Abstract
Direct injection of double-stranded adeno-associated virus type 2 (dsAAV2) with a μ-opioid receptor (MOR) mutant [S4.45(196)A], and a reporter protein (enhanced green fluorescent protein) into the spinal cord (S2/S3) dorsal horn region of ICR mice resulted in antinociceptive responses to systemic injection of opioid antagonist naloxone without altering the acute agonist morphine responses and no measurable tolerance or dependence development during subchronic naloxone treatment. To develop further such mutant MORs into a therapeutic agent in pain management, a less invasive method for virus delivery is needed. Thus, in current studies, the dsAAV2 was locally injected into the subarachnoid space of the spinal cord by intrathecal administration. Instead of using the MORS196A mutant, we constructed the dsAAV2 vector with the MORS196ACSTA mutant, a receptor mutant in which naloxone has been shown to exhibit full agonistic properties in vitro. After 2 weeks of virus injection, naloxone (10 mg/kg s.c.) elicited antinociceptive effect (determined by tail-flick test) without tolerance (10 mg/kg s.c., b.i.d. for 6 days) and significant withdrawal symptoms. On the other hand, subchronic treatment with morphine (10 mg/kg s.c., b.i.d.) for 6 days induced significant tolerance (4.8-fold) and withdrawal symptoms. Furthermore, we found that morphine, but not naloxone, induced the rewarding effects (determined by conditioned place preference test). These data suggest that local expression of MORS196ACSTA in spinal cord and systemic administration of naloxone has the potential to be developed into a new strategy in the management of pain without addiction liability.
Introduction
Although morphine is efficacious in the control of moderate and severe postoperative pain, it also induces adverse effects, such as respiratory depression, nausea, constipation, tolerance, physical dependence, and addiction. Frequently, nonopioid pharmacological strategies are used with opioid administration to alleviate the side effects while maintaining the analgesic potency of the opioids. For example, laxative or bulking agents are used to counteract opioid constipative effects. Dopaminergic or serotonergic (5-HT3) antagonists are used for postoperative nausea and vomiting caused by opioid analgesics. In addition, peripheral opioid antagonists are used in the elimination of opioid side effects. Unlike centrally acting opioid antagonists, such as naloxone or naltrexone, that could reverse the analgesic effects in postoperative patients (Abou-madi et al., 1976; Gairola et al., 1980; Lehmann et al., 1988), peripheral opioid antagonists such as methylnaltrexone (the quaternary derivative of naltrexone) or alvimopan [a zwitterionic form of trans-3,4-dimethyl-4-(3-hydroxyphenyl) piperidine], because of their inability to penetrate the blood-brain barrier, have been used successfully to prevent the constipation, nausea, or vomiting effects in postoperative pain treatment (Taguchi et al., 2001; Bates et al., 2004). Unfortunately, tolerance and addiction to morphine remains with peripheral antagonists.
Other approaches were used to address the problem of opioid tolerance and addiction. With the cloning of the three opioid receptor types, MOR, δ-opioid receptor, and κ-opioid receptor (KOR) (Evans et al., 1992; Kieffer et al., 1992; Chen et al., 1993, 1994; Meng et al., 1993), and the subsequent generation of respective receptor null mouse lines (Matthes et al., 1996; Sora et al., 1997; Loh et al., 1998; Zhu et al., 1999), MOR is shown to mediate the in vivo morphine effects. In the MOR(−/−) mice, the rewarding responses to morphine were not observed (Matthes et al., 1996). Clinical studies indicated that the mixed agonist–antagonists (agonist on KOR and antagonist on MOR), although having lower patient compliance because of their dysphoric properties from KOR, exhibit lower addictive liabilities (Rosow, 1987). Thus, the development of KOR agonists as pharmaceutical agents for pain control with low addiction liability has been the focus of many laboratories (Hasebe et al., 2004). This requires the development of ligands with specific binding to one opioid receptor type, which has been difficult even with the identification of putative receptor domains involved in MOR, δ-opioid receptor, and KOR ligand binding (Paterlini, 2005; Surratt and Adams, 2005).
Instead of developing more specific ligands for the opioid receptors, or opioid ligand “cocktails” to enhance the recycling and resensitization of MOR, leading to lowering of the tolerance and dependence responses (He and Whistler, 2005), we decided to focus on developing a therapeutic agent using a mutant MOR that could be activated by the opioid alkaloid antagonists naloxone and naltrexone. The initial identification of this mutant in which the conserved Ser in the fourth transmembrane domain (TM4) was mutated to Leu [S4.45(196)L] suggested that the mutation did not affect agonist receptor affinity or potency (Claude-Geppert et al., 2005). Rather, the S4.45(196)L mutation resulted in opioid antagonists activating Kir3 or inhibiting adenylyl cyclase activity (Claude-Geppert et al., 2005). The ability of antagonists to activate the mutant receptor in vivo was demonstrated by generating a S4.45(196)A knock-in mouse line (Yang et al., 2003). In addition to morphine, naloxone and naltrexone could elicit antinociceptive responses in the MORS196A mice. Chronic morphine treatment resulted in tolerance and dependence development in these mice. However, long-term treatment of these mice with naloxone or naltrexone did not produce tolerance, and the withdrawal signs were less severe (Yang et al., 2003). Thus antagonist-mediated activation of MORS196A in vivo could be a probable pain management paradigm.
The utility of this approach was demonstrated by delivering the MORS196A mutant to the dorsal horn area of the spinal cord (S2/S3) region by using double-stranded adeno-associated virus type 2 (dsAAV2). Mice injected with dsAAV2 exhibited a naloxone-mediated antinociceptive response that was not accompanied by tolerance or dependence development during subchronic antagonist treatment (Chen et al., 2007). These results suggest that opioid antagonist is activating the mutant but not the endogenous MOR. However, the exposure of spinal cord segment after partial dorsal laminectomy during dsAAV2 injection greatly reduces the utility of this approach.
To develop the mutant MOR as a therapeutic agent further, we decided to explore the possibility of introducing the virus at the subarachnoid space of the spinal cord by intrathecal injection. Instead of delivering MORS196A, a MOR with additional mutations at transmembrane 7 [T7.44(327)A] and C7.47(330)S in which opioid antagonists exhibit full agonistic properties (Claude-Geppert et al., 2005), was delivered by dsAAV2. We found that naloxone showed antinociceptive effects without the development of tolerance, physical dependence, and rewarding effect [determined by conditioned place preference (CPP)] in these mice after the expression of MORS196ACSTA by intrathecal injection of dsAAV2-MORS196ACSTA-EGFP. Therefore, naloxone has great potential to be developed into a new strategy in the management of pain by delivering the mutated MOR gene intrathecally.
Materials and Methods
Construction of the dsAAV2 Virus
The original dsAAV-cytomegalovirus-EGFP shuttle vector was obtained from Xiao Xiao (University of North Carolina, Chapel Hill, NC). The cloned MOR or the mutant MORS4.45AT7.44AC7.47A (MORS196ACSTA) with EGFP as reporter was cloned into the dsAAV2 expression cassette pV4.1c containing the cytomegalovirus promoter as described previously (Chen et al., 2007). The dsAAV2 was produced in human embryonic kidney 293 cells by using the adenovirus-free, triple-plasmid cotransfection method (Wang et al., 2003) and purified by heparin affinity column chromatography following previously published methods (Clark et al., 1999; Wang et al., 2003). The virus titer of the dsAAV2 was determined to be 1013 viral particles/ml.
Animals
Male ICR mice (30–35 g) were used in this study. All mice were kept in an animal room with a 12-h light/dark cycle at a temperature of 25 ± 2°C and humidity of 55%. A standard diet and water were provided ad libitum. The care of animals was carried out in accordance with institutional and international standards (Principles of Laboratory Animal Care, National Institutes of Health), and the protocol had the approval of the Institutional Animal Care and Use Committee of the National Defense Medical Center (Taiwan, Republic of China). There were at least eight mice in each group in the beginning of the experiments.
Injection of the dsAAV2 into the Subarachnoid Space of the Spinal Cord
The direct lumbar puncture method (Wigdor and Wilcox, 1987; Fairbanks, 2003) was applied in awake, conscious mice. Mice were covered with a soft cloth over the head and upper body and griped firmly by the pelvic girdle (iliac crest). Five microliters of the virus was injected with a 50-μl Hamilton syringe (Hamilton Co., Reno, NV) attached to a 30-gauge, 0.5-inch sterile disposable needle, which was inserted into the intrathecal space at the cauda equine region according to the method described by Wigdor and Wilcox (1987). Puncture of the dura was indicated by a flick of the tail.
Determination of the Antinociceptive Effect of Drugs
Drug-induced antinociception was evaluated by using the tail-flick test (D'Amour and Smith, 1941). Using a tail-flick apparatus (Ugo Basile, Comerio, Italy), the intensity of the heat source was set at 40, which resulted in the basal tail-flick latency to occur between 2.5 and 3.5 s for most of the animals. The tail-flick latency was recorded at 30, 60, 90, 120, and 180 min after drug administration. The percentage of maximum possible effect (%MPE) was calculated for each mouse at each time point according to the following formula: %MPE = [(postdrug latency − predrug latency)/(cutoff latency − predrug latency)] × 100. The predrug latency was determined from an average of three predrug determinations, and cutoff latency was selected at 10 s. The trapezoidal rule, without extrapolation to infinite time, was used to calculate area under the %MPE versus time curves (AUCs) for each individual animal as shown in Fig. 2. The ED50 value was measured by an up and down method (Dixon, 1965). In brief, the tail-flick response was tested at the peak effect of morphine or naloxone, which occurs approximately 20 to 30 min after drug administration. A series of trials (n = 6) was carried out following the rule of a decrease in drug dose after inhibition of the tail-flick response (%MPE ≥50%) and an increase in drug dose after no inhibition of the tail-flick response (%MPE <50%). Each rat was tested in only one trial. The ED50 value was determined from the relationship ED50 = Xf + k × d (Dixon, 1965), where Xf was the last dose administered, k was the tabular value outlined by Dixon, and d was the interval between doses.
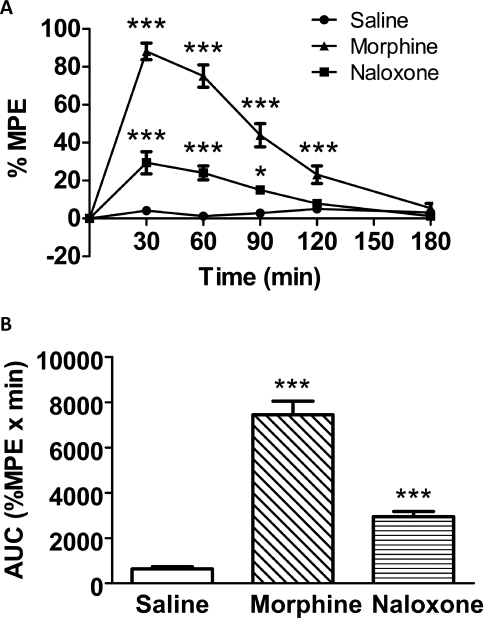
Fig. 2.
A, time-response curves of saline, naloxone (10 mg/kg, s.c.), and morphine (10 mg/kg s.c.) 2 weeks after intrathecal injection of dsAAV2-MORS196ACSTA. B, the area under curve values derived from A. Data are presented as mean %MPE ± S.E.M. (n = ∼7–10). One-way ANOVA and Newman-Keuls test were used to analyze the data. *, P < 0.05; ***, P < 0.001 compared with saline group at each time point.
Detection of the Expression of Mutated μ-Opioid Receptors
Mice were anesthetized with chlorohydrate (350 mg/kg i.p.) and perfused transcardially with Tyrode calcium-free buffer (116 mM NaCl, 5.36 mM KCl, 1.57 mM MgCl2 · 6H2O, 0.405 mM MgSO4, 1.23 mM NaH2PO4, 5.55 mM glucose, 26.2 mM NaHCO3, pH 7.4), followed by 4% paraformaldehyde in 0.1 M phosphate buffer (187.5 mM KH2PO4, 212.5 mM Na2HPO4 · 12H2O). The lumber spinal cord and L1–L4 dorsal root ganglion (DRG) were dissected and then put in 20% sucrose solution (in 0.1 M phosphate buffer) overnight at 4°C. The samples were then embedded in OCT compound and frozen immediately in a −80°C freezer. Serial transverse spinal cord slices and DRG (10 μm) were sectioned with a cryostat at −20°C. The slices were mounted on SuperFrost Plus slides (Menzel-Glaser, Braunschweig, Germany), and the EGFP expression of the mutated μ-opioid receptors was visualized with a fluorescence microscope. To investigate whether these mutated μ-opioid receptors were colocalized with calcitonin gene-related peptide (CGRP), a well characterized marker of the DRG sensory neurons and related afferent fiber terminals, immunohistochemistry was done in DRG at the L1–L4 level. Antibodies were diluted in blocking buffer (0.01 M phosphate-buffered saline containing 1% goat serum and 0.1% Triton X-100). Anti-CGRP antibody (AB5920; Millipore Bioscience Research Reagents, Temecula, CA) was used at a dilution of 1:2000. Secondary antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) was diluted 1:200 to against rabbit or mouse IgG that was coupled with rhodamine for immunofluorescence detection. Sections were then washed with phosphate-buffered saline, cleared, and mounted onto coverslips using mounting medium (HIS002B; Serotec, Oxford, UK). The processed sections were examined with an Olympus (Tokyo, Japan) fluorescence instrument (model BH2-RFL-T3) on an upright microscope and an Olympus BX50 camera with SPOT software (version 4.6; Diagnostic Instruments, Inc., Sterling Heights, MI).
Experiment 1.
To determine whether naloxone would induce antinociceptive effect in mice intrathecally injected with dsAAV2-MORS196ACSTA and whether this effect would last for the period (8 weeks) when we would carry out the acute and subchronic drug treatments, the tail-flick tests of naloxone (10 mg/kg s.c.) were done before gene transfer and at 1, 2, 3, and 8 weeks after gene transfer.
Experiment 2.
To determine subchronic drug treatment-induced tolerance and physical dependence, mice were separated into three groups. Mice in the control group were injected with saline intrathecally and tested with saline (intrathecal saline–saline); mice in the morphine or naloxone group were injected with dsAAV-MORS196ACSTA intrathecally and tested with morphine or naloxone. Before intrathecal injection of saline or virus, the antinociceptive effects of saline (10 ml/kg s.c.), naloxone (10 mg/kg s.c.), or morphine (10 mg/kg s.c.) were tested as pretest data (before intrathecal acute). The acute antinociceptive effects (including ED50 and AUC) of each drug were determined 2 weeks after intrathecal injection of saline or virus (after intrathecal acute). And then the mice were subchronically treated with saline (10 ml/kg s.c.), naloxone (10 mg/kg s.c.), or morphine (10 mg/kg s.c.) twice a day for 6 days. The AUC values (after intrathecal chronic) of saline or drug in each group were evaluated again in the morning of the sixth day. On the seventh day, the ED50 values were measured by the up and down method (Dixon, 1965) in the morning, and the required dose to make up to 10 mg/kg for each animal was injected right after the test. On the eighth day, mice were first injected with morphine or naloxone for 30 min and then injected with naltrexone (10 mg/kg i.p.) and placed into test chambers consisting of transparent round plastic boxes (20 cm diameter, 33 cm height). Naltrexone-induced precipitated withdrawal symptoms, such as the number of jumping and rearing moves, were counted for 15 min. The amount of feces and urine were weighed after the determination of withdrawal signs. The body weight losses were determined after 4 h of naltrexone injection. The natural withdrawal symptoms (jump, paw tremor, wet dog shaking, teeth chattering, diarrhea, ptosis, piloerection) were also counted at 14 and 38 h after the last drug treatment. Jump and paw tremor frequencies were recorded during the test time, and a score of 1 was assigned to every three jumps or five paw tremors. Diarrhea and ptosis events were recorded for every 5-min interval in which they occurred (maximal score = 6). The presence or absence of piloerection was noted. A global opiate withdrawal score was calculated by summing the values for each sign (Papaleo and Contarino, 2006).
Experiment 3.
To determine the rewarding effects induced by morphine or naloxone, the CPP test was carried out as reported previously (Chen et al., 2007).
In this study, a distinctive environment was paired repeatedly with administration of a drug, and a different environment was associated with the nondrugged state. The CPP test apparatus, made from an acrylic plastic box (33 × 15 × 15 cm), was divided into three compartments. Two identically sized compartments (15 × 15 × 15 cm) were constructed at both sides, separated by a narrower compartment (3 × 15 × 15 cm). The compartments were connected by guillotine doors (7 × 3.5 cm) in the central unit. One of the large compartments was covered by mosaic-type paper (3 × 3 cm black and white squares) on the three walls and as a visual cue; the other large compartment was covered by purely white paper. To give more visual cues, blue and red light bulbs were hung separately above the two large compartments. During the experiments, the CPP apparatus was kept in an isolated dark room, which was free from noise. After each behavioral test or place conditioning, the whole box was cleaned thoroughly to prevent the interference from the smell of feces and urine. For CPP conditioning, the mice were given saline in the morning and morphine (5 mg/kg i.p.) or naloxone (10 mg/kg i.p.) in the afternoon for 6 days. A distinctive environment was paired repeatedly with administration of saline, and a different environment was associated with drug injection. The animals were kept for 40 min in the corresponding compartment with the guillotine doors closed. CPP tests were performed on the day before conditioning and the day after conditioning (day 7). Place preference was determined by placing the mice into the central compartment of the apparatus with the guillotine doors opened for 15 min. The time that the mice stayed in each compartment was recorded to determine the place preference. The measurement of drug rewarding effect was determined by an increase in the time spent in the compartment previously paired with drug injection than the time spent in the saline-paired compartment.
Statistical Analysis
The data were expressed as means ± S.E.M. Student's t test, one-way ANOVA, repeated-measures ANOVA, and Newman-Keuls test were used to analyze the data. A difference was considered to be significant at P < 0.05.
Chemicals
Morphine hydrochloride was purchased from the National Bureau of Controlled Drugs, National Health Administration (Taipei, Taiwan, Republic of China). Naloxone, naltrexone, paraformaldehyde, and glucose were purchased from Sigma-Aldrich (St. Louis, MO). Chlorohydrate, MgSO4, H2PO4, and Na2HPO4 · 12H2O were purchased from Nacalai Tesque (Kyoto, Japan). KCl, KH2PO4, MgCl2 · 6H2O, NaCl, and NaHCO3 were purchased from Mallinckrodt Baker, Inc. (Phillipsburg, NJ).
Results
Expression of the Mutated MOR in Mice after Intrathecal Injection of dsAAV2-MORS196ACSTA-EGFP.
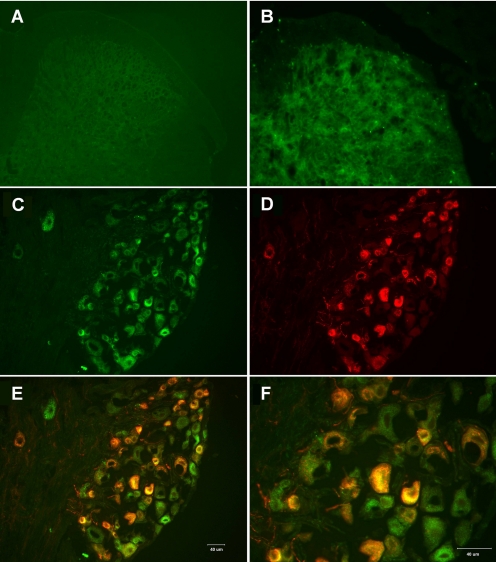
Our previous studies with the spinal cord dorsal horn injection of virus has validated the feasibility of using dsAAV2 as the delivery vehicle for the mutant opioid receptor. To develop a less invasive method for the transgene delivery, intrathecal injections of the dsAAV2 were attempted. Expression of the transgene was monitored by the EGFP fluorescence. Similar to previous studies (Chen et al., 2007), the intrathecal injection of the dsAAV2 resulted in the expression of the MORS196ACSTA at the L3–L5 spinal cord starting from 2 weeks after the virus injection. A representative picture of EGFP fluorescence in mice 8 weeks after the intrathecal injection is shown in Fig. 1B. Furthermore, the DRG located at the L3–L4 also exhibited EGFP fluorescence in all sizes of cells 8 weeks after the intrathecal injections (Fig. 1, C–F). When the DRGs were stained for CGRP (red; Fig. 1D), a marker for sensory neurons, colocalization of CGRP with the EGFP was observed (Fig. 1, E and F). The colocalization of the mutant MOR with CGRP suggests probable expression of the mutant opioid receptor within the sensory neurons associated with nociception.
Fig. 1.
A and B, fluorescence micrographs of the lumber spinal cord 8 weeks after intrathecal administration of saline (A) or dsAAV2-MORS196ACSTA-EGFP (B) are shown with the 20× objective. C to F, fluorescence micrographs of the L3 dorsal root ganglion 8 weeks after intrathecal administration of dsAAV2-MORS196ACSTA-EGFP are shown with the 20× objective (C–E) or the 40× objective (F). EGFP fluorescence (C), CGRP immunofluorescence (D), and the merging of EGFP (green) with CGRP immunofluorescence (red) (E and F) revealed colocalization (yellow) of MORS196ACSTA and CGRP.
The antinociceptive effects of saline, morphine, or naloxone 2 weeks after intrathecal injection of dsAAV2-MORS196ACSTA-EGFP are shown in Fig. 2A. The AUC values were calculated from the area under the time-response curve of Fig. 2A and are shown in Fig. 2B.
Naloxone Elicited Antinociceptive Responses in Mice Injected with dsAAV-MORS196ACSTA Intrathecally.
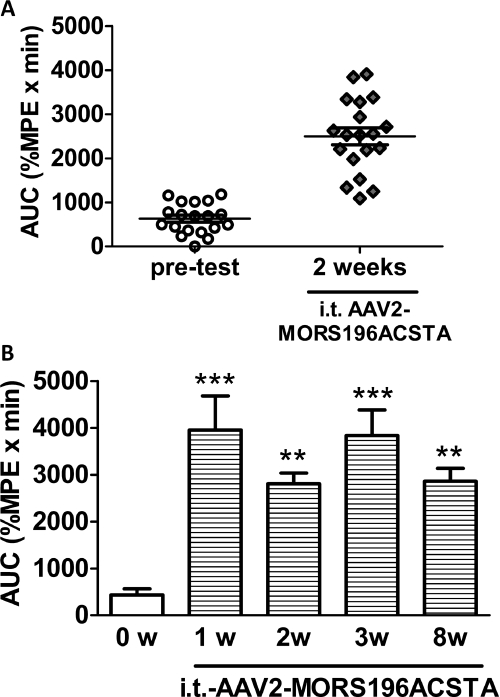
As shown in Fig. 3, naloxone (10 mg/kg s.c.) did not elicit any antinociceptive effect before gene transfer. The AUC value of naloxone was 434.6 ± 128.6 (%MPE × min), which was similar to that of saline [472.2 ± 81.3 (%MPE × min)]. In contrast, starting from 1 week after intrathecal administration of dsAAV2-MORS196ACSTA, 79% (15/19) of the mice responded to naloxone (10 mg/kg s.c.) with an average AUC value equal to 3951.2 ± 731.9 (%MPE × min) (Fig. 3). The naloxone-induced antinociceptive response remained constant when we measured 2, 3, or 8 weeks after the intrathecal injection of the virus. This effect of naloxone paralleled the observed EGFP immunofluorescence (Fig. 1).
Fig. 3.
The antinociceptive effects of naloxone in ICR mice after intrathecal administration of dsAAV2-MORS196ACSTA. A, the AUC values for naloxone (10 mg/kg s.c.) before and 2 weeks after MORS196ACSTA gene transfer (n = 19). B, the antinociceptive effects of naloxone (10 mg/kg s.c.) before (0w) and 1 week (1w), 2 weeks (2w), 3 weeks (3w), and 8 weeks (8w) after MORS196ACSTA gene transfer. Data are presented as mean ± S.E.M. (n = 6). Repeated-measures ANOVA and Newman-Keuls test were used to analyze the data. **, P < 0.01; ***, P < 0.001 compared with week 0.
Subchronic Drug Treatment-Induced Tolerance and Physical Dependence.
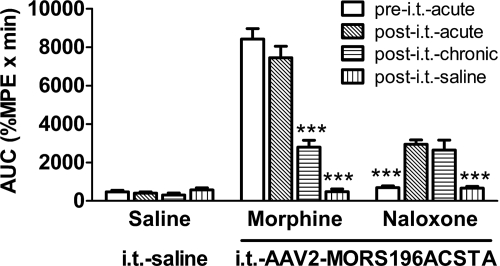
Two weeks after gene transfer, the naloxone-induced antinociceptive effect was determined. Only the mice that had AUC values more than 2000 (%MPE × min) were considered as responsible mice and were used in further studies. By calculating the subsequent values of AUC (Fig. 4) or ED50 (Table 1), it can be demonstrated that the ability of morphine (10 mg/kg s.c.) to elicit antinociceptive effect before or 2 weeks after intrathecal injection of dsAAV2 was not altered. The AUC values of morphine were 8417.1 ± 546.7 (%MPE × min) (before) and 7447.9 ± 600.6 (%MPE × min) (after), respectively. These values indicate that injection of the virus did not alter the endogenous μ-opioid receptor responses. Furthermore, intrathecal injection of dsAAV2 did not alter the subchronic morphine effect either. After the mice were subchronically treated with morphine (10 mg/kg s.c., b.i.d.) for 6 days, the AUC value was reduced to 2791.9 ± 364.3 (%MPE × min) (P < 0.001) and the ED50 value of morphine was increased from 1.3 ± 0.2 to 6.0 ± 0.9 mg/kg after 6 days of morphine treatment, reflecting a 5-fold tolerance development. In contrast to morphine-induced antinociceptive effect, subchronic naloxone activation of the mutated MOR (MORS196ACSTA) in the spinal cord did not result in tolerance. The ED50 of naloxone was 8.4 ± 1.2 mg/kg (Table 1) and the AUC was 2318.8 ± 436.7 (%MPE × min) (Fig. 4), which was not significantly different from the ED50 (8.4 ± 1.2 mg/kg) and AUC [2947.0 ± 225.1 (%MPE × min)] measured before subchronic drug treatment. To ensure that the antinociception effect was induced by morphine or naloxone but not nonspecific effect, the effect of saline was tested after subchronic drug treatment. As shown in Fig. 4, saline did not elicit any antinociception effect in each group.
Fig. 4.
Tolerance induced by morphine but not naloxone in mutated MOR gene-transferred mice. The acute antinociceptive effects of morphine (10 mg/kg s.c.) or naloxone (10 mg/kg s.c.) were determined before and after gene transfer. The subchronic effects of morphine or naloxone were determined again after 6 days of treatment (10 mg/kg s.c., b.i.d.). After subchronic drug treatment, the effect of saline (10 ml/kg) was tested (after intrathecal saline). The effects of saline injection acutely or subchronically in the intrathecal saline group (no gene transferred) were also determined as the control. Data are presented as mean ± S.E.M. (n = ∼7–10). Repeated-measures ANOVA and Newman-Keuls test were used to analyze the data. ***, P < 0.001 compared with after intrathecal acute effect of each group.
TABLE 1.
Tolerance induced by morphine or naloxone in mice injected with dsAAV2-MORS196ACSTA-EGFP intrathecally
The ED50 values of morphine or naloxone to inhibit the tail-flick responses were determined by the up-down method. After 2 weeks of gene transferred, animals were tested for the ED50 values of morphine or naloxone and then chronically treated with morphine (10 mg/kg s.c., b.i.d. for 6 days) or naloxone (10 mg/kg s.c., b.i.d. for 6 days). The ED50 values were then redetermined. The values represent the mean ± S.E.M. (n = ∼6–8).
| Treatment | AAV2-MORS196ACSTA Intrathecally |
|
|---|---|---|
| ED50 (mg/kg) of Morphine | ED50 (mg/kg) of Naloxone | |
| Before chronic treatment | 1.3 ± 0.2 | 8.4 ± 1.2 |
| After chronic treatment | 6.0 ± 0.9 (4.6-fold) | 8.4 ± 1.2 (1-fold) |
Another undesirable side effect of chronic opioid agonist is physical dependence. This is reflected in the appearance of withdrawal symptoms such as rearing, jumping, and weight loss upon the withdrawal of the agonist. In this study, a selective MOR antagonist, naltrexone (10 mg/kg i.p.), was administered to precipitate the withdrawal symptoms. Predictably, obvious rearing and jumping behaviors were seen in mutated MOR gene-transferred mice subchronically treated with morphine (10 mg/kg s.c., b.i.d. for 7 days). However, naloxone (10 mg/kg s.c., b.i.d. for 7 days) did not induce significant withdrawal signs as shown in Table 2. Although measurable loss in body weight was observed, there was no significant difference in these two groups of mice compared with the saline group.
TABLE 2.
Physical dependence induced by chronic morphine or naloxone in mutated MOR gene transferred mice by intrathecal injection of dsAAV-MORS196ACSTA
After chronical treatment with saline (10 ml/kg s.c., b.i.d.), morphine (10 mg/kg s.c., b.i.d.), or naloxone (10 mg/kg s.c., b.i.d.) for 7 days, withdrawal symptoms were precipitated by injection of naltrexone (10 mg/kg i.p.) 30 min after morphine (10 mg/kg s.c.) or naloxone (10 mg/kg s.c.) on day 8. Withdrawal signs (jumping, rearing, feces, and urine) were counted for 15 min. Body weight changes were recorded and calculated before and after 1 and 4 h of naltrexone injection. Data are presented as mean ± S.E.M. (n = ∼7–10). One-way ANOVA and Newman-Keuls test were used to analyze the data.
| Precipitated Withdrawal Symptoms | Control (Saline) | Morphine | Naloxone |
|---|---|---|---|
| Weight of feces and urine (g) | 0.12 ± 0.05 | 0.54 ± 0.09*** | 0 |
| Jumping | 0 | 15.3 ± 2.9** | 0.1 ± 0.1 |
| Rearing | 64.1 ± 4.7 | 256.6 ± 13.2*** | 79.1 ± 5.0 |
| Body weight loss (g) | |||
| 1 h after naltrexone injection | 0.66 ± 0.18 | 0.62 ± 0.25 | 0.60 ± 0.12 |
| 4 h after naltrexone injection | 1.74 ± 0.46 | 1.42 ± 0.49 | 2.00 ± 0.23 |
, P < 0.01;
, P < 0.001 compared with control group.
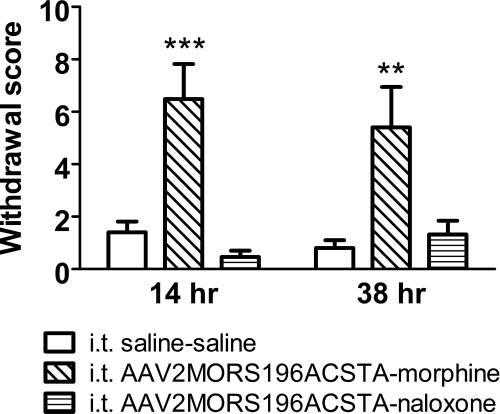
The natural withdrawal symptoms were also assessed in this study (see Fig. 6). At 14 and 38 h after the last morphine treatment, significant withdrawal signs were shown, and the global opiate withdrawal scores were 6.49 ± 1.34 (P < 0.001, vs. 1.40 ± 0.41 in control group) and 5.40 ± 1.54 (P < 0.01, vs. 0.80 ± 0.32 in control group), respectively. However, the withdrawal of naloxone after subchronic treatment did not induce significant withdrawal signs. The global opiate withdrawal scores in the naloxone groups were 0.47 ± 0.24 and 1.32 ± 0.53 at 14 and 38 h after the last naloxone treatment, respectively.
Fig. 6.
The global natural withdrawal scores after chronic saline (10 ml/kg s.c., b.i.d., 6 days), morphine (10 mg/kg s.c., b.i.d., 6 days), or naloxone (10 mg/kg s.c., b.i.d., 6 days) treatment in control (intrathecal saline) or gene-transferred mice (intrathecal AAV2MORS196ACSTA). Data are presented as mean ± S.E.M. (n = ∼7–10). One-way ANOVA and Newman-Keuls test were used to analyze the data. **, P < 0.01; ***, P < 0.001 compared with intrathecal saline–saline group.
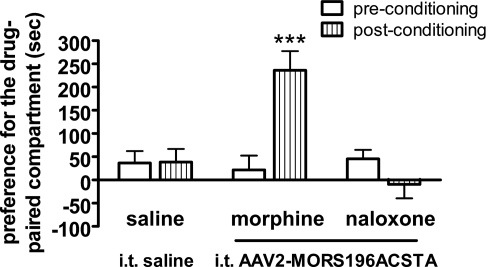
Rewarding Effects Induced by Morphine but Not Naloxone.
The rewarding effects of morphine or naloxone in mutated MOR gene-transferred mice were determined by CPP test (Chen et al., 2007). Our results showed that morphine (10 mg/kg s.c.) induced the drug rewarding effect as anticipated (Fig. 5). The time spent in the morphine-paired compartment was significantly increased after conditioning (P < 0.001). However, saline or naloxone (10 mg/kg s.c.) treatment did not induce the drug rewarding effects as shown in Fig. 5.
Fig. 5.
Morphine, but not naloxone, induced the rewarding effects in mice that had been gene-transferred by intrathecal administration of dsAAV2-MORS196ACSTA. Data are expressed as preference for the drug-paired compartment as determined by time spent in the drug-paired compartment minus time spent in the saline-paired compartment. Data are presented as mean ± S.E.M. (n = ∼7–10). Student's paired t test was used to analyze the data. ***, P < 0.001 compared with preconditioning.
Discussion
The results of our present study indicated that the intrathecal administration of dsAAV2-MORS196A can accomplish similar antinociceptive effects of naloxone as direct injection of the virus at the dorsal horn area of the spinal cord as reported previously (Chen et al., 2007). The expression of the transgene at the lumbar region of the spinal cord, with the retrograde transport of the transgene into the DRG neurons, resulted in the ability of opioid receptor antagonist to elicit antinociceptive activities after intrathecal injection of the virus.
There are obvious advantages when dsAAV2 is administered intrathecally rather than directly injected into dorsal horn area, such as ease, safety, and high percentage in the success rate (79%). These factors are critical if the mutant opioid receptor is to be developed further as a therapeutic agent. Although naloxone was shown to exhibit full agonistic properties in activating the MORS196ACSTA mutant in vitro (Claude-Geppert et al., 2005), our current AUC analyses of the tail-flick responses reflected that naloxone had less effect than morphine at the same dose (10 mg/kg s.c.). The reduced effect observed with naloxone could reflect the relatively low amount of mutant MOR being expressed in the spinal antinociceptive neurons compared with the normal MOR at the spinal or supraspinal level on which morphine could act.
The choice of dsAAV2 for current gene delivery vector is based mainly on its unique features such as safety, high titers, broad host range, transduction of quiescent cells, and vector integration (Xiao et al., 1997). Intrathecal gene transfer is an attractive approach for targeting spinal mechanisms of nociception. Several studies have shown that recombinant adeno-associated virus could successfully transfer genes into rats' spinal cord by intrathecal administration and the transgenes could be expressed in spinal cord and DRG (Milligan et al., 2005; Wang et al., 2005; Storek et al., 2006). The disadvantage of using dsAAV2 is the relative small size of the transgene that it could accommodate, i.e. <2 kb. To restrict the transgene expression within the nociceptive neurons, especially those normally expressing endogenous MOR, the 5′ untranslated region and elements that could control the expression of MOR must be included in the dsAAV expression cassette. With the minimal promoter of MOR that could regulate the spatial expression of the receptor to be ∼1.3 kb (Liang et al., 1995), dsAAV might not be the optimal gene transfer vehicle. Other viral vectors that could accommodate larger sizes of transgene and could infect neurons, such as lentivirus, or other methods for gene delivery, such as nanoparticles, must be developed for the eventual therapeutic delivery of the MOR mutant.
In this study, we assessed both the antagonist-precipitated withdrawal signs and natural withdrawal symptoms to determine the development of physical dependence. Because naltrexone also acts as an agonist when interacted with the mutated opioid receptors (Claude-Geppert et al., 2005), to assess precipitated withdrawal signs by injecting naltrexone seems not to be an ideal method to determine the development of physical dependence induced by naloxone. To rule out the effects resulted from naltrexone-induced activation of mutated opioid receptor, the natural withdrawal symptoms were also assessed in the present study. Our data indicated subchronic morphine, but not naloxone, induced significant natural withdrawal symptoms (Fig. 6).
Nausea, vomiting, and constipation are very common, irritating side effects of opiates in clinic patients. Opiate-induced nausea and vomiting are caused by activation of the μ-opioid receptors in the brainstem chemoreceptor trigger zone, and the constipating effects of opioids are mediated through an action on the μ-opioid receptors in the enteric nervous system (Schumacher and Basbaum, 2009). Because naloxone acts only on the mutated MOR expressed in the spinal cord, as an agonist if we transfer the mutated MOR by intrathecal injection, we can expect that naloxone will not cause these side effects of nausea, vomiting, and constipation. Furthermore, long-term use of opioid agonists to treat chronic pain may induce tolerance, physical dependence, and addiction, and our current intrathecal injection of dsAAV2-MORS196ACSTA and systemical administration of naloxone clearly demonstrate an alternative method for pain management without the side effects of tolerance and dependence development. Especially, the absence of place preference by naloxone indicates that this mutant receptor activated by naloxone could be a future therapeutic paradigm for the management of pain in the long term without addiction liability.
This work was supported by the National Science Council, Taiwan, Republic of China [Grant NSC97-2320-B-016-003]; and the National Institutes of Health National Institute on Drug Abuse [Grants R01-DA023905, R01-DA011806, K05-DA000513 (to P.Y.L.)].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.165399.
- dsAAV2
- double-stranded adeno-associated virus type 2
- MOR
- μ-opioid receptor
- KOR
- κ-opioid receptor
- CPP
- conditioned place preference
- EGFP
- enhanced green fluorescent protein
- ANOVA
- analysis of variance
- %MPE
- percentage of maximum possible effect
- AUC
- area under the curve
- DRG
- dorsal root ganglion
- CGRP
- calcitonin gene-related peptide.
References
- Abou-madi MN, Chin SP, Yaboub JM, Keszler H. (1976) Effects of naloxone on postoperative analgesia. Anesth Analg (Paris) 33:757–762 [PubMed] [Google Scholar]
- Bates JJ, Foss JF, Murphy DB. (2004) Are peripheral opioid antagonists the solution to opioid side effects? Anesth Analg 98:116–122 [DOI] [PubMed] [Google Scholar]
- Chen SL, Ma HI, Han JM, Tao PL, Law PY, Loh HH. (2007) dsAAV type 2-mediated gene transfer of MORS196A-EGFP into spinal cord as a pain management paradigm. Proc Natl Acad Sci USA 104:20096–20101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Fan Y, Liu J, Mestek A, Tian M, Kozak CA, Yu L. (1994) Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett 347:279–283 [DOI] [PubMed] [Google Scholar]
- Chen Y, Mestek A, Liu J, Hurley JA, Yu L. (1993) Molecular cloning and functional expression of a μ-opioid receptor from rat brain. Mol Pharmacol 44:8–12 [PubMed] [Google Scholar]
- Clark KR, Liu X, McGrath JP, Johnson PR. (1999) Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther 10:1031–1039 [DOI] [PubMed] [Google Scholar]
- Claude-Geppert PA, Liu J, Solberg J, Erickson-Herbrandson LJ, Loh HH, Law PY. (2005) Antagonist efficacy in MORS196L mutant is affected by the interaction between transmembrane domains of the opioid receptor. J Pharmacol Exp Ther 313:216–226 [DOI] [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. (1941) A method for determining loss of pain sensation. J Pharmacol Exp Ther 72:74–79 [Google Scholar]
- Dixon WJ. (1965) The up-and-down method for small samples. J Am Stat Assoc 60:967–978 [Google Scholar]
- Evans CJ, Keith DE, Jr, Morrison H, Magendzo K, Edwards RH. (1992) Cloning of a δ opioid receptor by functional expression. Science 258:1952–1955 [DOI] [PubMed] [Google Scholar]
- Fairbanks CA. (2003) Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev 55:1007–1041 [DOI] [PubMed] [Google Scholar]
- Gairola RL, Gupta PK, Pandley K. (1980) Antagonists of morphine-induced respiratory depression. A study in postoperative patients. Anaesthesia 35:17–21 [DOI] [PubMed] [Google Scholar]
- Hasebe K, Kawai K, Suzuki T, Kawamura K, Tanaka T, Narita M, Nagase H, Suzuki T. (2004) Possible pharmacotherapy of the opioid κ receptor agonist for drug dependence. Ann NY Acad Sci 1025:404–413 [DOI] [PubMed] [Google Scholar]
- He L, Whistler JL. (2005) An opiate cocktail that reduces morphine tolerance and dependence. Curr Biol 15:1028–1033 [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. (1992) The δ-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci USA 89:12048–12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann KA, Reichling U, Wirtz R. (1988) Influence of naloxone on the postoperative analgesic and respiratory effects of buprenorphine. Eur J Clin Pharmacol 34:343–352 [DOI] [PubMed] [Google Scholar]
- Liang Y, Mestek A, Yu L, Carr LG. (1995) Cloning and characterization of the promoter region of the mouse μ opioid receptor gene. Brain Res 679:82–88 [DOI] [PubMed] [Google Scholar]
- Loh HH, Liu HC, Cavalli A, Yang W, Chen YF, Wei LN. (1998) μ Opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Brain Res Mol Brain Res 54:321–326 [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, et al. (1996) Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid-receptor gene. Nature 383:819–823 [DOI] [PubMed] [Google Scholar]
- Meng F, Xie GX, Thompson RC, Mansour A, Goldstein A, Watson SJ, Akil H. (1993) Cloning and pharmacological characterization of a rat κ opioid receptor. Proc Natl Acad Sci USA 90:9954–9958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Sloane EM, Langer SJ, Cruz PE, Chacur M, Spataro L, Wieseler-Frank J, Hammack SE, Maier SF, Flotte TR, et al. (2005) Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol Pain 1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Contarino A. (2006) Gender- and morphine dose-linked expression of spontaneous somatic opiate withdrawal in mice. Behav Brain Res 170:110–118 [DOI] [PubMed] [Google Scholar]
- Paterlini MG. (2005) The function of the extracellular regions in opioid receptor binding: insights from computational biology. Curr Top Med Chem 5:357–367 [DOI] [PubMed] [Google Scholar]
- Rosow CE. (1987) The clinical usefulness of agonist-antagonist analgesics in acute pain. Drug Alcohol Depend 20:329–337 [DOI] [PubMed] [Google Scholar]
- Schumacher MA, Basbaum AI. (2009) Opioid analgesics and antagonists, in Basic and Clinical Pharmacology (Katzung BG, Masters SB, Trevor AJ. eds) pp 539–540, McGraw–Hill, Singapore [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. (1997) Opiate receptor knockout mice define μ receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci USA 94:1544–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storek B, Harder NM, Banck MS, Wang C, McCarty DM, Janssen WG, Morrison JH, Walsh CE, Beutler AS. (2006) Intrathecal long-term gene expression by self-complementary adeno-associated virus type 1 suitable for chronic pain studies in rats. Mol Pain 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surratt CK, Adams WR. (2005) G protein-coupled receptor structural motifs: relevance to the opioid receptors. Curr Top Med Chem 5:315–324 [DOI] [PubMed] [Google Scholar]
- Taguchi A, Sharma N, Saleem RM, Sessler DI, Carpenter RL, Seyedsadr M, Kurz A. (2001) Selective postoperative inhibition of gastrointestinal opioid receptors. N Engl J Med 345:935–940 [DOI] [PubMed] [Google Scholar]
- Wang X, Wang C, Zeng J, Xu X, Hwang PY, Yee WC, Ng YK, Wang S. (2005) Gene transfer to dorsal root ganglia by intrathecal injection: effects on regeneration of peripheral nerves. Mol Ther 12:314–320 [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X. (2003) Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther 10:2105–2111 [DOI] [PubMed] [Google Scholar]
- Wigdor S, Wilcox GL. (1987) Central and systemic morphine-induced antinociception in mice: contribution of descending serotonergic and noradrenergic pathways. J Pharmacol Exp Ther 242:90–95 [PubMed] [Google Scholar]
- Xiao X, Li J, McCown TJ, Samulski RJ. (1997) Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol 144:113–124 [DOI] [PubMed] [Google Scholar]
- Yang W, Law PY, Guo X, Loh HH. (2003) In vivo activation of a mutant μ-opioid receptor by antagonist: future direction for opiate pain treatment paradigm that lacks undesirable side effects. Proc Natl Acad Sci USA 100:2117–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. (1999) Retention of supraspinal δ-like analgesia and loss of morphine tolerance in δ opioid receptor knockout mice. Neuron 24:243–252 [DOI] [PubMed] [Google Scholar]