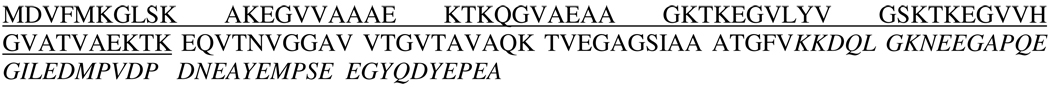Abstract
α-Synuclein (α-syn), a presynaptic protein believed to play an important role in neuropathology in Parkinson’s disease (PD), is known to bind Cu2+. Cu2+ has been shown to accelerate the aggregation of α-syn to form various toxic aggregates in vitro. Copper is also a redox active metal whose complexes with amyloidogenic proteins/peptides have been linked to oxidative stress in major neurodegenerative diseases. In this work, the formation of Cu2+ complex with α-syn or with an N-terminus peptide, α-syn(1–19), was confirmed with electrospray-mass spectrometry (ES–MS). The redox potentials of Cu2+ complex with α-syn (α-syn-Cu2+) and α-syn(1–19) were determined to be 0.018 and 0.053 V, respectively. Furthermore, the Cu2+ center(s) can be readily reduced to Cu+ and possible reactions of α-syn-Cu2+ with cellular species (e.g., O2, ascorbic acid, and dopamine) were investigated. The occurrence of a redox reaction can be rationalized by comparing the redox potential of the α-syn-Cu2+ complex to that of the specific cellular species. For example, ascorbic acid can directly reduce α-syn-Cu2+ to α-syn-Cu+, setting up a redox cycle in which O2 is reduced to H2O2 and cellular redox species is continuously exhausted. In addition, the H2O2 generated was demonstrated to reduce viability of the neuroblastoma SY-HY5Y cells. Although our results ruled out the direct oxidation of dopamine by α-syn-Cu2+, the H2O2 generated in the presence of α-syn-Cu2+ can oxidize dopamine. Our results suggest that oxidative stress is at least partially responsible for the loss of dopaminergic cells in PD brain and reveal the multifaceted role of the α-syn-Cu2+ complex in oxidative stress associated with PD symptoms.
Keywords: α-Synuclein, copper, electrochemistry, redox reaction, oxidative stress, hydrogen peroxide, cytotoxicity
Although many neurodegenerative diseases are manifested by the aggregation of the amyloidogenic proteins (e.g., β-amyloid or A β in Alzheimer’s disease (AD), α-synuclein (α-syn) in Parkinson’s disease (PD), and prion protein in Creutzfeldt-Jakob disease),(1, 2) oxidative stress has also been implicated in the pathogenesis of neurodegenerative diseases.(3, 4) Consequently, metal-induced oxidative damages have been an area under active pursuit.(5, 6) The implication of metal-induced oxidative stress is extremely broad, ranging from acceleration of the formation of reactive oxygen species (ROS) (7) in the presence of redox metals such as copper and iron,(8) mitochondria function impairment,(9, 10) and neuronal membrane damages through lipid peroxidation,(11) to depletion of vital intracellular species.(12) For example, in the senile plaques of AD patients, large amounts of redox active metal ions such as Cu2+ and Fe2+ have been found to coexist with the aggregates of Aβ peptides.(13) In vitro studies have firmly established that these metal ions can strongly bind Aβ peptides(14) and the resultant complexes can facilitate the generation of H2O2 by reacting with cellular species such as ascorbic acid (AA) and O2:(15, 16)
| (1) |
| (2) |
Recently, we measured the redox potentials of the Cu2+ complexes with several Aβ peptides.(12) Our results confirmed that the H2O2 generation can be catalyzed by the Cu2+ complexes of both the aggregation-prone, full-length Aβ(1–42) and the non-aggregating hydrophilic Aβ(1–16).(12) H2O2 is an important ROS because H2O2 itself can react with a variety of cellular species and is a precursor for or product from reactions of other ROS. For example, reaction 2 has been suggested to proceed by reduction of O2 to oxygen radical anion (O2•−, another ROS).(17) On the other hand, uncomplexed or free redox metal ions (e.g., redox active Cu2+ and Fe2+) can generate hydroxyl radicals (OH•) by reacting with H2O2 through the Harber-Weiss and Fenton reactions.(18, 19) Since the brain utilizes O2 for a variety of important functions, continuous reduction of O2 to generate ROS by trace Cu2+ complex with Aβ that is reduced by a cellular reductant (e.g., AA) could lead to pronounced deleterious effects.(5, 12) Postmortem chemical (20) and spectroscopic (21) analyses have also provided evidence for the possible involvement of these metals or their complexes with Aβ in producing neurotoxins, which eventually result in neuronal cell loss. Interestingly, Aβ has also been suggested to possibly serve as a protective agent through metal ion complexation, which ameliorates the free metal ion-induced hydroxyl radical generation.(22–24)
PD is pathologically marked by the progressive loss of neurons in the substantia nigra, a small brain region producing dopamine (DA).(25) A hallmark of PD is that surviving dopaminergic cells contain cytosolic filamentous inclusions known as the Lewy bodies (LBs).(26) A major component in LBs is the α-syn aggregates,(27, 28) whose monomeric constituent contains 140 amino-acid residues encompassing the positively charged N-terminus (residues 1–60), the aggregation-prone nonamyloid components (NAC, residues 60–95), and the negatively charged C-terminus (residues 96–140).(29)
α-Syn can also bind Cu2+ and several binding sites for Cu2+ in α-syn have been detected.(30) The binding mode contributed by the first four amino acids of N-terminus,(31) in which Met–1 serves as an anchor, has a dissociation constant of ~0.2 µM.(31) This binding mode is much stronger than the nonspecific binding by the C-terminus of α-syn (dissociation constant around millimolar level (30)) and also two orders of magnitude stronger than the binding sites centered around His–50.(30) The relatively high Cu2+ binding affinity at Met–1 explains why α-syn can sequester Cu2+ released from aberrant proteins (e.g., Cu/Zn superoxide dismutase) of PD patients.(32, 33) Together with the abnormal homeostasis of Cu2+ in PD patients,(34–36) the Cu2+ complex with α-syn (α-syn-Cu2+) has been suggested to play an important role in PD etiology.(37, 38) For example, Cu2+ is an effective ion in promoting the formation of α-syn oligomers,(39) which is cytotoxic due to its putative role in pore formation in the cell membrane.(38) However, given that α-syn is known to be redox-inactive,(40) the decreased level of redox-active species (e.g., glutathione (GSH) and DA(41)) and the increased level of protein oxidation(42, 43)in PD brain cannot be rationalized by the α-syn aggregation mechanism. Prompted by the thought that a mechanism similar to reactions 1 and 2 might be applicable, we hypothesize that easily oxidizable cellular species could react with α-syn-Cu2+. In fact, we recently discovered that the complex formed between α-syn and Fe2+ (44) can also take part in the generation of H2O2 in an analogous fashion.(45)
To verify our hypothesis, it is necessary to first measure the redox potential of α-syn-Cu2+, which will provide a better assessment of a reaction between the complex and a species that undergoes abnormal homeostasis (e.g., DA(46), GSH(41) or NADH(47)). Since H2O2 can cause neuronal cell death,(16) it is also intriguing to learn whether the α-syn-Cu2+ complex can participate in the generation of H2O2 in the same manner as Cu2+ complexes with Aβ (cf. reactions 1 and 2) and consequently impose toxicity to neuronal cells. In this work, α-syn-Cu2+ was characterized by mass spectrometry and voltammetry. To avoid possible α-syn aggregation during the measurements, we also synthesized an N-terminus α-syn(1–19) peptide, which encompasses the strongest Cu2+-anchoring site at Met–1 but does not contribute to the α-syn aggregation. The redox potentials of α-syn-Cu2+ and α-syn(1–19)-Cu2+ were determined to be 0.018 and 0.053 V, respectively. These potentials suggest that the complexes can oxidize certain cellular reductants (e.g., AA and GSH) and subsequently generate H2O2 through O2 reduction in a mechanism similar to that depicted by reactions 1 and 2. H2O2 was verified to be the product of O2 reduction and shown to be cytotoxic. The implication of the α-syn-Cu2+ complex in PD-related oxidative stress is discussed.
Materials and Methods
Materials
Wang resin, Fmoc-protected amino acids, diisopropylcarbodiimide, 1-hydroxylbenzotriazole, and piperidine were purchased from Anaspect Inc. (San Jose, CA). Potassium hydrogen phosphate, potassium hydroxide, sodium sulfate, ammonium sulfate, trifluoroacetic acid, copper sulfate, copper chloride, nickel chloride, isopropyl β-D-thiogalactopyranoside (IPTG), L-glutamine, and organic solvents were obtained from Thermo-Fisher Scientific Inc. (Pittsburgh, PA). DA and AA were acquired from Sigma-Aldrich (Milwaukee, WI). SH-SY5Y cells (human neuroblastoma) were from American Type Culture Collection Inc. (Manassas, VA) and fetal bovine serum (FBS) was from Omega (Tarzana, CA). Both Dulbecco’s Modified Eagle Medium (DMEM) and Ham’s F12 were acquired from Mediatech Inc. (Manassas, VA). E coli BL21 (DE3) and dithiothreitol (DTT) were purchased from Invitrogen Corp (Carlsbad, CA) and lysozyme was from EMD Inc. (Gibbstown, NJ). Both Aβ(1–16) and Aβ(1–42) were acquired from American Peptide Co. Inc. (Sunnyvale, CA). Water was purified by a Millipore system (Billerica, MA) to be 18 MΩ• cm and used for the preparation of all aqueous solutions and the mixture of penicillin and streptomycin for cytotoxicity study was also purchased from Millipore. Phosphate buffer was made by mixing 0.1 M potassium hydrogen phosphate and 0.1 M potassium hydroxide.
Peptide Synthesis
α-Syn(1–19) was synthesized via solid-phase Fmoc chemistry on a Symphony Quartet peptide synthesizer (Protein Technologies, Tucson, AZ). The Fmoc groups were deprotected with 20% piperidine in dimethylformamide (V/V) after the coupling reaction had proceeded for 30 min. Upon dehydration on a freeze-drier (VirTis Benchtop K, Warminster, PA), the crude product was purified by semi-preparative reversed-phase (RP) HPLC (Shimadzu 6AD, Columbia, MO) using a column (Jupiter-10-C18-300, 10 mm i.d. × 250 mm) from Phenomenex (Torrance, CA). The eluents were 0.1% trifluoroacetic acid in water (V/V, mobile phase A) and 0.1% trifluoroacetic acid in acetonitrile (V/V, mobile phase B). At a flow rate of 4.75 mL/min, purification of α-syn(1–19) was accomplished with an elution gradient of 25–65% phase B for 12 min. The purity of the synthesized peptides was verified by HPLC and electrospray-mass (ES–MS) spectrometry.
Expression and Purification of α-Syn
Expression and purification of α-syn followed the reported procedure.(48) In brief, E. coli BL21 (DE3) were transfected with pRK172/α-synuclein plasmids kindly donated by Prof. P. Lansbury (Harvard University). After the expression was induced by IPTG, cells were harvested, resuspended in 10 mM phosphate buffer solution (pH 7.4), and lysed by adding lysozyme. Following sonication, lysate was separated from the precipitate by centrifuge and the supernatant was mixed with 1 mM DTT. The crude product was purified by RP-HPLC and the elution gradient was 25–75% B for 20 min at a flow rate of 4.75 mL/min.
Mass Spectrometric Measurements
ES–MS was conducted on a Thermal Fisher LTQ linear ion-trap mass spectrometer (San Jose, CA). CuCl2 and α-syn(1–19) peptide were dissolved in water at 1 mM and 100 µM, respectively. Aliquots of the copper solution were then added into the peptide solution to form the complex. The mixture solution were subsequently diluted with a water/methanol (V/V = 1:1) solution to a final concentration of 10 µM for the peptide. The sampler capillary was kept at 200 °C and all the mass spectra were collected in the positive ion mode. The ES–MS measurement of the copper complexes with α-syn was performed in the same manner.
Electrochemical Measurements
Voltammetric measurements of the Cu2+ complexes with the two α-syn species were carried out on a CHI832 electrochemical workstation (CH Instruments, Austin, TX) using a homemade plastic electrochemical cell with an internal volume of ~400 µL. The three-electrode system is composed of a glassy carbon disk working electrode (3 mm in diameter), a platinum wire auxiliary electrode, and a Ag/AgCl reference electrode. The electrolyte solution was a 5 mM phosphate buffer (pH 7.4) in the presence of 0.1 M Na2SO4. Conducting voltammetric experiments under oxygen-free condition was achieved by transferring the electrochemical cell and solutions into a glove box (Plas Lab, Lansing, MI) that had been thoroughly purged with and kept under high-purity N2. The oxygen concentration in the glove box was measured to be less than 0.5 ppm by a portable conductivity meter (Orion 3-Star Plus, Thermo Electro Corp., MA).
Detection of Hydrogen Peroxide
H2O2 detection kit was purchased from Bioanalytical System Inc. (West Lafayette, IN) and calibrated as described in Supporting Information.(49) H2O2 was generated by two methods in this work. For the first method, the complexes of Cu2+ formed with α-syn and with α-syn(1–19) were electrolyzed at 0.04 V vs. Ag/AgCl for predefined times. The final solutions were injected through a six-port rotary valve (Valco, Houston, TX) into a flowing stream of phosphate buffer delivered by a syringe pump (Kd Scientific, Holliston, MA) at a flow rate of 10 mL/h. The amount of H2O2 was determined by comparing the measured current to that of a calibration curve constructed with H2O2 standard solutions (Figure S1 in Supporting Information). As for the second method, α-syn-Cu2+ or α-syn(1–19)-Cu2+ was mixed with 1 mM AA in aerated solution for a predetermined time. The concentration of H2O2 generated by AA reduction of these complexes was much higher than that produced by electrochemical reduction of these complexes. Therefore, the sample mixtures containing H2O2 were diluted by 25 times before the analysis with the abovementioned H2O2 detection kit.
Cell Culture and Cytotoxicity
SH-SY5Y cells were cultured in a medium of 44.5% DMEM containing 4 mM L-glutamine/Ham’s F12/FBS/mixture of penicillin and streptomycin (V:V:V:V = 44.5%:44.5%:10%:1%). For toxicity assay, cells collected at 45% confluence and resuspended in the same DMEM/F12 media with a smaller FBS content (5%) were added into a 96-well plate and the plate was placed in a humidified incubator at 37 °C and under 5% CO2. SH-SY5Y cells were treated with α-syn-containing solutions (vide infra) for 24 h. Viability of cells exposed to each solution was determined using the 3,[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay (EMD Inc., Gibbstown, NJ). Briefly, MTT was first dissolved in water to 5 mg/mL. Media from the wells in the plate were replaced with those containing 10% MTT and the well contents were subsequently incubated for 4 h. After removal of the MTT-comprising media, 150 µL of dimethyl sulfoxide was added into each well to dissolve the formazan precipitate. UV-vis absorption at 595 nm in each well was recorded by a plate reader (Tecan, San Jose, CA). For each type of solution, MTT assays in five separate wells were conducted, and the final viability is the average from MTT assays conducted on three different days.
Atomic Force Microscopy
We used atomic force microscopy (AFM) to investigate whether aggregations had formed with α-syn or α-syn-Cu2+ during the measurements of voltammetry, H2O2 detection, and cytotoxicity assays. AFM experiments were carried out on an MFP-3D-SA microscope (Asylum Research, Santa Barbara, CA) equipped with the tapping mode in air. The AFM cantilevers were purchased from MikroMasch (San Jose, CA). Freshly peeled mica was treated with Ni2+ by immersing the substrate in 10 mM NiCl2 for 15 min, followed by thoroughly rinsing with deionized water and purging with nitrogen. Aliquots of α-syn, α-syn/Cu2+ or α-syn/Cu2+/AA mixture at a predetermined incubation time were casted onto Ni2+-treated mica sheets for 15 min. The substrate was then rinsed with water to remove any residual salt and dried with nitrogen before AFM imaging.
Results
Cu2+ binds to α-syn protein/peptide and the resultant complexes are redox active
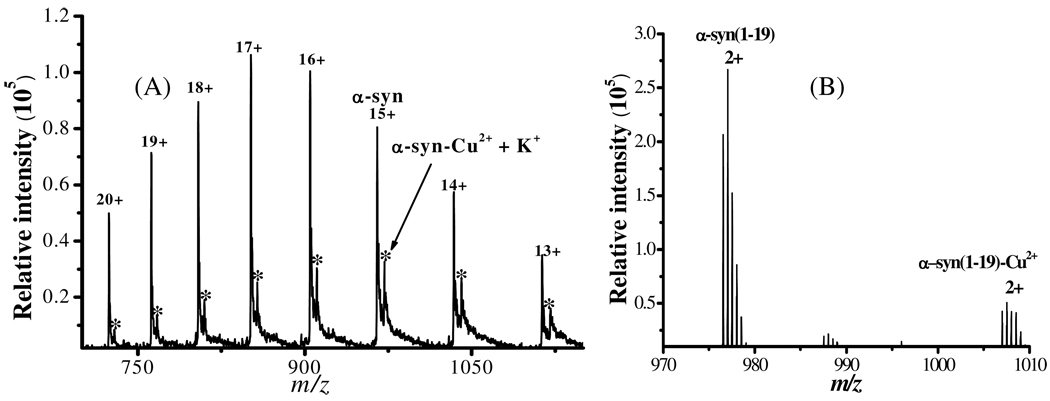
Figure 1 depicts representative electrospray mass spectra (ES–MS) collected from a solution containing Cu2+ and α-syn (panel A) and that comprising Cu2+ and the peptide segment α-syn(1–19) (panel B). The peaks in panel A correspond to α-syn and the α-syn complex with one Cu2+ center and one K+ adduct (given rise by K+ in the phosphate buffer used for the α-syn expression and preparation; peaks labeled with asterisks). No other peaks showing stoichiometric ratio higher than 1:1 between α-syn and Cu2+ were detected when more Cu2+ was mixed with the α-syn solution. This suggests that Cu2+ binds less strongly with and might have dissociated from other sites (e.g., the binding site around His-50 or the non-specific binding in the C-terminus).(30, 50, 51)
Figure 1.
Electrospray mass spectra from solutions of (A) α-syn and Cu2+, with peaks bearing 13+ to 20+ charges, and (B) α-syn(1–19) and Cu2+, with peaks corresponding to doubly charged peaks. The asterisks in (A) indicate the α-syn-Cu2+ +K+ adduct ions.
In Figure 1B, the peaks corresponding to the uncomplexed α-syn(1–19) and its 1:1 complex with Cu2+ have m/z centered around 977.0 and 1007.5, respectively. Notice that the number of charges on these peaks is much less than those on the whole α-syn protein, because the α-syn protein has much more ionizable amino acid residues. The binding stoichiometry also correlates well with a previous report showing that residues near the N-terminus can complex Cu2+ with Met-1 as the anchoring site.(31) Previously, it has been reported that residues of methionine and tyrosine can be oxidized in the presence of Cu2+.(16) Since the m/z values of the peaks in Figure 1A correspond to intact protein bound to Cu2+, we conclude that all of the amino acid residues are not chemically modified or oxidized upon the Cu2+ complexation.
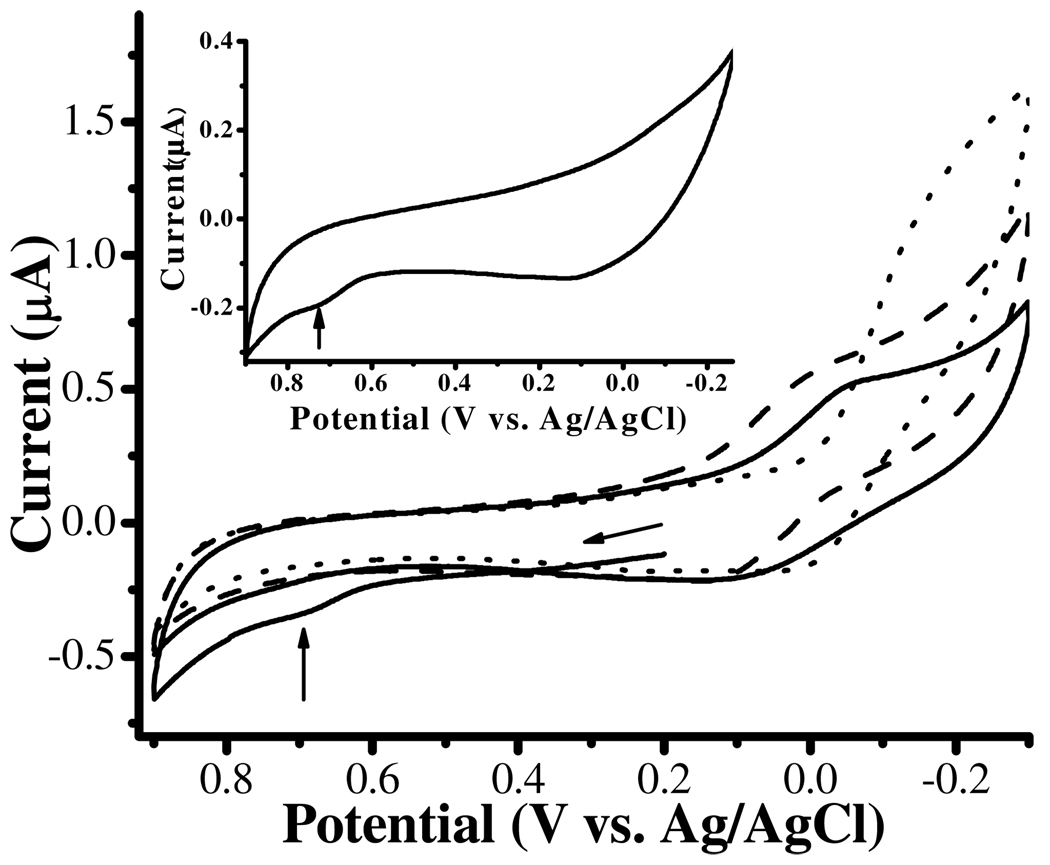
Cyclic voltammograms (CVs) of Cu2+ complexes formed with α-syn (solid line curve) and the α-syn(1–19) peptide (dashed line curve) are overlaid in Figure 2. To ensure extensive complexation, the concentrations of α-syn and α-syn(1–19) used are twice as high as that of Cu2+. The redox waves of α-syn-Cu2+ and α-syn(1–19)-Cu2+ both exhibit quasi-reversible behaviors, which are in contrast to that of the irreversible reduction peak of free Cu2+ (dotted line curve). Thus, we conclude that Cu2+ complexed by both α-syn and α-syn(1–19) can be reduced to Cu+, and the Cu+-containing complexes are stable. These characteristics are also similar to those of the complexes formed between Cu2+ and various Aβ peptides(12) or a Cu2+-histidine complex.(52) Furthermore, the redox potentials (approximated as the average of the anodic and cathodic peak potentials) are 0.018 V for α-syn-Cu2+ and 0.053 V for α-syn(1–19)-Cu2+. This implies that, similar to the Aβ-Cu2+ complex,(12) α-syn-Cu2+ should be able to facilitate the ROS generation (vide infra). Notice that the α-syn voltammogram without Cu2+ present shows a small irreversible oxidation peak at ca. 0.730 V (identified by the vertical arrow in the inset). This peak is due to the irreversible oxidation of the Tyr residue(s) at a high oxidation potential.(12) The oxidation peak of tyrosine (identified by the vertical arrow in the solid line curve) is still observable in the CV of the mixture of 100 µM α-syn and 50 µM Cu2+. This suggests that the Tyr residues in α-syn are not oxidized upon Cu2+ complexation, a point consistent with the aforementioned ES–MS results.
Figure 2.
Cyclic voltammograms of 100 µM α-syn and 50 µM Cu2+ (solid line curve), 100 µM α-syn(1–19) and 50 µM Cu2+ (dashed line curve), and 50 µM free Cu2+ (dotted line curve) in 5 mM phosphate buffer (pH 7.4) containing 0.1 M Na2SO4. The scan rate was 5 mV/s and the arrow indicates the initial scan direction. A voltammogram of 100 µM α-syn only is shown in the inset with the vertical arrow indicating the irreversible tyrosine oxidation peak.
For easy prediction of the likelihood of a redox reaction involving α-syn-Cu2+ and a redox molecule, we list the redox potentials of select cellular species(12) and α-syn-Cu2+ in Table 1. Note that the α-syn-Cu2+ complex has a redox potential higher than those of cellular species such as AA, GSH, and NADH. Therefore, thermodynamically α-syn-Cu2+ should be able to oxidize these cellular reductants. On the other hand, the redox potential of α-syn-Cu2+ is lower than that of DA, indicating that it is not possible for α-syn-Cu2+ to directly oxidize DA. To verify these predictions, we mixed α-syn-Cu2+ with AA or DA and collected differential pulse voltammograms (DPVs) to determine whether there is a change in the AA or DA concentration over a given period of time.
Table 1.
Redox potentials of the α-syn-Cu2+ and select biological redox couples
| System | E (V vs Ag/AgCl)a |
|---|---|
| Norepinephrine | 0.188 |
| Dopamine (DA) | 0.174 |
| O2/H2O2 | 0.099 |
| Cytochrome a | 0.094 |
| α-syn-Cu2+/α-syn-Cu+ | 0.018 |
| Hemoglobin | − 0.044 |
| CoQ/CoQH2 | − 0.096 |
| Ascorbic Acid (AA) | − 0.145 |
| Myoglobin | − 0.191 |
| Crotonyl-CoA/Butyryl-CoA | − 0.211 |
| FMN/FMNH2 | − 0.316 |
| Glutathione (GSH) | − 0.424 |
| Vitamin B12 | − 0.440 |
| NAD+/NADH | − 0.516 |
| FAD/FADH2 | − 0.523 |
The potential values are converted from those listed in Reference 12 in the scale of Ag/AgCl.
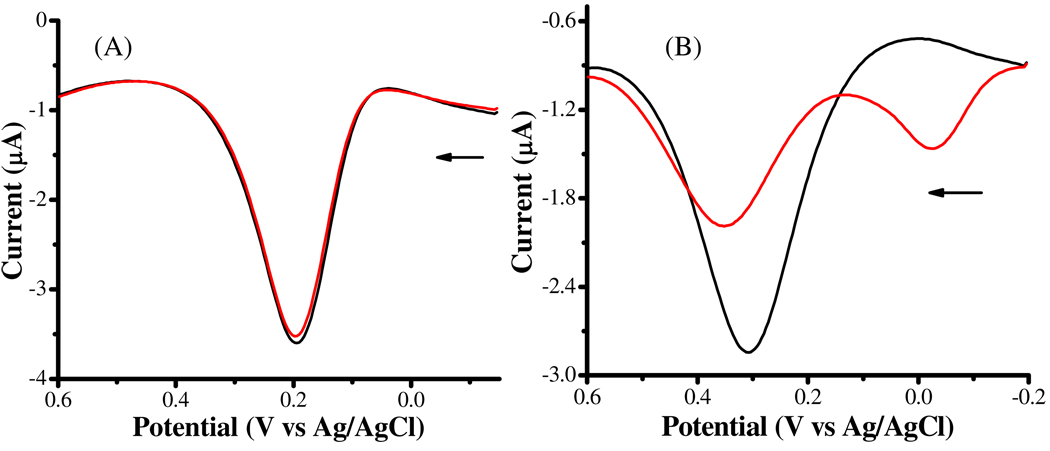
The black curve in Figure 3A is a DPV of a phosphate buffer containing only 100 µM DA. To avoid possible DA oxidation by O2 permeated into the solution, the entire electrochemical cell was again placed in a glove box under N2. Three hours after the addition of 50 µM Cu2+ and 100 µM α-syn, the height of the DA peak remained unchanged (red curve in Figure 3A). Thus, it is apparent that α-syn-Cu2+ does not directly oxidize DA. Interestingly, the presence of α-syn and Cu2+ significantly decreased the AA oxidation peak (Figure 3B) after 3 h. The red curve in Figure 3B has a peak height of only ~55% of that of the black curve, which corresponds to the DPV of AA in a solution that did not contain α-syn-Cu2+. Also, an oxidation peak emerged at ca. −0.06 V, which can be ascribed to the electrochemical oxidation of α-syn-Cu+ complex that had been generated in the presence of AA. The decrease in the peak height thus verifies that α-syn-Cu2+ can directly oxidize AA.(53)
Figure 3.
Differential pulse voltammograms of 100 µM dopamine (A) and 100 µM ascorbic acid (B) dissolved in deaerated phosphate buffer (pH 7.4) in the absence (black) and presence of 100 µM α-syn and 50 µM Cu2+ (red). The arrow indicates the initial scan direction.
Because the analyte concentration can affect its DPV peak potential for an irreversible redox reaction,(55) the anodic shift in Figure 3B after the addition of α-syn-Cu2+ can be attributed to the decrease in the AA concentration. We should point out that the peak potential corresponding to the DPV oxidation peak of AA (0.281 V in Figure 3B) is much more positive than the standard redox potential of AA (–0.145 V; see also Table 1).(54) The reason of this deviation is attributable to the fast irreversible chemical reaction that converts the AA radical cation to dehydroascorbic acid, which shifts the redox peak in the anodic direction.(55)
Generation of H2O2 facilitated by the Cu2+ complex of α-syn
Another prediction that can be made from the data in Table 1 is that O2 in solution should be able to oxidize the reduced form of α-syn-Cu2+ (i.e., α-syn-Cu+). As mentioned in the Introduction, this reaction is analogous to that occurring between O2 and Aβ-Cu+ (cf. eq. 2 given in the Introduction):(12)
| (3) |
In the presence of a cellular reductant (e.g., AA), α-syn-Cu2+ generated from the above reaction can be reduced, regenerating α-syn-Cu+
| (4) |
Reactions 3 and 4 set up a catalytic cycle in which α-syn-Cu2+ acts like a “catalyst” to continuously facilitate the O2 reduction to H2O2 and depletion of cellular reductant(s). Indeed, the redox behavior of α-syn-Cu2+ was found to be affected by O2 (cf. Figure S2 in Supporting Information), which indicates the feasibility of reaction 3. To verify the formation of H2O2 from the O2 reduction by α-syn-Cu+ (shown in reaction 3), we performed electrolyses of the α-syn-Cu2+ in an aerated solution for different times, because H2O2 generation in reaction 3 requires the Cu2+ center to be reduced to Cu+. H2O2 in these solutions were then quantified by the method described in Supporting Information (cf. Figure S1 and the detection scheme). As shown in Figure 4A, the amount of H2O2 increases with the electrolysis time. In addition, no H2O2 was detected if the α-syn-Cu2+ complex were not reduced. We also conducted a control experiment by holding the electrode potential at 0.04 V in a Cu2+-only solution, which generated little H2O2 (less than 1 nM). This is conceivable since free Cu+ is not stable in an aqueous solution.
Scheme 1.
The sequence of α-synuclein with the N-terminus underlined and the C-terminus expressed in Italics.
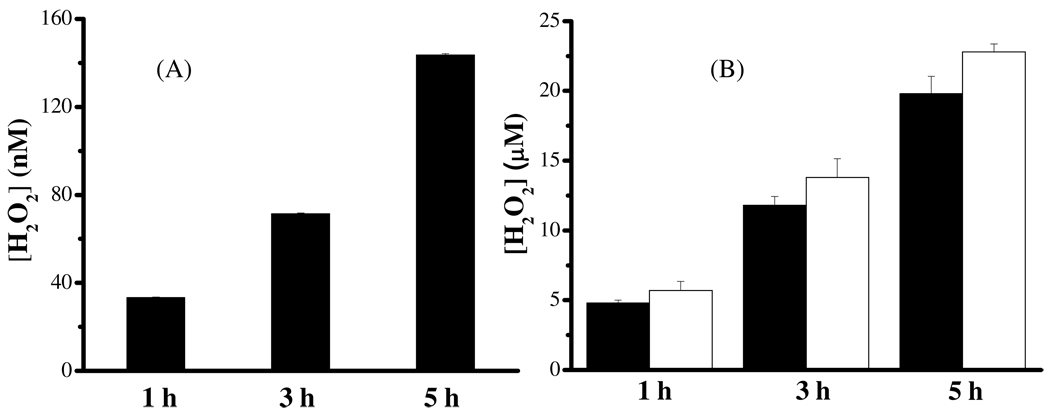
Figure 4.
Concentrations of H2O2 generated from solutions of: (A) 100 µM α-syn and 50 µM Cu2+ after electrolyses at 0.04 V and (B) 100 µM α-syn and 50 µM Cu2+ (black bars) or 100 µM α-syn(1–19) and 50 µM Cu2+ (white bars) mixed with 1 mM AA. Both of the measurements were made in ambient atmosphere for different period (1, 3, and 5 h) and the error bars correspond to relative standard deviation values of three replicates.
As illustrated in Figure 3B, AA can reduce α-syn-Cu2+ to α-syn-Cu+. We also determined the H2O2 content in an air-saturated solution containing α-syn-Cu2+ and AA at different reaction times. The results are shown in Figure 4B. Because free Cu2+ can also participate in the AA oxidation reaction,(56) the concentration of α-syn used was twice as high as that of Cu2+ so that little free Cu2+ remained in solution. Again it can be seen that the longer the reaction time, the greater the amount of H2O2 produced. A control experiment was also carried out by mixing 1 mM AA with 0.2 µM free Cu2+ (the equilibrium Cu2+ concentration predicted from the micromolar binding affinity constant(30) for a mixture of 100 µM α-syn and 50 µM Cu2+). Little H2O2 (less than 0.1 µM) was detected even after 5 h, suggesting that free Cu2+ does not significantly generate H2O2 when excess AA is present. We also determined H2O2 produced by an air-saturated mixture containing α-syn-(1–19)-Cu2+ and 1 mM AA (white bars in Figure 4B) and contrasted the results to those from the α-syn-Cu2+ solution. Because both α-syn-Cu+ and α-syn(1–19)-Cu+ complexes, instead of the α-syn species alone, are capable of producing H2O2, the binding of Cu2+ must occur within residues 1–19. This point is in line with the discussion of Figure 1. That a slightly higher (~15–19%) H2O2 amount was generated in the α-syn-(1–19)-Cu2+ solution can be rationalized by the fact that the Cu2+ center in the shorter and more hydrophilic α-syn-(1–19)-Cu2+ complex is more exposed.
Cytotoxicity of α-syn-Cu2+
The data presented in Figures 3 and 4 clearly indicate that α-syn-Cu2+ can be reduced by AA and its reduced form α-syn-Cu+ can be reoxidized by O2, producing H2O2 as a ROS. Because both AA and O2 are abundant in brain,(57) it is intriguing to know whether the amount of H2O2 formed is significant to cause neuronal cell death. To this end, we conducted cytotoxicity of α-syn-Cu2+ under various experimental conditions.
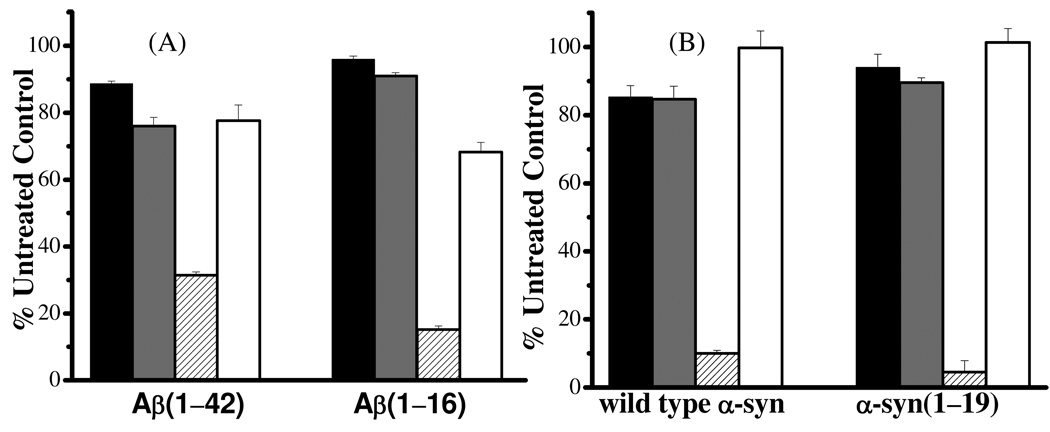
Previously, it has been shown that Aβ-Cu2+ in the presence of AA and O2 can result in the loss of PC12 cells,(16) presumably due to the oxidative damages imposed by H2O2 generated in the process shown by reactions 1 and 2. Since SH-SY5Y neuroblastoma cells are more commonly used in research concerning the neuropathology in PD, we first investigated whether H2O2 produced from the O2 reduction reaction that is “catalyzed” by Aβ-Cu2+ can similarly affect the viability of the SH-SY5Y cells. As shown in Figure 5A, Aβ(1–42) does not oppose apparent toxicity towards the SH-SY5Y cells, but its toxic effect on the SH-SY5Y cells is slightly greater in the presence of Cu2+. Interestingly, further addition of 1 mM AA substantially aggravates the cell viability, which decreased by 2.5 times with respect to a cell medium containing Aβ(1–42) and Cu2+. In a separate control experiment (data not shown), we found that 1 mM AA is not pernicious to the SH-SY5Y cells. Interestingly, when catalase, an enzyme that can decompose H2O2 with an extremely high efficiency,(58) was added, the cell viability dramatically improved (recovered back to ~80%). Bush and coworkers have also used catalase to scavenge H2O2 to enhance PC12 cell viability.(16) Thus, we have shown that H2O2 can inflict similar oxidative stress to the SH-SY5Y cells. To exclude the possibility that the toxicity might be originated from the Cu2+-induced Aβ(1–42) aggregates,(59) we also conducted a parallel experiment in which Aβ(1–16), which encompasses the metal-binding domain but not the aggregation-prone segment of Aβ(1–42). As shown in Figure 5A, a trend in the cell viability similar to that involving Aβ(1–42) was observed.
Figure 5.
Cytotoxicities of (A) Aβ-Cu2+ and (B) α-syn-Cu2+ in the following solutions: peptide/protein only (black bars), peptide/protein together with Cu2+ (gray bars), peptide/protein together with Cu2+ and 1 mM AA (patterned bars), and peptide/protein together with Cu2+, 1 mM AA, and 1000 units/mL catalase (white bars). For Aβ(1–42), the concentrations of Aβ(1–42) was kept at 10 µM to avoid significant aggregate formation. The concentrations of all other peptides/protein were 50 µM and the concentration of Cu2+ was half of those of the peptide/protein solutions.
As for the cytotoxicity of the α-syn-Cu2+ and α-syn(1–19)-Cu2+ complexes (Figure 5B), results remarkably analogous to those shown in Figure 5A were obtained. Again, the Cu2+ complexes with α-syn or α-syn(1–19) are not regarded to be toxic, unless a high concentration of a cellular reductant (AA in this case) is present. The fact that catalase largely abolishes the toxicity effects confirms our observation that H2O2 is a major product. Similar to the trend exhibited by Aβ(1–42) and Aβ(1–16), that α-syn(1–19) affects the cell viability slightly more than α-syn suggests that the former is capable of producing more H2O2, a point in line with the data shown in Figure 4B. The trend also suggests that aggregates of α-syn either did not form during the timeframe of the cell viability assay (24 h) or did not impose discernable toxic effects. In fact, we used AFM to identify possible aggregates at various times throughout the assay and did not find any forms of α-syn aggregates. This is not entirely surprising since it has been reported that α-syn aggregation is not significant in the first 24 h.(29, 60)
Discussion
The ES–MS (Figure 1) and electrochemical results (Figure 2) clearly indicate that Cu2+ can bind to α-syn and α-syn(1–19). The quasi-reversibility of the redox waves of the α-syn-Cu2+ complex suggests that cycling between Cu2+ and Cu+ in the complex is relatively facile. With the redox potential of the α-syn-Cu2+/α-syn-Cu+ couple measured, the likelihood of redox reactions between the α-syn-Cu2+/α-syn-Cu+ couple and cellular species (including amino acid residues on proteins) can be assessed. We demonstrated that AA, but not DA, can be directly oxidized by α-syn-Cu2+ (see Figure 3). Based on the fact that the oxidation potential of α-syn-Cu2+ is also more positive than the redox potential of the GSH/GSSG couple (−0.424 V),(61) it is likely that α-syn-Cu2+ can oxidize GSH to GSSG. It has been reported that the level of GSH in the substantia nigra of PD patients is lower than that in control tissues.(41) The declined level of GSH is strong evidence for the oxidative stress hypothesis.(3) Therefore, our data suggest that the α-syn-Cu2+ complex might be at least partially responsible for the depletion of GSH.
A physiologically relevant redox reaction closely examined in this work is the α-syn-Cu2+-triggered H2O2 formation. The redox potential we measured has accurately projected that, upon the α-syn-Cu2+ reduction, O2 can be reduced to H2O2 by α-syn-Cu+. As shown in Figure 4B, after 5 h of reaction, the concentration of H2O2 reached as high as ~20 µM. Due to the high concentrations of AA (10 mM),(57) GSH (2.5 mM),(57) O2 (20 µM)(62) and other cellular species (cf. Table1) in brain, the amount of H2O2 produced in vivo can be significant. Since α-syn-Cu2+ behaves as an enzyme for the catalytic reduction of O2 and is not consumed in the catalytic cycle depicted in reactions 3 and 4, the H2O2 production would not cease until O2 and cellular reductants have been completely exhausted. In vivo, in the presence of H2O2, many other aberrant processes can take place. As mentioned in the Introduction, H2O2 can participate in the Fenton reaction with free Fe2+ in the labile iron pool(63) within the neuronal cytosol to generate hydroxyl radical, a more reactive and potent ROS:
| (5) |
In cellular milieu, any rogue Cu2+ that is not readily complexed by copper-binding proteins (including α-syn), will also react with H2O2. The following Harber-Weiss reaction involving Cu2+ and H2O2 can also produce hydroxyl radicals:
| (6) |
| (7) |
| (8) |
The hydroxyl radical generation may explain the DA lesion and extensive protein oxidation in PD brains.(64) As described in the Results section, DA cannot be directly oxidized by α-syn-Cu2+. However, it has been shown that H2O2 is able to oxidize DA.(65) Similarly, the fact that the oxidation potential of α-syn-Cu2+ is lower than those of tyrosine (0.78 V vs AgCl/Ag)(12) and methionine (1.30 V vs AgCl/Ag)(12) indicates that complexation of Cu2+ by α-syn should not result in oxidation of these residues. This is consistent with the fact that these residues in the α-syn-Cu2+ complex have remained intact (see the mass spectra in Figure 1A). More importantly, H2O2 or OH• have been reported to result in oxidation of methionine residues in α-syn-Cu2+ to sulfoxide and sulfone groups.(66) Tyrosine residues are more susceptible to oxidation than methionine,(12) forming the quionone analogs (loss of protons).(67) Although not examined here in detail, we do not rule out the possibility that the production of ROS facilitated by the α-syn-Cu2+ complex (i.e., H2O2 and OH•), may ultimately lead to the oxidation of methionine and tyrosine residues of α-syn. Moreover, we did not examine the role of any uncomplexed Cu2+ in producing hydroxyl radicals in the presence of AA or other cellular redox species.(22–24)
It has been widely reported that α-syn aggregates, especially in the oligomeric forms, are highly neurotoxic.(68–70) The Cu2+-containing α-syn aggregates further exacerbate the cytotoxicity.(70) The role of the α-syn-Cu2+ complex in eliciting neuronal cell death (by depleting essential cellular species and/or generation of ROS) was implicated (cf. the cytotoxicity results shown in Figure 5B). Since no aggregation of α-syn was detected during our cytotoxcity studies, the oxidative stress/damage associated with α-syn-Cu2+ could have occurred long before aggregates of α-syn had been extensively produced. We should caution that our results do not exclude the possible toxic effects concertedly exerted by oxidative stress of the α-syn-Cu2+ complex and any damaging processes that are inherent in the α-syn aggregates. For instance, a hypothesis that has received wide acceptance is the pore formation within cell membrane that is induced by α-syn oligomers.(68–70) In such a process, to our knowledge, redox reactions of α-syn have not been invoked for the interpretation of the experimental results.
Finally, as revealed by data shown in Figure 4B, under the same experimental condition, the amount of H2O2 generation facilitated by α-syn(1–19)-Cu+ is more than that by α-syn-Cu+. Previously we found that the rate of H2O2 generation by the Cu+ complexes with different Aβ peptides also decreases with the peptide length.(12) If the accessibility of the Cu2+ center is crucial to the rate of the H2O2 generation or other redox reactions, it is plausible to suggest that the rate at which the various Cu2+-containing α-syn aggregates generate H2O2 should be slower than the Cu2+ complex formed with the monomeric α-syn. On the basis that the hydrophilic and/or metal binding domains of other amyloidogenic proteins/peptides are emanated from the hydrophobic cores (e.g., fibrils and bundles of fibrils,(71)) it is possible that the Cu2+-binding N-termini in the α-syn aggregates are also exposed to solution, albeit the amount of H2O2 produced might be lower. However, higher-ordered α-syn aggregates (e.g., insoluble oligomers, protofibrils, and fibrils) that also contain Cu2+ are not diffusible, and consequently the ROS accumulation over a period of time in a localized region can be substantial, rendering more severe damages to cell membranes and organelles.
Conclusion
The Cu2+ complexes with α-synuclein or an N-terminus peptide (i.e., α-syn(1–19)) was detected by ES–MS and the redox potentials of the two copper complexes have been measured electrochemically. The Cu2+ center(s) can be reduced to Cu+ readily and are well accessible to solution species. The occurrence of a particular redox reaction that might involve α-syn-Cu2+ can be predicted by comparing the redox potentials between the complex and other redox reactants. Because the redox potential of α-syn-Cu2+/α-syn-Cu+ is higher than that of AA/dehydroascorbic acid but lower than that of O2/H2O2, α-syn-Cu2+ was verified to facilitate the H2O2 generation in the presence of AA. On the other hand, the redox potential of DA is higher than that of α-syn-Cu2+/α-syn-Cu+, indicating that DA cannot be directly oxidized by α-syn-Cu2+. However, H2O2 and other ROS (e.g., hydroxyl radicals produced from Fenton reaction) can oxidize DA. We also demonstrate that the resultant H2O2 can cause neuronal cells death and the scavenging of it by catalase can retain the cell viability. These observations might provide new insight into the gradual loss of dopaminergic cells in PD brain. Taken together, our results about the depletion of oxidizable redox molecules (e.g., GSH DA, and AA), the reduction of O2 to H2O2 by α-syn-Cu+, and the cytotoxicity of the generated H2O2 have helped link the redox properties of the α-syn-Cu2+ complex to previous in vivo observations that are symptomatic of oxidative stress/damage in PD etiology.
Supplementary Material
Acknowledgement
We thank Renee Williams and Prof. Yinsheng Wang (University of California-Riverside) for their help on the ES–MS measurements and Shengmu Xiao and Prof. Howard Xu (CSULA) for their assistance on the α-synuclein expression/purification.
This work is supported by an NINDS grant (No. SC1NS070155-01) and the NIH-RIMI Program at California State University, Los Angeles (P20-MD001824-01 to FZ).
Abbreviations
- α-syn
α-synuclein
- PD
Parkinson’s disease
- ES–MS
electrospray-mass spectroscopy
- α-syn-Cu2+
Cu2+ complex of α-syn
- α-syn(1–19)-Cu2+
Cu2+ complex of α-syn(1–19)
- AD
Alzheimer’s disease
- ROS
reactive oxygen species
- Aβ
β-amyloid
- AA
ascorbic acid
- DA
dopamine
- CV
cyclic voltammogram
- DPV
differential pulse voltammogram
- LBs
Lewy bodies
- NAC
nonamyloid components
- GSH
glutathione
- IPTG
isopropyl β-D-thiogalactopyranoside
- FBS
fetal bovine serum
- DMEM
Dulbecco’s Modified Eagle Medium
- DTT
dithiolthreitol
- RP
reverse phase
- AFM
atomic force microscopy
- MTT
3, [4, 5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
Footnotes
Supporting Information Available
Additional experimental details about the H2O2 detection, and electrochemical and AFM studies of the complexes are described in the Supporting Information. These material are available free of charge via the internet at http://pubs.acs.org.
References
- 1.Gaggelli E, Kozlowski H, Valensin D, Valensin G. Copper homeostasis and neurodegenerative disorders (Alzheimer's, prion, and Parkinson's diseases and amyotrophic lateral sclerosis) Chem. Rev. 2006;106:1995–2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- 2.Aronoff-Spencer E, Burns CS, Avdievich NI, Gerfen GJ, Peisach J, Antholine WE, Ball HL, Cohen FE, Prusiner SB, Millhauser GL. Identification of the Cu2+ binding sites in the N-terminal domain of the prion protein by EPR and CD spectroscopy. Biochemistry. 2000;39:13760–13771. doi: 10.1021/bi001472t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenner P. Oxidative stress in Parkinson's disease. Ann. Neurol. 2003;53:S26–S36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 4.Varadarajan S, Yatin S, Aksenova M, Butterfield DA. Review: Alzheimer's amyloid β-peptide-associated free radical oxidative stress and neurotoxicity. J. Struct. Biol. 2000;130:184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- 5.Sigel A, Sigel H, Sigel RKO. Metal Ions in Life Sciences. West Sussex: John Wiley & Sons, Ltd; 2006. [Google Scholar]
- 6.Jakob-Roetne R, Jacobsen H. Alzheimer's disease: From pathology to therapeutic approaches. Angew. Chem. Int. Edit. 2009;48:3030–3059. doi: 10.1002/anie.200802808. [DOI] [PubMed] [Google Scholar]
- 7.Schrock DS, Baur JE. Chemical imaging with voltammetry-scanning microscopy. Anal. Chem. 2007;79:7053–7061. doi: 10.1021/ac071155t. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B. Reactive oxygen species and the central-nervous-system. J. Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 9.Crouch PJ, Barnham KJ, Duce JA, Blake RE, Masters CL, Trounce IA. Copper-dependent inhibition of cytochrome c oxidase by Aβ(1–42) requires reduced methionine at residue 35 of the Aβ peptide. J. Neurochem. 2006;99:226–236. doi: 10.1111/j.1471-4159.2006.04050.x. [DOI] [PubMed] [Google Scholar]
- 10.Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li Q, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, Masters CL, Trounce IA. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-β(1–42) J. Neurosci. 2005;25:672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu M, Qin Z, Hu D, Munishkina LA, Fink AL. α-synuclein can function as an antioxidant preventing oxidation of unsaturated lipid in vesicles. Biochemistry. 2006;45:8135–8142. doi: 10.1021/bi052584t. [DOI] [PubMed] [Google Scholar]
- 12.Jiang D, Men L, Wang J, Zhang Y, Chickenyen S, Wang Y, Zhou F. Redox reactions of copper complexes formed with different β-amyloid peptides and their neuropathalogical relevance. Biochemistry. 2007;46:9270–9282. doi: 10.1021/bi700508n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer's disease senile plaques. J. Neurol. Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 14.Karr JW, Kaupp LJ, Szalai VA. Amyloid-β binds Cu2+ in a mononuclear metal ion binding site. J. Am. Chem. Soc. 2004;126:13534–13538. doi: 10.1021/ja0488028. [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD, Tanzi RE, Bush AI. The Aβ peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38:7609–7614. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Cuajungco MP, Atwood CS, Hartshorn MA, Tyndall JDA, Hanson GR, Stokes KC, Leopold M, Multhaup G, Goldstein LE, Scarpa RC, Saunders AJ, Lim J, Moir RD, Glabe C, Bowden EF, Masters CL, Fairlie DP, Tanzi RE, Bush AI. Cu(II) potentiation of Alzheimer Aβ neurotoxicity - Correlation with cell-free hydrogen peroxide production and metal reduction. J. Biol. Chem. 1999;274:37111–37116. doi: 10.1074/jbc.274.52.37111. [DOI] [PubMed] [Google Scholar]
- 17.Hewitt N, Rauk A. Mechanism of hydrogen peroxide production by copper-bound amyloid beta peptide: A theoretical study. J. Phys. Chem. B. 2009;113:1202–1209. doi: 10.1021/jp807327a. [DOI] [PubMed] [Google Scholar]
- 18.Barb WG, Baxendale JH, George P, Hargrave KR. Reactions of ferrous and ferric ions with hydrogen peroxide. Part I–The ferrous ion reaction. Trans. Faraday Soc. 1951;47:462–500. [Google Scholar]
- 19.Jiang D, Li X, Liu L, Yagnik GB, Zhou F. Reaction rates and mechanism of the ascorbic acid oxidation by molecular oxygen facilitated by Cu(II)-containing amyloid-β complexes and aggregates. J. Phys. Chem. B. 2010;114:4896–4903. doi: 10.1021/jp9095375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith MA, Harris PLR, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. U. S. A. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong J, Atwood CS, Anderson VE, Siedlak SL, Smith MA, Perry G, Carey PR. Metal binding and oxidation of amyloid-β within isolated senile plaque cores: Raman microscopic evidence. Biochemistry. 2003;42:2768–2773. doi: 10.1021/bi0272151. [DOI] [PubMed] [Google Scholar]
- 22.Baruch-Suchodolsky R, Fischer B. Soluble amyloid β(1–28)–copper(I)/copper(II)/iron(II) complexes are potent antioxidants in cell-free system. Biochemistry. 2008;47:7796–7806. doi: 10.1021/bi800114g. [DOI] [PubMed] [Google Scholar]
- 23.Baruch-Suchodolsky R, Fischer B. Aβ1–40, soluble or aggregated, is a remarkably potent antioxidant in cell-free oxidative systems. Biochemistry. 2009;49:4354–4370. doi: 10.1021/bi802361k. [DOI] [PubMed] [Google Scholar]
- 24.Guilloreau L, Combalbert S, Sournia-Saquet A, Mazarguil H, Faller P. Rodox chemistry of copper-amyloid-β: the generation of hydroxyl radical in the presence of ascorbate is linked to redox-potentials and aggregation state. ChemBioChem. 2007;8:1317–1325. doi: 10.1002/cbic.200700111. [DOI] [PubMed] [Google Scholar]
- 25.Gibb WRG, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J. Neurol. Neurosurg. Psych. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forno LS. Neuropathology of Parkinson's disease. J. Neuropathol. Exp. Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. α-synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. α-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 29.Fink AL. The aggregation and fibrillation of α-synuclein. Acc. Chem. Res. 2006;39:628–634. doi: 10.1021/ar050073t. [DOI] [PubMed] [Google Scholar]
- 30.Binolfi A, Lamberto GR, Duran R, Quintanar L, Bertoncini CW, Souza JM, Cervenansky C, Zweckstetter M, Griesinger C, Fernandez CO. Site-specific interactions of Cu(II) with alpha and β-synuclein: Bridging the molecular gap between metal binding and aggregation. J. Am. Chem. Soc. 2008;130:11801–11812. doi: 10.1021/ja803494v. [DOI] [PubMed] [Google Scholar]
- 31.Jackson MS, Lee JC. Identification of the minimal copper(II)-binding α-synuclein sequence. Inorg. Chem. 2009;48:9303–9307. doi: 10.1021/ic901157w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim KS, Choi SY, Kwon HY, Woo MH, Kang TC, Kang JH. Aggregation of α-synuclein induced by the Cu,Zn-superoxide dismutase and hydrogen peroxide system. Free Radical Biol. Med. 2002;32:544–540. doi: 10.1016/s0891-5849(02)00741-4. [DOI] [PubMed] [Google Scholar]
- 33.Ceballos I, Lafon M, Javoyagid F, Hirsch E, Nicole A, Sinet PM, Agid Y. Superoxide dismutase and Parkinson's disease. Lancet. 1990;335:1035–1036. doi: 10.1016/0140-6736(90)91099-v. [DOI] [PubMed] [Google Scholar]
- 34.Dexter DT, Carayon A, Javoyagid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Alterations in the levels of iron, ferritin and other trace-metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114:1953–1975. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- 35.Pall HS, Blake DR, Gutteridge JM, Williams AC, Lunec J, Hall M, Taylor A. Raised cerebrospinal-fluid copper concentration in Parkinson's disease. Lancet. 1987;2:238–241. doi: 10.1016/s0140-6736(87)90827-0. [DOI] [PubMed] [Google Scholar]
- 36.Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MBH. Transition-metals, ferritin, glutathione, and ascorbic-acid in Parkinsonian brains. J. Neurochem. 1989;52:515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 37.Brown DR. Metal binding to alpha-synuclein peptides and its contribution to toxicity. Biochem. Biophys. Res. Commun. 2009;380:377–381. doi: 10.1016/j.bbrc.2009.01.103. [DOI] [PubMed] [Google Scholar]
- 38.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the innocent bystanders. Ann. Rev. Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 39.Paik SR, Shin HJ, Lee JH, Chang CS, Kim J. Copper(II)-induced self-oligomerization of α-synuclein. Biochemical J. 1999;340:821–828. [PMC free article] [PubMed] [Google Scholar]
- 40.Palecek E, Ostatna V, Masarik M, Bertoncini CW, Jovin TM. Changes in interfacial properties of alpha-synuclein preceding its aggregation. Analyst. 2008;133:76–84. doi: 10.1039/b712812f. [DOI] [PubMed] [Google Scholar]
- 41.Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoyagid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 42.Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J. Neurochem. 1997;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 43.Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B. Oxidative DNA damage in the parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- 44.Golts N, Snyder H, Frasier M, Theisler C, Choi P, Wolozin B. Magnesium inhibits spontaneous and iron-induced aggregation of α-synuclein. J. Biol. Chem. 2002;277:16116–16123. doi: 10.1074/jbc.M107866200. [DOI] [PubMed] [Google Scholar]
- 45.Peng Y, Wang C, Xu H, Liu Y, Zhou F. Binding of α-synuclein with Fe3+ and with Fe2+ and biological implications of the resultant complexes. J. Inorg. Biochem. 2010;104:365–370. doi: 10.1016/j.jinorgbio.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sulzer D, Bogulavsky J, Larsen KE, Behr G, Karatekin E, Kleinman MH, Turro N, Krantz D, Edwards RH, Greene LA, Zecca L. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11869–11874. doi: 10.1073/pnas.97.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dexter DT, Sian J, Rose S, Hindmarsh JG, Mann VM, Cooper JM, Wells FR, Daniel SE, Lees AJ, Schapira AHV, Jenner P, Marsen CD. Indexes of oxidative stress and Mitochondrial function in individuals with incidental Lewy body disease. Ann. Neurol. 1994;35:38–44. doi: 10.1002/ana.410350107. [DOI] [PubMed] [Google Scholar]
- 48.Ding T, Lee SJ, Rochet JC, Lansbury PT. Annular α-synuclein protofibrils are produced when spherical protofibrils are incubated in solution or bound to brain-derived membranes. Biochemistry. 2002;41:10209–10217. doi: 10.1021/bi020139h. [DOI] [PubMed] [Google Scholar]
- 49. [Accessed July 5th., 2010]; http://www.basinc.com/mans/PE-man.pdf.
- 50.Binolfi A, Rasia RM, Bertoncini CW, Ceolin M, Zweckstetter M, Griesinger C, Jovin TM, Fernandez CO. Interaction of α-synuclein with divalent metal ions reveals key differences: A link between structure, binding specificity and fibrillation enhancement. J. Am. Chem. Soc. 2006;128:9893–9901. doi: 10.1021/ja0618649. [DOI] [PubMed] [Google Scholar]
- 51.Rasia RM, Bertoncini CW, Marsh D, Hoyer W, Cherny D, Zweckstetter M, Griesinger C, Jovin TM, Fernandez CO. Structural characterization of copper(II) binding to α-synuclein: Insights into the bioinorganic chemistry of Parkinson's disease. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4294–4299. doi: 10.1073/pnas.0407881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weng Y, Fan FRF, Bard AJ. Combinatorial biomimetics. Optimization of a composition of copper(II) poly-L-histidine complex as an electrocatalyst for O2 reduction by scanning electrochemical microscopy. J. Am. Chem. Soc. 2005;127:17576–17577. doi: 10.1021/ja054812c. [DOI] [PubMed] [Google Scholar]
- 53.Scarpa M, Vianello F, Signor L, Zennaro L, Rigo A. Ascorbate oxidation catalyzed by bis(histidine)copper(II) Inorg. Chem. 1996;35:5201–5206. [Google Scholar]
- 54.Conway BE. Electrochemical Data. New York: Greenwood Press; 1969. [Google Scholar]
- 55.Bard AJ, Faulkner LR. Electrochemical methods. Fundamentals and applications. New York: John Wiley & Sons; 2001. [Google Scholar]
- 56.Guzman Barron ES, Demeio RH, Klemperer F. Studies on biological oxidations V. copper and hemochromogens as catalysts for the oxidation of ascorbic acid. The mechanism of the oxidation. J. Biol. Chem. 1936;112:925–940. [Google Scholar]
- 57.Rice ME, RussoMenna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neurosci. 1998;82:1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- 58.Greenwald RA. Superoxide-dismutase and catalase as therapeutic agents for human diseases, a critical review. Free Radical Biol. Med. 1990;8:201–209. doi: 10.1016/0891-5849(90)90092-w. [DOI] [PubMed] [Google Scholar]
- 59.Atwooda CS, Obrenovicha ME, Liu T, Chan H, Perry G, Smith MA, Martins RN. Amyloid-β: a chameleon walking in two worlds: a review of the trophic and toxic properties of amyloid-β. Brain Res. Rev. 2003;43:1–16. doi: 10.1016/s0165-0173(03)00174-7. [DOI] [PubMed] [Google Scholar]
- 60.Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human α-synuclein: A possible molecular link between Parkinson's disease and heavy metal exposure. J. Biol. Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- 61.Dryhurst G, Kadish KM, Scheller F, Renneberg R. Biological Electrochemistry. Vol. 1. New York and London: Academic Press; 1982. [Google Scholar]
- 62.Rolett EL, Azzawi A, Liu KJ, Yongbi MN, Swartz HM, Dunn JF. Critical oxygen tension in rat brain: a combined P-31-NMR and EPR oximetry study. Am. J. Physiol-Reg. I. 2000;279:9–16. doi: 10.1152/ajpregu.2000.279.1.R9. [DOI] [PubMed] [Google Scholar]
- 63.Crichton R. Iron Metabolism from Molecular Mechanisms to Clinical Consequences. 3rd ed. Vol. John Wiley & Sons Ltd.: West Sussex; 2009. pp. 224–226. [Google Scholar]
- 64.Tohgi H, Abe T, Takahashi S, Takahashi J, Hamato H. Alterations in the concentration of serotonergic and dopaminergic substances in the cerebrospinal-fluid of patients with Parkinson's disease, and their changes after L-dopa administration. Neurosci. Lett. 1993;159:135–138. doi: 10.1016/0304-3940(93)90817-5. [DOI] [PubMed] [Google Scholar]
- 65.Kawashima T, Ohkubo K, Fukuzumi S. Radical scavenging reactivity of catecholamine neurotransmitters and the inhibition effect for DNA cleavage. J. Phys. Chem. B. 2010;114:675–680. doi: 10.1021/jp909314t. [DOI] [PubMed] [Google Scholar]
- 66.Varadarajan S, Kanski J, Aksenova M, Lauderback C, Butterfield DA. Different mechanisms of oxidative stress and neurotoxicity for Alzheimer's Aβ(1–42) and Aβ(25–35) J. Am. Chem. Soc. 2001;123:5625–5631. doi: 10.1021/ja010452r. [DOI] [PubMed] [Google Scholar]
- 67.Sanaullah, Wilson S, Glass RS. The effect of pH and complexation of amino acid functionality on the redox chemistry of methionine and X-ray structure of [Co(en)2(L-Met)](ClO4)2.H2O. J. Inorg. Biochem. 1994;55:87–99. doi: 10.1016/0162-0134(94)85031-3. [DOI] [PubMed] [Google Scholar]
- 68.Kostka M, Hogen T, Danzer KM, Levin J, Habeck M, Wirth A, Wagner R, Glabe CG, Finger S, Heinzelmann U, Garidel P, Duan W, Ross CA, Kretzschmar H, Giese A. Single particle characterization of iron-induced pore-forming α-synuclein oligomers. J. Biol. Chem. 2008;283:10992–11003. doi: 10.1074/jbc.M709634200. [DOI] [PubMed] [Google Scholar]
- 69.Danzer KM, Haasen D, Karow AR, Moussaud S, Jakes R, Giese A, Kretzschmar H, Hengerer B, Kostka M. Different species of α-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 2007;27:9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wright JA, Wang X, Brown DR. Unique copper-induced oligomers mediate alpha-synuclein toxicity. FASEB J. 2009;23:2384–2393. doi: 10.1096/fj.09-130039. [DOI] [PubMed] [Google Scholar]
- 71.Kheterpal I, Williams A, Murphy C, Bledsoe B, Wetzel R. Structural features of the Aβ amyloid fibril elucidated by limited proteolysis. Biochemistry. 2001;40:11757–11767. doi: 10.1021/bi010805z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.