Abstract
Our work in dogs has revealed a U-shaped dose response between selenium status and prostatic DNA damage that remarkably parallels the relationship between dietary selenium and prostate cancer risk in men, suggesting that more selenium is not necessarily better. Herein, we extend this canine work to show that the selenium dose that minimizes prostatic DNA damage also maximizes apoptosis—a cancer-suppressing death switch used by prostatic epithelial cells. These provocative findings suggest a new line of thinking about how selenium can reduce cancer risk. Mid-range selenium status (.67–.92 ppm in toenails) favors a process we call “homeostatic housecleaning”—an upregulated apoptosis that preferentially purges damaged prostatic cells. Also, the U-shaped relationship provides valuable insight into stratifying individuals as selenium-responsive or selenium-refractory, based upon the likelihood of reducing their cancer risk by additional selenium. By studying elderly dogs, the only non-human animal model of spontaneous prostate cancer, we have established a robust experimental approach bridging the gap between laboratory and human studies that can help to define the optimal doses of cancer preventives for large-scale human trials. Moreover, our observations bring much needed clarity to the null results of the Selenium and Vitamin E Cancer Prevention Trial (SELECT) and set a new research priority: testing whether men with low, suboptimal selenium levels less than 0.8 ppm in toenails can achieve cancer risk reduction through daily supplementation.
Keywords: non-linear, dog, dietary supplements, cancer prevention, personalized medicine
INTRODUCTION
There is considerable interest among scientists and the public in determining whether daily supplementation with the essential trace mineral selenium can substantively reduce the incidence of prostate cancer. A catalogue of data from more than 400 animal studies (Combs and Gray 1998) provided strong rationale for venturing into large-scale human trials. In the Nutritional Prevention of Cancer (NPC) Trial, daily supplementation with selenium (200 micrograms daily in the form of high selenium yeast) significantly reduced prostate cancer risk in men (Clark et al. 1996). Despite these favorable results, the optimal intake of selenium for prostate cancer prevention remained unknown.
To address this knowledge gap, we conducted experiments in dogs to define the dose of selenium that minimizes DNA damage in the aging prostate. This work revealed an intriguing U-shaped dose response between toenail selenium concentration and prostatic DNA damage that remarkably paralleled the relationship between dietary selenium and prostate cancer risk in men in the NPC Trial (Waters et al. 2005). The canine dose-response curve helped to reconcile why men in the NPC Trial who had the highest plasma selenium concentration prior to supplementation did not benefit from additional selenium (Duffield-Lillico et al. 2002). More recently, null results from more than 34,000 men in the Selenium and Vitamin E Prostate Cancer Prevention Trial (SELECT) mirrored the null results of the oversupplemented non-responders in the NPC Trial (Lippman et al. 2009a). Clearly, when it comes to selenium and cancer prevention, more is not necessarily better. Not all men benefit from additional selenium.
Capitalizing on our experience that comparative studies in dogs can lead to novel insights into cancer biology (Waters and Wildasin 2006), we posited that the U-shaped relationship between selenium status and DNA damage in the dog prostate could guide inquiries into the mechanistic underpinnings of selenium’s dose-dependent anticancer effects. Herein, we document the intensity of prostatic epithelial cell apoptosis is greatest in mid-range toenail concentrations of selenium, compared to lower or higher selenium concentrations. Moreover, we utilize the dog dose response curve to shed light on the seemingly contradictory results of SELECT. By demonstrating a U-shaped relationship between selenium and the cancer-suppressing process of apoptosis, we provide further rationale for titrating dietary selenium intake as a prostate cancer risk reduction strategy.
MATERIALS AND METHODS
Methods and observations from this experimental cohort have been reported previously (Waters et al. 2003; Waters et al. 2005; Waters et al. 2007). Relevant details pertaining to study design, assessment of selenium status, DNA damage and apoptosis, and data analysis are described briefly here.
Study Design
Sixty-nine elderly (8 to 10.5 years old; physiologically equivalent to 62-to 69-year-old men) (Patronek et al. 1997) sexually intact male, beagle dogs weighing 8 to 21 kg were randomly assigned to either a nutritionally adequate control group (n = 20 dogs) or to receive daily supplementation with selenomethionine (Solgar Vitamin and Herb) or high-selenium yeast (Seleno Excell®, Cypress Systems) at 3 μg/kg/day (n = 29 dogs) or 6 μg/kg/day (n = 20 dogs) for 7 months. All dogs had nutritionally adequate selenium status confirmed by plasma selenium concentration prior to the start of the experiment. Throughout the feeding trial, all dogs received a selenium-adequate maintenance diet (0.3 ppm as fed basis; Science Diet® Canine Maintenance, Hills Pet Nutrition, Topeka, KS). Dogs were euthanized in accordance with guidelines set forth by the American Veterinary Medical Association Panel on Euthanasia.
Selenium Status Assessed by Selenium Concentration in Toenails
After seven months of treatment, toenail clippings were collected from dogs immediately after euthanasia. Specimens from control and selenium supplemented dogs were analyzed together, but in random order, with the supplementation status unknown to laboratory personnel. Nails from 69 dogs were analyzed for selenium by instrumental neutron activation analysis at the University of Missouri-Columbia Research Reactor Center (MURR), Columbia, MO using a modification of methods previously described (McKown and Morris 1978; Hunter et al. 1990; Cheng et al. 1994). Total selenium content in toenail clippings provides a reliable non-invasive measure of selenium status (Morris et al. 1983; Hunter et al. 1990; Garland et al. 1993; Longnecker et al. 1993).
Extent of Epithelial Cell Apoptosis Within the Prostate
At the end of the treatment period, the prostate was collected from each dog within 15 minutes after euthanasia. A modification of the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) method (Gavrieli et al. 1992) was used to determine the frequency of apoptosis within formalin-fixed prostate tissue sections. For each dog, the number of prostatic epithelial cells with positive nuclear staining was counted in randomly selected, noncontiguous, 200X microscopic fields. An average of 23 fields in one tissue section was evaluated for each dog. Immunopositive stromal cells, inflammatory cells, or epithelial cells that were shed into the acinar lumen were not counted. Microscopic fields that contained areas that displayed intense inflammation were not scored. The median number of apoptotic prostatic epithelial cells per 200X field was represented as an apoptotic index. The number of dogs with foci of increased apoptosis (apoptotic “hot spots”) was also determined. Apoptotic hot spots were defined as prostatic acini in which there were more than 30 apoptotic epithelial cells per 200X field. This cut-point represented a level of apoptosis that exceeded the mean number plus three standard deviations of apoptotic epithelial cells in prostate samples from unsupplemented control dogs (Waters et al. 2003).
Extent of Prostatic DNA Damage Measured by Alkaline Comet Assay
Prostate tissue (50–80mg) was harvested fresh to prepare prostate cell suspensions. Cytospin preparations indicated >90% of cells had epithelial cell morphology; mean percentage cell viability estimated by trypan blue exclusion was 80%. Histopathologic evaluation of formalin-fixed, step-sectioned prostate tissue sections revealed no foci of carcinoma. The extent of DNA damage in prostate cells, which is an index of oxidative stress and other genotoxic influences within the prostate, was measured by single cell gel electrophoresis (alkaline Comet assay) using a method previously described (Singh et al. 1988; Waters et al. 2005). Under the assay conditions used in this experiment, comet tails reflect the electrophoretic migration of DNA fragments that result from strand breaks, alkali-labile sites, crosslinks, or base excision repair sites (Singh et al. 1988). Extent of DNA damage was scored in 100 randomly selected cells from each sample by one examiner who was blinded to treatment group. SYBR Green 1 stained nucleoids were examined at 200X magnification with an epifluorescent microscope. Each cell was visually scored on a 0–4 scale using a method described by Duthie and Collins (1997) as follows: no damage (type 0); mild to moderate damage (type 1 & 2), and extensive DNA damage (type 3 & 4). The extent of DNA damage within prostate cells was expressed as the percentage of cells with extensive DNA damage (sum of type 3 & 4).
Data Analysis
To analyze results from the NPC Trial, SELECT, and other studies that reported selenium status as plasma selenium concentration, we converted plasma selenium concentration to an equivalent toenail selenium concentration using the equation: plasma selenium (μg/L) = toenail selenium (ppm)/0.0067 (Waters et al. 2005). This simple conversion ratio yielded results similar to the algorithmic approach described by Longnecker et al. (1996).
To compare the risk for high prostatic DNA damage and intensity of apoptosis at different selenium levels, dogs were divided into three ‘status’ groups based on their toenail selenium concentration: low, moderate, and high selenium status. Dogs with low selenium status had toenail concentration <.67 ppm (equivalent to 100 μg/L in plasma); this level has been demonstrated to be the threshold above which the selenoenzyme glutathione peroxidase (GPX3) is maximally expressed in humans (Thomson et al. 1993). Dogs with moderate selenium status had toenail selenium concentration in the range of .67–.92 ppm. The cut-point of .92 ppm (equivalent to 137 μg/L in plasma) was selected because it represents the toenail selenium concentration that is equivalent to one standard deviation above the mean plasma selenium level of U.S. men (Kafai and Ganji 2003). Dogs with high selenium status had toenail concentration that exceeded .92 ppm.
The mean apoptotic index from dogs with low, moderate, and high selenium status were compared using t- test. The chi square test was used to evaluate the association between selenium status and the proportion of dogs with apoptotic hot spots. Likelihood of intense apoptosis (hot spots) and risk for high DNA damage were evaluated for low, moderate, and high selenium groups by calculating odds ratios and 95% confidence intervals. For multivariate analysis, stepwise logistic regression was used to determine whether these odds ratios should be adjusted for potential confounders—factors that might influence DNA damage or apoptosis, such as age, change in body weight, serum testosterone, and sensitivity of peripheral blood lymphocytes to oxidative stress. Odds ratios were considered significant if the 95% confidence interval did not include 1.0. All data analyses were done using standard statistical software [SPSS (Version 16, Chicago, IL) and SAS System (Version 9.2, SAS Institute, Cary, NC)].
RESULTS
A randomized feeding trial design enabled us to create a study population that had a wide range of steady-state selenium levels after supplementation that mimicked those seen in U.S. men. Median toenail selenium concentration in the lowest, middle, and highest quintiles in dogs of this study were 0.51, 0.73, and 0.99 ppm, compared with 0.66, 0.82, and 1.14 ppm for men in the lowest, middle, and highest quintiles of the Health Professionals Follow-up Study (Yoshizawa et al. 1998). In terms of post-supplementation selenium status and extent of DNA damage, there were no differences noted in the distribution of dogs supplemented with different forms of selenium. This enabled us to combine in our analysis the results from control dogs and those receiving selenomethionine or selenium yeast.
The extent of prostatic epithelial cell DNA damage and apoptosis was compared between dogs with low selenium (<.67 ppm in toenails), moderate selenium (.67–.92 ppm), and high selenium status (>.92 ppm) (see Data Analysis section of Materials and Methods for the rationale for these cut-points). Dogs with moderate selenium status were 84% less likely to have high prostatic DNA damage than dogs in the low selenium group (OR, 95% CI = 0.16, 0.04–0.63) (Figure 1), whereas the extent of prostatic damage in the low and high selenium groups was not significantly different. Apoptosis was significantly higher in dogs with moderate selenium status than in dogs with low selenium (mean of 2.6 versus 1.0 apoptotic cells/200X field, p=.025) (Table 1). In contrast, mean apoptotic index in dogs with high selenium status was 2.2, a value which did not differ significantly from dogs with low selenium (p=0.06).
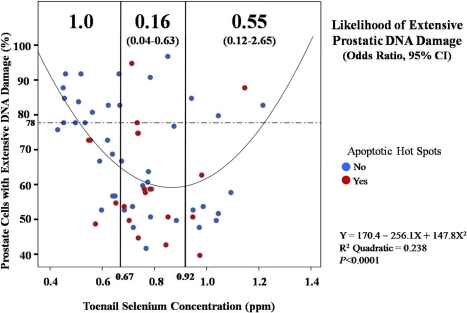
FIGURE 1.
U-shaped dose response relationship between toenail selenium concentration and prostatic DNA damage in 69 elderly dogs that were physiologically equivalent to 62–69 year-old men. Likelihood of extensive prostatic DNA damage, expressed as odds ratio and 95% confidence interval, was compared in dogs with low selenium (< .67 ppm), moderate selenium (.67 – .92), and high selenium (>.92 ppm) (see Data Analysis section of Materials and Methods for rationale for using these cut-points). Dogs with low selenium were chosen as the reference group, and odds ratios were considered significant if the 95% confidence interval did not include 1.0. Dogs with extensive prostatic DNA damage were those in the highest tertile of DNA damage in the study population, i.e., dogs with >78% of prostate cells scoring 3 or 4 on a 0 to 4 damage scale in alkaline Comet assay (see text for details). The U-shaped curve depicted here informs that prostatic DNA damage is highest at low and high selenium concentrations. Our new data suggest the intensity of apoptosis follows a selenium status dose-response curve that is inverse to that for DNA damage (see Table 1). Each RED data point represents a dog that had apoptotic hot spots, defined as prostatic acini in which there was intense apoptosis (> 30 apoptotic cells per 200X microscopic field). Each BLUE data point represents a dog that was negative for apoptotic hot spots within the prostate. Foci of intense apoptosis were seen 4.1 times more often in the moderate selenium group than in the low selenium group (p = .04, chi square); intensity of apoptosis did not differ significantly between dogs with low or high selenium status (p = .75). Taken together, these results define an optimal mid-range of toenail selenium concentration in which prostatic DNA damage is minimized and the intensity of apoptosis is maximized.
TABLE 1.
The association between selenium status and epithelial cell apoptosis within the prostate.
| Selenium Status1 |
|||
|---|---|---|---|
| Low (<0.67 ppm) | Moderate (0.67–0.92 ppm) | High (>0.92 ppm) | |
| Toenail selenium concentration (ppm) | |||
| Range | 0.43–0.66 | 0.67–0.88 | 0.94–1.22 |
| Mean | 0.56 | 0.76 | 1.03 |
| Number of dogs2 | 23 | 26 | 12 |
| Mean apoptotic index3 | 1.0 ± 1.3 | 2.6 ± 3.1* | 2.2 ± 2.1 |
| Likelihood of apoptotic hot spots4 | |||
| Odds Ratio (95% CI)5 | 1.0 (reference) | 4.1 (1.1–15.3)** | 1.6 (0.3–8.6) |
Selenium concentration in toenails of dogs in low, moderate and high selenium groups are equivalent to plasma concentrations of <100, 100–137, >137 μg/L, respectively, using the equation: plasma selenium (μg/L) = toenail selenium (ppm)/0.0067 (Waters et al. 2005). Rationale for using these cut-points is described in Data Analysis section of Materials and Methods.
Complete information on explanatory and response variables was available for 61 of 69 dogs.
Apoptotic index was defined as the median number of cells with positive nuclear staining per 200X microscopic field.
Apoptotic hot spots were defined as regions of prostate acini in which there were >30 apoptotic cells per 200X microscopic field.
Odds ratios are considered significant if 95% confidence interval (CI) does not include 1.0.
Different from the low selenium group, P = 0.025
Different from the low selenium group, P = 0.04
None of the 12 dogs with low selenium status and high prostatic DNA damage had foci of intense prostatic epithelial apoptosis, i.e., “hot spots” with >30 apoptotic cells/200X field (see upper left quadrant, Figure 1). Apoptotic hot spots were seen 4.1 times (95% CI, 1.1–15.3) more often in the moderate selenium group than in the low selenium group (Table 1) (Figure 1). However, the likelihood of intense apoptosis did not differ significantly between the high selenium and low selenium groups (OR, 95% CI = 1.6, 0.3–8.6; p = 0.75). To further evaluate the strength of association between selenium status and intensity of apoptosis, we evaluated other potential confounders, including age, body weight change, serum testosterone, and sensitivity of peripheral blood lymphocytes to oxidative stress. None of these variables were accepted in the stepwise logistic regression model, suggesting that the significant, non-linear association between selenium status and apoptosis in the prostate could not be attributed to these factors.
DISCUSSION
At first glance, it is puzzling why the null results of SELECT (Lippman et al. 2009a) should contradict more than two decades of evidence from cellular and animal models, and human epidemiological data showing that selenium exerts significant anticancer effects (Kok et al. 1987; Knekt et al. 1990; Combs and Gray 1998; Helzlsouer et al. 2000; Ip et al. 2000; Menter et al. 2000; Rayman 2000; Vinceti et al. 2000; El-Bayoumy, 2001; Klein et al. 2001; Nève 2002; Seo et al. 2002; Vogt et al. 2003; Li et al. 2008; Facompre and El-Bayoumy. 2009; Zeng 2009). But the apparent contradiction becomes far less troublesome if one posits that the anticancer effects of selenium are non-linearly dose-dependent. In our previous work, we discovered a U-shaped dose response between selenium status and prostatic DNA damage—DNA damage was greatest at lower and higher selenium levels (Waters et al. 2005). Dogs with mid-range selenium status have an 84% decreased likelihood of high prostatic DNA damage, compared to dogs with low selenium status (Waters et al. 2007). The provocative new finding revealed in this report is that the relationship between selenium status and apoptosis is also U-shaped, with the highest cell suicide in prostatic epithelial cells occurring in dogs with mid-range selenium status. Importantly, the intensity of apoptosis follows a selenium status-associated trajectory that is inverse of that for DNA damage, indicating an optimal range of selenium status in which DNA damage is minimized and apoptosis is maximized. Based upon our data and data from human studies (Clark et al. 1996; Yoshizawa et al. 1998; van den Brandt et al. 2003; Bleys et al. 2008; Lippman et al. 2009a), we estimate the optimal selenium status for prostate cancer risk reduction to be .8 to .92 ppm in toenails, equivalent to 119 to 137 μg/L in plasma.
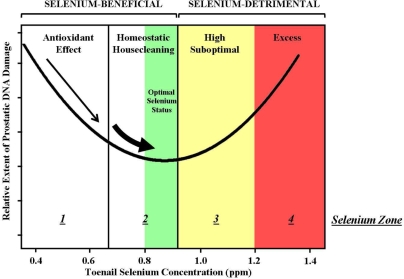
A working knowledge of the dose-response relationship between selenium status, DNA damage, and apoptosis can help to clarify the risk-benefit profile of daily selenium supplementation. To construct this profile, we categorized individuals into four different zones on the basis of their selenium status (Figure 2). Our goal was to identify those individuals most likely to derive benefit or detriment from increasing their selenium status. Zone 1 and Zone 2 correspond to the selenium-beneficial regions of the U-shaped dose-response curve, in which supplementation may be of value. Zone 3 and Zone 4 represent the selenium-detrimental regions of the dose-response curve, in which supplementation would likely be harmful.
FIGURE 2.
The U-shaped dose response curve defines a risk-benefit profile for predicting the consequences of dietary selenium supplementation. Individuals whose selenium status is within the selenium-beneficial region of the U-shaped curve (< .92ppm in toenails) are expected to benefit from additional selenium intake (< .8 ppm) or already reside within the proposed optimal selenium range for prostate cancer risk reduction (0.8 – 0.92)(GREEN). The selenium-beneficial region is subdivided into 2 selenium zones based upon proposed mechanism of selenium action: Zone 1 individuals (< .67 ppm) can reduce their prostatic DNA damage through selenium-dependent antioxidant protection; Zone 2 individuals, as they move from 0.67 to 0.92 ppm, can reduce their DNA damage through selenium-induced upregulation of apoptosis in damaged cells, a process called homeostatic housecleaning. Individuals whose selenium status is within the selenium-detrimental region of the U-shaped curve (> .92 ppm) are not expected to benefit from additional selenium intake. The selenium-detrimental region is subdivided into 2 selenium zones: Zone 3 individuals (.93 – 1.2 ppm) exceed the range of optimal selenium status, defined as 0.8 to 0.92 ppm selenium in toenails; Zone 4 individuals (>1.2 ppm) are at risk for adverse effects associated with excessive selenium, including type II diabetes mellitus (Stranges et al. 2007) and increased overall cancer risk (Duffield-Lillico et al. 2002).
Individuals in Zone 1 have low selenium status—a level below that which is necessary to maximize the expression of selenoenzymes, including the antioxidant glutathione peroxidase (GPX). It is expected that individuals in Zone 1 will benefit from selenium supplementation by increasing GPX expression, thereby upregulating antioxidant protection against DNA damage (Figure 2, narrow arrow). Zone 2 individuals either have low, suboptimal levels of selenium (< .8 ppm) or reside within the proposed optimal range for prostate cancer risk reduction (.8 – .92 ppm) (Figure 2, GREEN). It is expected that Zone 2 men, especially those individuals whose selenium status is less than 0.8 ppm, will benefit from increasing their selenium status (Figure 2, thick arrow). Consistent with this prediction, the vast majority of men in the NPC Trial who benefited from selenium supplementation resided in Zone 2 prior to receiving additional selenium (Duffield-Lillico et al. 2002). This benefit from selenium supplementation, however, cannot be attributed to increased antioxidant defense, because individuals in Zone 2 have selenium status which exceeds the level required for maximum antioxidant protection by GPX. Instead, our data suggest that Zone 2 individuals benefit from additional selenium due to an upregulation of apoptosis. We use the term “homeostatic housecleaning” to refer to this process by which selenium preferentially upregulates apoptosis in DNA damaged cells. We define homeostatic housecleaning as the ability of any intervention to promote homeostasis and reduce cancer risk by selectively deleting damaged cell populations.
The risk-benefit profile in Figure 2 shows that individuals in Zone 3 exceed the optimal range of selenium status for cancer risk reduction. Individuals in Zone 3 are not expected to benefit from further dietary selenium supplementation, with concerns for harm at the high end of the zone. Individuals in Zone 4 have high selenium status—high enough to render them vulnerable to adverse side effects. These adverse effects include an increased incidence of type II diabetes mellitus that was statistically significant in the NPC Trial (Stranges et al. 2007) and reported as a trend in the early-halted SELECT (Lippman et al. 2009a). Supplementation of men in the NPC trial who had greater than 0.81 ppm selenium in toenails—achieving a mean post-supplementation Zone 4 level equivalent to 1.27 ppm selenium in toenails—was associated with a statistically significant 88% increased overall cancer incidence, compared to Zone 1 men who received additional selenium (Duffield-Lillico et al. 2002).
It follows from this new understanding that selenium’s ability to promote antioxidant protection cannot fully explain the dose-dependent activity of selenium to minimize DNA damage. We propose that homeostatic housecleaning, the ability to preferentially induce apoptosis in DNA damaged cells, is a major contributor to the anticancer effect of supranutritional selenium supplementation. That selenium can induce higher levels of apoptosis in DNA damaged cells compared to undamaged cells is supported not only by our in vivo dog studies, but also by in vitro studies by other investigators (Hu et al. 2005; Li et al. 2007). Ongoing studies in our laboratory (ECC, DJW) are examining the signaling pathways involved in homeostatic housecleaning in prostatic cells. Future studies should determine whether selenium exerts homeostatic housecleaning in other organs, such as colon or brain. Further, it should be determined whether this mechanism is shared by other anticancer agents, including other cancer-fighting nutrients.
Taken together, our findings support the hypothesis that selenium’s anticancer action reflects this nutrient’s ability to orchestrate the response of cells to DNA damage, rather than protect cells from further damage (Samaha et al. 1997; Fenech 2001; Ames 2006; Halliwell 2007). This new thinking prepares the ground for a paradigm shift—a shift away from continuous, daily supplementation as a means of cellular protection and toward intermittent strategies that make possible the purging of damaged, pre-malignant and malignant cells. No man mows his lawn every day. Instead, he executes the task intermittently. The notion that, in the future, men could rely upon intermittent, yet highly effective homeostatic housecleaning approaches for prostate cancer risk reduction, deserves further exploration.
Demonstrating the U-shaped dose response between selenium and the cancer-suppressing process of apoptosis bolsters our confidence that mid-range selenium status is optimal for prostate cancer risk reduction. Lay press advice that men seeking to improve their health should “Pop selenium” (Mazzucchi 2007) appears senseless in light of the U-shaped dose response. Equally misleading is the recent message from scientists to the men who participated in SELECT: “We now know that selenium and vitamin E do not prevent prostate cancer” (Southwest Oncology Group, 2008). A more accurate message would have qualified such a statement in the context of selenium status and dose. Within the urology community, this flippant way of communicating the results of SELECT out-of-context has deflated enthusiasm for dietary selenium supplementation as a potential cancer prevention strategy. But today, in a post-SELECT world, the critical cancer prevention hypothesis remains untested: Will men with low, suboptimal selenium status benefit from selenium supplementation? SELECT was not designed to test this hypothesis—concede lead investigators—but instead tested whether men in the U.S. general population could benefit from selenium supplementation (Lippman et al. 2009b).
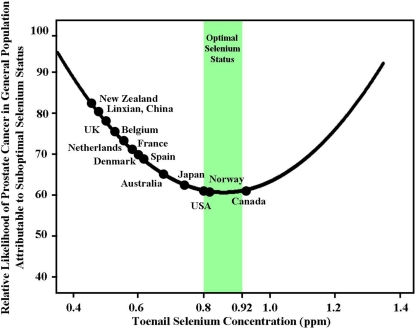
We believe the U-shaped dose response between selenium and cancer risk reduction offers a context of clarity, not contradiction, for interpreting the results of SELECT and has important implications for future selenium supplementation studies. Figure 3 plots the relative likelihood of prostate cancer in the general population attributable to low, suboptimal selenium status versus selenium concentration in toenails predicted by the U-shaped dog curve. By plotting the average selenium status of 13 countries on the U-shaped curve imputed from Figure 1, it is evident that the U.S. general population is not expected to benefit from daily selenium supplementation. Figure 3 renders graphically a compelling conclusion: the null results of SELECT are more expected then unexpected. Prior to supplementation, the average subject in SELECT already had optimal selenium status (equivalent to 0.91 ppm in toenails); after supplementation with 200 micrograms of selenium per day as selenomethionine the average SELECT subject reached excessive Zone 4 levels equivalent to 1.69 ppm in toenails (equivalent to 252 μg/L in plasma). In contrast to people living in the U.S., however, Figure 3 predicts the general population of several countries is more suitable for testing the critical hypothesis. Selenium supplementation is most likely to benefit individuals with toenail selenium levels less than .8 ppm, which we propose as a useful upper limit for eligibility in further studies. Intervention studies have been designed to evaluate daily supplementation with selenium in the United Kingdom, Denmark and Sweden (PRECISE), Australia (APPOSE), and New Zealand (Negative Biopsy Trial) (Rayman 2000; Costello 2001; Karunasinghe et al. 2004). Rather than dismissing the selenium-cancer prevention hypothesis on the basis of disappointing SELECT results, scientists and media should be watching carefully in the years ahead for the results of these and other studies—studies in which the enrolled participants are disadvantaged with respect to selenium status or specific genetic polymorphisms (Kumaraswamy et al. 2000; Hu and Diamond. 2003; Li et al. 2005; Méplan et al. 2007; Cooper et al. 2008; Rayman 2009). This would include careful follow-up of the men in SELECT who began the trial with low, suboptimal selenium status. It would seem logical that only by conducting clinical trials that tailor a nutritional intervention like selenium supplementation to those individuals who are disadvantaged for that nutrient, we will get a closer look at the promise, rather than pitfalls, of dietary supplementation (Davis 2007; Waters et al. 2008; Rayman et al. 2009).
FIGURE 3.
Selenium status in the general population of 13 countries: implications for optimal selenium intake for prostate cancer risk reduction. This figure considers the relative likelihood of prostate cancer in the general population attributable to low, suboptimal selenium status versus selenium concentration in toenails predicted by the U-shaped dog dose response curve. The curve is imputed from the canine data shown in Figure 1. For each country, an average selenium status was generated by converting reported average plasma selenium concentration in men and women to an equivalent toenail concentration using the equation: plasma selenium (μg/L) = toenail selenium (ppm)/0.0067 (Waters et al. 2005). Average selenium status in toenail equivalents (ppm) is as follows: New Zealand: 0.47 (Thomson and Robinson 1996); Linxian, China: 0.49 (Wei et al. 2004); UK: 0.51 (Allen et al. 2008); Belgium: 0.53 (Kornitzer et al. 2004); Netherlands: 0.55 (van den Brandt et al. 2003); France: 0.59 (Czernichow et al. 2006); Denmark:0.60 (Clausen et al. 1989); Spain: 0.62 (Navarro-Alarcon et al. 1998); Australia: 0.68 (Lux and Naidoo 1995); Japan: 0.74 (Imai et al. 1990); USA: 0.80 (Kafai and Ganji 2003); Norway: 0.82 (Meltzer et al. 1993); Canada: 0.94 (Morris et al. 2001). Optimal selenium status for prostate cancer risk reduction (.8 – .92 ppm in toenails) is shown in GREEN. The figure predicts that the general population of a majority of these countries (United States, Norway, and Canada are exceptions) is suitable for testing the critical selenium-cancer prevention hypothesis: Will men with low, suboptimal selenium status benefit from additional selenium intake? It is recognized, however, that health conscious men who enroll in clinical trials may have levels of selenium and other cancer-fighting nutrients that exceed the average level found in the general population (Lippman et al. 2009a; Wu et al. 2009). We contend that selenium supplementation is most likely to benefit individuals with toenail selenium less than 0.8 ppm, which we introduce here as a useful upper limit for eligibility in future studies.
In summary, the U-shaped dose response between selenium and DNA damage observed in dogs has led to a new line of thinking about how selenium can reduce cancer risk. Moderate supranutritional selenium status (0.67 – 0. 92 ppm in toenails) favors homeostatic housecleaning—an upregulated apoptosis that preferentially purges damaged prostatic cells. Our observations, together with the null results from the selenium replete men of SELECT, point to a new research priority: testing the hypothesis that men with low, suboptimal selenium less than 0.8 ppm in toenails can achieve cancer risk reduction through daily selenium supplementation. Clearly, not all men will benefit from selenium supplementation; Willett concluded this 26 years ago (Willett et al. 1983). Recognizing the U-shaped dose response offers prescient guidance not only for those seeking to titrate their selenium intake to optimize health (Waters et al. 2008), but for clinical trial design by categorizing individuals as selenium-responsive or selenium-refractory based upon the likelihood of reducing their cancer risk by additional selenium.
Acknowledgments
The authors thank D. Cooley, C. Oteham, and D. Schlittler for technical assistance. This work was supported by grant PC-970492 from the US Army Medical and Material Command Prostate Cancer Research Program.
REFERENCES
- Allen NE, Appleby PN, Roddam AW, Tjønneland A, Johnsen NF, et al. Plasma selenium concentration and prostate cancer risk: results from the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;88:1567–1575. doi: 10.3945/ajcn.2008.26205. [DOI] [PubMed] [Google Scholar]
- Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci U S A. 2006;103:17589–17594. doi: 10.1073/pnas.0608757103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168:404–410. doi: 10.1001/archinternmed.2007.74. [DOI] [PubMed] [Google Scholar]
- Cheng TP, Morris JS, Koirtyohann SR, Spate VL, Baskett CK. The analysis of human nails for 24 elements via k0 and cyclic neutron activation analysis. Nuclear Instrum Methods Phys Res. 1994;353:457–460. [Google Scholar]
- Clark LC, Combs GF, Jr, Turnbull BW, Slate E, Chalker DK, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- Clausen J, Nielsen SA, Kristensen M. Biochemical and clinical effects of an antioxidant supplementation of geriatric patients: A double blind study. Biol Trace Elem Res. 1989;20:135–151. doi: 10.1007/BF02919106. [DOI] [PubMed] [Google Scholar]
- Combs GF, Jr, Gray WP. Chemopreventive agents: selenium. Pharmacol Ther. 1998;79:179–192. doi: 10.1016/s0163-7258(98)00014-x. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Adami HO, Grönberg H, Wiklund F, Green FR, Rayman MP. Interaction between single nucleotide polymorphisms in selenoprotein P and mitochondrial superoxide dismutase determines prostate Cancer risk. Cancer Res. 2008;68:10171–10177. doi: 10.1158/0008-5472.CAN-08-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello AJ. A randomized, controlled chemoprevention trial of selenium in familial prostate cancer: Rationale, recruitment, and design issues. Urology. 2001;57:182–184. doi: 10.1016/s0090-4295(00)00969-9. [DOI] [PubMed] [Google Scholar]
- Czernichow S, Couthouis A, Bertrais S, Vergnaud AC, Dauchet L, Galan P, et al. Antioxidant supplementation does not affect fasting plasma glucose in the Supplementation with Antioxidant Vitamins and Minerals (SU.VI.MAX) study in France: association with dietary intake and plasma concentrations. Am J Clin Nutr. 2006;84:395–9. doi: 10.1093/ajcn/84.1.394. [DOI] [PubMed] [Google Scholar]
- Davis CD. Nutritional interactions: credentialing of molecular targets for cancer prevention. Exp Biol Med. 2007;232:176–183. [PubMed] [Google Scholar]
- Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF, Jr, Slate EH, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- Duthie SJ, Collins AR. The influence of cell growth, detoxifying enzymes and DNA repair on hydrogen peroxide-mediated DNA damage (measured using the comet assay) in human cells. Free Radic Biol Med. 1997;22:717–724. doi: 10.1016/s0891-5849(96)00421-2. [DOI] [PubMed] [Google Scholar]
- El-Bayoumy K. The protective role of selenium on genetic damage and on cancer. Mutat Res. 2001;475:123–139. doi: 10.1016/s0027-5107(01)00075-6. [DOI] [PubMed] [Google Scholar]
- Facompre N, El-Bayoumy K. Potential stages for prostate cancer prevention with selenium: implications for cancer survivors. Cancer Res. 2009;69:2699–2703. doi: 10.1158/0008-5472.CAN-08-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenech M. Recommended dietary allowances (RDAs) for genomic stability. Mutat Res. 2001;480–481:51–54. doi: 10.1016/s0027-5107(01)00168-3. [DOI] [PubMed] [Google Scholar]
- Garland M, Morris JS, Rosner BA, Stampfer MJ, Spate VL, et al. Toenail trace element levels as biomarkers: reproducibility over a six year period. Cancer Epidemiol Biomarkers Prev. 1993;2:493–497. [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- Helzlsouer KJ, Huang HY, Alberg AJ, Hoffman S, Burke A, et al. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J Natl Cancer Inst. 2000;92:2018–2023. doi: 10.1093/jnci/92.24.2018. [DOI] [PubMed] [Google Scholar]
- Hu H, Jiang C, Ip C, Rustum YM, Lü J. Methylseleninic acid potentiates apoptosis induced by chemotherapeutic drugs in androgen-independent prostate cancer cells. Clin Cancer Res. 2005;11:2379–2388. doi: 10.1158/1078-0432.CCR-04-2084. [DOI] [PubMed] [Google Scholar]
- Hu YJ, Diamond AM. Role of glutathione peroxidase 1 in breast cancer: loss of heterozygosity and allelic differences in the response to selenium. Cancer Res. 2003;63:3347–3351. [PubMed] [Google Scholar]
- Hunter DJ, Morris JS, Chute CG, Kushner E, Colditz GA, et al. Predictors of selenium concentration in human toenails. Am J Epidemiol. 1990;132:114–122. doi: 10.1093/oxfordjournals.aje.a115623. [DOI] [PubMed] [Google Scholar]
- Imai H, Suzuki T, Kashiwazaki H, Takemoto T, Izumi T, et al. Dietary habit and selenium concentrations in erythrocyte and serum in a group of middle-aged and elderly Japanese. Nutr Res. 1990;10:1205–1214. [Google Scholar]
- Ip C, Thompson HJ, Ganther HE. Selenium modulation of cell proliferation and cell cycle biomarkers in normal and premalignant cells of the rat mammary gland. Cancer Epidemiol Biomarkers Prev. 2000;9:49–54. [PubMed] [Google Scholar]
- Kafai MR, Ganji V. Sex, age, geographical location, smoking, and alcohol consumption influence serum selenium concentrations in the USA: third National Health and Nutrition Examination Survey, 1988–1994. J Trace Elem Med Biol. 2003;17:13–18. doi: 10.1016/S0946-672X(03)80040-8. [DOI] [PubMed] [Google Scholar]
- Karunasinghe N, Ryan J, Tuckey J, Masters J, Jamieson MJ, et al. DNA stability and serum selenium levels in a high-risk group for prostate cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:391–397. [PubMed] [Google Scholar]
- Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, et al. SELECT: the next prostate cancer prevention trial. Selenum and Vitamin E Cancer Prevention Trial. J Urol. 2001;166:1311–1315. doi: 10.1016/s0022-5347(05)65759-x. [DOI] [PubMed] [Google Scholar]
- Knekt P, Aromaa A, Maatela J, Alfthan G, Aaran RK, et al. Serum selenium and subsequent risk of cancer among Finnish men and women. J Natl Cancer Inst. 1990;82:864–868. doi: 10.1093/jnci/82.10.864. [DOI] [PubMed] [Google Scholar]
- Kok FJ, de Bruijn AM, Hofman A, Vermeeren R, Valkenburg HA. Is serum selenium a risk factor for cancer in men only? Am J Epidemiol. 1987;125:12–16. doi: 10.1093/oxfordjournals.aje.a114493. [DOI] [PubMed] [Google Scholar]
- Kornitzer M, Valente F, De Bacquer D, Neve J, De Backer G. Serum selenium and cancer mortality: a nested case-control study within an age- and sex-stratified sample of the Belgian adult population. Eur J Clin Nutr. 2004;58:98–104. doi: 10.1038/sj.ejcn.1601754. [DOI] [PubMed] [Google Scholar]
- Kumaraswamy E, Malykh A, Korotkov KV, Kozyavkin S, Hu Y, et al. Structure-expression relationships of the 15-kDa selenoprotein gene. Possible role of the protein in cancer etiology. J Biol Chem. 2000;275:35540–35547. doi: 10.1074/jbc.M004014200. [DOI] [PubMed] [Google Scholar]
- Li H, Kantoff PW, Giovannucci E, Leitzmann MF, Gaziano JM, et al. Manganese superoxide dismutase polymorphism, prediagnostic antioxidant status, and risk of clinical significant prostate cancer. Cancer Res. 2005;65:2498–2504. doi: 10.1158/0008-5472.CAN-04-3535. [DOI] [PubMed] [Google Scholar]
- Li GX, Lee HJ, Wang Z, Hu H, Liao JD, et al. Superior in vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite. Carcinogenesis. 2008;29:1005–1012. doi: 10.1093/carcin/bgn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhou Y, Wang R, Zhang H, Dong Y, et al. Selenium sensitizes MCF-7 breast cancer cells to doxorubicin-induced apoptosis through modulation of phospho-Akt and its downstream substrates. Mol Cancer Ther. 2007;6:1031–1038. doi: 10.1158/1535-7163.MCT-06-0643. [DOI] [PubMed] [Google Scholar]
- Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009a;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman SM, Klein EA, Goodman PJ. Author reply. JAMA. 2009b;301:1877. [Google Scholar]
- Longnecker MP, Stampfer MJ, Morris JS, Spate VL, Baskett C, et al. A one year trial of the effect of high selenium bread on selenium levels in blood and toenails. Am J Clin Nutr. 1993;57:408–413. doi: 10.1093/ajcn/57.3.408. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Stram DO, Taylor PR, Levander OA, Howe M, et al. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology. 1996;7:384–390. doi: 10.1097/00001648-199607000-00008. [DOI] [PubMed] [Google Scholar]
- Lux O, Naidoo D. The assessment of biological variation components of copper, zinc and selenium. J Nutr Biochem. 1995;6:43–47. [Google Scholar]
- Mazzucchi S. Cancer-proof your body. Men’s Health. 2007 Oct;:114–119. [Google Scholar]
- McKown DM, Morris JS. Rapid measurement of selenium in biological samples using instrumental neutron activation analysis. J Radioanal Chem. 1978;43:411–420. [Google Scholar]
- Meltzer HM, Bibow K, Paulsen IT, Mundal H, Norheim G, Holm H. Different bioavailability in humans of wheat and fish selenium as measured by blood platelet response to increased dietary Se. Biol Trace Elem Res. 1993;36:229–241. [Google Scholar]
- Menter DG, Sabichi AL, Lippman SM. Selenium effects on prostate cell growth. Cancer Epidemiol Biomarkers Prev. 2000;9:1171–1182. [PubMed] [Google Scholar]
- Méplan C, Crosley LK, Nicol F, Beckett GJ, Howie AF, et al. Genetic polymorphisms in the human selenoprotein P gene determine the response of selenoprotein markers to selenium supplementation in a gender-specific manner (the SELGEN study) FASEB J. 2007;21:3063–3074. doi: 10.1096/fj.07-8166com. [DOI] [PubMed] [Google Scholar]
- Morris JS, Willett WC, Stampfer M. Toenails as an indicator of dietary selenium. Biol Trace Element Res. 1983;5:529–537. doi: 10.1007/BF02988944. [DOI] [PubMed] [Google Scholar]
- Morris JS, Rohan T, Soskolne CL, Jain M, Horsman TL, et al. Selenium status and cancer mortality in subjects residing in four Canadian provinces. J Radioanal Nucl Chem. 2001;249:421–427. [Google Scholar]
- Navarro-Alarcon M, De La Serrana HL, Perez-Valero V, Lopez-Martinez C. Serum selenium levels as indicators of body status in cancer patients and their relationships with other nutritional and biochemical markers. Sci Total Environ. 1998;212:195–202. doi: 10.1016/s0048-9697(97)00343-4. [DOI] [PubMed] [Google Scholar]
- Nève J. Selenium as a ‘nutraceutical’: how to conciliate physiological and supra-nutritional effects for an essential trace element. Curr Opin Clin Nutr Metab Care. 2002;5:659–663. doi: 10.1097/00075197-200211000-00008. [DOI] [PubMed] [Google Scholar]
- Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol A Biol Sci Med Sci. 1997;52:B171–B178. doi: 10.1093/gerona/52a.3.b171. [DOI] [PubMed] [Google Scholar]
- Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- Rayman MP. Selenoproteins and human health: insights from epidemiological data. Biochim Biophys Acta. 2009;1790:1533–1540. doi: 10.1016/j.bbagen.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Rayman MP, Combs GF, Jr, Waters DJ. Selenium and vitamin E supplementation for cancer prevention. JAMA. 2009;301:1876. doi: 10.1001/jama.2009.625. [DOI] [PubMed] [Google Scholar]
- Samaha HS, Hamid R, el-Bayoumy K, Rao CV, Reddy BS. The role of apoptosis in the modulation of colon carcinogenesis by dietary fat and by the organoselenium compound 1,4-phenylenebis(methylene)selenocyanate. Cancer Epidemiol Biomarkers Prev. 1997;6:699–704. [PubMed] [Google Scholar]
- Seo YR, Kelley MR, Smith ML. Selenomethionine regulation of p53 by a ref1-dependent redox mechanism. Proc Natl Acad Sci U SA. 2002;99:14548–14553. doi: 10.1073/pnas.212319799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Southwest Oncology Group SELECT participant letter. 2008. Available at www.cancer.gov/SELECT-participant-letter.
- Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes. Ann Intern Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- Thomson CD, Robinson MF, Bulter JA, Whanger PD. Long-term supplementation with selenate and selenomethionine: selenium and glutathione peroxidase (EC 1.11 1.9) in blood components of New Zealand women. Br J Nutr. 1993;69:577–588. doi: 10.1079/bjn19930057. [DOI] [PubMed] [Google Scholar]
- Thomson CD, Robinson MF. The changing selenium status of New Zealand residents. Eur J Clin Nutr. 1996;50:107–114. [PubMed] [Google Scholar]
- van den Brandt PA, Zeegers MP, Bode P, Goldbohm RA. Toenail selenium levels and the subsequent risk of prostate cancer: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2003;12:866–871. [PubMed] [Google Scholar]
- Vinceti M, Rovesti S, Bergomi M, Vivoli G. The epidemiology of selenium and human cancer. Tumori. 2000;86:105–118. doi: 10.1177/030089160008600201. [DOI] [PubMed] [Google Scholar]
- Vogt TM, Ziegler RG, Graubard BI, Swanson CA, Greenberg RS, et al. Serum selenium and risk of prostate cancer in U.S. blacks and whites. Int J Cancer. 2003;103:664–670. doi: 10.1002/ijc.10866. [DOI] [PubMed] [Google Scholar]
- Waters DJ, Shen S, Cooley DM, Bostwick DG, Qian J, et al. Effects of dietary selenium supplementation on DNA damage and apoptosis in canine prostate. J Natl Cancer Inst. 2003;95:237–241. doi: 10.1093/jnci/95.3.237. [DOI] [PubMed] [Google Scholar]
- Waters DJ, Shen S, Glickman LT, Cooley DM, Bostwick DG, et al. Prostate cancer risk and DNA damage: translational significance of selenium supplementation in a canine model. Carcinogenesis. 2005;26:1256–1262. doi: 10.1093/carcin/bgi077. [DOI] [PubMed] [Google Scholar]
- Waters DJ, Wildasin K. Cancer clues from pet dogs. Sci Am. 2006;295:94–101. doi: 10.1038/scientificamerican1206-94. [DOI] [PubMed] [Google Scholar]
- Waters DJ, Shen S, Xu H, Kengeri SS, Cooley DM, et al. Noninvasive prediction of prostatic DNA damage by oxidative stress challenge of peripheral blood lymphocytes. Cancer Epidemiol Biomarkers Prev. 2007;16:1906–1910. doi: 10.1158/1055-9965.EPI-07-0034. [DOI] [PubMed] [Google Scholar]
- Waters DJ, Chiang EC, Bostwick DG. The art of casting nets: fishing for the prize of personalized cancer prevention. Nutr Cancer. 2008;60:1–6. doi: 10.1080/01635580701806699. [DOI] [PubMed] [Google Scholar]
- Wei WQ, Abnet CC, Qiao YL, Dawsey SM, Dong ZW, et al. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke and total death. Am J Clin Nutr. 2004;79:80–85. doi: 10.1093/ajcn/79.1.80. [DOI] [PubMed] [Google Scholar]
- Willett WC, Polk BF, Morris JS, Stampfer MJ, Pressel S, et al. Prediagnostic serum selenium and risk of cancer. Lancet. 1983;2:130–134. doi: 10.1016/s0140-6736(83)90116-2. [DOI] [PubMed] [Google Scholar]
- Wu J, Salisbury C, Graham R, Lyons G, Fenech M. Increased consumption of wheat biofortified with selenium does not modify biomarkers of cancer risk, oxidative stress, or immune function in healthy Australian males. Environ Mol Mutagen. 2009;50:489–501. doi: 10.1002/em.20490. [DOI] [PubMed] [Google Scholar]
- Yoshizawa K, Willett WC, Morris JS, Stampfer MJ, Spiegelman D, et al. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. J Natl Cancer Inst. 1998;90:1219–1224. doi: 10.1093/jnci/90.16.1219. [DOI] [PubMed] [Google Scholar]
- Zeng H. Selenium as an essential micronutrient: roles in cell cycle and apoptosis. Molecules. 2009;14:1263–78. doi: 10.3390/molecules14031263. [DOI] [PMC free article] [PubMed] [Google Scholar]