Abstract
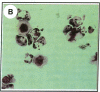
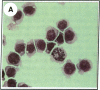
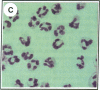
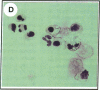
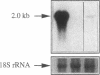
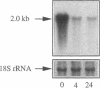
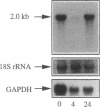
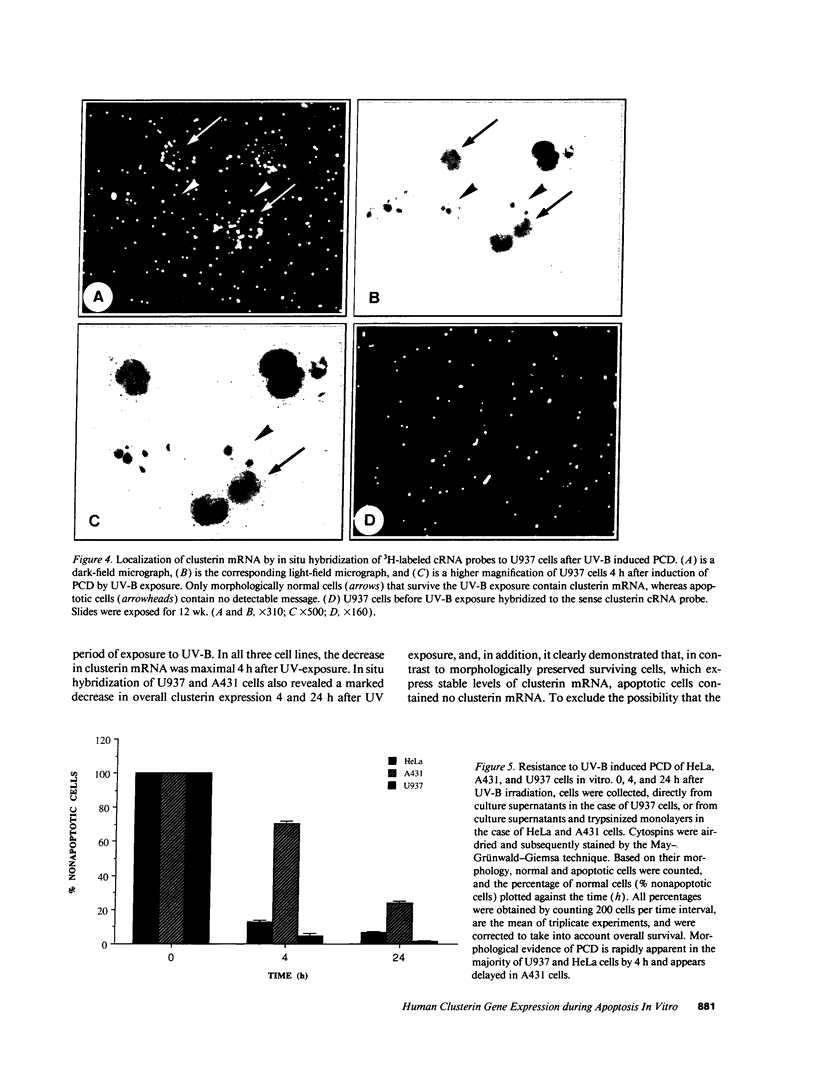
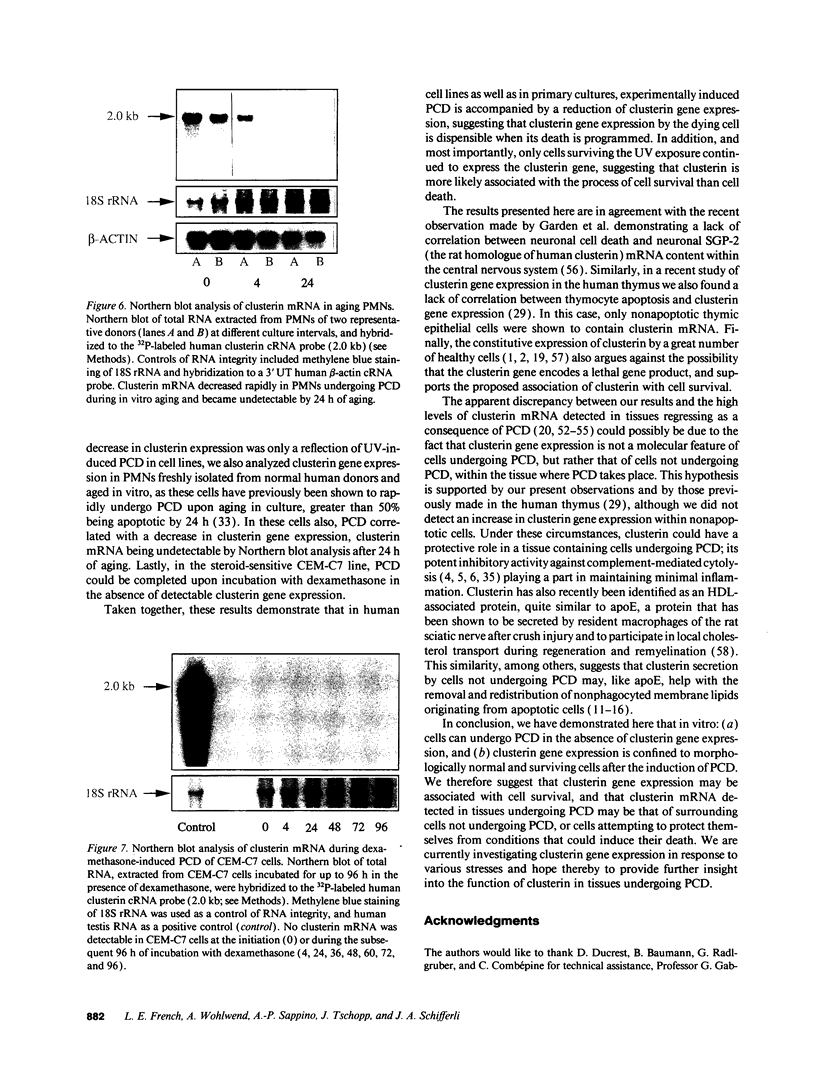
Clusterin is a serum glycoprotein endowed with cell aggregating, complement inhibitory, and lipid binding properties, and is also considered as a specific marker of dying cells, its expression being increased in various tissues undergoing programmed cell death (PCD). However, no study has so far directly shown that cells expressing clusterin in these tissues are actually apoptotic as defined by morphological and biochemical criteria. We have studied cellular clusterin gene expression in vitro using three different models of PCD: (a) ultraviolet B (UV-B) irradiation of human U937, HeLa, and A431 cell lines, (b) in vitro aging of human peripheral blood neutrophils (PMNs), and (c) dexamethasone-induced cell death of the human lymphoblastoid cell line CEM-C7. In all three models, the classical morphological and biochemical features of PCD observed did not correlate with an increase, but with either a marked decrease or an absence of clusterin gene expression as assessed by Northern blot analysis. In situ hybridization of U937 and A431 cells after UV-B irradiation revealed, in addition, that only morphologically normal cells that are surviving continue to express the clusterin gene. Our results demonstrate that in the human myeloid, lymphoid, and epithelial cell types studied, clusterin gene expression is not a prerequisite to their death by apoptosis. In addition, and most interestingly, in situ hybridization of U937 and A431 cells revealed that only surviving cells express the clusterin gene after the induction of PCD, thus providing novel evidence suggesting that clusterin may be associated with cell survival within tissues regressing as a consequence of PCD.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandyk M. G., Sawczuk I. S., Olsson C. A., Katz A. E., Buttyan R. Characterization of the products of a gene expressed during androgen-programmed cell death and their potential use as a marker of urogenital injury. J Urol. 1990 Feb;143(2):407–413. doi: 10.1016/s0022-5347(17)39975-5. [DOI] [PubMed] [Google Scholar]
- Bansal N., Houle A. G., Melnykovych G. Comparison of dexamethasone and lovastatin (mevinolin) as growth inhibitors in cultures of T-cell derived human acute leukemia lines (CEM). Leuk Res. 1989;13(10):875–882. doi: 10.1016/0145-2126(89)90040-4. [DOI] [PubMed] [Google Scholar]
- Bettuzzi S., Hiipakka R. A., Gilna P., Liao S. T. Identification of an androgen-repressed mRNA in rat ventral prostate as coding for sulphated glycoprotein 2 by cDNA cloning and sequence analysis. Biochem J. 1989 Jan 1;257(1):293–296. doi: 10.1042/bj2570293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschuk O., Burdzy K., Fritz I. B. Purification and characterization of a cell-aggregating factor (clusterin), the major glycoprotein in ram rete testis fluid. J Biol Chem. 1983 Jun 25;258(12):7714–7720. [PubMed] [Google Scholar]
- Boyles J. K., Zoellner C. D., Anderson L. J., Kosik L. M., Pitas R. E., Weisgraber K. H., Hui D. Y., Mahley R. W., Gebicke-Haerter P. J., Ignatius M. J. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J Clin Invest. 1989 Mar;83(3):1015–1031. doi: 10.1172/JCI113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkey B. F., deSilva H. V., Harmony J. A. Intracellular processing of apolipoprotein J precursor to the mature heterodimer. J Lipid Res. 1991 Jun;32(6):1039–1048. [PubMed] [Google Scholar]
- Buttyan R., Olsson C. A., Pintar J., Chang C., Bandyk M., Ng P. Y., Sawczuk I. S. Induction of the TRPM-2 gene in cells undergoing programmed death. Mol Cell Biol. 1989 Aug;9(8):3473–3481. doi: 10.1128/mcb.9.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi N. H., Mazda T., Tomita M. A serum protein SP40,40 modulates the formation of membrane attack complex of complement on erythrocytes. Mol Immunol. 1989 Sep;26(9):835–840. doi: 10.1016/0161-5890(89)90139-9. [DOI] [PubMed] [Google Scholar]
- Choi N. H., Nakano Y., Tobe T., Mazda T., Tomita M. Incorporation of SP-40,40 into the soluble membrane attack complex (SMAC, SC5b-9) of complement. Int Immunol. 1990;2(5):413–417. doi: 10.1093/intimm/2.5.413. [DOI] [PubMed] [Google Scholar]
- Collard M. W., Griswold M. D. Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry. 1987 Jun 16;26(12):3297–3303. doi: 10.1021/bi00386a008. [DOI] [PubMed] [Google Scholar]
- Connor J., Buttyan R., Olsson C. A., D'Agati V., O'Toole K., Sawczuk I. S. SGP-2 expression as a genetic marker of progressive cellular pathology in experimental hydronephrosis. Kidney Int. 1991 Jun;39(6):1098–1103. doi: 10.1038/ki.1991.139. [DOI] [PubMed] [Google Scholar]
- Cowan W. M., Fawcett J. W., O'Leary D. D., Stanfield B. B. Regressive events in neurogenesis. Science. 1984 Sep 21;225(4668):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Dugaiczyk A., Haron J. A., Stone E. M., Dennison O. E., Rothblum K. N., Schwartz R. J. Cloning and sequencing of a deoxyribonucleic acid copy of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry. 1983 Mar 29;22(7):1605–1613. doi: 10.1021/bi00276a013. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Cohen J. J. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986 Fall;5(4):289–299. [PubMed] [Google Scholar]
- Eddy A. A., Fritz I. B. Localization of clusterin in the epimembranous deposits of passive Heymann nephritis. Kidney Int. 1991 Feb;39(2):247–252. doi: 10.1038/ki.1991.29. [DOI] [PubMed] [Google Scholar]
- French L. E., Sappino A. P., Tschopp J., Schifferli J. A. Distinct sites of production and deposition of the putative cell death marker clusterin in the human thymus. J Clin Invest. 1992 Nov;90(5):1919–1925. doi: 10.1172/JCI116069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz I. B., Burdzy K., Sétchell B., Blaschuk O. Ram rete testis fluid contains a protein (clusterin) which influences cell-cell interactions in vitro. Biol Reprod. 1983 Jun;28(5):1173–1188. doi: 10.1095/biolreprod28.5.1173. [DOI] [PubMed] [Google Scholar]
- Garden G. A., Bothwell M., Rubel E. W. Lack of correspondence between mRNA expression for a putative cell death molecule (SGP-2) and neuronal cell death in the central nervous system. J Neurobiol. 1991 Sep;22(6):590–604. doi: 10.1002/neu.480220605. [DOI] [PubMed] [Google Scholar]
- Grima J., Zwain I., Lockshin R. A., Bardin C. W., Cheng C. Y. Diverse secretory patterns of clusterin by epididymis and prostate/seminal vesicles undergoing cell regression after orchiectomy. Endocrinology. 1990 Jun;126(6):2989–2997. doi: 10.1210/endo-126-6-2989. [DOI] [PubMed] [Google Scholar]
- Groux H., Torpier G., Monté D., Mouton Y., Capron A., Ameisen J. C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992 Feb 1;175(2):331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann K., Rauch J., Urban J., Parczyk K., Diel P., Pilarsky C., Appel D., Haase W., Mann K., Weller A. Molecular cloning of gp 80, a glycoprotein complex secreted by kidney cells in vitro and in vivo. A link to the reproductive system and to the complement cascade. J Biol Chem. 1991 May 25;266(15):9924–9931. [PubMed] [Google Scholar]
- Hochstrasser A. C., James R. W., Martin B. M., Harrington M., Hochstrasser D., Pometta D., Merril C. R. HDL particle associated proteins in plasma and cerebrospinal fluid: identification and partial sequencing. Appl Theor Electrophor. 1988;1(1):73–76. [PubMed] [Google Scholar]
- James R. W., Hochstrasser A. C., Borghini I., Martin B., Pometta D., Hochstrasser D. Characterization of a human high density lipoprotein-associated protein, NA1/NA2. Identity with SP-40,40, an inhibitor of complement-mediated cytolysis. Arterioscler Thromb. 1991 May-Jun;11(3):645–652. doi: 10.1161/01.atv.11.3.645. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Lowin B., Peitsch M. C., Böttcher A., Schmitz G., Tschopp J. Clusterin (complement lysis inhibitor) forms a high density lipoprotein complex with apolipoprotein A-I in human plasma. J Biol Chem. 1991 Jun 15;266(17):11030–11036. [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Clusterin: the intriguing guises of a widely expressed glycoprotein. Trends Biochem Sci. 1992 Apr;17(4):154–159. doi: 10.1016/0968-0004(92)90325-4. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Molecular structure and functional characterization of a human complement cytolysis inhibitor found in blood and seminal plasma: identity to sulfated glycoprotein 2, a constituent of rat testis fluid. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7123–7127. doi: 10.1073/pnas.86.18.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost A. Hormonal factors in the sex differentiation of the mammalian foetus. Philos Trans R Soc Lond B Biol Sci. 1970 Aug 6;259(828):119–130. doi: 10.1098/rstb.1970.0052. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Ravindranath Y., Peterson W. D., Jr T and B lymphocyte antigen-positive null cell leukemias. Blood. 1977 Mar;49(3):371–378. [PubMed] [Google Scholar]
- Kerr J. F., Harmon B., Searle J. An electron-microscope study of cell deletion in the anuran tadpole tail during spontaneous metamorphosis with special reference to apoptosis of striated muscle fibers. J Cell Sci. 1974 May;14(3):571–585. doi: 10.1242/jcs.14.3.571. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Isaacs J. T. Programmed cell death during regression of PC-82 human prostate cancer following androgen ablation. Cancer Res. 1990 Jun 15;50(12):3748–3753. [PubMed] [Google Scholar]
- Léger J. G., Montpetit M. L., Tenniswood M. P. Characterization and cloning of androgen-repressed mRNAs from rat ventral prostate. Biochem Biophys Res Commun. 1987 Aug 31;147(1):196–203. doi: 10.1016/s0006-291x(87)80106-7. [DOI] [PubMed] [Google Scholar]
- Martin D. P., Schmidt R. E., DiStefano P. S., Lowry O. H., Carter J. G., Johnson E. M., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988 Mar;106(3):829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. J., Cotter T. G. Ultraviolet B irradiation of human leukaemia HL-60 cells in vitro induces apoptosis. Int J Radiat Biol. 1991 Apr;59(4):1001–1016. doi: 10.1080/09553009114550891. [DOI] [PubMed] [Google Scholar]
- May P. C., Lampert-Etchells M., Johnson S. A., Poirier J., Masters J. N., Finch C. E. Dynamics of gene expression for a hippocampal glycoprotein elevated in Alzheimer's disease and in response to experimental lesions in rat. Neuron. 1990 Dec;5(6):831–839. doi: 10.1016/0896-6273(90)90342-d. [DOI] [PubMed] [Google Scholar]
- Murphy B. F., Davies D. J., Morrow W., d'Apice A. J. Localization of terminal complement components S-protein and SP-40,40 in renal biopsies. Pathology. 1989 Oct;21(4):275–278. doi: 10.3109/00313028909061073. [DOI] [PubMed] [Google Scholar]
- Murphy B. F., Kirszbaum L., Walker I. D., d'Apice A. J. SP-40,40, a newly identified normal human serum protein found in the SC5b-9 complex of complement and in the immune deposits in glomerulonephritis. J Clin Invest. 1988 Jun;81(6):1858–1864. doi: 10.1172/JCI113531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. F., Saunders J. R., O'Bryan M. K., Kirszbaum L., Walker I. D., d'Apice A. J. SP-40,40 is an inhibitor of C5b-6-initiated haemolysis. Int Immunol. 1989;1(5):551–554. doi: 10.1093/intimm/1.5.551. [DOI] [PubMed] [Google Scholar]
- Newman S. L., Henson J. E., Henson P. M. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982 Aug 1;156(2):430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman M. R., Thompson E. B. Characterization of a glucocorticoid-sensitive human lymphoid cell line. Cancer Res. 1977 Oct;37(10):3785–3791. [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappino A. P., Busso N., Belin D., Vassalli J. D. Increase of urokinase-type plasminogen activator gene expression in human lung and breast carcinomas. Cancer Res. 1987 Aug 1;47(15):4043–4046. [PubMed] [Google Scholar]
- Sappino A. P., Huarte J., Vassalli J. D., Belin D. Sites of synthesis of urokinase and tissue-type plasminogen activators in the murine kidney. J Clin Invest. 1991 Mar;87(3):962–970. doi: 10.1172/JCI115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. W., Jr Death in embryonic systems. Science. 1966 Nov 4;154(3749):604–612. doi: 10.1126/science.154.3749.604. [DOI] [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989 Mar;83(3):865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawczuk I. S., Hoke G., Olsson C. A., Connor J., Buttyan R. Gene expression in response to acute unilateral ureteral obstruction. Kidney Int. 1989 Jun;35(6):1315–1319. doi: 10.1038/ki.1989.128. [DOI] [PubMed] [Google Scholar]
- Sensibar J. A., Griswold M. D., Sylvester S. R., Buttyan R., Bardin C. W., Cheng C. Y., Dudek S., Lee C. Prostatic ductal system in rats: regional variation in localization of an androgen-repressed gene product, sulfated glycoprotein-2. Endocrinology. 1991 Apr;128(4):2091–2102. doi: 10.1210/endo-128-4-2091. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- de Silva H. V., Harmony J. A., Stuart W. D., Gil C. M., Robbins J. Apolipoprotein J: structure and tissue distribution. Biochemistry. 1990 Jun 5;29(22):5380–5389. doi: 10.1021/bi00474a025. [DOI] [PubMed] [Google Scholar]
- de Silva H. V., Stuart W. D., Duvic C. R., Wetterau J. R., Ray M. J., Ferguson D. G., Albers H. W., Smith W. R., Harmony J. A. A 70-kDa apolipoprotein designated ApoJ is a marker for subclasses of human plasma high density lipoproteins. J Biol Chem. 1990 Aug 5;265(22):13240–13247. [PubMed] [Google Scholar]
- de Silva H. V., Stuart W. D., Park Y. B., Mao S. J., Gil C. M., Wetterau J. R., Busch S. J., Harmony J. A. Purification and characterization of apolipoprotein J. J Biol Chem. 1990 Aug 25;265(24):14292–14297. [PubMed] [Google Scholar]