Abstract
Many studies have examined the relationship between polymorphisms in glutathione S-transferase genes and cancer in people exposed to polycyclic aromatic hydrocarbons (PAH) such as benzo[a]pyrene (BaP), but the results to date have been modest. Missing from these studies has been an exploration of the formation of the appropriate glutathione conjugates in humans. We incubated human hepatocytes from 10 donors with racemic anti-BaP-7,8-diol-9,10-epoxide (BPDE), believed to be a major ultimate carcinogen of BaP, or with the noncarcinogenic reverse diol epoxide, racemic anti-BaP-9,10-diol-7,8-epoxide (rev-BPDE). Incubations were carried out for 12 or 24 h. We used high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry-selected reaction monitoring at m/z 464 → m/z 317 to analyze the incubation mixtures for the mercapturic acid products that would result from glutathione conjugation. The standard mercapturic acids were synthesized by reaction of BPDE or rev-BPDE with N-acetylcysteine. We obtained convincing evidence in human hepatocytes for mercapturic acid formation from rev-BPDE in all 10 samples, in amounts up to 17 pmol/ml. However, we could detect mercapturic acids from BPDE in only 1 of 10 samples (0.05 pmol/ml). Taken together with our similar previous results of analyses of phenanthrene metabolites in human hepatocytes and human urine, the results of this study indicate that conjugation of BPDE with glutathione is a minor pathway in humans, indicating that glutathione S-transferase genotyping is not an effective method for assessing risk of PAH-induced cancer in humans, at least with respect to the diol epoxide pathway of PAH carcinogenesis.
Introduction
Polycyclic aromatic hydrocarbons (PAH) are well established environmental carcinogens that may play a significant role in the causation of skin and lung cancer in occupationally exposed humans and lung cancer in smokers (International Agency for Research on Cancer, 1983, 1984, 1985; Dipple et al., 1984; Luch, 2005; Straif et al., 2005). The prototypic PAH is benzo[a]pyrene (BaP), one of the most extensively studied of all carcinogens, and considered carcinogenic to humans by the International Agency for Research on Cancer (Straif et al., 2005). BaP and other PAH require metabolic activation to exert their carcinogenic effects (Dipple et al., 1984). One of the accepted pathways of metabolic activation of BaP proceeds through the formation of the carcinogenic “bay region diol epoxide,” anti-BaP-7,8-diol-9,10-epoxide (BPDE), illustrated in Fig. 1A (Conney, 1982; Cooper et al., 1983; Dipple et al., 1984). BPDE reacts easily with DNA to form adducts that cause miscoding and mutations. These adducts are crucial factors in its mechanism of carcinogenesis. There are opposing detoxification mechanisms, one of which is commonly believed to be conjugation with GSH. Many epidemiologic studies have examined the relationship between polymorphisms in glutathione S-transferase (GST) genes and the occurrence of cancer in people exposed to PAH and investigated the hypothesis that those deficient in GST activity, as determined by genotyping of variants in GSTM1 and GSTP1, should be at higher risk. Lung cancer is a thoroughly studied example of this effect, and the results have not shown consistent associations or were null (Hashibe et al., 2003; Vineis et al., 2004; Ye et al., 2006; Carlsten et al., 2008; Cote et al., 2009).
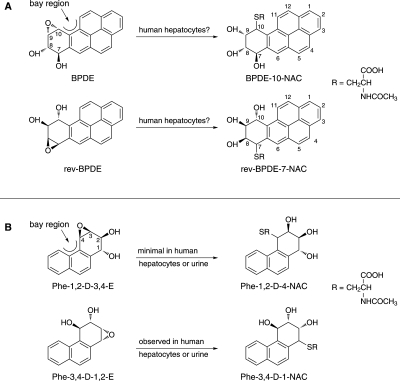
Fig. 1.
Overview of the question posed in this study (A) and our previous results in studies (B) of GSH conjugation of Phe diol epoxides (Hecht et al., 2008, 2009). The mercapturic acid metabolites illustrated are produced by normal metabolic processing of initially formed GSH conjugates by successive action of γ-glutamyltranspeptidase, cysteinylglycine dipeptidase, and cysteine S-conjugate N-acetyltransferase.
Our approach to investigating the role of GSTs in PAH carcinogenesis started with a phenotyping strategy in which we proposed to characterize and quantify the urinary mercapturic acids resulting from GST-catalyzed detoxification of PAH diol epoxides, followed by normal metabolic processing of the initially formed GST conjugates by γ-glutamyltranspeptidase, cysteinylglycine dipeptidase, and cysteine S-conjugate N-acetyltransferase (Hecht et al., 2008). In this work, we focused on phenanthrene (Phe), the simplest PAH with a bay region, a feature closely associated with PAH carcinogenicity; however, Phe is not generally considered to be carcinogenic. Human exposure to Phe is thousands of times higher than to BaP, thus facilitating analysis of its urinary metabolites. We analyzed human urine for the mercapturic acid 4-(N-acetylcysteinyl)-1,2,3-trihydroxy-1,2,3,4-tetrahydrophenanthrene (Phe-1,2-D-4-NAC) (Fig. 1B), which would be formed upon conjugation of the bay region diol epoxide anti-Phe-1,2-diol-3,4-epoxide (Phe-1,2-D-3,4-E). We found no evidence for the presence of this mercapturic acid in human urine, but we identified considerable amounts of 1-(N-acetylcysteinyl)-2,3,4-trihydroxy-1,2,3,4-tetrahydrophenanthrene (Phe-3,4-D-1-NAC), the mercapturic acid that resulted from the “reverse diol epoxide” anti-Phe-3,4-diol-1,2-epoxide (Phe-3,4-D-1,2-E). Reverse diol epoxides are not generally associated with strong mutagenicity or carcinogenicity, although there are some exceptions (Conney, 1982; Glatt et al., 1993). In further studies, we investigated the metabolism of Phe-1,2-diol, Phe-3,4-diol, Phe-1,2-D-3,4-E, and Phe-3,4-D-1,2-E in human hepatocytes (Hecht et al., 2009). Consistent with the results of our analyses of human urine, we found that GSH conjugation of the reverse diol epoxide Phe-3,4-D-1,2-E was strongly favored over conjugation of the bay region diol epoxide Phe-1,2-D-3,4-E (Hecht et al., 2009). Taken together, these results were quite surprising because they seemed to contradict the hypothesis that carcinogenic bay region diol epoxides, such as Phe-1,2-D-3,4-E and BPDE, were detoxified by GST-catalyzed conjugation. Many studies have investigated the conjugation of BPDE with GSH, catalyzed by GST-M1-1, GST-P1-1, and GST-A1-1, either as purified or expressed enzymes or in cellular systems engineered to overexpress these enzymes (Robertson et al., 1986; Jernström et al., 1989, 1996; Romert et al., 1989; Sundberg et al., 1997, 1998, 2001, 2002; Fields et al., 1998; Seidel et al., 1998; Hu et al., 1999; Srivastava et al., 1999; Kushman et al., 2007a,b). All of these studies demonstrate GSH conjugation of BPDE, and most show a decrease in BPDE-DNA binding and mutagenesis in tandem with the conjugation. Indeed, these studies reasonably form the basis for the hypothesis that individuals with lower GST activity are at higher risk for cancer upon exposure to PAH. However, we are not aware of any studies in the literature that demonstrate conjugation of BPDE with GSH in human cellular systems that have not been engineered to overexpress GSTs. Taken together, these data raised some important questions about the veracity of the BPDE GSH detoxification hypothesis. If BPDE is not conjugated by GSTs in humans, as we have seen for Phe-1,2-D-3,4-E, then this pathway is not as important as previously thought. Therefore, in this study, we examined the formation of mercapturic acids in human hepatocytes incubated with BPDE or the reverse diol epoxide anti-BaP-9,10-diol-7,8-epoxide (rev-BPDE) (Fig. 1A).
Materials and Methods
Chemicals.
10-(N-Acetylcysteinyl)-9-hydroxy-9,10-dihydrophenanthrene (Phe-9-hydroxy-10-NAC) was prepared as described previously (Upadhyaya et al., 2006). Racemic BPDE and rev-BPDE were obtained from the National Cancer Institute Chemical Carcinogen Reference Standard Repository (Midwest Research Institute, Kansas City, MO). N-Acetylcysteine (NAC) was purchased from Sigma-Aldrich (St. Louis, MO).
7-(N-Acetylcsyteinyl)-8,9,10-trihydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene (rev-BPDE-7-NAC) was prepared essentially as described previously (Hecht et al., 2008). In brief, a solution of racemic rev-BPDE (2.4 mg, 0.008 mmol) in 2.0 ml of dry tetrahydrofuran was added to 2 ml of NAC (3.0 M) (adjusted to pH 10.0). The mixture was stirred for 2 h, and the isomers were separated by high-performance liquid chromatography (HPLC) and collected using a 250 × 10 mm, 10μ, C18 Vydac 210TP column (Separations Group, Hesperia, CA) eluted at 3 ml/min with 30 mM NH4HCO3 (60%) and 40% CH3OH, overall yield, 1.6 mg, 45.3%. Isomer 1 (670 μg), retention time 29.92 min; UV λmax 202, 248, 328, 345 nm; MS (negative ion ESI) m/z 464 [M − H]− (100); MS/MS of m/z 464; m/z 446 (100), 317 (51); 1H NMR (D2O) δ8.19 (s, 1H, H11), 8.01 (m, 1H, H6), 7.66 − 7.45 (m, 6H, H1, H2, H3, H4, H5, and H12), 5.46 (m, 1H, H10), 4.40 (m, 1H, H9), 4.31 (m, 2H, H7 and H8), 3.95 (m, 1H, CHNH), 2.82 (m, 1H, SCH2a), 2.57 (m, 1H, SCH2b), 1.51 (s, 3H, CH3); 13C NMR (D2O) δ128.2, 127.9, 127.3, 126.2, 125.5, 125.5 (CH1, CH2, CH3, CH4, CH5, CH12), 125.8 (CH11), 123.1 (CH6), 72.9 (CH9), 68.9 (CH8), 68.6 (CH10), 54.3 (CHNH), 49.3 (CH7), 31.8 (SCH2), 21.8 (COCH3). Isomer 2 (1.0 mg), retention time 34.56 min; UV λmax 201, 248, 328, 345 nm; MS (negative ion ESI) m/z 464 [M − H]− (100); MS/MS of m/z 464; m/z 446 (100), 317 (51); 1H NMR (D2O) δ8.21 (s, 1H, H11), 8.13 (m, 1H, H6), 7.87 − 7.79 (m, 3H, H1, H5, and H3), 7.69 (dd, J = 7.2, 7.2 Hz, 1H, H2), 7.60 (m, 3H, H4, and H12), 5.51 (d, J = 3.6 Hz, 1H, H10), 4.43 (m, 1H, H9), 4.34 (m, 2H, H7 and H8), 4.27 (m, 1H, CHNH), 2.95 (dd, J = 4.8, 13.2 Hz, 1H, SCH2a), 2.60 (m, 1H, SCH2b), 1.81 (s, 3H, CH3); 13C NMR (D2O) δ127.6, 127.1, 126.7, 125.9, 125.2, 125.2 (CH1, CH2, CH3, CH4, CH5, CH12), 123.5 (CH11), 122.8 (CH6), 72.5 (CH8), 70.9 (CH10), 70.8 (CH9), 54.5 (CHNH), 46.0 (CH7), 34.4 (SCH2), 21.1 (COCH3).
10-(N-Acetylcysteinyl)-7,8,9-trihydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene (BPDE-10-NAC) was similarly prepared, 0.87 mg, 23.4% overall yield. Isomer 1 (254 μg), retention time 27.57 min; UV λmax 201, 248, 328, 345 nm; MS (negative ion ESI) m/z 464 [M − H]− (100); MS/MS of m/z 464; m/z 446 (100), 335 (58); 317 (53.5); 283 (13.6); 1H NMR (D2O) δ8.3 (s, 1H, H11), 8.19 (s, 1H, H6), 8.10 (m, 2H, H1, and H3), 8.01 (d, J = 7.2Hz, 1H, H5), 7.87 (dd, J = 7.8, 7.2 Hz, 1H, H2), 7.83 (m, 1H, H4), 7.67 (m, 1H, H12), 5.03 (m, 1H, H10), 4.64 (m, 1H, H7), 4.52 − 4.48 (m, 2H, H8 and H9), 4.19 (dd, J = 3.6, 6.6 Hz, 1H, CHNH), 3.17 (dd, J = 7.2, 13.2 Hz, 1H, SCH2a), 2.89 (m, 1H, SCH2b), 1.56 (s, 3H, CH3); 13C NMR (D2O) δ128.3, 127.9, 127.4, 126.7, 125.9, 125.9 (CH1, CH2, CH3, CH4, CH5, CH12), 123.7 (CH11), 123.8 (CH6), 73.8 (CH9), 70.9 (CH8), 71.1 (CH10), 71.0 (CH7), 54.8 (CHNH), 35.0 (SCH2), 22.0 (COCH3). Isomer 2 (622 μg), retention time 31.25 min; UV λmax 201, 248, 328, 345 nm; m/z 464 [M − H]− (100); MS/MS of m/z 464; m/z 446 (100), 335 (58); 317 (53.5); 283 (13.6); 1H NMR (D2O) δ7.78 (s, 1H, H11), 7.73 (s, 1H, H6), 7.48 − 6.96 (m, 6H, H1, H2, H3, H4, H5, and H12), 4.98 (d, J = 8.4 Hz, 1H, H10), 4.73 (m, 1H, H7), 4.57 (m, 1H, H9), 4.51 (m, 2H, H8, and CHNH), 3.29 (m, 1H, SCH2a), 2.80 (m, 1H, SCH2b), 1.76 (s, 3H, CH3); 13C NMR (D2O) δ128.8, 128.2, 127.0, 126,4, 125.5, 125.5 (CH1, CH2, CH3, CH4, CH5, CH12), 126.0 (CH11), 123.3 (CH6), 73.0 (CH9), 69.8 (CH8), 68.7 (CH10), 54.6 (CHNH), 49.2 (CH7), 32.6 (SCH2), 22.0 (COCH3).
Hepatocyte Incubations.
Primary human hepatocytes were purchased from CellzDirect (St. Louis, MO). In brief, freshly isolated hepatocytes were plated onto 12-well plates (7 × 105 cells/well) and overlaid with Matrigel 24 to 48 h after attachment. Cells were shipped overnight on cold preservation media, and, upon receipt, media were replaced with serum-free Williams' E media (without phenol red) and supplements as described previously (Hecht et al., 2009). Cells were allowed to recover from shipping for 10 h at 37°C in an atmosphere containing 5% CO2. Before incubation with substrate, the media were exchanged with 2 ml of fresh media per well. Hepatocytes (approximately 0.12 mg of protein per well) were incubated with BPDE or rev-BPDE, dissolved in 20 μl of dimethyl sulfoxide, for a final substrate concentration of 10 μM, except as noted in Table 1. Aliquots (0.4 ml) of media were removed 12 h after addition of substrate. At 24 h after addition, the remaining media were collected. Samples were stored at −20°C until analysis. Cell viability was assessed at 24 h by trypan blue exclusion staining. Expression of GSTM1 and GSTA1 and measurement of GST activity by conjugation of 1-chloro-2,4-dinitrobenzene (CDNB) were carried out as described previously (Hecht et al., 2009).
TABLE 1.
Characteristics of hepatocytes and their donors and formation of mercapturic acidsa
| Sample No. | Gender | Age | BMI | Race | Smoker | Alcohol User | CDNB Activity (nmol CDNB conjugate/min/mg total protein) | GSTM1 Expression | GSTA1 Expression | rev BPDE-7-NAC (pmol/ml)b |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 h |
24 h |
||||||||||||
| Peak 1 | Peak 2 | Peak 1 | Peak 2 | ||||||||||
| 1065 | F | 69 | 31 | Caucasian | No | No | 42 | + | + | ND | 0.34 | 0.28 | 10.1 |
| 1066 | F | 44 | 20 | Caucasian | No | Yes | 46 | + | ND | ND | 0.08 | 0.12 | |
| 1071 | M | 54 | 24 | African-American | No | No | 25 | + | 0.01 | 0.05 | 0.17 | 0.72 | |
| 1072 | F | 66 | 30 | Caucasian | Yes | No | 42 | + | 0.04 | 0.14 | 0.22 | 1.29 | |
| 1075 | F | 40 | 35 | Caucasian | No | No | 45 | + | + | ND | 0.24 | 0.1 | 2.4 |
| 1078 | M | 66 | 28 | Caucasian | No | No | 43 | + | ND | ND | 0.07 | 0.16 | |
| 1081 | F | 56 | 25 | Caucasian | No | No | 111 | + | ND | ND | 0.17 | 0.33 | |
| 1085 | F | 43 | 27 | Caucasian | No | No | 163 | + | + | 0.06 | 1.89 | 0.43 | 17 |
| 1088 | M | 58 | 22 | Caucasian | Yes | Yes | 74 | + | 0.04 | 0.06 | 0.07 | 0.18 | |
| 1091 | M | 47 | 30 | Caucasian | No | Yes | 61 | + | + | ND | 0.005 | ND | 0.19 |
| Mean ± S.D.c | 54 ± 11 | 27 ± 4.5 | 65.2 ± 41.8 | 0.017 ± 0.23 | 0.30 ± 0.61 | 0.18 ± 0.12 | 3.59 ± 5.95 | ||||||
BMI, body mass index; F, female; ND, not detected; M, male.
Hepatocytes were incubated with 10 μM BPDE or rev-BPDE, except sample 1091 (1 μM).
BPDE-10-NAC was detected in only one sample, no. 1085, 0.015 (12 h) and 0.035 (24 h) pmol/ml.
Means for rev-BPDE-7-NAC are based on the nine samples in which the concentration of rev-BPDE was 10 μM.
Analysis of Mercapturic Acids.
The internal standard Phe-9-hydroxy-10-NAC was added to a 0.2 ml of aliquots of incubation medium, and the mixture was applied to a Strata-X polymeric sorbent cartridge (33 μm, 30 mg/1 ml; Phenomenex, Torrance, CA) that had been activated with 1 ml of CH3OH and 1 ml of H2O. The cartridge was washed with 1 ml of H2O and the analyte was eluted with 1 ml of 90% CH3OH. The eluant was collected in a 2-ml silanized vial, and the solvents were removed on a SpeedVac. The residue was taken up in 250 μl of CH3OH and 5 μl of NH4OAc (4%), transferred to an insert vial, and concentrated to dryness. The residue was dissolved in 20 μl of aqueous NH4OAc (1%), and 8 μl was analyzed by liquid chromatography-electrospray ionization-tandem mass spectrometry-selected reaction monitoring (LC-ESI-MS/MS-SRM) on a Thermo Finnigan TSQ Quantum Discovery MAX instrument (Thermo Fisher Scientific, Waltham, MA) interfaced with an Agilent 1100 Capillary HPLC System (Agilent Technologies, Palo Alto, CA). The HPLC was equipped with a 5 μm, 150 × 0.5 mm Zorbax SB-C18 column (Agilent Technologies, Santa Clara, CA) and a KrudKatcher disposable precolumn filter (Phenomenex). The column was eluted at 10 μl/min with 30 mM NH4HCO3 (60%) in CH3OH. The analysis was carried out essentially as described previously (Hecht et al., 2009), except that the transition m/z 464→ m/z 317, negative ion mode, [M − 1]− → [(M − 1) − (CH2CH(CO2)NHCOCH3 + H2O)]−, was monitored for rev-BPDE-7-NAC or BPDE-10-NAC, and m/z 356 → m/z 209 was monitored for Phe-9-hydroxy-10-NAC.
Results
Each reaction of racemic BPDE or rev-BPDE with NAC produced a mixture of two isomers, separable by HPLC. An example of the reaction of rev-BPDE with NAC is illustrated in Fig. 2A. Each isomer had a UV spectrum, 1H and 13C-NMR spectra, and MS consistent with the expected products, BPDE-10-NAC and rev-BPDE-7-NAC (Fig. 1A). Analogous to previous work (Hecht et al., 2008), the first and second isomers probably resulted from cis and trans addition of NAC to the diol epoxides, but further characterization was not pursued.
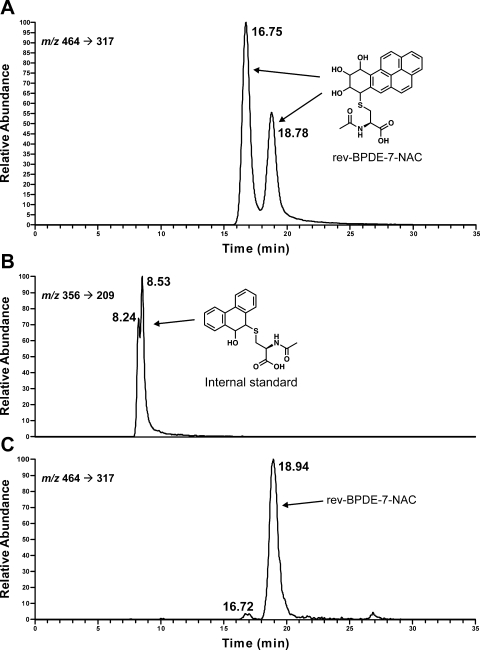
Fig. 2.
Chromatograms obtained upon LC-ESI-MS/MS-SRM analysis of products formed in the reaction of racemic rev-BPDE with NAC (A); internal standard Phe-9-hydroxy-10-NAC for the hepatocyte analysis (B); and medium from hepatocytes incubated with 10 μM rev-BPDE for 24 h (C).
Demographic data for the hepatocyte donors are summarized in Table 1. Each sample was positive for expression of GSTA1, and four were positive for GSTM1 expression. CDNB activity averaged 65.2 ± 41.8 nmol conjugate/(min · mg) protein. These data are consistent with previous results (Hecht et al., 2009).
A chromatogram obtained upon LC-ESI-MS/MS-SRM analysis of a sample in which rev-BPDE was incubated for 24 h with human hepatocytes is illustrated in Fig. 2C, along with the internal standard for the analysis (Fig. 2B). A clear peak corresponding to the second rev-BPDE-7-NAC isomer was observed. All samples in which rev-BPDE was added to human hepatocytes gave similar chromatograms, although the amounts of the two peaks varied. The results are summarized in Table 1. Mean levels of isomers 1 and 2 of rev-BPDE-7-NAC, respectively, in the nine samples incubated with 10 μM rev-BPDE were 0.017 ± 0.023 and 0.30 ± 0.61 pmol/ml in the 12-h incubations and 0.18 ± 0.12 and 3.59 ± 5.95 pmol/ml in the 24-h incubations. BPDE-10-NAC (isomer 2) was observed in only one of the 10 samples incubated with BPDE at levels of 0.015 (12 h) and 0.035 (24 h) pmol/ml.
Discussion
The results reported here demonstrate that the reverse diol epoxide rev-BPDE undergoes GSH conjugation in human hepatocytes, but the carcinogenic bay region diol epoxide, BPDE, does not (except for relatively small amounts in one sample). These results are completely consistent with our previous study of GSH conjugation of Phe diol epoxides in human hepatocytes, summarized in Fig. 1B. The reverse diol epoxide, Phe-3,4-D-1,2-E, was a far better substrate for GSH conjugation, as determined by analysis of Phe-3,4-D-1-NAC in the medium, than was the bay region diol epoxide Phe-1,2-D-3,4-E, for which only minimal conjugation to Phe-1,2-D-4-NAC was observed. Reverse diol epoxides such as rev-BPDE and their diol precursors generally have little or no carcinogenic or mutagenic activity in contrast to bay region diol epoxides (Conney, 1982; Glatt et al., 1993). Consequently, these results have substantial implications for molecular epidemiologic studies that have examined, using genotyping strategies, the relationship between GSTM1 null or GSTP1 low activity variants and cancer in people exposed to PAH. We find little or no evidence to support the assumption, inherent in many of these studies, that carcinogenic bay region diol epoxides of PAH are detoxified by GSH conjugation in unadulterated human cells.
Robertson and coworkers (Robertson et al., 1986) were apparently the first to study conjugation of BPDE and other PAH diol epoxides by human enzymes. This was followed by a series of studies using purified human GSTs (Robertson et al., 1986), cellular subfractions with GST activity (Jernström et al., 1989), recombinant GSTs and their variants (Jernström et al., 1996; Sundberg et al., 1997, 1998), and Chinese hamster V79 cells overexpressing human GSTs (Seidel et al., 1998; Sundberg et al., 2001, 2002). These studies demonstrated that bay region and fjord region diol epoxides can be converted to GSH conjugates by GST M1, GST P1, and GST A1, and that such conjugation was in most cases protective against DNA damage and mutagenicity. However, in one study using unadulterated MCF-7 human breast carcinoma cells incubated with BPDE, no activity was found (Romert et al., 1989). Another notable study found that only approximately 1 to 2% of the rate expected for GSH conjugation of BPDE was actually observed in cells, which indicated the importance of competing reactions of BPDE (Sundberg et al., 2002). Townsend and coworkers (Fields et al., 1998; Kushman et al., 2007a,b) also studied GSH conjugation of BPDE in cells overexpressing human GSTs and found protection against BPDE-DNA binding and mutagenicity. Singh and coworkers (Hu et al., 1999; Srivastava et al., 1999) obtained similar results and made the interesting observation that the GSH conjugate of BPDE inhibits its own formation. We are not aware of any studies in the literature that report the GSH conjugation of BPDE in unadulterated human cells or in humans.
The studies discussed above present a comprehensive and authoritative examination of PAH diol epoxide conjugation catalyzed by GSTs. One conclusion of these studies is that diol epoxide stereochemistry strongly affects conjugation. Our results are consistent with this finding. In BPDE, the 10-position of the diol epoxide, where conjugation would occur, is sterically more hindered because of its presence in the bay region than the 7-position of rev-BPDE. The major difference between the earlier studies and those presented here is that they used hamster V79 cells stably expressing different human GSTs, whereas our work was performed with human hepatocytes that were not engineered to affect GST conjugation, and this difference is undoubtedly critical in explaining the divergent results. However, one aspect that is not examined in this study is the possible differences in transport or secretion of the GST conjugates of rev-BPDE and BPDE into the medium.
In summary, the results presented here do not support the hypothesis that the carcinogenic bay region diol epoxide of BaP is detoxified by GSH conjugation in human hepatocytes. Therefore, a basic assumption underlying molecular epidemiology studies of GST variants and cancer in people exposed to PAH may be incorrect.
This work was supported by the National Institutes of Health National Cancer Institute [Grant CA-92025].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.034181.
- PAH
- polycyclic aromatic hydrocarbons
- BaP
- benzo[a]pyrene
- BPDE
- anti-BaP-7,8-diol-9,10-epoxide
- GST
- glutathione S-transferase
- Phe
- phenanthrene
- Phe-1,2-D-3,4-E
- anti-Phe-1,2-diol-3,4-epoxide
- Phe-3,4-D-1,2-E
- anti-Phe-3,4-diol-1,2-epoxide
- Phe-1,2-D-4-NAC
- 4-(N-acetylcysteinyl)-1,2,3-trihydroxy-1,2,3,4-tetrahydrophenanthrene
- Phe-3,4-D-1-NAC
- 1-(N-acetylcysteinyl)-2,3,4-trihydroxy-1,2,3,4-tetrahydrophenanthrene rev-BPDE, anti-BaP-9,10-diol-7,8-epoxide
- Phe-9-hydroxy-10-NAC
- 10-(N-acetylcysteinyl)-9-hydroxy-9,10-dihydrophenanthrene
- NAC
- N-acetylcysteine
- rev-BPDE-7-NAC
- 7-(N-acetylcsyteinyl)-8,9,10-trihydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene
- HPLC
- high-performance liquid chromatography
- MS
- mass spectrometry
- MS/MS
- tandem mass spectrometry
- ESI
- electrospray ionization
- BPDE-10-NAC
- 10-(N-acetylcysteinyl)-7,8,9-trihydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene
- CDNB
- 1-chloro-2,4-dinitrobenzene
- LC-ESI-MS/MS-SRM
- liquid chromatography-electrospray ionization-tandem mass spectrometry-selected reaction monitoring.
References
- Carlsten C, Sagoo GS, Frodsham AJ, Burke W, Higgins JP. (2008) Glutathione S-transferase M1 (GSTM1) polymorphisms and lung cancer: a literature-based systematic HuGE review and meta-analysis. Am J Epidemiol 167:759–774 [DOI] [PubMed] [Google Scholar]
- Conney AH. (1982) Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res 42:4875–4917 [PubMed] [Google Scholar]
- Cooper CS, Grover PL, Sims P. (1983) The metabolism and activation of benzo[a]pyrene. Prog Drug Metab 7:295–396 [Google Scholar]
- Cote ML, Chen W, Smith DW, Benhamou S, Bouchardy C, Butkiewicz D, Fong KM, Gené M, Hirvonen A, Kiyohara C, et al. (2009) Meta- and pooled analysis of GSTP1 polymorphism and lung cancer: a HuGE-GSEC review. Am J Epidemiol 169:802–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipple A, Moschel RC, Bigger CAH. (1984) Polynuclear aromatic hydrocarbons, in Chemical Carcinogens, 2nd ed, ACS Monograph 182, vol 1 (Searle CE. ed) pp 41–163, American Chemical Society, Washington, D.C. [Google Scholar]
- Fields WR, Morrow CS, Doss AJ, Sundberg K, Jernström B, Townsend AJ. (1998) Overexpression of stably transfected human glutathione S-transferase P1-1 protects against DNA damage by benzo[a]pyrene diol-epoxide in human T47D cells. Mol Pharmacol 54:298–304 [DOI] [PubMed] [Google Scholar]
- Glatt H, Wameling C, Elsberg S, Thomas H, Marquardt H, Hewer A, Phillips DH, Oesch F, Seidel A. (1993) Genotoxicity characteristics of reverse diol-epoxides of chrysene. Carcinogenesis 14:11–19 [DOI] [PubMed] [Google Scholar]
- Hashibe M, Brennan P, Strange RC, Bhisey R, Cascorbi I, Lazarus P, Oude Ophuis MB, Benhamou S, Foulkes WD, Katoh T, et al. (2003) Meta- and pooled analyses of GSTM1, GSTT1, GSTP1, and CYP1A1 genotypes and risk of head and neck cancer. Cancer Epidemiol Biomarkers Prev 12:1509–1517 [PubMed] [Google Scholar]
- Hecht SS, Berg JZ, Hochalter JB. (2009) Preferential glutathione conjugation of a reverse diol epoxide compared to a bay region diol epoxide of phenanthrene in human hepatocytes: relevance to molecular epidemiology studies of glutathione-S-transferase polymorphisms and cancer. Chem Res Toxicol 22:426–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, Villalta PW, Hochalter JB. (2008) Analysis of phenanthrene diol epoxide mercapturic acid detoxification products in human urine: relevance to molecular epidemiology studies of glutathione-S-transferase polymorphisms. Carcinogenesis 29:937–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Herzog C, Zimniak P, Singh SV. (1999) Differential protection against benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide-induced DNA damage in HepG2 cells stably transfected with allelic variants of pi class human glutathione S-transferase. Cancer Res 59:2358–2362 [PubMed] [Google Scholar]
- International Agency for Research on Cancer (1983) Polynuclear aromatic compounds, part 1, chemical, environmental, and experimental data, in IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol 32, pp 33–91, IARC, Lyon, FR: [PubMed] [Google Scholar]
- International Agency for Research on Cancer (1984) Polynuclear aromatic compounds, part 3. Industrial exposures in aluminum production, coal gasification, coke production, and iron and steel founding, in IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol 34, pp 65–131, IARC, Lyon, FR: [Google Scholar]
- International Agency for Research on Cancer (1985) Polynuclear aromatic compounds, part 4. Bitumens, coal-tars and derived products, shale oils and soots, in IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol 35, pp 83–241, IARC, Lyon, France: [PubMed] [Google Scholar]
- Jernström B, Dock L, Hall M, Mannervik B, Tahir MK, Grover PL. (1989) Glutathione transferase catalyzed conjugation of benzo[a]pyrene 7,8-diol 9,10-epoxide with glutathione in human skin. Chem Biol Interact 70:173–180 [DOI] [PubMed] [Google Scholar]
- Jernström B, Funk M, Frank H, Mannervik B, Seidel A. (1996) Glutathione S-transferase A1-1-catalysed conjugation of bay and fjord region diol epoxides of polycyclic hydrocarbons with glutathione. Carcinogenesis 17:1491–1498 [DOI] [PubMed] [Google Scholar]
- Kushman ME, Kabler SL, Ahmad S, Doehmer J, Morrow CS, Townsend AJ. (2007a) Protective efficacy of hGSTM1–1 against B[a]P and (+)- or (−)-B[a]P-7,8-dihydrodiol cytotoxicity, mutagenicity, and macromolecular adducts in V79 cells coexpressing hCYP1A1. Toxicol Sci 99:51–57 [DOI] [PubMed] [Google Scholar]
- Kushman ME, Kabler SL, Fleming MH, Ravoori S, Gupta RC, Doehmer J, Morrow CS, Townsend AJ. (2007b) Expression of human glutathione S-transferase P1 confers resistance to benzo[a]pyrene or benzo[a]pyrene-7,8-dihydrodiol mutagenesis, macromolecular alkylation and formation of stable N2-Gua-BPDE adducts in stably transfected V79MZ cells co-expressing hCYP1A1. Carcinogenesis 28:207–214 [DOI] [PubMed] [Google Scholar]
- Luch A. (2005) Polycyclic aromatic hydrocarbon-induced carcinogenesis-an introduction, in The Carcinogenic Effects of Polycyclic Aromatic Hydrocarbons (Luch A. ed) pp 1–18, Imperial College Press, London: [Google Scholar]
- Robertson IG, Guthenberg C, Mannervik B, Jernström B. (1986) Differences in stereoselectivity and catalytic efficiency of three human glutathione transferases in the conjugation of glutathione with 7 β,8 α-dihydroxy-9 α,10 α-oxy-7,8,9,10-tetrahydrobenzo(a)pyrene. Cancer Res 46:2220–2224 [PubMed] [Google Scholar]
- Romert L, Dock L, Jenssen D, Jernström B. (1989) Effects of glutathione transferase activity on benzo[a]pyrene 7,8-dihydrodiol metabolism and mutagenesis studied in a mammalian cell co-cultivation assay. Carcinogenesis 10:1701–1707 [DOI] [PubMed] [Google Scholar]
- Seidel A, Friedberg T, Löllmann B, Schwierzok A, Funk M, Frank H, Holler R, Oesch F, Glatt H. (1998) Detoxification of optically active bay- and fjord-region polycyclic aromatic hydrocarbon dihydrodiol epoxides by human glutathione transferase P1-1 expressed in Chinese hamster V79 cells. Carcinogenesis 19:1975–1981 [DOI] [PubMed] [Google Scholar]
- Srivastava SK, Hu X, Xia H, Awasthi S, Amin S, Singh SV. (1999) Metabolic fate of glutathione conjugate of benzo[a]pyrene-(7R,8S)-diol (9S,10R)-epoxide in human liver. Arch Biochem Biophy 371:340–344 [DOI] [PubMed] [Google Scholar]
- Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Cogliano V; WHO International Agency for Research on Cancer Monograph Working Group (2005) Carcinogenicity of polycyclic aromatic hydrocarbons. Lancet Oncol 6:931–932 [DOI] [PubMed] [Google Scholar]
- Sundberg K, Dreij K, Berntsen S, Seidel A, Jernström B. (2001) Expression of human glutathione transferases in V79 cells and the effect on DNA adduct-formation of diol epoxides derived from polycyclic aromatic hydrocarbons. Chem-Biol Interact 133:91–94 [Google Scholar]
- Sundberg K, Dreij K, Seidel A, Jernström B. (2002) Glutathione conjugation and DNA adduct formation of dibenzo[a,l]pyrene and benzo[a]pyrene diol epoxides in V79 cells stably expressing different human glutathione transferases. Chem Res Toxicol 15:170–179 [DOI] [PubMed] [Google Scholar]
- Sundberg K, Johansson AS, Stenberg G, Widersten M, Seidel A, Mannervik B, Jernström B. (1998) Differences in the catalytic efficiencies of allelic variants of glutathione transferase P1-1 towards carcinogenic diol epoxides of polycyclic aromatic hydrocarbons. Carcinogenesis 19:433–436 [DOI] [PubMed] [Google Scholar]
- Sundberg K, Widersten M, Seidel A, Mannervik B, Jernström B. (1997) Glutathione conjugation of bay- and fjord-region diol epoxides of polycyclic aromatic hydrocarbons by glutathione transferases M1-1 and P1-1. Chem Res Toxicol 10:1221–1227 [DOI] [PubMed] [Google Scholar]
- Upadhyaya P, Rao P, Hochalter JB, Li ZZ, Villalta PW, Hecht SS. (2006) Quantitation of N-acetyl-S-(9,10-dihydro-9-hydroxy-10-phenanthryl)-l-cysteine in human urine: comparison with glutathione-S-transferase genotypes in smokers. Chem Res Toxicol 19:1234–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vineis P, Veglia F, Anttila S, Benhamou S, Clapper ML, Dolzan V, Ryberg D, Hirvonen A, Kremers P, Le Marchand L, et al. (2004) CYP1A1, GSTM1 and GSTT1 polymorphisms and lung cancer: a pooled analysis of gene-gene interactions. Biomarkers 9:298–305 [DOI] [PubMed] [Google Scholar]
- Ye Z, Song H, Higgins JP, Pharoah P, Danesh J. (2006) Five glutathione S-transferase gene variants in 23,452 cases of lung cancer and 30,397 controls: meta-analysis of 130 studies. PLoS Med 3:e91 [DOI] [PMC free article] [PubMed] [Google Scholar]