rosenkranz S, et al. Population pharmacokinetics and pharmacodynamics of efavirenz, nelfinavir, and indinavir: Adult AIDS Clinical Trial Group Study 398. Antimicrob Agents Chemother. 2003 Jan;47(1):130–137. doi: 10.1128/AAC.47.1.130-137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stohr W, Back D, Dunn D, Sabin C, Winston A, Gilson R, et al. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir Ther. 2008;13(5):675–685. [PubMed] [Google Scholar]
- 50.Kwara A, Lartey M, Sagoe KW, Rzek NL, Court MH. CYP2B6 (c.516G-->T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients. Br J Clin Pharmacol. 2009 Apr;67(4):427–436. doi: 10.1111/j.1365-2125.2009.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haas DW, Ribaudo HJ, Kim RB, Tierney C, Wilkinson GR, Gulick RM, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. Aids. 2004 Dec 3;18(18):2391–2400. •• Landmark study that found that CYP2B6 516G→T predicts delayed clearance of efavirenz, which largely explains increased plasma exposure among persons of African descent.
- 52. Lang T, Klein K, Fischer J, Nussler AK, Neuhaus P, Hofmann U, et al. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics. 2001 Jul;11(5):399–415. doi: 10.1097/00008571-200107000-00004. • Reports the highly polymorphic nature of CYP2B6.
- 53. Tsuchiya K, Gatanaga H, Tachikawa N, Teruya K, Kikuchi Y, Yoshino M, et al. Homozygous CYP2B6 *6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem Biophys Res Commun. 2004 Jul 9;319(4):1322–1326. doi: 10.1016/j.bbrc.2004.05.116. • One of the early reports of the relationship between homozygous CYP2B6 *6 (Q172H and K262R) correlates with high plasma efavirenz concentrations.
- 54.Wyen C, Hendra H, Vogel M, Hoffmann C, Knechten H, Brockmeyer NH, et al. Impact of CYP2B6 983T>C polymorphism on non-nucleoside reverse transcriptase inhibitor plasma concentrations in HIV-infected patients. J Antimicrob Chemother. 2008 Apr;61(4):914–918. doi: 10.1093/jac/dkn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rotger M, Tegude H, Colombo S, Cavassini M, Furrer H, Decosterd L, et al. Predictive value of known and novel alleles of CYP2B6 for efavirenz plasma concentrations in HIV-infected individuals. Clin Pharmacol Ther. 2007 Apr;81(4):557–566. doi: 10.1038/sj.clpt.6100072. •• The authors report the predictive value of several CYP2B6 SNPs for efavirenz concentrations.
- 56.Nyakutira C, Roshammar D, Chigutsa E, Chonzi P, Ashton M, Nhachi C, et al. High prevalence of the CYP2B6 516G-->T(*6) variant and effect on the population pharmacokinetics of efavirenz in HIV/AIDS outpatients in Zimbabwe. Eur J Clin Pharmacol. 2008 Apr;64(4):357–365. doi: 10.1007/s00228-007-0412-3. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez-Novoa S, Barreiro P, Rendon A, Jimenez-Nacher I, Gonzalez-Lahoz J, Soriano V. Influence of 516G>T polymorphisms at the gene encoding the CYP450-2B6 isoenzyme on efavirenz plasma concentrations in HIV-infected subjects. Clin Infect Dis. 2005 May 1;40(9):1358–1361. doi: 10.1086/429327. [DOI] [PubMed] [Google Scholar]
- 58. Rotger M, Colombo S, Furrer H, Bleiber G, Buclin T, Lee BL, et al. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics. 2005 Jan;15(1):1–5. doi: 10.1097/01213011-200501000-00001. •• Authors show that intracellular efavirenz concentration and CNS side effects are associated with CYP2B6 516G→T polymorphism.
- 59. Saitoh A, Fletcher CV, Brundage R, Alvero C, Fenton T, Hsia K, et al. Efavirenz pharmacokinetics in HIV-1-infected children are associated with CYP2B6-G516T polymorphism. J Acquir Immune Defic Syndr. 2007 Jul 1;45(3):280–285. doi: 10.1097/QAI.0b013e318040b29e. • Authors show that efavirenz plasma concentrations are associated with CYP2B6 516G→T polymorphism in children.
- 60.ter Heine R, Scherpbier HJ, Crommentuyn KM, Bekker V, Beijnen JH, Kuijpers TW, et al. A pharmacokinetic and pharmacogenetic study of efavirenz in children: dosing guidelines can result in subtherapeutic concentrations. Antivir Ther. 2008;13(6):779–787. [PubMed] [Google Scholar]
- 61.Klein K, Lang T, Saussele T, Barbosa-Sicard E, Schunck WH, Eichelbaum M, et al. Genetic variability of CYP2B6 in populations of African and Asian origin: allele frequencies, novel functional variants, and possible implications for anti-HIV therapy with efavirenz. Pharmacogenet Genomics. 2005 Dec;15(12):861–873. doi: 10.1097/01213011-200512000-00004. [DOI] [PubMed] [Google Scholar]
- 62.Mehlotra RK, Bockarie MJ, Zimmerman PA. CYP2B6 983T>C polymorphism is prevalent in West Africa but absent in Papua New Guinea: implications for HIV/AIDS treatment. Br J Clin Pharmacol. 2007 Sep;64(3):391–395. doi: 10.1111/j.1365-2125.2007.02884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riska P, Lamson M, MacGregor T, Sabo J, Hattox S, Pav J, et al. Disposition and biotransformation of the antiretroviral drug nevirapine in humans. Drug Metab Dispos. 1999 Aug;27(8):895–901. [PubMed] [Google Scholar]
- 64.Lamson MJCS, Sabo JP, et al. Assessment of Nevirapine oral bioavailability in healthy volunteers following oral and intravenous administration. Pharmacol Res. 1995;12 S-415. [Google Scholar]
- 65.Lamson MJCS, Sabo JP, et al. Effects of gender on the single and multiple dose pharmacokinetics of nevirapine. Pharmacol Res. 1995;12 S-101. [Google Scholar]
- 66.Ofotokun I, Chuck SK, Hitti JE. Antiretroviral pharmacokinetic profile: a review of sex differences. Gend Med. 2007 Jun;4(2):106–119. doi: 10.1016/s1550-8579(07)80025-8. [DOI] [PubMed] [Google Scholar]
- 67.Combalbert J, Fabre I, Fabre G, Dalet I, Derancourt J, Cano JP, et al. Metabolism of cyclosporin A. IV. Purification and identification of the rifampicin-inducible human liver cytochrome P-450 (cyclosporin A oxidase) as a product of P450IIIA gene subfamily. Drug Metab Dispos. 1989 Mar–Apr;17(2):197–207. [PubMed] [Google Scholar]
- 68.Erickson DA, Mather G, Trager WF, Levy RH, Keirns JJ. Characterization of the in vitro biotransformation of the HIV-1 reverse transcriptase inhibitor nevirapine by human hepatic cytochromes P-450. Drug Metab Dispos. 1999 Dec;27(12):1488–1495. [PubMed] [Google Scholar]
- 69.Burman WJ, Jones BE. Treatment of HIV-related tuberculosis in the era of effective antiretroviral therapy. Am J Respir Crit Care Med. 2001 Jul 1;164(1):7–12. doi: 10.1164/ajrccm.164.1.2101133. [DOI] [PubMed] [Google Scholar]
- 70. Cohen K, van Cutsem G, Boulle A, McIlleron H, Goemaere E, Smith PJ, et al. Effect of rifampicin-based antitubercular therapy on nevirapine plasma concentrations in South African adults with HIV-associated tuberculosis. J Antimicrob Chemother. 2008 Feb;61(2):389–393. doi: 10.1093/jac/dkm484. • Nevirapine concentrations were significantly decreased by concomitant rifampicin-based antitubercular therapy and a high proportion of patients had subtherapeutic plasma concentrations.
- 71.Alexander CS, Asselin JJ, Ting LS, Montaner JS, Hogg RS, Yip B, et al. Antiretroviral concentrations in untimed plasma samples predict therapy outcome in a population with advanced disease. J Infect Dis. 2003 Aug 15;188(4):541–548. doi: 10.1086/376835. [DOI] [PubMed] [Google Scholar]
- 72.de Vries-Sluijs TE, Dieleman JP, Arts D, Huitema AD, Beijnen JH, Schutten M, et al. Low nevirapine plasma concentrations predict virological failure in an unselected HIV-1-infected population. Clin Pharmacokinet. 2003;42(6):599–605. doi: 10.2165/00003088-200342060-00009. [DOI] [PubMed] [Google Scholar]
- 73.Gonzalez de Requena D, Bonora S, Garazzino S, Sciandra M, D'Avolio A, Raiteri R, et al. Nevirapine plasma exposure affects both durability of viral suppression and selection of nevirapine primary resistance mutations in a clinical setting. Antimicrob Agents Chemother. 2005 Sep;49(9):3966–3969. doi: 10.1128/AAC.49.9.3966-3969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Veldkamp AI, Weverling GJ, Lange JM, Montaner JS, Reiss P, Cooper DA, et al. High exposure to nevirapine in plasma is associated with an improved virological response in HIV-1-infected individuals. AIDS. 2001 Jun 15;15(9):1089–1095. doi: 10.1097/00002030-200106150-00003. [DOI] [PubMed] [Google Scholar]
- 75.Kappelhoff BS, Crommentuyn KM, de Maat MM, Mulder JW, Huitema AD, Beijnen JH. Practical guidelines to interpret plasma concentrations of antiretroviral drugs. Clin Pharmacokinet. 2004;43(13):845–853. doi: 10.2165/00003088-200443130-00002. [DOI] [PubMed] [Google Scholar]
- 76.Dean GL, Back DJ, de Ruiter A. Effect of tuberculosis therapy on nevirapine trough plasma concentrations. AIDS. 1999 Dec 3;13(17):2489–2490. doi: 10.1097/00002030-199912030-00029. [DOI] [PubMed] [Google Scholar]
- 77.Elsherbiny D, Cohen K, Jansson B, Smith P, McIlleron H, Simonsson US. Population pharmacokinetics of nevirapine in combination with rifampicin-based short course chemotherapy in HIV- and tuberculosis-infected South African patients. Eur J Clin Pharmacol. 2009 Jan;65(1):71–80. doi: 10.1007/s00228-008-0481-y. [DOI] [PubMed] [Google Scholar]
- 78. Ramachandran G, Hemanthkumar AK, Rajasekaran S, Padmapriyadarsini C, Narendran G, Sukumar B, et al. Increasing nevirapine dose can overcome reduced bioavailability due to rifampicin coadministration. J Acquir Immune Defic Syndr. 2006 May;42(1):36–41. doi: 10.1097/01.qai.0000214808.75594.73. •• This study showed that reduced exposure of nevirapine due to concomitant rifampicin could be overcome by increasing the dose of nevirapine from 200mg to 300mg twice-daily.
- 79.Ribera E, Pou L, Lopez RM, Crespo M, Falco V, Ocana I, et al. Pharmacokinetic interaction between nevirapine and rifampicin in HIV-infected patients with tuberculosis. J Acquir Immune Defic Syndr. 2001 Dec 15;28(5):450–453. doi: 10.1097/00042560-200112150-00007. [DOI] [PubMed] [Google Scholar]
- 80. Boulle A, Van Cutsem G, Cohen K, Hilderbrand K, Mathee S, Abrahams M, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. Jama. 2008 Aug 6;300(5):530–539. doi: 10.1001/jama.300.5.530. •• This cohort study showed that co-administered rifampicin-based antitubercular therapy at antiretroviral therapy initiation resulted in higher probabilities of virological failure in the first 2 years of therapy in patients taking nevirapine-based antiretroviral therapy but not in patients who started efavirenz-based antiretroviral therapy.
- 81.Oliva J, Moreno S, Sanz J, Ribera E, Molina JA, Rubio R, et al. Co-administration of rifampin and nevirapine in HIV-infected patients with tuberculosis. AIDS. 2003 Mar 7;17(4):637–638. doi: 10.1097/00002030-200303070-00024. [DOI] [PubMed] [Google Scholar]
- 82.Autar RS, Wit FW, Sankote J, Mahanontharit A, Anekthananon T, Mootsikapun P, et al. Nevirapine plasma concentrations and concomitant use of rifampin in patients coinfected with HIV-1 and tuberculosis. Antivir Ther. 2005;10(8):937–943. [PubMed] [Google Scholar]
- 83.Manosuthi W, Ruxrungtham K, Likanonsakul S, Prasithsirikul W, Inthong Y, Phoorisri T, et al. Nevirapine levels after discontinuation of rifampicin therapy and 60-week efficacy of nevirapine-based antiretroviral therapy in HIV-infected patients with tuberculosis. Clin Infect Dis. 2007 Jan 1;44(1):141–144. doi: 10.1086/510078. [DOI] [PubMed] [Google Scholar]
- 84.Manosuthi W, Sungkanuparph S, Thakkinstian A, Rattanasiri S, Chaovavanich A, Prasithsirikul W, et al. Plasma nevirapine levels and 24-week efficacy in HIV-infected patients receiving nevirapine-based highly active antiretroviral therapy with or without rifampicin. Clin Infect Dis. 2006 Jul 15;43(2):253–255. doi: 10.1086/505210. [DOI] [PubMed] [Google Scholar]
- 85.Avihingsanon A, Manosuthi W, Kantipong P, Chuchotaworn C, Moolphate S, Sakornjun W, et al. Pharmacokinetics and 48-week efficacy of nevirapine: 400 mg versus 600 mg per day in HIV-tuberculosis coinfection receiving rifampicin. Antivir Ther. 2008;13(4):529–536. [PubMed] [Google Scholar]
- 86. Manosuthi W, Sungkanuparph S, Tantanathip P, Lueangniyomkul A, Mankatitham W, Prasithsirskul W, et al. A randomized trial comparing plasma drug concentrations and efficacies between 2 nonnucleoside reverse-transcriptase inhibitor-based regimens in HIV-infected patients receiving rifampicin: the N2R Study. Clin Infect Dis. 2009 Jun 15;48(12):1752–1759. doi: 10.1086/599114. •• This randomized clinical trial showed that antiretroviral regimens containing efavirenz were less compromised by concomitant use of rifampicin than were those that contained nevirapine, and that low drug exposure and low body weight were important predictors for treatment failure.
- 87.Soumya Swaminathan PC, Venkatesan P, et al. Once-daily nevirapine vs efavirenz in the treatment of HIV-infected patients with TB: A Randomized Clinical Trial. Abstract # 35. Presented at the 16th Conference on Retroviruses and Opportunistic Infections (CROI 2009).2009. [Google Scholar]
- 88.L'Homme R, Warris A, Gibb D, Burger D. Children with HIV are not small adults: what is different in pharmacology? Curr Opin HIV AIDS. 2007 Sep;2(5):405–409. doi: 10.1097/COH.0b013e3282ced13f. [DOI] [PubMed] [Google Scholar]
- 89. Anderson GD, Lynn AM. Optimizing pediatric dosing: a developmental pharmacologic approach. Pharmacotherapy. 2009 Jun;29(6):680–690. doi: 10.1592/phco.29.6.680. • A good review of developmental pharmacology of drugs and approaches to dosing in pediatrics.
- 90. Chokephaibulkit K, Plipat N, Cressey TR, Frederix K, Phongsamart W, Capparelli E, et al. Pharmacokinetics of nevirapine in HIV-infected children receiving an adult fixed-dose combination of stavudine, lamivudine and nevirapine. AIDS. 2005 Sep 23;19(14):1495–1499. doi: 10.1097/01.aids.0000183625.97170.59. • This study reported that the pharmcokinetics of nevirapine were satisfactory in HIV-infected children who received treatment with adult fixed dose formulations of antiretroviral drugs.
- 91. Ellis JC, L'Homme RF, Ewings FM, Mulenga V, Bell F, Chileshe R, et al. Nevirapine concentrations in HIV-infected children treated with divided fixed-dose combination antiretroviral tablets in Malawi and Zambia. Antivir Ther. 2007;12(2):253–260. •• This observational study reported that stunting led to lower plasma levels of nevirapine in HIV-infected children.
- 92.O'Brien DP, Sauvageot D, Zachariah R, Humblet P. In resource-limited settings good early outcomes can be achieved in children using adult fixed-dose combination antiretroviral therapy. AIDS. 2006 Oct 3;20(15):1955–1960. doi: 10.1097/01.aids.0000247117.66585.ce. [DOI] [PubMed] [Google Scholar]
- 93.L'Homme RF, Kabamba D, Ewings FM, Mulenga V, Kankasa C, Thomason MJ, et al. Nevirapine, stavudine and lamivudine pharmacokinetics in African children on paediatric fixed-dose combination tablets. AIDS. 2008 Mar 12;22(5):557–565. doi: 10.1097/QAD.0b013e3282f4a208. [DOI] [PubMed] [Google Scholar]
- 94. Saitoh A, Sarles E, Capparelli E, Aweeka F, Kovacs A, Burchett SK, et al. CYP2B6 genetic variants are associated with nevirapine pharmacokinetics and clinical response in HIV-1-infected children. AIDS. 2007 Oct 18;21(16):2191–2199. doi: 10.1097/QAD.0b013e3282ef9695. • This study reported for the first time, the impact of CYP2B6 G516T polymorphism on plasma nevirapine levels in HIV-infected children.
- 95.Kamateeka MML, Mudiope P, Mubiru M, Ajuna P, Lutajumwa M, Musoke P. Immunological and virological response to fixed-dose nevirapine based highly active antiretroviral therapy (HAART) in HIV-infected Ugandan children with concurrent active tuberculosis infection on rifampicin-based anti-TB treatment. Abstract MOPEB089 presented at the 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention, Cape Town, July 2009.2009. [Google Scholar]
- 96.Oudjik JMMH, Mulenga V, Chintu C, Merry C, Gibb D, Burger D. Pharmacokinetics of nevirapine in young children during combined ART and rifampicin-containing antituberculosis treatment. Abstract LBPEB10 presented at the 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention, Cape Town, July 2009.2009. [Google Scholar]
- 97.Penzak SR, Kabuye G, Mugyenyi P, Mbamanya F, Natarajan V, Alfaro RM, et al. Cytochrome P450 2B6 (CYP2B6) G516T influences nevirapine plasma concentrations in HIV-infected patients in Uganda. HIV Med. 2007 Mar;8(2):86–91. doi: 10.1111/j.1468-1293.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- 98. Ramachandran G, Ramesh K, Hemanth Kumar AK, Jagan I, Vasantha M, Padmapriyadarsini C, et al. Association of high T allele frequency of CYP2B6 G516T polymorphism among ethnic south Indian HIV-infected patients with elevated plasma efavirenz and nevirapine. J Antimicrob Chemother. 2009 Apr;63(4):841–843. doi: 10.1093/jac/dkp033. • This study showed a high T allele frequency of CYP2B6 G516T polymorphism in an ethnic population of HIV-infected patients in south India and the correlation of the mutant homozygous genotype with high plasma efavirenz levels.
- 99.Bakshi S, Ramachandran G, Ramesh K, Hemanthkumar AK, Anitha S, Padmapriyadarsini C, et al. Study of ABCB1 polymorphism (C3435T) in HIV-1-infected individuals from South India. Br J Clin Pharmacol. 2008 May;65(5):791–792. doi: 10.1111/j.1365-2125.2008.03093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bonnet MBN, Jani IV, Slawuski A, Silva C, Rouzioux C, Calmy A, Barre Furlan, Taburet AM. Pharmacokinetic parameters of nevirapine when given without 2-weeks leading dose in tuberculosis -HIV coinfected patients receiving rifamocin: substudy of the CARINE 12146 trial in Maputo. Abstract presented at the 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention, Cape Town, July 2009.2009. [Google Scholar]