Abstract
Objective
To present pre- and post-treatment language data for a nonfluent aphasia patient who received two treatment modalities: 1) Continuous Positive Airway Pressure (CPAP) for his sleep apnea, starting 1 year poststroke; and 2) repetitive transcranial magnetic brain stimulation (rTMS), starting 2 years poststroke.
Background
Language data were acquired beyond the spontaneous recovery period of 3–6 months poststroke onset (MPO). CPAP restores adequate oxygen flow throughout all stages of sleep, and may improve cognition. A series of slow, 1 Hz rTMS treatments to suppress a posterior portion of right pars triangularis has been shown to improve phrase length and naming in chronic nonfluent aphasia.
Method
The BDAE and Boston Naming Test (BNT) were administered pre- CPAP, and after 2–5 months of CPAP. These same tests were administered pre-TMS, and at 3 and 6 months post-TMS, and again 2.4 years later.
Results
Post-CPAP testing showed increased phrase length, auditory comprehension, and naming animals and tools/implements (BDAE). Testing at 3 and 6 months post-TMS showed significant increase in phrase length, auditory comprehension and BNT, compared to pre-TMS. These gains were retained at 2.4 years post-TMS. CPAP use continued throughout.
Conclusions
Physiological treatment interventions may promote language recovery in chronic aphasia.
Keywords: Aphasia, CPAP, TMS, Speech, Language, Stroke Rehabilitation
Introduction
Each year, there are approximately 80,000 new cases of adult aphasia due to stroke,1 and 50–60% of cases continue to have chronic, communicative impairment.2, 3 Approximately 20% have hesitant, poorly articulated, agrammatic speech with word-finding problems (nonfluent aphasia).4
The relative role of neuroplasticity in the right hemisphere (RH), or in the left hemisphere (LH) in aphasia recovery remains unknown. In functional imaging studies with nonfluent aphasia, an increased, possible “over-activation” in RH frontal regions (inferior frontal gyrus, IFG; and motor cortex, M1) has been observed.5–9 This possible RH over-activation may be related to transcallosal disinhibition from the damaged, dominant LH, leading only to partial, or incomplete recovery.
Heiss & Thiel10 have suggested that in long-term recovery, RH recruitment may be less efficient than restoring the LH network, where better recovery has been associated with higher activation in L superior temporal gyrus (STG) and L supplementary motor area (SMA).11, 12 Recovery of naming has been associated with reperfusion of L Brodmann area (BA) 37, particularly in acute stroke cases studied with perfusion weighted imaging.13 Winhuisen et al.14 also observed as early as 2 weeks poststroke onset, that better performance on a verbal fluency test (and better recovery) was associated with L IFG. After speech-language therapy with some chronic stroke patients, new LH activation has been associated with improvement in language.15–17 Richter et al.18 have observed therapeutic success following treatment with constraint-induced aphasia therapy to be correlated with relative decrease of activation in RH areas, including the IFG/insular cortex.
In some studies that included a variety of patients with aphasia, RH activation was considered to be compensatory.19–23 New RH activation has also been observed following speech-language therapy in patients with aphasia.23–26 Fernandez et al.27 suggested that RH participation in the acute recovery stage of LH stroke may be followed later, by LH activation corresponding to further recovery, and that the RH may play a larger role in supporting recovery when there is greater damage to LH language areas. Whether recovery in aphasia is mediated primarily from the LH, or from RH language homologues (or both), these studies suggest there is potential for brain reorganization and improved language in post-stroke aphasia.28 The underlying mechanisms remain elusive and there is need for additional treatment programs.
This case report presents language data acquired from 6 months poststroke onset (MPO) to 4.6 years poststroke, for a nonfluent aphasia patient who was treated with two physiological treatment modalities beginning at 12.5 MPO: 1) Continuous Positive Airway Pressure (CPAP) to treat his sleep apnea; and 2) repetitive transcranial magnetic brain stimulation (rTMS), previously reported to improve phrase length and picture-naming in patients with chronic nonfluent aphasia.29–31
Materials and Methods
Participant
The patient was a right-handed man who suffered a left middle cerebral artery (L MCA) embolic stroke at age 43. He received IV tissue plasminogen activator (tPA) for the L MCA clot with narrowing of the basilar artery. His history was significant for atrial septal defect that was repaired at age 9 with open heart surgery in Boston. He was born in Venezuela, premature in the 7th month, weighing 2 lbs. (his twin died). He considered English his native language, but also spoke fluent Spanish.
There was a residual right hemiparesis. He walked without a leg brace or a cane, and some finger flexion/spasticity was present. He was a graduate of a two-year junior college and had been a grocery store manager.
Structural MRI Scan, Left Hemisphere Lesion Sites
At 12 MPO, a 3-dimensional magnetization prepared rapid gradient echo (3D MPRAGE) MRI scan was obtained using a Siemens Vision Symphony/Quantum 1.5T scanner (FOV = 256 × 256, 0.5mm slice thickness, no gap; in plane resolution, 0.5 × 0.5mm). See Figure 1.
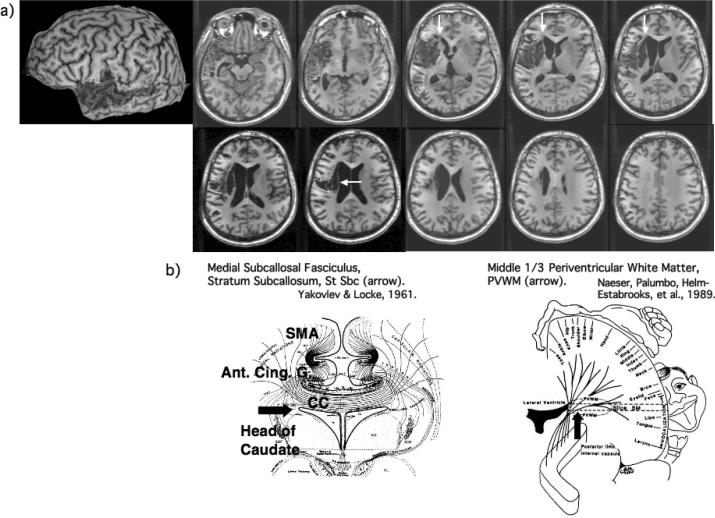
Figure 1.
a) Structural MRI scan (3-dimensional magnetization prepared rapid gradient echo) obtained at 1 year poststroke. Large cortical lesion was present in the left temporal lobe, including anterior portions of superior and middle temporal gyrus, with posterior extension across most of the middle temporal gyrus (BA 21). Only small lesion was present in the more anterior portion of Wernicke's area. Small cortical lesion was present in only the most inferior portions of Broca's area. The lesion was primarily subcortical, with lesion in the two, deep white matter areas near ventricle, associated with persistent nonfluent speech: 1) medial subcallosal fasciculus area, deep to Broca's area and adjacent to frontal horn (vertical white arrows); and 2) periventricular white matter area adjacent to body of lateral ventricle, deep to sensori-motor mouth area (horizontal white arrow). b) Diagrams showing location, and some pathways within each of these two white matter areas adjacent to ventricle: 1) medial subcallosal fasciculus area adjacent to frontal horn, showing pathways from SMA and anterior cingulate gyrus to head of caudate (horizontal black arrow); and 2) periventricular white matter (PVWM) adjacent to body of lateral ventricle (vertical black arrow). See also text, where additional pathways are listed.
Most of the cortical lesion was in the L temporal lobe, where lesion included the anterior portion of the STG and middle temporal gyrus (MTG). The STG lesion included only a small, anterior portion of Wernicke's area. This lesion was compatible with potential for some recovery of auditory comprehension.32, 33 Cortical lesion was also present in all of the L MTG (BA 21), including the superior temporal sulcus. In the left frontal lobe, small cortical lesion was present in only the most inferior part of Broca's area, and the sensory cortex area. No cortical lesion was present in the left supramarginal or angular gyrus areas. The lesion was primarily subcortical, centered over the putamen, with deep anterior and superior white matter lesion extension, near ventricle. Lesion in these deep white matter areas is associated with persistent nonfluent speech,34, 35 which otherwise would not be expected in a patient with primarily only temporal lobe lesion. These two areas are: 1) the medial subcallosal fasciculus (MScF) area, deep to Broca's area and located adjacent to the left frontal horn; and 2) the middle 1/3 periventricular white matter (PVWM) area, deep to the sensorimotor cortex area for mouth and located adjacent to body of caudate and body of the lateral ventricle. The MScF includes pathways from the SMA and the anterior cingulate gyrus area (BA 24) to head of the caudate, and these fibers may play a role in part, for the initiation of speech output. The PVWM area contains afferent and efferent pathways for mouth (and upper and lower limb), thalamo-cortical, callosal and occipito-frontal pathways; and these fibers may play a role in part, for motor-sensory aspects of speech production. Because this patient had lesion in less than half of the MScF and lesion in about half of the PVWM, there was potential for recovery of a longer phrase length. The right hemiparesis was likely associated with lesion in the PWVM, because there was no lesion in the posterior limb, internal capsule, or motor cortex. (Due to the presence of metal staples in the sternum, a 3T overt naming functional MRI scan could not be performed.)
Two Treatment Modalities
1. Continuous Positive Airway Pressure (CPAP)
At 6 MPO, when the patient was evaluated by the neurologist (RK), he displayed excessive sleepiness and fatigue. Later, following a sleep study, he was diagnosed with obstructive sleep apnea (OSA). CPAP, delivered by face mask throughout the night, has demonstrated benefit for the majority of those with mild to moderate sleep apnea.36 CPAP is continuous, to provide constant pressure, and it is positive, to prevent airway collapse, thus ensuring adequate oxygen flow throughout all stages of sleep.37 If left untreated, OSA can be associated with higher incidence of hypertension, myocardial arrhythmias, coronary artery and cerebrovascular disease; inadequate sleep has been linked to poor cognitive performance and increased rates of accidents.38
The patient began CPAP at 12.5 MPO, which is beyond the 3–6 month poststroke time period when most spontaneous recovery has occurred.39 Although CPAP was prescribed for medical reasons, and not planned as a treatment modality for aphasia, some limited language testing was available pre-CPAP at 6–8 MPO and at 12 MPO. Following 2–5 months of CPAP, language testing was performed three times (14–17 MPO). The post-CPAP testing was performed as part of baseline language testing prior to TMS treatment. Language testing included the Boston Diagnostic Aphasia Exam (BDAE) and the Boston Naming Test (BNT).40–42 (Table 1) He had received speech-language therapy only before 12 MPO; not during or after CPAP or TMS.
Table 1.
Language Test Scores pre- and post- CPAP and TMS Treatments. Boston Diagnostic Aphasia Examination (BDAE, 3rd ed., 2001; and 2nd ed.,1983, Auditory Comprehension subtests only) and Boston Naming Test (first 20 pictures)
| Pre-CPAP Testing | Start CPAP | Post-CPAP at 2–5 Months and Pre-TMS Baseline Testing | Pre-TMS Entry Baseline Means and SDs | Start Phase 2 TMS | Post-TMS at 3 and 6 Months | Post-TMS at 2.4 Years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline 1 | Baseline 2 | Baseline 3 | Ten, 20-min. Tx.'s to R Pars Triang., Post. | 3 Months | 6 Months | 29 Months | 29 Months | 29 Months | |||||||
| Months Poststroke Onset: | 6–8 | 12 | 12.5 | 14 | 17 | 17.5 | 27 | 30 | 33 | 56 | 56 | 56 | |||
| BDAE Subtest (Maximum Possible Score) | Score | Score | Score | Score | Score | MEAN | SD | MEAN +2 SD | Score | Score | Score | Score | Score | ||
| Spontaneous Speech (Picture Description) | |||||||||||||||
| Articulatory Agility (7) | 5 | 5 | 5 | 5 | 5.00 | 0.00 | 5 | 5 | 5 | 5 | 5 | ||||
| Phrase Length (7) | 2–4 | 2–4 | 6 | 4 | 5 | 5.00 CPAP | 1.00 | 7.00 | 11 * | 7 * | 6 | 9 * | 12 * | ||
| Grammatical Form (7) | 5 | 5 | 5 | 5 | 5.00 | 0.00 | 6 | 5 | 5 | 5 | 5 | ||||
| Auditory Comprehension (v.2, 1983) | |||||||||||||||
| Word Discrimination (72) | 52 | 60 | 60 | 69 | 70 | 66.33 CPAP | 5.50 | 77.33 | 67 | 65.5 | 64 | 69.5 | 69.5 | ||
| Body Part I.D. (20) | 12 | 10 | 13.5 | 12.5 | 14 | 13.33 CPAP | 1.78 | 16.89 | 13 | 15 | 16 | 16 | 14 | ||
| Commands (15) | 6 | 7 | 11 | 11 | 10 | 10.67 CPAP | 1.89 | 14.45 | 11 | 13 | 11 | 9 | 12 | ||
| Complex Ideational Material (12) | 7 | 5 | 8 | 5 | 6.00 | 1.50 | 9.00 | 6 | 5 | 2 | 4 | 5 | |||
| Repetition | |||||||||||||||
| Sentences (10) | 1 | 2 | 2 | 2 | 2.00 | 0.50 | 3.00 | 3 * | 5 * | 3 * | 3 * | 3 * | |||
| Naming | |||||||||||||||
| Boston Naming Test (first 20) | 8 | 6 | 9 | 8 | 9 | 8.67 | 1.41 | 11.50 | 12 * | 13 * | 15 * | 13 * | 15 * | ||
| Naming in Categories | |||||||||||||||
| Actions (12) | 3 | 5 | 4 | 1 | 3.33 | 1.71 | 6.75 | 4 | 5 | 2 | 4 | 3 | |||
| Animals (12) | 5 | 5 | 9 | 6 | 9 | 8.00 CPAP | 2.06 | 12.12 | 7 | 7 | 10 | 9 | 9 | ||
| Tools/Implements (12) | 2 | 6 | 5 | 6 | 5.67 CPAP | 1.89 | 9.45 | 5 | 2 | 5 | 6 | 6 | |||
CPAP = Improvement Post-CPAP
Note, inadequate number of pre- CPAP data points to test for a significant change post- CPAP
p<.05 = Score Increased by at least 2 SD from Pre-TMS Entry Baseline Mean
Results Following 2–5 months of CPAP
Post-CPAP, there was improvement in Phrase Length, Auditory Comprehension and some picture naming (BDAE). On pre-CPAP testing (6–8 MPO and 12 MPO), the longest phrase length on the BDAE cookie theft picture description was 2–4 words (e.g., “she's cookies”). Post- CPAP, across the three testings (14–17 MPO), the mean longest Phrase Length was 5 words (range, 4–6 words) - e.g., at testing one, the longest phrase length was 6 words: “she let it go with water;” at testing two, 4 words: “he got cookie jugs;” and at testing three, 5 words: “gave her and him some.”
An area of major improvement post-CPAP, was Auditory Comprehension (Table 1). Pre-CPAP, the Word Discrimination scores were 52 and 60/72; and post-CPAP, the mean across three testings was 66.3. For Body Part Identification, pre-CPAP the scores were 12 and 10/20; and post-CPAP, 13.33. For Commands, pre-CPAP, the scores were 6 and 7/15; and post-CPAP, 10.67.
Improvement was also observed post-CPAP on the BDAE subtests for naming Animals, and Tools/Implements. Pre-CPAP, the naming score for Animals was 5/12; and post-CPAP, 8. Pre-CPAP, the naming score for Tools/Implements was 2/12; and post-CPAP, 5.67. It is not possible to know if these changes were significant post-CAP, due to limited testing, pre-CPAP.
2. repetitive Transcranial Magnetic Brain Stimulation (rTMS)
As reviewed in the Introduction, functional imaging studies with nonfluent aphasia have observed unusually high, possible “over-activation,” in parts of R Broca's area and other R perisylvian language homologues during language tasks; this may represent maladaptive plasticity5–9. Repetitive TMS allows painless, noninvasive stimulation of human cortex (approximately 1 cc in size) from outside of the skull. Slow (1 Hz) rTMS decreases excitability in the targeted cortical region of interest (ROI)43, 44 that lasts beyond the duration of the train itself, 45 leading to measurable behavioral effects. Conversely, rapid rTMS (≥5 Hz) increases cortical excitability.45
In our TMS research with chronic nonfluent aphasia patients, we hypothesized that suppression of activity in a targeted RH ROI with slow, 1 Hz rTMS would have an overall modulating effect on functionally connected elements of the distributed neural network for speech and language.29–31 We have observed at 2 months following ten rTMS treatments applied to R posterior-inferior pars triangularis (PTr), there was significant improvement on three naming tests: 1) the BNT, first 20 items (p=.003); 2) the BDAE subtest, naming Animals (p=.02); and 3) the BDAE subtest, naming Tools/Implements (p=.04).31 At 8 months post- rTMS, all three naming test scores continued to improve relative to pre-rTMS testing, but only Tools/Implements was significant (p=.003). None of the four patients received any individual speech therapy during the TMS study, nor out to the 8-month follow-up testing.
rTMS Treatment Protocol in this Case
The patient continued to use CPAP throughout the study (and to date). He signed informed consent forms approved by institutional review boards at all three institutions where this research took place.
Baseline S&V Naming Ability
Prior to any rTMS, a baseline naming ability for Snodgrass & Vanderwart46 (S&V) pictures was established. This baseline S&V naming score was later used during Phase 1 TMS sessions (explained below), to help establish the best RH cortical ROI to suppress with rTMS, in order to improve picture naming. During the baseline S&V naming testing, ten, 20-item S&V picture lists were administered across separate testing sessions. His baseline mean S&V naming score was 9.6 (SD=2.67). The TMS treatment protocol consisted of two Phases.
Phase 1 of TMS
During Phase 1 of TMS, a best-response RH cortical ROI was located, which would later become the targeted location for TMS during Phase 2. During Phase 1, the effect of slow, 1 Hz rTMS for 10 min to suppress activity in each of five different RH frontal regions was examined across separate visits. In this case, the Phase 1 visits took place over a 10-month period, due to scheduling issues. Normally, these visits would take place within a few weeks prior to the first Phase 2 rTMS treatment.
During Phase 1 a total of 600 pulses [90% of motor threshold for the L first dorsal interosseus muscle (L FDI)] was delivered to each RH ROI using the Super-Rapid High Frequency MagStim Magnetic Stimulator (MagStim, NY). A figure-8 shaped rTMS coil with a 7 cm outside diameter on each wing was used. This allowed direct stimulation of an area that was about 1 cm × 1 cm. The interactive frameless stereotaxic system (Brainsight, Rogue Industries, Montreal) was used with the patient's 3D MPRAGE MRI scan to guide the position of the rTMS coil on the patient's scalp; the coil was held constant across sessions, at approximately 45 degrees. The location of the five R frontal ROIs studied during Phase 1 are shown in Figure 2. These five ROIs included the R M1, mouth (orbicularis oris muscle, as verified with MEP), and four subregions within R Broca's area. Each of the five RH ROIs was suppressed once, in separate sessions; and later, PTr posterior and PTr middle, were each suppressed again (similar results were obtained).
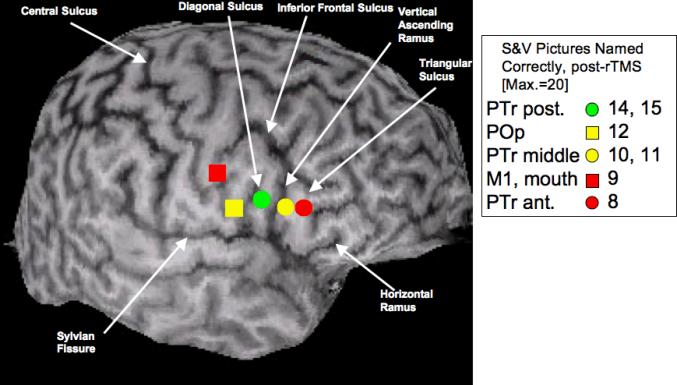
Figure 2.
Location of the five frontal ROIs that were each suppressed with 1 Hz rTMS for 10 min during Phase 1 TMS, to determine the best-response ROI. These five ROIs included R M1, mouth (orbicularis oris muscle, as verified with MEP), and four subregions within R Broca's area as defined in the text, using sulcal boundaries (arrows). The gyral location for each ROI that was suppressed with rTMS is marked in color, see legend box. The legend box also shows the number of Snodgrass & Vanderwart (S&V) pictures (Max.=20) named immediately after rTMS suppression of each ROI. The PTr posterior ROI (green symbol), was the best-response ROI - e.g., the area associated with a naming score that reached at least 2 SD above baseline S&V naming ability (e.g., 15). Note that the number of pictures named correctly immediately post-rTMS decreased for any given ROI as the distance from the best-response ROI increased by 1 or 2 cm, in a rostral or caudal direction. The PTr posterior ROI (green symbol) was used as the target for suppression with 1 Hz rTMS for ten, 20-min treatments during Phase 2 TMS. PTr, pars triangularis; POp, pars opercularis.
The subregions within R Broca's area on the patient's 3D MPRAGE MRI scan were labeled according to sulcal and gyral boundaries as defined by Amunts et al.47, 48 Broca's area is classically defined as the pars triangularis (PTr) and pars opercularis (POp) portions of the IFG, and these parts are often considered to correspond in a general manner with the cytoarchitectonic Brodmann areas (BA) 45 and 44, respectively. We are aware that without cytoarchitectonics, the issue of which anatomical landmark (sulcus) to use as a dividing marker between PTr and POp is not straightforward, especially when a diagonal sulcus (DS) is present, as it was in this case in the RH. When present, the DS is located caudal to the vertical ascending ramus (AR) and rostral to the inferior precentral sulcus. When this occurs, an “extra gyrus” within the posterior portion of the IFG is present. Amunts et al. (1999, 2004) examined 10 brains (20 hemispheres) both with surface anatomy, and with cytoarchitectonics. They observed large variation across the brains, and when a DS was present (occurring only in every second hemisphere), the DS could either mark the border between BA 45 and 44, or it could be inside BA 44. Thus, without cytoarchitectonics in the present study, it was not possible to know whether the DS was a border between PTr and POp, or if it was within the POp.
Taking the results of Amunts et al. (1999; 2004) into consideration for this case study, we arbitrarily chose to use the DS as the dividing marker between the POp and the PTr. The four subregions within R Broca's area (each about 1 cm apart) were defined as follows: 1) POp: gyrus that is rostral to the inferior precentral sulcus and caudal to the DS; 2) PTr posterior: gyrus that is rostral to the DS and caudal to the vertical ascending ramus; 3) PTr middle: gyrus that is rostral to the vertical ascending ramus and caudal to the triangular sulcus; 4) PTr anterior: gyrus that is rostral to the triangular sulcus and caudal to the horizontal ramus. See Figure 2.
Before and after each 10-min rTMS application to a specific RH ROI, a 20-item S&V set of pictures was presented for the patient to name. The best-response cortical ROI was defined as the ROI that was associated with a S&V naming score, at least 2 SD above the baseline mean S&V naming score. In this case, the S&V naming score after 10 min of rTMS to a RH ROI needed to reach at least 15 pictures named correctly (9.6+5.34=14.94), to meet the criterion for best-response RH ROI (baseline mean was 9.6; SD=2.67). The best-response RH ROI in this case was the gyrus located rostral to the DS and caudal to the vertical ascending ramus - e.g., R PTr posterior, in Figure 2. Figure 3 shows in bar graphs, the percent change from baseline for S&V pictures named correctly after 10 min of rTMS to suppress each of the five RH ROIs; the percent change in response time (RT) is also shown.
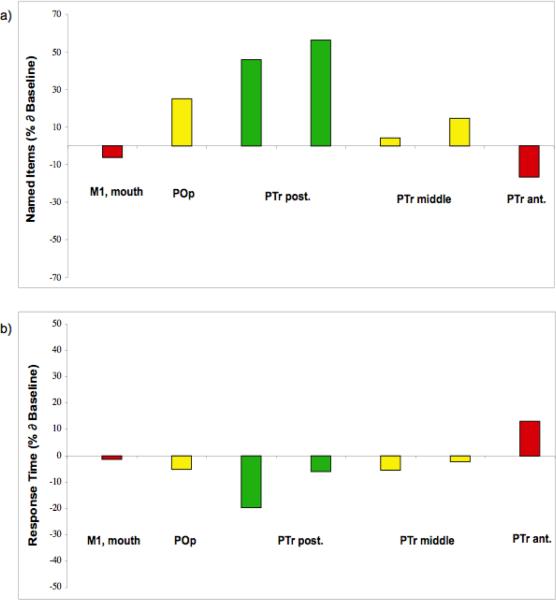
Figure 3.
a) Bar graph showing the percent change from baseline, for S&V pictures named correctly following 10 min of rTMS to suppress each of the five RH ROIs during Phase 1 TMS. b) Bar graph showing the percent change from baseline, for RT to name the pictures, for each of the five RH ROIs. Note, the PTr posterior ROI was associated with the greatest improvement in number of pictures named, on two occasions.
Figures 2 and 3 show that when a targeted ROI is located further away from the best-response ROI, naming accuracy decreases. The highest post-rTMS naming scores of 14 and 15 were obtained when R PTr posterior was suppressed (two separate rTMS sessions). When the coil was positioned 1 cm rostral to the PTr posterior, e.g., on the PTr middle, scores of only 10 or 11 were obtained. When the coil was positioned 1 cm caudal to PTr posterior, e.g., on the POp, the score of 12 was obtained.
The naming scores were even lower, when the coil was positioned 2 cm rostral to the best-response ROI, e.g. on the R PTr anterior (8 pictures named); or 2 cm caudal to best-response ROI, on the M1 mouth (9 pictures named). These scores show that the location of the best-response ROI in this case was precise, e.g., the R PTr posterior. These Phase 1 data emphasize the importance of testing the effect of rTMS to suppress several ROIs, in order to establish the location of a best-response ROI.
Phase 2 of TMS
During Phase 2, the best-response RH ROI from Phase 1 was suppressed for a longer treatment time (20 min), and over more days (five days a week, for two weeks). At 27 MPO, this patient received ten, 20-min rTMS treatments to suppress the R PTr posterior, as shown in Figure 2. On each day of treatment, the rTMS was applied at 1 Hz frequency for 20 min (1200 pulses) at 90% of motor threshold (L FDI), as tested each day before treatment. The same equipment used in Phase 1, was used in Phase 2. Our rTMS parameters are similar to those used in various studies where multiple rTMS treatments were given over time, to help treat depression.49–51 No negative side effects have been reported with these parameters.
Language testing was performed at 3 and 6 months post- Phase 2 TMS, and again at 2.4 years later. At the longest time post-TMS, testing was performed three times, 4 days apart each time.
Results following TMS Treatments
Significant improvements were observed on Phrase Length for the BDAE cookie theft picture description and naming pictures (BNT) at 3 and 6 months post- Phase 2 TMS; these significant gains remained stable at 2.4 years post-TMS. There was also significant improvement on two Auditory Comprehension subtests, Body Part Identification and Commands, at 6 months post-rTMS and at 2.4 years (Table 1).
On the BDAE cookie theft picture description, the pre-TMS baseline mean for longest phrase length was 5 words; and at 3 and 6 months post-TMS, it was 11 and 7 words, respectively. The 11-word phrase length at 3 months post-TMS was, “his mother wash the dish up and the water fall down.” At 6 months post-TMS the longest phrase was, “his mother was watching the paper plates.” At 2.4 years post-TMS, the longest phrase length at each of the three testing times was 6, 9 and 12 words – e.g., 6 words: “the mother was washing some dishes;” 9 words: “water was falling off the sink to the floor;” and 12 words: “she was getting her cookie jars and she started to fall back.” The complexity of his phrases, as well as length, increased. The BNT score improved from a mean of 8.67 pictures named at baseline, to 12 and 13 named at 3 and 6 months post-TMS. These gains in naming were stable at 2.4 years post-TMS, with scores of 15, 13 and 15, at the three testing times.
Discussion
This case report documented language improvement following each of two treatment modalities applied later than one year poststroke onset, in a patient with nonfluent aphasia. The patient began using the first treatment modality, CPAP, for sleep apnea beginning around 12.5 MPO. After 2–5 months of CPAP use, there was improved Phrase Length, Auditory Comprehension, and naming of Animals and Tools/Implements. The patient began treatment with the second modality, TMS, at 27 MPO. At 3 and 6 months post-TMS, as well as at 2.4 years post-TMS, there was significant improvement (p<.05, or 2 SD above pre-TMS baseline mean) in Phrase Length and the BNT. There was significant improvement at 6 months, and 2.4 years post-TMS, on two subtests of BDAE Auditory Comprehension.
Some areas of language improvement post-CPAP and post-TMS were similar, and some were unique to one treatment modality. For example, post-CPAP, a major area of improvement was in Auditory Comprehension (Word Discrimination, Body Part Identification, and Commands). Our prior TMS studies with aphasia patients have not observed improvement in Auditory Comprehension testing, post-TMS. In this case, however, there was additional significant improvement in Auditory comprehension for Body Part Identification and Commands, post-TMS at 6 months and 2.4 years later. Post-CPAP, there were also areas of improvement that overlapped with areas of improvement seen in our previous TMS studies – e.g., Phrase Length, and naming Animals and Tools/Implements.29–31
In addition to improvement in Phrase Length post-TMS, an area of significant improvement post-TMS that paralleled results from our previous TMS studies was naming pictures on the BNT. At 3 and 6 months post-TMS, this patient had BNT scores of 12 and 13. His baseline mean on this test was 8.67 (SD, 1.41; +2 SD=11.50). This significant gain on the BNT was retained at 2.4 years post-TMS, with scores of 13–15.
In this case, there was no additional improvement post-TMS on naming Animals or Tools/Implements, whereas significant improvement in these two naming areas had been observed in our previous TMS studies.30, 31 There had been improvement in naming Animals and Tools/Implements post-CPAP, however, in this case.
To our knowledge, there are no previous reports on the effect of CPAP on language behavior in stroke patients with aphasia who have sleep apnea. CPAP is a treatment applied for sleep apnea, and while it is not comparable to hyperbaric oxygen treatment in stroke patients (without sleep apnea), they each might have a potential, overall effect, on cerebral circulation and oxygenation. Sarno et al.52 studied the effect of a single hyperbaric oxygen treatment (100% oxygen at two atmospheres of pressure) on a group of 16 patients with aphasia. There was no significant effect on language, as tested immediately after the 1.5 hour treatment. In contrast, in the present study, CPAP was used almost nightly, for 2–5 months, after which time, language was again tested.
The specific effect of nightly CPAP use on cortex and brain activation is unknown without functional MRI, which could not be performed in this case. CPAP may have had a more general, overall cortical effect, including improved oxygenation to spared areas of L temporal lobe. Improvement was observed in Auditory Comprehension following CPAP use. This patient spared at least half of the L STG (Wernicke's area), which has been associated with potential for improvement in auditory comprehension.32,33
There was also improvement in naming Animals and Tools/Implements, post-CPAP. Naming ability in these categories has been associated with activation in the mesial occipital cortices and middle infero-temporal areas (animals), and posterior infero-temporal areas and anterior regions of the supramarginal gyrus (tools).53 This patient had also spared these regions of the left temporo-parietal cortex, and perhaps the repeated use of CPAP mediated some of these temporo-parietal networks associated with naming Animals and Tools/Implements.
The TMS treatments may have been more specific, primarily affecting neural networks associated more with speech output (vs. Auditory Comprehension), including Phrase Length and Naming (BNT). During the ten rTMS treatments in Phase 2, there was suppression of a specific part of R IFG - e.g., R PTr posterior. Suppression of this area was hypothesized to reduce the effect of transcallosal disinhibition from the damaged LH on R PTr, and thus reduce overactivation of R PTr (and likely exaggerated inhibition of R POp from R PTr posterior, via U-fibers) and in turn, promote better modulation of R POp and the bi-hemispheric neural network for speech and naming.
Components of this neural network in the LH, as reviewed by Gold and Buckner54 include in part, connections from L inferior prefrontal cortex (L IPC) to L BA 21 (for semantic processing); and connections from L IPC to an area near precentral L BA 6 and inferior parietal (L BA 40) (for phonological processing). While this patient did have lesion in most of L BA 21 (L MTG), there was no lesion in most of the other components of this neural network in the LH. Thus, while speech output did not become entirely normal in this patient post-TMS, there was apparently enough of this neural network remaining that could become active, to enable this patient to have significant improvement in at least two subtests requiring speech output – e.g., 1) Phrase Length; and 2) the BNT. Post-CPAP, the patient had also shown improvement in aspects of speech output, e.g., in Phrase Length, as well as in naming, although specific to BDAE Animals and Tools/Implements, not BNT.
The new, unique improvement post-CPAP had been in Auditory Comprehension, including Word Discrimination, Body Part Identification, and Commands. There was additional, significant improvement in Body Part Identification and Commands, at 6 months post-TMS, and this was retained at 2.4 years post-TMS. The patient continued to use CPAP, however, most nights, even out to 2.4 years post-TMS.
The overall positive response for language improvement in this patient following CPAP and TMS is encouraging. The use of both treatment modalities in this case suggests that the neural networks for several aspects of speech and language remain accessible in the chronic stage, poststroke onset. It is likely there are additional physiological interventions that could access this neuroplasticity.
Another physiological intervention that holds promise for improvement in patients with chronic aphasia includes transcranial direct current stimulation (cathodal to the left fronto-temporal area).55 Although the effect of only one session was examined, improved naming was observed with an increase of 33.6% (SEM 13.8%) in eight chronic nonfluent aphasia patients. Multiple tDCS sessions and the long-term language effects have not been reported.
The effect of high-frequency rTMS on naming Actions and Objects in mild and moderate-severe Alzheimer's disease (AD) has been recently studied.56 The 20 Hz, rTMS was applied to the left, or to the right dorsolateral prefrontal cortex areas, for 500 msec while the subject was asked to name a picture on the screen. The mild cases showed significant improvement in naming Actions, only; while the moderate-severe cases showed improvement in naming Actions and Objects. CPAP has been used to treat OSA in patients with AD, where comparison of pre- and post-treatment neuropsychological test scores after 3 weeks of therapeutic CPAP showed significant improvement in cognition.57
Results from these studies are similar to the present study, because they suggest a neural plasticity is present in chronic aphasia (and AD). Physiological intervention with CPAP (when OSA is present), and/or either slow or fast rTMS, or tDCS, when placed on the proper cortical target area with specific treatment parameters, can induce improvement in many aspects of language behavior.
Limitations of this Case Study
Limitations to this case report include: 1) It is unknown if the language improvements post-CPAP alone, were significant, because the patient was only tested twice, prior to CPAP treatment. 2) It is unknown if the improvement post-CPAP had plateaued after 2–5 months of use. The three testings over a 3-month period (at 14–17 MPO) following 2–5 months of CPAP use suggested stability in the language scores. However, it is not possible to know, if continued use of CPAP alone, and no intervention with TMS, would have yielded the same improvements in language behavior. 3) The relative contribution of rTMS to the significantly improved language scores post-TMS is also unknown, because the patient continued to use the CPAP mask, most nights, throughout the rTMS treatment periods and this continues to date. It is possible there was a synergistic contribution from each intervention, but this also is unknown.
Despite these limitations, results from the present study reinforce results from our previous TMS studies – e.g., improvement in Phrase Length, and picture-naming on the BNT following suppression of a posterior portion of R PTr (gyrus located immediately anterior to R POp) with 1 Hz rTMS.29–31 These TMS results also support the notion behind “paradoxical functional facilitation”58, where a new, temporary “virtual” lesion (as with 1 Hz rTMS) or a new real lesion (as with a second stroke in a specific location), can improve behavior in chronic stroke. For example, Vuilleumier et al.59 have reported the disappearance of L-sided neglect in a stroke patient with a R parietal infarct, following a new L frontal lobe lesion. There are also case studies where ambidextrous adults who had stuttered since childhood no longer stuttered, following unilateral brain damage in adulthood (e.g., stroke or head injury), even as soon as 10 days postonset.60
Additional studies with single, or multiple physiological interventions – e.g., low or high frequency rTMS, tDCS, and even hyperbaric oxygen could all be considered. Recently, transcranial, infra-red laser therapy was observed to significantly improve stroke outcome at 90 days (NIH Stroke Severity Scale), when applied once around 18 hours poststroke onset.61 With the exception of the rTMS protocol presented here (and in our previous TMS papers), these other studies with physiological interventions have applied only a single session of treatment. It is suggested that more treatment sessions be applied over longer periods of time. Also, the combination of a physiological intervention with speech therapy sessions provided immediately afterwards, might promote further language improvement in a variety of chronic aphasia patients.
Acknowledgments
Research supported by NIH grant RO1 DC05672 from the National Institute on Deafness and Other Communication Disorders, Bethesda, MD and a grant from the Medical Research Service, Department of Veterans Affairs, Washington, D.C. (to M.A.N.); a K24 NIH award (RRO18875, to A.P.-L) and the Harvard-Thorndike General Clinical Research Center (NCRR MO1 RR01032); and a P30 DC05207 grant to the Harold Goodglass BU Aphasia Research Center from the National Institute on Deafness and Other Communication Disorders.
References
- 1.Post-stroke rehabilitation . Clinical Practice Guideline. Aspen Publications; Gaithersburg, MD: 1996. [Google Scholar]
- 2.Kertesz A. Neurobiological aspects of recovery from aphasia in stroke. Int Rehabil Med. 1984;6:122–7. doi: 10.3109/03790798409165934. [DOI] [PubMed] [Google Scholar]
- 3.Kertesz A, McCabe P. Recovery patterns and prognosis in aphasia. Brain. 1977;100(Pt 1):1–18. doi: 10.1093/brain/100.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen PM, Vinter K, Olsen TS. Aphasia after stroke: type, severity and prognosis. The Copenhagen aphasia study. Cerebrovasc Dis. 2004;17:35–43. doi: 10.1159/000073896. [DOI] [PubMed] [Google Scholar]
- 5.Perani D, Cappa SF, Tettamanti M, et al. A fMRI study of word retrieval in aphasia. Brain Lang. 2003;85:357–68. doi: 10.1016/s0093-934x(02)00561-8. [DOI] [PubMed] [Google Scholar]
- 6.Belin P, Van Eeckhout P, Zilbovicius M, et al. Recovery from nonfluent aphasia after melodic intonation therapy: a PET study. Neurology. 1996;47:1504–11. doi: 10.1212/wnl.47.6.1504. [DOI] [PubMed] [Google Scholar]
- 7.Rosen HJ, Petersen SE, Linenweber MR, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55:1883–94. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- 8.Naeser MA, Martin PI, Baker EH, et al. Overt propositional speech in chronic nonfluent aphasia studied with the dynamic susceptibility contrast fMRI method. Neuroimage. 2004;22:29–41. doi: 10.1016/j.neuroimage.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Martin PI, Naeser MA, Ho M, et al. Overt Naming pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post- TMS. Brain Lang. 2007;103:248–49. doi: 10.1016/j.bandl.2009.07.007. abstract. and Brain Lang in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98:118–23. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Karbe H, Thiel A, Weber-Luxenburger G, et al. Brain plasticity in poststroke aphasia: what is the contribution of the right hemisphere? Brain Lang. 1998;64:215–30. doi: 10.1006/brln.1998.1961. [DOI] [PubMed] [Google Scholar]
- 12.Saur D, Lange R, Baumgaertner A, et al. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–84. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- 13.Hillis AE, Kleinman JT, Newhart M, et al. Restoring cerebral blood flow reveals neural regions critical for naming. J Neurosci. 2006;26:8069–73. doi: 10.1523/JNEUROSCI.2088-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winhuisen L, Thiel A, Schumacher B, et al. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36:1759–63. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
- 15.Small SL, Flores DK, Noll DC. Different neural circuits subserve reading before and after therapy for acquired dyslexia. Brain Lang. 1998;62:298–308. doi: 10.1006/brln.1998.1951. [DOI] [PubMed] [Google Scholar]
- 16.Leger A, Demonet JF, Ruff S, et al. Neural substrates of spoken language rehabilitation in an aphasic patient: an fMRI study. Neuroimage. 2002;17:174–83. doi: 10.1006/nimg.2002.1238. [DOI] [PubMed] [Google Scholar]
- 17.Cornelissen K, Laine M, Tarkiainen A, et al. Adult brain plasticity elicited by anomia treatment. J Cogn Neurosci. 2003;15:444–61. doi: 10.1162/089892903321593153. [DOI] [PubMed] [Google Scholar]
- 18.Richter M, Miltner WH, Straube T. Association between therapy outcome and right-hemispheric activation in chronic aphasia. Brain. 2008;131:1391–401. doi: 10.1093/brain/awn043. [DOI] [PubMed] [Google Scholar]
- 19.Weiller C, Isensee C, Rijntjes M, et al. Recovery from Wernicke's aphasia: a positron emission tomographic study. Ann Neurol. 1995;37:723–32. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- 20.Thulborn KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke. 1999;30:749–54. doi: 10.1161/01.str.30.4.749. [DOI] [PubMed] [Google Scholar]
- 21.Musso M, Weiller C, Kiebel S, et al. Training-induced brain plasticity in aphasia. Brain. 1999;122(Pt 9):1781–90. doi: 10.1093/brain/122.9.1781. [DOI] [PubMed] [Google Scholar]
- 22.Blasi V, Young AC, Tansy AP, et al. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron. 2002;36:159–70. doi: 10.1016/s0896-6273(02)00936-4. [DOI] [PubMed] [Google Scholar]
- 23.Peck KK, Moore AB, Crosson BA, et al. Functional magnetic resonance imaging before and after aphasia therapy: shifts in hemodynamic time to peak during an overt language task. Stroke. 2004;35:554–9. doi: 10.1161/01.STR.0000110983.50753.9D. [DOI] [PubMed] [Google Scholar]
- 24.Cherney LR, Small SL. Task-dependent changes in brain activation following therapy for nonfluent aphasia: discussion of two individual cases. J Int Neuropsychol Soc. 2006;12:828–42. doi: 10.1017/S1355617706061017. [DOI] [PubMed] [Google Scholar]
- 25.Crosson B, Moore AB, Gopinath K, et al. Role of the right and left hemispheres in recovery of function during treatment of intention in aphasia. J Cogn Neurosci. 2005;17:392–406. doi: 10.1162/0898929053279487. [DOI] [PubMed] [Google Scholar]
- 26.Raboyeau G, De Boissezon X, Marie N, et al. Right hemisphere activation in recovery from aphasia: lesion effect or function recruitment? Neurology. 2008;70:290–8. doi: 10.1212/01.wnl.0000287115.85956.87. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez B, Cardebat D, Demonet JF, et al. Functional MRI follow-up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke. 2004;35:2171–6. doi: 10.1161/01.STR.0000139323.76769.b0. [DOI] [PubMed] [Google Scholar]
- 28.Price CJ, Crinion J. The latest on functional imaging studies of aphasic stroke. Curr Opin Neurol. 2005;18:429–34. doi: 10.1097/01.wco.0000168081.76859.c1. [DOI] [PubMed] [Google Scholar]
- 29.Martin PI, Naeser MA, Theoret H, et al. Transcranial magnetic stimulation as a complementary treatment for aphasia. Semin Speech Lang. 2004;25:181–91. doi: 10.1055/s-2004-825654. [DOI] [PubMed] [Google Scholar]
- 30.Naeser MA, Martin PI, Nicholas M, et al. Improved naming after TMS treatments in a chronic, global aphasia patient--case report. Neurocase. 2005;11:182–93. doi: 10.1080/13554790590944663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naeser MA, Martin PI, Nicholas M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study. Brain Lang. 2005;93:95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Naeser MA, Helm-Estabrooks N, Haas G, et al. Relationship between lesion extent in `Wernicke's area' on computed tomographic scan and predicting recovery of comprehension in Wernicke's aphasia. Arch Neurol. 1987;44:73–82. doi: 10.1001/archneur.1987.00520130057018. [DOI] [PubMed] [Google Scholar]
- 33.Naeser MA, Palumbo CL. Neuroimaging and language recovery in stroke. J Clin Neurophysiol. 1994;11:150–74. doi: 10.1097/00004691-199403000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Naeser MA, Palumbo CL, Helm-Estabrooks N, et al. Severe nonfluency in aphasia. Role of the medial subcallosal fasciculus and other white matter pathways in recovery of spontaneous speech. Brain. 1989;112(Pt 1):1–38. doi: 10.1093/brain/112.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Duffau H, Capelle L, Sichez N, et al. Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo-functional study. Brain. 2002;125:199–214. doi: 10.1093/brain/awf016. [DOI] [PubMed] [Google Scholar]
- 36.Rose MW. Positive Airway Pressure Adherence: Problems an Interventions. Sleep Medicine Clinics. 2006;1:533–39. [Google Scholar]
- 37.Gast H, Schwalen S, Ringendahl H, et al. Sleep-Related Breathing Disorders and Continuous Positive Airway Pressure-Related Changes in Cognition. Sleep Medicine Clinics. 2006;1:499–511. [Google Scholar]
- 38.Reddy SS, Ryan MW. Obstructive Sleep Apnea. [online]. Available at: http://www.utmb.edu/otoref/Grnds/OSA-041215/osa-041215.htm. Accessed December 15, 2004.
- 39.Demeurisse G, Capon A. Language Recovery in Aphasic Stroke Patients: Clinical, CT and CBF Studies. Aphasiology. 1987;1:301–15. [Google Scholar]
- 40.Goodglass H, Kaplan E. Assessment of Aphasia and Related Disorders. Lea and Febiger; Philadelphia: 1983. [Google Scholar]
- 41.Goodglass H, Kaplan E, Barresi B. The Assessment of Aphasia and Related Disorders. 3rd Edition Lippincott, Williams and Wilkins; Philadelphia, PA: 2001. [Google Scholar]
- 42.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Lippincott, Williams and Wilkins; Philadelphia, PA: 2001. [Google Scholar]
- 43.Chen R, Classen J, Gerloff C, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 44.Maeda F, Keenan JP, Tormos JM, et al. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–5. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- 45.Pascual-Leone A, Tormos JM, Keenan J, et al. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333–43. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn] 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- 47.Amunts K, Schleicher A, Burgel U, et al. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–41. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Amunts K, Weiss PH, Mohlberg H, et al. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space--the roles of Brodmann areas 44 and 45. Neuroimage. 2004;22:42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 49.Klein E, Kreinin I, Chistyakov A, et al. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch Gen Psychiatry. 1999;56:315–20. doi: 10.1001/archpsyc.56.4.315. [DOI] [PubMed] [Google Scholar]
- 50.Kauffmann CD, Cheema MA, Miller BE. Slow right prefrontal transcranial magnetic stimulation as a treatment for medication-resistant depression: a double-blind, placebo-controlled study. Depress Anxiety. 2004;19:59–62. doi: 10.1002/da.10144. [DOI] [PubMed] [Google Scholar]
- 51.Padberg F, Zwanzger P, Thoma H, et al. Repetitive transcranial magnetic stimulation (rTMS) in pharmacotherapy-refractory major depression: comparative study of fast, slow and sham rTMS. Psychiatry Res. 1999;88:163–71. doi: 10.1016/s0165-1781(99)00092-x. [DOI] [PubMed] [Google Scholar]
- 52.Sarno MT, Sarno JE, Diller L. The effect of hyperbaric oxygen on communication function in adults with aphasia secondary to stroke. J Speech Hear Res. 1972;15:42–8. doi: 10.1044/jshr.1501.42. [DOI] [PubMed] [Google Scholar]
- 53.Damasio H, Tranel D, Grabowski T, et al. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–12. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- 55.Monti A, Cogiamanian F, Marceglia S, et al. Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry. 2008;79:451–3. doi: 10.1136/jnnp.2007.135277. [DOI] [PubMed] [Google Scholar]
- 56.Cotelli M, Manenti R, Cappa SF, et al. Transcranial magnetic stimulation improves naming in Alzheimer disease patients at different stages of cognitive decline. Eur J Neurol. 2008;15:1286–92. doi: 10.1111/j.1468-1331.2008.02202.x. [DOI] [PubMed] [Google Scholar]
- 57.Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076–81. doi: 10.1111/j.1532-5415.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kapur N. Paradoxical functional facilitation in brain-behaviour research. A critical review. Brain. 1996;119(Pt 5):1775–90. doi: 10.1093/brain/119.5.1775. [DOI] [PubMed] [Google Scholar]
- 59.Vuilleumier P, Hester D, Assal G, et al. Unilateral spatial neglect recovery after sequential strokes. Neurology. 1996;46:184–9. doi: 10.1212/wnl.46.1.184. [DOI] [PubMed] [Google Scholar]
- 60.Helm-Estabrooks N, Yeo R, Geschwind N, et al. Stuttering: disappearance and reappearance with acquired brain lesions. Neurology. 1986;36:1109–12. doi: 10.1212/wnl.36.8.1109. [DOI] [PubMed] [Google Scholar]
- 61.Lampl Y, Zivin JA, Fisher M, et al. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38:1843–9. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]