Abstract
Fusion proteins made up of glucagon-like peptide 1 (GLP-1) and exendin-4 (EX-4) fused to a nonglycosylated form of human transferrin (GLP-1-Tf or EX-4-Tf) were produced and characterized. GLP-1-Tf activated the GLP-1 receptor, was resistant to inactivation by peptidases, and had a half-life of approximately 2 days, compared with 1 to 2 min for native GLP-1. GLP-1-Tf retained the acute, glucose-dependent insulin-secretory properties of native GLP-1 in diabetic animals and had a profound effect on proliferation of pancreatic β-cells. In addition, Tf and the fusion proteins did not cross the blood-brain-barrier but still reduced food intake after peripheral administration. EX-4-Tf proved to be as effective as EX-4 but had longer lived effects on blood glucose and food intake. This novel transferrin fusion technology could improve the pharmacology of various peptides.
Introduction
According to recent Centers for Disease Control and Prevention data, 20.8 million people in the United States suffer from diabetes (Centers for Disease Control and Prevention, 2005), an increase of 2.6 million from 2002. Despite the availability of several new treatments, however, more than 60% of these patients have poor control of their blood glucose (Koro et al., 2004). Hence, more effective antihyperglycemic agents are needed. Disease-modifying treatments that would protect β-cells from progressive failure would also provide an advance in the arena of diabetes treatment.
Glucagon-like peptide-1 (GLP-1) is a 30/31-amino acid peptide derived from post-translational processing of the proglucagon gene in intestinal enteroendocrine L cells (Mojsov et al., 1986). Infusion of synthetic GLP-1 to humans suffering from type 2 diabetes mediates an insulinotropic effect in a glucose-dependent manner and improves total insulin secretion. This action results in a lowering of circulating glucose levels (Zander et al., 2002; Vilsboll and Holst, 2004). In addition to its insulinotropic effects, GLP-1 exerts multiple actions throughout the body, including a neuroprotective action in the brain (Perry and Greig, 2005; Martin et al., 2009), an increase in cardiac output (Nikolaidis et al., 2005), reduction of appetite (Zander et al., 2002), and inhibition of gastric emptying (Flint et al., 2001). Continuous GLP-1 infusions also lead to increases in insulin biosynthesis, β-cell proliferation, and β-cell mass in islets of Langerhans in rodents (Perfetti et al., 2000; Stoffers et al., 2000). One pharmacokinetic impediment, however, to the creation of GLP-1-based agents has been its short circulatory half-life (t1/2) of approximately 1 to 2 min. This minimal t1/2 is due to a rapid inactivation by dipeptidyl peptidase IV (DPP-IV). DPP-IV cleaves GLP-1 between alanine and glutamic acid in the second and third positions, respectively, at the N terminus of the peptide (Kieffer et al., 1995). The remaining peptide fragment of GLP-1 made up of 28/29 amino acids is no longer active and possesses no significant insulinotropic action (Knudsen and Pridal, 1996), and it is further hydrolyzed by circulating neutral endopeptidases (Plamboeck et al., 2005). Because a single subcutaneous injection of full-length GLP-1 disappears from the circulation within minutes (Deacon et al., 1995), long-acting degradation-resistant GLP-1 receptor agonists have been or are presently under development for routine use in humans. In this regard, exendin-4 (EX-4/Byetta; Amylin Pharmaceuticals, Inc., San Diego, CA), a potent GLP-1 receptor agonist that is an endogenous product in the salivary glands of the Gila monster (Heloderma suspectum) (Eng et al., 1992), was approved for treating type 2 diabetes. This peptide possesses the insulinotropic activity of GLP-1, inhibits food intake and gastric emptying, and stimulates islet β-cell proliferation (Aziz and Anderson, 2002; Egan et al., 2002, 2003a), yet it has a different primary amino acid sequence to GLP-1 (replacement of alanine by glycine at position 2 of its N terminus as well as changes in the body and C terminus of the peptide that serve to prevent its breakdown by DPP-IV and neutral endopeptidases). These substitutions increase the t1/2 of EX-4 to 2 to 4 h (Fineman et al., 2003), allowing a reduction of injection frequency (twice daily only; Fineman et al., 2003; Kolterman et al., 2003) to achieve effective lowering of blood glucose concentrations. In addition to EX-4 (Bachem, Torrance, CA), another GLP-1 analog has been approved for the treatment of type 2 diabetes, i.e., liraglutide (Victoza; Novo Nordisk, Princeton, NJ). Liralgutide possesses an extended plasma half-life, similarly to EX-4, via a fatty acid acylation mechanism (Chapter et al., 2010; Drucker et al., 2010). Another mechanism of GLP-1 modification that has demonstrated therapeutic potential is fusion of the GLP-1 dimer with recombinant human albumin, producing albiglutide (Baggio et al., 2004). This fusion extends plasma half-life but attenuates the central nervous system actions of GLP-1. Albiglutide is currently reported to be in phase III clinical trials (St Onge and Miller, 2010).
Unlike circulating small peptidergic hormones, such as GLP-1, there are proteins that use the circulation to transport agents throughout the body that possess tremendously long half-lives. For example, Tf, a large circulatory protein that transports iron from the intestine, reticuloendothelial system, and liver parenchymal cells to proliferative tissues, possesses a t1/2 in excess of 14 to 17 days. Glycosylation of Tf reduces this half-life to 7 to 10 days (Harford et al., 1994; Li and Qian, 2002). If one could create a hybrid molecule between GLP-1 and Tf (GLP-1-Tf) or any peptide and Tf, then perhaps the shuttling and stabilizing properties of Tf could be imbued to the peptide. Therefore, in this study, we have created fusion products of a DPP-IV-resistant GLP-1 analog to nonglycosylated human Tf and expressed them in yeast before harvesting and purification. Any potentiation of the effective t1/2 of these hybrids would facilitate a reduced frequency of parenteral injection, compared with what is currently required for existing GLP-1 receptor agonists. These hybrid molecules were then tested for effective euglycemic capacity in both nondiabetic and diabetic animals.
Materials and Methods
Synthesis of Human Nonglycosylated Tf, and GLP-1/ and EX-4/Human Nonglycosylated Transferrin (GLP-1-Tf and EX-4-Tf) in Yeast.
GLP-1-Tf is a fusion protein of GLP-1 attached to the N terminus of nonglycosylated human Tf, with an intervening linker peptide of 12 amino acids (two copies of PEAPTD). The GLP-1 moiety consists the 31 amino acids of mature, human GLP-1 (amino acids 7–37) in which the second amino acid is substituted (A to G) to prevent degradation by DPP-IV. EX-4-Tf is made up of full-length EX-4 fused to the N terminus of nonglycosylated transferrin using the same linker as in GLP-1-Tf. The transferrin sequence for both fusion proteins and Tf was modified to eliminate the two N-linked glycosylation sites in the C-lobe of Tf through two amino acid changes. Additional information concerning the construction of the GLP-1-Tf entities can be found at Sadegi et al. (2006). The encoding sequences for the proteins were inserted into a high copy number 2 μm-based plasmid (Sleep et al., 1991) and expressed at high levels in Saccharomyces cerevisiae. The secreted proteins were purified from high cell density fermentations using several chromatographic steps to a purity of >99%, as judged by SDS-PAGE, size-exclusion-high-performance liquid chromatography, and N-terminal sequencing.
Cell Culture.
Chinese hamster ovary (CHO) cells were stably transfected with the GLP-1 receptor (CHO/GLP-1R cells) and cultured with 5% CO2 at 37°C in Ham's F-12 medium (Cellgro; Mediatech, Manassas, VA), as described previously (Montrose-Rafizadeh et al., 1997). Rat insulinoma cell line (RIN1046-38 cells) was obtained from Dr. Samuel A. Clark (Betagene, Dallas, TX) and cultured with 5% CO2 at 37°C in M199 medium (Cellgro; Mediatech), as described previously (Wang et al., 2001).
Pancreatic Islet Preparation.
Islets from Sprague-Dawley rats were isolated. In brief, pancreata were digested by Collagenase Type XI (Sigma-Aldrich, St. Louis, MO), and islets were separated through a Ficoll-Paque gradient (GE Healthcare, Little Chalfont, Buckinghamshire, UK). The isolated islets were washed several times using Hanks' balanced salt solution (Biosource, Antwerp, Belgium) containing 0.2% bovine serum albumin (Sigma-Aldrich), hand-picked, and cultured overnight with 5% CO2 at 37°C in M199 medium supplemented with 5 mM glucose. Multiple human islet isolation preparations (5000 islets/batch) were obtained from the National Islet Cell Resource Center (Bethesda, MD) to perform GLP-1-Tf-related experiments.
In Vitro Experiments.
cAMP generation and insulin secretion. CHO/GLP-1R cells were rinsed with Krebs-Ringer buffer (Cellgro; Mediatech) and incubated for 1 h at 37°C to lower endogenous intracellular cAMP levels. This incubation was followed by a brief incubation in Krebs-Ringer buffer containing 4 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich) to inhibit intracellular phosphodiesterases that degrade cAMP. Triplicate wells of cells were treated with serial dilutions of GLP-1-Tf, GLP-1-(7-37), EX-4-Tf, and EX-4 for 30 min at 37°C. Afterward, the supernatants were removed, and cells were lysed in 0.1 N HCl. Cell lysates were assayed to determine intracellular cAMP concentrations using a competition-based chemiluminescent enzyme immunoassay (Assay Designs, Ann Arbor, MI). RIN1046-38 cells grown on 12-well plates that had reached 50 to 60% confluence were washed in glucose-free insulin secretion buffer (Biofluids, Rockville, MD), and the cells were incubated with the serial dilutions of GLP-1 and GLP-1-Tf for 1 h in insulin secretion buffer (5 mM glucose) at 37°C. Supernatant was collected, the protein content of the cells was subsequently quantified, and the amount of insulin secreted into the buffer was normalized to protein content. Insulin secretion assays from rat (in the presence of GLP-1 and GLP-1-Tf) and human (in the presence of EX-4-Tf and EX-4) islets were performed for 1 h at 37°C in insulin secretion buffer. The supernatant was then collected and saved at −80°C for determination of insulin concentration by ELISA [Crystal Chem, Downers Grove, IL (rodent insulin for RIN and rat islets) and Alpco, Salem, NH (human insulin for human islets)].
Animals.
Male diabetic db/db mice (C57BLKS/J-Leprdb/Leprdb) lacking a functioning leptin receptor and their nondiabetic heterozygous littermates were purchased at 4 weeks of age from The Jackson Laboratory (Bar Harbor, ME). Male Sprague-Dawley rats were purchased at 6 weeks of age from Harlan (Indianapolis, IN). Male cynomolgus monkeys (Macaca fascicularis) (n = 4) received intravenous and subcutaneous boluses of GLP-1-Tf (2.25 mg/kg), and blood was taken at the times indicated in Fig. 1D. The blood was centrifuged, and serum was stored at −80°C for assay of GLP-1-Tf. All animal experiments were carried out on approved protocols in accordance with the Animal Care and Use Committee of the National Institute on Aging.
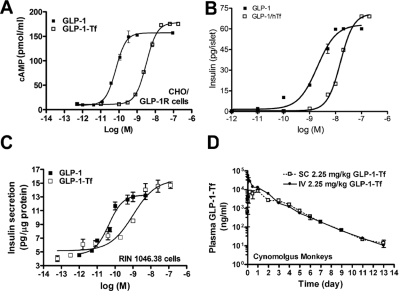
Fig. 1.
GLP-1-Tf exhibited similar efficacy but lower potency than native GLP-1, was insulinotropic, and had a prolonged half-life. A, stably transfected CHO/GLP-1R cells were treated with increasing concentrations of native GLP-1 and GLP-1-Tf for 30 min followed by assay of intracellular cAMP (n = 2 separate experiments). B, isolated rat islets (n = 40/treatment group) were treated with increasing concentrations of native GLP-1 or GLP-1-Tf for 1 h at 37°C, and the insulin secreted into the buffer was assayed (n = 4 different islet preparations). C, rat insulinoma (RIN1046-38) cells were treated with increasing concentrations of native GLP-1 and GLP-1-Tf for 1 h at 37°C, and the insulin secretion into the buffer was assayed (n = 4 separate experiments). D, cynomolgus monkeys were injected with GLP-1-Tf (2.25 mg/kg) subcutaneously and intravenously, and their serum was assayed at the time points shown (n = 4 animals).
Glucose Homeostasis Experiments and Plasma Insulin Determinations in Nondiabetic and db/db Mice.
An intraperitoneal glucose tolerance test (IPGTT) (0.5 g/kg body weight) was carried out after an overnight fast in nondiabetic mice. Tf and GLP-1-Tf were administered intraperitoneally 30 min before the glucose injection. Blood glucose levels (Glucometer Elite; Bayer Corp., Diagnostics Div., Tarrytown, NY) and plasma insulin levels were measured after the IPGTT. Tf and GLP-1-Tf were administered subcutaneously to another set of nondiabetic as well as to diabetic mice, and blood glucose was measured frequently for 48 h for prolonged effects. Plasma insulin levels were measured at 1 and 4 h. An IPGTT (0.5 g/kg body weight) also was performed after an overnight 12-h fast in diabetic mice that had received GLP-1-Tf or Tf subcutaneously at the beginning of the fast.
Food Intake and Blood Glucose Measurements after GLP-1-Tf Administration in Nondiabetic and db/db Mice.
Mice (n = 5/group) were conditioned to eat once daily (9–10 AM) for 5 days. On the morning of the sixth day, they received intraperitoneal Tf (10 mg/kg), GLP-1-Tf (0.1, 1, and 10 mg/kg), or EX-4 (1 nmol/kg), and then they were allowed to eat ad libitum. Animal food intake was measured manually, daily, by a consistent animal experimenter, and blood glucose levels were measured 2, 4, 7, and 24 or 48 h after peptide administration.
Food Intake and Blood Glucose Measurements after EX-4-Tf Administration in db/db Mice.
EX-4-Tf (0.01, 0.1, 0.3, and 1 mg/kg, respectively) and EX-4 (1 nmol/kg) were injected into 2 cohorts (n = 6/cohort) of ad libitum-fed db/db mice. In one cohort, food intake was measured 24, 48, 72, and 96 h after intraperitoneal peptide administration, and the other cohort was used for frequent blood glucose determinations after subcutaneous peptide administration.
Analysis of β-Cell Proliferation and β-Cell Area.
db/db mice, six per time point, received one intraperitoneal injection of Tf (10 mg/kg) and the bioactive peptides (10 mg/kg), and then they were euthanized 1, 2, and 3 days later for measurement of β-cell proliferation and 3, 5, 8, and 10 days later for measurement of islet area by using methods similar to those described previously (Perfetti et al., 2000).
Analysis of β-Cell Proliferation and β-Cell Area.
5-Bromo-2-deoxyuridine (BrdU, 60 mg/kg; Sigma-Aldrich) was injected intraperitoneally 6 h before euthanasia. Pancreata were removed, fixed overnight in 4% buffered formalin, and processed for embedding in paraffin; histological sections (4 μm) were mounted on poly-l-lysine-coated glass slides. BrdU was detected with a BrdU antibody (Sigma-Aldrich), 1:500) and BrdU staining kit (Zymed Laboratories, South San Francisco, CA). The number of islets that contained BrdU+ cells as well as the percentage of BrdU+ nuclei per total number of islet cells was quantified. For insulin staining and determination of β-cell area, histological sections were treated with a guinea pig insulin antibody (Linco, St. Charles, MO). Antibody binding was visualized with 3,3-diaminobenzidine, and sections were counterstained with hematoxylin (Vector Laboratories, Burlingame, CA). Sectioned tissue images were acquired using a phase contrast light microscope (Carl Zeiss GmbH, Jena, Germany) and digitized by means of a Sony Power HAD digital camera (20–30 images/section). Total pancreatic area and β-cell-positive area for every image were quantified using MetaMorph 4.6.3 software (Molecular Devices, Sunnyvale, CA). In another set of experiments, insulin and BrdU costaining was carried out with both insulin antibody (1:500; Linco) and monoclonal anti-BrdU antibody (1:500; Sigma-Aldrich). After the antibodies were applied to the slides and incubated at 4°C overnight, the slides were washed with phosphate-buffered saline (PBS). Secondary antibodies were then applied (1:200), and the slides were incubated for 2 h at 37°C. Next, the slides were washed with PBS and mounted. The immunofluorescence staining was observed using an LSM-410 confocal microscope (Carl Zeiss GmbH).
GLP-1-Tf ELISA.
A sandwich ELISA was developed to measure the concentration of GLP-1-Tf in body fluids. GLP-1 monoclonal antibody (200 ng; Antibody Shop, Gentofte, Denmark) was immobilized on microtiter plates coated with anti-mouse IgG. After washing, sample or standard was added to the plate that was then incubated at 37°C overnight. The plate was next washed and incubated with biotinylated chicken anti-human transferrin antibody (Fitzgerald Laboratories, Niagara Falls, NY). Washing was repeated, and the plate was incubated with horseradish-peroxidase (HRP)-streptavidin. The bound HRP was measured with Quanta Blu Fluorogenic Substrate (Pierce Chemical, Rockford, IL). Measurements were performed on a SpectraMax Gemini fluorescence plate reader and SoftMax Pro software (Molecular Devices). Buffer used for all steps was PBS containing 1% bovine serum albumin and 0.05% Tween 20.
Determination of Cerebrospinal Fluid Levels of hTf and GLP-1-Tf.
Sprague-Dawley rats were injected intraperitoneally with hTf and GLP-1-Tf. Animals were euthanized 2 and 24 h later for analysis of the presence of the proteins in plasma, cerebrospinal fluid (CSF), and brain. CSF was removed by insertion of a 30-gauge needle with syringe attached between the atlas and axis, and the CSF was gently aspirated. CSF used for analysis was without blood contamination. Rats were used in this experiment to obtain a sufficient quantity of CSF. The brains were removed, sonicated in SDS lysis buffer, and the homogenate was stored at −80°C for later use in Western blot analysis.
Western Blot Analysis of Plasma, CSF, and Brain Homogenates.
Plasma samples (0.1 μl) or 5 μl of CSF samples and brain homogenates were mixed with 10 μl of SDS-PAGE sample buffer and were subjected to 10% SDS-PAGE (Novex, San Diego, CA) according to the supplier's protocol. The proteins were transferred onto polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, CA) in 25 mM Tris + 192 mM glycine. The filter paper was equilibrated with 25 mM borate buffer (pH 8.0, + 150 mM NaCl + 5% nonfat dry milk). Rabbit anti-Tf (Rockland Immunochemicals, Gilbertsville, PA) was added to the blot, and then the blot was incubated overnight at 4°C. After extensive washing, HRP-conjugated goat anti-rabbit IgG (Pierce Chemical) was used as a secondary antibody. The bound antibodies to Tf and GLP-1-Tf bands were detected by SuperSignal West Pico chemiluminescent detection kit (Pierce Chemical).
Measurement of c-Fos Activation in Mouse Brain.
In brief, mice (n = 6/group) received intraperitoneal injections (100 μl) of either 1) PBS, 2) EX-4 (1 nmol/kg), 3) GLP-1-Tf (0.1, 1, and 10 mg/kg), or 4) Tf (10 mg/kg). Another three treatment groups were anesthetized by isoflurane inhalation and placed in a stereotaxic head-holder. The skull was exposed along the midline and 2 μl of the following treatments was injected unilaterally (intracerebroventricularly) into the lateral ventricle: 1) PBS, 2) 7.2 mg/ml GLP-1-Tf, or 3) 7 mg/ml Tf. Mice that received intraperitoneal injections were anesthetized with isoflurane 2 h later, and the mice that received intracerebroventricular injections were anesthetized 1.5 or 24 h later. Intracardiac perfusion with ice-cold 4% paraformaldehyde was immediately carried out. Brains were removed, stored in 4% paraformaldehyde for 72 h, and then transferred to a solution containing 4% paraformaldehyde and 25% sucrose for 12 h. Each brain was cut into 30-μm sections using a sliding microtome with a freezing stage. Brain sections from each animal were placed in a 12-well plate and then processed for immunocytochemistry. The free-floating sections from each animal were first incubated in 0.3% H2O2 for 30 min and then washed in PBS. Thereafter, the sections were incubated in blocking serum for 45 min at room temperature, followed by staining for c-Fos with a rabbit anti-human c-Fos antibody directed against the amino acid residues 4 to 17 (1:30,000; Calbiochem, San Diego, CA) for 48 h at 4°C. The sections were then washed with PBS and 0.1% Triton before incubation for 1.5 h at room temperature with biotinylated goat anti-rabbit IgG (1:600) in blocking serum (Vector Laboratories). Subsequently, the sections were visualized with 3,3-diaminobenzidine. Sections were mounted on Superfrost slides (Thermo Fisher Scientific, Waltham, MA), air-dried for 1 to 2 days, and coverslipped with Permount. Brain sections were analyzed using an BX51 microscope (Olympus, Melville, NY) to identify c-Fos-positive cells. The number of c-Fos-immunopositive cells was counted by using a constant area and scale grid position under a 10× objective. The position of the counting grid within each brain region was delineated through adjacent landmarks. Both sides of the brain at one representative level of each region were quantified.
Statistical Methods.
All results are given as means ± S.E. Student's t test was based on the results of the F-test that assessed the equality of variance of the two means. If the variances were statistically significantly different, then the t test was based on unequal variances. An analysis of variance test was used to calculate the significance of difference among multiple samples, followed by post hoc testing with Scheffé's test. P values <0.05 were considered statistically significant.
Results
GLP-1-Tf Activated the GLP-1 Receptor In Vitro and Was Absorbed When Given Subcutaneously.
The GLP-1 receptor (GLP-1R) is a membrane-associated G protein-coupled receptor, and upon ligand binding, adenylyl cyclase is activated, resulting in a concentration-dependent elevation in intracellular cAMP levels. We first examined whether GLP-1-Tf activated adenylyl cyclase in GLP-1R-transfected CHO cells (CHO/GLP-1R). It produced a dose-dependent increase in intracellular cAMP levels, with a similar (albeit slightly higher) maximal efficacy but reduced potency compared with native GLP-1 (GLP-1-Tf versus GLP-1: Emax of 157 versus 177 pmol/ml; EC50 of 2.28 versus 0.16 nM; Fig. 1A). We next determined that GLP-1-Tf stimulated insulin secretion from isolated rat islets and rat insulinoma cells in a concentration-dependent manner, with a lower potency than native GLP-1 (Fig. 1, B and C). After subcutaneous and intravenous administration of GLP-1-Tf (2.25 mg/kg) to cynomolgus monkeys, the concentration of GLP-1-Tf in serum was measured by a specific sandwich ELISA. The t1/2 of GLP-1-Tf, after both routes of administration, was approximately 44 h. Standard pharmacokinetic parameters [Cmax, Tmax and area under the curve (AUC)] recorded from the primate dosing are indicated in Table 1. Calculation of a classical subcutaneous versus intravenous bioavailability [(AUCSC/AUCIV) × (doseSC/doseIV) × 100] demonstrated a very high subcutaneous functional bioavailability for GLP-1-Tf, i.e., 82.83% [(1,630,070.35/1365895.06) × (2.25/2.25) × 100; Fig. 1D)]. By treating CHO/GLP-1R with the serum from the injected monkeys, we determined that the GLP-1-Tf measured by ELISA was biologically active and that the slope of decay of intracellular cAMP accumulation in response to the serum over time was similar to that of GLP-1-Tf in the ELISA (for further information, see Sadegi et al., 2006). The t1/2 in mice was determined to be 14 h. The antisera used in this ELISA were able to distinguish an amino-terminal epitope of GLP-1 indicative of its active form. The specific bioassay data are represented in the specific patent documentation (Sadegi et al., 2006). Based on these findings, a series of experiments in diabetic and nondiabetic mice were initiated to determine the duration of biological action, efficacy for controlling blood glucose in the diabetic state, effects on islet morphology, and central nervous system-activating properties of GLP-1-Tf.
TABLE 1.
Pharmacokinetic data of GLP-1-Tf dosing of cynomolgus monkeys (M. fascicularis)
The same bolus dose of GLP-1-Tf (2.25 mg/kg) was administered to the monkeys either via a subcutaneous or intravenous route. Plasma concentrations of GLP-1-Tf were assessed (by ELISA) at the time points after injection as depicted in Fig. 1D.
| GLP-1-Tf Route | Cmax | Tmax | AUC |
|---|---|---|---|
| ng/ml | h | h × ng/ml | |
| Intravenous | 634244 | 0.033 | 1649034.24 |
| Subcutaneous | 87212 | 0.4 | 1365895.06 |
GLP-1-Tf Lowered Blood Glucose and Increased Insulin Secretion in Nondiabetic Mice.
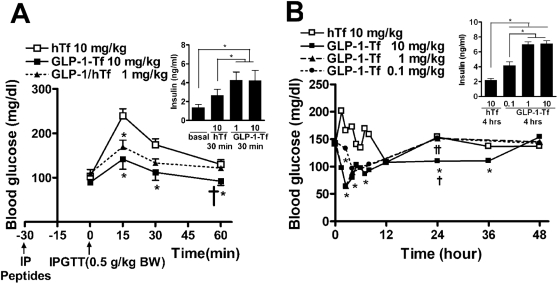
To determine whether GLP-1-Tf could activate GLP-1 receptors in vivo, the effect of the fusion protein on insulin secretion was evaluated. Intraperitoneal GLP-1-Tf (1 and 10 mg/kg) resulted in enhanced insulin secretion in nondiabetic mice in response to intraperitoneally injected glucose (IPGTT; 0.5 g/kg glucose). The peak blood glucose was decreased, coinciding with elevated insulin levels at 30 min (Fig. 2A). In addition, subcutaneous GLP-1-Tf dose-dependently reduced blood glucose concentration and increased insulin secretion in ad libitum-fed mice (Fig. 2B). In this experiment, blood glucose levels dropped to 70 mg/dl within 3 h of administration and gradually rose to the preadministration levels within 24 h. The lowered blood glucose persisted for up to 36 h with the higher dose (10 mg/kg).
Fig. 2.
GLP-1-Tf decreased peak glucose levels and increased insulin secretion after an IPGTT (0.5 g glucose/kg) and had a prolonged biological action in nondiabetic mice. A, blood glucose and plasma insulin levels after an IPGTT after an overnight fast. GLP-1-Tf and Tf were given intraperitoneally at the time indicated (30 min). B, blood glucose and plasma insulin levels in ad libitum-fed mice. GLP-1-Tf and Tf were given subcutaneously at time 0. *, p < 0.05, compared with hTf-treated animals alone; †, p < 0.05, compared with GLP-1-Tf (1 mg/kg)-treated animals; and ††, p < 0.05, compared with GLP-1-Tf (0.1 mg/kg)-treated animals, n = 4 to 6 animals per treatment group.
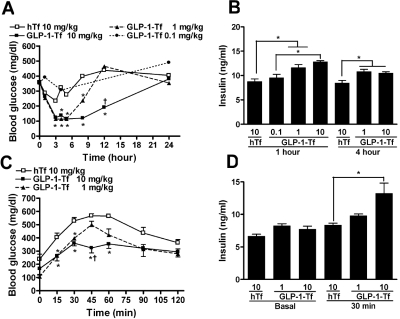
GLP-1-Tf Lowered Blood Glucose and Increased Insulin Secretion in Diabetic Mice.
Diabetic db/db (C57BLKS/J-Leprdb/Leprdb) mice are homozygous for a mutated nonfunctioning leptin receptor; consequently, they are hyperphagic; eat throughout the 24-h day (instead of the more usual feeding during the dark hours); become obese; and by 4 to 6 weeks of age, they have elevated blood glucose levels in the 300- to 400-mg/dl range (normal fasting blood glucose levels are 90–120 mg/dl). Their heterozygous littermates (nondiabetic mice) are not hyperphagic, lean, and do not develop diabetes. Both 1 and 10 mg/kg subcutaneous GLP-1-Tf lowered blood glucose to normal levels (from 358 ± 23 to 115 ± 18 mg/dl) in male ad libitum-fed db/db mice, with the effect beginning as early as 1 h after injection (Fig. 3A). Increased plasma insulin levels were present for at least 4 h after both 1 and 10 mg/kg GLP-1-Tf (Fig. 3B), and the highest dose sustained the effect on blood glucose, without causing hypoglycemia, for at least 12 h. A low dose of GLP-1-Tf (0.1 mg/kg) caused an acute, unsustained effect of lowering blood glucose for 4 h after administration, concomitant with increased plasma insulin levels at 1 h (Fig. 3, A and B). Next, db/db mice were given subcutaneous Tf or GLP-1-Tf at 9:00 PM, and fasted overnight, and an IPGTT was performed the next morning. As expected, the fasted Tf-treated animals had lower blood glucose than ad libitum-fed animals [238 ± 39 versus 358 ± 23 mg/dl; compare zero time of fasted animals (Fig. 3C) with zero time of fed animals (Fig. 3A)]. In addition, the animals given 1 or 10 mg of GLP-1-Tf had lower fasting blood glucose (132 ± 27 mg/dl) than those given Tf alone. After the IPGTT, the animals given GLP-1-Tf had lower blood glucose levels and higher plasma insulin levels, compared with hTf-treated animals (Fig. 3, C and D).
Fig. 3.
GLP-1-Tf had prolonged biological activity, normalized blood glucose, and increased insulin release in ad libitum-fed db/db mice after IPGTT (0.5 g glucose/kg). A and B, blood glucose and plasma insulin levels after subcutaneous administration at time 0 of GLP-1-Tf and Tf. C and D, blood glucose and plasma insulin levels after IPGTT in overnight-fasted mice that had received GLP-1-Tf and hTf 12 h before testing. *, p < 0.05, compared with Tf-treated animals alone; †, p < 0.05, compared with GLP-1-Tf (1 mg/kg)-treated animals. n = 6 animals per treatment group.
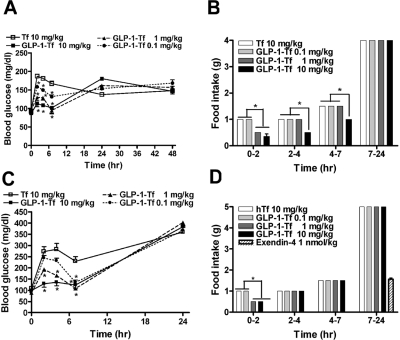
GLP-1-Tf Had Short-Lived Anorectic Properties.
In ad libitum-fed db/db mice, GLP-1-Tf, at concentrations that lowered blood glucose, did not decrease food intake when measured over 24 h. To assess anorectic effects of GLP-1-Tf more closely, 6-week-old nondiabetic and db/db mice were conditioned to feed once daily for 1 h from 9:00 AM to10:00 AM for 5 days. This prevented hyperphagia-related obesity and maintained normal fasting glucose levels in db/db animals (99 ± 9 mg/dl). During the feeding hour, both db/db and nondiabetic animals ate approximately 0.5 g of food. Two animals were euthanized on the morning of the sixth day, and no food was present in their stomachs or small bowels. Also on the sixth day, the remaining animals were administered GLP-1-Tf (0.1, 1, and 10 mg/kg), Tf (10 mg/kg), or EX-4 (1 nmol/kg to the db/db mice only) before having free access to food. Food intake was quantified, and blood glucose levels (to measure postprandial glucose elevations) were measured at intervals for the following 48 h. Food intake was decreased by approximately half in nondiabetic animals for up to 7 h after GLP-1-Tf (10 mg/kg) and for 2 h by GLP-1-Tf (1 mg/kg). Postprandial blood glucose levels were lower, in a concentration-dependent manner, with all three doses, compared with animals that received Tf only, reflecting both decreased food intake (Fig. 4, A and B) and increased insulin secretion (Fig. 3B). In the time segment 7 to 24 h after injection, GLP-1-Tf-treated animals had similar food intake to Tf-treated animals. In db/db animals, food intake was also decreased by approximately half for 2 h by GLP-1-Tf (1 and 10 mg/kg); and all three GLP-1-Tf doses, in a concentration-dependent manner, simultaneously lowered postprandial blood glucose levels, compared with Tf treatment (Fig. 4, C and D). EX-4 prevented all measurable food consumption for 7 h and caused a decrease in food intake for the remaining 17 h (Fig. 4D). We have shown previously that EX-4 (1 nmol/kg daily) causes a decrease in food intake for up to 4 days in this mouse model of diabetes (Greig et al., 1999).
Fig. 4.
Food intake was acutely decreased in nondiabetic and db/db mice that had been previously conditioned for 5 days to eat for 1 h daily, and postprandial glucose levels were blunted by GLP-1-Tf. A and B, blood glucose and food intake in nondiabetic mice measured during the time intervals shown after IP GLP-1-Tf and Tf at time 0. C and D, blood glucose and food intake in db/db mice measured during the time intervals shown after intraperitoneal GLP-1-Tf, Tf, and EX-4 at time 0. Values are expressed as means ± S.E., n = 5 animals per treatment group. *, p < 0.05, compared with hTf-treated animals alone.
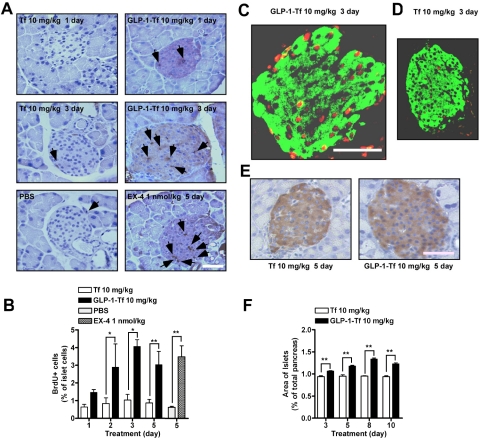
GLP-1-Tf Increased β-Cell Proliferation and Increased β-Cell Area.
db/db mice (n = 6/group) were given IP GLP-1-Tf (10 mg/kg), Tf (10 mg/kg), and BrdU (60 mg/kg; 6 h before euthanasia) to investigate cell turnover in islets. The GLP-1-Tf-treated animals had an increase in the number of BrdU+ nuclei in islets as early as 24 h after GLP-1-Tf injection, compared with Tf treatment (Fig. 5A). After 2 days of GLP-1-Tf treatment, there was a statistically significant increase in the number of BrdU+ nuclei (days 2 and 3, p < 0.05; day 5, p < 0.01; Fig. 5B). The majority of islets observed in GLP-1-Tf-treated animals had at least one or more BrdU+ nuclei (data not shown), whereas the Tf-only-treated animals failed to demonstrate any distinct increase in BrdU+ nuclei compared with vehicle-treated (PBS) animals (Fig. 5, A, C, and D). As shown in Fig. 5, A and D, it was common to note a single BrdU+ nuclei within an islet in Tf-treated animals, which probably represented a background rate of turnover. In contrast, with GLP-1-Tf treatment, a profound increase in the number of BrdU+ nuclei was seen, accounting for as much as 10% of the total islet cell nuclei (Fig. 5C). The BrdU+ nuclei were mainly in insulin-containing β cells (Fig. 5, C and D). After 3 days of treatment with GLP-1-Tf (10 mg/kg), total islet area was 1.0 ± 0.003%, compared with 0.94 ± 0.02% with Tf treatment; and by 5, 8, and 10 days, total islet area was increased even further (1.18 ± 0.017, 1.34 ± 0.02, and 1.23 ± 0.02%, respectively), with GLP-1-Tf (Fig. 5, E and F). The increase in BrdU incorporation was comparable with the increase in BrdU incorporation observed with EX-4 (1 nmol/kg daily; Fig. 5B).
Fig. 5.
Morphological and histological analysis of pancreatic islets from db/db mice after one intraperitoneal of GLP-1-Tf and Tf. A, representative sections from pancreata of mice treated with intraperitoneal GLP-1-Tf, Tf, EX-4, and PBS. Cell mitosis was measured by counting BrdU+ nuclei (brown in A, indicated by black arrows) per islet, BrdU having been injected 6 h before sacrifice. B, quantification of the number of BrdU+ cells from A. Values are expressed as means ± S.E. C and D, representative costaining for BrdU+ (red) and insulin+ (green) after one intraperitoneal dose of GLP-1-Tf- and Tf-treated mice. BrdU+ nuclei were almost always in insulin+ cells. Single staining for insulin (E) and quantification of total β-cell area as a percentage of the total pancreatic area (F). n = 5 to 6 animals per treatment group. F, total β-cell area values. *, p < 0.05; and **, p < 0.01. Magnification bar, 100 μm.
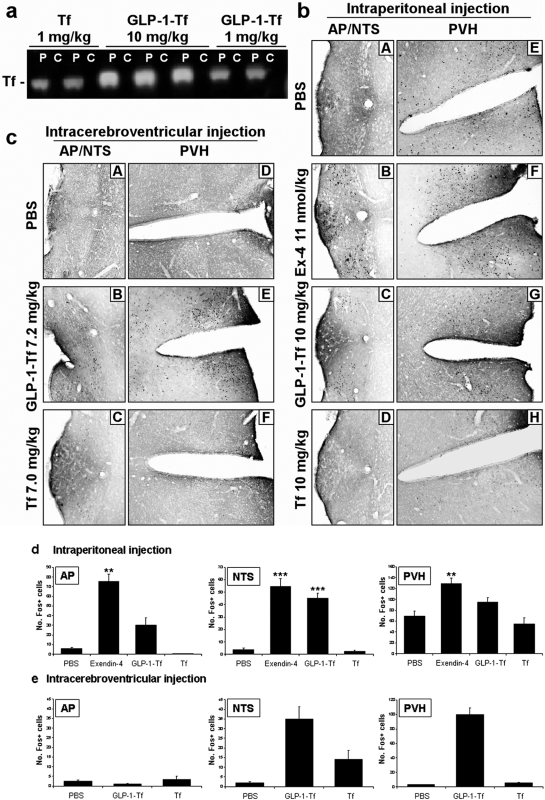
GLP-1-Tf-Mediated Central c-Fos Activation after Peripheral Administration Occurs in Conjunction with Minimal Cerebrospinal Fluid Detection.
Rats were administered intraperitoneal GLP-1-Tf and Tf. Plasma and CSF were obtained 2 and 24 h after injection, and brains were removed and homogenized. The presence of functional levels of GLP-1-Tf in the CSF could not be detected by ELISA after intraperitoneal administration at 2 or 24 h. Western blotting of plasma with human-specific anti-Tf antibodies demonstrated the presence of the expected 77-kDa band for Tf and the 79-kDa band for GLP-1-Tf, representing the full-length size; no other fragments were seen (either for Tf or GLP-1-Tf), and a concentration dependence was evident (Fig. 6A). Because our anti-Tf sera were human-specific, no human Tf was detected in plasma in the absence of Tf or GLP-1-Tf treatment (data not shown). In agreement with the ELISA, no immunoreactive Tf was seen in CSF by Western blot analysis. In addition, we incubated CHO/GLP-1R cells with CSF taken after 2 h from the GLP-1-Tf-treated rats, and no increase in intracellular cAMP occurred. Finally, homogenized brain slices from the treated animals did not contain Tf as assessed by Western blot analysis. Together, these data suggested that a profound blood-brain barrier penetrance by the GLP-1-Tf did not occur during our experimental paradigms. To determine whether peripherally administered GLP-1 analogs were capable of activating the central nervous system, we examined the pattern of c-Fos expression in the brain. We compared the central effects of intraperitoneally administered GLP-1-Tf (0.1, 1, and 10 mg/kg) to those of EX-4 (1 nmol/kg–4 μg/kg), which is known to activate specific brain areas after intraperitoneal injection (Baggio et al., 2004). As a control for our hybrid GLP-1 compound, we administered intraperitoneal Tf (10 mg/kg). In addition, we also administered intracerebroventricular Tf and GLP-1-Tf in another series of experiments and measured c-Fos immunoreactivity 1.5 and 24 h later. Peripherally administered intraperitoneal GLP-1-Tf (10 mg/kg) activated c-Fos expression in neurons of the area postrema (AP) (four of six animals), the nuclei of the solitary tract (NTS) (six of six animals), and the paraventricular nuclei of the hypothalamus (PVH) (six of six animals) 1.5 h after administration (Fig. 6, B and D). A lower dose of GLP-1-Tf (1 and 0.1 mg/kg) activated the NTS (six of six animals) and PVH (six of six animals) but not the AP region (zero of six animals) (data not shown). EX-4 activated similar areas to GLP-1-Tf in all injected animals, but the AP response was much more robust compared with GLP-1-Tf. Intraperitoneal injection of Tf (10 mg/kg) had no significant effect on c-Fos immunoreactivity in the AP, NTS, and PVH regions (Fig. 6B). One and a half hours after intracerebroventricular injection of GLP-1-Tf (7.2 mg/kg), c-Fos activation occurred in the PVH and NTS regions but not in the AP region (Fig. 6, C and E). c-Fos activation was not present in any of the three brain regions 24 h after intracerebroventricular injection (data not shown). Intracerebroventricular injection of Tf (7 mg/kg) had no significant effect on c-Fos immunoreactivity in the AP, NTS, and PVH regions (Fig. 6, C and E). Therefore, from our current data, it seems likely that despite demonstrating a minimal physical penetrance into the central nervous system, GLP-1-Tf could still engender the activation of c-Fos expression in the PVH and NTS. It is almost impossible to completely attribute these central effects of GLP-1-Tf to a peripheral-to-central signaling process, because even negligible levels of neuropeptides in the central nervous system can cause alterations in protein expression. Our data suggest, however, that with nearly undetectable levels of GLP-1-Tf blood-brain barrier penetrance, functional central responses to this ligand are effectively created. The relative contribution of peripheral and central actions may take many subsequent years of experimentation to resolve.
Fig. 6.
Peripherally administered GLP-1-Tf activated c-Fos in certain brain areas but was not present in CSF. a, Western blot analysis for Tf protein levels in plasma and CSF 2 h after IP GLP-1-Tf and Tf administration to rats. P, plasma; C, CSF. The number of c-Fos-immunopositive (Fos+) cells was counted in each field of view. b and d, intraperitoneal administration of EX-4 (1 nmol/kg) significantly increased c-Fos immunoreactivity in the AP, NTS, and PVH regions. However, intraperitoneal administration of GLP-1-Tf (10 mg/kg) caused a significant elevation of immunoreactivity in the NTS only. There was no significant increase in c-Fos immunoreactivity in the AP, NTS, and PVH regions after intraperitoneal administration of Tf (10 mg/kg). c and e, intracerebroventricular injection of GLP-1-Tf (7.2 mg/kg) caused a significant increase in c-Fos immunoreactivity in the NTS and PVH regions but not in the AP region. Intracerebroventricular administration of the Tf compound on its own (7 mg/kg) caused no significant changes in c-Fos immunopositivity in the AP, NTS, and PVH regions. n = 6 animals per treatment group. ***, p < 0.001 and **, p < 0.01.
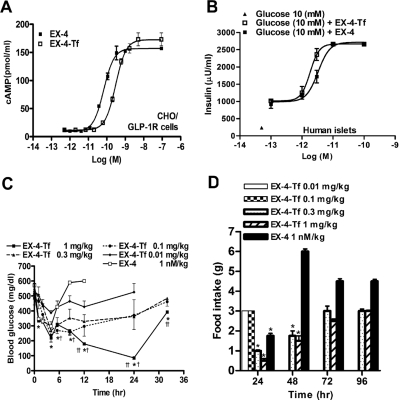
EX-4-Tf Was More Potent than GLP-1-Tf and Had Long-Lasting Effects on Food Intake.
Because EX-4 is already in clinical use and is reported to have significant effects on food intake and weight loss (Szayna et al., 2000), we next compared the activity of EX-4 to EX-4-Tf. In CHO/GLP-1R cells, EX-4-Tf produced a dose-dependent increase in intracellular cAMP levels. Unlike GLP-1-Tf compared with GLP-1, EX-4-Tf only demonstrated a slightly reduced potency compared with its non-Tf-conjugated form [EC50 of 0.06 nM (EX-4), 0.16 nM (GLP-1), and 0.3 nM (EX-4-Tf)]. However, in a similar manner to GLP1-Tf and GLP-1, EX-4-Tf again demonstrated a slight increase in maximal efficacy compared with EX-4 [Emax of 155 pmol/ml (EX-4), 157 pmol/ml (GLP-1), and 173 pmol/ml (EX-4-Tf)] (Figs. 1A and 7A). EX-4-Tf also stimulated insulin secretion from isolated human islets in a concentration-dependent manner and with similar potency to EX-4 (EC50 of 0.03 versus 0.02 nM for EX-4 versus EX-4-Tf) (Fig. 7B). EX-4-Tf (1 mg/kg) normalized blood glucose for at least 24 h in db/db mice, even in mice whose basal blood glucose was as high as 500 mg/dl. In addition, the compound was 10- to 20-fold more potent, and its dose-lowering effect was longer lasting than GLP-1-Tf (Figs. 3A and 7C): injection of EX-4 reduced blood glucose for just 8.5 h. Food intake was also significantly decreased by EX-4-Tf (0.3 and 1 mg/kg) for at least 48 h in ad libitum-fed db/db mice, again showing its effects to be longer lived than GLP-1-Tf and EX-4 (Fig. 7D).
Fig. 7.
Biological effects of EX-4-Tf on CHO/GLP-1R cells, human islets, and db/db mice. A, CHO/GLP-1R cells were treated with increasing concentrations of native EX-4 and EX-4-Tf for 30 min followed by assay of intracellular cAMP (n = 3 separate experiments). B, isolated human islets (n = 1000 per treatment group, one pancreatic isolation) were treated in triplicate with increasing concentrations of native EX-4 and EX-4-Tf for 1 h at 37°C, and the insulin secreted into the medium was assayed. C, blood glucose levels after subcutaneous administration at time 0 of EX-Tf and EX-4 in ad libitum-fed db/db mice. *, p < 0.05, compared with EX-4-treated animals; †, p < 0.05, compared with EX-4-Tf (0.01 mg/kg)-treated animals. n = 4 to 6 animals per treatment group. D, food intake in ad libitum-fed db/db mice was measured during the time intervals shown after IP EX-4-Tf and EX-4 at time 0. n = 5 animals per treatment group. *, p < 0.05, compared with EX-4-Tf (0.01 mg/kg-treated animals.
Discussion
Our data indicate that GLP-1-Tf and EX-4-Tf retain the pharmacological effects of native peptides, while extending their length of action. This work also illustrates an example of transferrin fusion technology using peptides with high therapeutic potential but poor practical utility, demonstrating the potential of this technology as a method of producing biopharmaceuticals with patient-friendly pharmacology. EX-4-Tf was a more potent hypoglycemic agent than GLP-1-Tf, and fusion of EX-4 to Tf had minimal deleterious effects on its biological activity.
The fused compounds also retained the insulinotropic properties of GLP-1 receptor agonists, and the robust and prolonged response to just one injection of GLP-1-Tf (10 mg/kg) exceeded what we and others have experienced with native GLP-1 and EX-4 (Perfetti et al., 2000; Stoffers et al., 2000; Egan et al., 2003b; Park et al., 2005). In an occasional islet of GLP-1-Tf-treated animals, up to 10% of its β cells showed evidence of mitosis 3 days after injection. In type 2 diabetes, β-cell area in postmortem pancreata is decreased compared with pancreata from subjects matched for obesity, but without diabetes (Butler et al., 2003). In addition, with increasing duration of diabetes, β-cell function continues to deteriorate (Clark et al., 2001; Butler et al., 2003), presumably because β-cell numbers decrease. Thus, transferrin fusion technology offers a promising therapeutic avenue for the treatment of type 2 diabetes.
GLP-1-Tf and EX-4-Tf also could potentially be used for the treatment of type 1 diabetes. If introduced to newly diagnosed patients who still have sufficient β-cells remaining, preservation and expansion of β-cell area may occur in conjunction with agents now under investigation for preventing the inexorable autoimmune β-cell destruction (Ogawa et al., 2004). Because injection of a fusion protein might be as infrequent as weekly, ease of use and lack of detrimental side effects in this vulnerable population would be added bonuses.
With respect to the therapeutic extension of serum half-life of biologically active peptides such as GLP-1, many diverse chemical strategies have been attempted (Lovshin and Drucker, 2009; Chapter et al., 2010). As a result of these multiple strategies, several modified GLP-1 compounds have recently demonstrated clinical promise. For example, liraglutide contains a S34R amino acid substitution and has a C16 palmitoyl fatty acid side chain at Lys26. In a controlled trial, once-daily injection seemed to be well tolerated and effective in treating type 2 diabetes (Seino et al., 2008). Lixisenatide (Christensen et al., 2009) is a modified exenatide molecule with six additional lysine residues in the carboxyl terminal; again, once-daily injections seemed to be specifically required for adequate antidiabetic efficacy (http://clinicaltrials.gov/ct2/show/NCT00975286). Additional compounds that have been developed with extended serum stability include albiglutide (two tandem copies of a modified GLP-1 in serum albumin); taspoglutide (a GLP-1-based molecule containing amino-isobutyric acid substitutions at positions 8 and 35); and exenatide-LAR, i.e., exenatide encapsulated in biodegradable polymer (d,l-lactic-coglycolic acid) microspheres. Other long-acting GLP-1 receptor agonists include CJC1134 (ConjuChem, Inc., Montreal, QC, Canada), a protein that contains an exenatide moiety covalently linked to human albumin through a chemical linkage (Baggio et al., 2008), and LY2428757, a pegylated GLP-1 molecule: long-term therapeutic efficacy of these promising molecules however is not yet fully known. In addition to their euglycemic activities, it is interesting to note that GLP-1-Tf analogs also have recently been shown to possess therapeutic benefit in cardiovascular disorders, because GLP-1-Tf can attenuate myocardial reperfusion injury (Matsubara et al., 2009).
In our experimental paradigms GLP-1-Tf treatment was associated with a significant, although short-lived decrease in food intake in animals that had not eaten for the proceeding 24 h (even in db/db animals, which lack functioning leptin receptors) and had been conditioned to eat for a specific time interval. EX-4 is a more potent and long-lasting inhibitor of food intake than native GLP-1, and this effect was again demonstrated in this report. However, EX-4-Tf inhibited food intake over a far longer timeframe than EX-4 even in db/db animals given free access to food. In free-feeding mice, one EX-4-Tf (0.3 and 1 mg/ml) injection had highly significant effects on food intake for 48 h. This effect was probably due to stimulation of enteric vagal afferents that terminate in the NTS, and neurons from the NTS that then project to satiety centers in the hypothalamus, in addition to effects on gastric emptying (Hornby, 2001). It is unlikely that the fusion proteins directly activated satiety centers; all evidence suggests that peripherally administered fusion proteins do not cross the blood-brain barrier because they could not be detected in brain tissue or in CSF by several assays, including a biological assay. This is unlike EX-4 and native GLP-1, which do cross the blood-brain barrier (Kastin et al., 2002; Kastin and Akerstrom, 2003).
EX-4 has been shown to activate GLP-1 receptors on tyrosine hydroxylase-containing neurons in the AP nucleus after intravenous but not intracerebroventricular administration (Yamamoto et al., 2003). Therefore, activation of AP neurons by peripheral EX-4 and GLP-1-Tf administration is due to their presence in circulation, and because EX-4 has a 3-fold higher affinity for the GLP-1 receptor than native GLP-1, it more readily activates the AP. We found that intracerebroventricularly administered fusion protein also did not activate the AP, corroborating that AP excitation after peripheral administration is probably due to the lack of a specific blood-brain barrier at that nucleus. Avoidance of food for 48 h by the animals after one EX-4-Tf injection could be a significant added bonus in the treatment of type 2 diabetes. The administration of GLP-1 or EX-4 as fusion proteins results in no “free” GLP-1 or EX-4 in the circulation at any time, unlike many other compounds under investigation (Agersø et al., 2002), and this again should lessen the gastrointestinal side effects. A caveat however, with respect to potential side effect reduction, should be made when considering the dosing regimens of transferrin bioconjugated peptides such as GLP-1, i.e., with the high molecular mass of the transferrin moiety higher drug doses are required to obtain comparable therapeutic molarities as unconjugated peptides.
Transferrin naturally has a long circulating half-life (7–10 days for glycosylated transferrin and 14–17 days for the nonglycosylated form). Nonglycosylated transferrin is normally present in the body (2–8% of total transferrin) and is amenable to large-scale production in a variety of expression systems, such as yeast and insect cells. The high concentration of endogenous transferrin (3–4 mg/ml serum) precludes any effect on iron homeostasis by exogenously added fusion protein. Transferrin fusion technology was investigated several years ago while attempting to target proteins to brain cells and intracellular targets, but these were not successful, because the Tf receptor is saturated with endogenous Tf concentrations at several orders of magnitude higher than its affinity constant (Li and Qian, 2002).
Our novel technology could be used to deliver other agents known to activate receptors on β cells. Furthermore, this method could be applicable to deliver an imaging agent attached to the C terminus of the Tf moiety into islets. This could allow monitoring of natural history, disease progression, and changes in islet area over time in humans, which is not currently possible. In addition, it could allow imaging of transplanted islets in the liver and hopefully help ascertain the contributions of functional abnormalities versus loss of transplanted islet area to the worsening hyperglycemia seen in transplanted patients. We propose that Tf-based fusion technology is a broadly applicable and promising approach for prolonging the half-life of many bioactive proteins that are now used to treat a myriad of human diseases.
Acknowledgments
We thank Titilola Iyun and Dr. Zhihong Guo for technical assistance.
This work was supported by the Intramural Research Program of the National Institutes of Health National Institute on Aging.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.166470.
- GLP-1
- glucagon-like peptide 1
- DPP-IV
- dipeptidyl peptidase IV
- EX-4
- exendin-4
- Tf
- transferrin
- GLP-1-Tf
- glucagon-like peptide 1 fused to human transferrin
- EX-4-Tf
- exendin-4 fused to human transferrin
- PAGE
- polyacrylamide gel electrophoresis
- CHO
- Chinese hamster ovary
- ELISA
- enzyme-linked immunosorbent assay
- IPGTT
- intraperitoneal glucose tolerance test
- BrdU
- 5-bromo-2-deoxyuridine
- PBS
- phosphate-buffered saline
- HRP
- horseradish peroxidase
- h
- human
- CSF
- cerebrospinal fluid
- GLP-1R
- glucagon-like peptide 1 receptor
- AUC
- area under the curve
- AP
- area postrema
- NTS
- nuclei of the solitary tract
- PVH
- paraventricular nuclei of the hypothalamus
- LY2428757
- polyethylene glycol-GLP-1.
References
- Agersø H, Jensen LB, Elbrønd B, Rolan P, Zdravkovic M. (2002) The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia 45:195–202 [DOI] [PubMed] [Google Scholar]
- Aziz A, Anderson GH. (2002) Exendin-4, a GLP-1 receptor agonist, modulates the effect of macronutrients on food intake by rats. J Nutr 132:990–995 [DOI] [PubMed] [Google Scholar]
- Baggio LL, Huang Q, Brown TJ, Drucker DJ. (2004) A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes 53:2492–2500 [DOI] [PubMed] [Google Scholar]
- Baggio LL, Huang Q, Cao X, Drucker DJ. (2008) An albumin-exendin-4 conjugate engages central and peripheral circuits regulating murine energy and glucose homeostasis. Gastroenterology 134:1137–1147 [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2005) National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2005. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- Chapter MC, White CM, DeRidder A, Chadwick W, Martin B, Maudsley S. (2010) Chemical modification of class II G protein-coupled receptor ligands: frontiers in the development of peptide analogs as neuroendocrine pharmacological therapies. Pharmacol Ther 125:39–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen M, Knop FK, Holst JJ, Vilsboll T. (2009) Lixisenatide, a novel GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus. IDrugs 12:503–513 [PubMed] [Google Scholar]
- Clark A, Jones LC, de Koning E, Hansen BC, Matthews DR. (2001) Decreased insulin secretion in type 2 diabetes: a problem of cellular mass or function? Diabetes 50 (Suppl 1):S169–S171 [DOI] [PubMed] [Google Scholar]
- Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. (1995) Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 44:1126–1131 [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Dritselis A, Kirkpatrick P. (2010) Liraglutide. Nat Rev Drug Discov 9:267–268 [DOI] [PubMed] [Google Scholar]
- Egan JM, Bulotta A, Hui H, Perfetti R. (2003a) GLP-1 receptor agonists are growth and differentiation factors for pancreatic islet beta cells. Diabetes Metab Res Rev 19:115–123 [DOI] [PubMed] [Google Scholar]
- Egan JM, Clocquet AR, Elahi D. (2002) The insulinotropic effect of acute exendin-4 administered to humans: comparison of nondiabetic state to type 2 diabetes. J Clin Endocrinol Metab 87:1282–1290 [DOI] [PubMed] [Google Scholar]
- Egan JM, Meneilly GS, Elahi D. (2003b) Effects of 1-mo bolus subcutaneous administration of exendin-4 in type 2 diabetes. Am J Physiol Endocrinol Metab 284:E1072–E1079 [DOI] [PubMed] [Google Scholar]
- Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. (1992) Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 267:7402–7405 [PubMed] [Google Scholar]
- Fineman MS, Bicsak TA, Shen LZ, Taylor K, Gaines E, Varns A, Kim D, Baron AD. (2003) Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care 26:2370–2377 [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Ersbøll AK, Holst JJ, Astrup A. (2001) The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord 25:781–792 [DOI] [PubMed] [Google Scholar]
- Greig NH, Holloway HW, De Ore KA, Jani D, Wang Y, Zhou J, Garant MJ, Egan JM. (1999) Once daily injection of exendin-4 to diabetic mice achieves long-term beneficial effects on blood glucose concentrations. Diabetologia 42:45. [DOI] [PubMed] [Google Scholar]
- Harford JB, Rouault TA, Huebers HA, Klausner RD. (1994) Molecular mechanisms of iron metabolism, in Molecular Basis of Blood Diseases (Stamatoyannopoulos G, Nienhuis AW, Majerus PW, Varmus H. eds) pp 351–378, W.B. Saunders Co., Philadelphia [Google Scholar]
- Hornby PJ. (2001) Central neurocircuitry associated with emesis. Am J Med 111 (Suppl 8A):106S–112S [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W. (2002) Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci 18:7–14 [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V. (2003) Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord 27:313–318 [DOI] [PubMed] [Google Scholar]
- Kieffer TJ, McIntosh CH, Pederson RA. (1995) Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 136:3585–3596 [DOI] [PubMed] [Google Scholar]
- Knudsen LB, Pridal L. (1996) Glucagon-like peptide-1-(9-36) amide is a major metabolite of glucagon-like peptide-1-(7-36) amide after in vivo administration to dogs, and it acts as an antagonist on the pancreatic receptor. Eur J Pharmacol 318:429–435 [DOI] [PubMed] [Google Scholar]
- Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, Taylor K, Kim D, Aisporna M, Wang Y, et al. (2003) Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 88:3082–3089 [DOI] [PubMed] [Google Scholar]
- Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. (2004) Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care 27:17–20 [DOI] [PubMed] [Google Scholar]
- Li H, Qian ZM. (2002) Transferrin/transferrin receptor-mediated drug delivery. Med Res Rev 22:225–250 [DOI] [PubMed] [Google Scholar]
- Lovshin JA, Drucker DJ. (2009) Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5:262–269 [DOI] [PubMed] [Google Scholar]
- Martin B, Golden E, Carlson OD, Pistell P, Zhou J, Kim W, Frank BP, Thomas S, Chadwick WA, Greig NH, et al. (2009) Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease. Diabetes 58:318–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara M, Kanemoto S, Leshnower BG, Albone EF, Hinmon R, Plappert T, Gorman JH, 3rd, Gorman RC. (2009) Single dose GLP-1-Tf ameliorates myocardial ischemia/reperfusion injury. J Surg Res doi:10.1016/j.jss.2009.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. (1986) Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem 261:11880–11889 [PubMed] [Google Scholar]
- Montrose-Rafizadeh C, Yang H, Rodgers BD, Beday A, Pritchette LA, Eng J. (1997) High potency antagonists of the pancreatic glucagon-like peptide-1 receptor. J Biol Chem 272:21201–21206 [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Elahi D, Shen YT, Shannon RP. (2005) Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 289:H2401–H2408 [DOI] [PubMed] [Google Scholar]
- Ogawa N, List JF, Habener JF, Maki T. (2004) Cure of overt diabetes in NOD mice by transient treatment with anti-lymphocyte serum and exendin-4. Diabetes 53:1700–1705 [DOI] [PubMed] [Google Scholar]
- Park S, Dong X, Fisher TL, Dunn S, Omer AK, Weir G, White MF. (2005) Exendin-4 uses Irs2 signaling to mediate pancreatic beta cell growth and function. J Biol Chem 281:1159–1168 [DOI] [PubMed] [Google Scholar]
- Perfetti R, Zhou J, Doyle ME, Egan JM. (2000) Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology 141:4600–4605 [DOI] [PubMed] [Google Scholar]
- Perry T, Greig NH. (2005) Enhancing central nervous system endogenous GLP-1 receptor pathways for intervention in Alzheimer's disease. Curr Alzheimer Res 2:377–385 [DOI] [PubMed] [Google Scholar]
- Plamboeck A, Holst JJ, Carr RD, Deacon CF. (2005) Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia 48:1882–1890 [DOI] [PubMed] [Google Scholar]
- Sadegi H, Turner AJ, Prior CP, Balance DJ. (2006), inventors; Biorexis Pharmaceutical Corp, assignee. Modified transferrin fusion proteins. World patent WO2006096515. 2006 Sept 14 [Google Scholar]
- Seino Y, Rasmussen MF, Zdravkovic M, Kaku K. (2008) Dose-dependent improvement in glycemia with once-daily liraglutide without hypoglycemia or weight gain: a double-blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 81:161–168 [DOI] [PubMed] [Google Scholar]
- Sleep D, Belfield GP, Ballance DJ, Steven J, Jones S, Evans LR, Moir PD, Goodey AR. (1991) Saccharomyces cerevisiae strains that overexpress heterologous proteins. Biotechnology 9:183–187 [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, Egan JM. (2000) Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes 49:741–748 [DOI] [PubMed] [Google Scholar]
- St Onge EL, Miller SA. (2010) Albiglutide: a new GLP-1 analog for the treatment of type 2 diabetes. Expert Opin Biol Ther 10:801–806 [DOI] [PubMed] [Google Scholar]
- Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RG, Greig NH, Egan JM. (2000) Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology 141:1936–1941 [DOI] [PubMed] [Google Scholar]
- Vilsbøll T, Holst JJ. (2004) Incretins, insulin secretion and Type 2 diabetes mellitus. Diabetologia 47:357–366 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhou J, Doyle ME, Egan JM. (2001) Glucagon-like peptide-1 causes pancreatic duodenal homeobox-1 protein translocation from the cytoplasm to the nucleus of pancreatic beta-cells by a cyclic adenosine monophosphate/protein kinase A-dependent mechanism. Endocrinology 142:1820–1827 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK. (2003) Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci 23:2939–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander M, Madsbad S, Madsen JL, Holst JJ. (2002) Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 359:824–830 [DOI] [PubMed] [Google Scholar]