Abstract
Recent data show that increases in bradykinin (BK) concentration contribute to the beneficial effects of angiotensin-converting enzyme inhibitor (ACEI) treatment in chronic kidney disease. However, the possible role of BK in attenuated proteinuria, often seen in ACEI-treated patients, is not well studied. Here, we report that BK decreases mouse podocyte permeability through rearrangement of the tight junction protein zonula occludens-1 (ZO-1) and identify some of the major signaling events leading to permeability change. We show that BK2 receptor (BK2R) stimulation transactivates the epidermal growth factor receptor (EGFR). EGFR transactivation is mediated by a disintegrin and metalloenzyme (ADAM) family members, which are required for both extracellular signal-regulated kinase (ERK) and EGFR activation by BK. Using a gene-silencing approach we observed that both BK-induced ERK activation and BK-induced permeability decrease in podocytes is attenuated by ADAM17 down-regulation, and we identified epiregulin (ER) as the EGFR ligand participating in ADAM-dependent BK2R-EGFR cross-talk. EGFR inhibition attenuated both ZO-1 rearrangement and BK-induced permeability decreases in podocyte. We propose that ZO-1 redistribution is an important element of BK-induced permeability change and the signaling events involved in ZO-1 rearrangement include transactivation of the EGFR via ADAM17 activation and ER shedding. Our data indicate that ADAM17 and the EGFR may be potential novel therapeutic targets in diabetic nephropathy and other chronic kidney diseases.
Introduction
The National Kidney Foundation's data show that one of every nine Americans currently has chronic kidney disease (http://www.kidney.org), and end-stage renal disease is increasing worldwide. The leading causes of chronic kidney disease are diabetes (diabetic nephropathy) and hypertension. An important predictor of glomerular function and disease progression is proteinuria. In the kidney, glomerular podocytes, endothelial cells, and the glomerular basement membrane provide the main filtration barrier for macromolecules (Pavenstädt, 2000). Recently, podocytes have gained substantial attention for their potential roles in glomerular function. Their distinctive “multilegged” structure enables them to encircle the vascular wall, and by regulating the slit diaphragm, the intercellular junction between their foot processes, they are capable of regulating molecular flow from the blood into the urinary space (Pavenstädt, 2000). Renal injury is characterized by foot process effacement, with concomitant changes in cellular and slit architecture that correlate with the development of proteinuria.
The mediators and the exact mechanisms underlying the progression of kidney disease are not fully understood; however, the role of angiotensin II (ANGII) in disease progression is well documented. Angiotensin II affects both inflammatory and fibrotic processes in the kidney (Ruiz-Ortega et al., 2006) and contributes to the development of proteinuria (Langham et al., 2004). Extensive clinical data support the renoprotective effects of angiotensin-converting enzyme inhibitors (ACEIs): they reduce proteinuria, delay renal fibrosis, and preserve kidney function (Macconi et al., 2000). ACEIs inhibit not only the formation of active ANGII, but also kinin degradation, thereby increasing the level of the potent vasodilator, bradykinin (BK). Despite some conflicting data (Nabokov et al., 1998), evidence is increasing that the renoprotective effects of ACEIs are mediated partly by BK B2 receptor (BK2R) activation (Yokota et al., 2003). BK2R activation reduced fibrosis in animal models of tubule-interstitial fibrosis (Seccia et al., 2006), and investigators observed increased albuminuria and glomerular sclerosis in a BK2R-deficient Akita mouse model of diabetes (Kakoki et al., 2004). Moreover, retrospective studies have shown that polymorphisms in the BK2R predispose individuals to increased urinary albumin excretion (Maltais et al., 2002) and certain kinin polymorphisms are more frequent among patients with end-stage renal disease (Zychma et al., 1999). Likewise, recent data suggest that BK exerts an important renoprotective effect through BK2R activation and could be an important therapeutic target in diabetic nephropathy [for review see Doggrell (2006)].
Inter-receptor cross-talk between angiotensin 1 receptor and the epidermal growth factor receptor (EGFR) has been implicated in ANGII-induced progression of chronic kidney disease, and ADAM17, a member of the “a disintegrin and metalloenzyme” family of matrix metalloproteases that process EGFR ligands to their mature form, was identified as a potential therapeutic target (Lautrette et al., 2005). Several in vivo studies showed that ACEIs preserve expression of glomerular nephrin (Benigni et al., 2001), which is one of the main components of the slit diaphragm. Nevertheless, signaling events associated with the regulation of the podocyte filtration barrier itself, thus with the development of proteinuria, are still poorly understood. It was shown that ANGII induces apoptosis through p38 mitogen-activated protein kinase activation in human podocytes (Lai et al., 2004). There is also evidence that BK increases intracellular Ca2+ concentrations in immortalized mouse podocytes (Mundel et al., 1997) and causes contraction of human podocytes (Langham et al., 2002). However, few studies describe the signaling mechanisms underlying the regulation of podocyte permeability. A transepithelial permeability study using fluorescein isothiocyanate (FITC)-labeled bovine serum albumin (BSA) showed that angiotensin II increases podocyte monolayer permeability through the angiotensin II type 1 receptor, Src family kinases, and phospholipase C activation (Macconi et al., 2006). However, no data are available to describe how kinins modulate podocyte permeability.
In this study, we sought to 1) determine the effect of BK on glomerular permeability and 2) characterize BK-induced signaling mechanisms that modulate podocyte permeability to identify potential therapeutic targets for the treatment of diabetic nephropathy and other chronic kidney diseases. We hypothesized that BK has a beneficial effect on glomerular function by decreasing glomerular permeability. We also hypothesized that, as a G protein receptor agonist, BK induces downstream signaling mechanisms through metalloenzyme activation and EGFR transactivation and ADAM17 (a disintegrin and metalloenzyme 17) is a key metalloenzyme regulating these events.
Materials and Methods
Chemicals and Antibodies.
All cell culture media, fetal bovine serum (FBS) and antibiotics, 4 to 12% and 3 to 8% acrylamide gels, SDS sample buffer, and sample reducing agent were purchased from Invitrogen (Carlsbad, CA). BSA, FITC-conjugated BSA, bradykinin, heparin, HOE140 (Icatibant; dArg-Arg-Pro-Hyp-Gly-Thi-Ser-dTic-Oic-Arg), and EGF were from Sigma-Aldrich (St. Louis, MO). GM6001 [N-[(2R)-2-(hydroxamidocarbonylmethyl)-4-methylpentanoyl]-l-tryptophan methylamide], the inactive GM negative control [N-t-butoxycarbonyl-l-leucyl-l-tryptophan methylamide], AG1478 [N-(3-chlorophenyl)-6,7-dimethoxy-4-quinazolinanine hydrochloride], and protease inhibitor cocktail set III were from EMD Biosciences (San Diego, CA). Antibodies against HB-EGF, TGF-α, and amphiregulin were purchased from R&D Systems (Minneapolis, MN). Epiregulin (ER) and zonula occludens-1 (ZO-1) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). ERK, phospho-ERK antibodies, EGFR, and phospho-EGFR (Tyr1068) antibodies were from Cell Signaling Technologies (Danvers, MA). ADAM17 antibody was purchased from R&D Systems. GAPDH was from Sigma-Aldrich, and α-actinin was from Santa Cruz Biotechnology, Inc. Chemiluminescence detection reagent was from Thermo Scientific (Rockford, IL). Nontargeting and mouse ADAM17 silencing RNA and Dharmafect transfection reagent were purchased from Dharmacon RNA Technologies (Lafayette, CO).
Cell Culture.
Immortalized murine podocytes were kindly provided by Dr. Peter Mundel (Mount Sinai School of Medicine, New York, NY). Undifferentiated cells were maintained in RPMI medium 1640 containing 10 units/ml of mouse recombinant interferon-γ, 10% (v/v) FBS, penicillin (100 units/ml), and streptomycin (100 μg/ml) in tissue culture dishes coated with collagen I at 33°C in 95% air and 5% CO2. To induce differentiation cells were placed at 37°C and cultured in the same medium without interferon-γ for 10 to 14 days. Differentiation of the cells was routinely tested by positive synaptopodin staining. Cells between passage numbers 17 and 26 were used in our studies. Immortalized human podocytes were kindly provided by Dr. Moin Saleem (Bristol University, Bristol, UK). Undifferentiated cells were maintained in RPMI medium 1640 containing 10 μg/ml insulin, 5.5 μg/ml transferrin, 5 ng/ml sodium selenite (Sigma-Aldrich), and 10% (v/v) FBS at 33°C in 95% air and 5% CO2. Differentiation was induced by placing the cells to 37°C for 14 days in collagen-coated dishes. Cells between passage numbers 17 and 20 were used in our studies.
Signaling Experiments.
For ERK phosphorylation and EGFR phosphorylation studies differentiated podocytes were plated into collagen-coated 12-well plates (∼100,000/well/1 ml) 12 days after thermoshift, serum-starved for 24 h in 0.5% (w/v) bovine serum albumin-containing RPMI medium, then pretreated for 1 h with the indicated inhibitors [10−5 M HOE140 or buffer; 10−7 M AG1478 or DMSO control (methylsulfoxide); and 10−5 M GM6001 or GM negative control compound] before treatment with the agonist (BK; 10−8 M or EGFR, 1 ng/ml) for 7 min. Cells were then lysed in Laemmli sample buffer and analyzed by Western blotting.
Western Blot Analysis of Podocyte Samples.
Protein samples were boiled for 3 min in reducing SDS-sample buffer and separated by 4 to 12% or 3 to 8% acrylamide gels. Resolved proteins were transferred to 0.45-μm polyvinylidene difluoride membranes (Millipore Corporation, Billerica, MA), blocked with 4% (w/v) nonfat dry milk or 3% (w/v) bovine serum albumin for 1 h, and incubated overnight at 4°C with the following antibodies: 1:1000 ERK and phospho-ERK, 1:1000 phospho-EGFR (Tyr1068), 1:1000 EGFR, 1:3000 α-actinin, 1:2000 ADAM17, or 1:1000 ZO-1 antibodies and 1:1000 GAPDH. Membranes were washed three times in phosphate-buffered saline containing 0.05% (v/v) Tween 20 (Polysorbate 20) and incubated for 1 h at room temperature in appropriate dilutions of secondary antibodies. Immunoreactive protein bands were visualized by using enhanced chemiluminescence (West Dura substrate; Thermo Fisher Scientific) and recorded on BioMax XR film (Eastman Kodak, Rochester, NY). Optimally exposed autoradiographs were digitally scanned and analyzed by using National Institutes of Health Image software. Unphosphorylated ERK or unphosphorylated EGFR was used to normalize the density of the phospho-ERK and phospho-EGFR bands, respectively.
RNA Interference.
Murine podocytes were seeded into collagen-coated 12-well plates (∼100,000/well/1 ml) or six-well plates (∼200,000/well/1 ml) 12 days after thermoshift. Cells (60–70% confluent) were transfected with 50 nM ADAM17 small interfering RNA (siRNA) or nontargeting siRNA (as control) using Dharmacon's Dharmafect 1 transfection reagent (1.5 or 3 μl/well, respectively) as suggested by the manufacturer. Cells were analyzed for successful ADAM17 silencing 48 h after transfection. For signaling experiments cells were serum-starved for 24 h in 0.5% (w/v) BSA containing RPMI medium before treatment with BK (10−8 M) or EGF (1 ng/ml).
Growth Factor Shedding Experiments.
Murine podocytes were seeded into collagen-coated six-well plates 12 days after thermoshift as described above. After transfection with ADAM17 siRNA or control, nontargeting siRNA cells were serum-starved for 24 h then treated with bradykinin (10−8 M) for 2 h. Cell supernatants were collected and concentrated by using Pierce concentrators (Thermo Fisher Scientific) with 3-kDa cutoff membranes. Concentrated supernatants were analyzed by Western blotting as described above using 1:100 of HB-EGF, amphiregulin, TGF-α, and epiregulin antibodies.
Permeability Assay.
Murine podocytes (∼60,000/well/400 μl) were seeded on the top of HTS Transwell inserts (24-well plate; Corning Life Sciences, Lowell, MA). Cells were serum-starved in 0.5% (w/v) bovine serum albumin-containing medium and incubated for 1 h with the indicated inhibitors (10−5 M HOE140, 10−7 M AG1478, or DMSO control; 10−5 M GM6001 or GM negative control compound, or alternatively ADAM17 silenced or control silenced cells were used) before treatment with bradykinin (10−8 M) for 1 h. FITC-labeled dextran or FITC-labeled bovine serum albumin was added to the upper chambers of the Transwells for 5 min at room temperature. Fluorescence activity of the medium in the lower chambers as reflection of the podocyte monolayer permeability was measured with a fluorescence plate reader (excitation: 495 nm, emission: 520 nm; Molecular Devices, Sunnyvale, CA).
ZO-1 Immunostaining.
Murine podocytes were grown on 35-mm lysine-coated glass-bottom culture dishes (MatTek, Ashland, MA). After overnight serum starving they were treated with either BK (10−8 M) or 10% (v/v) FBS (as positive control) then fixed with 2% (w/v) paraformaldehyde and 4% (w/v) sucrose in phosphate-buffered saline for 10 min at room temperature. Subsequently, the nonspecific binding sites were blocked with 2% (v/v) fetal calf serum, 2% (w/v) BSA, and 0.2% (w/v) gelatin in phosphate-buffered saline for 1 h. The cells were then incubated overnight at 4°C with 1:100 dilution of rabbit polylonal anti-ZO-1 antibody, followed by incubation for 1 h at room temperature with the appropriate Alexa Fluor-conjugated secondary antibodies (1:200 dilution; Invitrogen). Texas Red-X phalloidin (Invitrogen) at 1:40 dilution in 1% (w/v) BSA/phosphate-buffered saline for 20 min at room temperature was used to visualize actin. Confocal microscopy was performed by using a Zeiss LSM 510 Meta laser-scanning microscope (Carl Zeiss GmbH, Jena, Germany) equipped with a 60× objective, using the following laser wavelengths: excitation 488 nm, emission 505 to 530 nm; excitation 543 nm, emission 560 nm.
Statistical Analysis.
For analyzing permeability assay data, analysis of variance was used followed by Newman-Keul post hoc comparison. For Western blot image analyses Scion Corporation (Frederick, MD) Image software was used. For statistical analysis of ZO-1 immunostaining color images were converted to grayscale, then the percentage of white areas was calculated by using Scion Image software. Analysis of variance using Prism statistics software (GraphPad Software, Inc., San Diego, CA) was then performed to determine statistical significance. p values < 0.05 were regarded as statistically significant.
Results
BK Decreases Podocyte Monolayer Permeability through ZO-1 Rearrangement.
Because ANGII was shown previously to increase mouse podocyte monolayer permeability (Macconi et al., 2006), we first tested whether BK influences podocyte function by measuring the transepithelial flow of fluorescent albumin from the top chamber through the podocyte layer to the basolateral compartment in a transwell assay of monolayer permeability. We found that BK treatment (10−10 to 10−6 M; 1 h) dose-dependently and significantly decreased fluorescence activity by ∼10 to 50% in the bottom chambers compared with control (Fig. 1A). We also investigated the effect of BK on the permeability of a human podocyte cell line and found that 10−8 M BK decreased the cells permeability to 71% ± 3% (n = 4) of untreated control cells.
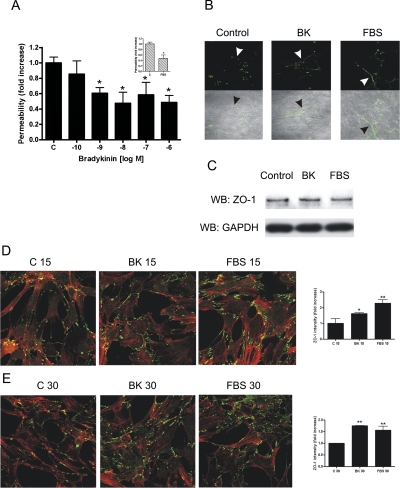
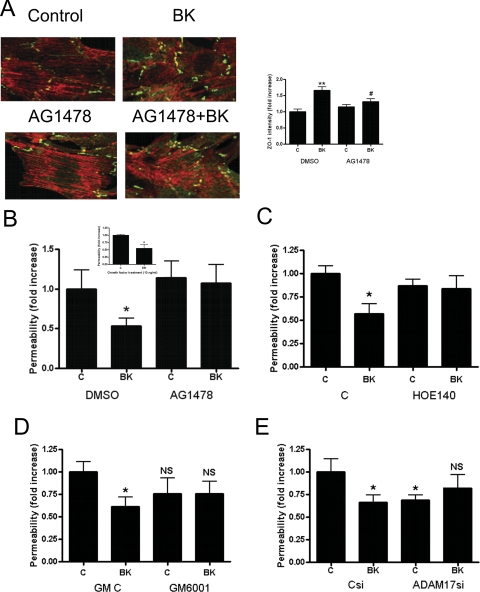
Fig. 1.
BK decreases podocyte monolayer permeability through ZO-1 rearrangement. A, transepithelial albumin permeability of podocyte monolayer was measured by using fluorescent BSA after incubating the cells for 1 h with various concentrations of BK (10−10 to 10−6 M) or 10% (v/v) FBS (inset). Data are means ± S.E. of four independent experiments. *, p < 0.05 versus control. B, ZO-1 redistribution (green, highlighted with arrowheads) is shown upon 10−8 M BK treatment and 10% (v/v) fetal bovine serum treatment (as positive control) without and with bright-field image overlay (bottom part of images, 60×). C, immunoblot (WB) analysis shows an equal amount of ZO-1 expression in control, 10−8 M BK-treated, and 10% (v/v) FBS-treated cell lysates. GAPDH immunoblot was used as loading control. D and E, ZO-1 redistribution was induced in podocytes by treatment with 10−8 M BK or FBS and visualized by immunostaining (green, 40×) after 15 min (D) and 30 min (E) of treatment. Texas Red-X phalloidin was used to visualize actin (red). Quantified ZO-1 immunostaining intensity is shown in bar graphs next to the images. *, p < 0.05; **, p < 0.01 versus corresponding controls, C 15 and C 30, respectively.
In vivo, podocyte permeability depends on the arrangement of slit diaphragm and cytoskeletal proteins. To investigate the mechanism through which BK decreases podocyte monolayer permeability, we studied changes in the arrangement of the cell-cell contact protein ZO-1. This protein is part of the slit diaphragm, and ZO-1 immunostaining has been shown to become fragmented in podocytes after ANGII treatment (Macconi et al., 2006). Figure 1B shows that subconfluent, untreated podocytes have positive ZO-1 immunostaining. However, this staining is not continuous. BK treatment (10−8 M) or 10% (v/v) FBS treatment (positive control) induced comparable increases in ZO-1 staining that became almost continuous around the cells (Fig. 1B, arrowheads). Increased staining intensity is possibly caused by rearrangement of ZO-1 and not by newly synthesized protein. The amount of ZO-1 protein remained the same in control, BK-treated, and FBS-treated cell lysates as assessed by Western blotting (Fig. 1C). Analysis of podocytes at lower magnification revealed that BK treatment for 15 and 30 min increased ZO-1 immunostaining 1.6-fold (Fig. 1D) and 1.75-fold (Fig. 1E), respectively, similar to exposure to the positive control, 10% (v/v) FBS.
BK2Rs Transactivate the EGFR in Podocytes.
BK is known to act through G protein-coupled receptors (GPCRs) that couple to Gq/11 and Gi family G proteins. In addition, some of its effects result from inter-receptor cross-talk between the BK2R and the EGFR (Göoz et al., 2006; Mukhin et al., 2006). In murine inner medullary collecting duct-3 cells, BK stimulates ERK1/2 activity by transactivating the EGFR (Mukhin et al., 2006). To characterize the signal transduction events behind the permeability changes, we first determined whether BK regulated EGFR and EGFR transactivation contributed to downstream BK signaling in podocytes. Figure 2, A and B shows concomitant activation of ERK (∼3-fold) and EGFR (∼2-fold) upon 10−8 M BK treatment. Pretreating the cells with the EGFR tyrosine kinase inhibitor AG1478 (10−7 M) almost completely blocked BK-induced EGFR and ERK activation. As expected, AG1478 inhibited EGF-induced phosphorylation of the EGFR and ERK, suggesting that most of the BK-induced ERK activation is mediated through transactivation of the EGFR in podocytes.
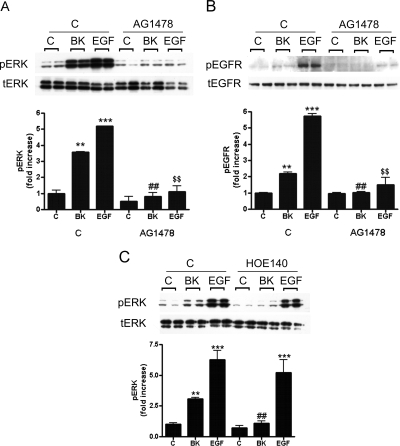
Fig. 2.
BK induces extracellular signal-regulated kinase phosphorylation (pERK) through EGFR and BK2 receptor activation. Podocytes were preincubated with AG1478 (EGFR tyrosine kinase inhibitor) for 1 h then stimulated with 10−8 M BK or 1 ng/ml EGF (as positive control) for 7 min. A and B, cell lysates were immunoblotted against phosphorylated ERK (pERK) and total ERK (tERK) (A) and against phosphorylated EGFR (pEGFR) and total EGFR (tEGFR) (B). C, podocytes were preincubated for 1 h either with control buffer or 10−5 M HOE140 (BK2R antagonist) and stimulated for 7 min with BK (10−8 M) or EGF (1 ng/ml). Cell lysates were immunoblotted against pERK and tERK. Results are mean ± S.E. (n = 3). **, p < 0.01 and ***, p < 0.001 versus control; ##, p < 0.01 versus BK alone; $$, p < 0.01 versus EGF alone. Quantified pERK and pEGFR immunostainings are shown in bar graphs; tERK and tEGFR were used as loading controls.
In mature podocytes, BK stimulates Ca2+ influx through BK2Rs (Ardaillou et al., 1996; Mundel et al., 1997). We used the BK2R antagonist HOE140 to determine whether ERK activation is also mediated by the BK2R. Figure 2C shows that 1-h pretreatment with the BK2R antagonist HOE140 (10−5 M) blocked 10−8 M BK-induced ERK phosphorylation in our cells. As control, we used 1 ng/ml EGF treatment. As expected, HOE140 treatment had no effect on direct EGF-induced ERK activation.
EGFR Transactivation Is ADAM Metalloenzyme-Dependent.
Matrix metalloproteinases (MMPs) are Zn2+-dependent endopeptidases. Because MMPs, including the ADAMs, have been shown to mediate cross-talk between GPCRs and receptor tyrosine kinases by promoting the shedding of endogenous EGFR ligands (Göoz et al., 2006; Mukhin et al., 2006), we investigated the possible involvement of these enzymes in BK-induced EGFR transactivation in podocytes. We found that metalloenzyme inhibition using the broad-range MMP inhibitor GM6001 (10−5 M) completely blocked 10−8 M BK-induced ERK (Fig. 3A) and EGFR phosphorylation (Fig. 3B). At the same time, metalloenzyme inhibition did not affect EGF-induced ERK activation and EGFR phosphorylation.
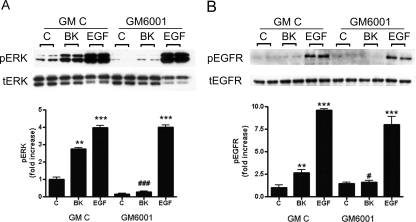
Fig. 3.
EGFR transactivation is metalloenzyme-dependent. Podocytes were preincubated for 30 min with 10−5 M GM6001 (broad-spectrum MMP inhibitor) or its inactive control compound GM C and treated for 7 min with BK (10−8 M) or EGF (1 ng/ml). Cell lysates were immunoblotted against pERK and tERK (A) and against phosphoTyr-1068 EGFR (pEGFR) and tEGFR (B). Results are mean ± S.E. (n = 3). **, p < 0.01 and ***, p < 0.001 versus GM control; #, p < 0.05 versus BK GM control; ###, p < 0.001 versus BK GM control. Quantified pERK and pEGFR immunostainings are shown in bar graphs; tERK and tEGFR were used as loading controls.
We have previously shown that ADAM17 contributes to GPCR and tyrosine kinase receptor cross-talk (Göoz et al., 2006), and ADAM17 was already identified as a potential new target in chronic kidney disease (Lautrette et al., 2005). To identify the metalloenzyme participating in BK2R-EGFR cross-talk, we transfected podocytes with siRNA (Dharmacon RNA Technologies) to down-regulate ADAM17 expression. As a negative control, we used nontargeting siRNA transfection. Figure 4A shows that ADAM17 silencing completely blocked BK-induced ERK phosphorylation but did not affect EGF-induced ERK activation (middle blot). Successful ADAM17 silencing was confirmed by Western blot (Fig. 4A, top blot) in each condition. To identify specific growth factors shed by ADAM17 in response to BK, we analyzed the concentrated supernatants of podocytes by Western blotting. We found that BK treatment significantly (∼1.6-fold) induced ER shedding of nontargeting siRNA-transfected cells (Fig. 4B). ADAM17 silencing significantly attenuated BK-induced ER shedding, indicating that ADAM17 has a significant role in BK-stimulated ER processing. The fact that we were unable to see shedding of HB-EGF, amphiregulin, or TGF-α (data not shown) could be caused by the low abundance of these proteins in the conditioned culture medium.
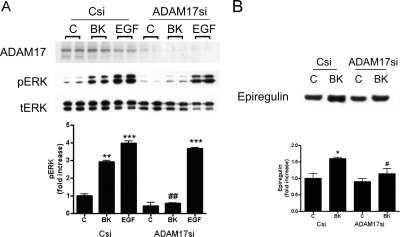
Fig. 4.
Silencing ADAM17 expression inhibits BK-induced ERK phosphorylation and shedding of the EGFR ligand epiregulin. Silencing RNAs against ADAM17 enzyme (ADAM17si) or nontargeting siRNA (Csi) were transfected into podocytes. A, Csi and ADAM17si cells were treated 48 h after transfection with BK (10−8 M) or EGF (1 ng/ml) for 7 min. The same cell lysates were immunoblotted concomitantly against ADAM17 (top), pERK (middle), and tERK (bottom). Results are presented as mean ± S.E. (n = 3). **, p < 0.01 and ***, p < 0.001 versus Csi control; ##, p < 0.01 versus Csi BK. B, Csi and ADAM17si cells were treated with 10−8 M BK for 2 h, and shedded ER was assessed by Western blotting from concentrated supernatant of the cells. Results are presented as mean ± S.E. (n = 3). *, p < 0.05 versus Csi control; #, p < 0.05 versus Csi BK. Quantified pERK, pEGFR, and ER immunostainings are shown in bar graphs; tERK and tEGFR were used as loading controls.
The BK-Induced ZO-1 Redistribution and Permeability Change Depends on EGFR Activation.
In the next set of experiments, we characterized how EGFR transactivation by BK contributes to the observed permeability changes. To test whether EGFR activity is necessary for BK-induced ZO-1 rearrangement, we pretreated subconfluent podocytes with the EGFR kinase inhibitor AG1478 before BK treatment. As shown in Fig. 5A, EGFR inhibition significantly inhibited (∼50%) BK-induced redistribution of ZO-1 immunostaining (AG1478+BK). At the same time, AG1478 did not significantly affect ZO-1 staining. We next tested whether EGFR activation has a role in BK-induced transepithelial permeability changes. Podocytes seeded on Transwell inserts were pretreated with AG1478 before being stimulated with BK. As shown in Fig. 5B, BK-induced decrease in podocyte permeability was completely blocked by AG1478. Because we identified ER as the EGFR ligand important for BK-induced EGFR activation in podocytes, we next investigated whether direct application of ER affects permeability. Using recombinant ER to test whether ER treatment is sufficient and necessary to induce a permeability change in podocytes, we found that ER (10 ng/ml) induced a significant (45%) decrease in podocyte monolayer permeability, similar to BK treatment (Fig. 5B, inset).
Fig. 5.
BK induces ZO-1 rearrangement and decreases podocyte monolayer permeability through EGFR activation. A, podocyte monolayer was pretreated for 1 h with 10−7 M AG1478 or DMSO as control and stimulated for 1 h with BK (10−8 M). ZO-1 distribution was visualized by immunostaining (green; 60×). Texas Red-X phalloidin was used to visualize actin. Quantified ZO-1 immunostaining intensity is shown in bar graphs next to the images. Results are means ± S.E. of three independent experiments. **, p < 0.05 versus DMSO-C (control); #, p < 0.05 versus DMSO-BK. B, transepithelial albumin permeability of podocyte monolayer was measured by using fluorescence BSA after cells were preincubated for 1 h with 10−7 M AG1478 (EGFR inhibitor) or DMSO control and stimulated for 1 h with 10−8 M BK. B, inset, effect of recombinant ER on the transepithelial albumin permeability of podocyte monolayer was measured after cells were preincubated with 10 ng/ml of epiregulin for 1 h. C, effect of BK2R inhibition was studied by using the BK2R inhibitor HOE140 (10−5 M) for 1 h before stimulating the cells for 1 h with 10−8 M BK. D, effect of metalloenzyme inhibition on BK-induced transepithelial albumin permeability was measured. Cells were preincubated for 30 min with 10−5 M GM6001 (broad-spectrum MMP inhibitor) or its inactive control compound GM C and treated for 1 h with 10−8 M BK. E, control silenced (Csi) and ADAM17 silenced (ADAMsi) cells were treated 48 h after transfection for 1 h with 10−8 M BK, and permeability of podocyte monolayer was measured by using fluorescence BSA. Data are means ± S.E. of three independent experiments. *, p < 0.05. NS, nonsignificant change versus untreated control.
To confirm the involvement of the BK2R in BK-induced permeability changes, podocytes on Transwell membrane were pretreated with HOE140 before being stimulated with BK. Figure 5C shows that BK2R inhibition significantly inhibited permeability decrease by BK.
Metalloenzyme Inactivation Changes Podocyte Permeability.
To test the effect of metalloenzyme/ADAM17 inhibition on podocyte monolayer permeability first we pretreated cells on Transwell membrane with the broad-spectrum MMP-inhibitor GM6001 or its control compound before treatment with BK. In subsequent experiments we treated cells transfected with ADAM17 silencing RNA or control RNA (Csi) with BK. Pretreatment with GM6001 had no significant effect on permeability, but completely inhibited BK-induced permeability changes (Fig. 5D). Similar results were obtained in cells treated with ADAM17-specific siRNA. ADAM17 silencing resulted in a modest, but significant, reduction in basal podocyte permeability in unstimulated cells and a complete loss of BK responsiveness (Fig. 5E).
Discussion
We report here for the first time that stimulation of the BK2R leads to rearrangement of the tight junction protein ZO-1 with a subsequent decrease in podocyte monolayer albumin permeability. Furthermore, we demonstrate that BK treatment induces a cascade of signals resulting in transactivation of EGFR through ADAM17 activation and ER shedding, and we provide evidence that these signaling events contribute to the observed permeability change.
Changes in podocyte permeability leading to albuminuria are often the first clinical sign of renal disease. Broadening of podocyte foot processes and decreases in the number of slit pores are often observed in diabetic nephropathy (Mifsud et al., 2001). Hypertrophy and degeneration of the filtration barrier can even lead to urinary excretion of podocytes and thus podocyte loss (Nakamura et al., 2000). Although there is evidence that podocytes can be replaced, to some extent, by stem cells (Prodromidi et al., 2006), the most important goal is to preserve podocytes and their function in the kidney glomerulus.
ACEIs are the most important therapeutic tool for preserving kidney function and delaying the progression of renal failure (Nakamura et al., 2000). Previous work has demonstrated that, in addition to changing kidney hemodynamics by lowering intracapillary hypertension, they modulate the intrarenal renin-angiotensin system, and by increasing intrarenal BK, attenuate macrophage-mediated mesangial cell scarring (Pawluczyk et al., 2004). There is increasing evidence of their effects on podocytes: ACEI treatment has been shown to preserve podocyte architecture (Mifsud et al., 2001) and prevent podocyte loss in diabetic animal models (Gross et al., 2003). Affecting the redistribution of ZO-1 seems to be one mechanism whereby ACEIs affect the progression of proteinuria (Macconi et al., 2000). In Munich Wistar Frömter rats, redistribution of ZO-1 protein in the glomerulus correlates with the age-dependent onset of proteinuria (Macconi et al., 2000). In mouse podocyte cultures ZO-1 immunostaining becomes more scattered in ANGII-treated cells (Macconi et al., 2000); ACEI preserved the distribution of ZO-1 in both models. In each case, the effect was on ZO-1 distribution, not expression; the total amount of ZO-1 did not change throughout these investigations (Macconi et al., 2000). Our observations agree with these previous studies: in our experiments BK increased ZO-1 immunostaining between neighboring cells' processes, and we did not observe changes in ZO-1 expression. Thus, ZO-1 distribution-redistribution has an important role in regulating glomerular permeability, and opposite to the previously observed effect of ANGII (Macconi et al., 2000), BK can decrease permeability by tightening cell connections between podocytes.
Paradoxically, BK is well known to increase paracellular permeability: it was shown to induce leakage in postcapillary venules of rat mesentery (Shigematsu et al., 2002) involving activation of protein kinase C and cytochrome P450 epoxygenase. In contrast, BK induced activation of endothelial nitric-oxide synthase in cardiac capillary endothelial cells through transactivation of the vascular endothelial growth factor receptor (Thuringer et al., 2002). In this cell model, EGFR kinase inhibitor had no effect on BK-induced endothelial nitric-oxide synthase production.
However, there is evidence that direct, ligand-induced activation of the EGFR and ERK can decrease permeability in epithelial cells. In Madin-Darby canine kidney epithelial cells, HB-EGF treatment increased transepithelial resistance and decreased permeability (Singh et al., 2007). Likewise, in primary canine gastric epithelial cells, EGF increased transepithelial resistance and decreased paracellular permeability of the cells (Chen et al., 2001). There is also evidence that EGF offers protection from oxidative stress-induced intestinal barrier damage (Banan et al., 2002). It is noteworthy that there are data to show that EGFR activation increases permeability. Several ErbB family inhibitors, including Herceptin, have been shown to reduce vascular permeability in a mouse model of human breast cancer (Izumi et al., 2002) and EGFR inhibition was shown to decrease vascular permeability in vivo in a lung cancer model (Wu et al., 2007). A novel finding of our studies is our evidence that EGFR activation contributes to BK-induced permeability decrease in podocytes through ADAM17-mediated ER shedding. The fact that EGFR activation can also increase permeability as shown by others in other cell models suggests that mechanisms other than EGFR activation may also be involved. The bidirectional effect of EGFR activation on permeability can be analogous to the effect of EGFR activation on proliferation and apoptosis; depending on the cellular context EGFR can induce either cell division or cell death (Jackson et al., 2003). Therefore direct activation of the EGFR by growth factors, oxidative stress or, in our case, inter-receptor cross-talk can have different effects.
ADAM17 previously was implicated in EGFR activation and permeability changes in cells other than podocytes. ADAM17 inhibition was effective in a mouse ischemia reperfusion model assessed by decreases in vascular permeability (Souza et al., 2007). Inhibiting ADAM17 impaired TNF-α mediated increases in broncho-alveolar permeability in a rat model of ischemia/reperfusion (Georgieva et al., 2007). Furthermore, oxidative stress-induced hyperpermeability was blocked by inhibitors of EGFR, ADAM17, TGF-α, and ERK1/2 in Caco-2 human colonic epithelial cells (Forsyth et al., 2007). In the present study we identified ER as a growth factor that is shed by ADAM17 upon BK treatment in podocytes, and we showed that ER is necessary and sufficient for the permeability changes seen during BK treatment. ER was already shown to participate in kidney development (Kim et al., 2007), promote proximal tubular epithelial cell migration by EGFR activation (Zhuang et al., 2007), and participate in kidney mesangial cell proliferation, as were HB-EGF and amphiregulin (Mishra et al., 2002). Cell-specific differences in ADAM17-induced permeability changes might be explained by the example of Caco-2 cells: ADAM17 cleaves TGF-α (Forsyth et al., 2007) and in our podocytes the enzyme sheds ER. We showed previously that different EGFR ligands initiate various signaling events in the same cell (Baldys et al., 2009); therefore, it is possible that different growth factors induce unique responses depending on the cell type. ADAM17-shedded growth factors or cytokines can cell-specifically activate not only the EGFR but other receptor tyrosine kinases by which they subsequently influence net cellular response. Our data showing that ADAM17 silencing decreases podocyte permeability and that in ADAM17-silenced cells BK is no longer capable of changing permeability confirm that ADAM17 has a crucial and complex role in podocyte permeability regulation and suggest that BK possibly regulates other signaling molecules besides EGFR through ADAM17. We are currently investigating these other BK-specific signaling events in podocytes.
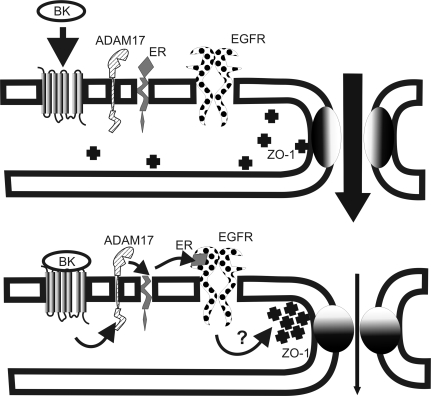
Taken together, we provide evidence in this article that BK is directly capable of decreasing podocyte permeability by rearranging ZO-1 distribution and tightening cell-cell contacts. Based on our results, we propose a model for BK-induced permeability decreases in podocytes in which the metalloenzyme ADAM17 plays a central role: BK2R engagement induces ADAM17 activation that contributes to ER shedding and subsequent activation of the EGFR, which in turn regulates permeability of the podocyte monolayer by rearranging ZO-1 protein (Fig. 6). Further investigations are needed to identify the signaling events leading from EGFR activation to ZO-1 rearrangements and learn how other slit diaphragm proteins are affected by BK.
Fig. 6.
Model of podocyte permeability regulation by BK. BK induces activation of ADAM17, which sheds the EGFR ligand ER. Activated EGFR induces downstream signaling events and ZO-1 rearrangement, which leads to decreased paracellular permeability.
However, the present study supports the hypothesis that BK can have antiproteinuric effects and provides strong evidence that the beneficial effect of ACEIs may arise from their amplification of kinin signals and inhibition of the angiotensin receptor in vivo. Furthermore, these data provide evidence of the importance of ADAM17 and EGFR in permeability regulation and identify them as potential novel therapeutic targets in diabetic nephropathy and other chronic kidney diseases.
Acknowledgments
We thank Dr. Jennifer G. Schnellmann at the Office of Scientific Editing and Publications at the Medical University of South Carolina for editing the manuscript.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants 5K01-DK070054, 3K01-DK070054-S1 (to M.G.), R01-DK055524 (to L.M.L.), R01-DK52448 (to J.R.R.)], the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM63909 ] (to J.R.R.); the Paul Teschan Research Fund of the Dialysis Clinic, Inc. (to M.G.); the Medical University of South Carolina Summer Health Professions Research Program (to D.B.S.); and Veteran Administration Research Services [Merit Award 1I0 1BX000182-01 and a Research Enhancement Award Program grant] (to J.R.R.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.168054.
- BK
- bradykinin
- BK2R
- BK B2 receptor
- ZO-1
- zonula occludens-1
- ADAM
- a disintegrin and metalloenzyme
- EGF
- epidermal growth factor
- EGFR
- EGF receptor
- pEGFR
- phosphorylated EGFR
- tEGFR
- total EGFR
- HB-EGF
- heparin-binding EGF
- ANGII
- angiotensin II
- ER
- epiregulin
- ERK
- extracellular signal-regulated kinase
- pERK
- phosphorylated ERK
- tERK
- total ERK
- FBS
- fetal bovine serum
- FITC
- fluorescein isothiocyanate
- BSA
- bovine serum albumin
- GPCR
- G protein-coupled receptor
- GM6001
- N-[(2R)-2-(hydroxamidocarbonylmethyl)-4-methylpentanoyl]-l-tryptophan methylamide
- GM negative control
- N-t-butoxycarbonyl-l-leucyl-l-tryptophan methylamide
- AG1478
- N-(3-chlorophenyl)-6,7-dimethoxy-4-quinazolinamine
- TGF-α
- transforming growth factor α
- ACE
- angiotensin-converting enzyme
- ACEI
- ACE inhibitor
- siRNA
- small interfering RNA
- MMP
- matrix metalloproteinase
- HOE140
- dArg-Arg-Pro-Hyp-Gly-Thi-Ser-dTic-Oic-Arg
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- DMSO
- dimethyl sulfoxide.
References
- Ardaillou N, Blaise V, Costenbader K, Vassitch Y, Ardaillou R. (1996) Characterization of a B2-bradykinin receptor in human glomerular podocytes. Am J Physiol Renal Physiol 271:F754–F761 [DOI] [PubMed] [Google Scholar]
- Baldys A, Göoz M, Morinelli TA, Lee MH, Raymond JR, Jr, Luttrell LM, Raymond JR., Sr (2009) Essential role of c-Cbl in amphiregulin-induced recycling and signaling of the endogenous epidermal growth factor receptor. Biochemistry 48:1462–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banan A, Fields JZ, Talmage DA, Zhang L, Keshavarzian A. (2002) PKC-ζ is required in EGF protection of microtubules and intestinal barrier integrity against oxidant injury. Am J Physiol Gastrointest Liver Physiol 282:G794–G808 [DOI] [PubMed] [Google Scholar]
- Benigni A, Tomasoni S, Gagliardini E, Zoja C, Grunkemeyer JA, Kalluri R, Remuzzi G. (2001) Blocking angiotensin II synthesis/activity preserves glomerular nephrin in rats with severe nephrosis. J Am Soc Nephrol 12:941–948 [DOI] [PubMed] [Google Scholar]
- Chen MC, Goliger J, Bunnett N, Soll AH. (2001) Apical and basolateral EGF receptors regulate gastric mucosal paracellular permeability. Am J Physiol Gastrointest Liver Physiol 280:G264–G272 [DOI] [PubMed] [Google Scholar]
- Doggrell SA. (2006) Bradykinin B2 receptors as a target in diabetic nephropathy. Curr Opin Investig Drugs 7:251–255 [PubMed] [Google Scholar]
- Forsyth CB, Banan A, Farhadi A, Fields JZ, Tang Y, Shaikh M, Zhang LJ, Engen PA, Keshavarzian A. (2007) Regulation of oxidant-induced intestinal permeability by metalloprotease-dependent epidermal growth factor receptor signaling. J Pharmacol Exp Ther 321:84–97 [DOI] [PubMed] [Google Scholar]
- Georgieva GS, Kurata S, Ikeda S, Eishi Y, Mitaka C, Imai T. (2007) Nonischemic lung injury by mediators from unilateral ischemic reperfused lung: ameliorating effect of tumor necrosis factor-α-converting enzyme inhibitor. Shock 27:84–90 [DOI] [PubMed] [Google Scholar]
- Göoz M, Göoz P, Luttrell LM, Raymond JR. (2006) 5-HT2A receptor induces ERK phosphorylation and proliferation through ADAM-17 tumor necrosis factor-α-converting enzyme (TACE) activation and heparin-bound epidermal growth factor-like growth factor (HB-EGF) shedding in mesangial cells. J Biol Chem 281:21004–21012 [DOI] [PubMed] [Google Scholar]
- Gross ML, El-Shakmak A, Szábó A, Koch A, Kuhlmann A, Münter K, Ritz E, Amann K. (2003) ACE-inhibitors but not endothelin receptor blockers prevent podocyte loss in early diabetic nephropathy. Diabetologia 46:856–868 [DOI] [PubMed] [Google Scholar]
- Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. (2002) Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature 416:279–280 [DOI] [PubMed] [Google Scholar]
- Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. (2003) Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J 22:2704–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakoki M, Takahashi N, Jennette JC, Smithies O. (2004) Diabetic nephropathy is markedly enhanced in mice lacking the bradykinin B2 receptor. Proc Natl Acad Sci USA 101:13302–13305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Kim MS, Hancock AL, Harper JC, Park JY, Poy G, Perantoni AO, Cam M, Malik K, Lee SB. (2007) Identification of novel Wilms' tumor suppressor gene target genes implicated in kidney development. J Biol Chem 282:16278–16287 [DOI] [PubMed] [Google Scholar]
- Lai XX, Ding GH, Huang CX, Shi M, Chen C. (2004) Angiotensin II-induced podocyte apoptosis: role of the MAPK subtypes. Beijing Da Xue Xue Bao 36:131–134 [PubMed] [Google Scholar]
- Langham RG, Kelly DJ, Cox AJ, Gow RM, Holthofer H, Gilbert RE. (2004) Angiotensin II-induced proteinuria and expression of the podocyte slit pore membrane protein, nephrin. Nephrol Dial Transplant 19:262–263 [DOI] [PubMed] [Google Scholar]
- Langham RG, Kelly DJ, Cox AJ, Thomson NM, Holthöfer H, Zaoui P, Pinel N, Cordonnier DJ, Gilbert RE. (2002) Proteinuria and the expression of the podocyte slit diaphragm protein, nephrin, in diabetic nephropathy: effects of angiotensin converting enzyme inhibition. Diabetologia 45:1572–1576 [DOI] [PubMed] [Google Scholar]
- Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. (2005) Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med 11:867–874 [DOI] [PubMed] [Google Scholar]
- Macconi D, Abbate M, Morigi M, Angioletti S, Mister M, Buelli S, Bonomelli M, Mundel P, Endlich K, Remuzzi A, et al. (2006) Permselective dysfunction of podocyte-podocyte contact upon angiotensin II unravels the molecular target for renoprotective intervention. Am J Pathol 168:1073–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macconi D, Ghilardi M, Bonassi ME, Mohamed EI, Abbate M, Colombi F, Remuzzi G, Remuzzi A. (2000) Effect of angiotensin-converting enzyme inhibition on glomerular basement membrane permeability and distribution of zonula occludens-1 in MWF rats. J Am Soc Nephrol 11:477–489 [DOI] [PubMed] [Google Scholar]
- Maltais I, Bachvarova M, Maheux P, Perron P, Marceau F, Bachvarov D. (2002) Bradykinin B2 receptor gene polymorphism is associated with altered urinary albumin/creatinine values in diabetic patients. Can J Physiol Pharmacol 80:323–327 [DOI] [PubMed] [Google Scholar]
- Mifsud SA, Allen TJ, Bertram JF, Hulthen UL, Kelly DJ, Cooper ME, Wilkinson-Berka JL, Gilbert RE. (2001) Podocyte foot process broadening in experimental diabetic nephropathy: amelioration with renin-angiotensin blockade. Diabetologia 44:878–882 [DOI] [PubMed] [Google Scholar]
- Mishra R, Leahy P, Simonson MS. (2002) Gene expression profiling reveals role for EGF-family ligands in mesangial cell proliferation. Am J Physiol Renal Physiol 283:F1151–F1159 [DOI] [PubMed] [Google Scholar]
- Mukhin YV, Gooz M, Raymond JR, Garnovskaya MN. (2006) Collagenase-2 and -3 mediate epidermal growth factor receptor transactivation by bradykinin B2 receptor in kidney cells. J Pharmacol Exp Ther 318:1033–1043 [DOI] [PubMed] [Google Scholar]
- Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R. (1997) Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236:248–258 [DOI] [PubMed] [Google Scholar]
- Nabokov A, Amann K, Gassmann P, Schwarz U, Orth SR, Ritz E. (1998) The renoprotective effect of angiotensin-converting enzyme inhibitors in experimental chronic renal failure is not dependent on enhanced kinin activity. Nephrol Dial Transplant 13:173–176 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Ebihara I, Koide H. (2000) Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant 15:1379–1383 [DOI] [PubMed] [Google Scholar]
- Pavenstädt H. (2000) Roles of the podocyte in glomerular function. Am J Physiol Renal Physiol 278:F173–F179 [DOI] [PubMed] [Google Scholar]
- Pawluczyk IZ, Patel SR, Harris KP. (2004) The role of bradykinin in the antifibrotic actions of perindoprilat on human mesangial cells. Kidney Int 65:1240–1251 [DOI] [PubMed] [Google Scholar]
- Prodromidi EI, Poulsom R, Jeffery R, Roufosse CA, Pollard PJ, Pusey CD, Cook HT. (2006) Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells 24:2448–2455 [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Rupérez M, Esteban V, Rodríguez-Vita J, Sánchez-López E, Carvajal G, Egido J. (2006) Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant 21:16–20 [DOI] [PubMed] [Google Scholar]
- Seccia TM, Belloni AS, Guidolin D, Sticchi D, Nussdorfer GG, Pessina AC, Rossi GP. (2006) The renal antifibrotic effects of angiotensin-converting enzyme inhibition involve bradykinin B2 receptor activation in angiotensin II-dependent hypertension. J Hypertens 24:1419–1427 [DOI] [PubMed] [Google Scholar]
- Shigematsu S, Ishida S, Gute DC, Korthuis RJ. (2002) Bradykinin-induced proinflammatory signaling mechanisms. Am J Physiol Heart Circ Physiol 283:H2676–H2686 [DOI] [PubMed] [Google Scholar]
- Singh AB, Sugimoto K, Dhawan P, Harris RC. (2007) Juxtacrine activation of EGFR regulates claudin expression and increases transepithelial resistance. Am J Physiol Cell Physiol 293:C1660–C1668 [DOI] [PubMed] [Google Scholar]
- Souza DG, Ferreira FL, Fagundes CT, Amaral FA, Vieira AT, Lisboa RA, Andrade MV, Trifilieff A, Teixeira MM. (2007) Effects of PKF242–484 and PKF241–466, novel dual inhibitors of TNF-α converting enzyme and matrix metalloproteinases, in a model of intestinal reperfusion injury in mice. Eur J Pharmacol 571:72–80 [DOI] [PubMed] [Google Scholar]
- Thuringer D, Maulon L, Frelin C. (2002) Rapid transactivation of the vascular endothelial growth factor receptor KDR/Flk-1 by the bradykinin B2 receptor contributes to endothelial nitric-oxide synthase activation in cardiac capillary endothelial cells. J Biol Chem 277:2028–2032 [DOI] [PubMed] [Google Scholar]
- Wu W, Onn A, Isobe T, Itasaka S, Langley RR, Shitani T, Shibuya K, Komaki R, Ryan AJ, Fidler IJ, et al. (2007) Targeted therapy of orthotopic human lung cancer by combined vascular endothelial growth factor and epidermal growth factor receptor signaling blockade. Mol Cancer Ther 6:471–483 [DOI] [PubMed] [Google Scholar]
- Yokota K, Kishida M, Ogura T, Suzuki J, Otsuka F, Mimura Y, Takeda M, Nakamura Y, Makino H. (2003) Role of bradykinin in renoprotective effects by angiotensin II type 1 receptor antagonist in salt-sensitive hypertension. Hypertens Res 26:265–272 [DOI] [PubMed] [Google Scholar]
- Zhuang S, Yan Y, Daubert RA, Schnellmann RG. (2007) Epiregulin promotes proliferation and migration of renal proximal tubular cells. Am J Physiol Renal Physiol 293:F219–F226 [DOI] [PubMed] [Google Scholar]
- Zychma MJ, Gumprecht J, Zukowska-Szczechowska E, Grzeszczak W. (1999) Polymorphisms in the genes encoding for human kinin receptors and the risk of end-stage renal failure: results of transmission/disequilibrium test. The End-Stage Renal Disease Study Group. J Am Soc Nephrol 10:2120–2124 [DOI] [PubMed] [Google Scholar]