Abstract
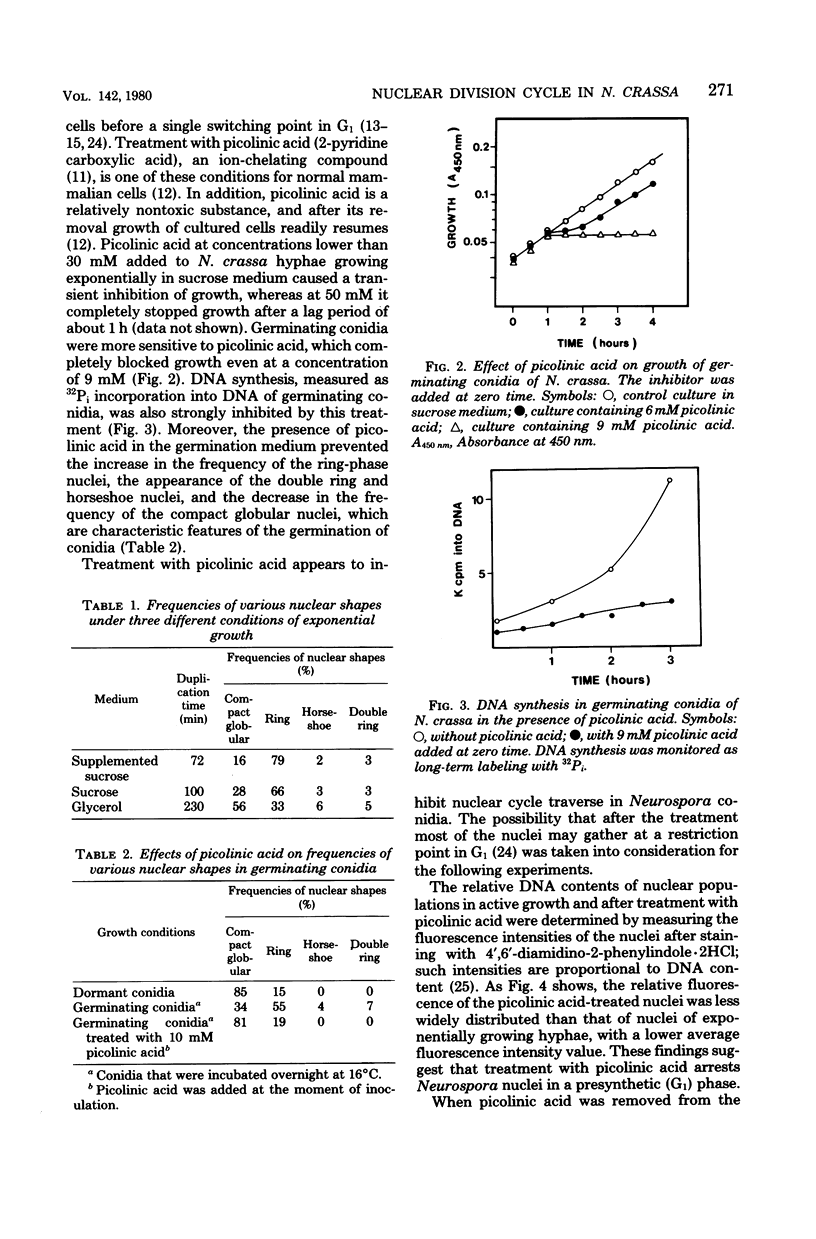
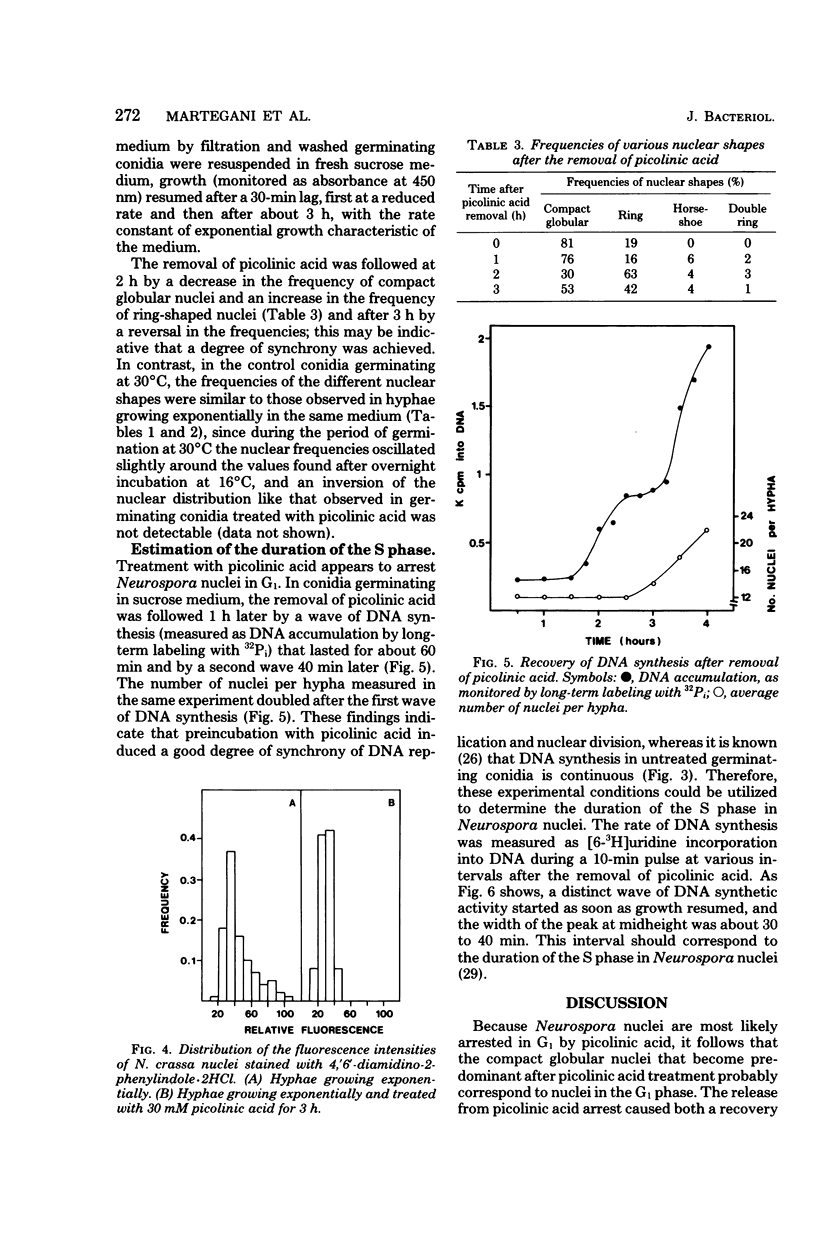
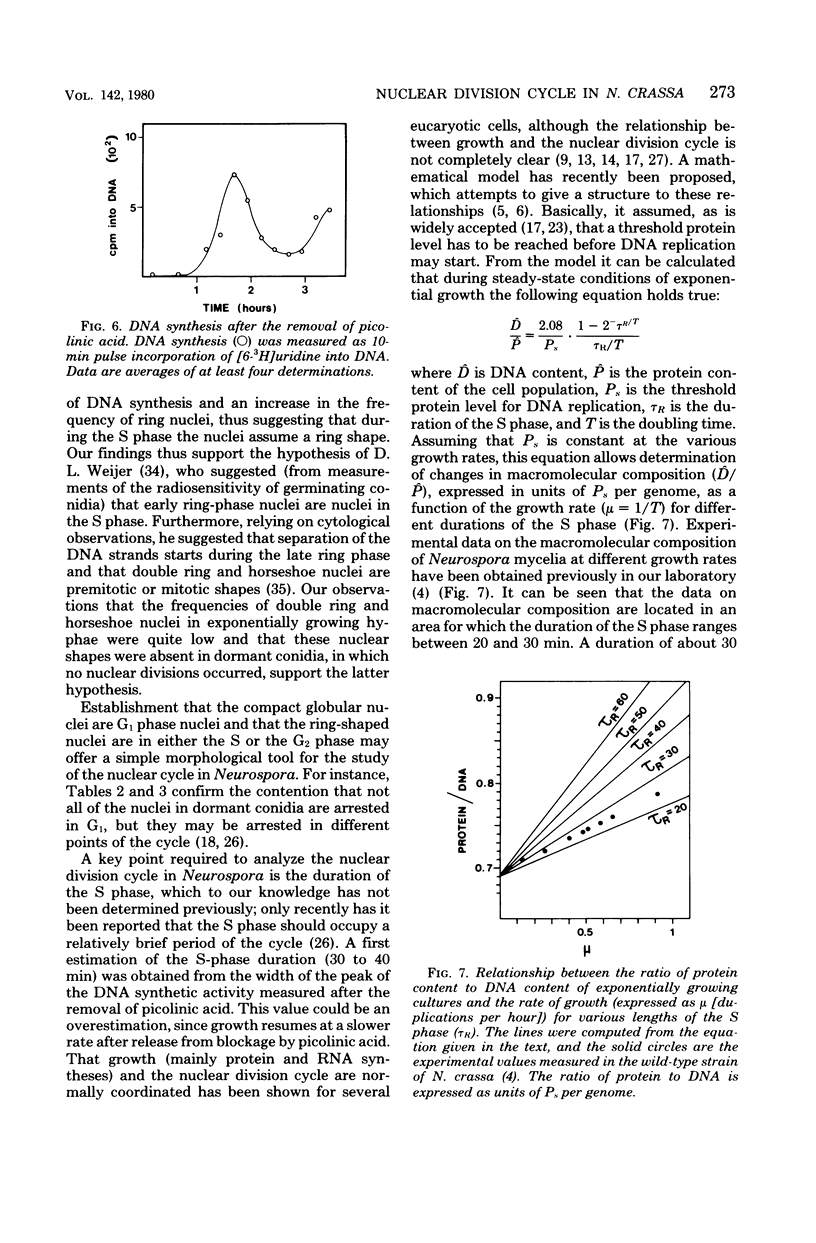
Treatment with picolinic acid blocked Neurospora crassa nuclei in G1, and recovery from the treatment allowed a synchronous wave of deoxyribonucleic acid synthesis to occur. Nuclei, which appeared as compact globular bodies during the period of blockage, assumed a ring shape during the following S phase, which was also maintained in the G2 phase. The proportion of compact globular nuclei was much higher in hyphae growing at lower rates, whereas that of ring nuclei increased when the hyphae were growing at higher rates. Horseshoe nuclei (probably mitotic nuclei) and double ring nuclei were also observed in growing hyphae, but their frequencies were low and fairly independent of the rate of growth. The length of the S phase of the Neurospora nuclear division cycle was determined to be about 30 min. From the frequencies of the phase-specific nuclear shapes, the durations of the G1 phase and the combined S plus G2 phases were calculated. The results showed that variations in the growth rates of the mycelia were mainly coupled with variations in the G1 phase of the nuclear division cycle. For mycelia growing in minimal sucrose, the lengths of all of the phases of the nuclear division cycle were estimated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberghina F. A. Growth regulation in Neurospora crassa. Effects of nutrients and of temperature. Arch Mikrobiol. 1973 Feb 5;89(2):83–94. doi: 10.1007/BF00425025. [DOI] [PubMed] [Google Scholar]
- Alberghina F. A., Schiaffonati L., Zardi L., Sturani E. Lack of guanosine tetraphosphate accumulation during inhibition of RNA synthesis in Neurospora crassa. Biochim Biophys Acta. 1973 Jun 23;312(2):435–439. doi: 10.1016/0005-2787(73)90389-4. [DOI] [PubMed] [Google Scholar]
- Alberghina F. A., Sturani E., Gohlke J. R. Levels and rates of synthesis of ribosomal ribonucleic acid, transfer ribonucleic acid, and protein in Neurospora crassa in different steady states of growth. J Biol Chem. 1975 Jun 25;250(12):4381–4388. [PubMed] [Google Scholar]
- Barford J. P., Hall R. J. Estimation of the length of cell cycle phases from asynchronous cultures of Saccharomyces cerevisiae. Exp Cell Res. 1976 Oct 15;102(2):276–284. doi: 10.1016/0014-4827(76)90043-4. [DOI] [PubMed] [Google Scholar]
- Bostock C. J. DNA synthesis in the fission yeast Schizosaccharomyces pombe. Exp Cell Res. 1970 Apr;60(1):16–26. doi: 10.1016/0014-4827(70)90484-2. [DOI] [PubMed] [Google Scholar]
- Fantes P. A., Nurse P. Control of the timing of cell division in fission yeast. Cell size mutants reveal a second control pathway. Exp Cell Res. 1978 Sep;115(2):317–329. doi: 10.1016/0014-4827(78)90286-0. [DOI] [PubMed] [Google Scholar]
- Fantes P., Nurse P. Control of cell size at division in fission yeast by a growth-modulated size control over nuclear division. Exp Cell Res. 1977 Jul;107(2):377–386. doi: 10.1016/0014-4827(77)90359-7. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pol J. A., Bono V. H., Jr, Johnson G. S. Control of growth by picolinic acid: differential response of normal and transformed cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2889–2893. doi: 10.1073/pnas.74.7.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Pol J. A. Iron: possible cause of the G1 arrest induced in NRK cells by picolinic acid. Biochem Biophys Res Commun. 1977 Sep 9;78(1):136–143. doi: 10.1016/0006-291x(77)91231-1. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Pringle J. R., Hartwell L. H. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1977 Mar 1;105(1):79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- Johnston G. C., Singer R. A. RNA synthesis and control of cell division in the yeast S. cerevisiae. Cell. 1978 Aug;14(4):951–958. doi: 10.1016/0092-8674(78)90349-5. [DOI] [PubMed] [Google Scholar]
- Kessel M., Rosenberger R. F. Regulation and timing of deoxyribonucleic acid synthesis in hyphae of Aspergillus nidulans. J Bacteriol. 1968 Jun;95(6):2275–2281. doi: 10.1128/jb.95.6.2275-2281.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killander D., Zetterberg A. A quantitative cytochemical investigation of the relationship between cell mass and initiation of DNA synthesis in mouse fibroblasts in vitro. Exp Cell Res. 1965 Oct;40(1):12–20. doi: 10.1016/0014-4827(65)90285-5. [DOI] [PubMed] [Google Scholar]
- Loo M. Some required events in conidial germination of Neurospora crassa. Dev Biol. 1976 Dec;54(2):201–213. doi: 10.1016/0012-1606(76)90299-2. [DOI] [PubMed] [Google Scholar]
- Martegani E., Alberghina L. Intracellular protein degradation in Neurospora crassa. J Biol Chem. 1979 Aug 10;254(15):7047–7054. [PubMed] [Google Scholar]
- Nurse P., Thuriaux P. Controls over the timing of DNA replication during the cell cycle of fission yeast. Exp Cell Res. 1977 Jul;107(2):365–375. doi: 10.1016/0014-4827(77)90358-5. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serna L., Stadler D. Nuclear division cycle in germinating conidia of Neurospora crassa. J Bacteriol. 1978 Oct;136(1):341–351. doi: 10.1128/jb.136.1.341-351.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields R., Brooks R. F., Riddle P. N., Capellaro D. F., Delia D. Cell size, cell cycle and transition probability in mouse fibroblasts. Cell. 1978 Oct;15(2):469–474. doi: 10.1016/0092-8674(78)90016-8. [DOI] [PubMed] [Google Scholar]
- Slater M. L., Sharrow S. O., Gart J. J. Cell cycle of Saccharomycescerevisiae in populations growing at different rates. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3850–3854. doi: 10.1073/pnas.74.9.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturani E., Costantini M. G., Martegani E., Alberghina L. Level and turnover of polyadenylate-containing ribonucleic acid in Neurospora crassa in different steady states of growth. Eur J Biochem. 1979 Aug 15;99(1):1–7. doi: 10.1111/j.1432-1033.1979.tb13224.x. [DOI] [PubMed] [Google Scholar]
- Sturani E., Costantini M. G., Zippel R., Alberghina F. A. Regulation of RNA synthesis in Neurospora crassa. An analysis of a shift-up. Exp Cell Res. 1976 May;99(2):245–252. doi: 10.1016/0014-4827(76)90580-2. [DOI] [PubMed] [Google Scholar]
- Sturani E., Magnani F., Alberghina F. A. Inhibition of ribosomal RNA synthesis during a shift-down transition of growth in Neurospora crassa. Biochim Biophys Acta. 1973 Aug 24;319(2):153–164. doi: 10.1016/0005-2787(73)90006-3. [DOI] [PubMed] [Google Scholar]
- WEIJER D. L. KARYOKINESIS OF SOMATIC NUCLEI OF NEUROSPORA CRASSA. I. THE CORRELATION BETWEEN CONIDIAL RADIOSENSITIVITY AND THEIR KARYOKINETIC STAGE. Can J Genet Cytol. 1964 Dec;6:383–392. doi: 10.1139/g64-049. [DOI] [PubMed] [Google Scholar]
- WEIJER J., KOOPMANS A., WEIJER D. L. KARYOKINESIS OF SOMATIC NUCLEI OF NEUROSPORA CRASSA: 3. THE JUVENILE AND MATURATION CYCLES (FEULGEN AND CRYSTAL VIOLET STAINING). Can J Genet Cytol. 1965 Mar;7:140–163. doi: 10.1139/g65-020. [DOI] [PubMed] [Google Scholar]



