Abstract
Clonidine is a centrally acting, α-2 adrenergic agonist used for the treatment of hypertension during pregnancy. The metabolic pathways of clonidine are poorly understood, and the quantitative contribution of specific human cytochrome P450 (P450) isoforms has not been systematically assessed. In this study, 17 cDNA-expressed P450 enzymes, in addition to pooled human liver microsomes, were evaluated for clonidine 4-hydroxylation activity in vitro. Five P450 enzymes—CYP2D6, 1A2, 3A4, 1A1, and 3A5—catalyzed measurable formation of 4-hydroxyclonidine. Selective inhibition studies in human liver microsomes confirmed that these isoforms are jointly responsible for 4-hydroxylation of clonidine in vitro, and CYP2D6 accounted for approximately two-thirds of the activity. The major role of CYP2D6 in clonidine metabolism might explain the increase in its nonrenal clearance during pregnancy.
Introduction
Clonidine [2-(2,6-dichloroanilino)-2-imidazoline] is an α-2 adrenergic agonist used extensively to treat hypertension. It is also used to treat withdrawal from opiate addiction, insomnia and Tourette's syndrome, and in conjunction with stimulants for the treatment of attention-deficit hyperactivity disorder (Dollery, 1991). By acting as a presynaptic α-2 receptor agonist in the brain, it inhibits the sympathetic outflow, causing lower heart rate and blood pressure (MacMillan et al., 1996).
Clonidine has been used for the treatment of hypertensive disorders in pregnant women and is well tolerated by the mothers (Horvath et al., 1985). However, clinical observations indicate that higher doses or more frequent dosing of clonidine may be required during pregnancy (Buchanan et al., 2009). Consistent with those observations, we have recently found that apparent oral clearance of clonidine in pregnant women is 80% higher compared with that reported in the literature for men and nonpregnant women (Buchanan et al., 2009). The renal component of clonidine clearance remained unchanged as we described previously (Buchanan et al., 2009), suggesting that alteration in bioavailability and/or nonrenal clearance is responsible for the observed change in clonidine pharmacokinetics during pregnancy. Limited studies have suggested that oral drug absorption is not altered in pregnancy (Anderson, 2005), which leaves modulation of nonrenal clearance as a more likely explanation for the change in oral clonidine clearance in pregnancy. In nonpregnant subjects, approximately 60% of orally administered clonidine is cleared unchanged by the kidneys (Davies et al., 1977), with the remainder undergoing hepatic metabolism to produce inactive metabolites, mainly 4-hydroxyclonidine (Dollery, 1991). However, phase I metabolism of clonidine is poorly understood, and no attempt has been made to assess the role of drug-metabolizing cytochrome P450 (P450) enzymes in clonidine metabolism. The aim of this study was to determine the contribution of P450 enzymes toward the 4-hydroxylation of clonidine, which, in turn, could offer insight into the mechanism of the pregnancy-induced alteration in the nonrenal clearance of clonidine.
Materials and Methods
Materials.
Clonidine hydrochloride and reduced NADPH were purchased from Sigma-Aldrich (St. Louis, MO). Human liver microsomes [(HLM) pool of 50 mixed-gender livers; lot no. 0710091] were obtained from XenoTech, LLC (Lenexa, KS). Recombinant P450 Supersomes (CYP3A4, 3A5, 2A6, 2B6, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 2J2, 1A1, 1A2, 1B1, 4F2, 4F3A and 4F3B) were obtained from BD Gentest (Woburn, MA). Puregene buccal cell kit was from Gentra Systems (Minneapolis, MN). The internal standard clonidine-d4 was from CDN Isotopes (Quebec, Canada). 4-Hydroxyclonidine and 4-hydroxyclonidine-d4 were synthesized at Fred Hutchinson Cancer Research Center (Mundla et al., 2000). All other reagents were from Sigma-Aldrich.
Microsomal Reaction for Clonidine Hydroxylation.
Either 0.1 mg of microsomal protein or 0.02 mg of recombinant enzyme in 0.1 M potassium phosphate buffer (pH 7.4) was added to 2 ml of microcentrifuge tubes. With all samples in a 37°C shaking water bath, clonidine (in methanol at <0.5% v/v final concentration) was added to initiate a 5-min preincubation, after which 1 mM NADPH was added. The final incubation volume was 200 μl. The reaction was allowed to proceed for 60 (HLM) or 30 min (Supersomes) and was terminated by the addition of an equal volume of ice-cold methanol, vortexing, and placing the samples on ice. A set of calibration standards (0.5–100 ng of 4-hydroxyclonidine) was processed along with the reaction incubates. Forty microliters of methanol containing 1 ng of each internal standard was added to all samples. Samples were vortexed and centrifuged at 13,000g for 5 min. The supernatant was injected directly onto the high-performance liquid chromatography column. Product formation was linear with respect to time and protein concentration for up to 2 h and up to 1 mg/ml protein, respectively.
Screening with Selective P450 Inhibition.
One hundred nanomolars quinidine (selective CYP2D6 inhibitor), 20 μM furafylline (selective CYP1A2 inhibitor), or 5 μM ketoconazole (selective CYP3A4 inhibitor) was added to a microsomal incubation with varying levels of substrate (0.1–10 μM clonidine) and corresponding controls. Contribution of the inhibited isoform to clonidine 4-hydroxylation in pooled HLM was measured as percent inhibition from control activity.
Liquid Chromatography-Mass Spectrometry Analysis.
Analysis of 4-hydroxyclonidine in the incubation samples was performed by liquid chromatography-mass spectrometry (Buchanan et al., 2009). Mass ions were monitored at m/z 230 (clonidine), m/z 236 (clonidine-d4), m/z 246 (4-hydroxyclonidine), and m/z 252 (4-hydroxyclonidine-d4). The fragmentor voltage was set to 160 V, and a dwell time of 192 ms was set for each ion.
Genotyping.
Genomic DNA was isolated from buccal swabs collected in our earlier clinical study in pregnant women (Buchanan et al., 2009) using Gentra Systems Buccal Cell Kit. DNA integrity was evaluated by agarose gel electrophoresis and A260/A280 ratio (1.8–2.0). The DNA samples were genotyped for CYP2D6 polymorphisms using Applied Biosystems (Foster City, CA) single nucleotide polymorphisms genotyping “Assays on Demand.” Validation was accomplished with internal control DNA samples of known genotype(s). The polymerase chain reaction (PCR) cycling conditions were 95°C for 10 min and 50 cycles of 95°C for 15 s and 60°C for 90 s. Post-PCR allelic discrimination analysis was performed on an ABI 7900HT instrument (Applied Biosystems). Specific polymorphisms examined were *2, *3, *4, *6, *9, *10, *17, *35, and *41, and the indicated location of the genetic variants were taken from the Human Cytochrome P450 Allele Nomenclature Committee website (http://www.cypalleles.ki.se/). CYP2D6 gene deletion/duplication (*5/CYP2D6UM) genotyping was performed using the Applied Biosystems “TaqMan Gene Copy Number Assay” reagents according to the manufacturer's protocol. Samples were run on an ABI 7900HT instrument at 95°C for 10 min and 40 cycles of 95°C for 15 s and 60°C for 1 min. The real-time PCR data were collected using ABI sequence detection system software (SDS version 2.3; Applied Biosystems). The cycle threshold values for the RNase P gene served as a reference when analyzing the samples for the presence or absence of CYP2D6 sequences. Each assay included controls known to have either 1, 2, or 3 copies of the CYP2D6 gene. Patient genotypes were called using CopyCaller software (version 1.0; Applied Biosystems).
Data Analysis.
Product formation rates for all incubations are presented as mean ± S.D. of quadruplicate determinations of activity for each substrate or inhibitor concentration. Km and Vmax values were estimated from a nonlinear regression analysis of the mean formation rates versus substrate concentration using Prism GraphPad software (La Jolla, CA).
Results and Discussion
Clonidine and its 4-hydroxy metabolite, along with deuterated internal standards of each compound, were fully resolved in the chromatographic system used. The lower limit of detection of 4-hydroxyclonidine was 0.125 ng/ml; the lower limit of quantitation was 0.25 ng/ml. Low (∼5 ng/ml) and high (∼100 ng/ml) quality controls exhibited intraday %CV of 1.4 and 1.8%, respectively. Interday %CV (n = 10) was 9.7 and 5.5% for low- and high-quality control samples, respectively.
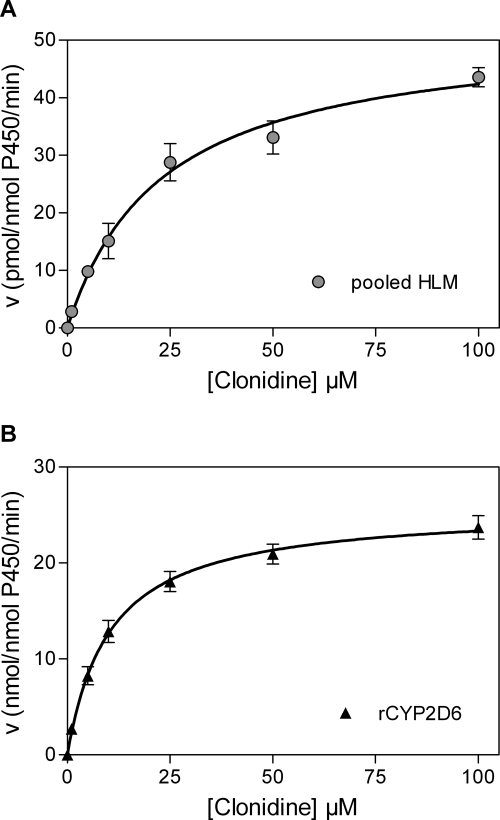
Figure 1 presents the rate of clonidine 4-hydroxylation versus substrate concentration in pooled HLM and recombinant CYP2D6. In HLM, clonidine 4-hydroxylation had a Km of 23.0 ± 2.6 μM and a Vmax of 52.1 ± 2.1 pmol/nmol P450/min; whereas recombinant CYP2D6 exhibited a Km of 10.3 ± 0.7 μM and a Vmax of 26.1 ± 0.5 nmol/nmol P450/min.
Fig. 1.
Saturation curves of clonidine 4-hydroxylation versus substrate concentration in pooled HLM (A) and recombinant CYP2D6 (B). Materials and Methods provides detailed assay information. Data points correspond to the mean ± S.D. of quadruplicate determinations in a single experiment. Activity expressed as pmol/nmol P450/min (A) or nmol/nmol P450/min (B).
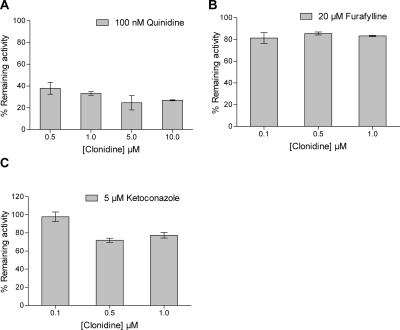
In the present study, 17 drug-metabolizing P450 enzymes were evaluated for 4-hydroxylation of clonidine, which was confirmed as the major metabolic pathway in vitro. In the initial screen, five of the P450 enzymes produced detectable quantities of 4-hydroxy metabolite under the conditions described, the rank order of formation rate per nanomoles P450 per minute being CYP2D6 (5.88 ± 3.27) > CYP1A2 (2.74 ± 0.10) > CYP3A4 (2.21 ± 0.12) > CYP1A1 (1.74 ± 0.08) > CYP3A5 (0.48 ± 0.06). In consideration of the known abundance of these five P450 enzymes in the human liver, further studies focused on CYP2D6, CYP1A2, and CYP3As. Selective inhibitors of CYP2D6 (quinidine), CYP1A2 (furafylline), and CYP3As (ketoconazole) were applied to quantify the contribution of each enzyme to the overall rate of clonidine 4-hydroxylation in pooled HLM (Fig. 2). Quinidine at 100 nM inhibited clonidine 4-hydroxylation by 66.9 ± 1.6% at a substrate concentration of 1 μM; whereas 5 μM ketoconazole and 20 μM furafylline inhibited formation by 22.6 ± 3.0% and 14.5 ± 1.5%, respectively. These results indicate that CYP2D6 is the dominant isoform responsible for 4-hydroyxlation of clonidine.
Fig. 2.
Effects on clonidine 4-hydroxylation in HLM from addition of quinidine [CYP2D6 inhibitor (A)], furafylline [CYP1A2 inhibitor (B)], and ketoconazole [CYP3A inhibitor (C)]. Quinidine was evaluated across a broader range of clonidine substrate concentrations than furafylline or ketoconazole.
To verify the in vivo importance of CYP2D6 in the nonrenal clearance of clonidine, we conducted a retrospective analysis of the relationship between CYP2D6 genotypes and the apparent nonrenal clearance of clonidine in our earlier cohort of pregnant mothers (Buchanan et al., 2009). Of the original 17 subjects, 14 of the buccal swabs provided good quality genotyping by real-time PCR. Two of the 14 genotyped subjects can be classified as poor metabolizer (PM), and both were homozygotes of CYP2D6*4. Of the remaining subjects with extensive metabolizer (EM) genotypes, seven were either homozygotes of wild-type alleles or heterozygotes of wild-type alleles and normal function or gain-of-function variants (*2 and *35), and five were heterozygotes with one copy of null or deleted allele or loss-of-function variant (*4, *5, and *10). The respective mean and 95% confidence interval for the apparent nonrenal clearance in the pregnant EMs were 311 ml/min and 211 to 411 ml/min. The apparent nonrenal clearance for the two PMs were 138 and 182 ml/min, both lying outside of the 95% confidence interval for the EMs. CYP2D6 EMs and PMs were clearly segregated with respect to their apparent nonrenal clonidine clearance, a strong indication that CYP2D6 plays a major role in the oxidative metabolism of clonidine in vivo.
The major involvement of CYP2D6 has several clinically important implications for clonidine pharmacokinetics in vivo. First, up-regulation of CYP2D6 activity has been suggested as the mechanism for the observed gestational changes in the pharmacokinetics of several drugs, including metoprolol (Högstedt et al., 1985) and dextromethorphan (Wadelius et al., 1997). Enhanced hepatic CYP2D6 activity is consistent with our earlier observation of an approximate 80% increase in apparent oral clearance of clonidine in pregnant women compared with nonpregnant subjects (440 ± 168 versus 245 ± 72 ml/min, respectively) (Buchanan et al., 2009). Gestational-induced changes in hepatic blood flow, plasma protein binding, or kidney function were ruled out as likely mechanisms, because clonidine was administered orally, its plasma protein binding is low, and the drug's renal clearance did not change (Buchanan et al., 2009). Thus, our current findings are highly suggestive of a role for CYP2D6 in the increased apparent oral clearance of clonidine during pregnancy. Second, the complex genetic polymorphism of CYP2D6 can lead to wide interindividual and ethnic differences in CYP2D6-mediated drug metabolism. Individual variation in the metabolic clearance of clonidine could explain the 6-fold range in the daily dose of clonidine (0.1–0.6 mg) for the treatment of hypertension.
Although it would have been desirable to determine the contribution of individual P450 isoforms to clonidine metabolism at therapeutically relevant concentrations, the clonidine concentrations used in our incubations ranged between 0.5 and 10 μM, which is approximately 100 to 1000 times the peak plasma concentration reported in the literature (Velasquez et al., 1983). Lower concentrations of clonidine could not be evaluated in the inhibitor experiments because of assay limitations for the metabolite. Because the contribution of P450 isoforms to metabolism of a drug may change depending on the concentration used (Lin and Lu, 1997), we cannot exclude the possibility that a very high-affinity (i.e., Km < 0.1 μM) and low Vmax enzyme(s) may have escaped detection because of saturation and much diminished contribution at concentrations near or above 0.5 μM. However, we are reasonably confident that CYP2D6 is the principal enzyme for clonidine metabolism in vivo based on our retrospective analysis of the relationship between CYP2D6 genotypes and clonidine oral clearance in our earlier cohort of pregnant mothers (Buchanan et al., 2009). The apparent nonrenal clearance of clonidine in the CYP2D6 PM subjects lie below the lower limit of the 95% confidence interval for the CYP2D6 EM group. This result confirms that CYP2D6 plays a major role in the oxidative metabolism of clonidine in vivo.
In conclusion, our findings indicate that 4-hydroxylation of clonidine is primarily mediated by CYP2D6 and to a lesser extent by CYP3A4/5 and CYP1A1/2. The major role of CYP2D6 in clonidine nonrenal clearance explains the previously observed apparent increase in clonidine oral clearance in pregnant women. Clonidine can now be added as another CYP2D6 substrate whose pharmacokinetics are altered in pregnancy.
Acknowledgments.
We thank Dr. Jeffrey Posakony (Fred Hutchinson Cancer Research Center) for synthesis of 4-hydroxyclonidine and 4-hydroxyclonidine-d4 and for technical consultation related to this project. We also thank Tot Bui Nguyen and Dr. Edward Kelly (University of Washington, School of Pharmacy DNA Sequencing and Gene Analysis Center) for their assistance in CYP2D6 genotyping.
This work was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant U10-HD047892] (in support of the Obstetric-Fetal Pharmacology Research Unit Network).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.033878.
- P450
- cytochrome P450
- HLM
- human liver microsomes
- PCR
- polymerase chain reaction
- %CV
- percent coefficient of variance
- PM
- poor metabolizer
- EM
- extensive metabolizer.
References
- Anderson GD. (2005) Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet 44:989–1008 [DOI] [PubMed] [Google Scholar]
- Buchanan ML, Easterling TR, Carr DB, Shen DD, Risler LJ, Nelson WL, Mattison DR, Hebert MF. (2009) Clonidine pharmacokinetics in pregnancy. Drug Metab Dispos 37:702–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DS, Wing AM, Reid JL, Neill DM, Tippett P, Dollery CT. (1977) Pharmacokinetics and concentration-effect relationships of intravenous and oral clonidine. Clin Pharmacol Ther 21:593–601 [DOI] [PubMed] [Google Scholar]
- Dollery SC. (1991) Clonidine (hydrochloride), in Therapeutic Drugs (Dollery SC. ed), pp C305–C311, Churchill Livingstone, New York: [Google Scholar]
- Högstedt S, Lindberg B, Peng DR, Regårdh CG, Rane A. (1985) Pregnancy-induced increase in metoprolol metabolism. Clin Pharmacol Ther 37:688–692 [DOI] [PubMed] [Google Scholar]
- Horvath JS, Phippard A, Korda A, Henderson-Smart DJ, Child A, Tiller DJ. (1985) Clonidine hydrochloride–a safe and effective antihypertensive agent in pregnancy. Obstet Gynecol 66:634–638 [PubMed] [Google Scholar]
- Lin JH, Lu AY. (1997) Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol Rev 49:403–449 [PubMed] [Google Scholar]
- MacMillan LB, Hein L, Smith MS, Piascik MT, Limbird LE. (1996) Central hypotensive effects of the alpha2a-adrenergic receptor subtype. Science 273:801–803 [DOI] [PubMed] [Google Scholar]
- Mundla SR, Wilson LJ, Klopfenstein SR, Seibel WL, Nikolaides NN. (2000) A novel method for the efficient synthesis of 2-arylamino-2-imidazolines. Tet Lett 41:6563–6566 [Google Scholar]
- Velasquez MT, Rho J, Maronde RF, Barr J. (1983) Plasma clonidine levels in hypertension. Clin Pharmacol Ther 34:341–346 [DOI] [PubMed] [Google Scholar]
- Wadelius M, Darj E, Frenne G, Rane A. (1997) Induction of CYP2D6 in pregnancy. Clin Pharmacol Ther 62:400–407 [DOI] [PubMed] [Google Scholar]