Abstract
Glyburide (GLB) is an oral sulfonylurea, commonly used for the treatment of gestational diabetes mellitus. It has been reported that the clearance of GLB in pregnant women is significantly higher than that in nonpregnant women. The molecular mechanism by which pregnancy increases the clearance of GLB is not known, but it may be caused by increased CYP3A activity. Because liver tissue from pregnant women is not readily available, in the present study, we investigated the mechanism of such pregnancy-related changes in GLB disposition in a mouse model. We demonstrated that the systemic clearance of GLB in pregnant mice was increased approximately 2-fold (p < 0.01) compared with nonpregnant mice, a magnitude of change similar to that observed in the clinical study. Plasma protein binding of GLB in mice was not altered by pregnancy. The half-life of GLB depletion in hepatic S-9 fractions of pregnant mice was significantly shorter than that of nonpregnant mice. Moreover, GLB depletion was markedly inhibited by ketoconazole, a potent inhibitor of mouse Cyp3a, suggesting that GLB metabolism in mice is primarily mediated by hepatic Cyp3a. These data suggest that the increased systemic clearance of GLB in pregnant mice is likely caused by an increase in hepatic Cyp3a activity during pregnancy, and they provide a basis for further mechanistic understanding and analysis of pregnancy-induced alterations in the disposition of GLB and drugs that are predominantly and extensively metabolized by CYP3A/Cyp3a.
Introduction
Glyburide (GLB), an oral sulfonylurea, hypoglycemic agent, has been used for the treatment of gestational diabetes mellitus because of its limited placental transfer, similar efficacy to insulin, and ease of administration (Langer et al., 2000). Our recent clinical study demonstrated that the apparent oral clearance of GLB in pregnant women with gestational diabetes mellitus was increased approximately 2-fold compared with that in the control nonpregnant women with type II diabetes mellitus (Hebert et al., 2009). The molecular mechanism by which pregnancy increases the oral clearance of GLB is not known. An increase in oral clearance of a drug (CL/F) could be attributable to an increase in hepatic and/or renal clearance (CL) or a decrease in oral bioavailability (F). GLB is extensively metabolized by the liver. There is no significant renal clearance of the drug (Hebert et al., 2009). GLB is well absorbed with an oral bioavailability of approximately 95% (Jonsson et al., 1994), indicating that the first-pass effect of GLB is likely to be minor. Consequently, we have hypothesized that the significant increase in apparent oral clearance of GLB during pregnancy is likely caused by an increase in hepatic clearance of the drug. This hypothesis is supported by the finding that the formation clearance of the major GLB metabolite, M1, in pregnant patients was increased by 130% compared with that in nonpregnant controls (Hebert et al., 2009).
GLB is a low hepatic extraction ratio (ER) drug (ER ∼0.1) in humans (Jonsson et al., 1994). For a low ER drug, based on the well stirred model, hepatic clearance of the drug is approximated by the product of fraction unbound in blood and intrinsic clearance of the drug in the liver (Rowland and Tozer, 1995). Because there is no observed change in plasma protein binding for GLB during pregnancy (Hebert et al., 2009), an increase in hepatic clearance of GLB can be explained by an increase in intrinsic clearance of GLB in the liver. Increased intrinsic clearance of GLB in the liver is likely caused by an increase in the activity of the major metabolizing enzyme of GLB. Our recent in vitro metabolism studies using human liver microsomes and the studies reported by others have revealed that CYP3A plays a major role in in vitro metabolism of GLB (Naritomi et al., 2004; Zharikova et al., 2009; Zhou et al., 2010). Hence, we further hypothesize that pregnancy induces the activity of hepatic CYP3A, resulting in an increase in oral clearance of GLB. To test this hypothesis, an animal model would be required, because liver tissue from pregnant women is not readily available. The mouse model was used in our study for two reasons. First, we have shown that both the protein levels of hepatic Cyp3a in pregnant mice and its activity measured using testosterone as the probe substrate are significantly increased compared with those in nonpregnant controls (Mathias et al., 2006; Zhang et al., 2008). Second, the activity of hepatic CYP3A in humans has also been shown to be induced by pregnancy, based on the observation of increased metabolism of probe drugs, such as midazolam, that are predominantly catalyzed by CYP3A (Hebert et al., 2008). This induction appears to be caused by increased expression of the CYP3A/Cyp3a gene, possibly mediated by pregnancy-related hormones or growth factors (Zhang et al., 2008).
Therefore, in the present study, we first investigated whether pregnancy increases the systemic clearance of GLB in wild-type FVB mice. We then determined whether the intrinsic clearance of GLB in the liver of pregnant mice was increased by measuring GLB depletion by hepatic S-9 fractions and if the increase in GLB depletion was caused by increased hepatic Cyp3a activity. We have previously shown that GLB is a substrate of human breast cancer resistance protein (BCRP) and its murine homolog Bcrp1 (Zhou et al., 2008). Because Bcrp1 expression in the liver and kidney is induced by pregnancy at midgestation (Wang et al., 2006), we also performed similar pharmacokinetic studies in Bcrp1(−/−) mice to assess the role of Bcrp1 in GLB disposition.
Materials and Methods
Animal Studies.
All of the materials and animals, which included GLB, [3H]GLB, polyethylene glycol 400, FVB wild-type mice, and Bcrp1(−/−) mice, were the same as those described previously (Zhou et al., 2008). The animal study protocol was approved by the Institutional Animal Care and Use Committee of the University of Washington. Feeding and maintenance of mice, weight and age of mice, mating, estimation of gestational age, and monitoring progression of pregnancy were essentially the same as those described previously (Zhou et al., 2008). Pregnant mice used were at day 15 of gestation. GLB was dissolved in a solvent [0.5% (v/v) dimethyl sulfoxide, 10% (v/v) ethanol, 39.5% (v/v) saline, and 50% (v/v) polyethylene glycol 400] at 0.5 mg/ml. Under anesthesia (isoflurane), GLB (1 mg/kg b.wt.) was administered to pregnant or nonpregnant mice by retro-orbital injection. At 0.5, 5, 10, 20, 30, 40, 60, 120, 180, and 240 min after drug administration, 3 to 5 mice per time point were sacrificed under anesthesia by cardiac puncture. Immediately thereafter, liver tissues were harvested and stored at −80°C until use. Blood was collected in heparinized microcentrifuge tubes (BD Biosciences, Franklin Lakes, NJ) and centrifuged at 1,500g at room temperature for 10 min. The harvested plasma samples were stored at −20°C until analysis. GLB concentrations in mouse plasma samples were determined using a validated high-performance liquid chromatography/mass spectrometry assay as described previously (Zhou et al., 2008).
Plasma Protein Binding.
Mouse plasma protein binding of GLB was determined by ultrafiltration using Millipore Centrifree ultrafiltration (Millipore Corporation, Billerica, MA) cartridges as described previously (Hebert et al., 2009). In brief, [3H]GLB (40 ng) in methanol was aliquoted into disposable culture tubes and evaporated to dryness. One milliliter of GLB-free blank plasma from pregnant or nonpregnant mice spiked with nonradioactive GLB was added to each tube. The samples were then mixed well and allowed to equilibrate at 37°C for at least 30 min. Three aliquots (0.3 ml each) of the samples from each tube were transferred to ultrafiltration cartridges, equilibrated at 37°C for 30 min, and centrifuged at 1,000g for 15 min at 37°C. Thirty microliters of the filtrates and unfiltered plasma were counted on a liquid scintillation counter. The fraction unbound (fu) of GLB was calculated as the percentage of the radioactivity of the filtrates to the radioactivity of the corresponding unfiltered plasma. Preliminary experiments showed constant GLB protein binding over the range of 138 to 6040 ng/ml. Nonspecific binding of [3H]GLB was determined by filtrating 0.3 ml of phosphate-buffered saline containing 40 ng/ml [3H]GLB. Nonspecific binding of [3H]GLB was approximately 20%.
GLB Depletion.
Mouse hepatic S-9 fractions were prepared as described previously (Mathias et al., 2006). GLB depletion reaction mixtures contained 100 mM phosphate-buffered saline (pH 7.4), 1 mg/ml S-9 fractions, 5 mM MgCl2, and 0.16 to 1.25 μM GLB dissolved in 1% (v/v) acetonitrile, in a final volume of 200 μl, in the absence or presence of 1 μM ketoconazole (KTZ). After prewarming for 5 min, reactions were initiated by adding the NADPH-regenerating system (1 mM NADP+, 10 mM glucose 6-phosphate, and 1 unit/ml glucose-6-phosphate dehydrogenase). Incubations with the regenerating system or 1% acetonitrile alone were used as negative controls. Reactions were stopped at 0, 5, 10, or 20 min by adding 2 ml of the mixed solvent (n-hexane/methylene chloride at a 1:1 ratio, v/v). Each sample was then acidified by adding 20 μl of HCl (2 M), and 20 μl of glipizide (2 μM) (internal standard) dissolved in acetonitrile was added. The samples were briefly vortexed at room temperature, and the upper organic phase was transferred to a disposable clean glass tube and dried under N2. The dried residue was reconstituted in 100 μl of a mixed solvent (methanol/ H2O at a 20:80 ratio with 0.5 mM ammonium formate). Fifteen microliters of each reconstituted sample were injected, and the GLB concentrations were determined using a validated high-performance liquid chromatography/mass spectrometry assay as described previously (Zhou et al., 2008). Half-life of GLB depletion was calculated according to the first-order decay kinetics by linear regression.
Pharmacokinetic Data Analysis.
Due to the nature of the data (one blood sample from each mouse), the Bailer's approach (Bailer, 1988) was used to estimate the mean and S.E. of the maternal plasma areas under plasma concentration-time curve (AUCs) and other pharmacokinetic parameters including the mean residence time (MRT), CL, and steady-state volume of distribution (Vss), and the normal hypothesis test was performed to assess the statistically significant difference of each parameter between two animal groups, as described previously (Zhou et al., 2008).
GLB was administered to pregnant or nonpregnant mice based on body weight. Because the body weight of a pregnant mouse is usually 1.5 times greater than that of a nonpregnant mouse, pregnant mice received a larger dose. Body weights (mean ± S.D.) of nonpregnant wild-type, pregnant wild-type, nonpregnant Bcrp1(−/−), and pregnant Bcrp1(−/−) mice were 22.9 ± 1.7, 31.2 ± 2.3, 21.2 ± 2.2, and 28.9 ± 3.6 g, respectively. We have previously shown that GLB in the fetuses of pregnant mice only accounts for a small fraction of the total amount of GLB in the body (Zhou et al., 2008), suggesting that the fetus is not a major site for GLB distribution. Hence, we estimated dose-normalized AUC and total plasma CL of GLB as follows.
CL = (mean actual dose of the respective mouse group)/AUC0.5–240 min
AUCdose-normalized = AUC0.5–240 min/(mean actual dose of the respective mouse group)
Statistical Analysis.
Except for pharmacokinetic parameters that were reported as means ± S.E., all other data were presented as means ± S.D. Differences between the two animal groups, pregnant mice versus nonpregnant mice, were analyzed by the normal hypothesis test or the Student's t test. Differences with p values <0.05 were considered statistically significant.
Results and Discussion
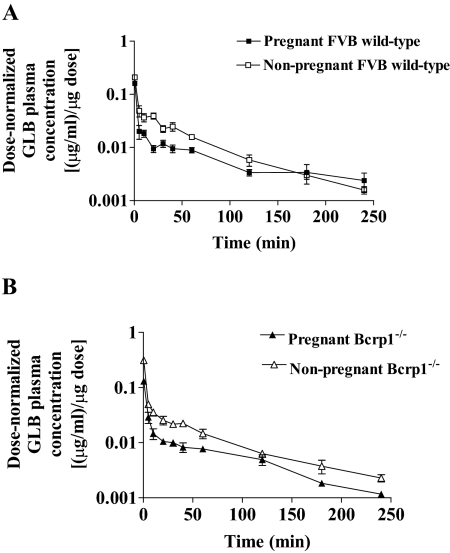
After intravenous administration, the dose-normalized maternal plasma concentrations of GLB in FVB wild-type pregnant mice tended to be lower than those in FVB wild-type nonpregnant mice (Fig. 1A). As a result, the dose-normalized AUC (AUCdose-normalized) in wild-type pregnant mice was 44% lower (p < 0.01) compared with that in wild-type nonpregnant mice (Table 1). Consequently, the CL of GLB was increased approximately 2-fold by pregnancy. Vss of GLB was 126% higher (p < 0.05) during pregnancy. MRT, terminal half-life (T1/2), and fu of GLB in plasma in wild-type pregnant and nonpregnant mice were not significantly different (Table 1). The magnitude of changes in dose-normalized AUC and CL was similar to those observed in humans (Hebert et al., 2009). Because GLB was administered intravenously, the effect of pregnancy on the systemic clearance probably reflects a change in the hepatic metabolism of GLB. For GLB, a low ER drug with no significant renal clearance, the increase in systemic clearance would be mainly caused by an increase in hepatic clearance, which is determined by the fraction unbound in plasma and the intrinsic clearance of GLB in the liver. Our data indicate that the increase in systemic clearance of GLB in pregnant mice is unlikely caused by the change in fraction unbound because there is no observed alteration in mouse plasma protein binding by pregnancy. Likewise, plasma protein binding of GLB in humans was not altered by pregnancy (Hebert et al., 2009).
Fig. 1.
Dose-normalized plasma concentration-time profiles of glyburide in FVB wild-type (A) and Bcrp1(−/−) (B) mice. Pregnant mice at day 15 of gestation and nonpregnant mice were administered GLB (1 mg/kg b.wt.) by retro-orbital injections. At 0.5 to 240 min after drug administration, plasma samples were collected. Dose-normalized GLB plasma concentrations were calculated by dividing the GLB plasma concentrations by the mean actual dose of the respective mouse group. Shown are mean ± S.D. (n = 3–5 mice per time point).
TABLE 1.
Pharmacokinetic parameters of glyburide in pregnant and nonpregnant FVB wild-type (WT) or Bcrp1(−/−) mice after retro-orbital administration at a dose of 1 mg/kg b.wt.
The pharmacokinetic parameters were estimated using Bailer's approach (Bailer, 1988). AUC, CL, MRT, T1/2, and Vss are presented as means ± S.E. (n = 3–5 mice per time point), and the fraction unbound fu (%) is presented as mean ± S.D. of 3 to 5 independent determinations. The differences between the pregnant and nonpregnant mice groups shown in this table were analyzed by normal hypothesis test as previously described (Zhou et al., 2008) for the pharmacokinetic data or the Student's t test for the unbound fraction data, and differences with p values <0.05 were considered to be statistically significant.
| Parameter | WT Pregnant | WT Nonpregnant | p Value | Bcrp1(−/−) Pregnant | Bcrp1(−/−) Nonpregnant | p Value |
|---|---|---|---|---|---|---|
| AUCdose-normalized [(μg · min/ml)/μg] | 1.8 ± 0.1 | 3.2 ± 0.1 | <0.01 | 1.7 ± 0.1 | 3.3 ± 0.1 | <0.01 |
| CL (ml/min) | 0.56 ± 0.03 | 0.31 ± 0.01 | <0.01 | 0.60 ± 0.03 | 0.31 ± 0.01 | <0.01 |
| MRT (min) | 63.2 ± 7.7 | 49.5 ± 3.3 | >0.05 | 56.7 ± 4.6 | 51.0 ± 3.9 | >0.05 |
| T1/2 (min) | 43.8 ± 8.8 | 34.3 ± 4.1 | >0.05 | 39.3 ± 6.0 | 35.4 ± 4.7 | >0.05 |
| Vss (ml) | 35.3 ± 4.6 | 15.6 ± 1.2 | <0.05 | 34.2 ± 3.2 | 15.6 ± 1.3 | <0.05 |
| fu (%) | 3.4 ± 0.3 | 3.2 ± 0.3 | >0.05 | 3.9 ± 0.4 | 3.6 ± 0.3 | >0.05 |
Similar results were obtained with Bcrp1(−/−) and wild-type mice, regardless of whether the mice were pregnant or nonpregnant (Fig. 1B; Table 1). Therefore, Bcrp1 appears to play only a minor role in the systemic clearance of GLB in mice. Consequently, the role of Bcrp1 in the systemic clearance of GLB was not further investigated. GLB is a substrate of other transporters such as P-glycoprotein (Golstein et al., 1999) and organic anion-transporting polypeptide OATP2B1 (Satoh et al., 2005). Whether these transporters play a role in the systemic clearance of GLB is not known. However, because the protein levels of P-glycoprotein in the mouse liver or kidney are not significantly altered by pregnancy (Zhang et al., 2008), we expect that P-glycoprotein-mediated elimination of GLB is not likely to be significantly changed by pregnancy.
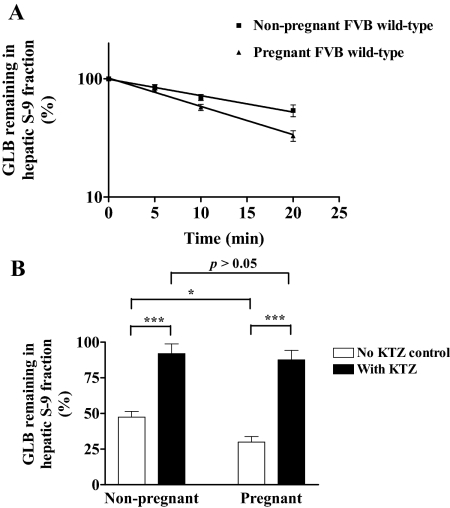
We next examined whether the increase in systemic clearance of GLB during pregnancy is possibly caused by an increase in hepatic depletion of the drug. We analyzed GLB depletion by S-9 fractions isolated from liver tissues of pregnant and nonpregnant wild-type mice. We first measured the yield of total hepatic protein recovered in the S-9 fractions. The protein contents in hepatic S-9 fractions normalized to liver weight were 285.4 ± 47.0 and 209.8 ± 3.1 mg/g liver for nonpregnant and pregnant mice, respectively. The liver weights per mouse for nonpregnant and pregnant mice were 0.64 ± 0.07 and 1.11 ± 0.09 g/mouse, respectively. The liver weights of nonpregnant and pregnant mice normalized to body weight were 27.8 ± 3.1 and 35.6 ± 2.8 g/kg b.wt., respectively. Therefore, the total hepatic protein contents in the S-9 fractions normalized to body weight were 7844.7 ± 849.2 and 7479.2 ± 644.4 mg/kg b.wt. for nonpregnant and pregnant mice, respectively. According to these data, there was approximately a 30% increase in total protein amount of hepatic S-9 fractions isolated from pregnant mice compared with nonpregnant mice. This difference is possibly caused by the increase in liver weight of pregnant mice compared with nonpregnant mice. However, the total protein amount in S-9 fractions normalized to body weight was not significantly affected by pregnancy. In preliminary depletion experiments, we determined the optimal protein concentration of S-9 fractions to be 1 mg/ml, which allows GLB depletion to follow the first-order decay kinetics. In addition, we found that incubation times of up to 20 min were optimal so that a sufficient amount of GLB was depleted for an accurate estimation of metabolic activity, and, at the same time, there was enough GLB remaining for accurate quantification of the drug. The half-life of GLB depletion was found to be unchanged over concentrations ranging from 0.16 to 1.25 μM. Therefore, all subsequent GLB depletion experiments were carried out with 0.625 μM GLB and 1 mg/ml S-9 fractions for incubation of up to 20 min. The half-life of GLB depletion in S-9 fractions of wild-type pregnant mice (13.0 ± 1.4 min) was 37% lower (p < 0.05) than that of wild-type nonpregnant mice (20.6 ± 4.6 min) (Fig. 2A). This result suggests that the intrinsic activity of GLB metabolism by hepatic S-9 fractions is significantly increased by pregnancy. Because the total protein yield in S-9 fractions was not significantly changed by pregnancy after normalization to body weight, we believe that the systemic clearance of GLB in pregnancy is increased by an induction in intrinsic clearance of GLB in the liver, rather than an increase in the amount of hepatic proteins in pregnant mice.
Fig. 2.
Time course of glyburide depletion by mouse hepatic S-9 fractions (A) and inhibition of glyburide depletion by KTZ (B). A, GLB depletion was performed by incubating GLB at 0.625 μM with S-9 fractions (1 mg/ml) of wild-type nonpregnant (solid squares, n = 7 per time point) or wild-type pregnant mice (solid triangles, n = 8 per time point) for up to 20 min. The amounts of GLB at time 0 were set as 100%. Shown are mean ± S.D. B, GLB depletion was performed as in A for 20 min in the absence (open bars) or presence (solid bars) of 1 μM KTZ. The amounts of GLB in the samples with no NADPH-regenerating system added were set as 100%. Shown are mean ± S.D. of six independent determinations. Significant differences: *, p < 0.05; ***, p < 0.005 by the Student's t test.
To determine whether GLB depletion by hepatic S-9 fractions is catalyzed by Cyp3a, we determined the effect of Cyp3a inhibition on GLB depletion. After incubation for 20 min, there was greater depletion of GLB in hepatic S-9 fractions of wild-type pregnant mice (∼70%) compared with wild-type nonpregnant mice (∼50%) (Fig. 2B). However, this depletion was significantly inhibited by 1 μM KTZ (Fig. 2B). Because KTZ is a potent inhibitor of mouse Cyp3a (Mathias et al., 2006), these data suggest that hepatic GLB depletion in mice is primarily mediated by Cyp3a, and the increased GLB depletion by hepatic S-9 fractions of pregnant mice is likely caused by an increase in intrinsic activity of hepatic Cyp3a.
We have previously shown that the activity of human CYP3A is elevated in vivo during pregnancy (Hebert et al., 2008). The activity of mouse hepatic Cyp3a, determined using testosterone 6β-hydroxylation as a marker activity, is similarly induced by pregnancy (Mathias et al., 2006; Zhang et al., 2008). Therefore, we postulate that the increase in systemic clearance of GLB in pregnant mice is likely caused by the increased intrinsic activity of hepatic Cyp3a during pregnancy. Approximately 15% of GLB was still depleted even in the presence of 1 μM KTZ, which is presumably sufficient to fully inhibit Cyp3a (Hickman et al., 1998). This finding suggests that other mouse hepatic cytochrome P450 (P450) isoforms and/or non-P450 enzymes may also contribute to GLB metabolism. We have shown that, in addition to CYP3A4, human CYP3A5, CYP2C19, CYP2C8, and CYP2C9 are also capable of metabolizing GLB (Zhou et al., 2010).
We also observed that pregnancy significantly increased Vss of GLB (Table 1) possibly due to the increase in both total body water and fat content during pregnancy, which suggests that distribution of GLB into maternal tissues is likely increased in pregnancy. Because the CL of GLB was also increased, this observation is consistent with the fact that T1/2 of GLB (T1/2 = 0.693 · Vss/CL) was not significantly altered by pregnancy (Table 1). In a clinical setting with multiple GLB oral dosing, this change in Vss would not be expected to affect the average steady-state plasma concentration (Css,ave) of GLB, because Css,ave of GLB is determined by its dosing rate and clearance [Css,ave = (dose/interval)/oral CL].
In summary, in the present study, we have illustrated that the increased systemic clearance of GLB in pregnant mice is likely due to induction of hepatic Cyp3a activity by pregnancy. Given that CYP3A4 is the major human P450 enzyme responsible for GLB metabolism (Naritomi et al., 2004; Zharikova et al., 2009; Zhou et al., 2010), these data support the hypothesis that the pregnancy-induced increase in the clearance of GLB in humans is also primarily caused by an increase in hepatic CYP3A activity. Such findings have significant clinical implications. For example, caution should be taken when CYP3A inducers or inhibitors are to be coadministered with GLB to avoid potential adverse drug-drug interactions in pregnant women (Lilja et al., 2007). Plasma concentrations of drugs that are CYP3A substrates with a narrow therapeutic index may fall below their effective therapeutic concentrations in pregnancy, and therefore dose adjustment may be required to maintain efficacy during pregnancy. On the other hand, if such medications are titrated to response during pregnancy, a dose reduction may be needed postpartum to avoid potential toxicity. Our data suggest that the FVB mouse may be an appropriate animal model to study the effect of pregnancy on the disposition of drugs that are predominantly and extensively metabolized by hepatic CYP3A/Cyp3a and to investigate the molecular mechanism by which pregnancy induces CYP3A/Cyp3a activity.
Acknowledgments.
We thank Drs. Honggang Wang, Zhanglin Ni, and Xiaokun Cai for technical assistance in GLB depletion and plasma protein binding experiments. We acknowledge Dr. Duane Bloedow for discussion of the pharmacokinetic data.
This work was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grants P50-HD044404, U10-HD047892]. Lin Zhou was the recipient of the William E. Bradley Endowed Fellowship from the School of Pharmacy, University of Washington.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.033837.
- GLB
- glyburide
- CL
- clearance
- F
- oral bioavailability
- ER
- extraction ratio
- BCRP
- breast cancer resistance protein
- fu
- fraction unbound
- KTZ
- ketoconazole
- AUC
- area under plasma concentration-time curve
- MRT
- mean residence time
- Vss
- steady-state volume of distribution
- T1/2
- terminal half-life
- P450
- cytochrome P450.
References
- Bailer AJ. (1988) Testing for the equality of area under the curves when using destructive measurement techniques. J Pharmacokinet Biopharm 16:303–309 [DOI] [PubMed] [Google Scholar]
- Golstein PE, Boom A, van Geffel J, Jacobs P, Masereel B, Beauwens R. (1999) P-glycoprotein inhibition by glibenclamide and related compounds. Pflugers Arch 437:652–660 [DOI] [PubMed] [Google Scholar]
- Hebert MF, Easterling TR, Kirby B, Carr DB, Buchanan ML, Rutherford T, Thummel KE, Fishbein DP, Unadkat JD. (2008) Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther 84:248–253 [DOI] [PubMed] [Google Scholar]
- Hebert MF, Ma X, Naraharisetti SB, Krudys KM, Umans JG, Hankins GD, Caritis SN, Miodovnik M, Mattison DR, Unadkat JD, et al. (2009) Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 85:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman D, Wang JP, Wang Y, Unadkat JD. (1998) Evaluation of the selectivity of in vitro probes and suitability of organic solvents for the measurement of human cytochrome P450 monooxygenase activities. Drug Metab Dispos 26:207–215 [PubMed] [Google Scholar]
- Jönsson A, Rydberg T, Ekberg G, Hallengren B, Melander A. (1994) Slow elimination of glyburide in NIDDM subjects. Diabetes Care 17:142–145 [DOI] [PubMed] [Google Scholar]
- Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. (2000) A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 343:1134–1138 [DOI] [PubMed] [Google Scholar]
- Lilja JJ, Niemi M, Fredrikson H, Neuvonen PJ. (2007) Effects of clarithromycin and grapefruit juice on the pharmacokinetics of glibenclamide. Br J Clin Pharmacol 63:732–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias AA, Maggio-Price L, Lai Y, Gupta A, Unadkat JD. (2006) Changes in pharmacokinetics of anti-HIV protease inhibitors during pregnancy: the role of CYP3A and P-glycoprotein. J Pharmacol Exp Ther 316:1202–1209 [DOI] [PubMed] [Google Scholar]
- Naritomi Y, Terashita S, Kagayama A. (2004) Identification and relative contributions of human cytochrome P450 isoforms involved in the metabolism of glibenclamide and lansoprazole: evaluation of an approach based on the in vitro substrate disappearance rate. Xenobiotica 34:415–427 [DOI] [PubMed] [Google Scholar]
- Rowland M, Tozer T. (1995) Elimination, in Clinical Pharmacokinetics: Concepts and Applications, 3rd ed, pp 165–168, Lippincott Williams & Wilkins, Philadelphia: [Google Scholar]
- Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H, Sawada Y. (2005) Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos 33:518–523 [DOI] [PubMed] [Google Scholar]
- Wang H, Wu X, Hudkins K, Mikheev A, Zhang H, Gupta A, Unadkat JD, Mao Q. (2006) Expression of the breast cancer resistance protein (Bcrp1/Abcg2) in tissues from pregnant mice: effects of pregnancy and correlations with nuclear receptors. Am J Physiol Endocrinol Metab 291:E1295–E1304 [DOI] [PubMed] [Google Scholar]
- Zhang H, Wu X, Wang H, Mikheev AM, Mao Q, Unadkat JD. (2008) Effect of pregnancy on cytochrome P450 3a and P-glycoprotein expression and activity in the mouse: mechanisms, tissue specificity, and time course. Mol Pharmacol 74:714–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zharikova OL, Fokina VM, Nanovskaya TN, Hill RA, Mattison DR, Hankins GD, Ahmed MS. (2009) Identification of the major human hepatic and placental enzymes responsible for the biotransformation of glyburide. Biochem Pharmacol 78:1483–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Naraharisetti SB, Liu L, Wang H, Lin YS, Isoherranen N, Unadkat JD, Hebert MF, Mao Q. (2010) Contributions of human cytochrome P450 enzymes to glyburide metabolism. Biopharm Drug Dispos 31:228–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Naraharisetti SB, Wang H, Unadkat JD, Hebert MF, Mao Q. (2008) The breast cancer resistance protein (Bcrp1/Abcg2) limits fetal distribution of glyburide in the pregnant mouse: an Obstetric-Fetal Pharmacology Research Unit Network and University of Washington Specialized Center of Research Study. Mol Pharmacol 73:949–959 [DOI] [PubMed] [Google Scholar]