Abstract
Background:
Indacaterol is a novel, inhaled once-daily ultra-long-acting β2-agonist for the treatment of chronic obstructive pulmonary disease (COPD).
Objectives:
This study compared the onset of action of single doses of indacaterol 150 and 300 μg with salbutamol 200 μg, salmeterol-fluticasone 50/500 μg, and placebo in moderate-to-severe COPD patients.
Methods:
This was a multicenter, randomized, double-blind, placebo-controlled crossover study. The primary variable was forced expiratory volume in one second (FEV1) at five minutes postdose.
Results:
Out of 89 patients randomized (mean age 62 years), 86 completed the study. At five minutes postdose, both indacaterol doses were statistically and clinically superior to placebo (P < 0.001), with treatment–placebo differences in FEV1 of 100 (95% confidence interval [CI] 70–130) mL and 120 (95% CI 90–150) mL for indacaterol 150 and 300 μg, respectively. FEV1 at five minutes postdose with both indacaterol doses was numerically higher than for salbutamol (10 and 30 mL for indacaterol 150 and 300 μg, respectively) and significantly higher than for salmeterol-fluticasone (50 mL, P = 0.003; 70 mL, P < 0.001, respectively). Moreover, both indacaterol doses showed significantly higher FEV1 than placebo (P < 0.001) at all postdose time points. The numbers of patients with an FEV1 increase of at least 12% and 200 mL at five minutes postdose were 16 (18.8%), 24 (27.6%), 20 (23.3%), 8 (9.1%), and 3 (3.4%) for indacaterol 150 and 300 μg, salbutamol 200 μg, salmeterol-fluticasone 50/500 μg, and placebo, respectively.
Conclusions:
Single doses of indacaterol 150 and 300 μg demonstrated a fast onset of action similar to that for salbutamol and faster than that for salmeterol-fluticasone.
Keywords: indacaterol, onset of action, chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD), being a condition associated with increased morbidity and mortality, causes a substantial and increasing burden to society in many countries.1,2 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline3 recommends regular use of bronchodilators for symptomatic management of COPD, including inhaled β2-agonists and anticholinergics.3 As with all chronic diseases, nonadherence to treatment is common in patients with COPD, potentially leading to adverse health outcomes and reduced quality of life.4 Characteristics of COPD medications and regimens may often contribute to nonadherence, and therefore should be considered when selecting the appropriate treatment for a patient. In general, more complex regimens result in suboptimal adherence,5 with frequency of dosing regarded as one of the principal factors that negatively affect adherence.6 In addition, adherence is lower for medications that do not have an immediate effect on symptoms.7
Indacaterol is a novel, inhaled once-daily ultra-long-acting β2-agonist (LABA)8 for the treatment of COPD. In preclinical studies, the onset of action of indacaterol was similar to that of salbutamol and of formoterol, and was significantly faster than that of salmeterol.9 In previous clinical studies, indacaterol has demonstrated 24-hour bronchodilation on once-daily dosing, along with a good overall safety and tolerability profile.10–12 The present study was conducted to evaluate the onset of action of single doses of indacaterol 150 and 300 μg in comparison with that of salbutamol 200 μg (the standard “fast-onset” bronchodilator), the combination of salmeterol 50 μg and fluticasone 500 μg (salmeterol-fluticasone 50/500 μg), and placebo in patients with moderate-to-severe COPD.
Methods
This was an international, multicenter, randomized, double-blind, placebo-controlled, crossover, single-dose study. Institutional review board or independent ethics committee approval was obtained from each participating study center. The study was conducted in accordance with the Declaration of Helsinki (1989) and local applicable laws and regulations. All patients provided written informed consent prior to participation in the study.
Study population
Patients of either gender, aged ≥40 years, with moderate-to-severe COPD (according to the GOLD 2005 guideline), smoking history of ≥20 pack years, postbronchodilator forced expiratory volume in one second (FEV1) ≥30% but <80% of the predicted normal value, and postbronchodilator FEV1/forced vital capacity (FVC) <70% were eligible for enrolment in the study.
Patients were excluded from the study if they had any concomitant pulmonary disease, type I diabetes or uncontrolled type II diabetes, uncontrolled hypertension, unstable ischemic heart disease, or malignancy. Patients who had a history of asthma, had been hospitalized for a COPD exacerbation within six weeks prior to screening or during the run-in period, or had experienced respiratory tract infection within six weeks prior to screening, were also excluded.
The following medications were prohibited at any time during the study (the minimum washout period is shown in brackets): long-acting anticholinergics (seven days), short-acting anticholinergics (eight hours), fixed-dose combination of a β2-agonist and an inhaled corticosteroid (ICS, 48 hours), fixed-dose combination of a short-acting β2-agonist and a short-acting anticholinergic (eight hours), other LABAs (48 hours), short-acting β2-agonists (other than those prescribed in the study, six hours), xanthine derivatives (one week), and parenteral or oral corticosteroids (one month). Patients on fixed-dose combinations of a β2-agonist and an ICS were to be switched to the equivalent ICS prior to the runin period, with the dose and dosage regimen to remain unchanged for the duration of the study. Patients on ICS at the screening visit were to continue ICS for the duration of the study, again at a dose and regimen to remain unchanged.
Study design and treatments
The study comprised a prescreening visit, a 14-day screening/run-in period, and five single-dose treatment periods. During the prescreening visit, patients’ ongoing COPD medications were reviewed and, if necessary, any prohibited medication was adjusted to an allowable COPD therapy. After the screening visit, patients entered into a 14-day run-in period, during which their eligibility was assessed. At baseline, patients were randomized equally (using a validated automated system) to one of five treatment sequences to receive indacaterol 150 μg and 300 μg delivered via a single-dose dry powder inhaler, salbutamol 200 μg via the manufacturer’s proprietary multidose dry powder inhaler, salmeterol-fluticasone 50/500 μg fixed-dose combination via the manufacturer’s proprietary multidose dry powder inhaler, and placebo. Each treatment sequence comprised five double-blind, single-dose treatment periods, with treatment periods separated by a 4- to 7-day washout period. At 4–7 days after the last treatment visit, patients were contacted by telephone for a study completion call.
Patients inhaled through three different devices on each visit (one inhalation from a single-dose dry powder inhaler and one from each of the two multidose dry powder inhalers used in the study), with the order of devices randomly assigned at the start of the study. Treatment allocation was concealed from the patients, investigating staff, and the clinical trial team by the use of matching placebo inhalers all identical in packaging, labeling, and schedule of administration. An unblinded pharmacist (or designee) prepared the individual patient-specific packs from supplied bulk medication. Salbutamol was the only rescue medication permitted throughout the study, although visits had to be rescheduled if salbutamol was taken within six hours prior to the start of the visit.
Assessments and outcomes
Efficacy
All efficacy evaluations were based on spirometric assessment of lung function, performed at 50 and 15 minutes predose and at 5, 15, and 30 minutes, and at 1 and 2 hours postdose. The postdose time was measured from the time the first device was used at each study visit (the three devices were used in a randomized order, as described above). All spirometry equipment and spirometric testing were in accordance with the American Thoracic Society/European Respiratory Society standards.13
The primary efficacy variable was FEV1 at five minutes postdose. The primary objective was to compare indacaterol 150 and 300 μg and placebo in terms of FEV1 at five minutes postdose. The secondary objectives were to compare indacaterol 150 and 300 μg with salbutamol 200 μg and salmeterol-fluticasone 50/500 μg, and to compare salmeterol-fluticasone 50/500 μg with salbutamol 200 μg in terms of FEV1 at five minutes postdose. The other efficacy variables were FEV1 at other scheduled time points; the proportion of patients with at least 10%, 12%, and 15% increase in FEV1 from baseline to each scheduled time point; and the proportion of patients with at least a 12% and 200 mL increase in FEV1 from baseline to each scheduled time point.
Safety
Safety assessments included recording of adverse events and serious adverse events, along with evaluation of their severity, duration, and relationship to study drug. In addition, samples were collected for hematology and blood chemistry (including serum potassium and blood glucose), and vital signs and electrocardiogram assessments were recorded.
Sample size calculation and statistical analysis
It was estimated that 53 patients were needed to complete the study, assuming a difference of 120 mL between each indacaterol dose and placebo in terms of FEV1 at five minutes postdose, a standard deviation of 240 mL, a two-sided significance level of 5% (adjusted to 2.5% using a Bonferroni correction for the two main treatment comparisons, that is, indacaterol [150 and 300 μg] versus placebo), and a power of 90%. As there were five treatment sequences and six inhaler orders for individual treatment sequences, a total of 60 patients with evaluable measurements were required in order to have an equal number of patients per inhaler order and per treatment sequence. Allowing for a 15% dropout rate and with the number inflated to ensure balance across the treatment sequences, it was calculated that 75 patients needed to be randomized.
The primary efficacy analysis was performed on a modified intent-to-treat population that included all randomized patients who received at least one dose of study drug (the modification was that patients were analyzed according to treatment received in each treatment period). The safety population, which was used in the analysis of all safety variables, included all patients who received at least one dose of study drug.
The primary efficacy variable, FEV1 at five minutes postdose, was analyzed using a mixed model, with treatment group and period modeled as fixed effects, patient as a random effect, and period baseline FEV1 as a covariate. Period baseline FEV1 was defined as the average of the FEV1 values measured 50 and 15 minutes prior to study drug administration in each treatment period. For the primary variable, estimated least squares means and treatment differences with associated 95% confidence intervals (CIs) are presented. A multiplicity adjustment for the two main treatment comparisons in the study was made using a step-down Dunnett’s test implemented in a closed test procedure. Similar mixed-effect models were used to analyze FEV1 at the other time points.
The proportion of patients with at least a 10%, 12%, and 15% increase in FEV1 from baseline at each scheduled time point, and the proportion of patients with at least a 12% and 200 mL increase in FEV1 from baseline at each scheduled time point, were summarized using descriptive statistics and analyzed by logistic regression for each time point. The logistic regression model included terms for period, treatment, and period baseline as fixed effects and patient as a random effect. The estimated odds ratios are presented along with the associated 95% CIs and P values. All analyses were performed using SAS statistical software (version 9.1.3 for Windows; SAS Institute Inc., Cary, NC).
Results
Patient disposition, demographics, and baseline characteristics
Of 114 patients screened, 89 were eligible to participate in the study. All eligible patients were randomized to treatment sequences, and no patient received the study drug in error; thus, the safety population was identical to the modified intent-to-treat population. Eighty-six (96.6%) patients completed the study, while three (3.4%) patients discontinued from the study (one each due to withdrawal of consent, loss to follow-up, and protocol deviation).
Demographic and baseline clinical characteristics are summarized in Table 1. More than half of the patients (61.8%) were aged <65 years, the majority was Caucasian, and more than half were male. All patients were current or former smokers.
Table 1.
Demographic summary and baseline clinical characteristics (safety population)
| Variable | Total, n = 89 |
|---|---|
| Age (years) | |
| Mean ± SD | 62.3 ± 8.37 |
| Range | 43–79 |
| Sex, n (%) | |
| Male | 54 (60.7) |
| Female | 35 (39.3) |
| Race, n (%) | |
| Caucasian | 88 (98.9) |
| Black | 1 (1.1) |
| Weight (kg) | |
| Mean ± SD | 80.1 ± 17.27 |
| Range | 49.0–120.0 |
| Duration of COPD (years) | |
| Mean ± SD | 6.8 ± 5.81 |
| Range | 0.1–27.0 |
| ICS use, n (%) | |
| Yes | 49 (55.1) |
| No | 40 (44.9) |
| COPD medication discontinued prior to start of study treatment, n (%) | |
| Long-acting anticholinergic | 31 (34.8) |
| β2-agonist plus steroid | 28 (31.5) |
| Long-acting β2-agonist | 23 (25.8) |
| β2-agonist plus anticholinergic | 14 (15.7) |
| Xanthine | 9 (10.1) |
| Short-acting anticholinergic | 4 (4.5) |
| Smoking history, n (%) | |
| Exsmoker | 40 (44.9) |
| Smoker | 49 (55.1) |
| Postbronchodilator FEV1 (L) at screening | |
| Mean ± SD | 1.48 ± 0.420 |
| Range | 0.77–2.60 |
| Postbronchodilator FVC (L) at screening | |
| Mean ± SD | 2.94 ± 0.851 |
| Range | 1.21–5.24 |
| Postbronchodilator FEV1 (% predicted) at screening | |
| Mean ± SD | 53.5 ± 12.73 |
| Range | 31.6–83.8 |
| Postbronchodilator FEV1/FVC at screening (%) | |
| Mean ± SD | 51.4 ± 9.08 |
| Range | 27.4–68.8 |
| FEV1 reversibility (% increase) at screening | |
| Mean ± SD | 9.1 ± 12.22 |
| Median | 6.1 |
| 25th–75th quartile | 0.9–16.5 |
| Range | −22.8 – 41.0 |
Note: FEV1 reversibility at screening was measured 15–30 minutes after inhalation of 400 μg salbutamol.
Abbreviations: SD, standard deviation; ICS, inhaled corticosteroids; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; COPD, chronic obstructive pulmonary disease.
Efficacy
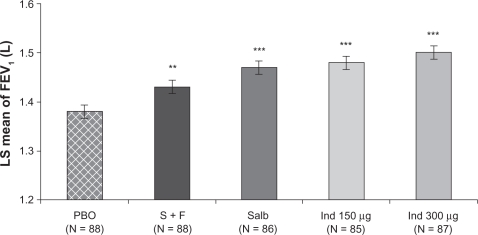
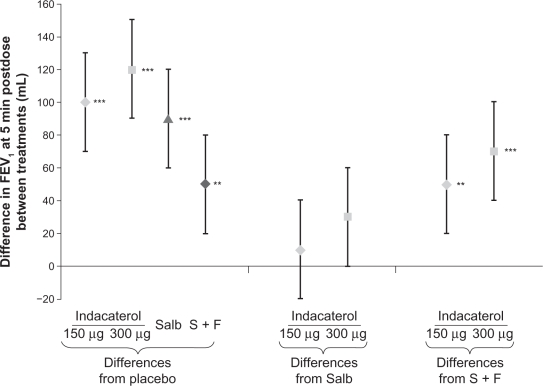
For the primary efficacy variable (FEV1 at five minutes postdose), the least squares mean values for indacaterol 150 and 300 μg were higher than that for placebo by 100 and 120 mL, respectively (P < 0.001, Figures 1 and 2). Both indacaterol doses showed a numerically higher FEV1 at five minutes postdose than salbutamol (differences of 10 and 30 mL for indacaterol 150 and 300 μg, respectively, versus salbutamol, Figure 2). Moreover, both indacaterol doses produced significantly higher FEV1 at five minutes postdose than salmeterol-fluticasone (50 mL, P = 0.003 and 70 mL, P < 0.001, Figure 2).
Figure 1.
Least squares mean FEV1 ± standard error of the mean at five minutes postdose.
Note: Differences versus placebo significant at **P < 0.01 and ***P < 0.001.
Abbreviations: Ind, indacaterol; LS, least squares; PBO, placebo; Salb, salbutamol; S + F, salmeterol-fluticasone.
Figure 2.
Least squares mean treatment differences (±95% confidence interval) in FEV1 at five minutes postdose.
Note: Differences significant at **P < 0.01 and ***P < 0.001.
Abbreviations: FEV1, forced expiratory volume in one second; Salb, salbutamol; S + F, salmeterol-fluticasone.
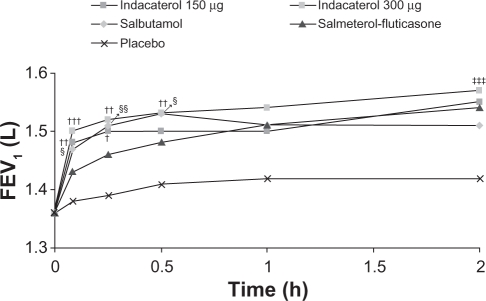
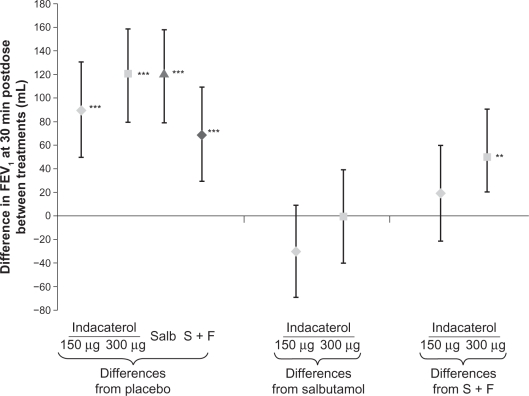
Serial measurements of FEV1 at all postdose time points are shown in Figure 3. Both indacaterol doses showed significantly higher FEV1 than placebo (P < 0.001) at all postdose time points, significantly higher than salmeterol-fluticasone (P < 0.05) at five and 15 minutes postdose, and numerically (150 μg) or statistically (300 μg) higher than salmeterol-fluticasone at 30 minutes postdose (Figure 4). The difference between indacaterol 300 μg and salbutamol was statistically significant at two hours postdose (P < 0.001).
Figure 3.
Serial least squares mean FEV1 over two hours.
Notes: P < 0.001 for both indacaterol doses versus placebo at all time points; P < 0.001 for salbutamol versus placebo at all time points; P < 0.01 for salmeterol-fluticasone at all time points; †P < 0.05; ††P < 0.01; †††P < 0.001 for indacaterol versus salmeterol-fluticasone; ‡‡‡P < 0.001 indacaterol versus salbutamol; §P < 0.05; §§P < 0.01 for salbutamol versus salmeterol-fluticasone.
Abbreviations: FEV1, forced expiratory volume in one second; h, hours.
Figure 4.
Least squares mean treatment differences (±95% confidence interval) in FEV1 at 30 minutes postdose.
Note: Differences significant at **P < 0.01 and ***P < 0.001.
Abbreviations: FEV1, forced expiratory volume in one second; Salb, salbutamol; S + F, salmeterol-fluticasone.
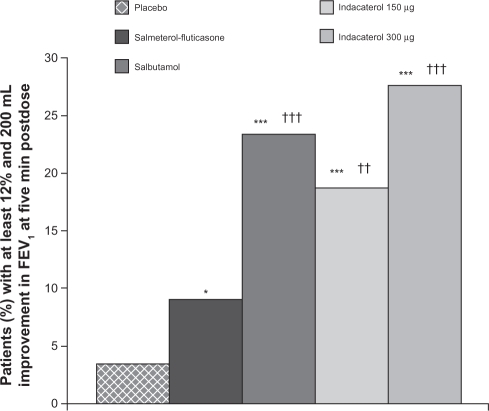
The proportion of patients with at least a 10%, 12%, or 15% increase in FEV1 from baseline at five minutes postdose was higher in both indacaterol groups compared with salmeterol-fluticasone (P < 0.01), and similar to salbutamol (Table 2). At 30 minutes postdose, the proportion of patients with at least a 10%, 12%, or 15% increase in FEV1 was higher in both indacaterol groups compared with placebo, with the proportions in the indacaterol 300 μg group higher than those in the salmeterol-fluticasone group (Table 3). The proportion was also higher in both indacaterol groups than placebo at five and 15 minutes, and at one and two hours postdose (P < 0.001 for all). The proportions of patients with at least a 12% and 200 mL increase in FEV1 from baseline at five minutes postdose in the indacaterol 150 μg, indacaterol 300 μg, and salbutamol 200 μg groups were higher than that in the salmeterol-fluticasone 50/500 μg and placebo groups (P < 0.05 for all, Figure 5).
Table 2.
Number and proportion of patients with at least 10%, 12%, or 15% increase in FEV1 at five minutes postdose
|
Percentage increase in FEV1 n (%) |
|||
|---|---|---|---|
| 10% | 12% | 15% | |
| Indacaterol 150 μg | 35 (41.2)***††† | 24 (28.2)***†† | 17 (20.0)***†† |
| Indacaterol 300 μg | 49 (56.3)***‡‡†††¶ | 40 (46.0)***†††¶¶ | 31 (35.6)***‡†††¶¶¶ |
| Salbutamol | 35 (40.7)***††† | 31 (36.0)***††† | 21 (24.4)***††† |
| Salmeterol-fluticasone | 15 (17.0)* | 12 (13.6) | 8 (9.1) |
| Placebo | 6 (6.8) | 5 (5.7) | 3 (3.4) |
Notes:
P < 0.05;
P < 0.001 versus placebo;
P < 0.05;
P < 0.01 versus salbutamol;
P < 0.01;
P < 0.001 versus salmeterol-fluticasone;
P < 0.05;
P < 0.01;
P < 0.001 versus indacaterol 150 μg.
Abbreviations: n, number of patients who achieved an improvement; FEV1, forced expiratory volume in one second.
Table 3.
Number and proportion of patients with at least 10%, at 30 minutes postdose 12%, or 15% increase in FEV1
|
Percentage increase in FEV1 n (%) |
|||
|---|---|---|---|
| 10% | 12% | 15% | |
| Indacaterol 150 μg | 39 (45.9)*** | 33 (38.8)*** | 26 (30.6)*** |
| Indacaterol 300 μg | 51 (58.6)***††¶ | 46 (52.9)***††¶ | 38 (43.7)***†††¶ |
| Salbutamol | 48 (55.8)***† | 43 (50.0)***† | 33 (38.4)***† |
| Salmeterol-fluticasone | 37 (42.0)*** | 32 (36.4)*** | 23 (26.1)*** |
| Placebo | 16 (18.4) | 12 (13.8) | 9 (10.3) |
Notes:
P < 0.001 versus placebo;
P < 0.05;
P < 0.01;
P < 0.001 versus salmeterol-fluticasone;
P < 0.05 versus indacaterol 150 μg.
Abbreviations: n, number of patients who achieved an improvement; FEV1, forced expiratory volume in one second.
Figure 5.
Proportion of patients with a ≥12% and 200 mL improvement in FEV1 at five minutes postdose.
Notes: *P < 0.05; ***P < 0.001 versus placebo; ††P ≤ 0.01; †††P ≤ 0.001 versus salmeterol-fluticasone for odds ratio (likelihood of achieving this improvement).
Abbreviations: FEV1, forced expiratory volume in one second.
Safety
Overall, adverse events were reported in 3/86 (3.5%), 3/87 (3.4%), 4/86 (4.7%), 6/88 (6.8%), and 4/87 (4.6%) patients taking indacaterol 150 μg, indacaterol 300 μg, salbutamol 200 μg, salmeterol-fluticasone 50/500 μg, and placebo, respectively. All reported adverse events were mild or moderate in severity, and none were suspected of being related to study drug. There were no deaths or serious adverse events during this study. No patient discontinued from the study due to adverse events. Notably, high pulse rate (>90 beats per minute) and systolic blood pressure (>140 mmHg) were more frequently reported for patients taking placebo than for those taking indacaterol. No patient in the study had an absolute corrected QT (Fridericia’s) value of >500 msec or a change from baseline of 60 msec. Furthermore, no patient had newly occurring or worsening clinically notable values for serum potassium (minimum postbaseline value <3.0 mmol/L) during this study, and only three patients (one patient each who took indacaterol 150 and 300 μg and salmeterol-fluticasone 50/500 μg) had newly occurring or worsening of clinically notable values for blood glucose (maximum postbaseline value >9.99 mmol/L).
Discussion
As with many chronic conditions, adherence to treatment is low in COPD, which is perhaps a reflection of the high rate of comorbid conditions and consequent polypharmacy. Although indacaterol is being developed for maintenance treatment of COPD, and not as rescue medication, fast onset on the first dose may help patients perceive an immediate symptomatic benefit. This, together with the sustained 24-hour duration of bronchodilation14 and a good overall safety and tolerability profile,12 which have been observed in other indacaterol clinical studies, may lead to improved patient adherence.15
This study was therefore designed to compare the onset of action of single doses of indacaterol 150 and 300 μg with salbutamol, salmeterol-fluticasone, and placebo. The study was conducted as planned, with 96.6% of randomized patients completing their assigned treatments. This study achieved all primary and secondary objectives. For the primary efficacy variable (FEV1 at five minutes postdose), both indacaterol doses were superior to placebo, with treatment–placebo differences of 100 and 120 mL for the 150 and 300 μg doses, respectively. Such differences (ie, ≥100 mL) have been described as ones that patients can perceive, and therefore can be considered clinically relevant.16 Furthermore, the bronchodilator efficacy of both indacaterol doses at this time point was similar to that observed with salbutamol, the ‘gold standard’ fast-acting rescue medication. Both indacaterol doses were statistically superior to placebo for the four other measures of onset of action, ie, the proportion of patients with at least a 10%, 12%, or 15% increase from baseline, or at least a 12% and 200 mL increase from baseline. The bronchodilator efficacy was at least as good as salbutamol for all of these endpoints, with the higher indacaterol dose (300 μg) reaching statistical significance versus salbutamol for some endpoints.
The second active comparator selected for this study was the fixed-dose combination of salmeterol and fluticasone. Both indacaterol doses demonstrated a faster onset of action than salmeterol-fluticasone, with treatment–placebo differences in FEV1 at five minutes postdose of 50 mL with salmeterol-fluticasone, compared with the 100–120 mL seen with indacaterol. In other studies, the combination of salmeterol and fluticasone has been shown to provide better efficacy (even after a single dose) than salmeterol alone.17 It is notable, therefore, that in the current study in addition to faster onset of action, both indacaterol doses demonstrated bronchodilator efficacy at least as good as salmeterol-fluticasone for the remainder of the two-hour assessment period.
There was a low incidence of adverse events, all of which were mild or moderate in severity and not suspected of being related to the study drug, as expected in a single-dose study. There were no deaths or discontinuations due to adverse events. There were no serious adverse events or significant adverse events reported and no clinically meaningful differences between the treatments with respect to the numbers and types of adverse events reported. The overall safety data collected indicate that single doses of indacaterol 150 and 300 μg have a good overall safety profile.
Conclusion
Single doses of indacaterol 150 and 300 μg demonstrated a fast onset of action, similar to that of salbutamol and faster than that of salmeterol-fluticasone.
Acknowledgments
The authors thank the patients who took part in the study and the staff at the participating clinical centers. The authors acknowledge Salil Bose, professional medical writer (Novartis), and David Young (Novartis) for assistance in the preparation of this manuscript.
Footnotes
Disclosure
This study was funded by Novartis Pharma AG, Basel, Switzerland. Mark Higgins, Roger Owen, Carolynn Amos, and Ben Kramer are employees of Novartis. Beatrix Balint and Henrik Watz have no actual or potential conflict of interest, including any financial relationships with industry (eg, employment, consultancies, stock ownership, honoraria, expert testimony), either directly or through immediate family.
References
- 1.Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: Current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global strategy for diagnosis, management, and prevention of COPD. Available at: http://www.goldcopd.com/Guidelineitem.asp?l1=2&l2=1&intId=2003. Accessed Mar 2, 2010.
- 4.DiMatteo MR. Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Med Care. 2004;42:200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 5.Rand CS. Non-adherence with asthma therapy: More than just forgetting. J Pediatr. 2005;146:157–159. doi: 10.1016/j.jpeds.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Adherence to long-term therapies: Policy for action. Meeting report 2001 Jun 4–5. Available at: http://www.who.int/chronic_conditions/adherence/en/. Accessed Jul 29, 2010. [Google Scholar]
- 7.Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63:831–838. doi: 10.1136/thx.2007.086041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cazzola M, Matera MG, Lotvall J. Ultra long-acting beta 2-agonists in development for asthma and chronic obstructive pulmonary disease. Expert Opin Investig Drugs. 2005;14:775–783. doi: 10.1517/13543784.14.7.775. [DOI] [PubMed] [Google Scholar]
- 9.Naline E, Trifilieff A, Fairhurst RA, Advenier C, Molimard M. Effect of indacaterol, a novel long-acting beta2-agonist, on isolated human bronchi. Eur Respir J. 2007;29:575–581. doi: 10.1183/09031936.00032806. [DOI] [PubMed] [Google Scholar]
- 10.Beeh KM, Derom E, Kanniess F, Cameron R, Higgins M, van As A. Indacaterol, a novel inhaled beta2-agonist, provides sustained 24-h bronchodilation in asthma. Eur Respir J. 2007;29:871–878. doi: 10.1183/09031936.00060006. [DOI] [PubMed] [Google Scholar]
- 11.Beier J, Chanez P, Martinot JB, et al. Safety, tolerability and efficacy of indacaterol, a novel once-daily beta(2)-agonist, in patients with COPD: A 28-day randomised, placebo controlled clinical trial. Pulm Pharmacol Ther. 2007;20:740–749. doi: 10.1016/j.pupt.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Rennard S, Bantje T, Centanni S, et al. A dose-ranging study of indacaterol in obstructive airways disease, with a tiotropium comparison. Respir Med. 2008;102:1033–1044. doi: 10.1016/j.rmed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 14.Bauwens O, Ninane V, van de Maele B, et al. 24-hour bronchodilator efficacy of single doses of indacaterol in subjects with COPD: Comparison with placebo and formoterol. Curr Med Res Opin. 2009;25:463–470. doi: 10.1185/03007990802675096. [DOI] [PubMed] [Google Scholar]
- 15.Cramer JA, Bradley-Kennedy C, Scalera A. Treatment persistence and compliance with medications for chronic obstructive pulmonary disease. Can Respir J. 2007;14:25–29. doi: 10.1155/2007/161652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2:111–124. doi: 10.1081/copd-200053377. [DOI] [PubMed] [Google Scholar]
- 17.Santus P, Matera MG, Carlucci P, et al. Onset of action of salmeterol/fluticasone (SFC) in single inhaler vs salmeterol (S) in patients with COPD. Eur Respir J. 2003;22(Suppl):285. [Google Scholar]