Abstract
Our previous studies have demonstrated that an increase in intracellular levels of Ca2+ in neurons is an important component of both the antinociception produced by morphine and morphine's tolerance. The present study tested the hypothesis that the Ca2+ signaling second messenger, cyclic ADP-ribose (cADPR), derived from CD38 activation participates in morphine antinociception and tolerance. We first showed that morphine's antinociceptive potency was increased by the intracerebroventricular injection of CD38 substrate β-NAD+ in mice. Furthermore, morphine tolerance was reversed by intracerebroventricular administration of each of three different inhibitors of the CD38–cADPR–ryanodine receptor Ca2+ signaling pathway. These inhibitors were the ADP–ribosylcyclase inhibitor nicotinamide, cADPR analog 8-bromo-cADPR, and a large dose of ryanodine (>50 μM) that blocks the ryanodine receptor. In CD38 gene knockout [CD38(−/−)] mice, the antinociceptive action of morphine was found to be less potent compared with wild-type (WT) mice, as measured by tail-flick response, hypothermia assay, and observations of straub tail. However, there was no difference in locomotor activation between CD38(−/−) and WT animals. It was also found that less tolerance to morphine developed in CD38(−/−) mice compared with WT animals. These results indicate that cADRP–ryanodine receptor Ca2+ signaling associated with CD38 plays an important role in morphine tolerance.
Introduction
Morphine is among the most prescribed opioid pain relievers in the United States. Despite its prevalence, tolerance to morphine is a major clinical side effect and roadblock to its chronic usage. Investigations in this laboratory and others have shown that intracellular calcium levels affect the development of tolerance. Although there are many pathways that mediate calcium concentration in the cell, one pathway involves the signaling nucleotide cADPR. CD38 is a transmembrane protein that is involved in cADPR production and calcium regulation, therefore potentially playing a role in the acute and chronic effects of morphine. The association between CD38 and morphine tolerance has not been addressed in the literature. To this end, we set out to characterize the role of CD38 in morphine tolerance in mice.
CD38, first identified as a leukocyte differentiation antigen, subsequently has been found in many tissues, including the brain (Hotta et al., 2000; Verderio et al., 2001; Ceni et al., 2003). CD38 is a type II transmembrane glycoprotein and a member of a family of enzymes with multiple functions, including ADP–ribosyl cyclase activity that cyclizes NAD+ into cADPR. CD38 is an enzyme detected in both plasma and intercellular compartments (De Flora et al., 2004). Recent studies in our laboratory found that CD38 on the cell membrane may be rapidly aggregated through lipid rafts clustering and moved into the inner side of the cell membrane, thereby intracellularly producing cADPR (Jia et al., 2008). Once cADPR is in the cell it can bind to ryanodine receptors (RyRs) or the ryanodine receptor's accessory proteins, leading to Ca2+ release from the endoplasmic reticulum (Li et al., 2003). The wide distribution in the cell and the variety of cell types in the brain where CD38 is found indicates that CD38 may play an important signaling role in neurons and astrocytes (Yamada et al., 1997). There is evidence that almost all ADP–ribosyl cyclase activity in the brain is linked to CD38. In CD38(−/−) knockout mice where no ADP–ribosyl cyclase activity could be detected cADPR levels were found to be nonexistent compared with wild-type (WT) control mice (Ceni et al., 2003).
It has been documented that the stimulation of receptors coupled to the Gi/Go G protein, such as the μ-opioid receptor, is associated with inhibition of voltage-operated Ca2+ channels in the plasma membrane and therefore prevents the elevation of intracellular free Ca2+ (Hille, 1994). However, there is also evidence that opioid stimulation can cause a transient increase in intracellular Ca2+ (Yeo et al., 2001). Many enzymes that regulate intracellular Ca2+ have been investigated for their potential role in opioids' actions; however, the role of CD38–cADPR in mediating the actions of opioids or tolerance to them has not yet been addressed.
Previous work in our laboratory and by others has shown that one can alter both the acute and chronic effects of morphine by inhibiting or stimulating various steps in the signal transduction system of neurons. Since the 1960s, it has been clear that manipulations of intracellular Ca2+ occur with morphine exposure and play an integral role in morphine-induced antinociception. The mechanism of morphine action is known to involve the phosphoinositol, adenylyl cyclase, and G protein-coupled receptor kinase pathways. However, their relative importance and order of activation have not been determined. In the present study, we proposed that the CD38–cADPR–RyR pathway is also involved in the mediation of morphine's antinociceptive actions. This hypothesis is also based on our previous findings that the RyR's Ca2+ release from the ER plays a role in morphine's antinociceptive actions (Smith and Stevens, 1995; Smith et al., 1999). To test this hypothesis, we examined whether administration of a substrate for CD38 altered acute morphine antinociception. We then investigated the effects of chemical inhibition of the CD38 pathway on both acute morphine antinociception and morphine tolerance. We found that acutely morphine's potency was increased by the in vivo application of the CD38 substrate. Furthermore, whereas morphine antinociception was unaffected, morphine tolerance was reversed by administration of each of three different inhibitors of the CD38–cADPR–RyR pathway. To further investigate the role of CD38 in morphine's actions we did a series of experiments in CD38 gene knockout [CD38(−/−)] mice. We found that some actions of morphine, including antinociception, were less potent in the knockout mice compared with WT mice, whereas there was no difference in locomotor activation between the knockout and WT mice. We also found that CD38(−/−) mice developed less tolerance to morphine compared with WT animals. These results indicate that the CD38–cADPR–RyR pathway's Ca2+ signaling plays an important role in morphine tolerance.
Materials and Methods
Drugs and Chemicals.
Morphine sulfate was obtained from the National Institute on Drug Abuse (Bethesda, MD). Morphine sulfate was dissolved in pyrogen-free isotonic saline (Hospira, Lake Forest, IL). Nicotinamide, β-NAD+, and 8-bromo-cADPR were obtained from Sigma-Aldrich (St. Louis, MO). Ryanodine was obtained from BIOMOL Research Laboratories, Plymouth Meeting, PA. All were dissolved in distilled water.
Animals.
Male Swiss-Webster mice (Harlan, Indianapolis, IN) weighing 25 to 30 g were housed six to a cage in animal care quarters and maintained at 22 ± 2°C on a 12-h light/dark cycle. CD38-deficient mice [CD38(−/−)] (Cockayne et al., 1998), backcrossed 12 generations to BALB/cBy mice, were obtained from the Trudeau Institute Breeding Facility (Saranac Lake, NY) and housed six to a cage in animal care quarters and maintained at 22 ± 2°C on a 12-h light/dark cycle. Food and water were available ad libitum. The mice were brought to a test room (22 ± 2°C, 12-h light/dark cycle), marked for identification, and allowed 18 h to recover from transport and handling. Protocols and procedures were approved by the Institutional Animal Care and Use Committee at the Virginia Commonwealth University Medical Center and comply with the recommendations of the International Association for the Study of Pain.
Intracerebroventricular Injections.
Intracerebroventricular injections were performed as described by Pedigo et al. (1975). Mice were anesthetized with isoflurane. The skin was cleansed with 10% providone iodine (General Medical Corp., Prichard, WV) before making a horizontal incision in the scalp. A free-hand 5-μl injection of drug or vehicle was made in the lateral ventricle (2 mm rostral and 2 mm lateral at a 45° angle from the bregma). Maintenance of a stringent aseptic surgical field minimized any potential contamination of the incision. The extensive experience of members of this laboratory has made it possible to inject drugs by this route of administration with more than 95% accuracy. Immediately after testing, the animals were euthanized to minimize any type of distress, according to Institutional Animal Care and Use Committee guidelines.
Seventy-Two-Hour Morphine Tolerance Model.
A 75-mg morphine pellet (MP) or placebo pellet (PP) was implanted according to Way et al. (1969). Mice were anesthetized with 2.5% isoflurane before the hair on the base of the neck was shaved. The skin was cleansed with 10% providone iodine (General Medical Corp.) and rinsed with alcohol before a 1-cm horizontal incision was made at the base of the neck. The underlying subcutaneous space toward the dorsal flanks was opened by using a sterile glass rod. Maintenance of a stringent aseptic surgical field minimized any potential contamination of the pellet, incision, and subcutaneous space. A placebo pellet or 75-mg morphine pellet was inserted in the space before closing the site with Clay Adams Brand, MikRon AutoClip 9-mm wound clips (BD Diagnostics, Sparks, MD) and again applying iodine to the surface. The animals were allowed to recover in their home cages where they remained throughout the experiment.
Tail Immersion Test.
The warm-water tail immersion test was performed according to Coderre and Rollman (1983) by using a water bath with the temperature maintained at 56 ± 0.1°C. Before injecting the mice, a baseline (control) latency was determined. Only mice with a control reaction time from 2 to 4 s were used. The test latency after drug treatment was assessed at 30 min after the subcutaneous administration of morphine with a 10-s maximum cutoff time imposed to prevent tissue damage. Antinociception was quantified according to the method of Harris and Pierson (1964) as the percentage of maximum possible effect (%MPE), which was calculated as: %MPE = [(test latency − control latency)/(10 − control latency)] × 100. %MPE was calculated for each mouse with at least six mice per treatment group.
Hotplate Test.
The hotplate test was performed as described by O'Callaghan and Holtzman (1975). The mice were placed on a Syscom model 35D hotplate set (Syscom, Woodland Hills, CA) at 55°C to obtain baseline latencies before drug administration. The mice were observed for licking either their fore or hind limb or jumping in response to the heat. The baseline latencies ranged between 5 and 6 s. Testing occurred 30 min after subcutaneous injection of morphine. A 30-s cutoff was used to prevent tissue damage. Antinociception was quantified according as the %MPE as described above.
Straub Tail.
The mice were observed for the development of straub tail 30 min after subcutaneous administration of morphine. The straub tail reaction was graded by using the numerical scoring system of Kameyama et al. (1978): 0 = 0°, 0.5 = 1 to 30°, 1.0 = 31 to 45°, 1.5 = 46 to 60°, 2.0 = 61 to 90°, and 2.5 = more than 90°. The angle was measured above the horizontal plane of the table.
Hypothermia.
Baseline rectal temperatures were obtained before exposure to morphine. Test rectal temperatures were obtained 30 min after morphine administration, and the change in body temperature (ΔTb) was calculated.
Statistical Analysis.
Opioid dose–response curves were generated for calculation of effective dose-50 (ED50) values using least-squares linear regression analysis followed by calculation of 95% confidence limits (95% CL) by the method of Bliss (1967). Tests for parallelism were conducted before calculating the potency-ratio values with 95% CL by the method of Colquhoun (1971), who noted that a potency ratio value of more than one, with the lower 95% CL more than one, is considered a significant difference in potency between groups.
Results
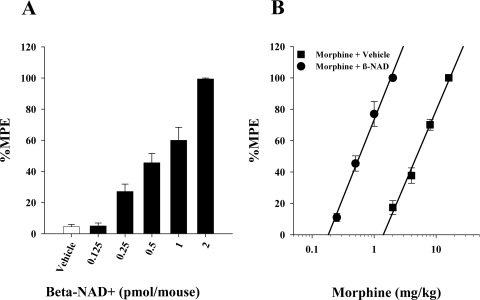
The overall goal of the present study was to determine what effect manipulation of CD38 in vivo would have on morphine antinociception and tolerance. We began by testing the effects of in vivo application of an ADP–ribosyl cyclase substrate, β-NAD+. When we administered β-NAD+ intracerebroventricularly in varying doses (Fig. 1A) there was a dose-dependent increase in the antinociceptive effects of a 2 mg/kg s.c. dose of morphine. The intracerebroventricular administration of β-NAD+ caused a seven times increase in morphine potency (Fig. 1B). This increase in acute morphine potency by β-NAD+ inhibited us from investigating the effects of β-NAD+ on morphine tolerance.
Fig. 1.
The CD38–ADP–ribosyl cyclase substrate βNAD+ increased morphine potency. A, mice treated with 2 mg/kg morphine subcutaneously were injected intracerebroventricularly with increasing doses of βNAD+ and tested in the 56°C tail immersion test. Each treatment group represents six mice. B, mice were treated with βNAD+ (2 nmol/mouse) and then various doses of morphine subcutaneously for construction of dose–response curves for calculation of ED50 values and potency ratios. ●, animals treated with vehicle and morphine; ■, animals treated with βNAD+ and morphine. Each data point represents six mice.
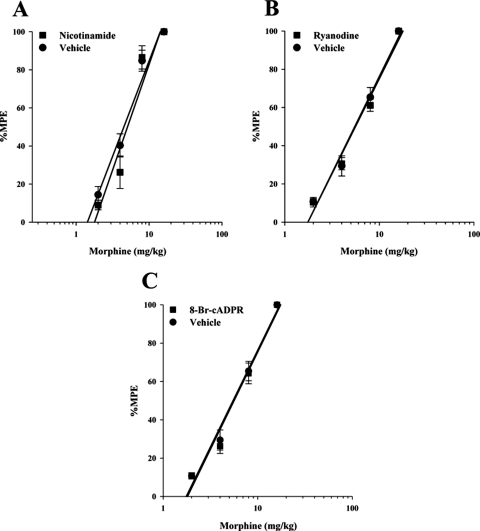
In the next series of experiments, we attempted to further elucidate the functional role of CD38 and explored whether it mediated the action of morphine in either acute antinociception or tolerance by inhibiting its enzymatic pathway. Nicotinamide, an inhibitor of CD38 ADP–ribosyl cyclase activity (Inageda et al., 1995; Berthelier et al., 1998), 8-bromo-cADPR, a chemical analog of cADPR and cell-permeable competitive antagonist of cADPR (Sethi et al., 1997), and ryanodine, which is an inhibitor of ryanodine receptors at a large doses (>50 μM) (Smith et al., 1999), were used to block the action of cADPR.
Animals were administered one of these compounds intracerebroventricularly followed immediately by varying doses of acute morphine. The warm-water tail immersion latencies were then measured 30 min later, and %MPE was calculated for the construction of the dose–response curves and the calculation of potency ratios. As shown in Table 1 and Fig. 2, none of the three inhibitors altered the acute antinociceptive effects of morphine. There was also no effect on acute morphine antinociception when higher doses of these compounds were administered (data not shown).
TABLE 1.
CD38–cADPR–ryanodine receptor pathway inhibitors in acute morphine antinociception
Mice were administered inhibitor intracerebroventricularly immediately followed by challenge doses of morphine. Thirty minutes after challenge doses were administered, tail immersion latencies were determined for construction of dose–response curves and calculation of ED50 values and potency ratios.
| Treatment | ED50 Value (95% CL) | Potency Ratio (95% CL) |
|---|---|---|
| mg/kg | ||
| Ryanodine + morphine | 5.59 (5.18, 6.03) | |
| Vehicle + morphine | 4.47 (3.91, 5.12) | vs. Ryanodine + morphine 1.32 (1.21, 1.43) |
| Nicotinamide + morphine | 5.07 (4.36, 5.90) | |
| Vehicle + morphine | 4.51 (3.99, 5.09) | vs. Nicotinamide + morphine 1.10 (0.94, 1.30) |
| 8-Br-cADPR + morphine | 5.62 (5.11, 6.17) | |
| Vehicle + morphine | 4.47 (3.91, 5.12) | vs. 8-Br-cADPR + morphine 1.33 (1.21, 1.44) |
Fig. 2.
CD38 inhibitors with acute morphine. Drugs that block the CD38–cADPR–ryanodine receptor pathway do not alter acute morphine antinociception. Mice were administered inhibitor intracerebroventricularly and various morphine doses subcutaneously. Antinociception was measured 30 min later, and %MPE was calculated and used to construct dose–response curves for calculation of ED50 values and potency ratios. All three inhibitors, nicotinamide (200 pmol/mouse) (A), ryanodine (1.0 nmol/mouse) (B), and 8-bromo-cADPR (10 nmol/mouse) (C), had no effect on acute morphine antinociception. Each data point represents six mice. ■, inhibitor injected intracerebroventricularly with morphine injected subcutaneously; ●, vehicle injected intracerebroventricularly with morphine injected subcutaneously.
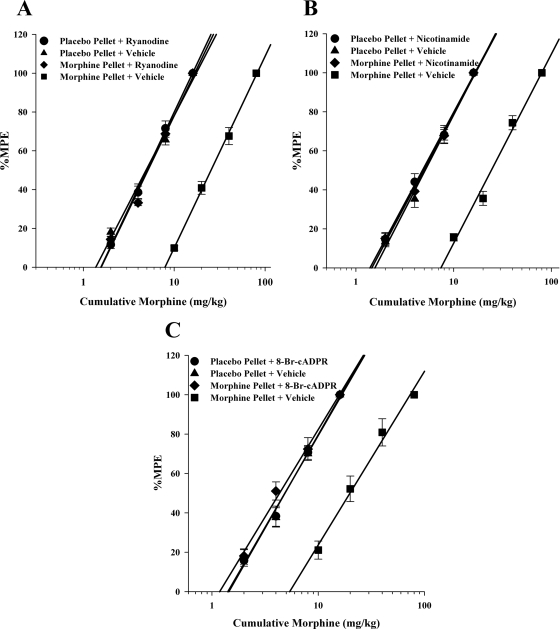
We then tested the effects of these compounds that inhibit CD38–cADPR–RyR signaling on morphine tolerance. Animals were implanted subcutaneously with 75-mg pellets of morphine or placebo pellets for 72 h. They were then administered one of the inhibitors mentioned above intracerebroventricularly followed immediately by varying doses of acute morphine. The warm-water tail immersion latencies were then measured 30 min later, and %MPE was calculated for the construction of the dose–response curves and the calculation of potency ratios. As shown in Table 2 and Fig. 3, all three inhibitors or blockers were fully effective at reversing 72-h morphine tolerance. The morphine dose–response curve in the presence of the inhibitors was similar to that of the acute morphine dose–response curve, indicating that the tolerance to morphine had been reversed. There were no signs of withdrawal observed in the animals.
TABLE 2.
CD38–cADPR–ryanodine receptor pathway inhibitors in morphine tolerance
Mice were pelleted with 75 mg of morphine for 72 h and then given the inhibitor and challenged with morphine. Thirty minutes after challenge doses were administered, tail immersion latencies were determined for construction of dose–response curves and calculation of ED50 values and potency ratios.
| Treatment | ED50 Value (95% CL) | Potency Ratio (95% CL) |
|---|---|---|
| mg/kg | ||
| Morphine pellet + ryanodine | 5.13 (4.87, 5.41) | |
| Morphine pellet + vehicle | 25.42 (23.80, 27.15) | vs. Morphine pellet + ryanodine 4.95 (4.59, 5.33) |
| Morphine pellet + nicotinamide | 4.94 (4.53, 5.39) | |
| Morphine pellet + vehicle | 24.28 (22.51, 26.19) | vs. Morphine pellet + nicotinamide 4.89 (4.39, 5.44) |
| Morphine pellet + 8-Br-cADPR | 4.32 (3.90, 4.81) | |
| Morphine pellet + vehicle | 19.86 (17.23, 27.90) | vs. Morphine pellet + 8-Br-cADPR 4.61 (3.86, 5.49) |
Fig. 3.
CD38 inhibitors with morphine 3-day tolerance. Drugs that block the CD38–cADPR–ryanodine receptor pathway reversed morphine tolerance. Mice were implanted with placebo or 75-mg morphine pellets for 72 h and then administered inhibitor intracerebroventricularly and various morphine doses subcutaneously. Antinociception was measured 30 min later, and %MPE was calculated and used to construct dose–response curves for calculation of ED50 values and potency ratios. All three inhibitors, ryanodine (1.0 nmol/mouse) (A), nicotinamide (200 pmol/mouse) (B), and 8-bromo-cADPR (10 nmol/mouse) (C), were able to fully reverse 72-h morphine tolerance. Each data point represents six mice. ●, placebo pellet with inhibitor; ▴, placebo pellet with vehicle; ♦, morphine pellet with inhibitor; ■, morphine pellet with vehicle.
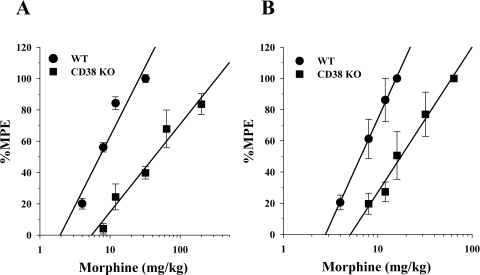
To further elucidate the role of CD38 in morphine's actions, experiments were performed in CD38(−/−) knockout animals. The CD38(−/−) mice were obtained from Dr. Francis E. Lund of the Trudeau Institute, Inc. This CD38(−/−) animal strain was developed as described in Cockayne et al. (1998). The acute antinociceptive effects of morphine in the CD38(−/−) animal were assessed by using warm-water tail immersion and hotplate tests. In the warm-water tail immersion, we found that morphine was only 25% as potent in the CD38(−/−) mice as it was in the WT animals (Fig. 4A). These results were replicated in the hotplate test where morphine was found to be only 17% as potent in the CD38(−/−) mice as in the WT mice (Fig. 4B).
Fig. 4.
Acute effects of morphine in antinociception assays in CD38 KO mice. A, acute effects of morphine in the warm-water tail immersion test. Mice were injected with various doses of morphine subcutaneously, and their tail immersion latencies were determined for construction of dose–response curves 30 min later. Data are expressed as mean %MPE ± S.E.M. Each data point represents 6 to 10 mice. The morphine ED50 values were as follows: wild type, 8.4 mg/kg (95% CL 6.7–10.6); CD38 KO, 34.3 mg/kg (95% CL 24.5–4.9). Morphine was found to be 4-fold less potent in the CD39 KO mice than in wild-type mice in this test. B, acute effects of morphine in the hotplate test. Mice were injected with various doses of morphine subcutaneously, and their hotplate test latencies were determined for construction of dose–response curves 30 min later. Data are expressed as mean %MPE ± S.E.M. Each data point represents 6 to 10 mice. The morphine ED50 values were as follows: wild type, 6.6 mg/kg (95% CL 5.6–7.8); CD38 KO, 39.8 mg/kg (95% CL 18.5–85.9). Morphine was found to be 6-fold less potent in the CD38 KO mice than in wild-type mice in this test.
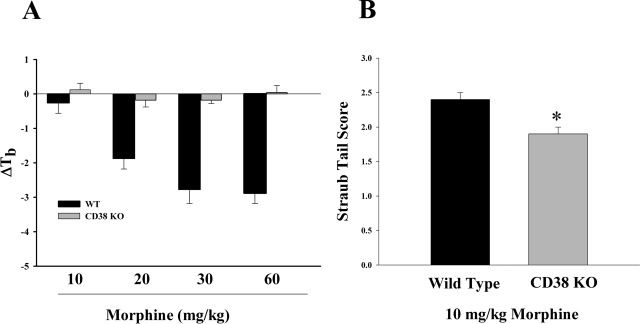
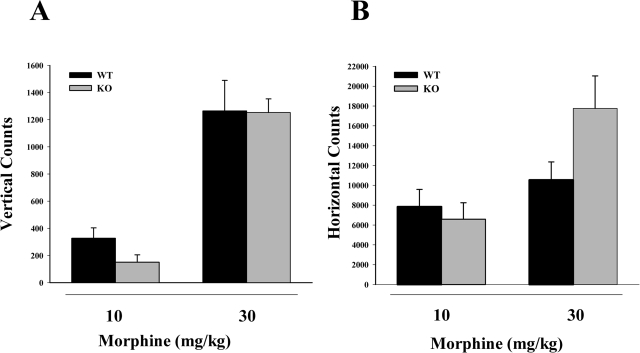
Other effects of acute administration of morphine were also observed in these knockout mice. In morphine-induced hypothermia and straub tail measurements, morphine was found to be less potent in the CD38(−/−) mice than in the WT animals (Fig. 5). However, in spontaneous motor activity (Fig. 6) there was no significant difference between the effects of a 10 or 30 mg/kg dose of morphine in the CD38(−/−) mice and the WT mice in either vertical counts (Fig. 6A) or lateral counts (Fig. 6B).
Fig. 5.
Morphine-induced hypothermia and straub tail. A, acute morphine-induced hypothermia. Mice were injected with various doses of morphine subcutaneously, and test rectal temperatures were obtained 30 min later. Results are expressed as the change in body temperature (ΔTb). In the wild-type animals morphine dose-dependently induced hypothermia. However, there was no hypothermia found in the CD38 KO animals even at doses where maximal hypothermia was found in wild-type animals. Each bar represents five mice. B, acute effects of morphine on straub tail. Mice were injected with 10 mg/kg morphine subcutaneously, and straub tail was measured 30 min later. Each bar represents 6 to 10 mice. There was a significant reduction in straub tail in the CD38 KO mice compared with wild type (p < 0.05). Each bar represents five mice.
Fig. 6.
Acute effects of morphine spontaneous motor activity. Mice were injected with 10 or 30 mg/kg morphine subcutaneously, and spontaneous activity was recorded. Each bar represents five mice. There was no significant difference between the wild-type animals and the CD38 KO animals at either 10 or 30 mg/kg morphine in either the vertical counts (p = 0.152 for 10 mg/kg morphine and p = 0.626 for 30 mg/kg morphine) (A) or the horizontal counts (p = 0.617 for 10 mg/kg morphine and p = 0.118 for 30 mg/kg morphine) (B).
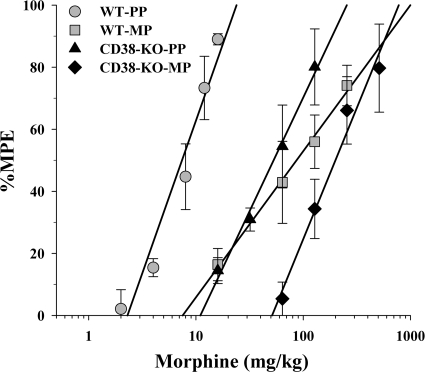
We also investigated whether there was any difference between CD38(−/−) and WT animals in morphine tolerance. The data presented in Fig. 7 illustrate the development of tolerance to morphine in both the CD38(−/−) and the WT mice. As expected from the acute antinociceptive assays, we found morphine to be less potent in the CD38(−/−) mice in both the placebo-treated animals and the morphine tolerance mice. In addition, although tolerance was developed in both CD38(−/−) and WT strains, the extent of tolerance developed was different between the strains of mice. In the WT animals 12-fold tolerance developed after the 72-h morphine treatment, whereas in the CD38(−/−) mice only 3-fold tolerance developed after the same treatment.
Fig. 7.
Tolerance to morphine in the warm-water tail immersion test. Mice were implanted with either a PP or 75-mg MP 72 h before they were challenged with morphine. Mice were then injected with various doses of morphine subcutaneously, and their tail immersion latencies were determined for construction of dose–response curves 30 min later. Data are expressed as mean %MPE ± S.E.M. Each data point represents 6 to 10 mice. The morphine ED50 values were as follows: wild-type PP ( ), 7.5 mg/kg (95% CL 6.4–8.9); wild-type MP (
), 7.5 mg/kg (95% CL 6.4–8.9); wild-type MP ( ), 86.8 mg/kg (95% CL 47.9–157.4); CD38 KO PP (▴), 54.9 mg/kg (95% CL 40.8–73.8); CD38 KO MP (♦), 167.7 mg/kg (95% CL 109.3–257.3). Both the wild-type mice and CD38 KO mice developed tolerance to morphine; however, morphine was less potent in the placebo-treated mice compared with the wild type and less potent in the tolerant mice compared with wild type. Wild-type mice developed approximately 12-fold tolerance in this model, whereas the CD38 KO mice developed only a 3-fold tolerance.
), 86.8 mg/kg (95% CL 47.9–157.4); CD38 KO PP (▴), 54.9 mg/kg (95% CL 40.8–73.8); CD38 KO MP (♦), 167.7 mg/kg (95% CL 109.3–257.3). Both the wild-type mice and CD38 KO mice developed tolerance to morphine; however, morphine was less potent in the placebo-treated mice compared with the wild type and less potent in the tolerant mice compared with wild type. Wild-type mice developed approximately 12-fold tolerance in this model, whereas the CD38 KO mice developed only a 3-fold tolerance.
Discussion
To our knowledge, there are no reports thus far discussing the role of CD38 in morphine antinociception or tolerance. Our previous results (Smith et al., 1999) indicated that RyR-mediated Ca2+ release from intracellular stores is involved in the action of morphine and the development of tolerance in mice. However, the endogenous second messenger to activate RyR was unknown at that time. Given current evidence that RyR is the target for the action of cADPR, a CD38 product, it is now imperative to know whether CD38–cADPR-mediated signaling is involved in the action of morphine and morphine tolerance. To this end, we investigated the role of the CD38–cADPR-RyR Ca2+ pathway in the acute antinociceptive actions of morphine and morphine tolerance.
There have been reports of an increase in intracellular Ca2+ after opioid receptor activation alone, but more commonly it has been reported to happen during concomitant activation of Gq-coupled receptors that cause the Ca2+ release from intracellular stores (Okajima et al., 1993; Connor and Henderson, 1996). Regardless of whether the increase of Ca2+ results from μ-opioid receptor activation alone or with another class of G protein-coupled receptor, it is clear that the majority of this elevation of intracellular Ca2+ is a result of the release of Ca2+ from stores in the ER rather than through an influx of Ca2+ across the plasma membrane (Werry et al., 2003). Opioids have been reported to stimulate the inositol phosphate turnover in many cell types (Dortch-Carnes and Potter, 2003), which is correlated with the release of Ca2+ from the inositol trisphosphate-mediated stores. However, there is also evidence that there may be other mechanisms by which the opioids increase the level of intracellular Ca2+, such as through activation of the ryanodine receptors (Allouche et al., 1996). It has been shown that the ryanodine receptor antagonist dantrolene blocks morphine-induced elevation in intracellular Ca2+ in isolated mouse astrocytes (El-Hage et al., 2005).
Opioid receptor activation and the resulting stimulation of the Gi/Go G proteins usually results in inhibition of neurotransmitter release (Christie et al., 2000). However, the brief elevations in presynaptic Ca2+ levels after opioid receptor stimulation can be enough to stimulate neurotransmitter release as well (McDonald et al., 1996). There have also been reports that the opioid receptor may be linked to Ca2+-sensitive K+ channels such as the BK(Ca) channel (Chin et al., 2002). If this is the case then the increase in Ca2+ caused by opioid stimulation could further enhance postsynaptic inhibition by opening these Ca2+-sensitive K+ channels.
The ability of opioids to cause elevations in intracellular Ca2+ implies that Ca2+ signaling molecules may be important in mediating opioid-induced antinociception (Samways and Henderson, 2006). It has been hypothesized that Ca2+ alters intracellular events to antagonize the antinociceptive effects of opioids (Chapman and Way, 1980). It is also known that a disruption in intracellular Ca2+ homeostasis contributes to the expression of antinociceptive tolerance. Opioid tolerance causes synaptosomal Ca2+ uptake to be increased (Chapman and Way, 1980), and basal free-intracellular Ca2+ concentrations are found to be higher in the brain and spinal cord compared with nontolerant animals (Welch and Olson, 1991). Opioid tolerance has also been shown to be affected by the alteration of intercellular Ca2+ (Antkiewicz-Michaluk et al., 1993). Previous work in our laboratory has shown that extracellular Ca2+ influx through voltage-sensitive Ca2+ channels and mobilization from Ca2+/caffeine-sensitive pools (RyR pools) are involved with morphine tolerance (Smith et al., 1999) and can be reversed with the application of ryanodine. It has also been demonstrated that when Ca2+ is injected into the periaqueductal gray region opioid antinociception from any type of injection, systemic or otherwise, is blocked (Munoz and Fearon, 1982). Previous work in our laboratory has shown that this block in antinociception by injecting Ca2+ is a result of the stimulation of Ca2+ influx into the cells and the subsequent Ca2+ release from ryanodine receptor pools through calcium-induced calcium release (Smith and Stevens, 1995).
We began our investigation with the intracerebroventricular administration of the CD38–ADP–ribosyl cyclase substrate β-NAD+. This resulted in an increase in the potency of morphine in antinociception. It is thought that exogenous application of β-NAD+ allows for increased generation of cADPR, leading to increased Ca2+ release from the ER. As discussed above, increased levels of intracellular Ca2+ have been linked to the development of tolerance to morphine, and the exogenous application of Ca2+ has been shown to block the antinociceptive action of morphine (Smith and Stevens, 1995). However, there are also reports of transient increases in intracellular Ca2+ after opioid administration unrelated to tolerance (Yeo et al., 2001). We did not observe any effect on antinociception after β-NAD+ application without morphine, and therefore we believe that in addition to increased levels of substrate it is necessary for morphine to stimulate the system for CD38 to be activated, causing the increase in cADPR and resultant transient increase in intracellular Ca2+. We hypothesize that it is this resultant increase in intracellular Ca2+ that increased the potency of morphine.
Our next goal was to investigate the effect of inhibition of the CD38–cADPR–RyR Ca2+ signaling pathway on morphine antinociception and its tolerance. In these experiments, the CD38–ADPR–RyR signaling pathway was blocked by the CD38–cADP–ribosyl cyclase inhibitor, nicotinamide; a cell-permeable cADPR antagonist, 8-bromo-cADPR; and a RyR blocker, a large dose of ryanodine.
None of these compounds that have been shown to alter the CD38 pathway had any effect on the acute antinociception induced by morphine. Given the enhancing effects of β-NAD on morphine antinociception, the reasons for the inability of the inhibition of CD38–cADPR signaling to blunt the acute action of morphine remain unclear.
In contrast to the lack of an effect of these inhibitors on the acute response to morphine, we found that they all fully reversed 72-h morphine tolerance. Opioid tolerance is known to correlate with increased basal levels of Ca2+ (Welch and Olson, 1991). Although we did not directly measure the intracellular Ca2+ levels in this in vivo study we hypothesize that inhibition of the CD38 product, cADPR, most probably leads to a decrease in intracellular Ca concentration, which resulted in the reversal of tolerance. It is clear from the data presented that alteration of the CD38 cADPR pathway in a number of ways significantly altered morphine tolerance but the same and even higher doses of these compounds did not alter the acute antinociceptive effects of morphine.
Because these pharmacological interventions may not be able to completely activate or block CD38 and may have nonspecific action, we felt it was necessary to use CD38(−/−) knockout animals to further explore the role of CD38 in morphine's actions. We examined both the stimulatory (straub tail and spontaneous activity) and suppressive (antinociception and hypothermia) effects of morphine. Our experiments in the CD38(−/−) mice confirmed that there is a functional role for the CD38 cascade in a variety of morphine actions such as antinociception, hypothermia, and straub tail. However, our results show that this signaling pathway is not involved in morphine's effect on spontaneous activity. Although not entirely conclusive, our results suggest that CD38 may play a more important role in the suppressive effects over the stimulatory effects of morphine.
Our hypothesis is that morphine antinociception is associated with alterations in the activity of CD38–ADP–ribosyl cyclase, leading to increases in cADPR. Ca2+ influx via voltage-sensitive Ca2+ channels has been demonstrated to stimulate ryanodine receptors, resulting in dramatic increases in cytosolic Ca2+ through calcium-induced calcium release (Solovyova et al., 2002). cADPR may bind to RyR or its accessory protein, such as FK506 proteins, and thereby stimulate Ca2+ mobilization from the ER in neurons. Some in vitro studies by others supported this hypothesis because RyR channel stimulation was found to activate neuronal physiological processes such as synaptic transmission and neurotransmitter release throughout the central nervous system (Simpson et al., 1996).
To our knowledge, the data presented here involving CD38–cADPR–RyR and morphine provide the first evidence of the essential role CD38 plays in morphine tolerance and possibly in acute actions as well. The use of CD38(−/−) helped us to more effectively define such actions of CD38–cADRP–RyR on morphine-induced antinociception and its tolerance. Further elucidation of related molecular mechanism will continue to enhance our understanding of the action of morphine in the production of morphine antinociception and, in particular, the mechanisms of morphine tolerance.
Acknowledgments
We thank Dr. Monica Chu for comments concerning the manuscript and general discussion.
This work was supported by the National Institutes of Health National Institute of Drug Abuse [Grants F31-DA022850, R01-DA020836, R01-DA024009].
These data are a part of the dissertation work of L.C.H., which was submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy at Virginia Commonwealth University Department of Pharmacology and Toxicology in July 2009.
Part of these data have been presented in poster form: Hull LC, Lee TT, Chen L, Zhang G, Li P, Dewey WL, and Smith FL (2006) CD38: novel interaction with morphine analgesic pathways in mice, at Experimental Biology; 2006 April 1–5; San Francisco, CA; Dewey WL, Smith FL, Li P, Hull LC, and Chen L (2006) The role of NAD(P)H oxidase and CD-38 in the actions of opioids, at International Narcotics Research Conference; 2006 July 9–14; St. Paul, MN; Hull LC, Li P, Smith FL, and Dewey WL (2007) Role of CD38 in morphine tolerance, at Experimental Biology; 2007 April 28-May 2; Washington, DC; and Hull LC, Rabender C, Smith FL, Li P, Gabra BH, and Dewey WL (2008) Evidence for an important role of CD38 in acute and chronic effects of morphine, at Neuroscience 2008; 2008 Nov 15–19; Washington, DC. Society for Neuroscience, Washington, DC.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.169243.
- cADPR
- cyclic ADP-ribose
- RyR
- ryanodine receptor
- ER
- endoplasmic reticulum
- WT
- wild type
- KO
- knockout
- PP
- placebo pellet
- MP
- morphine pellet
- %MPE
- percentage of maximum possible effect
- CL
- confidence limit.
References
- Allouche S, Polastron J, Jauzac P. (1996) The δ-opioid receptor regulates activity of ryanodine receptors in the human neuroblastoma cell line SK-N-BE. J Neurochem 67:2461–2470 [DOI] [PubMed] [Google Scholar]
- Antkiewicz-Michaluk L, Michaluk J, Romańska I, Vetulani J. (1993) Reduction of morphine dependence and potentiation of analgesia by chronic coadministration of nifedipine. Psychopharmacology (Berl) 111:457–464 [DOI] [PubMed] [Google Scholar]
- Berthelier V, Tixier JM, Muller-Steffner H, Schuber F, Deterre P. (1998) Human CD38 is an authentic NAD(P)+ glycohydrolase. Biochem J 330:1383–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss CI. (1967) Statistics in Biology, McGraw–Hill, New York [Google Scholar]
- Ceni C, Pochon N, Brun V, Muller-Steffner H, Andrieux A, Grunwald D, Schuber F, De Waard M, Lund F, Villaz M, et al. (2003) CD38-dependent ADP-ribosyl cyclase activity in developing and adult mouse brain. Biochem J 370:175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DB, Way EL. (1980) Metal ion interactions with opiates. Annu Rev Pharmacol Toxicol 20:553–579 [DOI] [PubMed] [Google Scholar]
- Chin JH, Harris K, MacTavish D, Jhamandas JH. (2002) Nociceptin/orphanin FQ modulation of ionic conductances in rat basal forebrain neurons. J Pharmacol Exp Ther 303:188–195 [DOI] [PubMed] [Google Scholar]
- Christie MJ, Connor M, Vaughan CW, Ingram SL, Bagley EE. (2000) Cellular actions of opioids and other analgesics: implications for synergism in pain relief. Clin Exp Pharmacol Physiol 27:520–523 [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Muchamuel T, Grimaldi JC, Muller-Steffner H, Randall TD, Lund FE, Murray R, Schuber F, Howard MC. (1998) Mice deficient for the ecto-nicotinamide adenine dinucleotide glycohydrolase CD38 exhibit altered humoral immune responses. Blood 92:1324–1333 [PubMed] [Google Scholar]
- Coderre TJ, Rollman GB. (1983) Naloxone hyperalgesia and stress-induced analgesia in rats. Life Sci 32:2139–2146 [DOI] [PubMed] [Google Scholar]
- Colquhoun D. (1971) Lectures in Biostatistics: An Introduction to Statistics with Applications in Biology and Medicine, Clarendon Press, London [Google Scholar]
- Connor M, Henderson G. (1996) δ- and μ-Opioid receptor mobilization of intracellular calcium in SH-SY5Y human neuroblastoma cells. Br J Pharmacol 117:333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Flora A, Zocchi E, Guida L, Franco L, Bruzzone S. (2004) Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system. Ann NY Acad Sci 1028:176–191 [DOI] [PubMed] [Google Scholar]
- Dortch-Carnes J, Potter DE. (2003) δ-Opioid agonist-stimulated inositol phosphate formation in isolated, rabbit iris-ciliary bodies: role of G(i/o) proteins and Gβγ-subunits. Exp Eye Res 77:647–652 [DOI] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. (2005) Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia 50:91–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LS, Pierson AK. (1964) Some narcotic antagonists in the benzomorphan series. J Pharmacol Exp Ther 143:141–148 [PubMed] [Google Scholar]
- Hille B. (1994) Modulation of ion-channel function by G protein-coupled receptors. Trends Neurosci 17:531–536 [DOI] [PubMed] [Google Scholar]
- Hotta T, Asai K, Fujita K, Kato T, Higashida H. (2000) Membrane-bound form of ADP-ribosyl cyclase in rat cortical astrocytes in culture. J Neurochem 74:669–675 [DOI] [PubMed] [Google Scholar]
- Inageda K, Takahashi K, Tokita K, Nishina H, Kanaho Y, Kukimoto I, Kontani K, Hoshino S, Katada T. (1995) Enzyme properties of Aplysia ADP-ribosyl cyclase: comparison with NAD glycohydrolase of CD38 antigen. J Biochem 117:125–131 [DOI] [PubMed] [Google Scholar]
- Jia SJ, Jin S, Zhang F, Yi F, Dewey WL, Li PL. (2008) Formation and function of ceramide-enriched membrane platforms with CD38 during M1-receptor stimulation in bovine coronary arterial myocytes. Am J Physiol Heart Circ Physiol 295:H1743–H1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama T, Nabeshima T, Ukai M, Yamaguchi K. (1978) Morphine-induced Straub tail reaction and spinal catecholamine metabolite content: antagonism of naloxone to morphine-induced effects in mice. Chem Pharm Bull 26:2615–2618 [DOI] [PubMed] [Google Scholar]
- Li PL, Lee HC, Nelson MT, Meininger GA, Van Breemen C. (2003) Novel Ca2+ signalling mechanisms in vascular myocytes: symposium overview. Acta Physiol Scand 179:339–352 [DOI] [PubMed] [Google Scholar]
- McDonald RL, Vaughan PF, Peers C. (1996) Neuropeptide Y elevates intracellular Ca2+ and evokes noradrenaline release in SH-SY5Y cells. Neuroreport 7:2913–2916 [DOI] [PubMed] [Google Scholar]
- Muñoz FG, Fearon Z. (1982) Opioids/opiates analgesic response modified by calcium. Life Sci 31:1237–1240 [DOI] [PubMed] [Google Scholar]
- O'Callaghan JP, Holtzman SG. (1975) Quantification of the analgesic activity of narcotic antagonists by a modified hot-plate procedure. J Pharmacol Exp Ther 192:497–505 [PubMed] [Google Scholar]
- Okajima F, Tomura H, Kondo Y. (1993) Enkephalin activates the phospholipase C/Ca2+ system through cross-talk between opioid receptors and P2-purinergic or bradykinin receptors in NG 108-15 cells. A permissive role for pertussis toxin-sensitive G-proteins. Biochem J 290:241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedigo NW, Dewey WL, Harris LS. (1975) Determination and characterization of the antinociceptive activity of intraventricularly administered acetylcholine in mice. J Pharmacol Exp Ther 193:845–852 [PubMed] [Google Scholar]
- Samways DS, Henderson G. (2006) Opioid elevation of intracellular free calcium: possible mechanisms and physiological relevance. Cell Signal 18:151–161 [DOI] [PubMed] [Google Scholar]
- Sethi JK, Empson RM, Bailey VC, Potter BV, Galione A. (1997) 7-Deaza-8-bromo-cyclic ADP-ribose, the first membrane-permeant, hydrolysis-resistant cyclic ADP-ribose antagonist. J Biol Chem 272:16358–16363 [DOI] [PubMed] [Google Scholar]
- Simpson PB, Nahorski SR, Challiss RA. (1996) Agonist-evoked Ca2+ mobilization from stores expressing inositol 1,4,5-trisphosphate receptors and ryanodine receptors in cerebellar granule neurones. J Neurochem 67:364–373 [DOI] [PubMed] [Google Scholar]
- Smith FL, Stevens DL. (1995) Calcium modulation of morphine analgesia: role of calcium channels and intracellular pool calcium. J Pharmacol Exp Ther 272:290–299 [PubMed] [Google Scholar]
- Smith FL, Dombrowski DS, Dewey WL. (1999) Involvement of intracellular calcium in morphine tolerance in mice. Pharmacol Biochem Behav 62:381–388 [DOI] [PubMed] [Google Scholar]
- Solovyova N, Veselovsky N, Toescu EC, Verkhratsky A. (2002) Ca2+ dynamics in the lumen of the endoplasmic reticulum in sensory neurons: direct visualization of Ca2+-induced Ca2+ release triggered by physiological Ca2+ entry. EMBO J 21:622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C, Bruzzone S, Zocchi E, Fedele E, Schenk U, De Flora A, Matteoli M. (2001) Evidence of a role for cyclic ADP-ribose in calcium signalling and neurotransmitter release in cultured astrocytes. J Neurochem 78:646–657 [DOI] [PubMed] [Google Scholar]
- Way EL, Loh HH, Shen FH. (1969) Simultaneous quantitative assessment of morphine tolerance and physical dependence. J Pharmacol Exp Ther 167:1–8 [PubMed] [Google Scholar]
- Welch SP, Olson KG. (1991) Opiate tolerance-induced modulation of free intracellular calcium in synaptosomes. Life Sci 48:1853–1861 [DOI] [PubMed] [Google Scholar]
- Werry TD, Wilkinson GF, Willars GB. (2003) Mechanisms of cross-talk between G-protein-coupled receptors resulting in enhanced release of intracellular Ca2+. Biochem J 374:281–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Mizuguchi M, Otsuka N, Ikeda K, Takahashi H. (1997) Ultrastructural localization of CD38 immunoreactivity in rat brain. Brain Res 756:52–60 [DOI] [PubMed] [Google Scholar]
- Yeo A, Samways DS, Fowler CE, Gunn-Moore F, Henderson G. (2001) Coincident signalling between the Gi/Go-coupled δ-opioid receptor and the Gq-coupled m3 muscarinic receptor at the level of intracellular free calcium in SH-SY5Y cells. J Neurochem 76:1688–1700 [DOI] [PubMed] [Google Scholar]