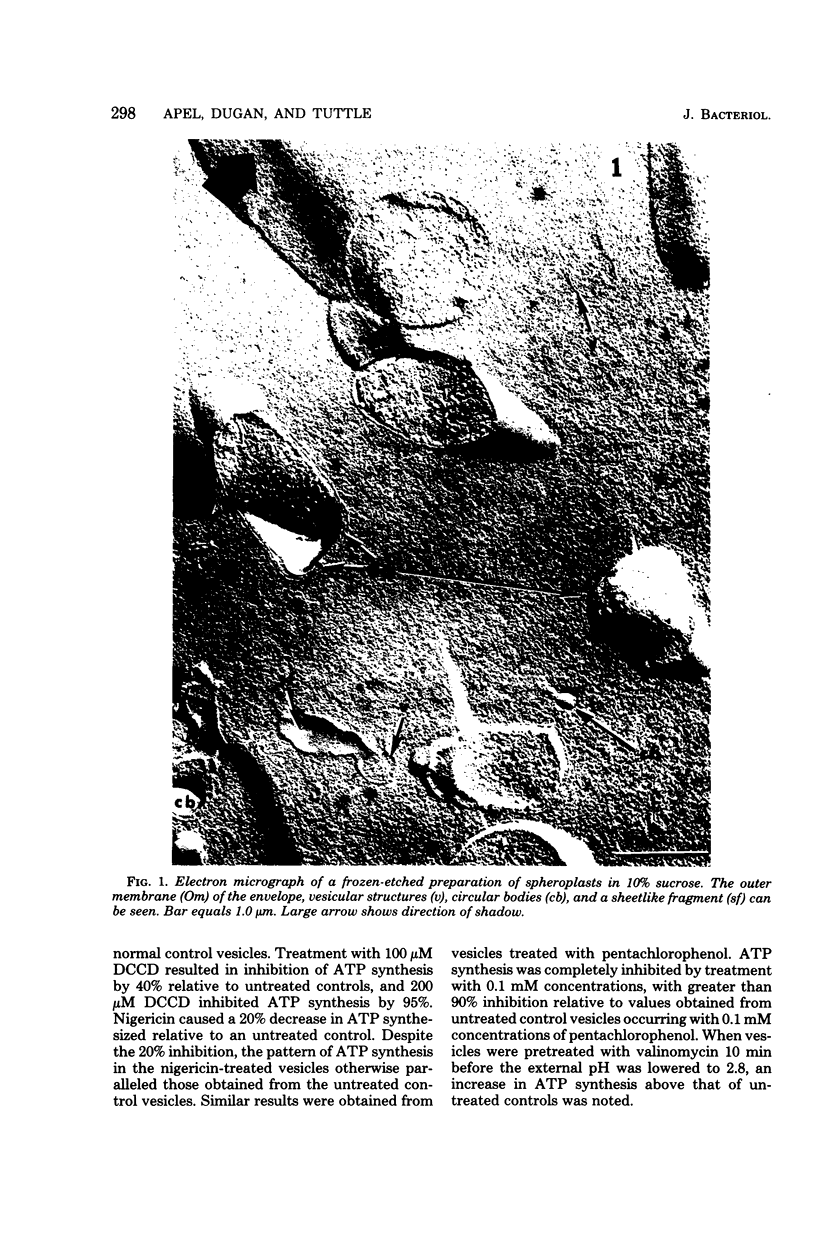
Abstract
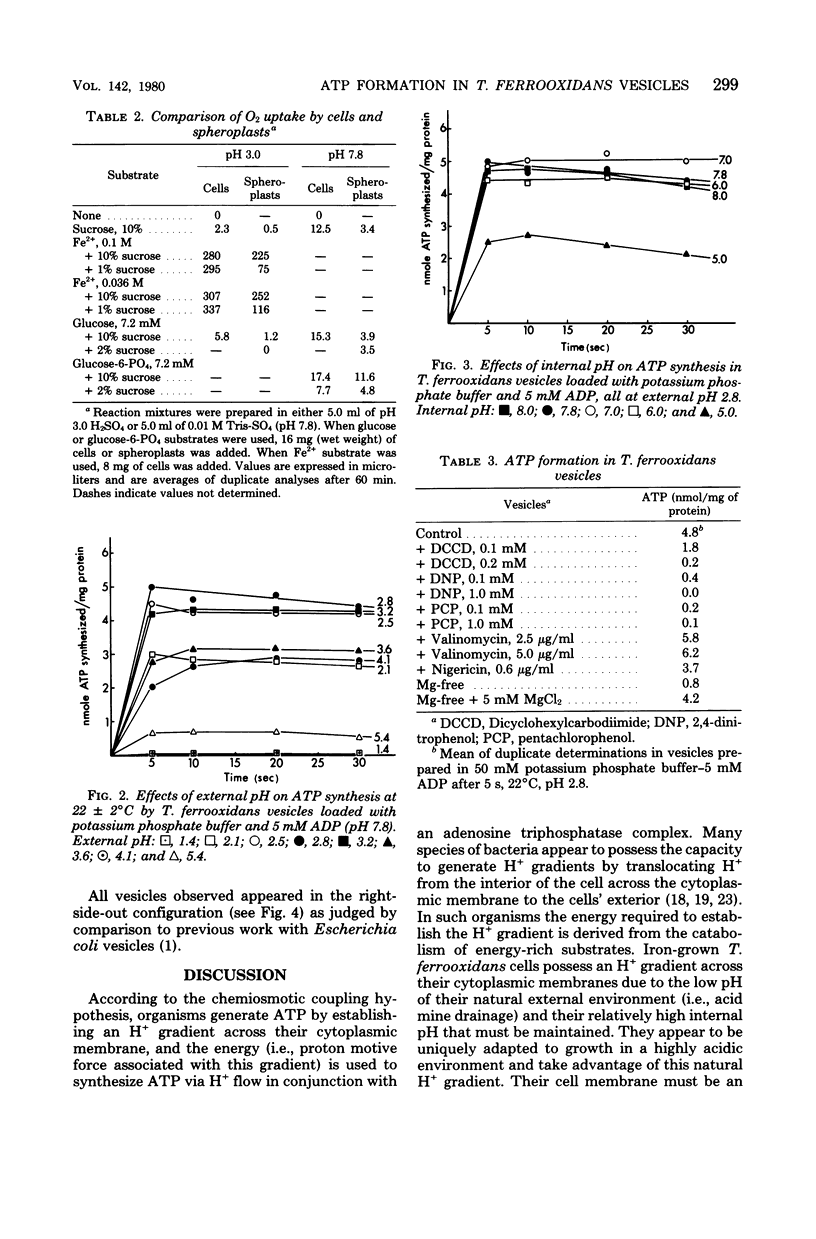
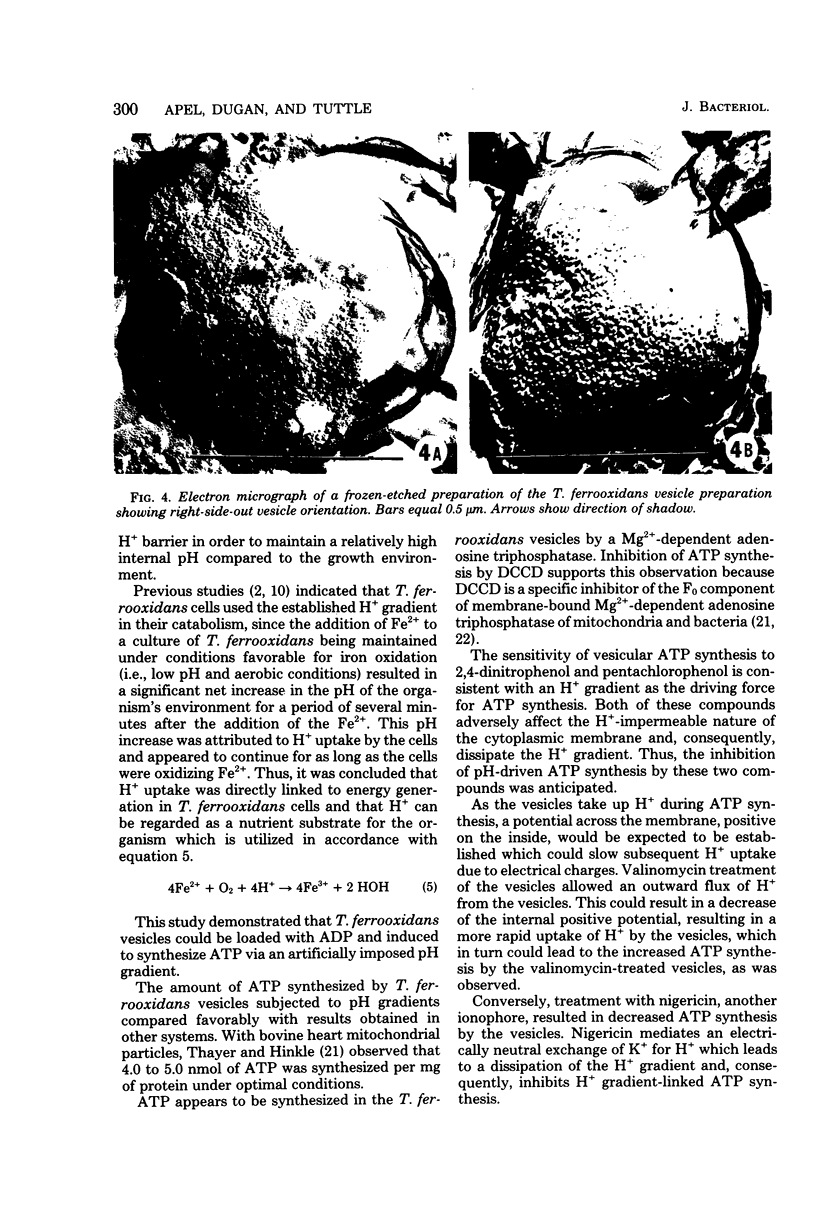
Vesicles prepared from iron-grown Thiobacillus ferrooxidans, and subsequently loaded with adenosine 5'-diphosphate and inorganic phosphate, produced adenosine 5'-triphosphate when subjected to H+ gradients comparable to those in the cells' normal environment (i.e., an internal pH in the range of 6.0 to 8.0 with an optimum of 7.0 to 7.8 and an external pH in the range of 2.1 to 4.1 with an optimum of 2.8). Nigericin, dicyclohexylcarbodiimide, and pentachlorophenol decreased adenosine 5'-triphosphate synthesis. Valinomycin at concentrations of 2.5 and 5.0 micrograms/ml increased adenosine 5'-triphosphate formation by 25 and 30%, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K. H., Staehelin L. A. Orientation of membrane vesicles from Escherichia coli as detected by freeze-cleave electron microscopy. J Bacteriol. 1974 Feb;117(2):888–899. doi: 10.1128/jb.117.2.888-899.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel W. A., Dugan P. R., Filppi J. A., Rheins M. S. Detection of Thiobacillus ferrooxidans in acid mine environments by indirect fluorescent antibody staining. Appl Environ Microbiol. 1976 Jul;32(1):159–165. doi: 10.1128/aem.32.1.159-165.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECK J. V. A ferrous-ion-oxidizing bacterium. I. Isolation and some general physiological characteristics. J Bacteriol. 1960 Apr;79:502–509. doi: 10.1128/jb.79.4.502-509.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLMER A. R., TEMPLE K. L., HINKLE M. E. An iron-oxidizing bacterium from the acid drainage of some bituminous coal mines. J Bacteriol. 1950 Mar;59(3):317–328. doi: 10.1128/jb.59.3.317-328.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer A. R., Hinkle M. E. The Role of Microorganisms in Acid Mine Drainage: A Preliminary Report. Science. 1947 Sep 19;106(2751):253–256. doi: 10.1126/science.106.2751.253. [DOI] [PubMed] [Google Scholar]
- Cox J. C., Nicholls D. G., Ingledew W. J. Transmembrane electrical potential and transmembrane pH gradient in the acidophile Thiobacillus ferro-oxidans. Biochem J. 1979 Jan 15;178(1):195–200. doi: 10.1042/bj1780195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Hypothesis: cation-translocating adenosine triphosphatase models: how direct is the participation of adenosine triphosphate and its hydrolysis products in cation translocation? FEBS Lett. 1973 Jul 15;33(3):267–274. doi: 10.1016/0014-5793(73)80209-1. [DOI] [PubMed] [Google Scholar]
- Mitchell P. The protonmotive Q cycle: a general formulation. FEBS Lett. 1975 Nov 15;59(2):137–139. doi: 10.1016/0014-5793(75)80359-0. [DOI] [PubMed] [Google Scholar]
- Reeves J. P. Transient pH changes during D-lactate oxidation by membrane vesicles. Biochem Biophys Res Commun. 1971 Nov;45(4):931–936. doi: 10.1016/0006-291x(71)90427-x. [DOI] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes P., Mitchell P., Moyle J. The polarity of proton translocation in some photosynthetic microorganisms. Eur J Biochem. 1969 Apr;8(3):450–454. doi: 10.1111/j.1432-1033.1969.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Thayer W. S., Hinkle P. C. Synthesis of adenosine triphosphate by an artificially imposed electrochemical proton gradient in bovine heart submitochondrial particles. J Biol Chem. 1975 Jul 25;250(14):5330–5335. [PubMed] [Google Scholar]
- Tsuchiya T., Rosen B. P. ATP synthesis by an artificial proton gradient in right-side-out membrane vesicles of Escherichia coli. Biochem Biophys Res Commun. 1976 Jan 26;68(2):497–502. doi: 10.1016/0006-291x(76)91173-6. [DOI] [PubMed] [Google Scholar]
- Tuovinen O. H., Nicholas D. J. Proton translocation in intack cells of Thiobacillus denitrificans. Arch Microbiol. 1977 May 13;113(1-2):11–16. doi: 10.1007/BF00428573. [DOI] [PubMed] [Google Scholar]
- Tuttle J. H., Dugan P. R., Apel W. A. Leakage of cellular material from Thiobacillus ferrooxidans in the presence of organic acids. Appl Environ Microbiol. 1977 Feb;33(2):459–469. doi: 10.1128/aem.33.2.459-469.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]