Abstract
Parkinson's disease (PD) is the second most common neurodegenerative disease characterized by a progressive loss of dopamine (DA) neurons in the substantia nigra. Accumulating evidence indicates that inhibition of microglia-mediated neuroinflammation may become a reliable protective strategy for PD. Resveratrol, a nonflavonoid polyphenol naturally found in red wine and grapes, has been known to possess antioxidant, anticancer, and anti-inflammatory properties. Although recent studies have shown that resveratrol provided neuroprotective effects against ischemia, seizure, and neurodegenerative disorders, the mechanisms underlying its beneficial effects on dopaminergic neurodegeneration are poorly defined. In this study, rat primary midbrain neuron-glia cultures were used to elucidate the molecular mechanisms underlying resveratrol-mediated neuroprotection. The results clearly demonstrated that resveratrol protected DA neurons against lipopolysaccharide (LPS)-induced neurotoxicity in concentration- and time-dependent manners through the inhibition of microglial activation and the subsequent reduction of proinflammatory factor release. Mechanistically, resveratrol-mediated neuroprotection was attributed to the inhibition of NADPH oxidase. This conclusion is supported by the following observations. First, resveratrol reduced NADPH oxidase-mediated generation of reactive oxygen species. Second, LPS-induced translocation of NADPH oxidase cytosolic subunit p47 to the cell membrane was significantly attenuated by resveratrol. Third and most importantly, resveratrol failed to exhibit neuroprotection in cultures from NADPH oxidase-deficient mice. Furthermore, this neuroprotection was also related to an attenuation of the activation of mitogen-activated protein kinases and nuclear factor-κB signaling pathways in microglia. These findings suggest that resveratrol exerts neuroprotection against LPS-induced dopaminergic neurodegeneration, and NADPH oxidase may be a major player in resveratrol-mediated neuroprotection.
Introduction
Parkinson's disease is among the most common and debilitating age-associated neurodegenerative disorder. It has been characterized by slow and progressive degeneration of dopamine (DA) neurons in the substantia nigra and a consequent severe decrease in DA levels in the striatum (Hirsch et al., 1988). Clinical symptoms of PD include tremor, rigidity, bradykinesia, and postural instability, which can be alleviated by restoring neurotransmission with the DA precursor levodopa or with DA agonists. Nevertheless, current therapeutic treatments cannot halt this degenerative process (Gao et al., 2003b).
Intensive studies have revealed that several molecular and cellular events, including oxidative stress, mitochondrial dysfunction, proapoptotic mechanisms, and abnormal protein handling, might be involved in the pathogenesis of PD (Nagatsu and Sawada, 2006). However, the mechanisms underlying the neuronal loss in PD are not fully understood. Recently, microglia-mediated neuroinflammation has been recognized to contribute to the cascade of events leading to PD (Hirsch and Hunot, 2009). Microglia are the resident immune cells in the brain and serve the role of immune surveillance. Once exposed to immunological challenges such as invading pathogens and neuronal injuries, microglia readily become activated and undergo changes in morphology (hypertrophy), number (proliferation), and function (phagocytosis). Activated microglia also produced a large number of proinflammatory factors including cytokines, reactive oxygen species (ROS), and reactive nitrogen species. Among these proinflammatory factors, NADPH oxidase (PHOX, Nox)-generated ROS play a key role in neurotoxicity elicited by various neurotoxins. DA neurons are known to be extremely sensitive to oxidative stress because of a lower antioxidant capacity, an increased accumulation of iron, and a high content of oxidation-prone DA and lipids (Jenner and Olanow, 1998; Greenamyre et al., 1999). Using in vitro midbrain neuron-glia cocultures, we have demonstrated a critical role of proinflammatory factor release from activated microglia in DA neurotoxicity (Liu et al., 2000).
Resveratrol (3,4′,5-trihydroxy-trans-stilbene) is a natural nonflavonoid polyphenol found in grapes and red wine and is recognized as a bioactive agent with a potential benefit for health. A large number of pharmacological properties including cardioprotective, antioxidant, and anticancer effects are believed to be associated with its beneficial effects (Saiko et al., 2008). Increasing interest has focused on its anti-inflammatory activities. Resveratrol attenuates the activation of immune cells and subsequent synthesis and release of inflammatory mediators through the inhibition of transcriptional factors such as nuclear factor-κB (NF-κB) (Das and Das, 2007). In addition, several lines of evidence show that resveratrol could exert neuroprotection against ischemia, seizure, and neurodegenerative diseases (Markus and Morris, 2008). Here, inflammation-induced dopaminergic neurodegeneration in midbrain neuron-glia cultures was used as an in vitro PD model for elucidating the molecular mechanisms underlying resveratrol-mediated neuroprotection. We show that resveratrol protected DA neurons against LPS-induced neurotoxicity through inhibiting the activation of microglia and the release of proinflammatory factors by microglia. Mechanistically, we observed that resveratrol-elicited anti-inflammatory and neuroprotective effects were mainly attributed to the inhibition of NADPH-oxidase activity and the consequent decrease in ROS production.
Materials and Methods
Animals.
Timed-pregnant Fisher F344 rats were obtained from Charles River Laboratories (Raleigh, NC). NADPH oxidase-deficient (gp91PHOX−/−) and wild-type C57BL/6J (gp91PHOX+/+) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Housing and breeding of the animals were performed in strict accordance with the National Institutes of Health guidelines.
Reagents.
Resveratrol, 1-methyl-4-phenylpyridinium (MPP+), cytosine β-d-arabinofuranoside, leu-leu methyl ester, and superoxide dismutase (SOD) were purchased from Sigma-Aldrich (St. Louis, MO). LPS (Escherichia coli strain O111:B4) and the fluorescence probe dichlorodihydrofluorescein diacetate (DCFH-DA) were obtained from Calbiochem (San Diego, CA). All of the materials of cell cultures were purchased from Invitrogen (Carlsbad, CA). [3H]DA (30 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). The polyclonal anti-tyrosine hydroxylase (TH) antibody was a gift from Dr. John Reinhard (GlaxoSmithKline, Research Triangle Park, NC). Anti-ionized calcium-binding adapter molecule-1 (Iba-1) antibody and rabbit anti-p47PHOX antibody were purchased from Wako Chemicals (Osaka, Japan) and Millipore Corporation (Billerica, MA), respectively. Mouse anti-gp91PHOX was obtained from BD Transduction Laboratories (San Jose, CA). All other primary antibodies came from Cell Signaling Technology (Danvers, MA). Anti-VECTASTAIN avidin-biotin complex kit and biotinylated horse anti-mouse and anti-rabbit secondary antibodies were obtained from Vector Laboratories (Burlingame, CA). Sources for other reagents included the following: WST-1 (Dojindo Laboratories, Gaithersburg, MD), TRIzol reagent (Invitrogen), RNeasy Kit (QIAGEN, Valencia, CA), SYBR green polymerase chain reaction (PCR) master mix (Applied Biosystems, Cheshire, UK), and enhanced chemiluminescence kit (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK).
Primary Rat Midbrain Neuron-Glia and Neuron-Astroglia Cultures.
Primary neuron-glia cultures were prepared from the ventral mesencephalic tissues of embryonic day 14 to 15 rats as described previously (Liu et al., 2000). In brief, dissociated cells were seeded at 5 × 105/well and 105/well in poly(d-lysine)-coated 24- and 96-well plates, respectively. The cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air in maintenance medium that was made up of minimum essential medium containing 10% heat-inactivated fetal bovine serum, 10% heat-inactivated horse serum, 1 g/l glucose, 2 mM l-glutamine, 1 mM sodium pyruvate, 100 μM nonessential amino acids, 50 U/ml penicillin, and 50 μg/ml streptomycin. Seven-day-old cultures were used for drug treatments. At the time of treatment, immunocytochemical analysis indicated that the rat neuron-glia cultures consisted of 10% microglia, 50% astrocytes, 40% neurons, and 1% tyrosine hydroxylase immunoreactive neurons. For treatment, cultures were changed to treatment medium composed of minimum essential medium, 2% fetal bovine serum, 2% horse serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 50 U/ml penicillin, and 50 μg/ml streptomycin. Primary neuron-astroglia cultures were obtained by suppressing microglial proliferation with 1.5 mM leu-leu methyl ester added to neuron-glia cultures 24 h after seeding the cells, as described previously (Zhang et al., 2006). Three days later, cultures were changed back to maintenance medium and used for treatment 7 days after initial seeding. The percentage of microglia in the cells was <1%.
Primary Microglia-Enriched Cultures.
Primary microglia-enriched cultures were prepared from the whole brains of 1-day-old rat pups as described previously (Zhang et al., 2006). After a confluent monolayer of glia cells had been obtained, microglia were shaken off, and immunocytochemical analysis indicated that the cultures were 95 to 98% pure for microglia. Cells were seeded at 5 × 105/well and 105/well in 24- and 96- well plates and used for treatment the following day.
Primary Midbrain Neuron-Enriched and Reconstituted Neuron-Microglia Cultures.
Midbrain neuron-enriched cultures were established as described previously. Cytosine β-d-arabinofuranoside was added to a final concentration of 6 to 8 μM 24 h after seeding to suppress glial proliferation. The 7-day-old neuron-enriched cultures were composed of 90% neurons, 10% astrocytes, and <0.1% microglia. The reconstituted cultures were established by adding 10% (5 × 104/well) of primary microglia back to neuron-enriched cultures as described previously (Liu et al., 2000).
[3H]DA Uptake Assay.
[3H]DA uptake assay was performed as described previously (Gao et al., 2002a). Cultures were incubated for 20 min at 37°C with 1 μM [3H]DA in Krebs-Ringer buffer (16 mU sodium phosphate, 119 mM NaCl, 4.7 mM KCl, 1.8 mM CaCl2, 1.2 mM MgSO4, 1.3 mM EDTA, and 5.6 mM Glc, pH 7.4). Cells were washed with ice-cold Krebs-Ringer buffer and then collected in 1 N NaOH. Radioactivity was determined by liquid scintillation counting. Nonspecific DA uptake observed in the presence of mazindol (10 μM) was subtracted.
Immunocytochemical Staining.
Immunostaining was performed as described previously (Liu et al., 2000). DA neurons were recognized with an anti-TH antibody, and microglia were detected with anti-Iba-1 antibody. In brief, 3.7% formaldehyde-fixed cells were treated with 1% hydrogen peroxide followed by sequential incubation with blocking solution, after which the cultures were incubated overnight at 4°C with primary anti-TH (1:5000) and anti-Iba-1 (1:1000) antibodies. Cells were incubated with biotinylated secondary antibody for 1 h followed by incubation with VECTASTAIN avidin-biotin complex reagents for 40 min, and then color was developed with 3,3′-diaminobenzidine. For morphological analysis, the images were recorded with a charge-coupled device camera and operated with the MetaMorph software (Molecular Devices, Sunnyvale, CA). For visual counting of TH-positive neurons, four representative areas per well of the 24-well plate were counted. In each condition, three wells were used for cell counting.
Nitrite, TNFα, and IL-1β Assay.
The production of NO was accessed by measuring the accumulated levels of nitrite in the culture supernatants with the Griess reagent. The release of TNFα and IL-1β was measured with the immunosorbent assay kits from R&D Systems (Minneapolis, MN).
Real-Time RT-PCR.
Total RNA was isolated using TRIzol reagent (Invitrogen) and purified with RNeasy kit (QIAGEN). The primers were designed with ABI Primer Express software (Applied Biosystems, Foster City, CA). The sequences of the primers were the following: β-actin, GTATGACTCCACTCACGGCAAA (forward), GGTCTCGCTCCTGGAAGATG (reverse); iNOS, ACATCAGGTCGGCCATCACT (forward), CGTACCGGATGAGCTGTGAATT (reverse); TNFα, GACCCTCACACTCAGATCATCTTCT (forward), CCTCCACTTGGTGGTTTGCT (reverse); and IL-1β, CTGGTGTGTGACGTTCCCATTA (forward), CCGACAGCACGAGGCTTT (reverse). Total RNA was reverse-transcribed with MuLV reverse transcriptase and oligo(dT) primers. The SYBR green PCR Master Mix was used for real-time PCR analysis. The relative differences in the expression of these inflammatory factors among groups were expressed using cycle time values as follows: the cycle time values of the interested genes were first normalized with β-actin of the same sample, and then the relative difference between control and each treatment group was calculated and expressed as a relative reduction, setting the LPS at 100%.
Superoxide Assay.
The production of extracellular superoxide was accessed by measuring the SOD-inhibitable reduction of the tetrazolium salt WST-1 (Tan and Berridge, 2000). Primary microglia-enriched cultures in 96-well plate were washed twice with Hanks' balanced salt solution (HBSS) without phenol red. Cells were then incubated at 37°C for 30 min with vehicle control or resveratrol in HBSS (50 μl/well). Thereafter, 50 μl of HBSS with and without SOD (50 U/ml) was added to each well along with 50 μl of WST-1 (1 mM) in HBSS and 50 μl of vehicle or LPS (10 ng/ml). The absorbance at 450 nm was read with a SpectraMax Plus microplate spectrophotometer (Molecular Devices) every 5 min for 1 h. The different absorbance observed in the presence and absence of SOD was considered to be the amount of produced superoxide.
Intracellular ROS Assay.
Intracellular ROS were determined by using the DCFH-DA assay. Primary microglia-enriched cultures were seeded in 96-well plate and then exposed to DCFH-DA for 1 h, followed by pretreatment with resveratrol for 30 min and then treatment with HBSS containing LPS. After incubation at 37°C for 30 min, the fluorescence was read at 485 nm for excitation and 530 nm for emission using a SpectraMax Gemini XS fluorescence microplate reader.
Western Blot Analysis.
For subcellular fractions, primary microglia-enriched cultures were lysed in hypotonic lysis buffer (1 mM EGTA, 1 mM EDTA, 10 mM β-glycerophosphate, 10 mM NaF, 1 mM sodium orthovanadate, 2 mM MgCl2, 10 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 10 μg/ml each of leupeptin, aprotinin, and pepstatin A), incubated on ice for 30 min, and then subjected to Dounce homogenization. The lysates were loaded onto a sucrose gradient in lysis buffer (0.5 M) and centrifuged at 1600g for 15 min. The supernatant above the sucrose gradient was used as the cytosolic fraction after centrifugation at 150,000g for 1 h. The pellet was solubilized in 1% Nonidet P-40 hypotonic lysis buffer and was used as the membranous fraction. For extracting the whole-cell lysates, microglia-enriched cultures were washed with cold phosphate-buffered saline and lysed with radioimmunoprecipitation assay cell lysis buffer consisting of 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 5 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, 1% Triton X-100, 1% sodium deoxycholate, and 1 mM Na3VO4. The lysates were incubated on ice for 30 min and then centrifuged at 12,000g for 25 min. Protein levels were quantified using BCA assay. Equal amounts of total protein (30–50 μg/lane) were separated on 4 to 12% Bis-Tris ν-PAGE gel and transferred onto polyvinylidene difluoride membranes. Membranes were blocked with 5% nonfat milk and then incubated with the following primary antibodies: anti-p47PHOX, anti-gp91PHOX, anti-phospho-ERK1/2, anti-ERK1/2, anti-phospho-p38, anti-p38, anti-phospho-JNK, anti-JNK, anti-phospho-p65, anti-p65, anti-phospho-IKKβ, anti-IKKβ, anti-phospho-IκBα, anti-IκBα, anti-β-actin, anti-iNOS, and anti-Iba-1 at 1:1000 dilution followed by horseradish peroxidase-conjugated secondary antibodies at 1:2500 dilution. The blots were developed with enhanced chemiluminescence reagent.
Statistical Analysis.
Data were expressed as mean ± S.E.M. Statistical significance was analyzed by one- or two-way ANOVA with treatment or time/dose/culture type as the independent factors. When ANOVA showed significant differences, pair-wise comparisons between means were tested by Bonferroni's post test with correction. A Pearson's coefficient test was conducted using GraphPad Prism software for correlation analysis (GraphPad Software Inc., San Diego, CA). The coefficient of determination (r2) and p value (two-tailed) were used to show the correlation between groups. Overall, a value of p < 0.05 or < 0.01 was considered statistically significant.
Results
Resveratrol Protected DA Neurons against LPS- Induced Neurotoxicity.
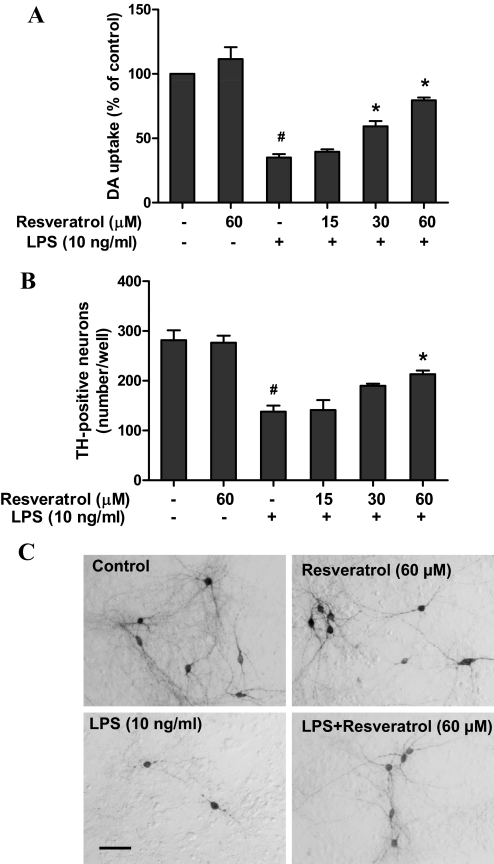
Mesencephalic neuron-glia cultures were pretreated with resveratrol (15–60 μM) for 30 min and then stimulated with LPS (10 ng/ml). Seven days later, the degeneration of DA neurons was assessed by determining [3H]DA uptake and counting TH-positive neurons. As shown in Fig. 1A, significant treatment effect of resveratrol against LPS-induced neurotoxicity were found by [3H] DA uptake [F(5, 18) = 49.46, p < 0.0001]. Post hoc analysis showed that LPS reduced DA uptake capacity by approximately 60% compared with the vehicle-treated control cultures (t = 10.24, p < 0.01 versus control). Pretreatment of resveratrol significantly restored LPS-induced reduction of DA uptake capacity at 30 μM (t = 3.821, p < 0.01) and 60 μM (t = 7.012, p < 0.01) Similar to the [3H]DA uptake assay, cell count revealed that pretreatment of resveratrol (60 μM) significantly attenuated the LPS-induced decrease in the number of TH-positive neurons [ANOVA: F(5, 12) = 19.45, p < 0.0001; post hoc analysis: t = 3.742, p < 0.01 versus LPS] (Fig. 1B). Morphologically, in addition to the reduction in the abundance of DA neurons in LPS-treated cultures, neurites of the remaining TH-positive neurons became shorter and fragmented compared with the vehicle control cultures. In resveratrol-treated cultures, not only more DA neurons survived, but their neurites were also less affected compared with the LPS-treated cultures (Fig. 1C). These morphological changes were consistent with the results from the functional assay of [3H]DA uptake.
Fig. 1.
Resveratrol protected DA neurons against LPS-induced neurotoxicity. Rat primary mesencephalic neuron-glia cultures were seeded in 24-well culture plates at 5 × 105/well and then pretreated with various concentrations of resveratrol (15–60 μM) for 30 min before the addition of 10 ng/ml LPS. Seven days later, the LPS-induced dopaminergic neurotoxicity was determined by [3H]DA uptake assay (A) and the quantification of TH-positive neurons after immunostaining of DA neurons with an anti-TH antibody (B). Representative images of immunostaining from three independent experiments are shown (C). Scale bar, 100 μm. Results are the mean ± S.E.M. from three independent experiments performed in triplicate, and data from [3H]DA uptake assay are expressed as a percentage of the control cultures. #, p < 0.05 compared with control cultures; *, p < 0.05 compared with LPS-treated cultures.
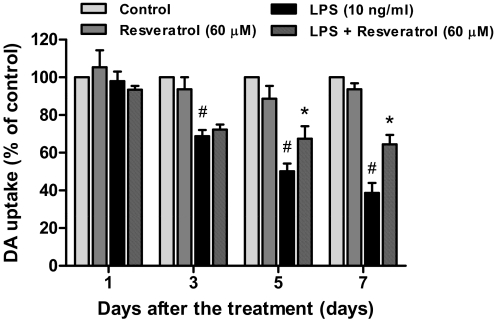
To determine whether resveratrol-mediated neuroprotection was time-dependent, [3H]DA uptake assay was determined 1, 3, 5, and 7 days after LPS addition. As shown in Fig. 2, LPS treatment caused a significant reduction of DA uptake capacity in a time-dependent manner; the LPS-induced progressive decrease of DA uptake was attenuated by pretreatment with resveratrol. The two-way ANOVA indicated a significant effect for treatment [F(3, 32) = 65.46, p < 0.0001] and for time [F(3, 32) = 28.21, p < 0.0001]. Thereafter, Bonferroni post test analysis revealed that although an initial 30% decrease of DA uptake was not prevented by resveratrol 3 days after LPS treatment (t = 0.5934, p > 0.05), a significant protection in resveratrol-treated cultures was observed after longer treatment periods (t = 3.047, p < 0.05 for 5-day treatment; t = 4.322, p < 0.001 for 7-day treatment). Taken together, these results indicate that resveratrol exerted neuroprotection against LPS-induced dopaminergic neurodegeneration in both concentration- and time-dependent manners.
Fig. 2.
Resveratrol afforded neuroprotection against LPS-induced dopaminergic neurotoxicity in a time-dependent manner. Primary neuron-glia cultures were pretreated with resveratrol (60 μM) and then stimulated with LPS (10 ng/ml). The neurotoxicity was quantified by [3H]DA uptake assay 1, 3, 5, and 7 days after LPS treatment. Results are expressed as a percentage of the control cultures and are the mean ± S.E.M. from three independent experiments performed in triplicate. #, p < 0.05 compared with control cultures; *, p < 0.05 compared with LPS-treated cultures.
Microglia Were Essential for Resveratrol-Mediated Neuroprotection.
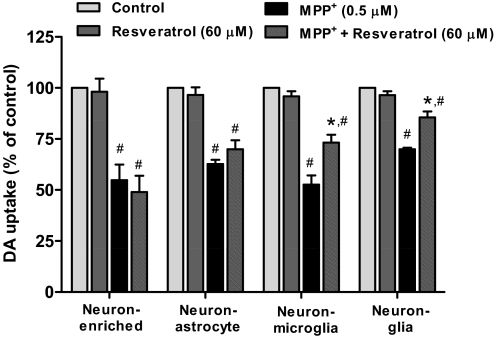
To determine whether the neuroprotective effects of resveratrol were dependent on the presence of glia cells, four types of cultures, including neuron-glia, neuron-microglia, neuron-astrocyte, and neuron-enriched cultures, were prepared and treated with 0.5 μM MPP+, the active metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. MPP+ was used in this experiment because this toxin exerts DA neuronal damage in dual manners: 1) through the inhibition of mitochondrial complex I after its uptake by DA neurons; and 2) through the induction of reactive microgliosis (secondary microglial activation to neuronal damages) and the consequent secretion of proinflammatory factors (Gao et al., 2003a; Gao and Hong, 2008). Seven days after the treatment, MPP+-induced neurotoxicity was determined by [3H]DA uptake assay. The pretreatment of resveratrol significantly protected MPP+-induced neurotoxicity in neuron-glia (t = 4.243, p < 0.001) and neuron-microglia cultures (t = 4.709, p < 0.001) but not in neuron-astrocyte (t = 1.344, p > 0.05) or neuron-enriched cultures (t = 1.604, p > 0.05) (Fig. 3). The two-way ANOVA indicated a significant effect for cell type [F(3, 32) = 5.607, p = 0.0033]. These results together indicate that microglia are the target of resveratrol-mediated neuroprotection.
Fig. 3.
Microglia are essential for resveratrol-mediated neuroprotection. Four types of cultures including neuron-glia, neuron-enriched, neuron-astrocyte, and neuron-microglia reconstituted cultures were pretreated with resveratrol (60 μM) followed by the treatment of 0.5 μM MPP+. The [3H]DA uptake analysis was performed 7 days after MPP+ addition. Results are expressed as a percentage of the control cultures and are the mean ± S.E.M. from three independent experiments performed in triplicate. #, p < 0.05 compared with control cultures; *, p < 0.05 compared with MPP+-treated cultures.
Resveratrol Attenuated LPS-Induced Activation of Microglia and Production of Proinflammatory Factors.
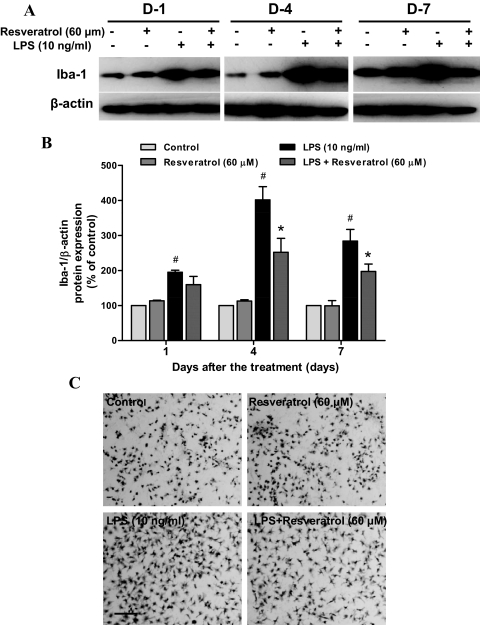
Microglia activation and the subsequent release of various proinflammatory factors were the key source of the damage to DA neurons (Gao and Hong, 2008). To investigate the basis of the neuroprotective effects of resveratrol on LPS-induced neurotoxicity, we evaluated the ability of resveratrol to inhibit LPS-induced microglia activation. Neuron-glia cultures were pretreated with resveratrol for 30 min followed by the stimulation with LPS. At 1, 4, and 7 days after LPS treatment, the whole-cell lysis was harvested The two-way ANOVA indicated a significant effect for treatment [F(3, 8) = 79.06, p < 0.0001] and for time [F(2, 16) = 13.36, p < 0.001] on the expression of Iba-1, a marker of activated microglia. Specifically, the level of Iba-1 was initially increased 1 day after LPS treatment (t = 3.409, p < 0.01) and enhanced by up to 4-fold of control cultures 4 days later (t = 10.83, p < 0.001) and then maintained at 3-fold level at day 7 (t = 6.846, p < 0.001) (Fig. 4, A and B). The pretreatment of resveratrol significantly inhibited LPS-induced elevation of Iba-1 expression (t = 5.383, p < 0.001 for day 4; t = 2.997, p < 0.05 for day 7). In addition, morphological assay of Iba-1-positive microglia indicated that resveratrol significantly attenuated the activation of microglia. LPS-stimulated microglia exhibited an enlarged cell body and irregular shapes, a transformation from resting round and small cells to the highly activated amoeboid status (Fig. 4C).
Fig. 4.
Resveratrol attenuated LPS-induced microglia activation. Rat primary mesencephalic neuron-glia cultures were pretreated with resveratrol (60 μM) for 30 min before LPS (10 ng/ml) stimulation. After 1, 4, and 7 days of LPS treatment, the total cell protein was harvested, respectively, and the level of Iba-1 protein was determined by Western blotting analysis (A). The ratio of densitometry values of Iba-1 and β-actin was accessed and normalized to each respective control group (B). Results were the mean ± S.E.M. from three independent experiments performed in triplicate. #, p < 0.05 compared with control cultures; *, p < 0.05 compared with LPS-treated cultures. Seven days after LPS treatment, the cultures were immunostained with anti-Iba-1 antibody (C). Activated microglia exhibited an enlarged cell body and irregular shapes, a shift from resting round and small cells to the highly activated amoeboid status. Images presented were representative of three independent experiments. Scale bar, 200 μm.
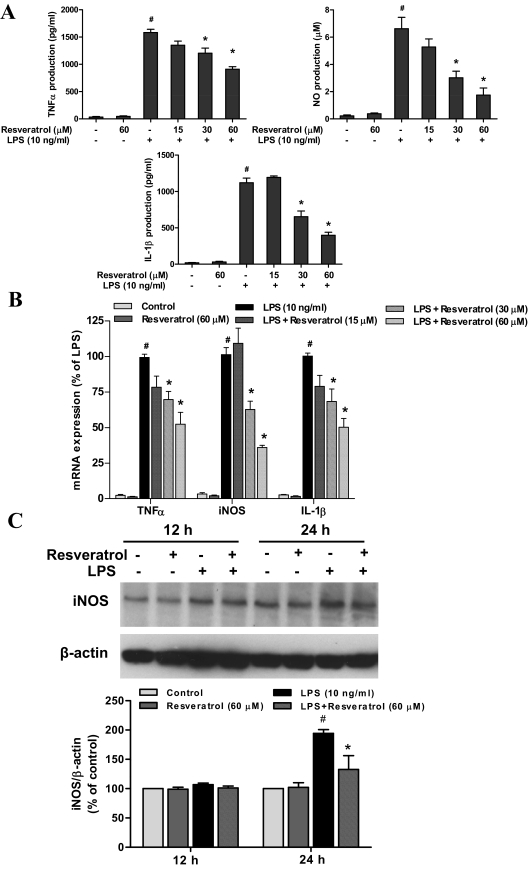
We further investigated whether resveratrol was capable of reducing the production of microglia-derived proinflammatory factors. Because the proinflammatory factors released from microglia vary in terms of time and quantities, different time points were adjusted for the measurement of each proinflammatory factor: TNFα at 3 h, whereas NO and IL-1β at 24 h after LPS treatment. The results showed that resveratrol treatment significantly affected LPS-induced production of proinflammatory factors measured from the supernatant of neuron-glia cultures [TNFα: F(5, 12) = 118.0, p < 0.0001; NO: F(5, 12) = 26.49, p < 0.0001; IL-1β: F(5, 12) = 52.83, p < 0.0001] (Fig. 5A). Post hoc test revealed that resveratrol significantly reduced LPS-induced secretion of TNFα (t = 4.338, p < 0.05), IL-1β (t = 4.915, p < 0.05), and NO (t = 4.997, p < 0.05) at 30 μM concentration, whereas 60 μM resveratrol greatly suppressed the production of TNFα (t = 6.557, p < 0.05), IL-1β (t = 8.291, p < 0.05), and NO (t = 6.766, p < 0.05) (Fig. 5A). As shown by real-time RT-PCR analysis, LPS treatment led to marked increase in mRNA expression of TNFα, iNOS, and IL-1β in neuron-glia cultures. However, the pretreatment of resveratrol apparently inhibited LPS-induced mRNA expression of these proinflammatory factors [ANOVA: TNFα, F(5, 12) = 60.28, p < 0.0001; iNOS: F(5, 12) = 86.44, p < 0.0001; IL-1β, F(5, 12) = 67.47, p < 0.0001; Post hoc test, TNFα t = 4.079, p < 0.05 for 30 μM; t = 6.410, p < 0.05 for 60 μM; iNOS t = 5.274, p < 0.05 for 30 μM; t = 9.041, p < 0.05 for 60 μM; IL-1β t = 4.517, p < 0.05 for 30 μM; t = 8.035, p < 0.05 for 60 μM] (Fig. 5B). Moreover, the expression of iNOS protein was determined by Western blotting assay. As shown in Fig. 5C, the treatment with resveratrol for 24 h significantly inhibited LPS-induced increase of iNOS protein [ANOVA, F(3, 8) = 20.10, p < 0.001; post hoc test, t = 4.899, p < 0.05].
Fig. 5.
Resveratrol inhibited the levels of proinflammatory factors produced by LPS-activated microglia. Primary neuron-glia cultures were pretreated with resveratrol for 30 min before 10 ng/ml LPS stimulation. The supernatant was collected in the following time point after LPS treatment: 3 h for TNFα assay; and 24 h for nitrite (an indicator of NO production) and IL-1β assays. The release of TNFα and IL-1β was detected by enzyme-linked immunosorbent assay and the production of NO was accessed by Griess reagent (A). The mRNA expression of proinflammatory factors was measured by real-time RT-PCR at different time after LPS treatment: 1 h for TNFα and 3 h for IL-1β and iNOS (B). Twelve and 24 h after LPS treatment, the total protein was harvested, respectively, to detect the levels of iNOS using Western blotting assay (C). Results were the mean ± S.E.M. from three independent experiments performed in triplicate. #, p < 0.05 compared with control cultures; *, p < 0.05 compared with LPS-treated cultures.
NADPH Oxidase Participated in Resveratrol-Mediated Neuroprotection against LPS-Induced Neurotoxicity.
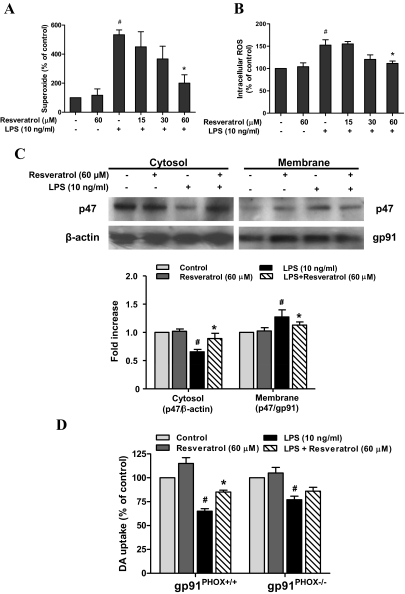
Among the proinflammatory and cytotoxic factors released by activated microglia, superoxide has been shown to be a key element in contributing to the oxidative stress in PD (Gao et al., 2003b). To determine whether resveratrol was able to decrease the LPS-induced production of ROS, primary microglia-enriched cultures were used to measure the production of extracellular superoxide and intracellular ROS. The results showed that notable release of superoxide was detected in LPS-treated cultures; resveratrol significantly inhibited LPS-induced production of superoxide [ANOVA, F(5, 12) = 7.911, p = 0.0017; post hoc test, t = 3.651, p < 0.05] and intracellular ROS [ANOVA, F(5, 12) = 11.77, p < 0.001; post hoc test: t = 4.130, p < 0.05] (Fig. 6).
Fig. 6.
NADPH oxidase participated in resveratrol-mediated neuroprotection against LPS-induced neurotoxicity. Primary microglia-enriched cultures were pretreated with resveratrol for 30 min before LPS treatment. The production of extracellular superoxide was detected by SOD-inhibitable reduction of WST-1 (A), and the levels of intracellular ROS were measured with DCFH-DA (B). After LPS treatment for 15 min, subcellular fractions were isolated to perform Western blot analysis for p47PHOX levels in membrane and cytosolic fractions of microglia. β-Actin and gp91PHOX were used as internal cytosolic and membrane controls, respectively (C). Primary neuron-glia cultures from PHOX+/+ and PHOX−/− mice were pretreated with resveratrol (60 μM) for 30 min and then stimulated with 10 ng/ml LPS. Seven days later, the neurotoxicity was accessed by [3H]DA uptake assay (D). Results are expressed as a percentage of the control cultures and are the mean ± S.E.M. from three independent experiments performed in triplicate. #, p < 0.05 compared with control cultures; *, p < 0.05 compared with LPS-treated cultures.
Because NADPH oxidase is the key enzyme required for the production of ROS in activated microglia, studies were performed to determine whether resveratrol inhibited the activation of NADPH oxidase induced by LPS. It has been reported that the activation of NADPH oxidase requires the translocation of phosphorylated cytosolic subunits (p47PHOX, p67PHOX, and p40PHOX) to the cell membrane and binding to the catalytic subunit cytochrome b558 composed of p22PHOX and gp91PHOX (Groemping and Rittinger, 2005). Western blotting analysis showed that LPS caused a significant increase of p47PHOX in the membrane of microglia and this increase was blocked by the pretreatment of resveratrol [ANOVA, F(3, 8) = 15.92, p = 0.001; post hoc test, t = 4.338, p < 0.05] (Fig. 6C). Thus, we concluded that resveratrol decreased LPS-induced ROS production through the inhibition of NADPH oxidase activation.
Additional evidence further supporting the critical role of NADPH oxidase in mediating the neuroprotective effects of resveratrol came from an experiment using NADPH oxidase-deficient (gp91PHOX−/−) cultures. The midbrain neuron-glia cultures from gp91PHOX−/− and wild-type (p91PHOX+/+) mice were prepared and pretreated with resveratrol for 30 min followed by LPS treatment for 7 days. As shown in Fig. 6D, similar to the rat midbrain neuron-glia cultures (Fig. 1A), LPS treatment caused a 40% reduction in the cultures from wild-type mice; resveratrol significantly attenuated this LPS-induced neurotoxicity [ANOVA, F(3, 8) = 37.50, p < 0.0001; post hoc test, t = 4.054, p < 0.05]. A smaller but significant reduction of DA uptake capacity was also discerned in LPS-treated cultures from gp91PHOX−/− mice [ANOVA, F(3, 8) = 10.13, p < 0.01; post hoc test: t = 4.034, p < 0.05], whereas no neuroprotective effects were seen in these cultures pretreated with resveratrol (post hoc test, t = 1.579, p > 0.05). These results strongly suggested that NADPH oxidase was a major target of resveratrol-mediated neuroprotection against LPS-induced neurotoxicity.
Resveratrol Inhibited LPS-Induced Activation of MAPKs and NF-κB in Microglia.
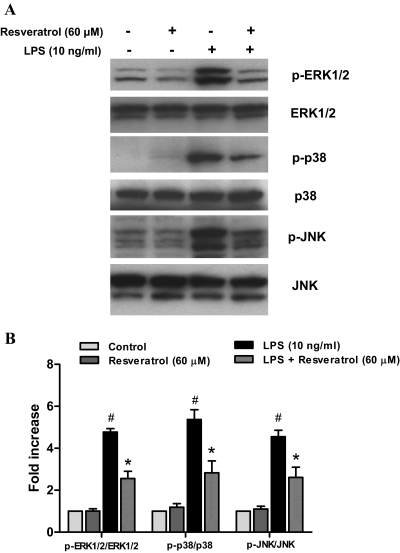
Previous studies have shown that MAPKs were associated with the activation of NADPH oxidase and the consequent regulation of immune responses (Bardwell, 2006). Therefore, we evaluated inhibitory effects of resveratrol on LPS-induced activation of MAPKs, including ERK1/2, p38, and JNK. Primary microglia-enriched cultures were pretreated with resveratrol for 30 min and then incubated with LPS for another 15 min. Western blot analysis indicated that the pretreatment of resveratrol had significant effects on LPS-induced phosphorylation of ERK1/2 [F(3, 8) = 81.21, p < 0.0001], p38 [F(3, 8) = 28.54, p = 0.0001], and JNK [F(3, 8) = 31.54, p < 0.0001] (Fig. 7); the phosphorylation of MAPKs was significantly reduced by the pretreatment of resveratrol, as indicated by post hoc test: ERK1/2 (t = 7.915, p < 0.05); p38 (t = 4.741, p < 0.05); and JNK (t = 4.637, p < 0.05) (Fig. 7).
Fig. 7.
Resveratrol inhibited LPS-induced MAPKs activation in microglia. Primary microglia-enriched cultures were pretreated with resveratrol (60 μM) for 30 min followed by LPS (10 ng/ml) treatment for 15 min. The whole-cell protein was harvested to perform Western blotting assay (A). The ratio of densitometry values of phosphorylated MAPKs compared with total MAPKs was analyzed and normalized to each respective control group (B). Results are the mean ± S.E.M. from three independent experiments. #, p < 0.05 compared with control cultures; *, p < 0.05 compared with LPS-treated cultures.
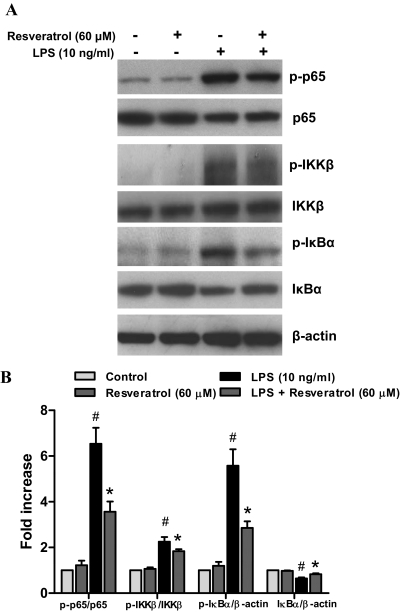
We next investigated whether resveratrol influenced the activation of NF-κB signaling pathways. In the resting conditions, NF-κB is mainly located in the cytoplasm in an inactive form through the binding to its inhibitor IκBs. In response to inflammatory stimuli, IκBs are rapidly phosphorylated via IKK complex and then degraded, which allows the release and translocation of NF-κB dimers (p50 and p65) to the nucleus and thereby regulates the expression of target genes. Resveratrol pretreatment significantly influenced LPS-induced phosphorylation of IKKβ [F(3, 8) = 39.91, p < 0.0001], IκBα [F(3, 8) = 28.42, p = 0.0001], and p65 [F(3, 8) = 36.21, p < 0.0001] (Fig. 7); Post hoc test indicated that such elevated phosphorylation was greatly attenuated by resveratrol: IKKβ (t = 3.609, p < 0.05); IκBα (t = 4.844, p < 0.05); and p65 (t = 4.911, p < 0.05) (Fig. 8). Furthermore, a Pearson's coefficient test (Table 1) indicated a positive correlation between the suppression of MAPKs and NF-κB pathway activation (Figs. 7B and 8B) and the attenuated production of proinflammatory factors (Fig. 5A). Thus, the inhibition of LPS-elicited MAPKs and NF-κB activation by resveratrol is well correlated to the attenuated release of TNFα, IL-1β, and NO (Fig. 5). In addition, a positive correlation between the attenuation of membrane translocation of p47PHOX and the inhibition of MAPKs and NF-κB activation suggests a cross-talk between these signaling pathways.
Fig. 8.
Resveratrol prevented LPS-induced activation of microglia NF-κB signaling pathway. Primary microglia-enriched cultures were pretreated with resveratrol (60 μM) for 30 min and then incubated with LPS (10 ng/ml) for 15 min. The whole-cell lysates were analyzed by Western blotting assay (A). The ratio of densitometry values of phosphorylated p65 and IKKβ compared with total p65 and IKKβ and phosphorylated IκBα and total IκBα relative to β-actin was analyzed and normalized to each respective control group (B). Results are the mean ± S.E.M. from three independent experiments. #, p < 0.05 compared with control cultures; *, p < 0.05 compared with LPS-treated cultures.
TABLE 1.
Correlation between MAPKs and NF-κB activation and proinflammatory factor production as well as p47 activation
Primary microglia-enriched cultures were pretreated with resveratrol (60 μM) for 30 min and then incubated with LPS (10 ng/ml) for 15 min. The activation of various molecular signaling molecules (Figs. 7B, 8B, and 6C) and the production of proinflammatory factors (Fig. 5A) were described in detail in corresponding figure legends. A Pearson's coefficient test was conducted using GraphPad Prism software. The coefficient of determination (r2; square of the correlation coefficient, r) and P value (two-tailed) were displayed in the table to indicate the correlation between groups. P < 0.05 was considered statistically significant. P < 0.05 and r > 0 (first four columns, data not shown) indicate a positive correlation, whereas P < 0.05 and r < 0 indicate a negative correlation.
|
r2/P |
r/r2/P Cytosol p47/β-Actin | ||||
|---|---|---|---|---|---|
| TNFα | IL-1β | NO | Membrane p47/gp91 | ||
| p-p65/p65 | 0.9674/0.0164 | 0.9671/0.0166 | 0.9459/0.0274 | 0.9646/0.0179 | 0.9891/0.9783/0.0109 |
| p-Erk/Erk | 0.9522/0.0242 | 0.9806/0.0098 | 0.9627/0.0188 | 0.9664/0.0170 | 0.9906/0.9813/0.0094 |
| p-p38/p38 | 0.9485/0.0261 | 0.9815/0.0093 | 0.9651/0.0176 | 0.9800/0.0100 | 0.9935/0.9870/0.0065 |
| p-JNK/p-JNK | 0.9656/0.0173 | 0.9696/0.0153 | 0.9488/0.0259 | 0.9664/0.0170 | 0.9906/0.9813/0.0094 |
Discussion
In this study, we demonstrate that resveratrol produced significant neuroprotection on DA neurons against LPS-induced neurotoxicity. Using multiple primary cell cultures, we have elucidated novel mechanisms and advanced our understanding of the relationship between the anti-inflammatory and the neuroprotective effects of resveratrol. First, our study showed that resveratrol protected DA neurons against LPS-induced neurotoxicity in the midbrain neuron-glia cultures through inhibiting the activation of microglia and the production of proinflammatory factors. Second, our study is the first report indicating that microglial NADPH oxidase is a major target mediating resveratrol-elicited anti-inflammation and neuroprotection. Further mechanistic study showed that resveratrol reduced the ROS production through suppressing the translocation of cytosolic subunit p47PHOX to the cell membrane. Third, our results also suggest that inhibition of NADPH oxidase by resveratrol may link to the inhibition of attenuated activation of MAPKs and NF-κB and subsequent decreases of proinflammatory factors, TNFα and NO. These findings strongly suggest that anti-inflammatory actions underlie resveratrol-mediated neuroprotection.
It is interesting to note that resveratrol afforded neuroprotection against both LPS- and MPP+-induced dopaminergic neurotoxicity, two toxins with different modes of actions. LPS directly induced microglial activation and the subsequent release of various proinflammatory factors, which eventually caused DA neuronal damage. In contrast, MPP+ directly led to dopaminergic neuronal damage initially and then damaged neurons released toxic soluble factors such as α-synuclein, which in turn induced microglial activation termed reactive microgliosis (Gao et al., 2003a). We have reported previously that these reactivated microglia also produced proinflammatory factors and contributed to additional neuronal damage (Gao and Hong, 2008). Here, we propose that resveratrol prevented MPP+-induced neurotoxicity through the inhibition of reactive microgliosis. Either direct microglial activation induced by LPS or reactive microgliosis induced by MPP+ caused DA neuronal damage. Based on the requirement of microglia in resveratrol-mediated neuroprotection (Fig. 3), we concluded that microglia were the target of resveratrol action and resveratrol exerted neuroprotection through the inhibition of microglial activation (Figs. 4–8).
Among various neurotoxic factors produced by activated microglia, superoxide reacts with NO to form highly reactive intermediates peroxynitrite to causes dopaminergic neurodegeneration (Gao et al., 2002b). Recent studies have suggested that ROS serve as secondary messengers to enhance the gene expression, encoding a variety of proinflammatory factors (Qin et al., 2004). Although resveratrol has been reported to attenuate oxidative insults by functioning as an ROS scavenger (Leonard et al., 2003), a new finding from the present study was that resveratrol inhibited LPS-induced activation of microglial NADPH oxidase and consequent production of superoxide. The LPS-induced neurotoxicity was prevented by resveratrol in neuron-glia cultures from wild-type mice but not from NADPH oxidase-deficient mice, indicating that the inhibition of NADPH oxidase-derived superoxide and its down-stream products was critical for resveratrol-elicited neuroprotective effects. Further molecular mechanistic studies revealed that resveratrol inhibited LPS-induced translocation of p47PHOX in microglia from the cytosol to the membrane, leading to the decrease of superoxide production. Previously, Spanier et al. (2009) reported that resveratrol down-regulated mRNA expression of Nox4, a homolog of gp91phox and the most abundant NADPH oxidase catalytic subunit in human umbilical vein endothelial cells and human umbilical vein endothelial cell-derived EA.hy 926 cells. Likewise, resveratrol has been shown to reduce LPS-induced ROS generation in Raw 246.7 macrophage cell line, in which resveratrol seems to suppress the mRNA expression of Nox1, a homolog of gp91phox (Park et al., 2009). However, although it is unclear whether resveratrol affects Nox2 (gp91phox), the dominant NADPH oxidase and the major superoxide-generating enzyme in inflamed macrophages and microglia, the action of resveratrol on the protein expression of NADPH oxidase subunits to influence NADPH oxidase activity remains to be confirmed and to be further investigated. Moreover, resveratrol protects vascular endothelial cells from oxidative damages induced by oxidized low-density lipoproteins through both direct ROS scavenging and inhibition of NADPH oxidase activity by reducing the membrane association of gp91phox and Rac1 (Chow et al., 2007). Nevertheless, overwhelming evidence has confirmed that NADPH oxidase plays an important role in inflammation-induced neurodegeneration and may become an ideal therapeutic target for the treatment of PD (Gao et al., 2003b).
Further analysis of cascade signaling events mediating the anti-inflammatory effects and the consequent neuroprotection of resveratrol indicates the involvement of the MAPK pathway. MAPKs are a highly conserved family of serine/threonine kinases including ERK1/2, p38, and JNK subtypes and activate many enzymatic pathways, contributing to oxidative stress and inflammatory responses (Bardwell, 2006). These signals are responsive to different environmental stimuli. ERK1/2 can be activated by oxidative stress, LPS, and cytokines (Miller et al., 2009). Myriad studies show that ERK1/2 plays a pivotal role on NADPH oxidase activation based on the following findings: 1) ERK1/2 is a crucial mediator of granulocyte macrophage–colony-stimulating factor-induced activation of p47PHOX in neutrophils (Dang et al., 2006); 2) ERK1/2 becomes a central contributor in LPS-induced phosphorylation of p47PHOX in microglia (Qian et al., 2007); and 3) a specific ERK inhibitor, U0126, produces significant neuroprotective effects against LPS-induced neurodegeneration by inhibiting p47PHOX translocation and subsequent superoxide production (Qian et al., 2008). Therefore, these results suggest that ERK1/2 is an ideal target for developing anti-inflammatory drugs via the inhibition of p47PHOX activation. In this study, we observed that resveratrol suppressed LPS-induced phosphorylation of ERK1/2. Based on the mechanistic studies mentioned above, we suggest that resveratrol blocked the activation of NADPH oxidase through the regulation of the ERK1/2 signaling pathway.
We next investigated the effects of resveratrol on LPS-induced activation of p38 and JNK as well as NF-κB pathways. Because the key signaling pathway mediating inflammatory responses, from MAPKs to NF-κB transcription factor, has been established in various inflammatory diseases (Akira et al., 2001), attempts to develop drugs that target these signaling pathways is an area of great interest. It has been reported that JNK is an essential mediator of relevant proinflammatory functions in microglia, and the intervention of JNK pathway may be a therapeutic approach for treating inflammatory neurological diseases (Jang et al., 2008). Likewise, p38 has also been postulated to play an important role in the regulation of NO and TNFα production after LPS stimulation (Bhat et al., 1998). In addition, NF-κB is the key transcription factor regulating cell survival, immunity, and inflammation. It is broadly expressed in the central nervous system, including neurons and glia. Once activated, in collaboration with other proinflammatory factors, NF-κB drives the transcription of several proinflammatory factors such as iNOS, TNFα, IL-1β in microglia, which have been documented in patients with PD (Sriram et al., 2002). Increasing evidence has shown that intracellular ROS can induce the activation of MAPKs cascades and NF-κB pathway in activated microglia (Ramanan et al., 2008). In agreement with these studies, the inhibitor of NADPH oxidase (diphenylene iodonium) and the ROS-scavenging enzyme catalase significantly inhibit the activation of MAPKs as well as NF-κB, thereby linking NADPH oxidase-derived ROS with LPS-induced signaling transduction and transcriptional activation (Pawate et al., 2004). Moreover, Pearson's coefficient test indicated that the activation of both MAPKs and NF-κB pathways correlated positively with membrane p47PHOX and negatively with cytosol p47PHOX (table 1). Thus, the membrane translocation of p47PHOX and consequent activation of NADPH oxidase (Fig. 6C) positively correlated with the activation of MAPKs and NF-κB pathways (Figs. 7 and 8). Although this correlation did not necessarily mean a direct causative relationship between these two events, the inhibitory effects of resveratrol on LPS-induced activation of p38 and JNK and NF-κB cascade pathways would probably result from the decreased production of ROS via the inhibition of NADPH oxidase activity.
Furthermore, we found that resveratrol showed potent inhibitory effects on LPS-induced production of NO, TNFα, and IL-1β in neuron-glia cultures. These findings agree with the previous studies in which resveratrol inhibited LPS-induced production of these three proinflammatory factors in primary microglia or microglial cell lines (Bi et al., 2005; Candelario-Jalil et al., 2007; Meng et al., 2008). Of the numerous neurotoxic factors released by activated microglia, the consequences of production of NO, TNFα, and ROS have been relatively well studied (McGuire et al., 2001). In addition, increased levels of cytokines such as TNFα, IL-1β, and interferon-γ have been demonstrated in the substantia nigra of patients with PD (Nagatsu et al., 2000). Hence, the inhibition of the accumulation of these proinflammatory factors conferred resveratrol-mediated significant neuroprotection on DA neurons.
In summary, our study has demonstrated that resveratrol protects DA neurons against LPS-induced neurotoxicity through inhibiting the activation of microglia and the production of proinflammatory factors. These neuroprotective effects of resveratrol are at least mediated by suppressing the activity of NADPH oxidase and further decreasing ROS production and inhibiting the activation of MAPKs and NF-κB cascade signaling pathways. This study extends our understanding of anti-inflammatory activities mediated by resveratrol and suggests that NADPH oxidase is an important action site for its neuroprotection.
This work was supported by the Intramural Research Program of the National Institutes of Health National Institute of Environmental Health Sciences [Grant XXX]; the National Natural Science Foundation of China [Grant 30960447]; and the Science and Technology Foundation of Guizhou Province of China [Grant 20107030].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.064535.
- DA
- dopamine
- PD
- Parkinson's disease
- SN
- substantia nigra
- ROS
- reactive oxygen species
- ERK
- extracellular signal-regulated kinase
- LPS
- lipopolysaccharide
- NF-κB
- nuclear factor-κB
- iNOS
- inducible nitric-oxide synthase
- MAPK
- mitogen-activated protein kinase
- PCR
- polymerase chain reaction
- MPP+
- 1-methyl-4-phenylpyridinium
- Nox
- NADPH oxidase
- DCFH-DA
- dichlorodihydrofluorescein diacetate
- SOD
- superoxide dismutase
- TH
- tyrosine hydroxylase
- RT-PCR
- reverse transcription-polymerase chain reaction
- TNFα
- tumor necrosis factor-α
- IL-1β
- interleukin-1β
- HBSS
- Hanks' balanced salt solution
- JNK
- c-Jun N-terminal kinase
- IκB
- inhibitor of κB
- IKK
- inhibitor of κB kinase
- Iba-1
- ionized calcium-binding adapter molecule-1
- ANOVA
- analysis of variance
- U0126
- 1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene.
References
- Akira S, Takeda K, Kaisho T. (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2:675–680 [DOI] [PubMed] [Google Scholar]
- Bardwell L. (2006) Mechanisms of MAPK signalling specificity. Biochem Soc Trans 34:837–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat NR, Zhang P, Lee JC, Hogan EL. (1998) Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci 18:1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi XL, Yang JY, Dong YX, Wang JM, Cui YH, Ikeshima T, Zhao YQ, Wu CF. (2005) Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. Int Immunopharmacol 5:185–193 [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, de Oliveira AC, Gräf S, Bhatia HS, Hüll M, Muñoz E, Fiebich BL. (2007) Resveratrol potently reduces prostaglandin E2 production and free radical formation in lipopolysaccharide-activated primary rat microglia. J Neuroinflammation 4:25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow SE, Hshu YC, Wang JS, Chen JK. (2007) Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol 102:1520–1527 [DOI] [PubMed] [Google Scholar]
- Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, Hayem G, Jensen ON, Gougerot-Pocidalo MA, El-Benna J. (2006) A specific p47phox-serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest 116:2033–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Das DK. (2007) Anti-inflammatory responses of resveratrol. Inflamm Allergy Drug Targets 6:168–173 [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS. (2008) Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol 29:357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. (2002a) Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 22:782–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. (2002b) Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem 81:1285–1297 [DOI] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. (2003a) Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson's disease. FASEB J 17:1954–1956 [DOI] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. (2003b) Novel anti-inflammatory therapy for Parkinson's disease. Trends Pharmacol Sci 24:395–401 [DOI] [PubMed] [Google Scholar]
- Greenamyre JT, MacKenzie G, Peng TI, Stephans SE. (1999) Mitochondrial dysfunction in Parkinson's disease. Biochem Soc Symp 66:85–97 [DOI] [PubMed] [Google Scholar]
- Groemping Y, Rittinger K. (2005) Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J 386:401–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Graybiel AM, Agid YA. (1988) Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature 334:345–348 [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. (2009) Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol 8:382–397 [DOI] [PubMed] [Google Scholar]
- Jang S, Kelley KW, Johnson RW. (2008) Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci USA 105:7534–7539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P, Olanow CW. (1998) Understanding cell death in Parkinson's disease. Ann Neurol 44 (3 Suppl 1):S72–S84 [DOI] [PubMed] [Google Scholar]
- Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, Shi X. (2003) Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun 309:1017–1026 [DOI] [PubMed] [Google Scholar]
- Liu B, Du L, Hong JS. (2000) Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther 293:607–617 [PubMed] [Google Scholar]
- Markus MA, Morris BJ. (2008) Resveratrol in prevention and treatment of common clinical conditions of aging. Clin Interv Aging 3:331–339 [PMC free article] [PubMed] [Google Scholar]
- McGuire SO, Ling ZD, Lipton JW, Sortwell CE, Collier TJ, Carvey PM. (2001) Tumor necrosis factor alpha is toxic to embryonic mesencephalic dopamine neurons. Exp Neurol 169:219–230 [DOI] [PubMed] [Google Scholar]
- Meng XL, Yang JY, Chen GL, Wang LH, Zhang LJ, Wang S, Li J, Wu CF. (2008) Effects of resveratrol and its derivatives on lipopolysaccharide-induced microglial activation and their structure-activity relationships. Chem Biol Interact 174:51–59 [DOI] [PubMed] [Google Scholar]
- Miller RL, James-Kracke M, Sun GY, Sun AY. (2009) Oxidative and inflammatory pathways in Parkinson's disease. Neurochem Res 34:55–65 [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. (2000) Cytokines in Parkinson's disease. J Neural Transm Suppl 58:143–151 [PubMed] [Google Scholar]
- Nagatsu T, Sawada M. (2006) Cellular and molecular mechanisms of Parkinson's disease: neurotoxins, causative genes, and inflammatory cytokines. Cell Mol Neurobiol 26:781–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DW, Baek K, Kim JR, Lee JJ, Ryu SH, Chin BR, Baek SH. (2009) Resveratrol inhibits foam cell formation via NADPH oxidase 1- mediated reactive oxygen species and monocyte chemotactic protein-1. Exp Mol Med 41:171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawate S, Shen Q, Fan F, Bhat NR. (2004) Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res 77:540–551 [DOI] [PubMed] [Google Scholar]
- Qian L, Tan KS, Wei SJ, Wu HM, Xu Z, Wilson B, Lu RB, Hong JS, Flood PM. (2007) Microglia-mediated neurotoxicity is inhibited by morphine through an opioid receptor-independent reduction of NADPH oxidase activity. J Immunol 179:1198–1209 [DOI] [PubMed] [Google Scholar]
- Qian L, Wei SJ, Zhang D, Hu X, Xu Z, Wilson B, El-Benna J, Hong JS, Flood PM. (2008) Potent anti-inflammatory and neuroprotective effects of TGF-beta1 are mediated through the inhibition of ERK and p47phox-Ser345 phosphorylation and translocation in microglia. J Immunol 181:660–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. (2004) NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. The Journal of biological chemistry 279:1415–1421 [DOI] [PubMed] [Google Scholar]
- Ramanan S, Kooshki M, Zhao W, Hsu FC, Robbins ME. (2008) PPARalpha ligands inhibit radiation-induced microglial inflammatory responses by negatively regulating NF-kappaB and AP-1 pathways. Free Radic Biol Med 45:1695–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiko P, Szakmary A, Jaeger W, Szekeres T. (2008) Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat Res 658:68–94 [DOI] [PubMed] [Google Scholar]
- Spanier G, Xu H, Xia N, Tobias S, Deng S, Wojnowski L, Forstermann U, Li H. (2009) Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J Physiol Pharmacol 60 (Suppl 4):111–116 [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O'Callaghan JP. (2002) Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson's disease. FASEB J 16:1474–1476 [DOI] [PubMed] [Google Scholar]
- Tan AS, Berridge MV. (2000) Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods 238:59–68 [DOI] [PubMed] [Google Scholar]
- Zhang W, Shin EJ, Wang T, Lee PH, Pang H, Wie MB, Kim WK, Kim SJ, Huang WH, Wang Y, et al. (2006) 3-Hydroxymorphinan, a metabolite of dextromethorphan, protects nigrostriatal pathway against MPTP-elicited damage both in vivo and in vitro. FASEB J 20:2496–2511 [DOI] [PubMed] [Google Scholar]