Abstract
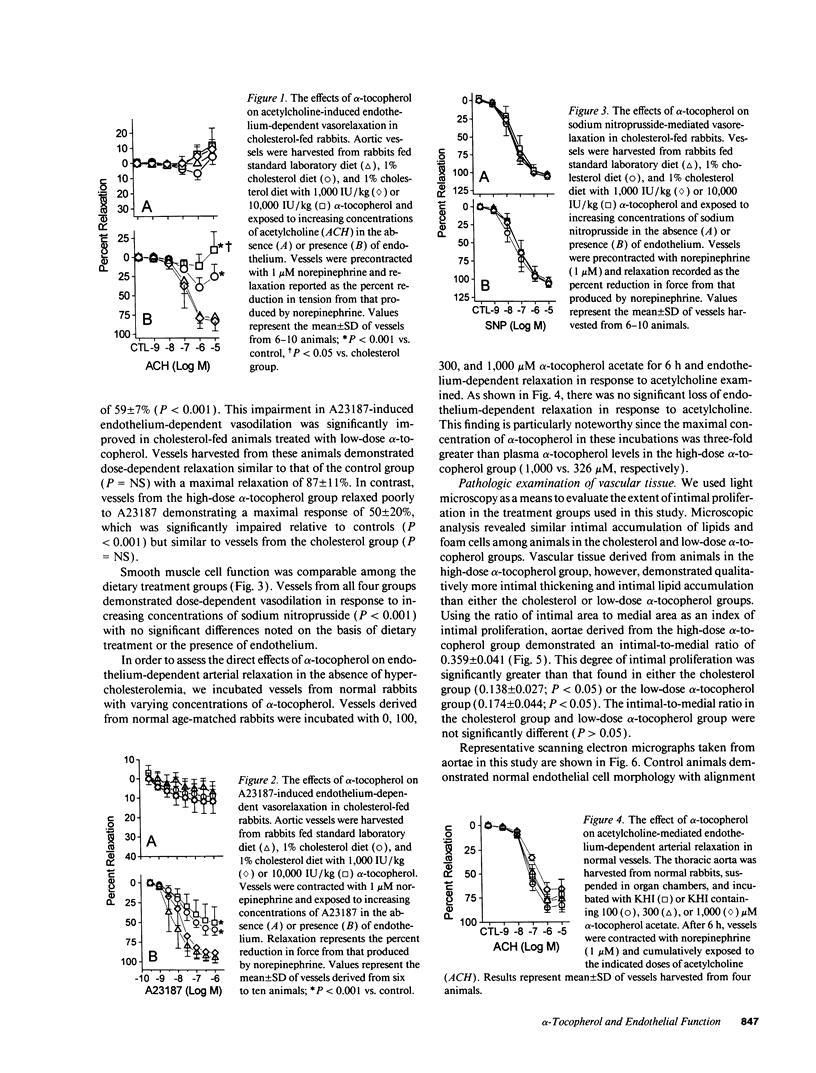
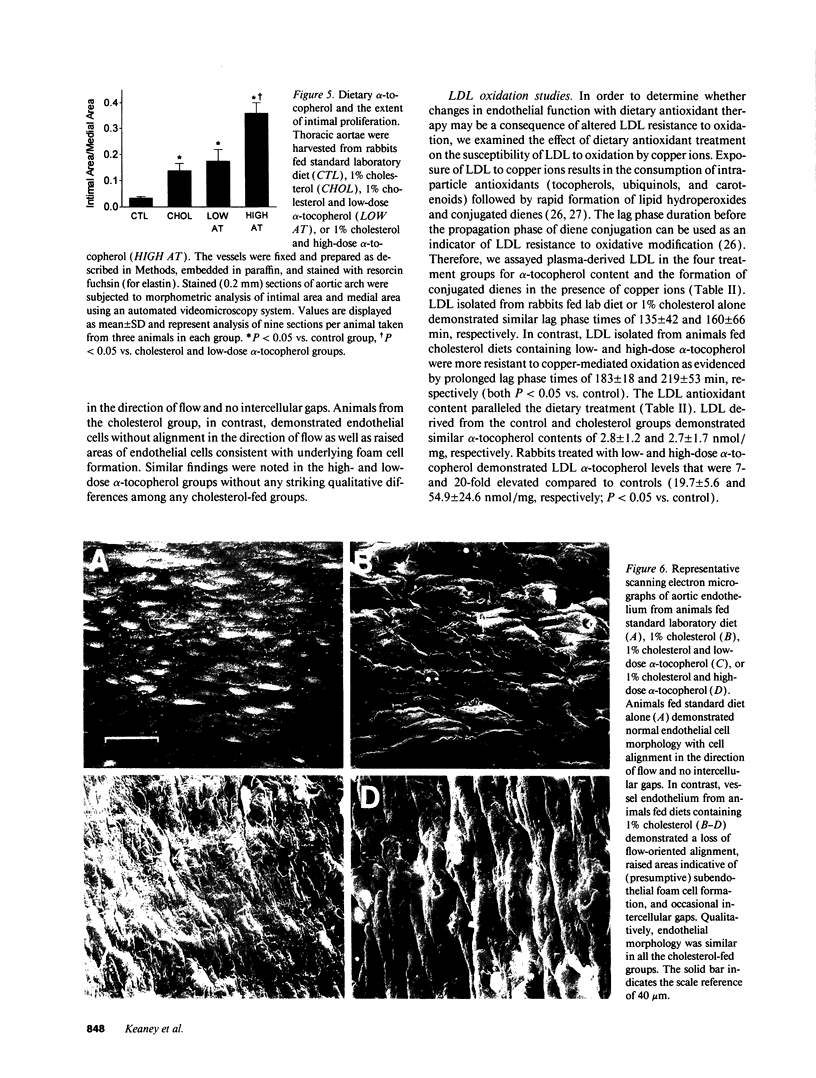
Abnormalities in endothelium-dependent arterial relaxation develop early in atherosclerosis and may, in part, result from the effects of modified low-density lipoprotein (LDL) on agonist-mediated endothelium-derived relaxing factor (EDRF) release and EDRF degradation. alpha-Tocopherol (AT) is the main lipid-soluble antioxidant in human plasma and lipoproteins, therefore, we investigated the effects of AT on endothelium-dependent arterial relaxation in male New Zealand White rabbits fed diets containing (a) no additive (controls), (b) 1% cholesterol (cholesterol group), or 1% cholesterol with either (c) 1,000 IU/kg chow AT (low-dose AT group) or (d) 10,000 IU/kg chow AT (high-dose AT group). After 28 d, we assayed endothelial function and LDL susceptibility to ex vivo copper-mediated oxidation. Acetylcholine-and A23187-mediated endothelium-dependent relaxations were significantly impaired in the cholesterol group (P < 0.001 vs. control), but preserved in the low-dose AT group (P = NS vs. control). Compared to the control and cholesterol groups, vessels from the high-dose AT group demonstrated profound impairment of arterial relaxation (P < 0.05) and significantly more intimal proliferation than other groups (P < 0.05). In normal vessels, alpha-tocopherol had no effect on endothelial function. LDL derived from both the high- and low-dose AT groups was more resistant to oxidation than LDL from control animals (P < 0.05). These data indicate that modest dietary treatment with AT preserves endothelial vasodilator function in cholesterol-fed rabbits while a higher dose of AT is associated with endothelial dysfunction and enhanced intimal proliferation despite continued LDL resistance to ex vivo copper-mediated oxidation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- Ames B. N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann G., Schriewer H., Schmitz G., Hägele E. O. Quantification of high-density-lipoprotein cholesterol by precipitation with phosphotungstic acid/MgCl2. Clin Chem. 1983 Dec;29(12):2026–2030. [PubMed] [Google Scholar]
- Azuma H., Ishikawa M., Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986 Jun;88(2):411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaller C., Habib G. B., Yamamoto H., Williams C., Wells S., Henry P. D. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 5'-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J Clin Invest. 1987 Jan;79(1):170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowry V. W., Ingold K. U., Stocker R. Vitamin E in human low-density lipoprotein. When and how this antioxidant becomes a pro-oxidant. Biochem J. 1992 Dec 1;288(Pt 2):341–344. doi: 10.1042/bj2880341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucolo G., David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973 May;19(5):476–482. [PubMed] [Google Scholar]
- Burton G. W., Joyce A., Ingold K. U. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochem Biophys. 1983 Feb 15;221(1):281–290. doi: 10.1016/0003-9861(83)90145-5. [DOI] [PubMed] [Google Scholar]
- Chin J. H., Azhar S., Hoffman B. B. Inactivation of endothelial derived relaxing factor by oxidized lipoproteins. J Clin Invest. 1992 Jan;89(1):10–18. doi: 10.1172/JCI115549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B. H., Segrest J. P., Ray M. J., Brunzell J. D., Hokanson J. E., Krauss R. M., Beaudrie K., Cone J. T. Single vertical spin density gradient ultracentrifugation. Methods Enzymol. 1986;128:181–209. doi: 10.1016/0076-6879(86)28068-4. [DOI] [PubMed] [Google Scholar]
- Cooney R. V., Franke A. A., Harwood P. J., Hatch-Pigott V., Custer L. J., Mordan L. J. Gamma-tocopherol detoxification of nitrogen dioxide: superiority to alpha-tocopherol. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1771–1775. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieber-Rotheneder M., Puhl H., Waeg G., Striegl G., Esterbauer H. Effect of oral supplementation with D-alpha-tocopherol on the vitamin E content of human low density lipoproteins and resistance to oxidation. J Lipid Res. 1991 Aug;32(8):1325–1332. [PubMed] [Google Scholar]
- Esterbauer H., Striegl G., Puhl H., Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun. 1989;6(1):67–75. doi: 10.3109/10715768909073429. [DOI] [PubMed] [Google Scholar]
- Frei B., England L., Ames B. N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Godfried S. L., Combs G. F., Jr, Saroka J. M., Dillingham L. A. Potentiation of atherosclerotic lesions in rabbits by a high dietary level of vitamin E. Br J Nutr. 1989 May;61(3):607–617. doi: 10.1079/bjn19890148. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N., Hirata K., Yamada M., Hamamori Y., Matsuda Y., Akita H., Yokoyama M. Lysophosphatidylcholine inhibits bradykinin-induced phosphoinositide hydrolysis and calcium transients in cultured bovine aortic endothelial cells. Circ Res. 1992 Dec;71(6):1410–1421. doi: 10.1161/01.res.71.6.1410. [DOI] [PubMed] [Google Scholar]
- Kelm M., Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990 Jun;66(6):1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- Kugiyama K., Kerns S. A., Morrisett J. D., Roberts R., Henry P. D. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature. 1990 Mar 8;344(6262):160–162. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mangin E. L., Jr, Kugiyama K., Nguy J. H., Kerns S. A., Henry P. D. Effects of lysolipids and oxidatively modified low density lipoprotein on endothelium-dependent relaxation of rabbit aorta. Circ Res. 1993 Jan;72(1):161–166. doi: 10.1161/01.res.72.1.161. [DOI] [PubMed] [Google Scholar]
- Minor R. L., Jr, Myers P. R., Guerra R., Jr, Bates J. N., Harrison D. G. Diet-induced atherosclerosis increases the release of nitrogen oxides from rabbit aorta. J Clin Invest. 1990 Dec;86(6):2109–2116. doi: 10.1172/JCI114949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel D. W., Hessler J. R., Chisolm G. M. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983 Aug;24(8):1070–1076. [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Mügge A., Elwell J. H., Peterson T. E., Hofmeyer T. G., Heistad D. D., Harrison D. G. Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ Res. 1991 Nov;69(5):1293–1300. doi: 10.1161/01.res.69.5.1293. [DOI] [PubMed] [Google Scholar]
- Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991 Dec;88(6):2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara Y., Peterson T. E., Harrison D. G. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993 Jun;91(6):2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Barnett J. Phospholipase A2 activity of low density lipoprotein: evidence for an intrinsic phospholipase A2 activity of apoprotein B-100. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9741–9745. doi: 10.1073/pnas.87.24.9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S. Evidence for an additional intracellular site of action of probucol in the prevention of oxidative modification of low density lipoprotein. Use of a new water-soluble probucol derivative. J Clin Invest. 1992 May;89(5):1618–1621. doi: 10.1172/JCI115757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Young S. G., Witztum J. L., Pittman R. C., Steinberg D. Probucol inhibits oxidative modification of low density lipoprotein. J Clin Invest. 1986 Feb;77(2):641–644. doi: 10.1172/JCI112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Fong L. G., Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987 May;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retsky K. L., Freeman M. W., Frei B. Ascorbic acid oxidation product(s) protect human low density lipoprotein against atherogenic modification. Anti- rather than prooxidant activity of vitamin C in the presence of transition metal ions. J Biol Chem. 1993 Jan 15;268(2):1304–1309. [PubMed] [Google Scholar]
- Riemersma R. A., Wood D. A., Macintyre C. C., Elton R. A., Gey K. F., Oliver M. F. Risk of angina pectoris and plasma concentrations of vitamins A, C, and E and carotene. Lancet. 1991 Jan 5;337(8732):1–5. doi: 10.1016/0140-6736(91)93327-6. [DOI] [PubMed] [Google Scholar]
- Riemersma R. A., Wood D. A., Macintyre C. C., Elton R., Gey K. F., Oliver M. F. Low plasma vitamins E and C. Increased risk of angina in Scottish men. Ann N Y Acad Sci. 1989;570:291–295. doi: 10.1111/j.1749-6632.1989.tb14928.x. [DOI] [PubMed] [Google Scholar]
- Rimm E. B., Stampfer M. J., Ascherio A., Giovannucci E., Colditz G. A., Willett W. C. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med. 1993 May 20;328(20):1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- Saran M., Michel C., Bors W. Reaction of NO with O2-. implications for the action of endothelium-derived relaxing factor (EDRF). Free Radic Res Commun. 1990;10(4-5):221–226. doi: 10.3109/10715769009149890. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Stampfer M. J., Hennekens C. H., Manson J. E., Colditz G. A., Rosner B., Willett W. C. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993 May 20;328(20):1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher U. P., Pritchard P. H. Hydrolysis of phosphatidylcholine during LDL oxidation is mediated by platelet-activating factor acetylhydrolase. J Lipid Res. 1989 Mar;30(3):305–315. [PubMed] [Google Scholar]
- Stocker R., Bowry V. W., Frei B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does alpha-tocopherol. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1646–1650. doi: 10.1073/pnas.88.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita J. A., Treasure C. B., Nabel E. G., McLenachan J. M., Fish R. D., Yeung A. C., Vekshtein V. I., Selwyn A. P., Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990 Feb;81(2):491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- Williams R. J., Motteram J. M., Sharp C. H., Gallagher P. J. Dietary vitamin E and the attenuation of early lesion development in modified Watanabe rabbits. Atherosclerosis. 1992 Jun;94(2-3):153–159. doi: 10.1016/0021-9150(92)90240-h. [DOI] [PubMed] [Google Scholar]
- Wilson R. B., Middleton C. C., Sun G. Y. Vitamin E, antioxidants and lipid peroxidation in experimental atherosclerosis of rabbits. J Nutr. 1978 Nov;108(11):1858–1867. doi: 10.1093/jn/108.11.1858. [DOI] [PubMed] [Google Scholar]
- Witztum J. L., Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991 Dec;88(6):1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M., Hirata K., Miyake R., Akita H., Ishikawa Y., Fukuzaki H. Lysophosphatidylcholine: essential role in the inhibition of endothelium-dependent vasorelaxation by oxidized low density lipoprotein. Biochem Biophys Res Commun. 1990 Apr 16;168(1):301–308. doi: 10.1016/0006-291x(90)91708-z. [DOI] [PubMed] [Google Scholar]
- de Groot H., Hegi U., Sies H. Loss of alpha-tocopherol upon exposure to nitric oxide or the sydnonimine SIN-1. FEBS Lett. 1993 Jan 4;315(2):139–142. doi: 10.1016/0014-5793(93)81150-x. [DOI] [PubMed] [Google Scholar]




