Abstract
Organic anion transporter 6 (Oat6; Slc22a20), a member of the OAT family, was demonstrated previously to mediate the transport of organic anions (Am J Physiol Renal Physiol 291:F314–F321, 2006). In the present study, we sought to further delineate the function of murine Oat6 (mOat6) by analyzing the effect of select organic anions on mOat6-mediated transport by using a Chinese hamster ovary (CHO) cell line stably expressing mOat6 (CHO-mOat6). When examined, kinetic analysis demonstrated that the mechanism of inhibition of mOat6 and mOat3 was competitive. Homovanillic acid, 5-hydroxyindole acetic acid, 2,4-dihydroxyphenylacetate, hippurate, and dehydroepiandrosterone sulfate (DHEAS) each significantly reduced mOat6 activity with inhibitory constant (Ki) values of 3.0 ± 0.5, 48.9 ± 10.3, 61.4 ± 7.1, 59.9 ± 4.9, and 38.8 ± 3.1 μM, respectively. Comparison to Ki values determined for mOat3 (67.8 ± 7.2, 134.5 ± 27.0, 346.7 ± 97.9, 79.3 ± 4.0, and 3.8 ± 1.1 μM, respectively) revealed that there are significant differences in compound affinity between each transporter. Fluoroquinolone antimicrobials and reduced folates were without effect on mOat6-mediated uptake. Investigation of testicular cell type-specific expression of mOat6 by laser capture microdissection and quantitative polymerase chain reaction revealed significant mRNA expression in Sertoli cells, but not in Leydig cells or spermatids. Overall, these data should aid further refinements to the interpretation and modeling of the in vivo disposition of OAT substrates. Specifically, expression in Sertoli cells suggests Oat6 may be an important determinant of blood-testis barrier function, with Oat6-mediated transport of estrone sulfate and DHEAS possibly representing a critical step in the maintenance of testicular steroidogenesis.
Introduction
The solute carrier (SLC) superfamily is comprised of transporter families involved in the cellular uptake and secretion of endogenous and xenobiotic molecules. It includes the proton-dependent oligopeptide transporter (SLC15) family, the organic cation/anion/zwitterion transporter (SLC22) family, the nucleoside transporter (SLC29) family, and the multidrug and toxin extrusion (SLC47) family, among others. The organic anion transporters (OATs; SLC22) are centrally involved in the distribution and elimination of small (∼150–500 Da) organic compounds that exist as anions at physiological pH (Sweet et al., 2001; Russel et al., 2002). Eleven OATs (OATs 1–10 and Urat1) have been cloned and characterized, and their substrates encompass diuretics, antibiotics, antivirals, plant polyphenols, mycotoxins, uremic toxins, and hormone and neurotransmitter metabolites (Sweet, 2005; VanWert et al., 2010). It is now widely established that transporters (including OATs) frequently affect the clinical pharmacokinetics, efficacy, and toxicity of substrate drugs. In fact, concern over transporter-based drug interactions prompted the U.S. Food and Drug Administration to participate in the drafting of guidances to help establish and standardize tools and protocols to appropriately assess transporter involvement in these parameters during the drug development process (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072101.pdf; Zhang et al., 2008; Giacomini et al., 2010). Furthermore, whereas most OATs are expressed in multiple barrier epithelia, some exhibit organ-specific patterns, e.g., expression of Oat5 and Urat1 is kidney-specific, Oat6 is confined to olfactory mucosa and testis, and OAT7 seems to be exclusively hepatic (Enomoto et al., 2002a; Monte et al., 2004; Youngblood and Sweet, 2004; Shin et al., 2007). These patterns of expression are indicative of a role for OATs in organ-specific functions and/or organ-specific toxicities associated with environmental toxins (aflatoxin B1, aristolochic acid), endogenous toxins (hippurate, indoxyl sulfate), heavy metals (mercury), and therapeutics (fluoroquinolones and antivirals) (Sweet, 2005; VanWert et al., 2010).
As alluded to above, Oat6 is expressed in testis; however, at the molecular level very little is known about organic solute transporter expression or activity in this tissue. Two main functions of the testis are production of spermatozoa and steroidogenesis, both of which are complex processes that require membrane-impermeable substances. Within the architecture of the testis are two membrane barriers, the blood-testis barrier (BTB) and the blood-epididymal barrier (BEB). Studies with P-gp or Bcrp knockout mice found increased testicular accumulation of transporter substrates, and increased cytotoxicity of anticancer drugs was observed in the seminiferous tubules of Mrp1 knockout mice (Schinkel et al., 1994; Wijnholds et al., 1998; Enokizono et al., 2007). These data indicate that Pgp, Bcrp, and Mrp1 serve to limit testicular permeation of substrates and that they are a vital component of testicular barrier function. In addition, the Slc22 family members OctN2 and OctN3 were detected in murine epididymal spermatozoa by immunohistochemistry and proposed to mediate delivery of carnitine, which is required for the maturation and activation of spermatazoa (Kobayashi et al., 2007). Further information about testicular expression of organic solute transporters is limited mostly to detection of mRNA transcripts in preparations from whole testis or partially purified Sertoli cells, including Mdr2, Mrp5, Mrp7, Mrp8, Oatp3, Ent1–3, Cnt1–3, Oat2, and Oct3 (Monte et al., 2004; Augustine et al., 2005; Kato et al., 2005). Oat6 expression in testis, and its ability to handle sulfated steroids such as estrone sulfate (ES), suggests that Oat6 also may be an important component of the BTB and/or BEB. Initial investigation found Oat6 activity to be subject to trans-stimulation by glutarate, suggesting Oat6 is an organic anion/dicarboxylate exchanger (Schnabolk et al., 2006). Oat6 could therefore contribute to the maintenance of testis homeostasis either by limiting testicular permeation of harmful endogenous and/or exogenous substrates or promoting uptake of different endogenous and/or exogenous substrates required for spermatogenesis. To properly define the role Oat6 plays in the function of the testis, it is also necessary to define its substrate specificity and establish its localization at the cellular level within the testis.
Toward that end, the present study sought to expand our knowledge of the classes of substrates, including reduced folates, fluoroquinolones, uremic toxins, and neurotransmitter and steroid metabolites that interact with Oat6. In addition, because protein sequence comparisons revealed that Oat6 shares the highest degree of homology with Oat1 and Oat3, and previous investigations established that estrone sulfate is a substrate shared by Oat6 and Oat3 with similar affinity (Schnabolk et al., 2006; VanWert et al., 2008), we derived inhibitory constants (Ki values) for compounds interacting with Oat6 and Oat3, using the same heterologous expression system for each transporter. Finally, laser capture microdissection combined with quantitative polymerase chain reaction was used to determine the distribution of Oat6 expression among testicular cell-type populations.
Materials and Methods
Chemicals.
The radiolabeled chemicals para-aminohippurate (PAH), 2,4-dichlorophenoxyacetate (2,4-D), and [3H]salicylate were purchased from American Radiolabeled Chemicals (St. Louis, MO); benzylpenicillin ([3H]penicillin G) was purchased from GE Healthcare (Little Chalfont, Buckinghamshire, UK); and ES was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). Unlabeled ES, PAH, 2,4-D, penicillin G, probenecid, homovanillic acid (HVA), 2,4-dihydroxyphenylacetic acid (DOPAC), dehydroepiandrosterone sulfate (DHEAS), 5-hydroxyindoleacetic acid (5-HIAA), folic acid, folinic acid (leucovorin), and 5-methyltetrahydrofolate (5-CH3-THF) were purchased from Sigma-Aldrich (St. Louis, MO). Salicylate, hippuric acid (HA), and ciprofloxacin were purchased from Thermo Fisher Scientific (Waltham, MA). Methotrexate, norfloxacin, and ofloxacin were purchased from MP Biomedicals (Solon, OH), and gatifloxacin was purchased from LKT Labs (St. Paul, MN). All chemicals were of reagent grade.
Tissue Culture.
Derivation of the CHO-mOat3 and CHO-mOat6 stably transfected cell lines has been described previously (Schnabolk et al., 2006; VanWert and Sweet, 2008). Cell lines were maintained at 37°C with 5% CO2 in Ham's F-12 medium (Mediatech, Herndon, VA) containing 10% serum, 1% penicillin/streptomycin, and 125 μg/ml hygromycin B.
Cell Transport Assays.
Accumulation assay protocols were adapted from those published previously (Schnabolk et al., 2006; VanWert and Sweet, 2008; VanWert et al., 2008). Two days before uptake assays, cell lines were plated in 24-well tissue culture plates (2 × 105 cells per well). Medium containing antibiotics was replaced with fresh medium without antibiotics ∼24 to 36 h before the uptake assays were performed. Before the transport experiments, cells were equilibrated with 500 μl of transport buffer [Hanks' balanced salt solution containing 10 mM HEPES (Sigma-Aldrich), pH 7.4] for 10 min. After equilibration, the buffer was removed and replaced with 500 μl of fresh transport buffer containing 1 to 200 μM [3H]ES (0.25 μCi/ml), 50 μM [3H]PAH (0.25 μCi/ml), 50 μM [3H]salicylate (0.25 μCi/ml), 25 μM [14C]2,4-D (0.25 μCi/ml), or 25 μM [3H]penicillin G (0.25 μCi/ml) in the presence or absence of inhibitors as indicated. Cells were incubated for 1, 2, or 15 min, the medium was removed, and cells were immediately washed three times with ice-cold transport buffer. Substrate concentrations and accumulation times used for kinetic analyses (mOat6: 5 μM ES for 2 min, Km = 45 μM; mOat3: 1 μM ES for 1 min, Km = 14 μM) were based on previous determinations (Schnabolk et al., 2006; VanWert et al., 2008). Cells were dissolved with 500 μl of 1 M NaOH and neutralized with 50 μl of 10 M HCl, and aliquots were removed for liquid scintillation counting by using Ecoscint H (National Diagnostics, Atlanta, GA) and protein determination by using a Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA). Substrate accumulation was calculated as picomole of substrate per microgram of protein, and data were analyzed by nonlinear regression using mixed-model inhibition with Prism software (GraphPad Software Inc., San Diego, CA). Inhibition constant values (Ki) were determined by using nonlinear regression and the one-site competition model in Prism software. Lineweaver-Burk plots are presented solely for the purpose of visually displaying the mode of inhibition data. Results were confirmed by performing experiments three times in triplicate (three wells of cells/determination/experiment).
mOat6 Testicular Expression.
Three male C57BL6/J mice (12–16 weeks) were euthanized, and whole testis and epididymis were freshly isolated, snap-frozen in liquid nitrogen, and stored at −80°C before being processed for laser capture microdissection (LCM). For LCM, sections ∼8 μm thick were cut from the frozen tissues and stained with hematoxylin and eosin. Clusters of Sertoli cells, Leydig cells, spermatids, and epididymal duct cells were then isolated according to their histological location in the tissue, using the Veritas laser capture microdissection system (Molecular Devices, Sunnyvale, CA). Total RNA was extracted from captured cells by using the RNAqueous-micro extraction kit (Ambion, Austin, TX), including DNase-I digestion to remove genomic DNA contamination. RNA purity and concentration was assessed by RNA integrity numbers using the Bioanalyzer RNA pico assay (Agilent Technologies, Santa Clara, CA). Samples were prepared by using TaqMan Reverse Transcription and Universal PCR Master Mix Reagents and analyzed with the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). Gene expression levels of Oat6 and Gapdh were determined by using TaqMan chemistry and real-time quantitative reverse transcriptase polymerase chain reaction (QPCR). Because of the limited amount of RNA, the LCM samples were run in duplicate. The probes and primer sets used for detection of mOat6 (Mm01227885_m1 for Slc22a20) were obtained from inventoried assays (Applied Biosystems). For all samples, Gapdh from the Predeveloped TaqMan Assay Reagents (Applied Biosystems) was used as endogenous control. Thus, gene-specific probes labeled at the 5′ end with 6-carboxyfluorescein and at the 3′ end with a dark quencher were used for the detection of the target genes of interest. The cycling conditions were 48°C for 30 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The 2−ΔΔCt method was used to calculate fold changes in the expression levels of the genes of interest (Livak and Schmittgen, 2001).
Statistics.
Data are presented as mean ± S.E. The unpaired Student's t test was used to make comparisons. Mean value differences were considered significant at p ≤ 0.05.
Results
Selectivity of mOat6 Inhibition.
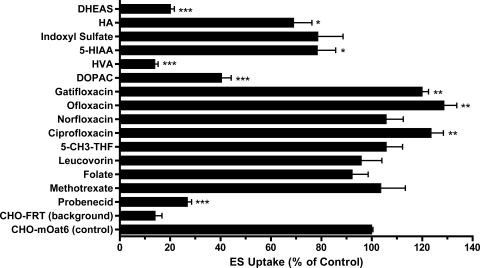
The effect of various classes of organic anions on mOat6-mediated uptake of ES was analyzed in stably transfected CHO cells (Fig. 1). These organic anions included reduced folates, fluoroquinolones, neurotransmitters, uremic toxins, and steroids. Mock-transfected CHO-FRT cells exhibited a low, probenecid-insensitive background for ES that was equal to ∼15% of the total accumulation obtained in transporter-expressing cells (CHO-FRT: 0.061 ± 0.013 pmol/μg protein/15 min versus CHO-mOat6: 0.428 ± 0.010 pmol/μg protein/15 min). The prototypical OAT inhibitor probenecid blocked ∼90% of mOat6-mediated uptake when present at 100-fold excess concentration (Fig. 1). Methotrexate, folate, leucovorin, and 5-CH3-THF failed to produce a significant effect on mOat6-mediated ES uptake. Of the fluoroquinolones, only norfloxacin failed to affect ES uptake, whereas ciprofloxacin, ofloxacin, and gatifloxacin all induced a slight increase (∼20–30%; Fig. 1). Conversely, the catecholamine neurotransmitter metabolites 5-HIAA, DOPAC, and HVA all produced significant inhibition of mOat6-mediated ES uptake at levels of approximately 35, 75, and 100%, respectively. The uremic toxins HA and indoxyl sulfate both inhibited ES uptake (∼36 and ∼25%, respectively); however, the effect of indoxyl sulfate (Oat3 substrate) did not reach significance. The steroid metabolite DHEAS virtually abolished ES uptake.
Fig. 1.
Inhibition profile of mOat6. Inhibition of mOat6-mediated uptake of [3H]ES (5 μM) by DHEAS, HA, indoxyl sulfate, 5-HIAA, HVA, DOPAC, gatifloxacin, ofloxacin, norfloxacin, ciprofloxacin, 5-CH3-THF, leucovorin, folate, methotrexate, and probenecid (500 μM) was measured in CHO-mOat6 cells (15 min). Background ES accumulation was measured in CHO-FRT cells in the absence of inhibitor and is shown to provide a clear gauge of the low background noise in the experimental system. Raw accumulation values for CHO-FRT and CHO-mOat6 cells were 0.061 ± 0.013 versus 0.428 ± 0.010 pmol/μg protein/15 min, respectively. Values are mean ± S.E. of triplicate values. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Mode of Inhibition.
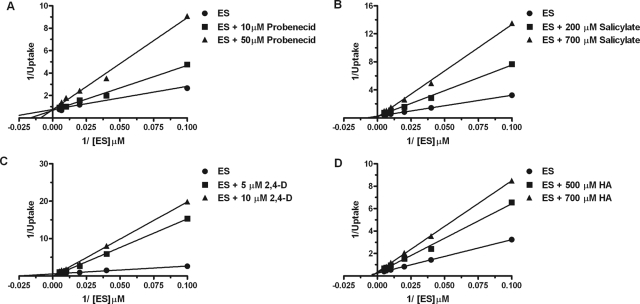
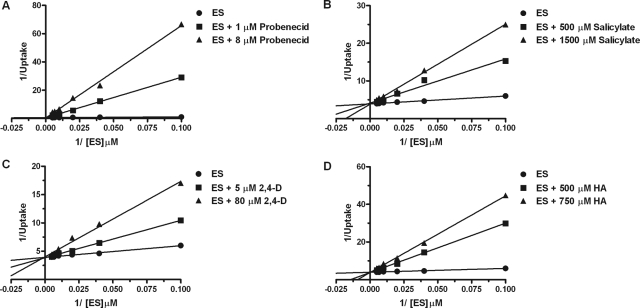
The mechanism of inhibition of mOat6- and mOat3-mediated transport of estrone sulfate was investigated for inhibitory compounds identified in Fig. 1, such as probenecid, salicylate, 2,4-D, and hippuric acid. Time course evaluations in CHO-mOat6 and CHO-mOat3 cells indicated ES accumulation was linear through at least the first 5 min (data not shown; VanWert et al., 2008). Nonlinear regression analysis of background-corrected data using mixed-model inhibition revealed that probenecid, salicylate, 2,4-D, and hippuric acid inhibited both mOat6- and mOat3-mediated uptake of estrone sulfate in a competitive manner. Mode of inhibition for each compound was determined in this manner; however, Lineweaver-Burk plots were used to graphically present the data. In the Lineweaver-Burk plots these results are visualized as a changing x-intercept (increasing Km values) and consistent y-intercept (steady Vmax values) in the presence of increasing inhibitor concentrations for both mOat6 and mOat3 (Figs. 2 and 3).
Fig. 2.
Competitive inhibition of mOat6-mediated transport. Two-minute cellular accumulation assays were performed with 10, 25, 50, 100, 150, and 200 μM [3H]ES in the absence and presence of varying concentrations of probenecid (A), salicylate (B), 2,4-D (C), and hippuric acid (D). Data were corrected for nonspecific background measured in the CHO-FRT (control) cells, and saturation curves were generated. Kinetic constant determination was done by using nonlinear regression analysis; however, Lineweaver-Burk transformations of the data are displayed to aid visualization of the mode of inhibition as competitive. Each experiment was repeated three times in triplicate. Data shown are mean values ± S.E. (n = 3) from a representative experiment.
Fig. 3.
Competitive inhibition of mOat3-mediated estrone sulfate transport. Two-minute cellular accumulation assays were performed with 10, 25, 50, 100, 150, and 200 μM [3H]ES in the absence and presence of varying concentrations of probenecid (A), salicylate (B), 2,4-D (C), and hippuric acid (D). Data were corrected for nonspecific background measured in the CHO-FRT (control) cells, and saturation curves were generated. Kinetic constant determination was done by using nonlinear regression analysis; however, Lineweaver-Burk transformations of the data are displayed to aid visualization of the mode of inhibition as competitive. Each experiment was repeated three times in triplicate. Data shown are mean values ± S.E. (n = 3) from a representative experiment.
Inhibition Potencies for mOat6 and mOat3.
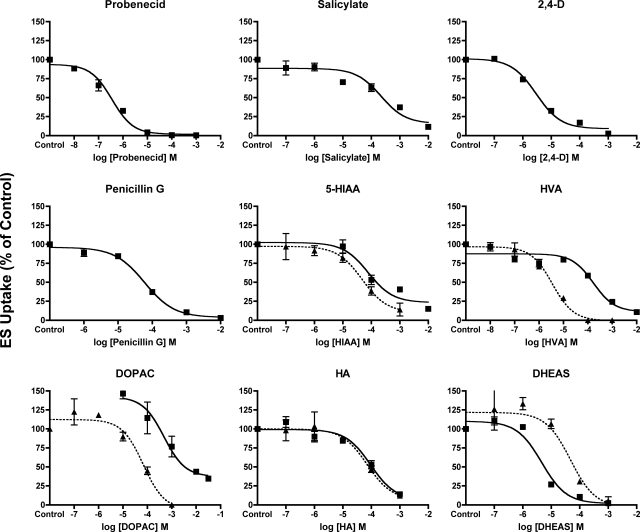
To allow direct comparisons of transporter–substrate interactions between Oat6 and Oat3, experiments were conducted to determine the inhibition potency (Ki) of select organic anions known to interact with mOat6 and mOat3 (Fig. 4; Table 1). Using increasing concentrations of unlabeled test compounds (10−8 to 10−2 M) inhibition of mOat6- and mOat3-mediated transport of ES was measured. Because competitive inhibition of mOat6- and mOat3-mediated transport was directly demonstrated for probenecid, salicylate, 2,4-D, and hippuric acid, subsequent Ki analysis was performed by using competitive inhibition. Inhibition constants for the organic anions probenecid (0.8 ± 0.2 μM), salicylate (342.7 ± 93.1 μM), 2,4-D (2.0 ± 0.4 μM), and penicillin G (60.7 ± 5.9 μM), previously analyzed in the CHO-mOat6 cell line (Schnabolk et al., 2006), were determined in CHO-mOat3 cells (Fig. 4; Table 1). In addition, Ki values for the newly identified mOat6 inhibitors HVA, 5-HIAA, DOPAC, DHEAS, and HA were obtained in the CHO-mOat6 and CHO-mOat3 cell lines, assuming competitive inhibition. The neurotransmitter metabolite 5-HIAA demonstrated similar affinities for both mOat6 and mOat3 (Ki = 48.9 ± 10.3 versus 67.8 ± 7.2 μM, respectively), as did the uremic toxin HA (Ki = 59.9 ± 4.9 versus 79.3 ± 4.0). Values in the CHO-mOat6 cell line for HVA (Ki = 3.0 ± 0.5 μM) and DOPAC (Ki = 61.4 ± 7.1 μM) indicated high affinity for mOat6, whereas values determined in the CHO-mOat3 cell line (HVA Ki = 134.5 ± 27.0 μM; DOPAC Ki = 346.7 ± 97.9 μM) indicated moderate affinity for mOat3 (Fig. 4; Table 1). Conversely, DHEAS exhibited an order of magnitude higher affinity for mOat3 than mOat6 (Ki = 3.8 ± 1.1 versus 38.8 ± 3.1 μM, respectively).
Fig. 4.
Inhibition constant (Ki) determination for select organic anions in the CHO-mOat6 and CHO-mOat3 cell lines. One-minute uptake of [3H]ES (1 μM) in CHO-mOat3 cells (■) was measured in the presence of increasing concentrations (10−8 to 10−2 M) of probenecid, salicylate, 2,4-D, penicillin G, 5-HIAA, HVA, DOPAC, HA, and DHEAS. Two-minute uptake of [3H]ES (5 μM) in CHO-mOat6 cells (▴) was measured in the presence of increasing concentrations (10−7 to 10−3 M) of 5-HIAA, HVA, DOPAC, HA, and DHEAS. Data were corrected for nonspecific background measured in the CHO-FRT (control) cells before kinetic analysis. Ki values were determined by using nonlinear regression and the “one-site competition” model in Prism software. Experiments were repeated three times in triplicate with the mean Ki ± S.E. reported in Table 1. Graphs shown are mean ± S.E. (n = 3) from representative experiments.
TABLE 1.
Estimated Ki (μM) values for murine Oat6- and Oat3-mediated estrone sulfate transport
Oat6 and Oat3 values are reported as mean ± S.E. Significance between Oat6 and Oat3 Ki values is indicated as *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
| Compound | Oat6 | Oat3 | Ki Ratio (Oat6/Oat3) |
|---|---|---|---|
| Probenecid | 8.3 ± 2.5a | 0.8 ± 0.2 | 10.4 |
| Salicylate | 49.0 ± 4.4a | 342.7 ± 93.1* | 0.1 |
| 2,4-D | 15.7 ± 2.0a | 2.0 ± 0.4** | 7.9 |
| Penicillin G | 1450 ± 480a | 60.7 ± 5.9* | 23.9 |
| HVA | 3.0 ± 0.5 | 134.5 ± 27.0** | 0.02 |
| HIAA | 48.9 ± 10.3 | 67.8 ± 7.2 | 0.7 |
| DOPAC | 61.4 ± 7.1 | 346.7 ± 97.9* | 0.2 |
| DHEAS | 38.8 ± 3.1 | 3.8 ± 1.1*** | 10.2 |
| HA | 59.9 ± 4.9 | 79.3 ± 4.0* | 0.8 |
Values were taken from Schnabolk et al. (2006).
Substrate Specificity of mOat6.
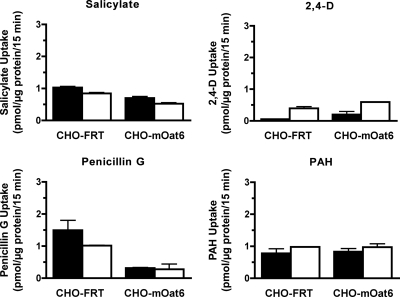
Previous analysis of mOat6-mediated ES uptake identified salicylate and 2,4-D as strong mOat6 inhibitors and PAH and penicillin G as weak inhibitors (Schnabolk et al., 2006). To determine whether any of these inhibitory compounds are actual mOat6 substrates, intracellular accumulation of radiolabeled 2,4-D, salicylate, penicillin G, and PAH was investigated in the CHO-mOat6 cells (Fig. 5). No significant accumulation of any of the compounds was observed. These data suggest that, although these compounds are effective at blocking mOat6-mediated substrate translocation (tight binding), they are not efficient (slow turnover) substrates themselves, i.e., no detectable transporter-mediated cellular accumulation occurs. Independent positive controls verified transporter function (data not shown).
Fig. 5.
Determination of mOat6-mediated transport. Fifteen-minute uptake of [3H]PAH (50 μM), [3H]salicylate (50 μM), [14C]2,4-D (25 μM), and [3H]penicillin G (25 μM) was measured in CHO-FRT and CHO-mOat6 cells in the absence (filled columns) or presence (open columns) of probenecid (1 mM). No significant mOat6-mediated uptake was observed for any of these inhibitory compounds. Independent controls verified cell transport function (data not shown). Experiments were repeated three times in triplicate, and data shown are mean values ± S.E. (n = 3) from representative experiments.
Testicular Expression of mOat6.
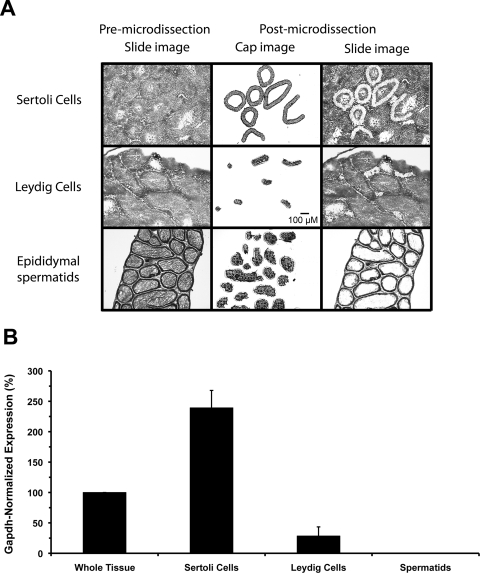
To determine which testicular cell types express Oat6, QPCR was performed on RNA samples isolated from Leydig cells, Sertoli cells, and spermatids by LCM (Fig. 6). Oat6 expression levels were normalized to Gapdh levels within each sample. Oat6 message expression was readily detectable in whole testis, and the Gapdh-normalized value was set to 100%. When examined in isolation, the Sertoli cell-specific Oat6 expression level, 239 ± 29%, was significantly higher than that from any other sample (Fig. 6). Oat6 signal associated with Leydig cells was inconsistent, 28 ± 15%, and markedly below the expression level observed in whole testis. No Oat6 signal was detected in spermatids or the ductal epithelium of the epididymis (ductal data not shown).
Fig. 6.
Laser capture microdissection and testicular expression of mOat6. Expression of Oat6 mRNA in different murine testicular cell types was examined by QPCR on samples isolated by LCM. A, histological images depicting the LCM process. The premicrodissection slide image shows individual ∼8-μm sections used for identification and capture of the desired cell types. Sertoli and Leydig cell populations were isolated from sections through the testis proper, whereas spermatids were harvested from sections of the epididymis. Epididymal spermatids were used to avoid potential cross-contamination with Sertoli cell fragments in spermatids isolated from the testis proper. The postmicrodissection cap image shows the various populations of cells isolated for QPCR analysis. The postmicrodissection slide image, compared with the premicrodissection slide image, shows where within each section the LCM samples were collected. All images are 10×. Scale bar, 100 μm. B, Oat6 mRNA expression levels in testicular cell types. Total RNA was purified from the cells isolated by LCM, and from whole testis, and reverse-transcribed. Gene expression levels of Oat6 and Gapdh were determined by using TaqMan chemistry and real-time QPCR. Oat6 signal (normalized to Gapdh expression) was readily detectable in intact testis, and this level was set to 100%. The Oat6 expression level in Sertoli cells (250%) was significantly higher than that detected in whole tissue. A few random Leydig cell replicates exhibited sporadic signal in the final cycles of a run. This was never a consistent result and never involved all sample replicates; thus this was interpreted to be the result of slight contamination with Sertoli cells during the LCM process and not as extremely low Leydig cell expression. No signal was ever detected in epididymal spermatids. Data are presented as mean values ± S.E. (n = 3 animals).
Discussion
Similar to the blood-brain and blood-cerebrospinal fluid barriers that insulate the central nervous system from systemic fluctuations in toxicant concentrations, the BTB and BEB serve to protect and nourish the maturing gametes (Waites and Gladwell, 1982; Cyr et al., 1995). However, despite this critical function, virtually nothing is known at the cellular and molecular levels about the mechanisms and players (including transporters) that govern testicular permeation of charged organic solutes. The only Slc22 family members to have protein expression in testis confirmed are mOctN2 and mOctN3, which were restricted to the epididymal spermatozoa (Kobayashi et al., 2007). In human testis, detection of P-gp and MRP1 via immunohistochemistry was reported, and experiments with knockout mice confirmed these known efflux transporters serve to limit testicular permeation of their substrates (Schinkel et al., 1994; Wijnholds et al., 1998; Bart et al., 2002). Recently, the transporter mOat6 was proven to function as an organic anion transporter and to be expressed in testis, suggesting it, too, may be involved in BTB and/or BEB function (Monte et al., 2004; Schnabolk et al., 2006).
To begin to better understand what Oat6's role in testis might be and how Oat6 transport specificity and activity compares with other OAT family members, interaction of mOat6 with known OAT substrates and inhibitors was investigated (Fig. 1). The chemotherapeutic methotrexate is of interest because it significantly inhibited mOat3 activity and exhibited reduced plasma clearance in Oat3 knockout mice (VanWert and Sweet, 2008). Furthermore, it is known that testicular permeation of several chemotherapeutics is blocked or limited by BTB function, allowing the testis to serve as an origination site for cancer relapse, even after complete remission (Miyazaki et al., 2003). A recent clinical investigation found that inclusion of methotrexate therapy in the treatment of patients with advanced testicular cancer was of no benefit (Miyazaki et al., 2003). Herein, it was determined that methotrexate produced no effect on mOat6 activity. Thus, perhaps one contributing factor to the lack of efficacy of methotrexate therapy in the treatment of testicular cancer is lack of expression of functional Oat3 and lack of recognition of methotrexate by Oat6.
Similar to murine and human Oat1 and Oat3, mOat6 activity was greatly attenuated by the serotonin and dopamine metabolites 5-HIAA, HVA, and DOPAC (Alebouyeh et al., 2003). What, if any, significance this property would have on testicular mOat6 function is unclear. However, mOat6 is also highly expressed in the olfactory mucosa, which is an epithelial barrier between the nasal cavity and the central nervous system, and perhaps this property is relevant to Oat6 function in that tissue (Monte et al., 2004; Kaler et al., 2006). The uremic toxins hippuric acid and indoxyl sulfate also reduced mOat6 function, implying renal pathophysiology resulting in elevated plasma levels of uremic toxins could lead to impaired testicular organic anion transport. Finally, the steroid metabolite DHEAS significantly inhibited Oat6-mediated ES uptake (∼95%). DHEAS has low membrane permeability and is highly protein-bound in the circulation, indicating specific transport systems are required for its efficient movement across cell membranes. Thus, the interaction observed for DHEAS (and ES) on mOat6 in the present study suggests that mOat6 may be involved in the import of steroidogenic precursors from the blood into the testis across the BTB and, thus, play a pivotal role in the homeostasis of gonadal steroid metabolism.
Four major cell types found in the testis are Sertoli cells, Leydig cells, spermatids, and epididymal ductal cells. The BTB is principally comprised of Sertoli cells linked by tight junctions and effectively excludes entry of toxicants while allowing entry of essential nutrients such as amino acids, glucose, nucleosides, and hormones into the seminiferous space via specialized transport systems (Griswold, 1995; Lui et al., 2003). The ductal cells of the epididymis form the BEB, which is critical for maintaining the proper environment for spermatid maturation and activation (Cyr et al., 1995). Dysfunction of these barriers can lead to impaired sperm formation and maturation, and ultimately male infertility, thus integrity of these barriers is essential. Leydig cells both synthesize and secrete androgens, including testosterone and DHEA, both of which are required in the seminiferous tubules to stimulate spermatogenesis. Thus, Oat6 could potentially aid accumulation of steroidogenic precursors in Leydig cells, secretion of androgens from Leydig cells, movement of testosterone across the BTB into the seminiferous space, regulation of luminal fluid composition in the epididymis, and/or accumulation of maturation and activation factors in the spermatids, depending on which cell type it is expressed in. When surveyed by QPCR, it seemed that mOat6 signal detectable in whole testis was the result of Sertoli cell-specific expression of mOat6 (Fig. 6). These data strongly indicate that mOat6 plays a role in BTB function. However, whether mOat6 is positioned to mediate substrate movement into or out of the seminiferous space cannot be determined until its membrane targeting (basolateral versus apical) is known.
To assess the impact of OATs on drug disposition, elimination, and organ-specific accumulation in vivo, and the potential for OAT-mediated drug–drug and drug–xenobiotic interactions, determination of individual OAT inhibitor-binding affinities (Ki values) is essential. Therefore, we first investigated the type of interaction that was occurring between the probe substrate, ES, and selected mOat6 and mOat3 inhibitors. In every instance examined the nature of the interaction was competitive. These findings are in agreement with previous investigations wherein human OAT1–4 and rat and mouse Oat3 were found to be competitively inhibited by probenecid, penicillin G, 2,4-D, or ciprofloxacin (Jariyawat et al., 1999; Enomoto et al., 2002b; Hashimoto et al., 2004; Nagata et al., 2004; VanWert et al., 2008). Subsequently, Ki values were calculated by using competitive inhibition (Fig. 4; Table 1). With the exception of 5-HIAA and HA, notable differences in substrate affinity were evident. Examination of Oat6/Oat3 Ki ratios revealed that penicillin G, probenecid, 2,4-D, and even DHEAS greatly favor Oat3; whereas HVA, DOPAC, and salicylate show a greater preference for Oat6. Thus, despite having extensive overlap in their substrate profiles in terms of compounds with which they interact, when compared directly mOat6 and mOat3 often exhibit broad differences in affinity, reaching one or more orders of magnitude. In addition to these differences in compound specificity and affinity between transporter paralogs, similar differences exist between transporter orthologs (i.e., across species) (VanWert et al., 2010). For example, PAH is readily transported by human OAT3, but rat and mouse Oat3 exhibit little to no interaction with PAH (VanWert et al., 2010). These differences undoubtedly lead to profound species variations in the pharmacokinetics, efficacy, and toxicity of substrates.
Probenecid, salicylate, 2,4-D, penicillin G, and PAH (prototypical OAT substrate) were identified previously as inhibitors of mOat6 (Schnabolk et al., 2006). However, despite strong inhibition of all known OATs by probenecid, OAT-mediated cellular accumulation of probenecid has not been observed, i.e., although it is an effective (tight binding) inhibitor, it is not efficiently translocated (slow turnover) into the cell. Therefore, we determined whether significant mOat6-mediated accumulation of salicylate, 2,4-D, penicillin G, or PAH occurred when they served directly as substrates. Similar to the action of probenecid on OATs, these compounds effectively inhibited mOat6, but were not efficient substrates (Fig. 5). Cumulatively, these and other results demonstrate that even though OATs possess overlapping specificities the nature of the interaction between compound and individual OAT can be distinct. For example, 2,4-D inhibits Oat1, Oat3, and Oat6, but only Oat1 and Oat3 mediate its accumulation; estrone sulfate is transported by Oat3 and Oat6, but not by Oat1; and penicillin G is a substrate for Oat3, but not Oat1 or Oat6 (Sweet et al., 1997, 2002, 2005; Schnabolk et al., 2006). One possible explanation for these varying interactions comes from recent analysis of structural determinants influencing substrate discrimination by Oat1, Oat3, and Oat6 (Truong et al., 2008). It was concluded that, despite having overlapping substrate specificities and the highest degree of shared sequence homology, these three transporters have distinctly different binding pockets (Truong et al., 2008). Identifying compounds that are strong transporter inhibitors, but that are not translocated, provides invaluable information in terms of drug design. Clearly, a desirable feature of an effective transport inhibitor might be the ability to engage the transporter and prevent mediated movement of a drug substrate, but not accumulate within the cell itself, thus avoiding any potential complications associated with cytotoxicity of the inhibitor.
Broadening our knowledge of the differences in substrate preferences between the closely related OATs, both in terms of chemical class and structure, should aid drug design efforts aimed at targeting or avoiding OAT-mediated translocation or at developing organ-specific drug entities, in the case of Oat6, Oat1, and Oat3 perhaps designing drugs that would exhibit testis-specific permeation while avoiding renal elimination. Such knowledge also will increase our understanding of testis-specific accumulation of endogenous and therapeutic compounds. Together, mOat6 expression in Sertoli cells (BTB) and transport of sulfated steroid conjugates suggest that mOat6 function in the testis might contribute to hormone balance, steroidogenesis, and/or spermatogenesis.
Acknowledgments
We thank members of the Virginia Commonwealth University Laser Microscopy Laboratory and Dr. Amy Ladd, Director, for performing the LCM procedure and members of the Virginia Commonwealth University Molecular Diagnostics Laboratory and Dr. Catherine I. Dumur, Associate Director, for carrying out the QPCR analysis.
This research was supported in part by a National Institutes of Health Training Grant in Environmental Stress Signaling [Grant T32ES012878] (to G.W.S.) and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01DK067216] (to D.H.S.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.168765.
- OAT
- organic anion transporter
- mOat
- murine Oat
- BEB
- blood-epididymal barrier
- BTB
- blood-testis barrier
- 5-CH3-THF
- 5-methyltetrahydrofolate
- CHO
- Chinese hamster ovary
- 2,4-D
- 2,4-dichlorophenoxyacetate
- DHEAS
- dehydroepiandrosterone sulfate
- DOPAC
- 2,4-dihydroxyphenylacetic acid
- ES
- estrone sulfate
- HA
- hippuric acid
- 5-HIAA
- 5-hydroxyindoleacetic acid
- HVA
- homovanillic acid
- PAH
- para-aminohippurate
- QPCR
- real-time quantitative polymerase chain reaction
- SLC
- solute carrier
- LCM
- laser capture microdissection
- Gapdh
- glyceraldehyde 3-phosphate dehydrogenase
- FRT
- flippase recognition target.
References
- Alebouyeh M, Takeda M, Onozato ML, Tojo A, Noshiro R, Hasannejad H, Inatomi J, Narikawa S, Huang XL, Khamdang S, et al. (2003) Expression of human organic anion transporters in the choroid plexus and their interactions with neurotransmitter metabolites. J Pharmacol Sci 93:430–436 [DOI] [PubMed] [Google Scholar]
- Augustine LM, Markelewicz RJ, Jr, Boekelheide K, Cherrington NJ. (2005) Xenobiotic and endobiotic transporter mRNA expression in the blood-testis barrier. Drug Metab Dispos 33:182–189 [DOI] [PubMed] [Google Scholar]
- Bart J, Groen HJ, van der Graaf WT, Hollema H, Hendrikse NH, Vaalburg W, Sleijfer DT, de Vries EG. (2002) An oncological view on the blood-testis barrier. Lancet Oncol 3:357–363 [DOI] [PubMed] [Google Scholar]
- Cyr DG, Robaire B, Hermo L. (1995) Structure and turnover of junctional complexes between principal cells of the rat epididymis. Microsc Res Tech 30: 54–66 [DOI] [PubMed] [Google Scholar]
- Enokizono J, Kusuhara H, Sugiyama Y. (2007) Effect of breast cancer resistance protein (Bcrp/Abcg2) on the disposition of phytoestrogens. Mol Pharmacol 72:967–975 [DOI] [PubMed] [Google Scholar]
- Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, et al. (2002a) Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417:447–452 [DOI] [PubMed] [Google Scholar]
- Enomoto A, Takeda M, Shimoda M, Narikawa S, Kobayashi Y, Kobayashi Y, Yamamoto T, Sekine T, Cha SH, Niwa T, et al. (2002b) Interaction of human organic anion transporters 2 and 4 with organic anion transport inhibitors. J Pharmacol Exp Ther 301:797–802 [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, et al. (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MD. (1995) Interactions between germ cells and Sertoli cells in the testis. Biol Reprod 52:211–216 [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Narikawa S, Huang XL, Minematsu T, Usui T, Kamimura H, Endou H. (2004) Characterization of the renal tubular transport of zonampanel, a novel α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor antagonist, by human organic anion transporters. Drug Metab Dispos 32:1096–1102 [DOI] [PubMed] [Google Scholar]
- Jariyawat S, Sekine T, Takeda M, Apiwattanakul N, Kanai Y, Sophasan S, Endou H. (1999) The interaction and transport of β-lactam antibiotics with the cloned rat renal organic anion transporter 1. J Pharmacol Exp Ther 290:672–677 [PubMed] [Google Scholar]
- Kaler G, Truong DM, Sweeney DE, Logan DW, Nagle M, Wu W, Eraly SA, Nigam SK. (2006) Olfactory mucosa-expressed organic anion transporter, Oat6, manifests high affinity interactions with odorant organic anions. Biochem Biophys Res Commun 351:872–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato R, Maeda T, Akaike T, Tamai I. (2005) Nucleoside transport at the blood-testis barrier studied with primary-cultured sertoli cells. J Pharmacol Exp Ther 312:601–608 [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Tamai I, Sai Y, Yoshida K, Wakayama T, Kido Y, Nezu J, Iseki S, Tsuji A. (2007) Transport of carnitine and acetylcarnitine by carnitine/organic cation transporter (OCTN) 2 and OCTN3 into epididymal spermatozoa. Reproduction 134:651–658 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Lui WY, Mruk D, Lee WM, Cheng CY. (2003) Sertoli cell tight junction dynamics: their regulation during spermatogenesis. Biol Reprod 68:1087–1097 [DOI] [PubMed] [Google Scholar]
- Miyazaki J, Kawai K, Hayashi H, Onozawa M, Tsukamoto S, Miyanaga N, Hinotsu S, Shimazui T, Akaza H. (2003) The limited efficacy of methotrexate, actinomycin D and cisplatin (MAP) for patients with advanced testicular cancer. Jpn J Clin Oncol 33:391–395 [DOI] [PubMed] [Google Scholar]
- Monte JC, Nagle MA, Eraly SA, Nigam SK. (2004) Identification of a novel murine organic anion transporter family member, OAT6, expressed in olfactory mucosa. Biochem Biophys Res Commun 323:429–436 [DOI] [PubMed] [Google Scholar]
- Nagata Y, Kusuhara H, Imaoka T, Endou H, Sugiyama Y. (2004) Involvement of rat organic anion transporter 3 in the uptake of an organic herbicide, 2,4-dichlorophenoxyacetate, by the isolated rat choroid plexus. J Pharm Sci 93:2724–2732 [DOI] [PubMed] [Google Scholar]
- Russel FG, Masereeuw R, van Aubel RA. (2002) Molecular aspects of renal anionic drug transport. Annu Rev Physiol 64:563–594 [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP. (1994) Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 77:491–502 [DOI] [PubMed] [Google Scholar]
- Schnabolk GW, Youngblood GL, Sweet DH. (2006) Transport of estrone sulfate by the novel organic anion transporter Oat6 (Slc22a20). Am J Physiol Renal Physiol 291:F314–F321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HJ, Anzai N, Enomoto A, He X, Kim do K, Endou H, Kanai Y. (2007) Novel liver-specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology 45:1046–1055 [DOI] [PubMed] [Google Scholar]
- Sweet DH. (2005) Organic anion transporter (Slc22a) family members as mediators of toxicity. Toxicol Appl Pharmacol 204:198–215 [DOI] [PubMed] [Google Scholar]
- Sweet DH, Bush KT, Nigam SK. (2001) The organic anion transporter family: from physiology to ontogeny and the clinic. Am J Physiol Renal Physiol 281:F197–F205 [DOI] [PubMed] [Google Scholar]
- Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. (2002) Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 [Oat3 (Slc22a8)] knockout mice. J Biol Chem 277:26934–26943 [DOI] [PubMed] [Google Scholar]
- Sweet DH, Wolff NA, Pritchard JB. (1997) Expression cloning and characterization of ROAT1. The basolateral organic anion transporter in rat kidney. J Biol Chem 272:30088–30095 [DOI] [PubMed] [Google Scholar]
- Truong DM, Kaler G, Khandelwal A, Swaan PW, Nigam SK. (2008) Multi-level analysis of organic anion transporters 1, 3, and 6 reveals major differences in structural determinants of antiviral discrimination. J Biol Chem 283:8654–8663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanWert AL, Sweet DH. (2008) Impaired clearance of methotrexate in organic anion transporter 3 (Slc22a8) knockout mice: a gender specific impact of reduced folates. Pharmacol Res 25:453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanWert AL, Gionfriddo MR, Sweet DH. (2010) Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos 31:1–71 [DOI] [PubMed] [Google Scholar]
- VanWert AL, Srimaroeng C, Sweet DH. (2008) Organic anion transporter 3 (Oat3/Slc22a8) interacts with carboxyfluoroquinolones, and deletion increases systemic exposure to ciprofloxacin. Mol Pharmacol 74:122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites GM, Gladwell RT. (1982) Physiological significance of fluid secretion in the testis and blood-testis barrier. Physiol Rev 62:624–671 [DOI] [PubMed] [Google Scholar]
- Wijnholds J, Scheffer GL, van der Valk M, van der Valk P, Beijnen JH, Scheper RJ, Borst P. (1998) Multidrug resistance protein 1 protects the oropharyngeal mucosal layer and the testicular tubules against drug-induced damage. J Exp Med 188:797–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood GL, Sweet DH. (2004) Identification and functional assessment of the novel murine organic anion transporter Oat5 (Slc22a19) expressed in kidney. Am J Physiol Renal Physiol 287:F236–F244 [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang YD, Strong JM, Reynolds KS, Huang SM. (2008) A regulatory viewpoint on transporter-based drug interactions. Xenobiotica 38:709–724 [DOI] [PubMed] [Google Scholar]