Abstract
β-Adrenergic receptor induces cAMP/Protein kinase A (PKA) activation to regulate cardiac contraction. Using real-time fluorescence resonance energy transfer imaging for highly sensitive detection of cAMP and PKA activities, we show two distinct phases in isoproterenol dose-dependent responses in cardiomyocytes: a transient and dose-dependent increase in cAMP and PKA activities at lower concentrations from 10−12 to 10−8 M; and a saturated initial increases at higher concentrations from 10−8 to 10−5 M followed by a rapid decrease to different levels that were later sustained in a dose-dependent manner. The dose-dependent temporal responses are patterned by equilibrium between receptor-activated adenylyl cyclase (AC) and phosphodiesterase (PDE). At lower concentrations, cAMP is produced in an agonist dose-dependent manner with AC as a rate-limiting factor. However, the cAMP activities are confined within local domains for phosphorylation of PDE isoforms in the receptor complex but not for phosphorylation of phospholamban and troponin I. At higher concentrations, isoproterenol promotes a dose-dependent selective dissociation of PDE4D but not ACVI from the receptor complex, which shifts the equilibrium between AC and PDE. This shifted balance leads to sustained cAMP accumulation and diffusion for PKA phosphorylation of phospholamban and troponin I, and for myocyte contraction. Pharmacological inhibition or overexpression of either ACVI or PDE4D8 disrupts the balance and shapes the temporal responses in cAMP accumulation. Together, our data reveal a new paradigm for adrenergic agonist dose-dependent cAMP/PKA activities for substrate-specific phosphorylation dictated by dual regulation of AC and PDE in cardiomyocytes.
Introduction
Activation of adrenergic receptors (ARs) represents the primary mechanism to increase cardiac performance under stress. Activated ARs couple to Gs proteins, which leads to AC-dependent increases in secondary-messenger cAMP to activate PKA (Lefkowitz, 2007). The increased PKA activities promote phosphorylation of diversified substrates ranging from the receptor and its associated partners, to ryanodine receptor, phospholamban (PLB), and contractile myofibril proteins such as troponin I (TnI) and troponin T, which eventually leads to increases in contractility and heart rate (Xiang and Kobilka, 2003; Xiao et al., 2006). Although cAMP/PKA activation plays an essential role in controlling physiological responses, accumulating evidence indicates that changes in cAMP/PKA activities exert distinct cellular effects via substrate-specificity in highly differentiated cardiomyocytes. For example, β2AR displays a high sensitivity to PKA phosphorylation under stimulation with subnanomolar concentrations of isoproterenol in human embryonic kidney (HEK) 293 cells and mouse neonatal cardiomyocytes (Tran et al., 2004; Liu et al., 2009). In contrast, a minimal concentration of 1 nM isoproterenol is required to promote increases in myocyte contraction rate and contractility (De Arcangelis et al., 2008).
The concept of spatiotemporal regulation of cellular cAMP and PKA activities provides new insights into understanding how cAMP/PKA signaling is translated into physiological contraction response in highly organized muscle cells (Cooper, 2005; Zaccolo, 2006). PKA is anchored on distinct subcellular structures through a family of proteins named A-kinase anchoring proteins. In contrast, correlating to the distribution of most ACs, cellular cAMP is primarily confined along the plasma membrane under neurohormonal stimulation (Cooper, 2005). Despite being a diffusible small molecule, the distribution and diffusion of cAMP is rather limited because of cAMP degradation mediated by phosphodiesterases (PDEs) (Mongillo and Zaccolo, 2006; Zaccolo, 2006; Houslay et al., 2007). Under a specific hormonal stimulation, individual PKAs anchored at different subcellular compartments will be selectively activated to conduct the phosphorylation of local proteins for specific cellular processes (McConnachie et al., 2006; Jarnaess and Taskén, 2007). A spatial distribution of cAMP/PKA signaling regulated by ACs and PDEs is therefore essential for selective phosphorylation of substrates for myocyte contraction.
Consistent with this notion, PDE 4D (PDE4D) has been shown to be significant in regulating the adrenergic receptor subtype-induced myocyte contraction rate response (Xiang et al., 2005). Recent evidence indicates that PDE4D splicing isoforms selectively bind β-adrenergic receptors (De Arcangelis et al., 2008; Richter et al., 2008). Specifically, PDE4D8 binds to β1AR in HEK293 cells and dissociates from the receptor upon stimulation with incremental doses of agonist (Richter et al., 2008). In contrast, PDE4D9 and to a lesser extent PDE4D8 bind to β2AR in cardiomyocytes (De Arcangelis et al., 2009). These receptor-associated PDE4Ds play critical roles in controlling cAMP/PKA activities in the vicinity of the receptors for differential cellular responses under stimulation (Zaccolo and Pozzan, 2002; Xiang et al., 2005; De Arcangelis et al., 2008; Richter et al., 2008). Inhibition of PDE4 significantly enhances propagation of cAMP/PKA activities for increasing PKA phosphorylation of PLB and myocyte contraction response under low doses of isoproterenol stimulation (De Arcangelis et al., 2008). This results in saturated responses becoming equivalent to those induced by saturating doses of isoproterenol (De Arcangelis et al., 2008).
We hypothesized that cardiomyocyte cAMP/PKA signaling is differentially regulated through a balance between AC-dependent cAMP production and PDE-dependent cAMP degradation in an agonist dose-dependent manner. By using high-sensitive fluorescence resonance energy transfer (FRET)-based biosensors for cAMP and PKA activities in living-cell imaging, we found that cAMP/PKA activities displayed two distinct phases in isoproterenol dose-dependent fashion: a transient and dose-dependent increase in FRET ratio at concentration from 10−12 to 10−8 M, and a saturated initial increase in FRET ratio from 10−8 to 10−5 M, which was followed by a rapid decrease to different levels that were later sustained in a dose-dependent manner. The transient and sustained cAMP/PKA signals are patterned by a shifting balance between AC-dependent cAMP production and PDE-dependent cAMP degradation at increasing concentration of isoproterenol, which also dictates substrate-specificity for PKA phosphorylation and myocyte contraction responses.
Materials and Methods
Neonatal and Adult Cardiac Myocyte Contraction Assays.
Neonatal and adult myocytes were isolated from newborn or 2- to 4-month-old wild-type FVB mice, respectively. Spontaneously beating neonatal cardiac myocytes were prepared from newly born wild-type or mice lacking β1AR or β2AR, or both genes as described previously (Devic et al., 2001). Measurement of spontaneous contraction rate was carried out as described previously (Devic et al., 2001). Adult myocytes were placed in a dish with HEPES buffer and were electrically stimulated at 30 V/cm at 1 Hz at room temperature. Cell length was recorded with a charge-coupled device camera. Cell contraction shortening was analyzed by Metamorph software (Molecular Devices, Sunnyvale, CA) and normalized as the increase over the basal levels after being fitted to a sigmoidal curve. The maximal shortening was normalized to the baseline value or plotted as a percentage of the maximal response stimulated by 10 μM forskolin.
Drug Treatment.
Neonatal myocytes were treated with rolipram (10−5 M; Calbiochem, San Diego, CA), a PDE4 inhibitor, for 10 min (Alvarez et al., 1995) or with 2′,5′-dideoxyadenosisne triphosphate (2′,5′-DDA; 10−4 M, Sigma-Aldrich, St. Louis, MO), a selective adenylyl cyclase inhibitor, for 40 min before stimulation. Cells were stimulated with isoproterenol (Iso, 10−12 to 10−5 M; Sigma-Aldrich) or an AC agonist, forskolin (10−5 M; Sigma-Aldrich) (Wang et al., 2008).
Immunoprecipitation and Western Blotting.
Neonatal cardiac myocytes from wild-type or β1β2AR gene knockout (KO) pups were infected with recombinant adenovirus expressing HA-tagged mouse β1AR, PDE4D8-mCherry, and/or ACVI as indicated. After Iso stimulation for 10 min at different concentrations, cells were rinsed in ice-cold phosphate-buffered saline and lysed in coimmunoprecipitation buffer as described previously (De Arcangelis et al., 2009). In brief, HA-β1AR-infected lysates were immunoprecipitated using anti-HA affinity matrix (Roche Diagnostics, Indianapolis, IN). The total immunoprecipitated proteins and 5% of lysates were resolved by 4 to 20% Tris-HCl precast gel (Bio-Rad, Hercules, CA) and plotted with the following antibodies: anti-HA (HA.11; BAbCO, Richmond, CA), anti-ACV/VI (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-pan PDE4 antibody (Abcam Inc, Cambridge, MA), anti-RFP-mCherry (Rockland Immunochemicals, Gilbertsville, PA).
Wild-type neonatal cardiac myocytes were stimulated with Iso for 15 min at different concentration (10−5 or 10−9 M) as indicated. The lysates were separated by SDS-polyacrylamide gel electrophoresis for Western blotting with antibodies against PLB, phospho-Ser16-PLB (p-PLB), TnI, and phospho-TnI (Badrilla Ltd., Leeds, UK), phospho-(Ser/Thr)-PKA substrate (Cell Signaling Technology, Danvers, MA), and anti γ-tubulin (Sigma-Aldrich). The primary antibodies were revealed with IRDye 680CW goat-anti mouse or IRDye 800CW goat-anti rabbit secondary antibodies using an Odyssey scanner (LI-COR Biosciences, Lincoln, NE).
Fluorescence Resonance Energy Transfer Recording.
Neonatal cardiac myocytes from wild type or β1β2AR-KO pups were infected with viruses to express either A-kinase activity reporter AKAR3 (Allen and Zhang, 2006) or cAMP probe ICUE3 (Allen et al., 2006) as described previously (Soto et al., 2009). Living myocytes were imaged on an Axiovert 200M microscope (Carl Zeiss, Thornwood, NY) with a ×40/1.3 numerical aperture oil-immersion objective lens and a charge-coupled device camera controlled by a Metafluor software (Molecular Devices). Dual-emission recording of both cyan direct (440/480 nm, excitation/emission) and FRET (440/535 nm, excitation/emission) was coordinated by Lambda 10-3 filter shutter controller (Sutter Instrument Company, Novato, CA). Exposure time was 100 ms, and recording interval was 20 s. Images in both channels were subjected to background subtraction, and ratios of yellow-to-cyan color were calculated at different time points. After the PKA phosphorylation on the consensus site in AKAR3, the ratio yellow-to-cyan fluorescent protein displayed increases. However, the binding cAMP to ICUE3 led to decreases in the ratio of yellow-to-cyan fluorescent protein (Allen et al., 2006), which were plotted with an inverted y-axis.
Statistical Analysis.
Curve-fitting and statistical analyses were performed using Prism (GraphPad Software, Inc., San Diego, CA).
Results
FRET-Based Living Cell Imaging Assays Reveal Distinct Feature in Initial and Sustained Responses in cAMP/PKA Activities upon Adrenergic Stimulation in Cardiac Myocytes.
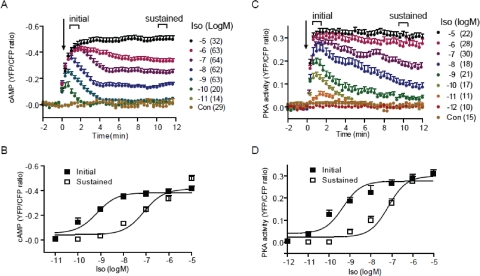
To investigate mechanisms that control the spatiotemporal regulation of cAMP/PKA signaling for substrate phosphorylation and physiological myocyte contraction response, we explored real-time FRET-based living cell-imaging analysis of cAMP and PKA activities. Minimal increases in cAMP and PKA activities were detected at 10−10 and 10−11 M isoproterenol, respectively (Fig. 1, A and C), in contrast to a minimal 10−9 M isoproterenol required for a detectable increase in myocyte contraction rate (De Arcangelis et al., 2008). From 10−12 to 10−8 M, both cAMP and PKA activities displayed a transient and dose-dependent increase in FRET ratio (Fig. 1, A and C). From 10−8 to 10−5 M, the initial peak increases in cAMP and PKA FRET ratios were equivalent but were followed by a rapid decrease to different levels that were later sustained (Fig. 1, A and C). At the saturating dose of 10−5 M, the increase was sustained after reaching peak levels (Fig. 1, A and C). The EC50 values of initial peak increases in cAMP and PKA FRET ratio were 6.86 × 10−10 and 4.53 × 10−10 M, respectively (Fig. 1, B and D). In contrast, the sustained increases in cAMP/PKA FRET ratio have much higher EC50 values (7.99 × 10−8 M for cAMP and 6.77 × 10−8 M for PKA; Fig. 1, B and D). The EC50 concentrations for sustained increases are in correlation with the constants of ligand binding (Kd) to βARs or the EC50 concentrations of the isoproterenol-induced increases in myocyte contraction rate reported previously (Pike and Lefkowitz, 1978; Insel et al., 1983; De Arcangelis et al., 2008).
Fig. 1.
Activation of βARs induces a dose-dependent increase in cAMP ICUE3 and PKA AKAR3 FRET ratio in cardiomyocyte. A and B, the cAMP biosensor ICUE3 was expressed in wild-type myocytes. Cells were treated with isoproterenol at different concentrations. Changes in cAMP ICUE3 FRET ratio (an indication of cAMP activity) were measured. A, time courses of changes in cAMP FRET ratio were calculated and normalized against the baseline levels. B, the initial peak increases (EC50 6.86 × 10−10 M) and the sustained increases (EC50 7.99 × 10−8 M) in cAMP FRET ratio were plotted. C and D, the PKA biosensor AKAR3 was expressed in wild-type myocytes. Cells were treated with isoproterenol at different concentrations. Changes in PKA AKAR3 FRET ratio (an indication of PKA activity) were calculated and normalized against the baseline levels. C, time courses of changes in PKA FRET ratio were plotted. D, the initial peak increases (EC50 4.53 × 10−10 M) and the sustained increases (EC50 6.77 × 10−8 M) in PKA FRET ratio were plotted.
Phosphorylation of Phospholamban and Troponin I, and Myocyte Contraction Responses Display Agonist Dose-Dependent Increases upon Adrenergic Stimulation.
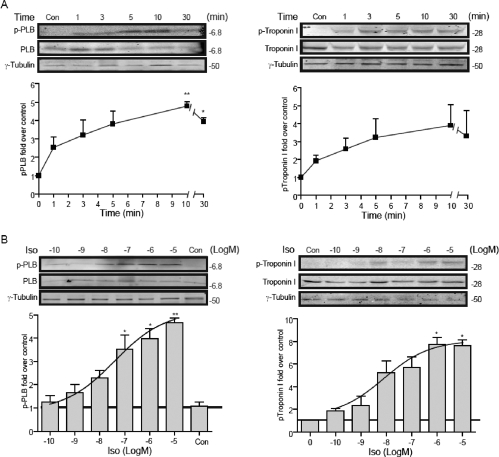
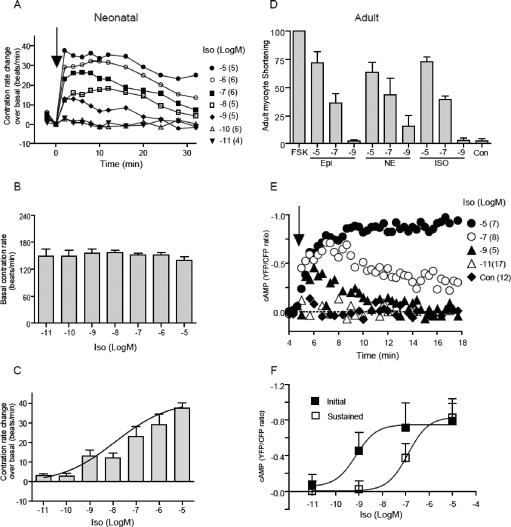
To understand how the temporal cAMP/PKA signal affects physiological function of cardiac myocytes, we examined PKA phosphorylation of PLB and TnI, and myocyte contraction rate response under stimulation with different doses of isoproterenol. At a saturating dose of 10−5 M, both PLB and TnI displayed rapid increases in PKA phosphorylation (Fig. 2A). The increases were maintained during 30 min of stimulation (Fig. 2A), consistent with the βAR-induced sustained increases in cAMP/PKA activities under the same stimulation condition (Fig. 1, A and C). At peak levels after 10 min of stimulation, the increases in PKA phosphorylation of PLB and TnI displayed an agonist dose-dependent manner (EC50, 3.38 × 10−8 for PLB and 7.41 × 10−9 for TnI; Fig. 2B), which is consistent with the EC50 of sustained cAMP/PKA activities measured by FRET assays (Fig. 1, B and D). We then carried out neonatal myocyte contraction rate assay; a minimal 10−9 M isoproterenol was required for a detectable increase in contraction rate (Fig. 3A). Whereas the baseline contraction rates were equivalent, upon increasing the concentration of isoproterenol, the contraction rate displayed an agonist dose-dependent increase (EC50, 4.33 × 10−8; Fig. 3, B and C). The contraction rate response also displays a high correlation with sustained cAMP and PKA activities (Fig. 1).
Fig. 2.
Activation of βARs induces a dose-dependent increase in PKA phosphorylation in cardiomyocyte. A, wild-type myocytes were stimulated with isoproterenol for different time as indicated. Time courses of PKA phosphorylation of PLB (left) and troponin I (right) induced by isoproterenol (10−5 M), *, p < 0.05, and **, p < 0.01 by one-way ANOVA compared with unstimulated controls. B, at 10 min of stimulation, isoproterenol dose-dependent increases in PKA phosphorylation of PLB (left, EC50 3.38 × 10−8) and troponin I (right, EC50 7.41 × 10−9) were plotted. The intensity of each band was quantified and normalized against the total PLB or TnI in the same sample and plotted in the bar graph.
Fig. 3.
Activation of βARs induces a dose-dependent increase in myocyte contraction in both neonatal and adult cardiomyocytes. A, upon isoproterenol stimulation, time courses of changes in spontaneous myocyte contraction rate over baseline level were plotted. The baseline contraction rate (B) and the maximal increase (C, EC50 4.33 × 10−8 M) in contraction rate upon stimulation with isoproterenol were plotted. D, wild-type adult myocytes were paced at 1 Hz and stimulated with forskolin, isoproterenol, norepinephrine, or epinephrine as indicated. The maximal myocyte shortening were normalized against the baseline, and plotted as a percentage of those induced by forskolin. E, the cAMP biosensor ICUE3 was expressed in wild-type adult myocytes. Cells were treated with isoproterenol at different concentrations. Changes in cAMP ICUE3 FRET ratio (an indication of cAMP activity) were calculated and normalized against the baseline levels. F, the initial peak increases (EC50 7.46 × 10−10 M) and the sustained increases (EC50 9.04 × 10−8 M) in cAMP FRET ratio were plotted.
We also examined the βAR-induced cAMP/PKA activities and contraction responses in more physiologically relevant adult myocytes. In adult myocyte shortening assay, stimulation with isoproterenol, norepinephrine, or epinephrine all induced an agonist dose-dependent increase (Fig. 3D). At 10−9 M isoproterenol and epinephrine, minimal myocyte shortening was detected, whereas at 10−9 M norepinephrine, a small but significant myocyte shortening was detected. At 10−5 M, all three drugs induced similar maximal shortening in adult myocytes. We then examined cAMP activities in adult myocytes with ICUE3 FRET assay. Stimulation of myocyte with isoproterenol induced a dose-dependent ICUE3 FRET response (Fig. 3E). Similar to those observed in neonatal myocytes, the responses were transient at submaximal doses but were sustained at the saturated dose. The EC50 values for initial peak increases and sustained increases were 7.46 × 10−10 and 9.04 × 10−8 M, respectively (Fig. 3F).
ACs Control the Initial Peak of cAMP/PKA Activities under Adrenergic Stimulation in Cardiomyocytes.
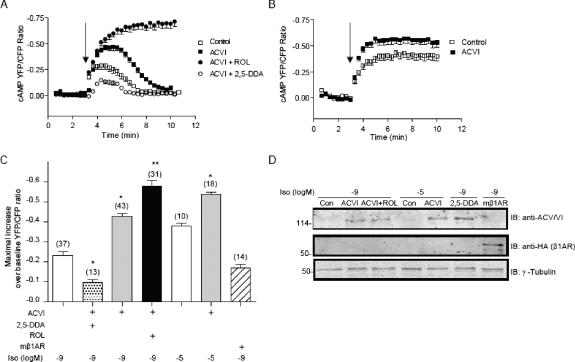
To understand the relative contribution of β1AR and β2AR, the two major subtypes expressed in cardiac myocytes, we used myocytes isolated from mice lacking either β1AR or β2AR gene (β1AR-KO and β2AR-KO, respectively). Stimulation of endogenous β1AR with isoproterenol in β2AR-KO myocytes induced responses similar to those in WT myocytes, a transient increase at 10−8 M, and a sustained increase at 10−5 M isoproterenol, respectively (Supplemental Fig. S1, A and B). Inhibition of PDE4 with rolipram enhanced the initial peak increases, which also became sustained at both concentrations (Supplemental Fig. S1, A and B). In contrast, stimulation of endogenous β2AR with isoproterenol in β1AR-KO myocytes induced smaller and transient responses at both 10−8 and 10−5 M isoproterenol. Inhibition of PDE4 with rolipram enhanced the initial peak increases, which also became sustained at both concentrations (Supplemental Fig. S1, C and D). We then aimed to determine the roles of two key components in the system, ACs and PDEs, in controlling the temporal cAMP/PKA activities induced by adrenergic stimulation. ACs have been implicated as a rate-limiting factor in the βAR/Gs/AC signaling pathway (Ostrom et al., 2000). Inhibition of AC with 2′,5′-DDA, a selective AC inhibitor, significantly reduced the cAMP FRET response induced by 10−9 M isoproterenol (Fig. 4, A and D). ACV and ACVI are highly expressed in cardiac muscle cells; overexpression of ACVI alone significantly enhanced the increases of cAMP activity upon stimulation with either minimal 10−9 M or saturating 10−5 M isoproterenol (Fig. 4, A–D). Inhibition of PDE4 with rolipram further enhanced the initial peak increases induced by 10−9 M isoproterenol (Fig. 4, A, C, and D). In contrast, overexpression of β1AR, the major adrenergic subtype to stimulate cardiac contraction, failed to promote higher initial peak increase in cAMP FRET ratio than those by endogenous βARs in wild-type myocytes (Fig. 4, C and D). These data suggest that AC is the determining factor for the initial peak increase in cAMP induced by adrenergic stimulation.
Fig. 4.
AC determines initial peak increases in cAMP FRET ratio upon adrenergic stimulation in cardiomyocytes. ACVI or HA-β1AR was expressed together with the cAMP biosensor ICUE3 in wild-type cardiomyocytes. A, effects of inhibition of AC with 2′,5′-DDA (10−4 M) or overexpression of ACVI on increases in cAMP FRET ratio induced by 10−9 M isoproterenol alone or by 10−9 M isoproterenol with additional inhibition of PDE4 with inhibitor rolipram (10−6 M). B, effects of overexpression of ACVI on increases in cAMP FRET ratio induced by 10−5 M isoproterenol. C, the maximal increases in cAMP FRET ratio in A and B and the maximal increase in cAMP FRET ratio induced by 10−9 M isoproterenol on β1β2AR-KO myocytes with HA-β1AR overexpression were plotted. *, p < 0.05, and **, p < 0.01 by one-way ANOVA compared with controls stimulated with the same concentration of isoproterenol. D, the expression of ACVI, HA-β1AR, and ICUE3 was detected in Western blot.
PDEs Dissociate with βAR and Control the Duration of cAMP/PKA Activities in Cardiomyocytes.
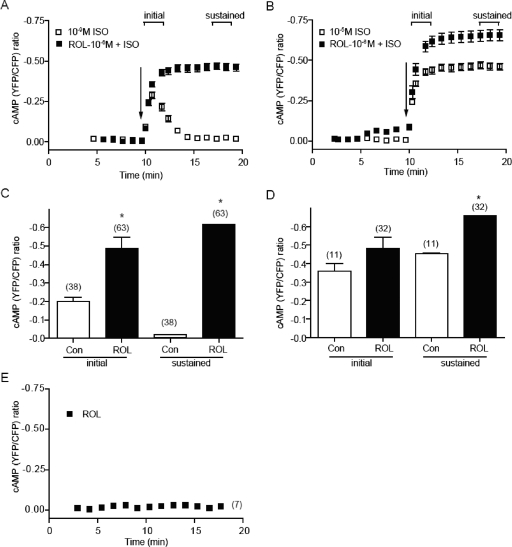
In contrast, when the concentration of isoproterenol was increased from the nanomolar to the micromolar range, the βAR-induced initial increases in cAMP FRET ratio were saturated (Fig. 1A). However, these cAMP signals underwent rapid attenuation to different levels, which were later sustained in a dose-dependent fashion (Fig. 1A). Recent studies show that PDE4D is the major PDE gene that associates with adrenergic stimulation for cardiac myocyte contraction responses (Xiang et al., 2005), and PDE4D isoforms display preferential association with βAR subtypes (Richter et al., 2008; De Arcangelis et al., 2009). In agreement, inhibition of PDE4 with the specific inhibitor rolipram significantly increased both the initial and sustained responses in cAMP FRET ratios induced by 10−9 M isoproterenol but was less effective in enhancing the initial and sustained increases in cAMP induced by 10−5 M isoproterenol (Fig. 5, A and B). After inhibition of PDE4, the responses induced by isoproterenol at both doses were equivalent (Fig. 5, C and D). As a control, inhibition of PDE4 alone did not affect the basal cAMP levels in myocytes (Fig. 5E). PDE4D can be activated through PKA phosphorylation to act as a negative-feedback mechanism that attenuates cAMP signal upon receptor activation (Alvarez et al., 1995; Baillie et al., 2001). Indeed, endogenous PDE4D was phosphorylated by receptor-induced PKA activity at both 10−9 and 10−5 M isoproterenol (Fig. 6A). These data support the role of PDE4D activity in shaping the dose-dependent sustained increases in cAMP/PKA activities in myocytes.
Fig. 5.
Inhibition of PDE4 enhances increases in cAMP FRET ratio induced by submaximal dose of isoproterenol. The cAMP biosensor ICUE3 was expressed in wild-type cardiomyocytes. Cells were treated with isoproterenol in the presence or absence of PDE4-selective inhibitor rolipram (10−6 M). Effects of inhibition of PDE4 with rolipram on increases in cAMP FRET ratio induced by isoproterenol at 10−9 M (A) or 10−5 M (B). The initial increases and the sustained increases induced by isoproterenol at 10−9 M (C) or 10−5 M (D) were plotted. E, effect of inhibition of PDE4 with rolipram (10−6 M) alone on cAMP FRET ratio. *, P < 0.05 by one-way ANOVA.
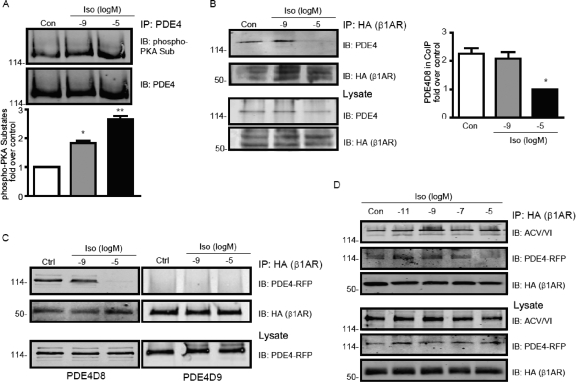
Fig. 6.
Agonist dose-dependent dissociations of PDE4D8 but not ACVI from β1AR under stimulation of isoproterenol. A, β2AR-KO cardiomyocytes were stimulated with isoproterenol at either 10−9 or 10−5 M for 10 min. The endogenous PDE4 proteins were immunoisolated with anti-PDE antibody, and the isoproterenol-induced PKA phosphorylation of PDE4 proteins was detected in Western blotting. B, β1β2AR-KO cardiomyocytes expressing HA-β1AR were stimulated with isoproterenol at either 10−9 or 10−5 M for 10 min. The endogenous PDE4 proteins were coimmunoprecipitated with anti-HA affinity beads before Western blotting. C, β1β2AR-KO cardiomyocytes expressing HA-β1AR together with either PDE4D8-GFP or PDE4D9-GFP were stimulated with either 10−9 or 10−5 M isoproterenol for 10 min. The receptor-associated PDE4D isoforms were immunoprecipitated with anti-HA affinity beads before Western blotting. D, β1β2AR-KO cardiomyocytes expressing HA-β1AR, ACVI, and PDE4D8-RFP were stimulated with isoproterenol at different concentrations for 10 min. The receptor-associated ACVI and PDE4D8 were immunoprecipitated with anti-HA affinity beads before Western blotting. *, p < 0.05, and **, p < 0.01 by one-way ANOVA compared with unstimulated controls.
Because β1AR is the major βAR subtype responsible for adrenergic stimulation of cardiac contraction, we then examined the association between PDE4D isoforms and β1AR upon adrenergic stimulation. Endogenous PDE4D8 bound the β1AR in cardiac myocytes at resting state (Fig. 6B), consistent with the binding of PDE4D8 to β1AR in HEK293 cells (Richter et al., 2008). Moreover, the PDE4D8/β1AR complex was stable under stimulation with 10−9 M isoproterenol; however, the enzyme was dissociated from the receptor under stimulation with a saturating 10−5 M isoproterenol (Fig. 6B). We also examined the association between individual PDE4D isoforms and β1AR upon adrenergic stimulation. PDE4D8, but not a closely related PDE4D9, bound the β1AR in cardiac myocytes at resting state but selectively dissociated from the receptor upon stimulation with a saturating 10−5 M isoproterenol (Fig. 6C). Further examination showed that PDE4D8 displayed an agonist-dependent dissociation from the receptor (Fig. 6D). In contrast, ACVI remained in the receptor complex under stimulation with increasing concentrations of isoproterenol (Fig. 6D). These data indicate that selective dissociation of PDE4D8 shifts the balance between AC-dependent cAMP production and PDE-dependent cAMP degradation at increasing concentrations of isoproterenol and patterns the agonist dose-dependent temporal responses in cAMP/PKA activities in cardiomyocytes.
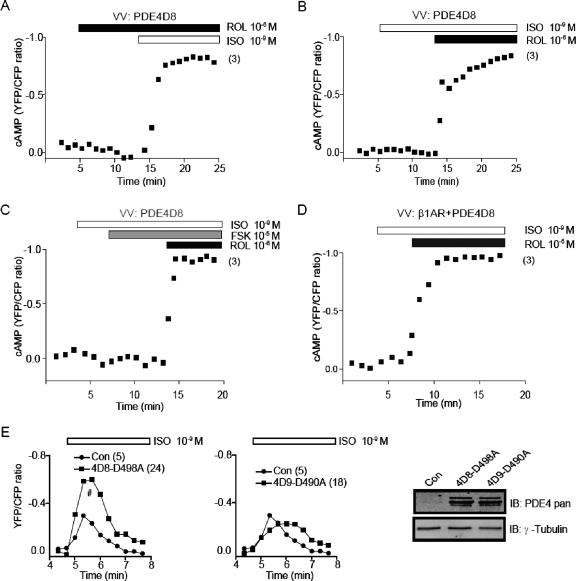
We further probed the role of PDE4D8 in shaping the sustained responses induced by βAR activation. Overexpression of PDE4D8 completely blocked the increases in cAMP FRET ratio induced by activation of βAR at a saturating concentration 10−5 M isoproterenol or by activation of ACs with 10−5 M forskolin (Fig. 7, A–C). However, the inhibitory effect of PDE4D8 was readily released by pretreatment with rolipram (Fig. 7A) or by the addition of rolipram (Fig. 7, B and C), which promoted the saturated increases in the cAMP FRET ratio. With overexpressed PDE4D8, even activation of overexpressed β1AR failed to induce any significant increase in cAMP FRET ratio at 10−9 M isoproterenol. However, additional inhibition of PDE4 with rolipram led to the saturated increases in cAMP FRET ratio (Fig. 7D). In addition, we further dissected the role of individual PDE4D isoforms with overexpression of dominant-negative PDE4Ds containing a mutation destroying catalytic activities. The overexpressed dominant-negative inhibits the endogenous PDE4D isoforms by displacing them from the correct association with receptor complexes. Overexpression of PDE4D8 dominant-negative selectively enhanced the cAMP increase induced by of 10−9 M isoproterenol but did not change the transient feature of cAMP response (Fig. 7E). In contrast, overexpression of dominant-negative PDE4D9, an isoform that does not bind to β1AR did not affect the maximal increase in cAMP FRET ratio but slightly delayed the decrease of cAMP FRET responses (Fig. 7E). These data solidify the functional association of a dominant PDE4D8 activity with the β1AR for tuning the cAMP equilibrium upon isoproterenol stimulation in cardiomyocytes.
Fig. 7.
Overexpression of PDE4D8 blocks the cAMP FRET responses induced by either isoproterenol or forskolin. A to C, PDE4D8 and the cAMP biosensor ICUE3 were coexpressed in wild-type cardiomyocytes. A, changes in cAMP ICUE3 FRET ratio were measured after inhibition of PDE4 with rolipram (10−6 M) followed by additional stimulation with isoproterenol (10−9 M). B, changes in cAMP ICUE3 FRET ratio were measured after stimulation with isoproterenol (10−9 M) followed by additional inhibition of PDE4 with rolipram (10−6 M). C, changes in cAMP ICUE3 FRET ratio were measured after stimulation with isoproterenol (10−9 M), followed by additional stimulation with forskolin (10−5 M) before additional inhibition of PDE4 with rolipram (10−6 M). D, PDE4D8, HA-β1AR, and ICUE3 were coexpressed in β1β2AR-KO cardiomyocytes. Changes in cAMP ICUE3 FRET ratio were measured after stimulation with isoproterenol (10−9 M) followed by additional inhibition of PDE4 with rolipram (10−6 M). E, dominant-negative PDE4D8 (498A) and PDE4D9 (490A) were coexpressed together with ICUE in β2AR-KO cardiomyocytes. Changes in cAMP ICUE3 FRET ratio were measured after stimulation with isoproterenol (10−9 M). The expression of dominant-negative PDE4D isoforms was detected in Western blot. #, p < 0.05 by two-way ANOVA compared with control.
Discussion
A typical monoexponential dose-dependent cellular response has been a widely accepted pharmacological principle for most G-protein-coupled receptor actions. However, there is lack of correlation between cAMP/PKA activities and myocyte contraction responses under the same stimulation condition (Zhu et al., 2005; De Arcangelis et al., 2008). In this study, we have used sensitive FRET-based living-cell imaging to analyze cellular cAMP/PKA signals induced by the adrenergic agonist isoproterenol. Our data indicate that isoproterenol induced two distinct phases in dose-dependent responses in cAMP/PKA activities in cardiac myocytes: a transient and dose-dependent increase of initial response under concentrations from 10−12 to 10−8 M isoproterenol; and a saturated initial increase under concentration from 10−8 to 10−5 M isoproterenol followed by a dose-dependent decrease to different levels that are later sustained (Figs. 1 and 8). The sustained but not the initial cAMP/PKA activities display a high correlation to the substrate phosphorylation and myocyte contraction-rate response. Moreover, the agonist dose-dependent temporal increases in cAMP/PKA activities are patterned by a shifting equilibrium between two distinct mechanisms, AC-dependent cAMP production and PDE-dependent cAMP degradation because of the selective dissociation of PDE but not AC from the activated receptor at higher concentrations. This shifting equilibrium allows cAMP accumulation and propagation in cardiomyocytes, which dictates PKA substrate-specificity and cardiac contraction response (Fig. 8).
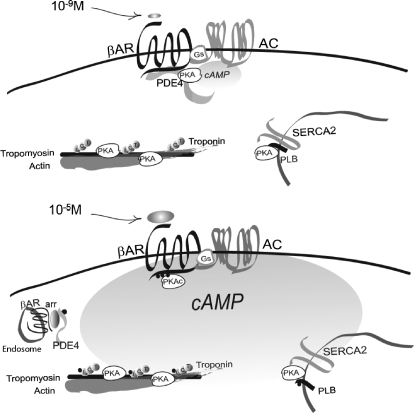
Fig. 8.
Model of dual mechanistic regulation of cAMP/PKA activities by AC and PDE4D under different doses of adrenergic stimulation. At 10−9 M isoproterenol, the βAR-activated AC induces significant production of cAMP (the gas pedal is on), which is transient and restricted at the vicinity of the receptor for local PKA activation. The activated PKA has access to the receptor and receptor-associated PDE that negatively feeds back to confine and attenuate cAMP signaling at local domains (the brake is still on). At 10−5 M isoproterenol, the AC-produced cAMP (the gas pedal is on) can propagate to access to PKA in different subcellular compartments because of dissociation of PDE4D from the activated receptors (the brake is off). The activated PKA phosphorylates both local (near the receptor) and distant substrates such as PLB and TnI for myocyte contraction responses.
Among βAR subtypes, the β1AR is the major subtype expressed in myocytes and induces stronger cAMP/PKA activities compared with those by the β2AR, consistent with our previous studies showing the β1AR signaling induces stronger contraction rate responses than that induced by the β2AR in cardiac myocytes (Devic et al., 2001). In addition, a minor role of β3AR in negatively controlling cAMP/PKA activities and myocyte contraction rate has been detected previously (Devic et al., 2001; Mongillo et al., 2006). However, because of the minimal expression of this subtype in cardiac myocytes, it should have no effect on the cAMP/PKA activities at low doses of isoproterenol and probably a limited effect to modify the cAMP signaling at high doses of isoproterenol. The transient initial increases of cAMP/PKA activities display a high sensitivity to isoproterenol stimulation at low concentrations, which has a very low EC50 compared with the binding constants (Kd) (EC50 is not a binding constant, although it is usually proportional to it) of isoproterenol to βARs (Pike and Lefkowitz, 1978; Insel et al., 1983). Because receptors are more abundant than G proteins and ACs (Gao et al., 1998; Ostrom et al., 2001), activation of a small number of receptors may be sufficient in evoking the receptor/Gs/AC system for cAMP production. Thus, the initial peak increases may be a reflection of the available pool of ACs activated in the receptor/G protein/AC system, supporting the notion that the quantity of ACs is the rate-limiting factor in producing cellular cAMP (Gao et al., 1998; Ostrom et al., 2001). In agreement, overexpression of ACVI, but not β1ARs significantly enhances the maximal increases in cAMP accumulation (Fig. 4).
Alternatively, it has been reported that adrenergic receptors can form precoupled complexes with Gs proteins, which display a much higher binding affinity to isoproterenol (Green et al., 1992). At concentrations from 10−12 to 10−8 M, the dose-dependent maximal increases of cAMP/PKA activities may be influenced by binding of isoproterenol to the high-affinity sites of the precoupled receptors. In this scenario, the maximal responses are probably due to agonist occupancy at the precoupled receptors, which seems to be sufficient to promote the maximal cAMP production via receptor/Gs/AC axis (Figs. 1 and 8). However, under these low concentrations, the agonist-induced cAMPs are rapidly degraded by the PKA-activated PDE4D within receptor complexes, a negative-feedback mechanism to attenuate cAMP/PKA signaling (Mongillo et al., 2004; Willoughby et al., 2006; Leroy et al., 2008). The equilibrium between AC-dependent cAMP production and PDE-dependent cAMP degradation is dominated by the powerful PDE activities, which also functions as a “gating/braking” mechanism to ensure cAMP activities are restricted within the receptor complex or the vicinity for local activation of PKA. Such PKA activation can only have access to the activated receptors (Tran et al., 2004; Liu et al., 2009) and receptor-associated downstream signaling components such as PDE4D (Fig. 6A) but not to the substrate in distance, such as phospholamban and troponin I for cardiac contraction.
This scenario is totally different when the concentration of isoproterenol is increased from 10−8 to 10−5 M. At these concentrations, the AC-mediated cAMP production seems to be maximized. The saturation of cAMP production can be due to many factors, including activation of AC by either Gαs or Gβγ subunits inside or outside of caveolae, receptor desensitization, G protein hydrolysis, and negative regulation of AC activities by either kinases or Gi proteins (Hanoune and Defer, 2001; Violin et al., 2008; Dessauer, 2009; Sadana and Dessauer, 2009). In contrast, the receptor-associated PDE4D isoforms display an agonist dose-dependent dissociation from the receptor complex, which results in a shifting equilibrium between AC-dependent cAMP production and PDE-dependent cAMP degradation and promotes sustained increases in cAMP in a dose-dependent manner. The dissociation of PDE4D isoforms also functions as releasing the “gate/brake” to allow propagation of cAMP signal to potentiate PKA phosphorylation of phospholamban and troponin I and cardiac contraction. Perturbation of the balance by altering the expression levels of either AC or PDE or by inhibition of either of them drastically changes the temporal profiles of cAMP activities (Figs. 4, 5, and 7), dictating the substrate specificity by PKA (De Arcangelis et al., 2008). Therefore, at higher concentrations, although the AC-dependent cAMP production remains constant within receptor complexes, the dissociation of PDE4D from the receptor seems to open the gate/release the brake for cAMP diffusion and plays a critical role in shaping the dose-dependent cAMP signaling propagation for myocyte contraction (De Arcangelis et al., 2008).
Together, using real-time FRET-based biosensors, we have revealed biphasic dose-dependent cAMP and PKA activities under adrenergic stimulation in cardiomyocytes: a transient and dose-dependent increase in initial peak responses at picomolar doses, and saturated initial increases followed by dose-dependent sustained increases at nanomolar doses. These data underscore an elegant integration of dual mechanistic regulation of cAMP/PKA activities by βAR-associated AC and PDE in an agonist-dose-dependent manner, which shapes the temporal responses in cAMP/PKA activities for substrate-specificity and physiological myocyte contraction-rate responses. Our data provide a new paradigm for further investigation of cAMP/PKA signaling for cardiac responses under different physiological and clinical conditions.
Supplementary Material
Acknowledgments
We thank Dr. Paul Insel of the University of California at San Diego for providing ACVI adenovirus.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This study was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL082646] and the American Heart Association [Grant 0635331N].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.064444.
- AR
- adrenergic receptor
- FRET
- fluorescence resonance energy transfer
- PKA
- protein kinase A
- βAR
- β-adrenergic receptor
- AC
- adenylyl cyclase
- PDE
- phosphodiesterase
- RyR
- ryanodine receptor
- PLB
- phospholamban
- TnI
- troponin I
- HEK
- human embryonic kidney
- 2′,5′-DDA
- 2′,5′-dideoxyadenosine triphosphate
- Iso
- isoproterenol
- KO
- knockout
- HA
- hemagglutinin
- GFP
- green fluorescent protein
- RFP
- red fluorescent protein
- ANOVA
- analysis of variance.
References
- Allen MD, DiPilato LM, Rahdar M, Ren YR, Chong C, Liu JO, Zhang J. (2006) Reading dynamic kinase activity in living cells for high-throughput screening. ACS Chem Biol 1:371–376 [DOI] [PubMed] [Google Scholar]
- Allen MD, Zhang J. (2006) Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem Biophys Res Commun 348:716–721 [DOI] [PubMed] [Google Scholar]
- Alvarez R, Sette C, Yang D, Eglen RM, Wilhelm R, Shelton ER, Conti M. (1995) Activation and selective inhibition of a cyclic AMP-specific phosphodiesterase, PDE-4D3. Mol Pharmacol 48:616–622 [PubMed] [Google Scholar]
- Baillie G, MacKenzie SJ, Houslay MD. (2001) Phorbol 12-myristate 13-acetate triggers the protein kinase A-mediated phosphorylation and activation of the PDE4D5 cAMP phosphodiesterase in human aortic smooth muscle cells through a route involving extracellular signal regulated kinase (ERK). Mol Pharmacol 60:1100–1111 [DOI] [PubMed] [Google Scholar]
- Cooper DM. (2005) Compartmentalization of adenylate cyclase and cAMP signalling. Biochem Soc Trans 33:1319–1322 [DOI] [PubMed] [Google Scholar]
- De Arcangelis V, Liu R, Soto D, Xiang Y. (2009) Differential association of phosphodiesterase 4D isoforms with beta2-adrenoceptor in cardiac myocytes. J Biol Chem 284:33824–33832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Arcangelis V, Soto D, Xiang Y. (2008) Phosphodiesterase 4 and phosphatase 2A differentially regulate cAMP/protein kinase a signaling for cardiac myocyte contraction under stimulation of beta1 adrenergic receptor. Mol Pharmacol 74:1453–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessauer CW. (2009) Adenylyl cyclase–A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol 76:935–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devic E, Xiang Y, Gould D, Kobilka B. (2001) Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Mol Pharmacol 60:577–583 [PubMed] [Google Scholar]
- Gao M, Ping P, Post S, Insel PA, Tang R, Hammond HK. (1998) Increased expression of adenylylcyclase type VI proportionately increases beta-adrenergic receptor-stimulated production of cAMP in neonatal rat cardiac myocytes. Proc Natl Acad Sci USA 95:1038–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Holt BD, Liggett SB. (1992) Beta 1- and beta 2-adrenergic receptors display subtype-selective coupling to Gs. Mol Pharmacol 41:889–893 [PubMed] [Google Scholar]
- Hanoune J, Defer N. (2001) Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol 41:145–174 [DOI] [PubMed] [Google Scholar]
- Houslay MD, Baillie GS, Maurice DH. (2007) cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res 100:950–966 [DOI] [PubMed] [Google Scholar]
- Insel PA, Mahan LC, Motulsky HJ, Stoolman LM, Koachman AM. (1983) Time-dependent decreases in binding affinity of agonists for beta-adrenergic receptors of intact S49 lymphoma cells. A mechanism of desensitization. J Biol Chem 258:13597–13605 [PubMed] [Google Scholar]
- Jarnaess E, Taskén K. (2007) Spatiotemporal control of cAMP signalling processes by anchored signalling complexes. Biochem Soc Trans 35:931–937 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. (2007) Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf) 190:9–19 [DOI] [PubMed] [Google Scholar]
- Leroy J, Abi-Gerges A, Nikolaev VO, Richter W, Lechêne P, Mazet JL, Conti M, Fischmeister R, Vandecasteele G. (2008) Spatiotemporal dynamics of beta-adrenergic cAMP signals and L-type Ca2+ channel regulation in adult rat ventricular myocytes: role of phosphodiesterases. Circ Res 102:1091–1100 [DOI] [PubMed] [Google Scholar]
- Liu R, Ramani B, Soto D, De Arcangelis V, Xiang Y. (2009) Agonist dose-dependent phosphorylation by protein kinase A and G protein-coupled receptor kinase regulates beta2 adrenoceptor coupling to G(i) proteins in cardiomyocytes. J Biol Chem 284:32279–32287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnachie G, Langeberg LK, Scott JD. (2006) AKAP signaling complexes: getting to the heart of the matter. Trends Mol Med 12:317–323 [DOI] [PubMed] [Google Scholar]
- Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, Huston E, Hannawacker A, Lohse MJ, Pozzan T, et al. (2004) Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ Res 95:67–75 [DOI] [PubMed] [Google Scholar]
- Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, et al. (2006) Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res 98:226–234 [DOI] [PubMed] [Google Scholar]
- Mongillo M, Zaccolo M. (2006) A complex phosphodiesterase system controls beta-adrenoceptor signalling in cardiomyocytes. Biochem Soc Trans 34:510–511 [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Gregorian C, Drenan RM, Xiang Y, Regan JW, Insel PA. (2001) Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J Biol Chem 276:42063–42069 [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Violin JD, Coleman S, Insel PA. (2000) Selective enhancement of beta-adrenergic receptor signaling by overexpression of adenylyl cyclase type 6: colocalization of receptor and adenylyl cyclase in caveolae of cardiac myocytes. Mol Pharmacol 57:1075–1079 [PubMed] [Google Scholar]
- Pike LJ, Lefkowitz RJ. (1978) Agonist-specific alterations in receptor binding affinity associated with solubilization of turkey erythrocyte membrane beta adrenergic receptors. Mol Pharmacol 14:370–375 [PubMed] [Google Scholar]
- Richter W, Day P, Agrawal R, Bruss MD, Granier S, Wang YL, Rasmussen SG, Horner K, Wang P, Lei T, et al. (2008) Signaling from beta1- and beta2-adrenergic receptors is defined by differential interactions with PDE4. EMBO J 27:384–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadana R, Dessauer CW. (2009) Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals 17:5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D, De Arcangelis V, Zhang J, Xiang Y. (2009) Dynamic protein kinase a activities induced by beta-adrenoceptors dictate signaling propagation for substrate phosphorylation and myocyte contraction. Circ Res 104:770–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TM, Friedman J, Qunaibi E, Baameur F, Moore RH, Clark RB. (2004) Characterization of agonist stimulation of cAMP-dependent protein kinase and G protein-coupled receptor kinase phosphorylation of the beta2-adrenergic receptor using phosphoserine-specific antibodies. Mol Pharmacol 65:196–206 [DOI] [PubMed] [Google Scholar]
- Violin JD, DiPilato LM, Yildirim N, Elston TC, Zhang J, Lefkowitz RJ. (2008) beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem 283:2949–2961 [DOI] [PubMed] [Google Scholar]
- Wang Y, De Arcangelis V, Gao X, Ramani B, Jung YS, Xiang Y. (2008) Norepinephrine- and epinephrine-induced distinct beta2-adrenoceptor signaling is dictated by GRK2 phosphorylation in cardiomyocytes. J Biol Chem 283:1799–1807 [DOI] [PubMed] [Google Scholar]
- Willoughby D, Wong W, Schaack J, Scott JD, Cooper DM. (2006) An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. EMBO J 25:2051–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Kobilka BK. (2003) Myocyte adrenoceptor signaling pathways. Science 300:1530–1532 [DOI] [PubMed] [Google Scholar]
- Xiang Y, Naro F, Zoudilova M, Jin SL, Conti M, Kobilka B. (2005) Phosphodiesterase 4D is required for beta2 adrenoceptor subtype-specific signaling in cardiac myocytes. Proc Natl Acad Sci USA 102:909–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao RP, Zhu W, Zheng M, Cao C, Zhang Y, Lakatta EG, Han Q. (2006) Subtype-specific alpha1- and beta-adrenoceptor signaling in the heart. Trends Pharmacol Sci 27:330–337 [DOI] [PubMed] [Google Scholar]
- Zaccolo M. (2006) Phosphodiesterases and compartmentalized cAMP signalling in the heart. Eur J Cell Biol 85:693–697 [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T. (2002) Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295(5560):1711–1715 [DOI] [PubMed] [Google Scholar]
- Zhu WZ, Chakir K, Zhang S, Yang D, Lavoie C, Bouvier M, Hébert TE, Lakatta EG, Cheng H, Xiao RP. (2005) Heterodimerization of beta1- and beta2-adrenergic receptor subtypes optimizes beta-adrenergic modulation of cardiac contractility. Circ Res 97:244–251 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.