Abstract
Testosterone modulates seizure susceptibility, but the underlying mechanisms are obscure. Recently, we demonstrated that testosterone affects seizure activity via its conversion to neurosteroids in the brain. Androstanediol (5α-androstan-3α,17β-diol) is an endogenous neurosteroid synthesized from testosterone. However, the molecular mechanism underlying the seizure protection activity of androstanediol remains unclear. Here, we show that androstanediol has positive allosteric activity as a GABAA receptor modulator. In whole-cell recordings from acutely dissociated hippocampus CA1 pyramidal cells, androstanediol (but not its 3β-epimer) produced a concentration-dependent enhancement of GABA-activated currents (EC50 of 5 μM). At 1 μM, androstanediol produced a 50% potentiation of GABA responses. In the absence of GABA, androstanediol has moderate direct effects on GABAA receptor-mediated currents at high concentrations. Systemic doses of androstanediol (5–100 mg/kg), but not its 3β-epimer, caused dose-dependent suppression of behavioral and electrographic seizures in mouse hippocampus kindling, which is a model of temporal lobe epilepsy. The ED50 value for antiseizure effects of androstanediol was 50 mg/kg, which did not produce sedation/motor toxicity. At high (2× ED50) doses, androstanediol produced complete seizure protection that lasted for up to 3 h after injection. The estimated plasma concentrations of androstanediol producing 50% seizure protection in the kindling model (10.6 μM) are within the range of concentrations that modulate GABAA receptors. These studies suggest that androstanediol could be a neurosteroid mediator of testosterone actions on neuronal excitability and seizure susceptibility via its activity as a GABAA receptor modulator and that androstanediol may play a key role in men with epilepsy, especially during the age-related decline in androgen levels.
Introduction
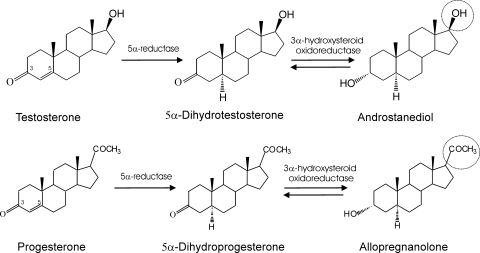
Steroid hormones play a key role in the neuroendocrine control of neuronal excitability and behavior. There is strong evidence that circulating steroid hormones serve as precursors for the synthesis of neurosteroids in the brain (Schumacher et al., 2003). Neurosteroids are endogenous modulators of neuronal excitability. Neurosteroids such as the progesterone-derived allopregnanolone (5α-pregnan-3α-ol-20-one) and the deoxycorticosterone-derived allotetrahydrodeoxycorticosterone (5α-pregnane-3α,21-diol-20-one) are potent positive modulators of GABAA receptors with anxiolytic and anticonvulsant properties (Harrison et al., 1987; Belelli et al., 1989; Kokate et al., 1994; Reddy, 2003). These neurosteroids have been shown to play a significant role in the pathophysiology of epilepsy, anxiety, depression, and stress (Purdy et al., 1991; Smith et al., 1998; Reddy, 2009). Synthetic neurosteroids have been proposed as treatments for epilepsy (Reddy and Rogawski, 2010). Testosterone has been shown to modulate seizure susceptibility, but the underlying mechanisms are obscure. Recently, we showed that testosterone affects seizure activity via its conversion to neurosteroids estradiol and androstanediol in the brain (Reddy, 2004a, b, 2008). Androstanediol (5α-androstan-3α,17β-diol) is synthesized from testosterone by the 5α-reductase pathway (Fig. 1). Androstanediol is a neurosteroid because it is produced locally by glial cells and principal neurons in the hippocampus, which have 5α-reductase and 3α-hydroxysteroid oxidoreductase enzymes (Martini et al., 1993; Mensah-Nyagan et al., 1999; Agís-Balboa et al., 2006). Androstanediol has anticonvulsant and neuroprotective properties (Frye and Reed, 1998; Reddy, 2004a; Frye et al., 2010) and therefore may mediate the cellular actions of testosterone in the brain. However, the molecular mechanism underlying the protective activity of androstanediol remains unclear.
Fig. 1.
Biosynthesis and structural similarity between neurosteroids androstanediol and allopregnanolone. Androstanediol is synthesized from testosterone by reduction at the 5- and 3-positions of the steroid A-ring. Allopregnanolone is derived from progesterone by reduction at the 5- and 3-positions of the steroid A-ring. Androstanediol differs from allopregnanolone by a 17β-hydroxyl group instead of 17β-methyl-carbonyl group. 3β-Androstanediol, the 3β-epimer of 3α-androstanediol, has a 3-hydroxyl group in the β-orientation.
GABAA receptors, the main mediators of inhibition in the brain, are the major target for endogenous neurosteroids. GABAA receptors are pentameric chloride-selective ion channels. The major isoforms consist of the 2α, 2β, and 1γ2 subunits, which are localized to synapses. In some channels, the δ-subunit substitutes for the γ2-subunit in the hippocampus and thalamus. At nanomolar concentrations, allopregnanolone-like neurosteroids potentiate GABA currents (Harrison et al., 1987), whereas at micromolar concentrations, they directly activate the receptor (Majewska et al., 1986; Reddy, 2003). Neurosteroids modulate most GABAA receptor isoforms (Puia et al., 1990; Belelli et al., 2002). The modulating effects of neurosteroids occur by their binding to discrete sites on the GABAA receptor that are located within the transmembrane domains of the α- and β-subunits (Hosie et al., 2006). Although the exact location of androstanediol binding sites is currently unknown, it has been shown that a highly conserved glutamine at position 241 in the M1 domain of the α-subunit plays a key role in neurosteroid modulation (Hosie et al., 2009). The δ-subunit containing GABAA receptors, located perisynaptically/extrasynaptically and that mediate tonic current activated by ambient GABA levels, are more sensitive to neurosteroids (Belelli et al., 2002; Wohlfarth et al., 2002). To regulate GABAA receptors, neurosteroids require a C3α-hydroxyl group on their A-ring and a C20-ketone in the D-ring (Fig. 1; Harrison et al., 1987). These groups are important for the binding of neurosteroids to the transmembrane domains of the receptor, which mediate the potentiating and direct activation effects (Hosie et al., 2006). Androstanediol is a C19–5α,3α-reduced steroid with C17β-hydroxyl group in the D-ring (Fig. 1). A structural homology exists between androstanediol and allopregnanolone. Because of these features, there are indications that androstanediol is able to interact with GABAA receptors (Frye et al., 1996; Park-Chung et al., 1999). However, androstanediol has not been previously characterized for allosteric potentiation and direct modulation of GABAA receptors in the hippocampus. Despite strong protective activity against experimental seizures (Frye and Reed, 1998; Reddy, 2004a), the physiological role of androstanediol in epilepsy and other brain conditions is unclear.
Here, we sought to demonstrate that androstanediol, like allopregnanolone, has activity as a positive modulator of GABAA receptors and protects against seizures induced by hippocampus kindling, which is a widely used model of human temporal lobe epilepsy. Our results suggest for the first time that androstanediol is a positive allosteric modulator of GABAA receptors, supporting the possibility that the modulation of GABAergic inhibition could play an important role in its antiseizure activity. Androstanediol has comparable efficacy but is less potent than the prototype neurosteroid allopregnanolone.
Materials and Methods
In Vitro Studies
Hippocampus Slice Preparation.
Transverse slices (400 μm in thickness) of hippocampus were prepared using standard techniques from adult (3- to 4-month-old) mice and were used for dissociation of CA1 pyramidal neurons. Mice of the C57BL/6J strain were obtained from the institutional breeding facility. Mice were anesthetized with isoflurane. Then, the brain was rapidly removed and chilled with artificial cerebrospinal fluid (ACSF) plus 0.3 mM kynurenic acid bubbled with carbogen gas (95% O2, 5% CO2). Several 400-μm slices were cut with a Vibratome (model 1500 with 900 Refrigeration System; Leica Microsystems, Inc., Bannockburn, IL). Brain slices were equilibrated in ACSF at 24°C on a mesh surface, in a small beaker in a waterbath (Thermo Fisher Scientific, Waltham, MA) continuously with oxygen (95% O2, 5% CO2). The composition of the ACSF was 126 mM NaCl, 3 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 26 mM NaHCO3, 1.25 mM NaH2PO4, 11 mM glucose, osmolarity, 305–315 mOsm/kg. All procedures were performed as per the protocol approved by the Institutional Animal Care and Use Committee.
Dissociation of Pyramidal Neurons.
Hippocampal CA1 pyramidal cells were prepared by the standard dissociation technique described previously (Kay and Wong, 1986). The hippocampal pieces with the CA1 region were microdissected carefully under the microscope (model SMZ 647; Nikon, Tokyo, Japan) and incubated in ACSF for 1 h at 24°C. The isolated slices were transferred into an enzymatic solution consisting of ACSF with 3 mg/ml protease XXIII (Sigma-Aldrich, St. Louis, MO). The slices were then incubated for precisely 23 to 25 min at 24°C. Next, the slices were washed and returned to ACSF. To separate the cells, the ACSF solution with slices was triturated through three different sizes of glass pipettes. For this step, three fire-polished Pasteur pipettes with increasingly smaller tips were used. The first tip was just fire-polished, the second tip was fire-polished until it was approximately half closed, and the third tip was fire-polished until it was approximately three-fourths closed. For each batch, slices were triturated five or six times with each pipette with approximately 0.5 to 1 ml of ACSF in it. Then, the solution was allowed 1 min for the tissue to settle down, and the suspended cells were carefully pipetted into the recording chamber (Warner Instruments, Hamden, CT). We consistently obtained a great yield of cells from young adult mice that were sufficient for recording. Older animals gave lower yields. The incubation time (23–25 min) and temperature (24°C) were strictly followed for a great yield of healthy cells.
Recording of GABA-Evoked Currents.
Electrophysiological recordings were performed in the whole-cell patch-clamp configuration at room temperature. The recording chamber was fixed into the stage of an inverted microscope with phase-contrast and differential interference contrast optics (model IX71; Olympus, Tokyo, Japan). The bath solution in the chamber was composed of 140 mM NaCl, 3 mM KCl, 10 mM HEPES, 2 mM MgCl2, 2 mM CaCl2, and 16 mM glucose, pH 7.4, with NaOH, osmolarity, 315–325 mOsm/kg. Cells were visualized and images were acquired through videocamera CCD-100 (Dage-MTI, Michigan City, IN) with FlashBus Spectrim 1.2 software (Pelco, Clovis, CA). Recording pipettes were pulled from capillary glass tubes (King Precision Glass, Claremont, CA) using a P-97 Flaming-Brown horizontal puller (Sutter Instrument Company, Novato, CA). The pipette tip resistance was 2 to 3.5 MΩ. The pipette solution was composed of 124 mM CsCl, 20 mM tetraethylammonium, 2 mM MgCl2, 10 mM EGTA, 10 mM HEPES, 0.1 mM GTP, 4 mM ATP, pH 7.2, with CsOH, osmolarity, 295–305 mOsm/kg. Currents were recorded by using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). The membrane capacitance, series resistance, and input resistance of the recordings were monitored by applying a 5-mV (100-ms) depolarizing voltage step from a holding potential of −70 mV. Signals were low-pass filtered at 1 kHz and digitized at 2 kHz with Digidata 1440A interface and pClamp 10.2 software (Molecular Devices). Peak currents were measured by using Clampfit 10.2 software (Molecular Devices).
Test Drug Applications.
All of the chemicals used in the electrophysiology studies were purchased from Sigma-Aldrich unless otherwise specified. GABA was dissolved in water and stock solutions (1–100 mM) of androstanediol and 3β-androstanediol (Steraloids, Newport, RI) were prepared in dimethyl sulfoxide. Stock solutions were diluted in the external solution to the desired concentration. The concentration of dimethyl sulfoxide was less than 1%. The solvent has no effect on GABA currents. GABA, bicuculline, and picrotoxin were prepared daily from stock solutions (100, 10, and 20 mM, respectively) that were dissolved in distilled water. GABA and drug solutions were applied using an eight-channel perfusion system (AutoMate Scientific, Inc., Berkeley, CA) that allowed rapid switching among the bath solution and the drug solutions. The cells were continuously perfused with the external solution. The perfusion pipette tip (diameter, 500 μm) was positioned near the isolated cell. To reduce desensitization with repeated application, a 5-s application was used to examine the response of GABAA receptors. To test the modulation of steroid, a 5-s preapplication of steroid solution was followed by a 5-s application of GABA plus steroid. To examine the direct actions of the androstanediol, the steroid was continuously perfused for 10 s. Applications of GABA and steroid either alone or in combination were separated by a time interval of at least 1 min to minimize desensitization.
Data Analysis.
Fractional potentiation produced by the steroid was calculated as IS/IGABA, where IGABA is the peak amplitude of the control GABA response and IS is the response of the coapplication of GABA and the test neurosteroid. The GABA concentration-response curve was obtained by different concentration of GABA (0.1–1000 μM), with a washout interval of 0.5 to 5 min. The neurosteroid response data was expressed as a percentage of maximal response to 3 μM GABA that evoked 10% of the maximal inhibitory currents (EC10). The concentration of androstanediol producing half of the maximal increase in the amplitude of the GABA response (EC50) was determined by fitting the concentration-response relationships to the following sigmoid function: I/IMAX = [1+ (EC50/A)n]−1, where A is the neurosteroid concentration, IMAX is the current evoked by GABA in the presence of a maximal potentiating concentration of the steroid, I is the current produced by GABA in the presence of a concentration A of neurosteroid, EC50 is the concentration of the neurosteroid required to produce half of its own maximal GABA potentiating effect, and n is the Hill coefficient. Concentration-response data for direct activation of Cl− currents were subjected to logistic fitting. Data are presented as the mean ± S.E.M. Statistical analysis for mean difference was performed by using one-way analysis of variance followed by Tukey's post hoc test or Student's t test. The criterion for statistical significance was p < 0.05.
In Vivo Studies
Animals.
Adult male mice of the C57BL6 strain weighing approximately 25 to 30 g were used in the study. The mice were housed in an environmentally controlled animal facility under a normal 12-h light/dark cycle and allowed free access to food and water, except during the experimental sessions. The experimental groups consisted of six to eight animals. The experiments were performed during the light phase of the light/dark cycle between 8:00 AM and 5:00 PM. All procedures were performed in strict compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals under a protocol approved by the Institutional Animal Care and Use Committee.
Hippocampus Kindling Model of Epilepsy.
To study seizure protection activity of androstanediol, we used the hippocampus kindling model, which is the best model of human temporal lobe epilepsy characterized by progressive complex partial seizures with secondary generalization (Albright and Burnham, 1980). A mild focal, nonconvulsant electrical stimulus to the hippocampus on a daily basis leads to the development of a kindled state, exhibiting electrographic and behavioral seizures. In the mouse kindling, the focal electroencephalogram afterdischarge models complex partial seizures, whereas the behavioral motor seizure stages 4/5 models generalized seizures. Electrode implantation and stimulation procedures for mouse hippocampus kindling were performed as described previously (Reddy and Rogawski, 2002). Mice were anesthetized by an intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). A twisted bipolar stainless-steel wire electrode (model MS303/1; Plastic Products, Roanoke, VA) was stereotaxically implanted in the right hippocampus (2.9 mm posterior and 3.0 mm lateral to bregma and 3.0 mm below the dorsal surface of the skull) using the Franklin and Paxinos atlas (Franklin and Paxinos, 1997) and anchored with dental acrylic to three jeweler's screws placed in the skull. A period of 7 to 10 days was allowed for recovery. The stimulation paradigm consisted of 1-ms duration, bipolar, square current pulses delivered at 60 Hz for 1 s using a kindling stimulator (A-M Systems, Sequim, WA). The afterdischarge threshold was determined by stimulating at 5-min intervals beginning with an intensity of 25 μA and increasing in steps of 25 μA until an afterdischarge of at least 5 s was obtained. Stimulation on subsequent days used a stimulation intensity 125% of the threshold value. Seizure activity after each stimulation was rated according to the criterion of Racine (1972) as modified for the mouse: stage 0, no response or behavior arrest; stage 1, chewing or head nodding; stage 2, chewing and head nodding; stage 3, forelimb clonus; stage 4, bilateral forelimb clonus and rearing; and stage 5, falling. The afterdischarge was recorded from the hippocampus electrode with a Grass CP511 preamplifier (Astro-Med, West Warwick, RI) and stored in digital form using Axoscope 8.1 (Axon Instruments, Foster City, CA). Afterdischarge duration was the total duration of hippocampal electrographic spike activity (amplitude >2× baseline) occurring in a rhythmic pattern at a frequency >1 Hz. The day of afterdischarge threshold determination was considered day 1 of kindling. Kindling stimulation was delivered daily until stage 5 seizures were elicited on 3 consecutive days. Stimulation was continued on a 5-day/week schedule each afternoon. Mice were used for drug testing when they consistently exhibited stage 5 seizures after stimulation, which is considered the “fully kindled” state.
Test Drug Administration and Kindling Protocol.
To examine the ability of androstanediol to suppress the expression of kindled seizures, the kindled mice underwent a 3-day test protocol. On day 1, they were verified to exhibit stimulation-induced stage 5 seizures. On day 2, a subcutaneous androstanediol injection (5–100 mg/kg) was administered 15 min before stimulation. On day 3, the animals were stimulated again without drug pretreatment. During each test session, the behavioral seizure score and the afterdischarge duration were noted. The seizure scores and afterdischarge durations on days 1 and 3 were averaged and taken as the control values.
Horizontal Screen Test.
Androstanediol was evaluated for motor toxicity by using a modification of the horizontal screen test as described previously (Kokate et al., 1994; Kaminski et al., 2005). Mice were placed on a horizontally oriented grid (consisting of parallel 1.5-mm-diameter rods situated 1 cm apart), and the grid was inverted. Animals that fell from the grid within 10 s were scored as impaired. The dose producing impairment in 50% of mice (TD50) and the 95% confidence limits were estimated by log-probit analysis using the Litchfield and Wilcoxon method as described previously (Reddy et al., 2004).
Determination of Plasma Androstanediol Levels.
Animals were anesthetized with isoflurane and ∼0.5 ml of carotid blood was collected in heparinized tubes. The plasma was separated by centrifugation at 12,000g for 10 min and stored at −20°C in 10-ml glass tubes containing 7.5% EDTA solution (68 μl). The concentration of androstanediol was analyzed by liquid chromatography-mass spectroscopy as described previously (Reddy et al., 2005). In brief, a 0.2-ml plasma sample was added to a tube containing evaporated internal standard. The steroid and internal standard were extracted with 4 ml of hexane. Each sample was analyzed by using the atmospheric pressure chemical ionization technique under acidic conditions. A standard curve was plotted by using pure androstanediol in methanol mixed with 0.2 ml of blank mouse plasma.
Data Analysis.
Group data were expressed as the mean ± S.E.M.; difference in seizure stages between groups was compared with the nonparametric Kruskal-Wallis test followed by the Mann-Whitney U test. Comparison of means of the afterdischarge duration between groups was made with one-way analysis of variance, followed by unpaired two-tailed Student's t test. Comparison of the mean percentage inhibition of seizure stage and afterdischarge duration in fully kindled animals was made by Wilcoxon signed ranks test and paired two-tailed Student's t test, respectively. To construct dose-effect curves, androstanediol was tested at several doses spanning the dose producing 50% protection (ED50). ED50 values were determined by nonlinear curve fitting using the Levenberg-Marquardt algorithm to a logistic equation where the maximal inhibition was assumed to be 100%. The androstanediol dose producing 50% sedation/motor toxicity (TD50) with 95% confidence limits were determined by log-probit analysis using the Litchfield and Wilcoxon procedure. A logistic model was used to fit plots of seizure protection versus plasma androstanediol levels. In all statistical tests, the criterion for statistical significance was p < 0.05.
Drugs.
Stock solutions of androstanediol (Steraloids) and other drugs for injection were made in 30% β-cyclodextrin in water, and additional dilutions were made using normal saline. By itself, β-cyclodextrin at concentrations as high as 50% failed to affect kindled seizures. Drug solutions were administered subcutaneously in a volume equaling 1% of the animal's body weight. Cyclodextrin and flutamide were procured from Sigma-Aldrich.
Results
Androstanediol Potentiates GABA Responses in Acutely Dissociated CA1 Neurons.
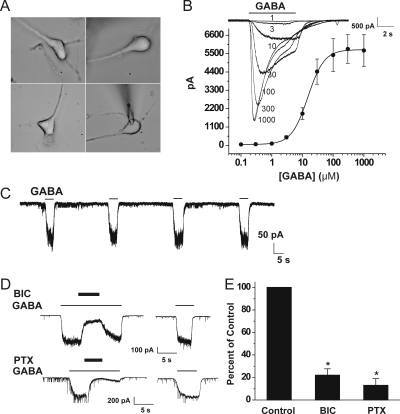
To investigate the physiological actions of androstanediol on GABAA receptor responses, we first characterized the GABAA receptor-mediated Cl− currents in acutely dissociated CA1 neurons from adult mice. An acutely dissociated CA1 pyramidal neuron had a clear primary apical dendrite with a typical cell body (Fig. 2A). Application of GABA (0.1–1000 μM) for 5 s evoked brief inward currents in a concentration-dependent manner (Fig. 2B). At 3 μM concentration, GABA evoked significant currents with the average value of 479 ± 98 pA, which is ∼10% of the maximal current at high GABA application (EC10). The desensitization was minimal at 30-s intervals between applications. Thus, we chose 3 μM GABA for further experiments. Repeated application of 3 μM GABA induced similar and consistent Cl− currents without desensitization when applied with 30-s intervals (Fig. 2C). Like cells from the enzymatic method, mechanically dissociated neurons showed similar GABA responses but showed high background noise (data not shown). Next, we sought to confirm the GABAA receptor component of inhibition in CA1 neurons (Fig. 2D). The GABA-evoked currents were blocked by the GABAA receptor competitive antagonist bicuculline and the GABAA receptor channel blocker picrotoxin (Fig. 2E), indicating that these currents were mediated by the GABAA receptors.
Fig. 2.
Isolation and characterization of GABA-activated whole-cell currents in acutely dissociated hippocampal CA1 pyramidal neurons from adult mice. A, morphology of acutely dissociated hippocampal CA1 pyramidal neurons. Cells chosen for recording appeared with clean cell body and a clear primary apical dendrite. A recording pipette with the cell is shown at the bottom right. B, concentration-response curves for GABA (0.1–1000 μM)-evoked currents in CA1 neurons (EC50 of 15 μM). Sample traces showing Cl− currents evoked during perfusion with 1 to 1000 μM GABA for 5 s in the same cell are illustrated in the inset. C, repeated application of 3 μM GABA at 30-s interval caused similar responses with little desensitization. GABA current traces shown in B and C are from different cells. D, currents evoked by GABA are blocked by the GABAA receptor antagonists bicuculline (BIC; 10 μM) and picrotoxin (PTX; 20 μM). Representative current traces showing competitive blockade of currents activated by 3 μM GABA by bicuculline and noncompetitive channel blockade by picrotoxin. The picrotoxin-induced inhibition could not be washed immediately and full recovery was evident after ∼20 min. E, fractional block of currents evoked by 3 μM GABA by bicuculline and picrotoxin in experiments similar to those shown in D. Peak current amplitudes in the presence of PTX and BIC are normalized to the peak control current amplitudes in the absence of antagonist. Each point or bar represents the mean ± S.E.M. of data from four to nine cells. *, p < 0.01 versus control.
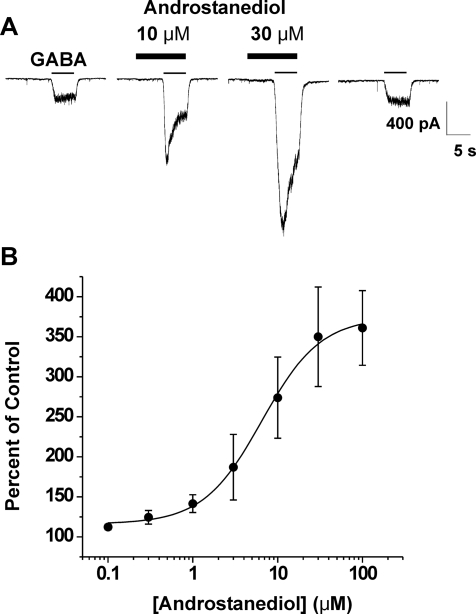
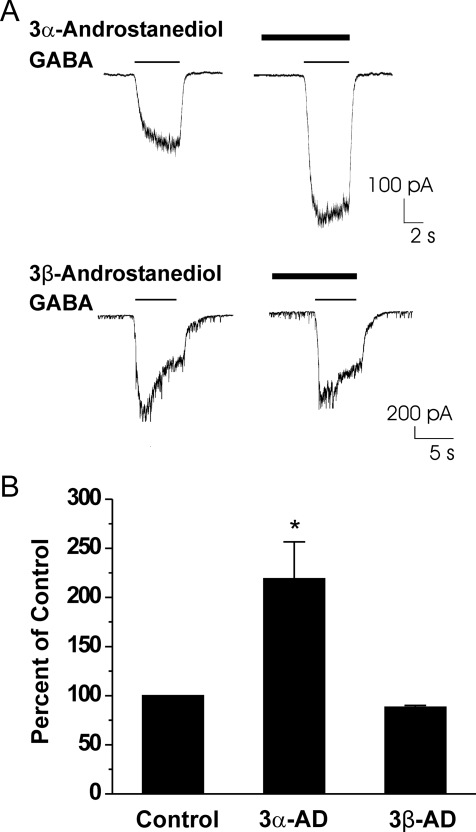
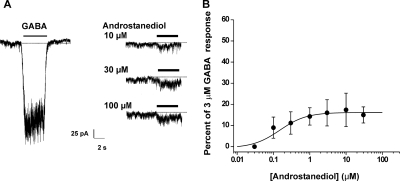
We next assessed the ability of androstanediol to potentiate currents generated by 3 μM GABA. Neurons were preapplied with androstanediol for 5 s and then GABA was coapplied with androstanediol for 5 s. Androstanediol caused a concentration-dependent increase in peak-current responses evoked by GABA, and at high concentrations it seemed to enhance desensitization (Fig. 3A). The effect of androstanediol was fully reversible but required long wash times at higher concentrations (Fig. 3A). At a concentration of 10 nM, the mean percentage of control value for androstanediol was 109 ± 2 (n = 4). At higher concentrations, androstanediol caused up to a 4-fold potentiation of control responses (Fig. 3B). The estimated concentration that produced a 50% potentiation of the control GABA current was 1.0 μM. The concentration of androstanediol producing half of the maximal enhancement of the GABA-evoked current (EC50) was 5 μM. To determine whether the potentiating effect is structurally specific, we next compared the response to androstanediol with that of its 3β-epimer (Fig. 1). As shown in Fig. 4, only the natural 3α-isomer was active at a concentration of 10 μM (2× EC50). The 3β-epimer at 10 μM failed to increase GABA-activated currents in acutely dissociated CA1 neurons.
Fig. 3.
Androstanediol reversibly potentiates GABA-activated whole-cell currents in acutely dissociated hippocampal CA1 pyramidal neurons. A, representative traces showing androstanediol increase in GABA-activated Cl− currents at 10 and 30 μM concentrations in a single neuron. The neurosteroid was preapplied for 5 s before the onset of the GABA coapplication. Gray bars indicate the period of application of 3 μM GABA; black bars indicate preapplication/coapplication of androstanediol at the concentrations noted. Recovery from androstanediol response was obtained at 100 s with continuous washing. B, concentration-response curves for androstanediol derived from experiments similar to those shown in A. Peak amplitude values during coapplication of GABA and neurosteroid were compared with the amplitude of 3 μM GABA responses in the same cells. Each point represents the mean ± S.E.M. of fractional increase values from five to eight cells. The curve represents logistic fits to the mean values; the estimated EC50 value of androstanediol from these responses was 5 μM. The Hill coefficient was 1.255.
Fig. 4.
Stereoselective effects of the 3-hydroxy epimers of androstanediol on GABA-activated currents in acutely dissociated hippocampal CA1 neurons. A, representative recordings showing 3α-androstanediol (10 μM)-induced increase in GABA response (3 μM). 3β-Androstanediol (10 μM) had no significant effect on the GABA response (3 μM). B, grouped data showing the percentage potentiation of control values by 3α-androstanediol (3α-AD; 10 μM) and 3β-androstanediol (3β-AD; 10 μM) calculated from the peak GABA-activated current values obtained in a series of experiments similar to those shown in A. Each bar represents the mean ± S.E.M. of data from four to seven cells. *, p < 0.01 versus control.
The efficacy and potency of androstanediol potentiation of GABA-mediated inhibitory currents were compared with the prototype neurosteroid allopregnanolone. In acutely dissociated CA1 pyramidal neurons, allopregnanolone caused a concentration-dependent increase in peak-current responses evoked by 3 μM GABA. At 10 nM, the mean percentage of control value for allopregnanolone was 161 ± 17 (n = 4). At higher concentrations (0.1–10 μM), the maximal peak amplitude obtained was markedly greater (200–550% potentiation of the control GABA), as is the degree of fluctuation. The data could not be fit because no response plateau was achieved even at high concentrations; the maximal (4- to 5-fold) potentiation at higher concentrations (1–10 μM) was comparable with androstanediol (Fig. 3B). Values for percentage of potentiation at 1 μM were 482 ± 73 and 49 ± 11 for allopregnanolone and androstanediol, respectively, indicating a 10-fold higher potency of allopregnanolone compared with androstanediol.
Direct Activation of GABAA Receptor Currents by Androstanediol in Acutely Dissociated CA1 Neurons.
At high concentrations, neurosteroids, in the absence of GABA, directly activate GABAA receptor Cl− currents (Kokate et al., 1994; Reddy and Rogawski, 2002). To investigate the direct actions of androstanediol on GABA-A receptor currents, we tested androstanediol at several higher concentrations in acutely dissociated CA1 pyramidal neurons. As illustrated in Fig. 5, perfusion of hippocampal neurons with 1 to 100 μM androstanediol (in the absence of GABA) resulted in activation of inward current responses that were very small compared with the response evoked by GABA. The androstanediol response amplitudes were normalized to the mean amplitude of the GABA response (Fig. 5A). Androstanediol caused a moderate concentration-dependent increase in the peak inward currents (Fig. 5B). The EC50 value could not be derived because responses were saturated at concentrations <10 μM. The direct effect of the neurosteroid was picrotoxin-sensitive. The maximal amplitudes of direct androstanediol responses at the highest concentration (100 μM) were approximately 25% of the 3 μM GABA response amplitude recorded in the same neuron, indicating that androstanediol has marginal efficacy in directly activating GABAA receptors relative to GABA.
Fig. 5.
Direct activation of GABAA receptor-mediated inward currents by androstanediol in acutely dissociated CA1 pyramidal neurons. A, representative traces comparing responses of GABA (3 μM) and androstanediol (10–100 μM) in the same cell. The interval between the GABA and androstanediol perfusions was 30 s. B, peak direct androstanediol (0.1–100 μM)-induced current expressed as a fraction of the mean current amplitude evoked by 3 μM GABA. There was modest (<25%) direct activation even at highest concentrations (100 μM). The curve show arbitrary logistic fits to the mean values; parameters could not be derived because responses were largely saturated at concentrations <10 μM, and this is probably due to slow onset and duration of the currents. Each point represents the mean ± S.E.M. of data from four to six cells.
Antiseizure Activity of Androstanediol in the Hippocampus Kindling Model in Mice.
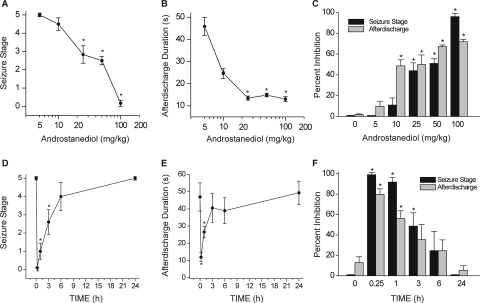
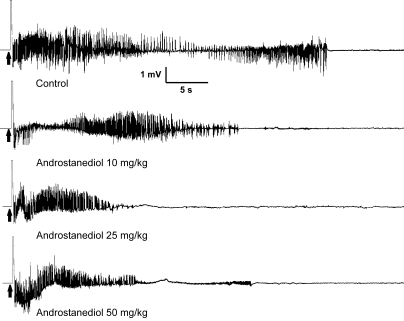
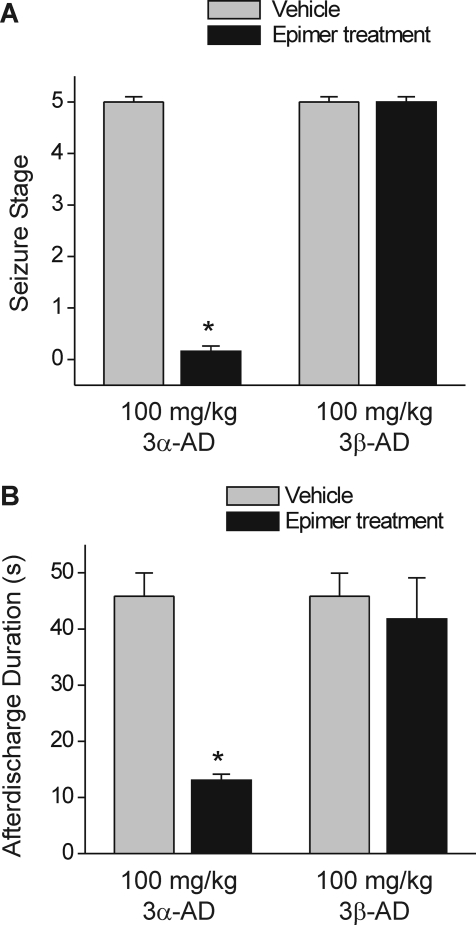
Neurosteroids with GABAA receptor modulatory activity have protective effects in the kindling model of epilepsy (Reddy and Rogawski, 2002, 2010). Therefore, we selected the kindling model for characterization of the antiseizure activity of androstanediol and correlation of the effects of androstanediol in vitro on GABA currents with its systemic seizure protection. To evaluate the systemic activity of androstanediol in protecting against kindled seizures, first we prepared the fully kindled mice with stage 5 seizures by stimulation of the right hippocampus once daily at 125% of their afterdischarge threshold. The overall average value of the initial afterdischarge threshold was 58 ± 9 μA. There was a progressive increase in seizure stage, with all animals achieving consistent stage 5 seizures after 15 stimulations. Then, we used these fully kindled mice for drug testing. The fully kindled mice were treated with various doses of androstanediol 15 min before stimulation. As shown in Fig. 6, androstanediol produced a dose-dependent suppression of behavioral seizure activity (Fig. 6A) and afterdischarge duration (Fig. 6B) with significant effects on both at 25, 50, and 100 mg/kg. The percentage inhibition at the various doses is shown in Fig. 6C. At the highest dose tested, behavioral seizures were nearly completely suppressed. The estimated ED50 value for suppression of seizure stage and afterdischarge duration was 50 ± 4.5 and 22 ± 3.4 mg/kg, respectively. The potency in the kindling model was marginally less than that in the chemoconvulsant models (Table 1). Sample recordings of afterdischarge from fully kindled mice are shown in Fig. 7. Androstanediol pretreatment markedly reduced the afterdischarge duration in a dose-dependent manner. The stereoselective effects of the 3-hydroxy epimers of androstanediol against kindling seizures are shown in Fig. 8. Unlike the 3α-androstanediol that at a dose of 100 mg/kg (2× ED50) conferred complete protection against seizures, the 3β-epimer at a dose of 100 mg/kg failed to protect seizures in the kindling model.
Fig. 6.
Inhibition of hippocampus kindling seizures by androstanediol in mice. A–C, dose-response curves for behavioral seizure stage (A), afterdischarge duration (B), and percentage of inhibition (C) with varying doses of androstanediol (5–100 mg/kg s.c.) in fully kindled mice. D–F, time course for behavioral seizure stage (D), afterdischarge duration (E), and percentage of inhibition (F) with 2× ED50 dose of androstanediol (100 mg/kg s.c.) in fully kindled mice. Mice that were fully kindled with consistent stage 5 seizures were injected subcutaneously with vehicle or androstanediol 15 min before stimulation or other time points as specified. Percentage of inhibition of seizure stage was calculated as 100 × (1 − S/5), where S is the seizure stage after drug treatment. Percentage of inhibition of afterdischarge was calculated as 100 × (1 − D/Dc), where D is the afterdischarge duration after drug treatment and Dc is the average control afterdischarge duration without any drug treatment. The overall mean control afterdischarge duration was 45.3 ± 4.1 s. Each point or bar represents the mean ± S.E.M. of data from six to eight animals. *, p < 0.05 versus control.
TABLE 1.
Antiseizure ED50 values of androstanediol in mouse models of epilepsy
Values in parentheses are 95% confidence limits.
| Seizure Model | ED50 | Reference |
|---|---|---|
| mg/kg | ||
| Kindling model | ||
| Hippocampus kindling | 50 (36–64) | This study |
| GABAA receptor antagonists | ||
| Pentylenetetrazol | 40 (27–60) | Reddy (2004b) |
| Bicuculline | 44 (24–81) | Reddy (2004a) |
| Picrotoxin | 39 (21–74) | Reddy (2004a) |
| Glutamate receptor agonists | ||
| Kainic acid | >200 (N.D.) | Reddy (2004a) |
| N-Methyl-d-aspartate | >200 (N.D.) | Reddy (2004a) |
| 4-Aminopyridine | >200 (N.D.) | Reddy (2004a) |
| Status epilepticus model | ||
| Pilocarpine | 81 (45–143) | D.S. Reddy, unpublished data |
| Electroshock models | ||
| Maximal electroshock | N.D. | |
| 6 Hz | N.D. |
N.D., not determined.
Fig. 7.
Representative traces illustrating androstanediol inhibition of afterdischarge in fully kindled mice. Traces show depth electroencephalogram recordings from a stimulating electrode in the right hippocampus. Arrows indicate onset of the 1-s kindling stimulus, which is followed by the stimulus artifact (mostly blanked to prevent saturation of the amplifier circuitry). In each trace, a slow negative wave after stimulation was digitally subtracted. Androstanediol was administered subcutaneously 15 min before the stimulation; control trace was obtained without drug treatment.
Fig. 8.
Stereoselective protective effects of the 3-hydroxy epimers of androstanediol in the kindling seizure model. Comparative effects of 100 mg/kg 3β-androstanediol (3β-AD) with the natural 3α-isomer (3α-AD) at the same dose on behavioral seizure stage (A) and afterdischarge duration (B) in fully kindled mice. Androstanediol epimers or vehicle was administered subcutaneously 15 min before stimulations. Kindling experimental conditions are similar as described in Fig. 6. Each bar represents the mean ± S.E.M. of data from six to eight animals. *, p < 0.01 versus control.
Time Course for Seizure Protection by Androstanediol in Fully Kindled Mice.
The time courses for seizure protection after a 2× ED50 dose of androstanediol are shown in Fig. 6, D–F. Androstanediol (100 mg/kg s.c.) exhibited a rapid onset for seizure protection. The protection was maximal at 15 min and diminished during the 180-min period after the injection as evident by its time-dependent decrease in behavioral seizures (Fig. 6D), afterdischarge duration (Fig. 6E), and percentage of protection of behavioral and electrographic seizures (Fig. 6F). On the day after androstanediol treatment, all mice exhibited stage 5 seizures, indicating that androstanediol treatment suppresses the expression of kindled seizures but does not alter the kindled state. These results are compatible with the possibility that androstanediol protection is due to potentiation of GABA-A receptor inhibition that occur rapidly within few minutes.
Effect of Flutamide on Antiseizure Activity of Androstanediol.
There are two potential mechanisms by which androstanediol causes seizure protection: potentiation of GABAA receptor-mediated inhibition and direct interaction with intracellular androgen receptors (ARs), which are the major targets for endocrine effects of testosterone. Although androstanediol binds poorly to AR, it may indirectly affect ARs by intracellular reverse reduction to dihydrotestosterone, a potent AR agonist with moderate antiseizure activity (Reddy, 2004b). Thus, it is possible that the protective activity of androstanediol could be due to its interaction with ARs. To test this conjecture, the antiseizure activity of androstanediol was examined in the presence of flutamide, a competitive AR antagonist. Pretreatment with flutamide (100 mg/kg i.p.) produced little or no effect on the antiseizure activity of androstanediol as reflected by similar behavioral seizure stage (0.2 ± 0.16 androstanediol versus 0.3 ± 0.26 flutamide + androstanediol) and afterdischarge durations (13 ± 2 androstanediol versus 14 ± 5 flutamide + androstanediol) in fully kindled mice. Flutamide alone had no significant effect on the kindling seizure stage (4.8 ± 0.16 vehicle versus 4.7 ± 0.33 flutamide alone) or the afterdischarge duration (45 ± 4 vehicle versus 43 ± 5 flutamide alone). These results indicate that androstanediol does not appear to protect against kindling seizures through classical steroid hormone receptors.
Effects of Androstanediol in the Motor Toxicity Test.
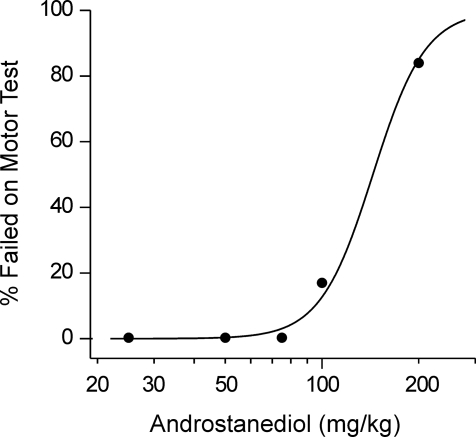
Agents modulating GABAA receptors are expected to produce sedation and motor impairment at very high doses (Reddy, 2003). The horizontal screen test was used to determine the sedative/motor-impairing action of androstanediol. The neurosteroid was administered 15 min before testing to groups of six to eight mice at doses of 25 to 200 mg/kg. As shown in Fig. 9, androstanediol caused a dose-dependent increase in percentage of animals failing in the screen test; one of the six animals receiving the 100-mg/kg dose of androstanediol scored positive in the test, whereas at 200 mg/kg, five of six animals showed impairment. The TD50 (95% confidence limit) value was 148 (96–226) mg/kg. The protective index of androstanediol (TD50/ED50) was 3, indicating a clear separation of androstanediol doses producing seizure protection from doses that produce sedation/motor impairment in the horizontal screen test.
Fig. 9.
Androstanediol effects on sedation/motor toxicity in mice. Androstanediol (25–200 mg/kg) was administered subcutaneously 15 min before the horizontal screen test. Values represent the percentage animals failed in the motor test (n = 6–8 animals).
Androstanediol Plasma Levels: Correlation with Seizure Protection and Motor Toxicity.
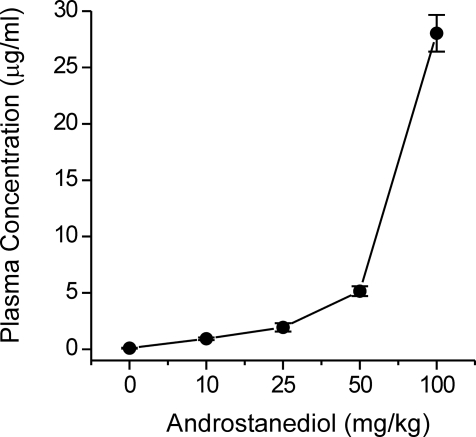
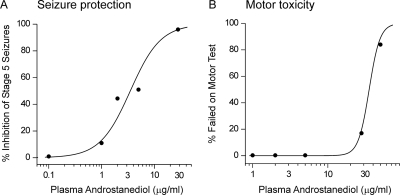
To correlate whether plasma levels at doses that protect against kindling seizures relate to the actions on GABAA receptors, androstanediol plasma concentrations were determined 15 min after administration of various doses of androstanediol (10–100 mg/kg s.c.) in mice. As shown in Fig. 10, androstanediol plasma levels increased in a dose-dependent manner with increasing neurosteroid dose given to mice. Figure 11 shows logistic fits to plots of seizure protection or motor toxicity against plasma androstanediol concentration. These graphs were used to estimate the plasma concentrations of androstanediol producing 50% seizure protection (EC50). The EC50 value of androstanediol for seizure protection was 3.1 μg/ml (10.6 μM), which is within the range of concentrations that cause positive allosteric potentiation of GABAA receptors in the hippocampal CA1 neurons (EC50 of 5 μM; Fig. 3B). It is likely that target concentrations in the brain do not fully reflect the plasma concentrations due to local synthesis and distribution factors. Nevertheless, these results indicate that the protective effect of androstanediol against the kindling seizures is caused by potentiation of GABAA receptor-mediated inhibition in the hippocampus.
Fig. 10.
Plasma androstanediol levels in mice treated with androstanediol (10–100 mg/kg s.c.). Plasma samples were collected 15 min after administration of the indicated dose of neurosteroid. Values represent the mean ± S.E.M. of levels in four to five animals.
Fig. 11.
Correlation between plasma androstanediol levels and seizure protection in the kindling model. A, plasma androstanediol levels after various doses of androstanediol (from Fig. 10) were plotted against kindling seizure protection values from experiment in Fig. 6A. The data points were fit with a logistic function (EC50 = 3.1 μg/ml). B, similar analysis from the sedative/motor toxicity experiment in Fig. 9 (EC50 = 29 μg/ml).
Discussion
The principal observation of this study is that the testosterone-derived neurosteroid androstanediol has GABAA receptor-modulating activity in the adult hippocampus. Consistent with GABAA receptor-modulating properties, androstanediol has powerful antiseizure activity in the mouse hippocampus kindling model of epilepsy. Plasma androstanediol levels associated with seizure protection are found to be within the range of concentrations that potentiate GABAA receptors, confirming that the target for the antiseizure effects is probably the GABAA receptor. These results support the possibility that androstanediol modulation of GABAergic inhibition could play an important role in men with epilepsy and sex differences in seizure susceptibility.
Androstanediol is an endogenous neurosteroid produced from testosterone. The mechanism of androstanediol actions has not been completely elucidated, although it meets the structural requirements for a steroid allosteric modulator of GABAA receptors (Majewska et al., 1986; Kokate et al., 1994). Our results demonstrate that androstanediol exhibits functional actions on GABAA receptors similar to that of allopregnanolone. We found that the neurosteroid markedly potentiates responses to GABA in acutely dissociated CA1 pyramidal neurons in a concentration-dependent manner (EC50 of 5 μM), with a 4-fold increase in current amplitude at high concentrations. The maximal potentiation of androstanediol is comparable with the prototypic neurosteroid allopregnanolone, but androstanediol is less potent, indicating that the C17 side chain drastically alters neurosteroid potency (Peters et al., 1988; Puia et al., 1990; Wohlfarth et al., 2002). A critical structural characteristic of GABAA receptor-modulating neurosteroids is that they are reduced at the 5- and 3-positions of the A-ring (Reddy, 2003). Among such structures, maximal potency is obtained when the substituents at the 3- and 5-positions are in the α-configuration. In contrast to androstanediol, the 3β-epimer failed to potentiate responses to exogenous GABA, which is extremely consistent with the stereoselective effects of neurosteroids at GABAA receptors (Gee et al., 1988; Kokate et al., 1994). Overall, these studies strongly support that androstanediol is a positive modulator of GABAA receptors.
Androstanediol is a neurosteroid because it is synthesized within the brain (Reddy, 2008). In addition, the peripherally produced androstanediol is able to access the brain because it could readily cross the blood-brain barrier. Androstanediol is produced from testosterone de novo by glial cells and principal neurons (Martini et al., 1993; Agís-Balboa et al., 2006). Neurosteroid synthesis occurs in various regions of the brain such as cortex, hippocampus, and amygdala. Within these brain regions, neurosteroid synthesis enzymes are localized to glutamatergic principal neurons and not GABAergic inhibitory neurons (Agís-Balboa et al., 2006); thus, androstanediol may be synthesized within the same neurons that express its GABAA receptor targets. Because neurosteroids mainly influence the decay of synaptically generated currents, androstanediol, at these local sites, could have a neurosteroid role in the modulation of neuronal excitability.
The modulating effects of neurosteroids occur by binding to discrete sites on the GABAA receptor that are located within the transmembrane domains of the α- and β-subunits (Hosie et al., 2006). Like allopregnanolone, androstanediol is believed to bind to these sites. The binding site for androstanediol is proposed to be distinct from that of the GABA, benzodiazepine, and barbiturate sites (Frye et al., 1996; Reddy, 2004a). The precise nature of the androstanediol binding site remains unclear. Exposure to neurosteroids enhances the open probability of the GABAA receptor, resulting in chloride influx and ultimately resulting in a reduced neuronal excitability.
Neurosteroids modulate most GABAA receptor isoforms but are specifically more potent at δ-subunit-containing GABAA receptors. Although we did not estimate the subunit composition of GABAA receptors in adult CA1 pyramidal neurons used in the study, GABAA receptors composed of the α1β2γ2- and α2β2γ2-subunits are abundant subunit combinations in the CA1 region, whereas the neurosteroid-sensitive α1β2δ-subunit composition is generally present in the dentate granule cells. Androstenol, an androstanediol-like steroid, had comparable efficacy in potentiating GABA currents in human embryonic kidney-293 cells transfected with α1β2γ2 or α2β2γ2-subunit isoforms to that obtained with native receptors in hippocampus neurons (Kaminski et al., 2006). Because δ-subunit-containing receptors are not usually present in the adult CA1 pyramidal cells used in the present study, the effect of androstanediol on such receptors isoforms expressed in granule cells remains to be determined in future studies. Moreover, androstanediol at higher concentrations (30 μM) directly activated the GABAA receptors, as has been observed previously for other neurosteroids (Kokate et al., 1994; Wohlfarth et al., 2002). However, such responses are moderate (<25% of control GABA response), suggesting that androstanediol is less efficacious for direct actions than allopregnanolone and allotetrahydrodeoxycorticosterone. Nevertheless, it is possible that high levels of androstanediol could elicit direct currents and may modulate tonic currents mediated by δ-subunit-containing extrasynaptic GABAA receptors.
Our results show that systemic administration of androstanediol has antiseizure effects in the hippocampus kindling model. We found that androstanediol has dose-dependent antiseizure activity, with an ED50 value of 50 mg/kg. The protective activity of androstanediol is stereoselective, because the 3β-epimer of androstanediol has no protective activity. These results are consistent with our previous studies of androstanediol in other seizure models (Reddy, 2004a,b). Androstanediol protects mice against seizures induced by GABAA receptor antagonists and pilocarpine (Frye and Reed, 1998; Reddy, 2004a,b). The potency in the kindling model was comparable with that of the pentylenetetrazol model, but more than the pilocarpine model (Table 1). Androstanediol has longer protection than other neurosteroids in the kindling model. In accordance with the limited protective efficacy of neurosteroids against seizures induced by kainic acid (Kokate et al., 1996), androstanediol does not fully protect against seizures induced by glutamate receptor agonists (Table 1). Overall, the antiseizure profile of androstanediol is highly consistent with other GABAA receptor modulating neurosteroids and may be due to its interaction with GABAA receptors in the hippocampus.
The measurements of plasma neurosteroid levels allowed us to estimate the plasma concentrations associated with seizure protection in the kindling model. The EC50 value of androstanediol in the kindling model (10.6 μM; Fig. 11A) is within the range of concentrations that modulate GABAA receptors in acutely dissociated CA1 neurons (EC50 of 5 μM; Fig. 3B). Although androstanediol levels near the receptor sites in the hippocampus are not known, brain levels are expected to rise with plasma levels due to high lipophilicity of this neurosteroid. It seems likely that hippocampus concentrations may be higher due to local synthesis. It has been previously shown that neurosteroids, like other GABAA receptor modulators, can produce sedation and motor impairment at higher doses (Kokate et al., 1994). At very high doses, androstanediol caused gross impairment of motor function, as is also typical of allopregnanolone.
Age-related changes in androstanediol levels may profoundly affect anxiety, cognitive function and seizure susceptibility (Reddy, 2003; Pinna et al., 2005). Aging is associated with low levels of testosterone that could lead to declining synthesis of androstanediol in the brain (Frye et al., 2010). The incidence of epilepsy is approximately 15% higher in men than in women, and men with epilepsy exhibit testosterone deficiency. Studies in animals have shown that decreased testosterone is associated with higher incidences of seizures and replacement with testosterone attenuates seizures (Thomas and McLean, 1991; Reddy, 2008). Because testosterone elevates seizure threshold via androstanediol (Reddy, 2004b), a decrease in testosterone levels, such as occur in hypogonadism and enzyme-inducing drug therapy (Herzog et al., 2006), may therefore contribute to seizure exacerbation. The introduction of finasteride, a 5α-reductase inhibitor that blocks androstanediol synthesis, for the treatment of male pattern baldness led to recurrent seizures. In addition, inhibition of 5α-reductase by finasteride in humans or a genetic deficiency in the 5α-reductase type I enzyme in mice can result in alterations in seizure susceptibility (Frye et al., 2001). Anxiolytic effect of testosterone also requires its conversion to androstanediol. Replacement of androstanediol in aged animals is shown to prevent cognitive deficits and anxiety behavior (Frye et al., 2010). Overall, androstanediol may be a key neurosteroid in age-related brain disorders.
Androstanediol may play a role in sex differences in brain disorders. The incidence of epilepsy is generally higher in men than in women (Christensen et al., 2005). Sex differences in seizure susceptibility have long been attributed to sex steroid hormones. Like allopregnanolone (Reddy et al., 2004), androstanediol has sex-dependent antiseizure activity, which is not related to pharmacokinetic factors (D. S. Reddy, unpublished data). Androstanediol is also present in abundant amounts in females. Serum levels of androstanediol in men and women are 3 to 5 nM, which is in the same range as the concentrations of allopregnanolone in women in the luteal phase of the menstrual cycle (Galli et al., 2001). Therefore, it is conceivable that sex differences in the molecular targets or action of androstanediol could be involved in its gender-specific actions.
In conclusion, our results demonstrate for the first time that the testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors and has powerful antiseizure activity in the mouse hippocampus kindling model of epilepsy. Thus, it is possible that androstanediol could play a key role in men with epilepsy, especially during age-related decline in androgen levels and also in gender-related differences in epilepsy, anxiety, and other brain disorders.
Acknowledgments
We thank Drs. William Griffith, Gerald Frye, David Murchison (Texas A&M Health Science Center), and Hugh Criswell (University of North Carolina at Chapel Hill) for advice in patch-clamp studies.
This research was supported in part by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS051398].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.169854.
- ACSF
- artificial cerebrospinal fluid
- AR
- androgen receptor.
References
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. (2006) Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci USA 103:14602–14607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright PS, Burnham WM. (1980) Development of a new pharmacological seizure model: effects of anticonvulsants on cortical- and amygdala-kindled seizures in the rat. Epilepsia 21:681–689 [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. (2002) The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology 43:651–661 [DOI] [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. (1989) Anticonvulsant profile of the progesterone metabolite 5α-pregnan-3α-ol-20-one. Eur J Pharmacol 166:325–329 [DOI] [PubMed] [Google Scholar]
- Christensen J, Kjeldsen MJ, Andersen H, Friis ML, Sidenius P. (2005) Gender differences in epilepsy. Epilepsia 46:956–960 [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. (1997) The Mouse Brain in Stereotaxic Coordinates. Academic Press, San Diego, CA [Google Scholar]
- Frye CA, Van Keuren KR, Erskine MS. (1996) Behavioral effects of 3α-androstanediol. I: Modulation of sexual receptivity and promotion of GABA-stimulated chloride flux. Behav Brain Res 79:109–118 [DOI] [PubMed] [Google Scholar]
- Frye CA, Reed TA. (1998) Androgenic neurosteroids: anti-seizure effects in an animal model of epilepsy. Psychoneuroendocrinology 23:385–399 [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Walf AA, Harney JP. (2001) Testosterone reduces pentylenetetrazol-induced ictal activity of wildtype mice but not those deficient in type I 5alpha-reductase. Brain Res 918:182–186 [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger K, Lephart ED, Walf AA. (2010) 3α-Androstanediol, but not testosterone, attenuates age-related decrements in cognitive, anxiety, and depressive behavior of male rats. Front Aging Neurosci 2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli R, Luisi M, Pizzanelli C, Monteleone P, Casarosa E, Iudice A, Murri L. (2001) Circulating levels of allopregnanolone, an anticonvulsant metabolite of progesterone, in women with partial epilepsy in the postcritical phase. Epilepsia 42:216–219 [DOI] [PubMed] [Google Scholar]
- Gee KW, Bolger MB, Brinton RE, Coirini H, McEwen BS. (1988) Steroid modulation of the chloride ionophore in rat brain: structure-activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther 246:803–812 [PubMed] [Google Scholar]
- Harrison NL, Majewska MD, Harrington JW, Barker JL. (1987) Structure-activity relationships for steroid interaction with the γ-aminobutyric acidA receptor complex. J Pharmacol Exp Ther 241:346–353 [PubMed] [Google Scholar]
- Herzog AG, Drislane FW, Schomer DL, Pennell PB, Bromfield EB, Dworetzky BA, Farina EL, Frye CA. (2006) Differential effects of antiepileptic drugs on neuroactive steroids in men with epilepsy. Epilepsia 47:1945–1948 [DOI] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. (2009) Conserved site for neurosteroid modulation of GABA-A receptors. Neuropharmacology 56:149–154 [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. (2006) Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 444:486–489 [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Marini H, Ortinski PI, Vicini S, Rogawski MA. (2006) The pheromone androstenol (5α-androst-16-en-3α-ol) is a neurosteroid positive modulator of GABAA receptors. J Pharmacol Exp Ther 317:694–703 [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Marini H, Kim WJ, Rogawski MA. (2005) Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia 46:819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR, Wong RK. (1986) Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J Neurosci Methods 16:227–238 [DOI] [PubMed] [Google Scholar]
- Kokate TG, Cohen AL, Karp E, Rogawski MA. (1996) Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology 35:1049–1056 [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. (1994) Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther 270:1223–1229 [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. (1986) Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232:1004–1007 [DOI] [PubMed] [Google Scholar]
- Martini L, Melcangi RC, Maggi R. (1993) Androgen and progesterone metabolism in the central and peripheral nervous system. J Steroid Biochem Mol Biol 47:195–205 [DOI] [PubMed] [Google Scholar]
- Mensah-Nyagan AG, Do-Rego JL, Beaujean D, Luu-The V, Pelletier G, Vaudry H. (1999) Neurosteroids: expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol Rev 51:63–81 [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. (1999) Sulfated and unsulfated steroids modulate GABAA receptor function through distinct sites. Brain Res 830:72–87 [DOI] [PubMed] [Google Scholar]
- Peters JA, Kirkness EF, Callachan H, Lambert JJ, Turner AJ. (1988) Modulation of the GABAA receptor by depressant barbiturates and pregnane steroids. Br J Pharmacol 94:1257–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. (2005) Changes in brain testosterone and allopregnanolone biosynthesis elicit aggressive behavior. Proc Natl Acad Sci USA 102:2135–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. (1990) Neurosteroids act on recombinant human GABAA receptors. Neuron 4:759–765 [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Paul SM. (1991) Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA 88:4553–4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294 [DOI] [PubMed] [Google Scholar]
- Reddy DS. (2003) Pharmacology of endogenous neuroactive steroids. Crit Rev Neurobiol 15:197–234 [DOI] [PubMed] [Google Scholar]
- Reddy DS. (2004a) Anticonvulsant activity of the testosterone-derived neurosteroid 3α-androstanediol. Neuroreport 15:515–518 [DOI] [PubMed] [Google Scholar]
- Reddy DS. (2004b) Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3α-androstanediol and 17β-estradiol. Neuroscience 129:195–207 [DOI] [PubMed] [Google Scholar]
- Reddy DS. (2008) Mass spectrometric quantification and physiological-pharmacological activity of androgenic neurosteroids. Neurochem Int 52:541–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. (2009) The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res 85:1–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. (2002) Stress-induced deoxycorticosterone-derived neurosteroids modulate GABAA receptor function and seizure susceptibility. J Neurosci 22:3795–3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. (2010) Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Res 89:254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Castaneda DC, O'Malley BW, Rogawski MA. (2004) Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther 310:230–239 [DOI] [PubMed] [Google Scholar]
- Reddy DS, Chien B, Ramu K. (2005) A high-performance liquid chromatography-tandem mass spectrometry assay of the androgenic neurosteroid 3-androstanediol (5-androstan-3,17-diol) in plasma. Steroids 70:879–885 [DOI] [PubMed] [Google Scholar]
- Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJ, Garcia-Segura LM, Lambert JJ, Mayo W, Melcangi RC, Parducz A, et al. (2003) Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol 71:3–29 [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. (1998) GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 392:926–930 [DOI] [PubMed] [Google Scholar]
- Thomas J, McLean JH. (1991) Castration alters susceptibility of male rats to specific seizures. Physiol Behav 49:1177–1179 [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. (2002) Enhanced neurosteroid potentiation of ternary GABA-A receptors containing the δ subunit. J Neurosci 22:1541–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]