Abstract
The aim of the present study was to develop nanoparticles of tamoxifen citrate, a non-steroidal antiestrogenic drug used for the treatment of breast cancer. Biodegradable poly (D, L- lactide-co-glycolide)-85:15 (PLGA) was used to develop nanoparticles of tamoxifen citrate by multiple emulsification (w/o/w) and solvent evaporation technique. Drug-polymer ratio, polyvinyl alcohol concentrations, and homogenizing speeds were varied at different stages of preparation to optimize the desired size and release profile of drug. The characterization of particle morphology and shape was performed by field emission scanning electron microscope (FE-SEM) and particle size distribution patterns were studied by direct light scattering method using zeta sizer. In vitro drug release study showed that release profile of tamoxifen from biodegradable nanoparticles varied due to the change in speed of centrifugation for separation. Drug loading efficiency varied from 18.60% to 71.98%. The FE-SEM study showed that biodegradable nanoparticles were smooth and spherical in shape. The stability studies of tamoxifen citrate in the experimental nanoparticles showed the structural integrity of tamoxifen citrate in PLGA nanoparticles up to 60°C in the tested temperatures. Nanoparticles containing tamoxifen citrate could be useful for the controlled delivery of the drug for a prolonged period.
Keywords: biodegradable, nanoparticles, PLGA, stability, tamoxifen citrate
Introduction
Cancer is a major human health problem worldwide and it is the second leading cause of death in USA.1,2 Breast cancer is the most fatal disease especially for women worldwide. tamoxifen, a non-steroidal antiestrogen and a selective estrogen receptor modulator, has been the clinical choice for the antiestrogenic treatment of metastasis or advanced breast cancer for more than 20 years.2 It is used as adjuvant or additional therapy following primary treatment for early stage breast cancer3–5 and to treat post menopausal breast cancer.6,7 Nanoparticles as drug delivery systems are rapidly expanding area in drug delivery sciences. Tremendous opportunities1 exist for using nanoparticles as controlled drug delivery systems for cancer treatment.8,9 Results of scientific efforts to develop tamoxifen citrate-loaded nanoparticles/microparticles using various polymers, both natural and synthetic, have been available in the literature.10–15 PLGA or poly (lactic-co-glycolic acid) is one of approved polymers by the US Food and Drug Administration (FDA) for human use as surgical sutures, implantable devices, and drug delivery systems, owing to its biodegradability and biocompatibility.16 Depending on the ratio of lactides to glycolides used for the polymerization, different forms of PLGA can be obtained and it undergoes hydrolysis in the body to produce the original monomers, lactic acid, and glycolic acid. These two monomers under normal physiological conditions are byproducts of various metabolic pathways in the body. Since the body effectively deals with the two monomers, there is very minimal systemic toxicity associated with using PLGA for drug delivery or biomaterial applications.17 PLGA-based nanoparticles containing several types of drugs including anticancer drugs have been reported as unique to this type of formulation.1 Enhanced systemic stability of drugs or therapeutics, continuous and controlled drug release, reduced dosage and decrease in systemic side effects, reduced possibility of dose dumping, reduced frequency of administration and therefore increased patient compliance are some of the advantages of sustained release nanoparticles based on biodegradable polymers such as PLGA.18 PLGA has different varieties with different characteristics based on the molar ratio of L-lactides and glycolides in PLGA. Presence of more lactides and less glycolides in PLGA (85:15) as compared to the other varieties of PLGA such as PLGA (75:25), PLGA (65:35), and PLGA (50:50), copolymer PLGA (85:15) showed slower release because of its high molecular weight and less hydrophilicity as compared to the other varieties of PLGA. Increased entrapment efficiency was observed for particles of PLGA (85:15) than the PLGA with lower L-lactide moiety,19 which ultimately could suggest more loading of drug in the co-polymer. Further, nanoparticles made of PLGA (85:15) without drug were showed to produce nanoparticles of lower polydispersity index than PLGA with less L-lactide content.19 Because of the higher molecular weight, PLGA (85:15) was found to produce particles of much smaller sizes as compared to the PLGA of other available varieties, using the same preparation technique.20 Therefore an effort was made here to develop and evaluate (in vitro) biodegradable PLGA-(85:15) based nanoparticles for providing sustained release of tamoxifen citrate for breast cancer therapy.
Materials
Tamoxifen citrate was obtained as a gift sample from Khandelwal Pharmaceutical (Mumbai, India). Biodegradable polymer poly (D, L-lactide-co-glycolide)-85:15 (PLGA) from Sigma-Aldrich (Bangalore, India) and dichloromethane and methanol from Merck (Mumbai, India) were purchased. Polyvinyl alcohol (M.W.1,25,000) (PVA) was procured from SD Fine-Chem Pvt Ltd (Mumbai, India). All other chemicals used were of analytical grade.
Methodology
The biodegradable polymer poly (D, L-lactide-co-glycolide)-85:15 (PLGA) based nanoparticles containing tamoxifen citrate was prepared by water-in-oil-in-water (w/o/w) emulsification and solvent evaporation method.21,22 Initially numbers of different formulations were prepared by varying different manufacturing parameters and few selective successful formulations in terms of physicochemical evaluation data have been reported here. At first, the weighed amount of the polymer PLGA was dissolved in dichloromethane and tamoxifen citrate was solubilized in methanol. The drug and polymer solutions were mixed in a 10 mL beaker and then aqueous phase containing polyvinyl alcohol with specific concentration was added drop-wise into the oil phase and homogenized in a high speed homogenizer in specific optimized speed for 4 minutes (Tables 1 and 2). The primary emulsion (w/o) was formed. The primary emulsion was then added drop-wise into the aqueous phase containing PVA with specific concentrations and homogenized in the same speed for 6 minutes and a multiple emulsion (w/o/w) was formed. The prepared emulsion was placed on a magnetic stirrer and stirred for 12 hours at room temperature for the evaporation of organic solvent such as dichloromethane and methanol. Nanoparticles obtained were f iltered, washed in a cooling centrifuge (Remi, Mumbai, India) at 4°C and kept in an ultra-low freezer (So-Low, Environmental Equipment, Cincinnati, Ohio, USA) at −80°C and dried in a freeze drier (IIC Industrial Corporation, Kolkata, India) at −42°C for 8 hours.
Table 1.
Polymer–drug composition of nanoparticles
| Batch nr | Tamoxifen citrate (mg) | PLGA (mg) |
|---|---|---|
| BS-1 | 5 | 238 |
| BS-2 | 5 | 240 |
| BS-3 | 10 | 512 |
Table 2.
Percentage yield and loading efficiency of the experimental formulations
| Formulation code | Speed of homogenization (rpm) | Speed of centrifugation for separation (rpm) | Yield (%) | Loading efficiency (%) (mean ± SD, n = 3) | Polydispersity index (PDI) |
|---|---|---|---|---|---|
| BS-1LS | 16000 | 5000 | 44.07 | 33.16 ± 0.16 | 0.02 |
| BS-2LS | 16000 | 5000 | 44.86 | 36.35 ± 0.87 | 0.03 |
| BS-3LS | 16000 | 5000 | 88.86 | 71.98 ± 1.58 | 0.02 |
| BS-1HS | 16000 | 14000 | 21.17 | 18.60 ± 0.16 | 0.03 |
| BS-2HS | 16000 | 14000 | 25.56 | 20.84 ± 0.17 | 0.03 |
| BS-3HS | 16000 | 14000 | 58.19 | 50.75 ± 0.85 | 0.04 |
Nanoparticle evaluation and characterization
Drug–excipient interaction study using FTIR spectroscope
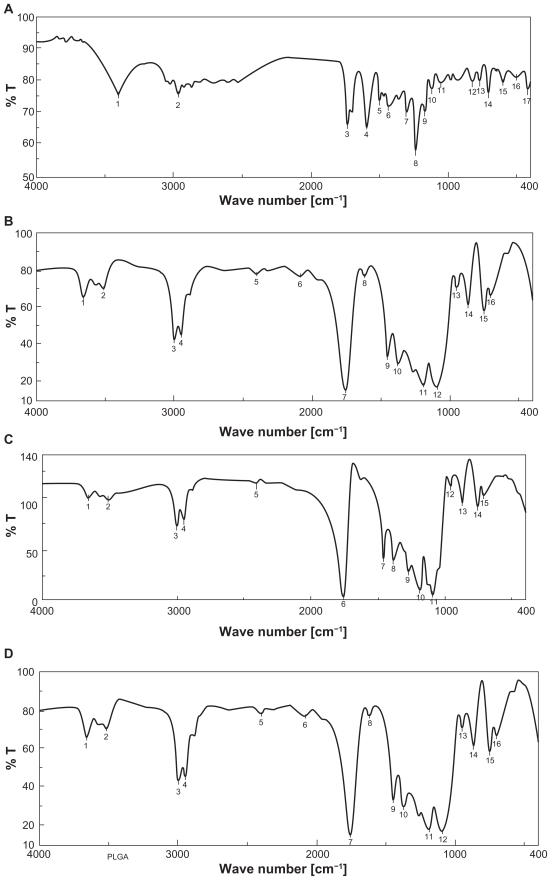
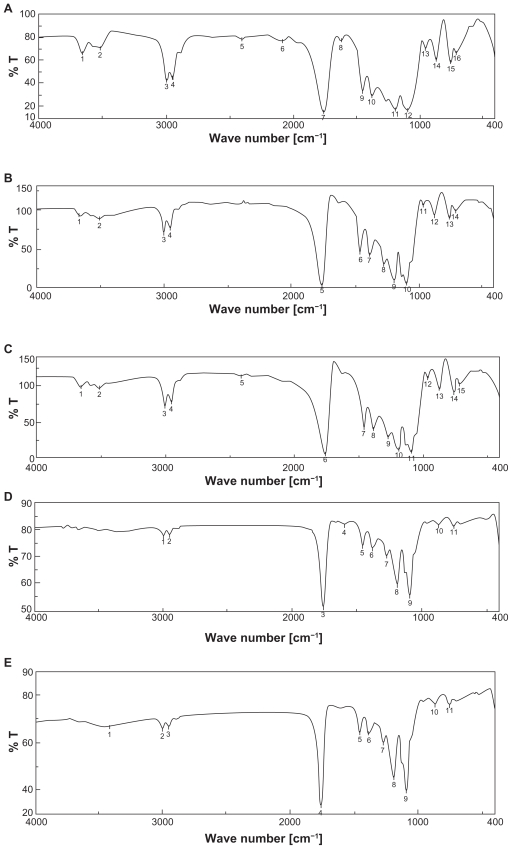
The pure drug tamoxifen citrate, PLGA-85:15, PVA, a mixture of PLGA and PVA, and a mixture of tamoxifen citrate, PLGA, and PVA; and a freshly prepared formulation were mixed separately with IR grade KBr in the ratio of 1:100 and corresponding pellets were prepared by applying 5.5 metric ton pressure with a hydraulic press. The pellets were scanned in an inert atmosphere over a wave number range of 4000–400 cm−1 in Magna IR 750 series II, FTIR instrument (Nicolet, Madison, WI, USA). However, due to space constraint, we have shown here only the important spectra (Figure 1).
Figure 1.
FTIR spectra of A) tamoxifen citrate; B) PLGA; C) mixture of drug and excipients; D) freshly prepared nanoparticles in the formulation (BS-3HS).
Percentage of yield
The lyophilized nanoparticles from each formulation were weighed and the respective percentage yield was calculated using the following formula.
Drug content study
Nanoparticles (2 mg) were dispersed in 1 mL of 0.1 M NaOH aqueous solution containing 5% w/v sodium dodecyl sulphate (SDS) and the mixture was shaken for 2–3 hours in an incubator shaker at 37°C. Drug from nanoparticles was extracted. The extracts were filtered by centrifugation and 0.8 mL of supernatant was taken with the help of micropipette and absorbance was noted using spectrophotometer at 275 nm. The drug entrapment efficiency was determined from the calibration curve prepared earlier using the following formula.
Surface morphology of nanoparticles
The surface morphology of nanoparticles was investigated using Field Emission Scanning Electron Microscope (FESEM). Nanoparticle samples were mounted on the stubs using double-sided adhesive tapes. The stubs were then vacuum-coated with platinum using JEOL JFC 1600 autofine coater (JEOL, Tokyo, Japan). The nanoparticles were examined with FESEM, JEOL JSM 6700F (JEOL, Tokyo, Japan).
Particle size distribution
The characterization of particle size distribution was performed by using Zetasizer nano ZS with DTS software (Malvern Instruments Limited, UK). NIBS® (non-invasive backscatter optics) technology was used for measurements of particles. The formulations were taken in lyophilized form in microcentrifuge tubes, suspended in phosphate buffer, pH 7.4 and introduced in the instrument to read the results.
In vitro drug release study
Nanoparticles (3 mg) containing tamoxifen citrate were taken in 1 mL phosphate buffer, pH 7.4, in 2 mL Eppendorf tube and kept in an incubator shaker at 37°C. Numbers of such tubes were kept for analysis of drug release at different time points. At a particular time point the tubes intended for analysis (at that point) were taken out and the remaining tubes to be analyzed at their respective time points were left in the incubator shaker. Then the tubes were centrifuged at 5000 rpm for 10 minutes; the supernatant was taken and analyzed spectrophotometrically at 275 nm. Amount of drug released was determined from the calibration curve prepared earlier.
Stability study
Following the ICH guidelines, nanoparticles containing tamoxifen citrate samples were stored at elevated temperatures and relative humidity (25 ± 2°C/60% RH, 45 ± 2°C/75% RH, 60 ± 2°C/75% RH ) in a stability analysis chamber (Darwin Chambers Company, St Louis, USA) over a period of 3 months.23 Samples stored at 2–8°C were used as control. Samples were kept for 3 months for stability analysis and after 3 months, FTIR spectra of drug of the nanoparticles were compared with those of the control formulations.
Results
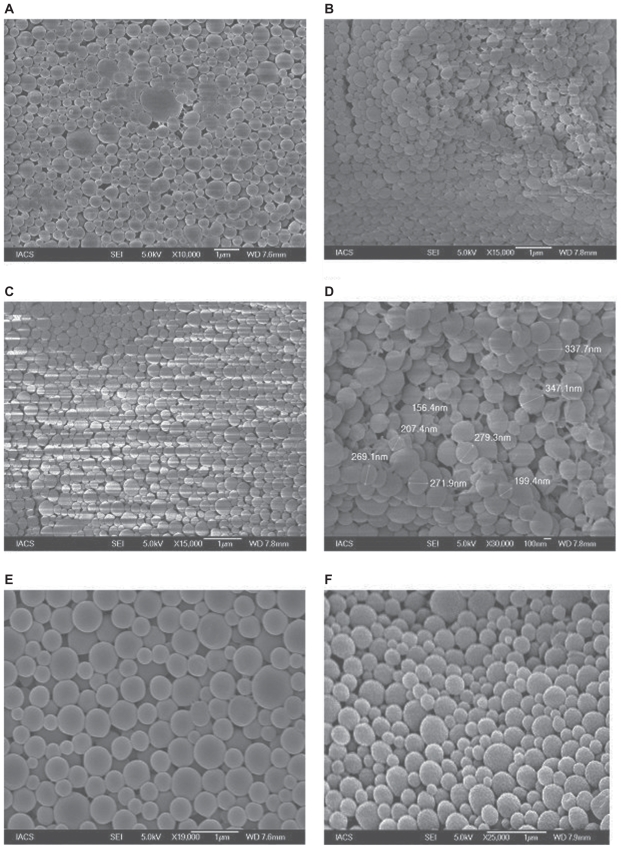
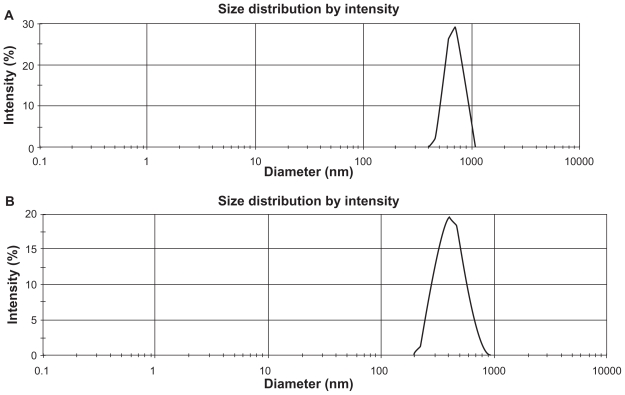
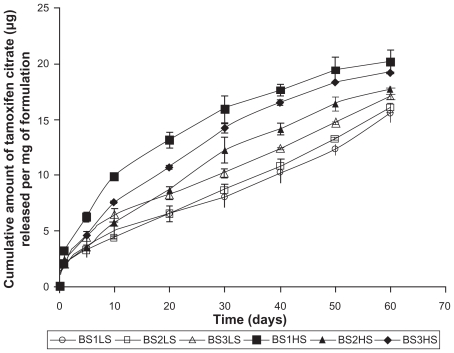
Yield percentage and loading efficiency were found to vary with a variation in speed of centrifugation (Table 2). Yields were more in case of the formulations (BS-1LS, BS-2LS, and BS-3LS) exposed to lower speed of centrifugation. Highest respective yield (%) values were obtained (both in high and low centrifugal speeds) in case of the formulations BS-3LS and BS-3HS. A similar trend was noticed in case of the loading efficiency also. Highest loading efficiency percentage (both in high and low centrifugal speeds of separation) was obtained for BS-3LS and BS-3HS in the respective category as compared to the other experimental formulations. Drug–excipient interactions as determined by FTIR spectra (Figures 1, A–D) suggest that there were physical interactions between drug and excipients at the level of functional groups between 1300 and 1200 cm−1 wave numbers. SEM photographs (Figures 2, A–F) show that the nanoparticles were densely dispersed with a narrow range of dispersion. Particles were of nano size with smooth surface. With the particle size analyzer the average diameter of particles for BS-3LS and BS-3HS were found to be 550 nm and 276 nm (Figure 3, A and B). In vitro drug release patterns were similar in all the formulations (Figure 4). Initial burst release was followed by sustained release of drug with the progress of time. Further the nanoparticles prepared with low speed centrifugation released the drug in slower patterns as compared to those exposed to higher centrifugal force. Drug stability in the formulations as assessed by comparing the FTIR spectra of stored formulations (Figures 5, A–E) with the freshly prepared formulation (Figure 1D) revealed that there was no physicochemical interaction due to storage occurred in the formulations stored up to 25°C. However, at 45°C and 60°C mild physical interactions as compared to the freshly prepared samples were detected.
Figure 2.
Scanning electron microscopy of PLGA nanoparticles A) BS-1LS; B) BS-1HS; C) BS-2LS; D) BS-2HS; E) BS-3LS; F) BS-3HS.
Figure 3.
Particle size distribution pattern of experimental nanoparticles A) BS-3LS; B) BS-3HS.
Figure 4.
In vitro drug release profile in phosphate buffer solution at Ph 7.4.
Figure 5.
FTIR spectra of experimental nanoparticles A) freshly prepared nanoparticles; B) stored at 2–8°C for 90 days; C) stored at 25°C for 90 days; D) stored at 45°C for 90 days; E) stored at 60°C for 90 days.
Discussion
Yield (%) of various experimental nanoparticles were between 21.17% and 88.86% (Table 2). Therefore, the loss of yield was observed in nanoparticle preparations using PLGA and tamoxifen citrate. It might be due to recovery problem. The loss of yield might be due to adherence of highly viscous primary emulsion (w/o) (which was also sticky in nature) on the surface of the inner wall of the homogenizer and was not fully recovered. Loading efficiency of the experimental nanoparticles was found to vary significantly (P < 0.05) as assessed by ANOVA followed by Tukey HSD Test. The variation in loading efficiency might be due to separation of smaller particles at higher centrifugal speed, which could entrap a smaller amount of drug.
Batches (BS-1LS, BS-2LS, and BS-3LS) separated at lower centrifugal speed (5000 rpm) had higher drug entrapment as compared to those (BS-1HS, BS-2HS, and BS-3HS) separated at higher centrifugal speed (14,000 rpm). In reality the speeds of centrifugation were determined by several experiments using the speeds of centrifugation ranging from 4000 rpm to 20,000 rpm. Similar trends have been obtained throughout. However we have reported here the effect of two different speeds of centrifugation, which influenced all the parameters of the formulations predominantly.
Drug–excipient interaction
Figure 1 (A–D) shows the FTIR spectra of tamoxifen citrate, PLGA and PVA (excipients), and mixture of drug and excipients and a freshly prepared formulation. Variation in peaks was noticed between 1300 and 1200 wave numbers (cm−1) in nanoparticles. The region is the IR stretching vibration zone of functional groups such as C-O (alcohol) and C-N.21 Formation of weak physical bonds such as hydrogen bonding, bond due to Van der Waals forces or dipole moment might take place between C-N present in the drug molecule and –OH present in PLGA. The data suggest that there was no chemical interaction between the drug and the excipients since no shifting of characteristic peaks of drug as well as excipients was noticed as the shifting of characteristic peak is claimed as chemical interaction.24
Scanning electron microscopic study
SEM photographs (Figures 2, A–F) show the particle size distribution pattern of nanoparticle formulations. The figure shows that PLGA nanoparticles containing tamoxifen citrate were spherical and of smooth surface.
Particle size distribution
Although we showed data of all six different formulations so far, we here now limit ourselves to formulations BS-3LS and BS-3HS, since they had the highest amount of loading and yield values. Figure 3 shows that the average particle size was about 550 nm (Figure 3A) with a polydispersity index (PDI) of 0.02 of the sample formulation obtained from low speed centrifugation (5000 rpm). Figure 3B shows that the average particle size of the nanoparticles was 276 nm for the formulation BS-3HS (PDI 0.04). Thus, the high speed of centrifugation used here was able to separate nanoparticles of smaller range with a lower polydispersion. The polydispersity indices suggest that particles were in nano-size range only with a narrow range of particle size distribution.
In vitro drug release study
Tamoxifen citrate was released from PLGA nanoparticles (Figure 4) following ‘bi-phasic’ pattern with an initial ‘burst release’ of loosely bound tamoxifen citrate on/or near the surface of particles and then release of the remaining drug from the core due to diffusion and as the polymer matrix disintegrated. The release profile of the various samples were similar, ie, the primary burst release was followed by a sustained release. The differences in sustained release of the various samples might also be due to the nanoparticles with variable sizes, which varied surface areas as well as tortuosity of drug diffusion pathways in the nanoparticles. Drug release patterns were more or less linear as the duration of release progressed. BS-1LS showed the slowest drug release whereas BS-1HS showed the fastest drug release among the formulations studied. Similar trends of drug release were also obtained for the other two (LS and HS) formulations. It could be that due to the exposure of high centrifugal force in BS-1HS, loosely bound drug molecules had come more from the core towards the surface and this resulted in a faster drug release.
Stability study
For stability study of the drug in formulations, the samples were kept at different temperatures (at 25°C, 45°C, and 60°C) for 90 days following ICH guidelines23 and spectra of the formulation were determined by FTIR spectroscopy and compared with those of the freshly prepared formulations. Figure 5 (A–E) represents the IR spectra of the formulations that were kept at different temperatures for 90 days. Figure 1D represents the spectrum of freshly prepared formulation and Figures 1D and 5A and B were used as controls. Figures 5, C–E were compared with Figure 5B. No variation of spectra of the samples stored at 25°C suggests that the drug remained stable in the formulations at those storage conditions and there was no physicochemical interaction between the drug and the excipients of nanoparticles under the storage condition. Variation in the FTIR spectra of the samples stored at 45°C and 60°C suggests that in those formulations drug might have physically interacted with the excipients. This may help the slower release of drug from the formulations. However, this would not degrade the drug chemically in the nanoparticles since physical interactions in presence of humid environment at those temperatures are believed to occur due to the formation of weak physical bonds such as hydrogen bonding, bond due to Van der Waals forces or dipole-dipole interactions.
Conclusion
The prepared nanoparticles had good entrapment and reasonable loading of the drug with controlled and prolonged release of tamoxifen citrate for the betterment of breast cancer therapy. Thus the mentioned nanoparticles could be beneficial to deliver tamoxifen citrate in a long-term controlled release pattern for the treatment of breast cancer. It may be a potential alternative dosage form of the drug for the treatment of breast cancer and further studies are warranted.
Acknowledgment
The work was supported by University Grants Commission (Government of India); grant nr P-1/RS/404/07.
Footnotes
Disclosure
No conflicts of interest were declared in relation to this paper.
References
- 1.Orive G, Harnandez RM, Gascon AR, et al. Micro and nano drug delivery systems in cancer therapy. Cancer Ther. 2005;3:131–138. [Google Scholar]
- 2.Bilensoy EM, Vural I, Bochot A, et al. Tamoxifen citrate loaded amphiphilic ß-cyclodextrin nanoparticles; in-vitro characterization and cytotoxicity. J Control Release. 2005;104(3):489–496. doi: 10.1016/j.jconrel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Reagan RMO, Jordan C. The evolution of tamoxifen therapy in breast cancer: selective estrogen receptor modulators and down regulators. Lancet Oncol. 2002;3(4):207–214. doi: 10.1016/s1470-2045(02)00711-8. [DOI] [PubMed] [Google Scholar]
- 4.Pappas SG, Jordan VC. Chemoprevention of breast cancer; current and future prospects. Cancer Metastasis Rev. 2002;21:3–4. 311–321. doi: 10.1023/a:1021219212638. [DOI] [PubMed] [Google Scholar]
- 5.Powles TJ. Anti-estrogenic chemoprevention of breast cancer-the need to progress. Eur J Cancer. 2003;39(5):572–579. doi: 10.1016/s0959-8049(02)00771-2. [DOI] [PubMed] [Google Scholar]
- 6.Petero-Engl C, Frank W, Danamayr E, et al. Association between endometrial cancer and tamoxifen treatment of breast cancer. Breast Cancer Treat. 1999;54(3):255–260. doi: 10.1023/a:1006126411210. [DOI] [PubMed] [Google Scholar]
- 7.Cohen I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol. 2004;94(2):256–266. doi: 10.1016/j.ygyno.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 8.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissues. Adv Drug Deliv Rev. 2003;55(3):329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 9.Birnbaum DT, Brannon-Peppas L. Microparticle drug delivery systems. In: Brown DM, editor. Drug delivery systems in cancer therapy (cancer drug discovery) Totowa, NJ: Humana Press; 2003. pp. 117–135. [Google Scholar]
- 10.Reddy LH, Vivek K, Bakshi N, et al. Tamoxifen citrate loaded solid lipid nanoparticles: preparation, characterization, in vitro drug release, and pharmacokinetic evaluation. Pharm Dev Technol. 2006;11(2):167–177. doi: 10.1080/10837450600561265. [DOI] [PubMed] [Google Scholar]
- 11.Sarmah JK, Bhattacharjee SK, Maganta R, et al. Preparation of crosslinked guar gum nanospheres containing tamoxifen citrate by single step emulsion in situ polymer crosslinking method. J Inclusion Phenomena Macrocyclic Chem. 2009;65:3–4. 329–334. [Google Scholar]
- 12.Gerelli Y, Di Bari MT, Deriu A, et al. Structure and organization of phospholipid/polysaccharide nanoparticles. J Phys Condens Matter. 2008;120:104211. [Google Scholar]
- 13.Yezhelyev M, Yakoub R, O’Regan R. Inorganic nanoparticles for predictive oncology of breast cancer. Nanomedicine. 2009;4(1):83–103. doi: 10.2217/17435889.4.1.83. [DOI] [PubMed] [Google Scholar]
- 14.Bilensoy E, DoA L, Hancal A. Complexation behavior of antiestrogen drug tamoxifen citrate with natural and modified cyclodextrins. J Inclusion Phenomena Macrocyclic Chem. 2007;57:1–4. 651–655. [Google Scholar]
- 15.Sehra S, Dhake AS. Formulation and evaluation of sustained release microspheres of poly-lactide-co-glycolide containing tamoxifen citrate. J Microencapsulation. 2005;22(5):521–528. doi: 10.1080/02652040500162170. [DOI] [PubMed] [Google Scholar]
- 16.Mainardes RM, Silva LP. Drug delivery systems: past, present and future. Curr Drug Targets. 2004;5(5):449–455. doi: 10.2174/1389450043345407. [DOI] [PubMed] [Google Scholar]
- 17.Kumari A, Yadav SK, Yadav SC. Biodegradable, polymeric nanoparticles based drug delivery systems. Colloids Surfaces B: Biointerfaces. 2009;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Betancourt T, Brown B, Brannon-Peppas L. Doxorubicin-loaded PLGA nanoparticles by nanoprecipitation: preparation, characterization and in vitro evaluation. Nanomedicine. 2007;2(2):219–232. doi: 10.2217/17435889.2.2.219. [DOI] [PubMed] [Google Scholar]
- 19.Mittal G, Sahana DK, Bhardwaj V, et al. Estradiol loaded PLGA nanoparticles for oral administration: Effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J Control Release. 2007;119(1):77–85. doi: 10.1016/j.jconrel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Sinha VR, Trehan A. Formulation, characterization, and evaluation of ketorolac tromethamine-loaded biodegradable microspheres. Drug Delivery. 2005;12:133–139. doi: 10.1080/10717540590925726. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee B, Santra K, Pattnaik G, et al. Preparation, characterization and in-vitro evaluation of sustained release protein-loaded nanoparticles based on biodegradable polymers. Int J Nanomed. 2008;3(4):487–496. doi: 10.2147/ijn.s3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemoine D, Preat V. Polymeric nanoparticles as delivery system for influenza virus glycoproteins. J Control Release. 1998;54(1):15–27. doi: 10.1016/s0168-3659(97)00241-1. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh S, Roy G, Mukherjee B. Dental mold: a novel formulation to treat common dental disorders. AAPS Pharm Sci Tech. 2009;10(2):692–702. doi: 10.1208/s12249-009-9255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckett AH, Stenlake JB. Practical pharmaceutical chemistry. Fourth edition. part two. CBS Publishers; New Delhi, India: 1997. Infrared spectrophotometry; pp. 384–390. [Google Scholar]