Abstract
Constitutive androgen receptor (CAR) is activated by several chemicals and in turn regulates multiple detoxification genes. Our research demonstrates that parathion is one of the most potent, environmentally relevant CAR activators with an EC50 of 1.43 μM. Therefore, animal studies were conducted to determine whether CAR was activated by parathion in vivo. Surprisingly, CAR-null mice, but not wild-type (WT) mice, showed significant parathion-induced toxicity. However, parathion did not induce Cyp2b expression, suggesting that parathion is not a CAR activator in vivo, presumably because of its short half-life. CAR expression is also associated with the expression of several drug-metabolizing cytochromes P450 (P450). CAR-null mice demonstrate lower expression of Cyp2b9, Cyp2b10, Cyp2c29, and Cyp3a11 primarily, but not exclusively in males. Therefore, we incubated microsomes from untreated WT and CAR-null mice with parathion in the presence of esterase inhibitors to determine whether CAR-null mice show perturbed P450-mediated parathion metabolism compared with that in WT mice. The metabolism of parathion to paraoxon and p-nitrophenol (PNP) was reduced in CAR-null mice with male CAR-null mice showing reduced production of both paraoxon and PNP, and female CAR-null mice showing reduced production of only PNP. Overall, the data indicate that CAR-null mice metabolize parathion slower than WT mice. These results provide a potential mechanism for increased sensitivity of individuals with lower CAR activity such as newborns to parathion and potentially other chemicals due to decreased metabolic capacity.
Introduction
Because of a continuously growing human population, modern agriculture has relied heavily on pesticides to produce high crop yields, prevent diseases, and control pests. Organophosphates are some of the most widely used pesticides (Singh and Walker, 2006), accounting for 38% of total worldwide pesticide use for agricultural purposes and household pest control (Parliamentary Office of Science and Technology, 1998). In the United States, these pesticides accounted for 70% of the total insecticides used as of 2001 (Kiely et al., 2004). The Food Quality Protection Act severely restricts organophosphate use in the United States (Environmental Protection Agency, 2002); however, we are increasingly consuming imported fruits and vegetables that may come from countries that presently use organophosphates such as parathion. A total of 36% of fresh fruits are imported into the United States from countries such as Ecuador, Honduras, Panama, Costa Rica, Colombia, and Guatemala, and Canada and Mexico supply 83% of the fresh vegetables imported (Huang and Huang, 2007). A study in Mexico found organophosphate residues in 87% of the broccoli analyzed (Perez et al., 2009). In Colombia every sample of several crops contained residues of at least two different organophosphates (Murcia and Stashenko, 2008). Recent studies have reported the indoor and outdoor application of the methyl ortholog of parathion in southern Texas (Saller et al., 2007). Thus, organophosphates are still routinely used worldwide and are still entering the U.S. marketplace.
Some of the health effects of organophosphate poisoning include several nerve and muscular effects, primarily caused by inhibition of the breakdown of the neurotransmitter acetylcholine (Ragnarsdottir, 2000). Of the organophosphates, parathion has been classified by the Environmental Protection Agency as pesticide Toxicity Category 1 (highly toxic) and Group C (possible human carcinogen) as well as being labeled as one of the most acutely toxic pesticides registered.
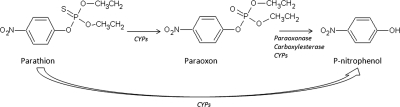
Parathion toxicity is caused by its bioactivation into a toxic metabolite, paraoxon, by cytochromes P450 (P450s) through an oxidative desulfuration event (Gallow and Lawryk, 1991) (Fig. 1). The thionosulfur atom of the paraoxon metabolite covalently binds to the active site of acetylcholinesterase, resulting in inactivation of the enzyme and leading to the overstimulation of cholinergic neurons (Gallow and Lawryk, 1991). Esterases, such as paraoxonases and carboxylesterases, are important detoxifiers of the toxic-oxon metabolite into the nontoxic p-nitrophenol (PNP) metabolite. P450s can also mediate the formation of PNP and are therefore critical in the detoxification of parathion (Fig. 1). CYP2B6, a key enzyme regulated by CAR (NR1I3) in humans, is considered crucial in the metabolism of parathion as are CYP2D6, and CYP2C and CYP3A isoforms, but to a lesser degree than CYP2B6 (Mutch and Williams, 2006; Foxenberg et al., 2007).
Fig. 1.
Parathion is metabolized by P450s (CYPs) into the toxic metabolite paraoxon and detoxified by P450s, paraoxonases, and carboxylesterases from paraoxon to PNP or directly from parathion to PNP.
We recently demonstrated that a number of pesticides, including several organophosphate insecticides, activated CAR in transactivation assays. Pesticides that activate CAR include fenitrothion, S,S,S-tributylphosphorotrithioate, chlorpyrifos, endosulfan, monosodium acid methane arsenate, cypermethrin, butylate, methoxychlor, and parathion. Most chemicals are partial activators, but the organophosphates parathion, S,S,S-tributylphosphorotrithioate, and chlorpyrifos are full activators. We hypothesized that these organophosphates are CAR ligands (Baldwin and Roling, 2009). Research by Küblbeck et al. (2008) in part provides further evidence that organophosphates may actually be ligands, as human CAR is ligand-activated by a number of sulfur-containing chemicals such as substituted sulfonamides and thiazolidin-4-one derivatives, indicating a potential preference of CAR for thiols.
CAR is a key regulator of several detoxification enzymes, including CYP2B6 in humans, Cyp2b10 in mouse, and several other P450s in families 1 to 3 (Sueyoshi and Negishi, 2001; Hernandez et al., 2009a). CAR also basally regulates several P450s, and loss of CAR from mice causes subsequent alterations in the expression of Cyp2c29, Cyp2b13, and maybe Cyp2b10 (Hernandez et al., 2009b). Furthermore, CAR expression is associated with the expression of several detoxification enzymes and transporters in human liver, including CYP2A6, CYP2B6, CYP2C8, P450 oxidoreductase, and multidrug resistance protein 2, indicating basal regulation of P450s by CAR in humans (Wortham et al., 2007). The expression of CAR and PXR is also significantly lower in children younger than 6 months of age, and in turn they show lower expression of several P450s involved in xenobiotic detoxification (Vyhlidal et al., 2006), suggesting potential susceptibility of children to chemical toxicity due to limited metabolic capacity (Padilla et al., 2000; Sheets, 2000).
We confirmed that parathion is a CAR activator in transactivation assays and determined its EC50. Furthermore, we tested whether P450s are induced by parathion in a CAR-dependent manner in vivo and investigated changes in P450-mediated parathion metabolism in untreated CAR-null and WT mice. Our data indicate that CAR is important in the basal regulation of P450s, and parathion is metabolized slower and is more toxic to CAR-null mice than to WT mice.
Materials and Methods
Chemicals.
Parathion (C10H14NO5PS; CAS No. 56-38-2), paraoxon (C10H14NO6P; CAS No. 311-45-5), PNP (C6H5NO3; CAS No. 100-02-7), and chlorpyrifos (C9H11Cl3NO3PS; CAS No. 2921088-2) were obtained from Chem Service (West Chester, PA). Dihydroandrosterone (DHA) (C19H32O2; CAS No. 1852-53-5), tetraisopropyl pyrophosphoramide (iso-OMPA) (C12H32N4O3P2; CAS No. 513-00-8), and 1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) (C16H8Cl4N2O2; CAS No. 76150-91-9) were obtained from Sigma-Aldrich (St. Louis, MO). 4-Nonylphenol 85% p-isomers (C15H24O; CAS No. 84852-15-3) were obtained from Fluka Chemical Corp. (Seelze, Germany). Acetonitrile (C2H3N; CAS No. 75-05-8) and methanol (CH4O; CAS No. 67-56-1) were obtained from B&J Brand (Honeywell Burdick and Jackson, Muskegon, MI), and EDTA (C10H16N2O8; CAS No. 60-00-4) and Tween 20 (C58H114O26; CAS No. 9005-64-5) were obtained from Thermo Fisher Scientific (Waltham, MA).
Transactivation Assay.
A detailed protocol for our mCAR transactivation assay with HepG2 cells has been published previously (Baldwin and Roling, 2009). The Steady-Glo luciferase assay system (Promega, Madison, WI) was used to measure reporter activity 24 h after pesticide or TCPOBOP treatments, and data were normalized to treatments with the inverse agonist, DHA, as 0% activity and TCPOBOP-treated cells as 100% activity with GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA). EC50 values and 95% confidence intervals were also determined with the GraphPad Prism 4.0 software package.
Animals.
All studies were performed in agreement with National Institutes of Health guidelines for the humane use of research animals and approved by The University of Texas at El Paso or Clemson University's Animal Care and Use Committee. Mice were provided with food and water ad libitum. Male and female 8- to 10-week-old WT and CAR-null mice (Wei et al., 2000) were randomly split into six groups each (n = 4–6). The mice were injected with 100 μl of 5 or 20 mg/kg parathion mixed with 50:50 DMSO-corn oil based on previously published studies (Weitman et al., 1983; Sobarzo and Bustos-Obregón, 2000; Kim et al., 2005). Mice were euthanized, and livers were excised 6 h after the last treatment. As a positive control, male and female WT and CAR-null mice were injected with 100 μl of 3 mg/kg TCPOBOP mixed with DMSO and corn oil for 2 days and euthanized 6 h after the last injection. All samples were stored at −80°C.
Liver Sample Preparation.
Livers from euthanized mice were excised, snap-frozen, weighed, and diced into several pieces for RNA extraction and microsome preparation. Total RNA was isolated using a modified guanidinium thiocyanate-phenol-chloroform extraction protocol with TRI reagent (Sigma-Aldrich) according to the manufacturer's specifications followed by DNase (Promega) treatment to remove residual genomic DNA. RNA was quantified spectrophotometrically at 260/280 nm and stored at −80°C. Reverse transcription was performed to make cDNA using Moloney murine leukemia virus-reverse transcriptase, a dNTP mixture, and random hexamers. cDNA was stored at −20°C. Microsomes were isolated by differential centrifugation in the presence of aprotinin, leupeptin, and phenylmethylsulfonyl fluoride as protease inhibitors. Protein concentrations were quantified using commercially available reagents and microsomes were stored at −80°C (Bio-Rad Laboratories, Hercules, CA).
Quantification of P450 Expression.
Q-PCR was performed as described previously by us (Hernandez et al., 2007, 2009b) and others (Muller et al., 2002) using previously published primers (Wiwi et al., 2004; Hernandez et al., 2009b). PCR efficiency was determined from standard curves developed from 1:1, 1:10, 1:100, and 1:1000 dilutions of a cDNA composite of all samples. Amplifications were performed in triplicate using a 96-well iQ5 multicolor real-time PCR detection system (Bio-Rad Laboratories) with 0.25× SYBR Green to quantify gene expression (SABiosciences, Frederick, MD). All samples were diluted 1:10. Q-PCR results were normalized to the expression of a housekeeping gene, 18S rRNA. A minimum of 40 cycles were run on all real-time samples to ensure a log-based growth curve. Quantification was done by taking the efficiency curve of the Q-PCR reaction to the power of the threshold cycle (Ct) over the housekeeping gene (Muller et al., 2002).
P450 protein levels were quantified by immunoblotting. Because each antibody probably recognizes more than one mouse isoform in a subfamily, we refer to the quantification of mouse P450s only by subfamily. Mouse 2b, 2c, and 3a subfamilies were quantified using antibodies from several sources. Cyp2c and Cyp3a were quantified using human CYP2C8/9/19 or rat CYP3A1 antibodies, respectively, from Millipore Bioscience Research Reagents (Temecula, MA). Induction of Cyp2b by parathion was performed using a Cyp2b10 antibody from Randy Rose (North Carolina State University, Raleigh, NC) (Hernandez et al., 2007). In addition, we quantified constitutive Cyp2b levels using a new and more sensitive antibody that we developed. In brief, the GenScript antigen design tool (GenScript USA Inc., Piscataway, NJ) was used to design a basic peptide with high antigenicity. A cysteine was added to the C terminus of the designed peptide (LHDPQYFEQPDSFN-C) and conjugated to keyhole limpet hemocyanin. This peptide is poorly conserved between Cyp2b and other P450s and therefore should be specific for only Cyp2b subfamily members. Two rabbits were injected 4× each, and approximately 160 ml of blood was collected and pooled from the rabbits. The antibody was purified by affinity purification through a column containing conjugated peptide and is specific for Cyp2b. The Cyp2b antibody was diluted 1:500 for immunoblotting.
Immunoblots were run with 30 to 50 μg of microsomal protein separated electrophoretically on a 10% polyacrylamide gel (SDS-polyacrylamide gel electrophoresis), transferred to a nitrocellulose membrane (Bio-Rad Laboratories), blocked in TBST (Tris-buffered saline, pH 7.4 and 0.1% Tween 20) containing 2.5 to 5% dried milk for 30 min to 1 h, and incubated with antibodies at room temperature for 2 h and overnight at 4°C. The nitrocellulose was then incubated at room temperature for 2 h with goat anti-mouse or goat anti-rabbit (1:500; Bio-Rad Laboratories) secondary antibody, depending on the source of the primary antibody. β-Actin (Sigma-Aldrich) was used as a housekeeper. The protein on the blots was detected by chemiluminescence using the Immun-Star AP Chemiluminescent Protein Detection System and quantified by Chemi Doc XRS HQ using Quantity One 4.6.5 software (Bio-Rad Laboratories).
Parathion Metabolism.
P450-mediated parathion metabolism was examined in vitro with microsomes from untreated WT and CAR-null mice as described previously (Foxenberg et al., 2007). Protein from mouse liver microsomes (250 μg) was incubated in 0.1 M Tris-HCl and 5 mM MgCl2 at pH 7.4 with a final concentration of 20 μM parathion and in the presence of the esterase inhibitors 1 mM EDTA (A esterase inhibitor) and 50 μM iso-OMPA (B esterase inhibitor) (Reiner et al., 1993). Samples were incubated at 37°C in a shaking water bath; the reaction was initiated with 1 mM NADPH and stopped after 60 min with 500 μl of methanol and 0.1% phosphoric acid. Samples were filtered (0.22 μm PTFE filter; Thermo Fisher Scientific), and metabolite concentrations were measured using reverse-phase HPLC. Standards for all compounds were prepared in methanol and stored at −20°C before analysis. The metabolites of parathion, paraoxon and PNP, were analyzed using a Waters 1525 binary reverse-phase high-performance liquid chromatography pump (HyperSelect Gold C18, 5 μm, 120 Å particle size, 250 mm × 4.6 mm with guard column; ES Industries Chromega Columns, West Berlin, NJ). The mobile phase consisted of 60% acetonitrile and 40% water over 18 min at a flow rate of 1 ml/min. Chemical detection was determined at 275 nm for parathion and paraoxon and at 310 nm for PNP. The detection limits of paraoxon and PNP were 0.0275 and 0.0139 μg/ml, respectively.
Statistical Analysis.
A Mann-Whitney rank-sum test was performed to test the significance of toxicity observations, where 1 = not toxic, 2 = eye leakage, 3 = slow or tremors, and 4 = death. Student's t tests were performed on immunoblot data when treated mice were compared with untreated mice and during Q-PCR to compare differences in P450 expression between males and females or WT and CAR-null mice. Differences in parathion metabolism were also determined by Student's two-tailed t tests. Values of p < 0.05 were considered to be significant. All statistical analyses were performed using GraphPad Prism 4.0.
Results
Transactivation Assay.
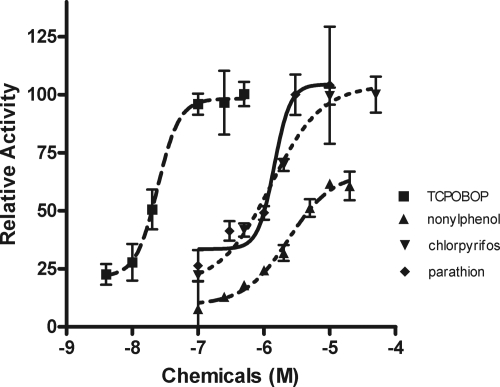
Previous research in our laboratory demonstrated that mCAR is activated by a variety of organophosphate insecticides. Parathion and chlorpyrifos were the most efficacious CAR activators of the environmental chemicals tested (Baldwin and Roling, 2009). Furthermore, computational models as well as hCAR assays showed that thiol-containing compounds can activate mouse and human CAR through ligand binding (Küblbeck et al., 2008). Therefore, we compared mCAR activation by parathion, chlorpyrifos, nonylphenol (a partial activator) (Hernandez et al., 2007), and TCPOBOP (positive control) (Tzameli et al., 2000) in dose-response transactivation assays and determined their EC50 values. With the exception of TCPOBOP, the organophosphates, parathion, and chlorpyrifos were the most efficacious and potent full CAR activators we have tested to date with EC50 values of 1.426 and 1.288 μM, respectively (Fig. 2). In comparison, TCPOBOP has an EC50 of 0.024 μM and the partial agonist, nonylphenol, has an EC50 of 2.386 μM (Table 1).
Fig. 2.
Dose-response curves of organophosphate insecticides in transactivation assays. HepG2 cells were cotransfected with a mCAR expression plasmid and a luciferase reporter plasmid containing the CYP2B6 PBREM and then were cotreated with the inverse agonist, DHA plus the mCAR activators. Data were normalized to cells treated with only DHA (0% activity) and TCPOBOP (100% full agonist activity), and sigmoidal dose-response curves and EC50 values were determined using GraphPad Prism 4.0 (n = 3).
TABLE 1.
EC50 and 95% CI for parathion compared with other mCAR activators
| Chemical | EC50 a | 95% CI a | Agonist b |
|---|---|---|---|
| μM | |||
| Parathion | 1.426 | 0.481–4.233 | Full |
| Chlorpyrifos | 1.288 | 0.809–2.049 | Full |
| Nonylphenol | 2.386 | 1.357–4.195 | Partial |
| TCPOBOP | 0.0248 | 0.0147–0.040 | Full |
CI, confidence interval.
EC50 values and 95% CI were determined using GraphPad Prism 4.0 as described under Materials and Methods.
Chemicals that show reduced efficacy compared with TCPOBOP are considered partial agonists.
CAR-Null Mice Are Sensitive to Parathion.
WT and CAR-null male mice were treated with 5 or 20 mg/kg/day parathion based on previously published studies (Weitman et al., 1983; Sobarzo and Bustos-Obregón, 2000; Kim et al., 2005) to determine whether parathion is a CAR activator in vivo in addition to activating CAR in vitro. We were surprised to find that the CAR-null mice but not the WT mice showed toxicity at 5 mg/kg/day (Table 2). All of the mice treated at 20 mg/kg/day died. Therefore, in the subsequent study with female mice, the 20 mg/kg/day dose was eliminated. The parathion-treated male and female CAR-null mice that showed only minor symptoms such as increased lacrimation (tears and mucus eye leakage) recovered, but the other mice were euthanized or died.
TABLE 2.
Differential toxicity of CAR-null mice compared with wild-type mice treated with 5 mg/kg/day parathion for 2 consecutive days
Data are shown as number of mice showing toxicity/number of mice treated.
| Treatment | WT (M) | CAR-Null (M) | WT (F) | CAR-Null (F) |
|---|---|---|---|---|
| 0 mg/kg/day | 0/5 | 0/5 | 0/5 | 0/5 |
| 3 mg/kg TCPOBOP | 0/4 | 0/5 | 0/4 | 0/5 |
| 5 mg/kg/day parathion | 0/5* | 4/4* | 2/5* | 5/5* |
| 20 mg/kg/day parathion | 4/4 | 4/4 |
M, male; F, female.
Statistical significance was assessed by Mann-Whitney rank-sum tests using toxicity rankings depending on the severity: 1 = not toxic; 2 = eye leakage; 3 = lethargy; slow movements or tremors; and 4 = death. Male and female data, p = 0.0079.
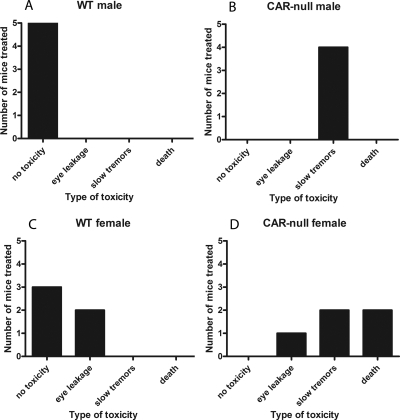
The severity of toxicity was quantified based on these symptoms: no toxicity = 1; eye leakage = 2; slow, lethargic, weak tremors = 3; and death = 4 (Fig. 3). WT male mice showed no toxicity after parathion treatment (Fig. 3A), in contrast to CAR-null male mice that showed a significantly higher degree of toxicity (assessed as lethargic or slow with weak tremors) (Fig. 3B). WT female mice showed no toxicity or a significantly lower severity of toxicity (assessed as eye leakage) (Fig. 3C) compared with CAR-null female mice that showed eye leakage, lethargy, tremors, and even death (Fig. 3D). Overall, both male and female CAR-null mice showed significantly (p = 0.0079) greater sensitivity to parathion than WT mice, indicating a protective role for CAR in parathion toxicity.
Fig. 3.
Increased toxicity of parathion in CAR-null mice compared with WT mice. A, WT male mice treated with parathion. B, CAR-null male mice treated with parathion. C, WT female mice treated with parathion. D, CAR-null female mice treated with parathion. A significant increase in toxicity to parathion was observed in the CAR-null male and female mice as assessed by degrees of toxicity using the Mann-Whitney ranked sum test: 1 = not toxic; 2 = eye leakage; 3 = lethargic (slow) or tremors; and 4 = death (males p = 0.0079; females p = 0.0079).
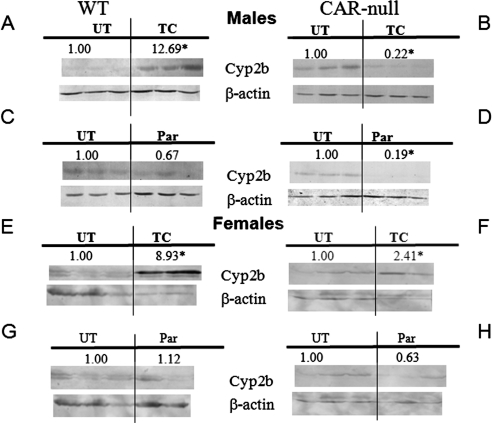
Immunoblots of hepatic microsomes from parathion-treated mice showed no induction of Cyp2b (Fig. 4), 2c, or 3a (data not shown) in WT or CAR-null mice. This result suggests that parathion did not activate CAR in vivo. In contrast, Cyp2b was decreased by parathion and TCPOBOP treatment in CAR-null males probably because of toxicity. We had previously observed a decrease in Cyp2b after TCPOBOP treatment performed over 2 days in CAR-null mice (Hernandez et al., 2007). Overall, these data suggest that parathion does not activate CAR in vivo, which might be attributed to the short half-life of parathion (Sultatos and Minor, 1986); therefore, parathion may not have reached the required hepatic concentrations to activate CAR.
Fig. 4.
Immunoblots of Cyp2b as a biomarker of CAR activation in vivo. Cyp2b and β-actin (housekeeper) immunoblots from hepatic microsomes of untreated (UT), TCPOBOP-treated (TG), and parathion-treated (Par) WT and CAR-null male and female mice were performed and quantified. A, WT male mice treated with TCPOBOP. B, CAR-null male mice treated with TCPOBOP. C, WT male mice treated with parathion. D, CAR-null male mice treated with parathion. E, WT female mice treated with TCPOBOP. F, CAR-null female mice treated with TCPOBOP. G, WT female mice treated with parathion. H, CAR-null female mice treated with parathion. *, statistically significant difference compared with untreated mice as performed by Student's t test.
We also observed weak induction of Cyp2b protein in CAR-null female mice after TCPOBOP treatment. This finding was not expected, and we consider that it is probably an anomaly caused by one sample. Alternatively, it could be TCPOBOP activation of PXR, although other groups have not observed this previously. More likely, it may be caused by reduced food intake because FoxA2 (hepatic nuclear factor 3β) positively regulates Cyp2b9, especially in females, and insulin reduces Cyp2b9 expression by deactivating FoxA2 (Hashita et al., 2008).
Basal Regulation of P450s by CAR.
CAR has been shown to basally regulate several drug-metabolizing P450s in mice (Hernandez et al., 2009b) and humans (Wortham et al., 2007), including CYP2B6, CYP2C19, P450 oxidoreductase, multidrug resistance protein 2, Cyp2c29, and potentially Cyp2b10. Furthermore, some of these P450s such as CYP2B6 in humans are critical in the metabolism of parathion (Mutch and Williams, 2006; Foxenberg et al., 2007). Therefore, we hypothesized that CAR-null mice may be sensitive to parathion because of differences in constitutive expression of P450s, especially Cyp2b subfamily members, between CAR-null and WT mice. Q-PCR comparisons of WT and CAR-null mice demonstrated that Cyp2b9, Cyp2b10, Cyp2c29, and Cyp3a11 were all reduced in male CAR-null mice relative to WT mice (Table 3). Female mice showed similar trends, but the effects were smaller and only Cyp3a11 was significant (Table 4). Overall, these data confirm that CAR is important in the basal regulation of several P450s, and CAR-null mice may show lower P450-mediated parathion metabolism as a result.
TABLE 3.
Basal regulation of P450s by CAR in male mice
Results are shown as mean relative activity ± S.E.M. (n = 5–6).
| WT Male | CAR-Null Male | |
|---|---|---|
| Cyp2b9 | 1.00 + 0.52 | 0.03 + 0.02* |
| Cyp2b10 | 1.00 ± 0.50 | 0.08 ± 0.07* |
| Cyp2b13 | 1.00 ± 0.93 | 3.99 ± 3.57 |
| Cyp2c29 | 1.00 ± 0.41 | 0.03 ± 0.02* |
| Cyp3a11 | 1.00 ± 0.26 | 0.37 ± 0.13* |
Statistical significance using Student's t test (p < 0.05).
TABLE 4.
Basal regulation of P450s by CAR in female mice
Results are shown as mean relative activity ± S.E.M. (n = 5–6).
| WT Female | CAR-Null Female | |
|---|---|---|
| Cyp2b9 | 1.00 ± 0.30 | 2.07 ± 0.23 |
| Cyp2b10 | 1.00 ± 0.31 | 0.69 ± 0.27 |
| Cyp2b13 | 1.00 ± 0.12 | 1.14 ± 0.34 |
| Cyp2c29 | 1.00 ± 0.27 | 0.53 ± 0.28 |
| Cyp3a11 | 1.00 ± 0.24 | 0.47 ± 0.16* |
Statistical significance using Student's t test (p < 0.05).
Differential Parathion Metabolism in CAR-Null Mice.
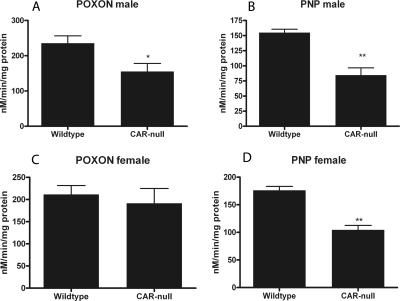
Differences in parathion metabolism between untreated WT and CAR-null mice were assessed by measuring the formation of the parathion metabolites, paraoxon and PNP, via reverse-phase-HPLC in the presence of carboxylesterase inhibitors so that P450s would be the primary, if not only, enzymes responsible for the metabolism of parathion. The standard peaks for each metabolite, their retention times, and a reverse-phase-HPLC sample run at 275 and 310 nm are shown in the Supplemental Data. Microsomes from CAR-null male mice showed significant reductions in paraoxon (34%) and PNP (45%) production compared with WT male mice (Fig. 5, A and B), indicating their reduced capacity for metabolizing parathion. CAR-null female mice also showed reduced metabolic capacity; however, paraoxon formation was not significantly reduced (Fig. 5C). PNP formation was reduced 41% (Fig. 5D) in female CAR-null mice compared with WT mice. Therefore, the ratio of PNP/paraoxon production in female mice was changed from 0.86 in WT mice to 0.61 in CAR-null mice, a significant difference (p = 0.03; Student's t test), indicating lower production of the nontoxic PNP in the CAR-null female mice. Greater production of PNP compared with paraoxon is consistent with decreased toxicity (Mutch and Williams, 2006). Males did not show a significant difference in PNP/paraoxon ratio. WT males had a ratio of 0.68, and CAR-null mice had a ratio of 0.58 (p = 0.41). Overall, P450-mediated parathion metabolism was reduced in both male and female CAR-null mice relative to WT mice.
Fig. 5.
Parathion metabolites from male and female WT and CAR-null mice. HPLC was performed to quantify the formation of the toxic metabolite, paraoxon (POXON), and the nontoxic metabolite, PNP. A, paraoxon formation in male mice. B, PNP formation in male mice. C, paraoxon formation in female mice. D, PNP formation in female mice. *, significant difference between the two genotypes by Student's t test (p < 0.05).
Discussion
CAR is an important transcription factor that regulates a number of key detoxification genes, including P450s. CAR activation induces several CYP1-3 family members (Sueyoshi and Negishi, 2001; Hernandez et al., 2009a), and CAR exhibits some basal activity because untreated CAR-null mice show differential P450 expression compared with untreated WT mice (Hernandez et al., 2009b). This may leave CAR-null mice sensitive to some toxicants. In this study, a clear difference in toxicity was exhibited between CAR-null and WT mice after parathion treatment. Both male and female CAR-null mice treated with parathion showed greater sensitivity to parathion than WT mice, as observed by symptoms consistent with organophosphate poisoning and activation of the parasympathetic nervous system such as mucus discharge from the eyes, lacrimation, slow stiff, tremorous movements, lethargy, and, in some cases, death. This research demonstrates a protective role of CAR in parathion toxicity.
Studies have shown that low CAR and PXR expression and, in particular, low CAR expression, is associated with reduced P450 expression in humans (Wortham et al., 2007) and mice (Hernandez et al., 2009b). Because there was no significant induction of P450s by parathion, we conclude that the basal regulation of P450s by CAR is crucial in the P450-mediated metabolism of parathion and potentially in the sensitivity of CAR-null mice to parathion. Therefore, individuals with low CAR expression may be sensitive to parathion. Newborn children younger than the age of 6 months have low CAR and PXR expression (Vyhlidal et al., 2006). They also show lower metabolic capacity, including reduced P450 activity and are more sensitive to a number of chemicals including organophosphate insecticides (Padilla et al., 2000; Sheets, 2000).
Data indicate that CYP2B6 is a high-affinity enzyme for parathion (Foxenberg et al., 2007). CAR is a key regulator of CYP2B6 in humans and Cyp2b10 in mice (Wei et al., 2000; Sueyoshi and Negishi, 2001) so changes in parathion metabolism and parathion toxicity are consistent with regulation of Cyp2b subfamily members by CAR. For example, phenobarbital pretreatment increases the production of paraoxon and PNP from parathion (Kim et al., 2005), confirming the importance of P450s such as Cyp2b10 induced by CAR activation in parathion metabolism in vivo. However, CYP2B6 exhibits two times higher production of the toxic metabolite paraoxon than the detoxification product PNP and a higher intrinsic clearance for paraoxon formation than for PNP (Foxenberg et al., 2007). In contrast, another study found that CYP2B6 preferentially produced PNP and little paraoxon (Mutch and Williams, 2006). Both studies indicate that CYP2B6 is a key enzyme in parathion detoxification. Several P450s including Cyp2b9, Cyp2b10, Cyp3a11, and Cyp2c29 were repressed in CAR-null males, and in turn both paraoxon and PNP production were reduced. However, Cyp2b (and Cyp2c) subfamily members were not repressed in CAR-null female mice, and in turn only PNP production was reduced. Taken together, our data suggest that Cyp2bs and potentially Cyp2c29 preferentially produce paraoxon in mice. Overall, Cyp2bs seem to be crucial P450s in the activation and detoxification of parathion in mice and humans (Kim et al., 2005; Mutch and Williams, 2006; Foxenberg et al., 2007).
Other P450s are also important in parathion metabolism. Human CYP3A members metabolize parathion to paraoxon and PNP, and both CYP3A4 and CYP3A5 show a preference for producing the nontoxic PNP over the toxic metabolite, paraoxon (Mutch and Williams, 2006). Cyp3a11 is the only P450 that we examined that is significantly decreased in CAR-null female mice; therefore, the decreased PNP production in CAR-null females is consistent with Cyp3a11 playing a role in parathion detoxification. Given the high expression levels of CYP3A enzymes in rodent and human liver and the reduced PNP production in association with reduced Cyp3a11 expression in this study, CYP3A members in mice and humans are almost certainly key P450s in parathion detoxification.
A higher PNP/paraoxon ratio indicates increased production of PNP relative to the production of paraoxon and should indicate greater detoxification. The PNP/paraoxon ratio was 41% higher in WT female mice than in CAR-null female mice, suggesting a reduced capacity to produce the nontoxic parathion metabolite, PNP, in the CAR-null female mice. This may play a role in the greater toxicity observed in the CAR-null mice. However, males did not show a significant difference in the PNP/paraoxon ratio but still demonstrated greater toxicity in the CAR-null mice.
In addition, paraoxonases and carboxylesterases are involved in the metabolism of and protection from organophosphate insecticides (Costa et al., 1990; Maxwell, 1992). They are key enzymes that can detoxify the toxic metabolite of parathion, paraoxon, into the nontoxic metabolite, PNP. Recent reports indicate that TCPOBOP activation of CAR, but not phenobarbital activation of CAR regulates mouse carboxylesterase 6 (Xu et al., 2009). This suggests that repression of carboxylesterase expression in CAR-null mice may also be associated with increased sensitivity to parathion.
Current in vitro research, as well as our data, found parathion to be one of the most potent and efficacious CAR activators compared with several environmental pollutants (Baldwin and Roling, 2009) (Fig. 2). However, parathion was not a CAR activator in vivo; although significant toxicity was observed in CAR-null male and female mice. Some sublethal toxicity was also observed in WT female mice. No toxicity was observed in WT male mice; however, it is possible that undetected toxicity may have perturbed the ability of WT mice to respond to parathion.
The lack of in vivo CAR activation by parathion could also be attributed to rapid metabolism of parathion and paraoxon. Therefore, oral dosing of parathion may lead to a sufficiently short half-life in mice primarily due to a first-pass effect (Sultatos and Minor, 1986). However, low bioavailability due to parathion binding to serum proteins cannot be excluded. Taken together, these results indicate that parathion may not have reached the necessary concentrations to activate CAR in vivo. Lower doses provided over a longer period of time may bioconcentrate and activate CAR. However, the half-life of parathion is reported to be 6.2 min (Sultatos and Minor, 1986). Other chemicals known to activate CAR in vivo, such as phenobarbital and nonylphenol, have significantly longer half-lives of approximately 7.5 h for phenobarbital (Iven and Feldbusch, 1983) and 2 to 3 h for nonylphenol in humans (Muller et al., 1998) and 3 to 13 h in rodents, depending on the dose and sex (Green et al., 2003). Other organophosphates have longer half-lives, such as chlorpyrifos for which the half-life through oral treatment was 15.5 h and the half-life through dermal treatment was 30 h (Griffin et al., 1999). The methyl ortholog of parathion was found to have a half-life in rat liver of 19.3 h (Abu-Qare et al., 2000); therefore, these organophosphates may be more likely to activate CAR in vivo.
A clear connection was shown for the protective role of CAR in parathion toxicity, for which CAR-null mice demonstrated an increased degree of toxicity compared with WT mice. Parathion is detoxified by P450s, paraoxonases, and carboxylesterases. We examined changes in P450 expression in CAR-null mice relative to those in WT mice because of increased toxicity of CAR-null mice to parathion. Based on the lower expression of several P450s including Cyp2b and Cyp3a subfamily members in CAR-null mice, we attribute the increased parathion toxicity in CAR-null mice in part to decreased P450-mediated parathion metabolism because CAR is important in the basal regulation of P450s that metabolize parathion. However, we cannot fully discount the role of paraoxonases or carboxylesterases in the sensitivity of CAR-null mice to parathion. It is interesting to consider the potential human implications of low CAR expression to parathion toxicity, especially in susceptible populations such as children. Research has shown an increased sensitivity to pesticides in children caused by limited metabolic capacity, and newborns are very susceptible (Padilla et al., 2000; Sheets, 2000). Because CAR expression is associated with P450 expression in humans (Wortham et al., 2007), and children younger than 6 months of age have low CAR expression (Vyhlidal et al., 2006), our data may indicate a potential mechanism for increased toxicity to parathion and other pesticides in newborn children.
Supplementary Material
Acknowledgments.
We thank Lisa Bain, Kristen Gaworecki, and Peter van den Hurk for their help and technical expertise.
This work was supported in part by the National Institutes of Health National Institute of Environmental Health Sciences [Grant R15-ES017321]; the National Institutes of Health National Institute of General Medical Sciences [S06-GM008012]; and Clemson University.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.032961.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
- P450
- cytochrome P450
- PNP
- p-nitrophenyl
- CAR
- constitutive androstane receptor
- PXR
- pregnane X receptor
- WT
- wild-type
- DHA
- dihydroandrosterone
- iso-OMPA
- tetraisopropyl pyrophosphoramide
- TCPOBOP
- 1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene
- mCAR
- mouse CAR
- Q
- quantitative
- PCR
- polymerase chain reaction
- HPLC
- high-performance liquid chromatography
- Ct
- threshold cycle.
References
- Abu-Qare AW, Abdel-Rahman AA, Kishk AM, Abou-Donia MB. (2000) Placental transfer and pharmacokinetics of a single dermal dose of [14C]methyl parathion in rats. Toxicol Sci 53:5–12 [DOI] [PubMed] [Google Scholar]
- Baldwin WS, Roling JA. (2009) A concentration addition model for the activation of the constitutive androstane receptor by xenobiotic mixtures. Toxicol Sci 107:93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, McDonald BE, Murphy SD, Omenn GS, Richter RJ, Motulsky AG, Furlong CE. (1990) Serum paraoxonase and its influence on paraoxon and chlorpyrifos-oxon toxicity in rats. Toxicol Appl Pharmacol 103:66–76 [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency (2002) Organophosphate Pesticides: Revised Cumulative Risk Assessment: Executive Summary, p 11, Environmental Protection Agency, Washington, DC: [Google Scholar]
- Foxenberg RJ, McGarrigle BP, Knaak JB, Kostyniak PJ, Olson JR. (2007) Human hepatic cytochrome P450-specific metabolism of parathion and chlorpyrifos. Drug Metab Dispos 35:189–193 [DOI] [PubMed] [Google Scholar]
- Gallow MA, Lawryk NJ. (1991) Organophosphorus Pesticides. Academic Press, San Diego: [Google Scholar]
- Green T, Swain C, Van Miller JP, Joiner RL. (2003) Absorption, bioavailability, and metabolism of para-nonylphenol in the rat. Regul Toxicol Pharmacol 38:43–51 [DOI] [PubMed] [Google Scholar]
- Griffin P, Mason H, Heywood K, Cocker J. (1999) Oral and dermal absorption of chlorpyrifos: a human volunteer study. Occup Environ Med 56:10–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashita T, Sakuma T, Akada M, Nakajima A, Yamahara H, Ito S, Takesako H, Nemoto N. (2008) Forkhead box A2-mediated regulation of female-predominant expression of the mouse Cyp2b9 gene. Drug Metab Dispos 36:1080–1087 [DOI] [PubMed] [Google Scholar]
- Hernandez JP, Huang W, Chapman LM, Chua S, Moore DD, Baldwin WS. (2007) The environmental estrogen, nonylphenol, activates the constitutive androstane receptor. Toxicol Sci 98:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Mota LC, Baldwin WS. (2009a) Activation of CAR and PXR by dietary, environmental and occupational chemicals alters drug metabolism, intermediary metabolism, and cell proliferation. Curr Pharmacogenomics Person Med 7:81–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Mota LC, Huang W, Moore DD, Baldwin WS. (2009b) Sexually dimorphic regulation and induction of P450s by the constitutive androstane receptor (CAR). Toxicology 256:53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Huang K. (2007) Increased U.S. imports of fresh fruits and vegetables, in A Report from the Economic Research Service, p 20, U.S. Department of Agriculture, Washington, DC: [Google Scholar]
- Iven H, Feldbusch E. (1983) Pharmacokinetics of phenobarbital and propylhexedrine after administration of barbexaclone in the mouse. Naunyn Schmiedebergs Arch Pharmacol 324:153–159 [DOI] [PubMed] [Google Scholar]
- Kiely T, Donaldson D, Grube R. (2004) Pesticide Industry Sales and Usage: 2000–2001 Market Estimates, p 33, Biological and Economic Analysis Division Office of Pesticide Programs, Office of Prevention, Pesticides, and Toxic Substances. U.S. Environmental Protection Agency, Washington, DC: [Google Scholar]
- Kim DO, Lee SK, Jeon TW, Jin CH, Hyun SH, Kim EJ, Moon GI, Kim JA, Lee ES, Lee BM, et al. (2005) Role of metabolism in parathion-induced hepatotoxicity and immunotoxicity. J Toxicol Environ Health A 68:2187–2205 [DOI] [PubMed] [Google Scholar]
- Küblbeck J, Jyrkkärinne J, Poso A, Turpeinen M, Sippl W, Honkakoski P, Windshügel B. (2008) Discovery of substituted sulfonamides and thiazolidin-4-one derivatives as agonists of human constitutive androstane receptor. Biochem Pharmacol 76:1288–1297 [DOI] [PubMed] [Google Scholar]
- Maxwell DM. (1992) The specificity of carboxylesterase protection against the toxicity of organophosphorus compounds. Toxicol Appl Pharmacol 114:306–312 [DOI] [PubMed] [Google Scholar]
- Muller PY, Janovjak H, Miserez AR, Dobbie Z. (2002) Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 32:1372–1379 [PubMed] [Google Scholar]
- Muller S, Schmid P, Schlatter C. (1998) Pharmacokinetic behavior of 4-nonylphenol in humans. Environ Toxicol Pharmacol 5:257–265 [DOI] [PubMed] [Google Scholar]
- Murcia AM, Stashenko E. (2008) Determinacion de plaguicidas organofosforados en vegetales producidos en Colombia. Agro Sur 36:71–81 [Google Scholar]
- Mutch E, Williams FM. (2006) Diazinon, chlorpyrifos and parathion are metabolised by multiple cytochromes P450 in human liver. Toxicology 224:22–32 [DOI] [PubMed] [Google Scholar]
- Padilla S, Buzzard J, Moser VC. (2000) Comparison of the role of esterases in the differential age-related sensitivity to chlorpyrifos and methamidophos. Neurotoxicology 21:49–56 [PubMed] [Google Scholar]
- Perez MA, Segura A, Garcia R, Colinas T, Perez M, Vazquez A, Navarro H. (2009) Residuos de plaguicidas organofosforados en cabezuela de brocoli (Brassica oleracea) determinados por cromatografia de gases. Rev Int Contam Ambient 25:103–110 [Google Scholar]
- Parliamentary Office of Science and Technology (1998) Post Note 122: Organophosphates, Parliamentary Office of Science and Technology, London: [Google Scholar]
- Ragnarsdottir KV. (2000) Environmental fate and toxicology of organophosphate pesticides. J Geol Soc 157:859–876 [Google Scholar]
- Reiner E, Pavković E, Radić Z, Simeon V. (1993) Differentiation of esterases reacting with organophosphorus compounds. Chem Biol Interact 87:77–83 [DOI] [PubMed] [Google Scholar]
- Saller J, Reyes P, Maldonado PA, Gibbs SG, Byrd TL. (2007) Children's exposure to pesticides used in homes and farms. J Environ Health 69:27–31 [PubMed] [Google Scholar]
- Sheets LP. (2000) A consideration of age-dependent differences in susceptibility to organophosphorus and pyrethroid insecticides. Neurotoxicology 21:57–63 [PubMed] [Google Scholar]
- Singh BK, Walker A. (2006) Microbial degradation of organophosphorus compounds. FEMS Microbiol Rev 30:428–471 [DOI] [PubMed] [Google Scholar]
- Sobarzo C, Bustos-Obregón E. (2000) Sperm quality in mice acutely treated with parathion. Asian J Androl 2:147–150 [PubMed] [Google Scholar]
- Sueyoshi T, Negishi M. (2001) Phenobarbital response elements of cytochrome P450 genes and nuclear receptors. Annu Rev Pharmacol Toxicol 41:123–143 [DOI] [PubMed] [Google Scholar]
- Sultatos LG, Minor LD. (1986) Factors affecting the biotransformation of the pesticide parathion by the isolated perfused mouse liver. Drug Metab Dispos 14:214–220 [PubMed] [Google Scholar]
- Tzameli I, Pissios P, Schuetz EG, Moore DD. (2000) The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol Cell Biol 20:2951–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyhlidal CA, Gaedigk R, Leeder JS. (2006) Nuclear receptor expression in fetal and pediatric liver: correlation with CYP3A expression. Drug Metab Dispos 34:131–137 [DOI] [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. (2000) The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature 407:920–923 [DOI] [PubMed] [Google Scholar]
- Weitman SD, Vodicnik MJ, Lech JJ. (1983) Influence of pregnancy on parathion toxicity and disposition. Toxicol Appl Pharmacol 71:215–224 [DOI] [PubMed] [Google Scholar]
- Wiwi CA, Gupte M, Waxman DJ. (2004) Sexually dimorphic P450 gene expression in liver-specific hepatocyte nuclear factor 4a-deficient mice. Mol Endocrinol 18:1975–1987 [DOI] [PubMed] [Google Scholar]
- Wortham M, Czerwinski M, He L, Parkinson A, Wan YJ. (2007) Expression of constitutive androstane receptor, hepatic nuclear factor 4 α, and P450 oxidoreductase genes determines interindividual variability in basal expression and activity of a broad scope of xenobiotic metabolism genes in the human liver. Drug Metab Dispos 35:1700–1710 [DOI] [PubMed] [Google Scholar]
- Xu C, Wang X, Staudinger JL. (2009) Regulation of tissue-specific carboxylesterase expression by pregnane X receptor and constitutive androstane receptor. Drug Metab Dispos 37:1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.