Abstract
An article by Jerome Puskin attempts to justify the continued use of the linear no-threshold (LNT) assumption in radiation protection and risk assessment. In view of the substantial and increasing amount of data that contradicts this assumption; it is difficult to understand the reason for endorsing this unscientific behavior, which severely constrains nuclear energy projects and the use of CT scans in medicine. Many Japanese studies over the past 25 years have shown that low doses and low dose rates of radiation improve health in living organisms including humans. Recent studies on fruit flies have demonstrated that the original basis for the LNT notion is invalid. The Puskin article omits any mention of important reports from UNSCEAR, the NCRP and the French Academies of Science and Medicine, while citing an assessment of the Canadian breast cancer study that manipulated the data to obscure evidence of reduced breast cancer mortality following a low total dose. This commentary provides dose limits that are based on real human data, for both single and chronic radiation exposures.
Jerome Puskin’s perspective on the use of the linear no-threshold (LNT) assumption for radiation protection and risk assessment (Puskin 2009) raises the question: does the U.S. Environmental Protection Agency (EPA) really protect the public or only the established worldwide practice of protecting people from radiation, which costs hundreds of billions of dollars a year? EPA exposure limits are many orders of magnitude below the levels where there is evidence of harm (Jaworowski 1999, Sanders 2010), leading to inappropriate restrictions on the use of nuclear energy to generate electricity and on the use of ionizing radiation in medicine to diagnose serious illnesses. Harmless and beneficial doses should not be regulated. Living organisms can adapt and have adapted to natural radiation, which ranges in intensity from about 0.1 to more than 70 rem per year.
The assumptions and models employed by the EPA are not based on modern biological science. The LNT assumption of radiation carcinogenesis, formulated more than 50 years ago, was originally based on experiments that were carried out on fruit flies in the mid-1920s (Muller 1954). At that time, it appeared to be reasonable for estimating cancer risk because this risk was considered to be proportional to mutation rate, which was found to be proportional to radiation dose in high dose ranges. Radiobiologists now know that organisms have defenses against DNA damage and that these can be stimulated by low doses. Although the LNT assumption is still widely accepted, it does not reflect reality, and its continued use is causing great social harm, particularly by constraining wider use of nuclear energy and CT diagnostic scans (Scott et al. 2008).
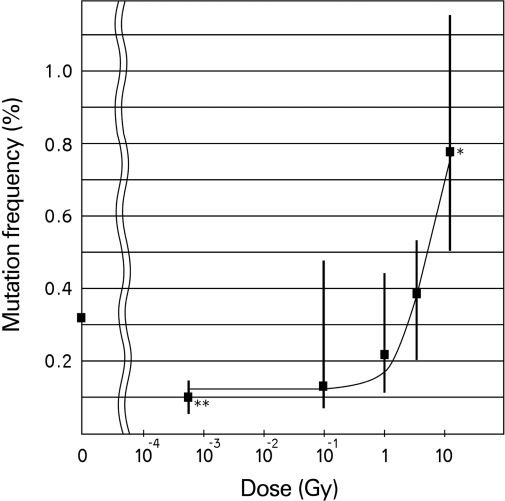
Since the mid-1980s, the Central Research Institute of the Electric Power Industry in Japan has been carrying out remarkable studies on health effects of radiation. Their recent research has demonstrated a threshold at about 1 Gy† for x-ray-induced DNA mutations in fruit flies and activation of repair by low-dose irradiation, which reduced background mutation (Koana et al. 2004, Koana et al. 2007). Gamma ray irradiation of fruit flies at a dose rate of 22.4 mGy per hour reduced lethal mutation frequency below that in the control flies (Ogura et al. 2009), as shown in Figure 1. The original basis for the LNT assumption has therefore been shown to be invalid.
FIGURE 1.
In selecting reports from scientific advisory bodies, the EPA appears to have omitted Scientific Annex B in UNSCEAR 1994, which assessed 192 scientific publications that provide evidence of beneficial health effects of low doses or low dose rates of radiation. The EPA also did not select the report of the French Academy of Science (Académie des sciences 1997), or the joint report of the French Academies of Medicine and Science (Tubiana et al. 2005) both of which raise doubts about the validity of the LNT hypothesis at low doses. A more recent publication in Radiology points out that the LNT relationship is inconsistent with data (Tubiana et al, 2009).
Lauriston Taylor, former president of the National Council on Radiation Protection and Measurements (Taylor 2010), denounced the use of a procedure to calculate the expected number of deaths per year resulting from x-ray diagnoses, as follows (Taylor 1980): “These are deeply immoral uses of our scientific heritage.” Unfortunately, this advice was ignored when scientists assessing the Chernobyl accident projected up to 28,000 excess cancer deaths using the LNT assumption and high-dose Hiroshima-Nagasaki data (Catlin et al. 1987). “No one has been identifiably injured by radiation while working within the first numerical standards set by the ICRP in 1934 (safe dose limit: 0.2 rad per day)” (Taylor 1980). Yet members of the U.S. public are limited to 0.5 rem per year.
The LNT methodology, as it is generally applied by radiation protection organizations, was tested by a comprehensive study of radon levels in U.S. homes. It failed the test (Cohen 1995).
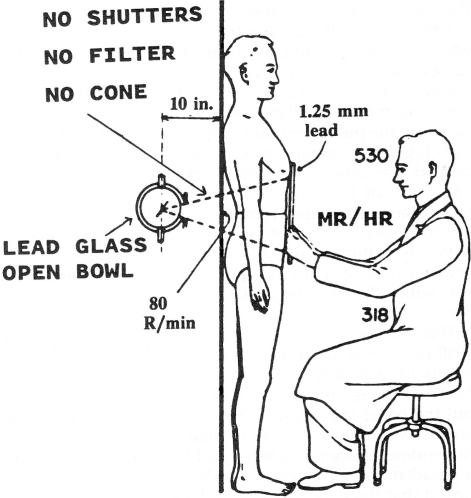
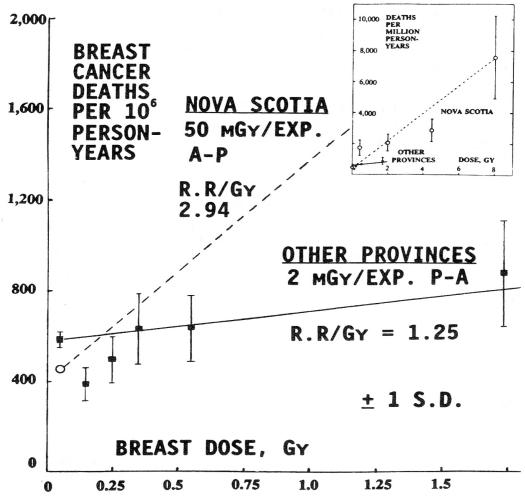
Puskin cites the Howe and McLaughlin 1996 assessment of the Canadian breast cancer study of tuberculosis (TB) patients (Miller et al. 1989) as support for the LNT model, which has been fitted to the Hiroshima-Nagasaki life span study data. However, this assessment manipulated the breast cancer mortality data in a manner that concealed the evidence of protection by low doses that Edward Webster revealed in his Lauriston S. Taylor lecture to the NCRP (Webster 1992). Figure 2 shows the configuration for the fluoroscopy examinations. Figure 3 is Webster’s graph of the Miller et al. data for patients treated for TB between 1930 and 1952. The Nova Scotia patients received a breast dose of 50 mGy (5 rad) per exposure. The patients in the other provinces received a dose of 2 mGy per exposure. Webster fitted straight lines to the high dose data points, and he extended the lines to the breast cancer death rate of the unexposed subjects. The number of exposed subjects in the “other provinces” is 12,094, while the number of unexposed subjects is 17,557. The graph suggests that women who received a total breast dose of 0.15 Gy (15 rad) have a death rate one-third lower than the breast cancer death rate for unexposed women.
FIGURE 2.
FIGURE 3.
The Howe-McLaughlin study combined three low-dose data ranges, averaging risk over the wide dose interval 0.01 to 0.49 Gy, and thus obscured the evidence that low doses of radiation provide the benefit of reduced breast cancer mortality. This evidence is highly relevant to the risk of mammography performed repeatedly over a long period of time. This manipulation of low-dose data is one of several “tricks” that epidemiologists have been using over the years to obscure evidence of radiation hormesis (Scott et al. 2008, Scott 2008).
A recent review of nuclear energy and health (Cuttler and Pollycove 2009) concludes: “Based upon human data, a single whole body dose of 150 mSv (15 rem) is safe. The high background of 700 mSv/year (70 rem/year) in the city of Ramsar, Iran is also a safe dose limit for continuous chronic exposure. Both dose limits are also beneficial.”
Footnotes
1 Gy (joules/kg) = 100 rad = 100 rem for x rays
References
- Académie des sciences Problems Associated with the Effects of Low Doses of Ionizing Radiation. French Academy of Sciences. 1997 Report No. 38. [Google Scholar]
- Catlin RJ, Goldman M, Anspaugh LR. Projected Global Health Impacts from Severe Nuclear Accidents: Conversion of Projected Doses to Risks on a Global Scale: Experience from Chernobyl Releases. 1987. US DOE report UCRL-96542. IAEA report CN-48/273. Available at: http://www.osti.gov/bridge/product.biblio.jsp?query_id=3&page=0&osti_id=5720088.
- Cohen BL. Test of the Linear-No Threshold Theory of Radiation Carcinogenesis for Inhaled Radon Decay Products. Health Phys. 1995;68:157–174. doi: 10.1097/00004032-199502000-00002. [DOI] [PubMed] [Google Scholar]
- Cuttler JM, Pollycove M. Nuclear Energy and Health: And the Benefits of Low-Dose Radiation Hormesis. Dose-Response. 2009;7:52–89. doi: 10.2203/dose-response.08-024.Cuttler. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2664640/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GR, McLaughlin J. Breast Cancer Mortality between 1950 and 1987 after Exposure to Fractionated Moderate-Dose-Rate Ionizing Radiation in the Canadian Fluoroscopy Cohort Study and a Comparison with Breast Cancer Mortality in the Atomic Bomb Survivors Study. Radiat Res. 1996;145:694–707. [PubMed] [Google Scholar]
- Jaworowski Z. Radiation Risk and Ethics. American Institute of Physics. Physics Today. 1999;52(9):24–29. Available at: http://www.riskworld.com/nreports/1999/jaworowski/NR99aa01.htm. [Google Scholar]
- Koana T, Takashima Y, Okada MO, Ikehata M, Miyakoshi J, Sakai K. A Threshold Exists in the Dose-Response Relationship for Somatic Mutation Frequency Indicated by X Irradiation of Drosophila. Radiat Res. 2004;161:391–396. doi: 10.1667/rr3152. [DOI] [PubMed] [Google Scholar]
- Koana T, Okada MO, Ogura K, Tsujimura H, Sakai K. Reduction of the Background Mutation by Low-Dose X Irradiation of Drosophila Spermatocytes at a Low Dose Rate. Radiat Res. 2007;167:217–221. doi: 10.1667/rr0705.1. [DOI] [PubMed] [Google Scholar]
- Miller AB, Howe GR, Sherman GJ, Lindsay JP, Yaffe MJ, Dinner PJ, Risch HA, Preston DL. Mortality from Breast Cancer after Irradiation during Fluoroscopic Examinations in Patients being Treated for Tuberculosis. N Eng J Med. 1989;321:1285–1289. doi: 10.1056/NEJM198911093211902. [DOI] [PubMed] [Google Scholar]
- Muller HJ. Radiation Biology. Vol. 1. McGraw-Hill Book Co. Inc; 1954. The Manner of Production of Mutations by Radiation; pp. 475–626. Chap. 8: [Google Scholar]
- Ogura K, Magae J, Kawakami Y, Koana T. Reduction in Mutation Frequency by Very Low-Dose Gamma Irradiation of Drosophila Melanogaster Germ Cells. Radiat Res. 2009;171:1–8. doi: 10.1667/RR1288.1. [DOI] [PubMed] [Google Scholar]
- Puskin JS. Perspective on the use of LNT for radiation protection and risk assessment by the U.S. Environment Protection Agency. Dose-Response. 2009;7:284–291. doi: 10.2203/dose-response.09-005.Puskin. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2790313/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders CL. Radiation Hormesis and the Linear-No-Threshold Assumption. Springer Verlag; Berlin Heidelberg: 2010. [Google Scholar]
- Scott BR, Sanders CL, Mitchel REJ, Boreham DR. CT Scans May Reduce Rather than Increase the Risk of Cancer. J Am Phys & Surg. 2008;13:8–11. Available at: http://www.jpands.org/vol13no1/scott.pdf. [Google Scholar]
- Scott BR. It’s Time for a New Low-Dose-Radiation Risk Assessment Paradigm—One that Acknowledges Hormesis. Dose-Response. 2008;6:333–351. doi: 10.2203/dose-response.07-005.Scott. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2592992/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor LS. Some Non-Scientific Influences on Radiation Protection Standards and Practice in Radiation Protection: A Systematic Approach to Safety; Proc. 5th Congress of the International Radiation Society; Jerusalem. Pergamon Press; 1980. Mar, pp. 3–15.pp. 851–874. See also Health Phys. [Google Scholar]
- Taylor LS. Health Physics Society testimonial. 2010. Available at: http://hps.org/aboutthesociety/people/inmemoriam/LauristonTaylor.html.
- Tubiana M, Aurengo A, Averbeck D, Bonnin A, Le Guen B, Masse R, Monier R, Valleron A-J, de Vathaire F, editors. Dose-Effect Relationships and the Estimation of the Carcinogenic Effects of Low Doses of Ionizing Radiation. Academy of Medicine; Paris: 2005. and Academy of Science (Paris). Joint Report No. 2. [Google Scholar]
- Tubiana M, Feinendegen LE, Yang C, Kaminski JM. The Linear No-Threshold Relationship is Inconsistent with Radiation Biologic and Experimental Data. Radiology. 2009;251:13–22. doi: 10.1148/radiol.2511080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNSCEAR . Annex B. Adaptive Response to Radiation in Cells and Organisms. United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation. Report to the General Assembly with Scientific Annexes. New York, NY: 1994. pp. 185–272. Annex B: [Google Scholar]
- Webster EW. Dose and Risk in Diagnostic Radiology: How Big? How Little? National Council on Radiation Protection and Measurements. 1992. LS Taylor Lecture No. 16.